Canine Mammary Neoplasia Induces Variations in the Peripheral Blood Levels of CD20, CD45RA, and CD99
Abstract
1. Introduction
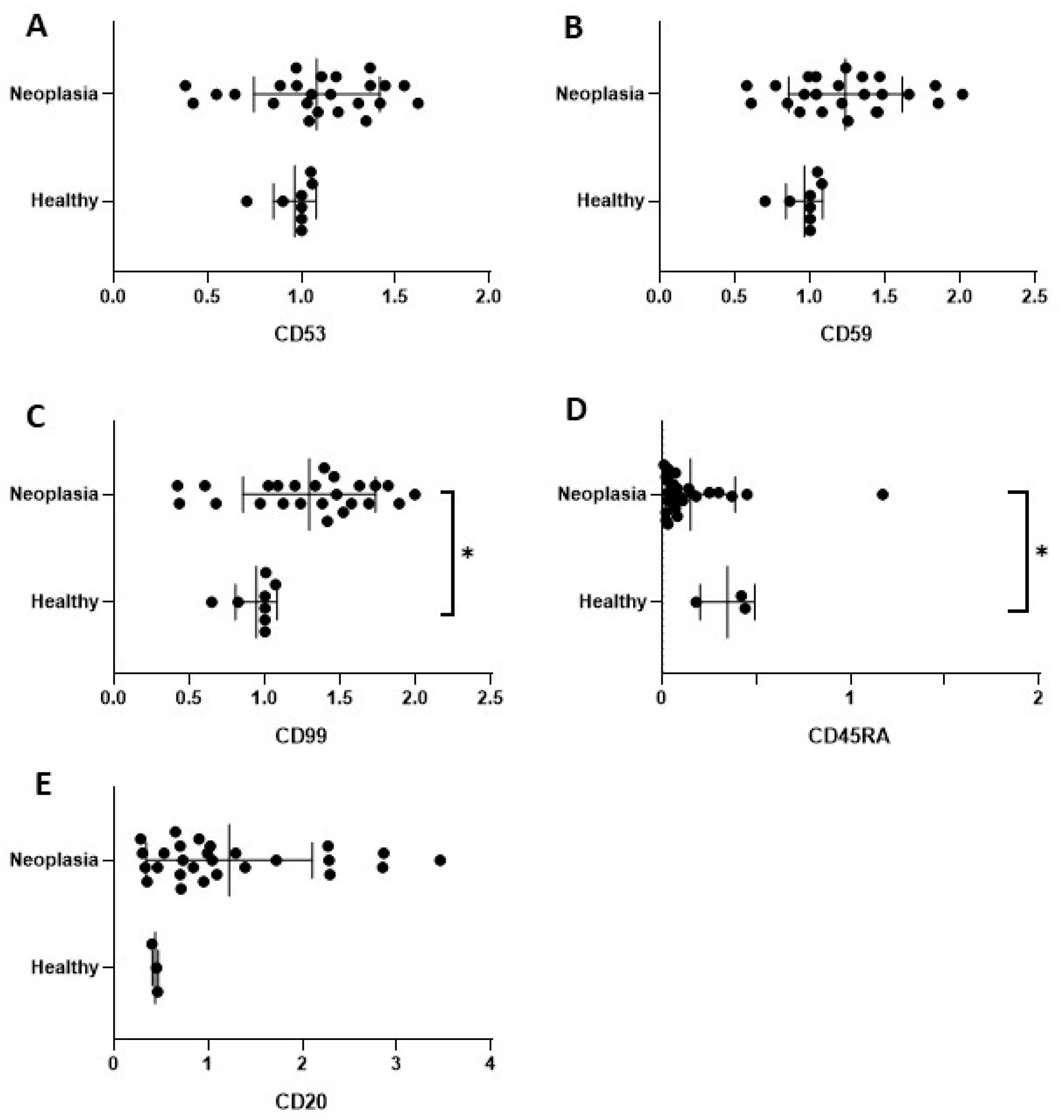
2. Results
2.1. Animals and Histopathology
2.2. Microarrays
2.3. Immunoblotting
3. Discussion
4. Materials and Methods
4.1. Animals and Sampling
4.2. Microarrays Technique
4.3. Immunoblotting
4.4. Primary Antibodies
4.5. Secondary Antibodies
4.6. Image Analysis
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| ANCA | CD45RO | CYTL |
| BDNF | CD46 | Eotaxin-1 |
| CD1a | CD47 | GDC |
| CD2 | CD48 | GM-CSF |
| CD3 | CD49d | HCD137 |
| CD4 | CD50 | HLA |
| CD5 | CD52 | HLA-ABC |
| CD6 | CD53 | HLA-DP |
| CD7 | CD54 | HLA-DR |
| CD8 | CD55 | HLA-I |
| CD9 | CD56 | IFN |
| CD10 | CD57 | IFN alpha |
| CD11a | CD58 | IFNg |
| CD11b | CD59 | IFNy |
| CD11c | CD61 | IgE |
| CD13 | CD62L | IL-1 |
| CD14 | CD62p | IL-10 |
| CD15 | CD63 | IL-12B |
| CD16 | CD66e | IL-12p70 |
| CD17 | CD69 | IL-13 |
| CD18 | CD71 | IL-15 |
| CD19 | CD72 | IL-16 |
| CD20 | CD79a | IL-18 |
| CD21 | CD80 | IL-1a |
| CD22 | CD86 | IL-1b |
| CD23 | CD95 | IL-37 |
| CD24 | CD97 | IL-4 |
| CD25 | CD98 | IL-6 |
| CD27 | CD99 | IL-7 |
| CD28 | CD99R | IL-8 |
| CD29 | CD105 | MICP-2 |
| CD30 | CD106 | MCP-E |
| CD31 | CD116 | MIP-1a |
| CD33 | CD117 | MIP-4 |
| CD34 | CD123 | MPO |
| CD35 | CD139 | NT-4 |
| CD36 | CD147 | NTAL |
| CD37 | CD162 | P53 |
| CD38 | CD177 | P72Syk |
| CD40 | CD222 | Pan |
| CD41 | CD235a | PD1 |
| CD41a | CD235ab | PDL1 |
| CD41b | CDw131 | RANTES |
| CD42b | CEACAM1 | TNFa |
| CD43 | CEACAM3 | TRAIL |
| CD44 | CEACAM5 | TSLP |
| CD45 | CEACAM6 | TSLPR |
| CD45RA | CEACAM8 | tTG |
| CD45RB | COX1 | CYTL |
References
- Gupta, K.; Sood, N.K.; Uppal, S.K.; Mohindroo, J.; Mahajan, S.; Raghunath, M.; Singh, K. Epidemiological studies on canine mammary tumour and its relevance for breast cancer studies. IOSR J. Pharm. 2012, 2, 322–333. [Google Scholar] [CrossRef]
- Sharma, N.; Gupta, A.K.; Bhat, R.A.; Yatoo, M.I.; Oveas, R.; Parray, O.R. Epidemiology and treatment of canine mammary tumours in Jammu region of India. J. Dairy Vet. Anim Res. 2018, 7, 59–62. [Google Scholar] [CrossRef]
- Irac, S.E.; Oksa, A.; Jackson, K.; Herndon, A.; Allavena, R.; Palmieri, C. Cytokine expression in canine lymphoma, osteosarcoma, mammary gland tumour and melanoma: Comparative aspects. Vet. Sci. 2019, 6, 37. [Google Scholar] [CrossRef]
