The Effect of Statins on Male Reproductive Parameters: A Mechanism Involving Dysregulation of Gonadal Hormone Receptors and TRPV1
Abstract
1. Introduction
2. Results