- Gelaleti, G.B.; Jardim, B.V.; Leonel, C.; Moschetta, M.G.; Zuccari, D.A. Interleukin-8 as a prognostic serum marker in canine mammary gland neoplasias. Vet. Immunol. Immunop. 2012, 146, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Tamura, D.; Nakamura, T.; Makita, Y.; Ariyama, H.; Komiyama, K.; Yoshihara, T.; Asano, R. 4-Methylumbelliferone leads to growth arrest and apoptosis in canine mammary tumor cells. Oncol. Rep. 2013, 29, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Kabir, F.M.L.; Alvarez, C.E.; Bird, R.C. Canine mammary carcinomas: A comparative analysis of altered gene expression. Vet. Sci. 2015, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Sorenmo, K. Canine mammary gland tumors. Vet. Clin. N. Am. Small Anim. Pract. 2003, 33, 573–596. [Google Scholar] [CrossRef]
- Sleeckx, N.; de Rooster, H.; Veldhuis Kroeze, E.J.; Van Ginneken, C.; Van Brantegem, L. Canine mammary tumours, an overview. Reprod. Domest. Anim. 2011, 46, 1112–1131. [Google Scholar] [CrossRef]
- Diao, H.; Cheng, N.; Zhao, Y.; Xu, H.; Dong, H.; Thamm, D.H.; Zhang, D.; Lin, D. Ivermectin inhibits canine mammary tumor growth by regulating cell cycle progression and WNT signaling. BMC Vet. Res. 2019, 15, 276. [Google Scholar] [CrossRef]
- Varallo, G.R.; Jardim-Perassi, B.V.; Alexandre, P.A.; Fukumasuc, H.; Zuccar, D.A.P.C. Global gene expression profile in canine mammary carcinomas. Vet. J. 2019, 254, 105393. [Google Scholar] [CrossRef]
- Kaszak, I.; Ruszczak, A.; Kanafa, S.; Kacprzak, K.; Król, M.; Jurka, P. Current biomarkers of canine mammary tumors. Acta Vet. Scand. 2018, 60, 66. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, S.; Kim, W.H. Expression and prognostic value of TRPM7 in canine mammary tumours. Vet. Comp. Oncol. 2021, 19, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.I.; Silva-Carvalho, R.; Pires, I.; Prada, J.; Bianchini, R.; Jensen-Jarolim, E.; Queiroga, F.L. A comparative approach of tumor-associated inflammation in mammary cancer between humans and dogs. BioMed. Res. Int. 2016, 2016, 4917387. [Google Scholar] [CrossRef]
- Morrison, W.B. Inflammation and cancer: A comparative view. J. Vet. Intern. Med. 2012, 26, 18–31. [Google Scholar] [CrossRef]
- Balkwill, F.; Mantovani, A. Inflammation and Cancer: Back to Virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef]
- de Visser, K.E.; Coussens, L.M. The inflammatory tumor microenvironment and its impact on cancer development. Contrib. Microbiol. 2006, 13, 118–137. [Google Scholar]
- DeNardo, D.G.; Andreu, P.; Coussens, L.M. Interactions between lymphocytes and myeloid cells regulate pro-versus anti-tumor immunity. Cancer Metas. Rev. 2010, 29, 309–316. [Google Scholar] [CrossRef]
- Amirkhani Namagerdi, A.; d’Angelo, D.; Ciani, F.; Iannuzzi, C.A.; Napolitano, F.; Avallone, L.; De Laurentiis, M.; Giordano, A. Triple-negative breast cancer comparison with canine mammary tumors from light microscopy to molecular pathology. Front. Oncol. 2020, 10, 563779. [Google Scholar] [CrossRef]
- Manuali, E.; De Giuseppe, A.; Feliziani, F.; Forti, K.; Casciari, C.; Marchesi, M.C.; Pacifico, E.; Pawłowski, K.M.; Majchrzak, K.; Król, M. CA 15–3 cell lines and tissue expression in canine mammary cancer and the correlation between serum levels and tumour histological grade. BMC Vet. Res. 2012, 8, 86. [Google Scholar] [CrossRef]
- Kaszak, I.; Witkowska-Piłaszewicz, O.; Domrazek, K.; Jurka, P. The novel diagnostic techniques and biomarkers of canine mammary tumors. Vet. Sci. 2022, 9, 526. [Google Scholar] [CrossRef] [PubMed]
- Pastor, N.; Ezquerra, L.J.; Santella, M.; Caballé, N.C.; Tarazona, R.; Durán, M.E. Prognostic significance of immunohistochemical markers and histological classification in malignant canine mammary tumours. Vet. Comp. Oncol. 2020, 18, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.K.; Ng, C.S.; Hui, P.K. A simple guide to the terminology and application of leukocyte monoclonal antibodies. Histopathology 1988, 12, 461–480. [Google Scholar] [CrossRef] [PubMed]
- Rezaeeyan, H.; Shahrabi, S.; McKee, T.D.; Saki, N. The expression of CD markers in solid tumors: Significance in metastasis and prognostic value. Histol. Histopathol. 2018, 33, 1005–1012. [Google Scholar] [PubMed]
- Degnim, A.C.; Brahmbhatt, R.D.; Radisky, D.C.; Hoskin, T.L.; Stallings-Mann, M.; Laudenschlager, M.; Mansfield, A.; Frost, M.H.; Murphy, L.; Knutson, K.; et al. Immune cell quantitation in normal breast tissue lobules with and without lobulitis. Breast Cancer Res. Treat. 2014, 144, 539–549. [Google Scholar] [CrossRef]
- Zumwalde, N.A.; Haag, J.D.; Sharma, D.; Mirrielees, J.A.; Wilke, L.G.; Gould, M.N.; Gumperz, J.E. Analysis of immune cells from human mammary ductal epithelial organoids reveals Vδ2+ T cells that efficiently target breast carcinoma cells in the presence of bisphosphonate. Cancer Prev. Res. 2016, 9, 305–316. [Google Scholar] [CrossRef]
- Goff, S.L.; Danforth, D.N. The role of immune cells in breast tissue and immunotherapy for the treatment of breast cancer. Clin. Breast Cancer 2020, 21, e63–e73. [Google Scholar] [CrossRef]
- Fonsatti, E.; Jekunen, A.P.; Kairemo, K.J.; Coral, S.; Snellman, M.; Nicotra, M.R.; Natali, P.G.; Altomonte, M.; Maio, M. Endoglin is a suitable target for efficient imaging of solid tumors: In vivo evidence in a canine mammary carcinoma model. Clin. Cancer Res. 2000, 6, 2037–2043. [Google Scholar]
- Arce, C.; Moreno, A.; Pérez de la Lastra, J.M.; Garrido, J.J.; Barbancho, M.; De Andrés, D.F.; Morera, L.; Llanes, D. Expression of CD61 (beta3 integrin subunit) on canine cells. Platelets 2001, 12, 69–73. [Google Scholar] [CrossRef]
- Duff, S.E.; Chenggang, L.; Garland, J.M.; Kumar, S. CD105 is important for angiogenesis: Evidence and potential applications. FASEB J. 2003, 17, 984–992. [Google Scholar] [CrossRef]
- Trowbridge, I.S.; Thomas, M.L. CD45: An emerging role as a protein tyrosine phosphatase required for lymphocyte activation and development. Annu. Rev. Immunol. 1994, 12, 85–116. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, K.; Okumura, M.; Shiono, H.; Inoue, M.; Kadota, Y.; Miyoshi, S.; Matsuda, H. A study on CD45 isoform expression during T-cell development and selection events in the human thymus. Hum. Immun. 2002, 63, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.A.; Rozzo, S.J.; Cambier, J.C. Aging-dependent exclusion of antigen-inexperienced cells from the peripheral b cell repertoire. J. Immunol. 2002, 168, 5014–5023. [Google Scholar] [CrossRef]
- Vidal, S.; Bellido-Casado, J.; Granel, C.; Crespo, A.; Plaza, V.; Juarez, C. Flow cytometry analysis of leukocytes in induced sputum from asthmatic patients. Immunobiology 2012, 217, 692–697. [Google Scholar] [CrossRef]
- Sylvester, T.T.; Parsons, S.D.C.; van Helden, P.D.; Miller, M.A.; Loxton, A.G. A pilot study evaluating the utility of commercially available antibodies for flow cytometric analysis of Panthera species lymphocytes. BMC Vet. Res. 2018, 14, 410. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Choi, M.R.; Park, H.; Kim, M.; Hong, J.E.; Lee, J.Y.; Chun, H.S.; Lee, K.W.; Yoon Park, J.H. Dietary fat increases solid tumor growth and metastasis of 4T1 murine mammary carcinoma cells and mortality in obesity-resistant balb/c mice. Breast Cancer Res. 2011, 13, R78. [Google Scholar] [CrossRef]
- Negus, R.P.; Stamp, G.W.; Hadley, J.; Balkwill, F.R. Quantitative assessment of the leukocyte infiltrate in ovarian cancer and its relationship to the expression of C-C chemokines. Am. J. Pathol. 1997, 150, 1723–1734. [Google Scholar]
- Ma, D.; Wang, Y.; Du, G.; Yang, J.; Tang, Q.; Zhou, L. CD41 and CD45 expression marks the angioformative initiation of neovascularization in human haemangioblastoma. Tumour Biol. 2016, 37, 3765–3774. [Google Scholar] [CrossRef]
- Pasello, M.; Manara, M.C.; Scotlandi, K. CD99 at the crossroads of physiology and pathology. J. Cell Commun. Signal. 2018, 12, 55–68. [Google Scholar] [CrossRef]
- Lou, O.; Alcadie, P.; Luscinskas, F.W.; Muller, W.A. CD99 is a key mediator of the transendothelial migration of neutrophils. J. Immunol. 2007, 178, 1136–1143. [Google Scholar] [CrossRef]
- Schenkel, A.R.; Mamdouh, Z.; Chen, X.; Liebman, R.M.; Muller, W.A. CD99 plays a major role in the migration of monocytes through endothelial junctions. Nat. Immunol. 2002, 3, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Milanezi, F.; Pereira, E.M.; Ferreira, F.V.; Leitão, D.; Schmitt, F.C. CD99/MIC-2 surface protein expression in breast carcinomas. Histopathology 2001, 39, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Lee, H.J.; Hahn, J.H.; Park, S.; Lee, H. Expression of a Spliced Variant of CD99 Membrane Protein Increases Motility, Matrix Degradation, and Invasiveness of Human Breast Carcinoma Cells. Proc. Am. Assoc. Cancer Res. 2000, 41, 231–232. [Google Scholar]
- Lee, H.J.; Kim, E.; Jee, B.; Hahn, J.H.; Han, K.; Jung, K.C.; Park, S.H.; Lee, H. Functional involvement of Src and focal adhesion kinase in a CD99 splice variant-induced motility of human breast cancer cells. Exp. Mol. Med. 2002, 34, 177–183. [Google Scholar] [CrossRef]
- Baccar, A.; Ferchichi, I.; Troudi, W.; Marrakchi, R.; Ben Hmida, N.; Jebini, S.; Mrad, K.; Ben Romdhane, K.; Benammar Elgaaied, A. CD99 and HLA-II immunostaining in breast cancer tissue and their correlation with lymph node metastasis. Dis. Markers 2013, 34, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Manara, M.C.; Pasello, M.; Scotlandi, K. CD99: A cell surface protein with an oncojanus role in tumors. Genes 2018, 9, 159. [Google Scholar] [CrossRef]
- Kreppel, M.; Aryee, D.N.; Schaefer, K.L.; Amann, G.; Kofler, R.; Poremba, C.; Kovar, H. Suppression of KCMF1 by constitutive high CD99 expression is involved in the migratory ability of Ewing’s sarcoma cells. Oncogene 2006, 25, 2796–2800. [Google Scholar] [CrossRef]
- Rochhi, A.; Manara, M.C.; Sciandra, M.; Zambelli, D.; Nardi, F.; Nicoletti, G.; Garofalo, C.; Meschini, S.; Astolfi, A.; Colombo, M.P.; et al. CD99 inhibits neural differentiation of human Ewing sarcoma cells and thereby contributes to oncogenesis. J. Clin. Investig. 2010, 120, 668–680. [Google Scholar] [CrossRef]
- Benini, S.; Gamberi, G.; Cocchi, S.; Garbetta, J.; Alberti, L.; Righi, A.; Gambarotti, M.; Picci, P.; Ferrari, S. Detection of circulating tumor cells in liquid biopsy from Ewing sarcoma patients. Cancer Manag. Res. 2018, 10, 49–60. [Google Scholar] [CrossRef]
- Zakzok, O.; Elshanshory, M.; Zekri, W.; Elsharkawy, N.; Zaky, I.; Salama, A.; Kamel, A.; Elantably, I.; Said, S. Prognostic value of detection of CD99+, CD45- cells in peripheral blood by flow cytometry in children with Ewing sarcoma. Pediatr. Blood Cancer 2022, 69, e29298. [Google Scholar] [CrossRef]
- Sullivan, D.P.; Muller, W.A. Neutrophil and monocyte recruitment by PECAM, CD99 and other molecules via the LBRC. Semin. Immunopathol. 2014, 36, 193–209. [Google Scholar] [CrossRef] [PubMed]
- Tedder, T.F.; Engel, P. CD20: A regulator of cell-cycle progression of B lymphocytes. Immunol. Today 1994, 15, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Kanzaki, M.; Shibata, H.; Mogami, H.; Kojima, I. Expression of calcium-permeable cation channel CD20 accelerates progression through the G1 phase in Balb/c 3T3 cells. J. Biol. Chem. 1995, 270, 13099–13104. [Google Scholar] [CrossRef] [PubMed]
- Hamed, M.M.; Gouida, M.S.; Abd El-Aziz, S.R.; El-Sokkary, A.M.A. Evaluation PD-L1, CD8 and CD20 as early predictor and tracking markers for breast cancer (BC) in Egypt. Heliyon 2022, 8, e09474. [Google Scholar] [CrossRef]
- Rismanchi, S.; Muhammadnejad, S.; Amanpour, S.; Muhammadnejad, A. First pathological study of canine primary breast lymphoma and the description of its clinicopathological characteristics as an animal model for human primary breast lymphoma. Biomed. Rep. 2015, 3, 75–77. [Google Scholar] [CrossRef]
- Schmidt, M.; Böhm, D.; von Törne, C.; Steiner, E.; Puhl, A.; Pilch, H.; Lehr, H.A.; Hengstler, J.G.; Kölbl, H.; Gehrmann, M. The humoral immune system has a key prognostic impact in node-negative breast cancer. Cancer Res. 2008, 68, 5405–5413. [Google Scholar] [CrossRef]
- Olkhanud, P.B.; Damdinsuren, B.; Bodogai, M.; Gress, R.E.; Sen, R.; Wejksza, K.; Malchinkhuu, E.; Wersto, R.P.; Biragyn, A. Tumor-evoked regulatory B cells promote breast cancer metastasis by converting resting CD4+ T Cells to T-regulatory cells. Cancer Res. 2011, 71, 3505–3515. [Google Scholar] [CrossRef]
- Schnellhardt, S.; Erber, R.; Büttner-Herold, M.; Rosahl, M.C.; Ott, O.J.; Strnad, V.; Beckman, M.W.; King, L.; Hartmann, A.; Fietkau, R.; et al. Tumour-infiltrating inflammatory cells in early breast cancer: An underrated prognostic and predictive factor? Int. J. Mol. Sci. 2020, 21, 8238. [Google Scholar] [CrossRef]
- Jia, Y.; Xu, L.; Lin, Q.Q.; Zhu, M.; Ding, L.; Wu, K.; Lu, Y. Levels of lymphocyte subsets in peripheral blood prior treatment are associated with aggressive breast cancer phenotypes or subtypes. Med. Oncol. 2014, 31, 981. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Hageman, T.; Lawrence, T.; McNeish, I.; Charles, K.A.; Kulbe, H.; Thompson, R.G.; Robinson, S.C.; Balkwill, F.R. “Re-educating” tumor-associated macrophages by targeting NF-KappaB. J. Exp. Med. 2008, 205, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.R.; Hassan, H.I. Analysis of the mononuclear inflammatory cell infiltrate in the normal breast, benign proliferative breast disease, in situ and infiltrating ductal breast carcinomas: Preliminary observation. J. Clin. Pathol. 2006, 59, 972–977. [Google Scholar] [CrossRef] [PubMed]
- Ruffell, B.; Au, A.; Rugo, H.S.; Esserman, L.J.; Hwang, E.S.; Coussens, L.M. Leukocyte composition of human breast cancer. Proc. Natl. Acad. Sci. USA 2012, 109, 2796–2801. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.A. A Rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–256. [Google Scholar] [CrossRef]
- Sirois, J.; Dore, M. The late induction of prostaglandin G/H synthase-2 in equine preovulatory follicles support its role as a determinant of the ovulatory process. Endocrinology 1997, 138, 4427–4434. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]


| Protein | Antibody ID | log FC | AveExp | Adj. p-Val | Uniport-Link |
|---|---|---|---|---|---|
| CD20 | S0288 | 1.51 | 13.45 | 0.270 | P11836 |
| ICAM1 | S0333 | 1.25 | 11.50 | 0.920 | P05362 |
| CD53 | S0332 | 1.23 | 13.91 | 0.650 | P19397 |
| ICAM1 | S0334 | 1.22 | 12.28 | 0.920 | P05352 |
| DAF | S0335 | 1.08 | 9.49 | 0.370 | P08174 |
| MCP | S0325 | 1.05 | 12.60 | 0.960 | P15529 |
| CD20 | S0286 | −0.96 | 11.50 | 0.039 | P11836 |
| CD139 | S0438 | −1.01 | 14.31 | 0.950 | |
| PDCD1 | S0003 | −1.01 | 12.57 | 0.960 | Q15116 |
| IL1β | S0387 | −1.04 | 14.53 | 0.950 | P01584 |
| IL6 | S0530 | −1.06 | 10.01 | 0.490 | P05231 |
| CCL3 | S0393 | −1.07 | 14.54 | 0.920 | P10147 |
| IL16 | S0529 | −1.09 | 11.88 | 0.720 | Q14005 |
| CD86 | S0356 | −1.10 | 10.98 | 0.960 | P42081 |
| TGM2 | S0405 | −1.14 | 9.07 | 0.650 | P21980 |
| CD79A | S0354 | −1.15 | 11.66 | 0.920 | P11912 |
| ITAX | S0268 | −1.22 | 11.04 | 0.036 | P20702 |
| KIT | S0001 | −1.24 | 13.51 | 0.960 | P10721 |
| CD24 | S0294 | −1.33 | 10.84 | 0.960 | P25063 |
| CD177 | S0368 | −1.34 | 10.62 | 0.039 | Q8N6Q3 |
| MPRI | S0369 | −1.42 | 11.61 | 0.090 | P11717 |
| IFNA1 | S0402 | −2.10 | 13.94 | 0.720 | P01562 |
| Protein | Antibody ID | log FC | AveExp | Adj. p-Val | Uniport-Link |
|---|---|---|---|---|---|
| CD20 | S0288 | 2.57 | 13.45 | 0.002 | P11836 |
| MCP | S0325 | 2.05 | 12.60 | 0.820 | P15529 |
| CD3 | S0407 | 2.05 | 12.86 | 0.290 | P07766 |
| PD1L1 | S002 | 1.96 | 12.88 | 0.820 | Q9NZQ7 |
| CD53 | S0332 | 1.92 | 13.91 | 0.040 | P19397 |
| AMPN | S0378 | 1.54 | 13.32 | 0.710 | P15144 |
| ICAM1 | S0.334 | 1.47 | 12.28 | 0.480 | P05362 |
| CD44 | S0424 | 1.43 | 14.10 | 0.190 | P16070 |
| CD22 | S0292 | 1.40 | 11.35 | 0.420 | P20273 |
| CD99 | S0360 | 1.31 | 11.75 | 0.430 | P14209 |
| CD8A | S0254 | 1.15 | 12.13 | 0.480 | P01732 |
| CD44 | S0315 | 1.12 | 12.40 | 0.520 | P16070 |
| EGLN | S0363 | 1.02 | 11.72 | 0.820 | P17813 |
| CD20 | S0286 | −0.87 | 11.50 | 0.049 | P11836 |
| CEAM6 | S0503 | −1.00 | 12.45 | 0.630 | P40199 |
| IL7 | S0388 | −1.01 | 13.42 | 0.910 | P13232 |
| IL16 | S0529 | −1.02 | 11.88 | 0.470 | Q14005 |
| CEAM1 | S050 | −1.02 | 12.76 | 0.480 | P13688 |
| IL8 | S0389 | −1.08 | 11.41 | 0.190 | P10145 |
| LFA3 | S0340 | −1.10 | 12.29 | 0.910 | P19256 |
| CEAM5 | S0348 | −1.12 | 12.35 | 0.500 | P06731 |
| IL8 | S0475 | −1.15 | 10.33 | 0.420 | P10145 |
| ITAX | S0268 | −1.16 | 11.04 | 0.019 | 020702 |
| CD24 | S0294 | −1.16 | 10.84 | 0.820 | P25063 |
| NTF4 | S0397 | −1.22 | 13.88 | 0490 | P34130 |
| CCL7 | S0394 | −1.22 | 13.59 | 0.710 | P80098 |
| FCG3A | S0277 | −1.23 | 10.50 | 0.011 | P08637 |
| CD177 | S0368 | −1.24 | 10.62 | 0.044 | Q8N6Q3 |
| CD53 | S0331 | −1.35 | 12.03 | 0.250 | P19397 |
| TGM2 | S0405 | −1.36 | 9.07 | 0.230 | P21980 |
| CCL11 | S0382 | −1.37 | 14.23 | 0.370 | P51671 |
| IL15 | S0391 | −1.39 | 14.48 | 0.290 | P40933 |
| CD139 | S0438 | −1.42 | 14.31 | 0.480 | |
| IL1b | S0387 | −1.43 | 14.53 | 0.480 | P01584 |
| BDNF | S0381 | −1.50 | 14.15 | 0.400 | P23560 |
| IL18 | S0392 | −1.52 | 14.34 | 0.290 | Q14116 |
| MPRI | S069 | −1.65 | 11.61 | 0.017 | P11717 |
| CCL3 | S0393 | −1.75 | 14.54 | 0.230 | P10147 |
| CD86 | S0356 | −2.12 | 10.98 | 0.390 | P42081 |
| Protein | Antibody ID | log FC | AveExp | Adj. p-Val | Uniport-Link |
|---|---|---|---|---|---|
| PDCD1 | S0003 | 1.82 | 12.57 | 0.012 | Q15116 |
| IFNA1 | S0402 | 1.72 | 13.94 | 0.008 | P01562 |
| CD44 | S0315 | 1.31 | 12.40 | 0.007 | P16070 |
| KIT | S0001 | 1.24 | 13.51 | 0.370 | P10721 |
| CD3 | S0407 | 1.11 | 12.86 | 0.170 | P07766 |
| PD1L1 | S0002 | 1.08 | 12.88 | 0.550 | Q9NZQ7 |
| CD20 | S0288 | 1.07 | 13.45 | 0.011 | P11836 |
| MCP | S0325 | 1.00 | 12.60 | 0.640 | P15529 |
| IL37 | S0473 | −0.42 | 10.27 | 0.012 | Q9NHZ6 |
| PTPRC | S0323 | −068 | 11.29 | 0.012 | P08575 |
| CD38 | S0308 | −0.78 | 10.15 | 0.011 | P28907 |
| IL8 | S0475 | −0.80 | 10.33 | 0.021 | P10145 |
| NCAM1 | S0337 | −0.94 | 11.60 | 0.013 | P13591 |
| CD86 | S0356 | −1.03 | 10.98 | 0.200 | P42081 |
| CD15 | S0273 | −1.04 | 11.41 | 0.012 | |
| LFA3 | S0340 | −1.04 | 12.29 | 0.550 | P19256 |
| IL37 | S0474 | −1.11 | 11.36 | 0.008 | Q9NZH6 |
| TNR8 | S0302 | −1.13 | 12.34 | 0.021 | P28908 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galadima, M.; Kotova, I.; Schmidt, R.; Pastor, J.; Schröder, C.; Rodríguez-Gil, J.E.; del Alamo, M.M.R. Canine Mammary Neoplasia Induces Variations in the Peripheral Blood Levels of CD20, CD45RA, and CD99. Int. J. Mol. Sci. 2023, 24, 9222. https://doi.org/10.3390/ijms24119222
Galadima M, Kotova I, Schmidt R, Pastor J, Schröder C, Rodríguez-Gil JE, del Alamo MMR. Canine Mammary Neoplasia Induces Variations in the Peripheral Blood Levels of CD20, CD45RA, and CD99. International Journal of Molecular Sciences. 2023; 24(11):9222. https://doi.org/10.3390/ijms24119222
Chicago/Turabian StyleGaladima, Makchit, Iuliia Kotova, Ronny Schmidt, Josep Pastor, Christoph Schröder, Joan Enric Rodríguez-Gil, and Maria Montserrat Rivera del Alamo. 2023. "Canine Mammary Neoplasia Induces Variations in the Peripheral Blood Levels of CD20, CD45RA, and CD99" International Journal of Molecular Sciences 24, no. 11: 9222. https://doi.org/10.3390/ijms24119222
APA StyleGaladima, M., Kotova, I., Schmidt, R., Pastor, J., Schröder, C., Rodríguez-Gil, J. E., & del Alamo, M. M. R. (2023). Canine Mammary Neoplasia Induces Variations in the Peripheral Blood Levels of CD20, CD45RA, and CD99. International Journal of Molecular Sciences, 24(11), 9222. https://doi.org/10.3390/ijms24119222







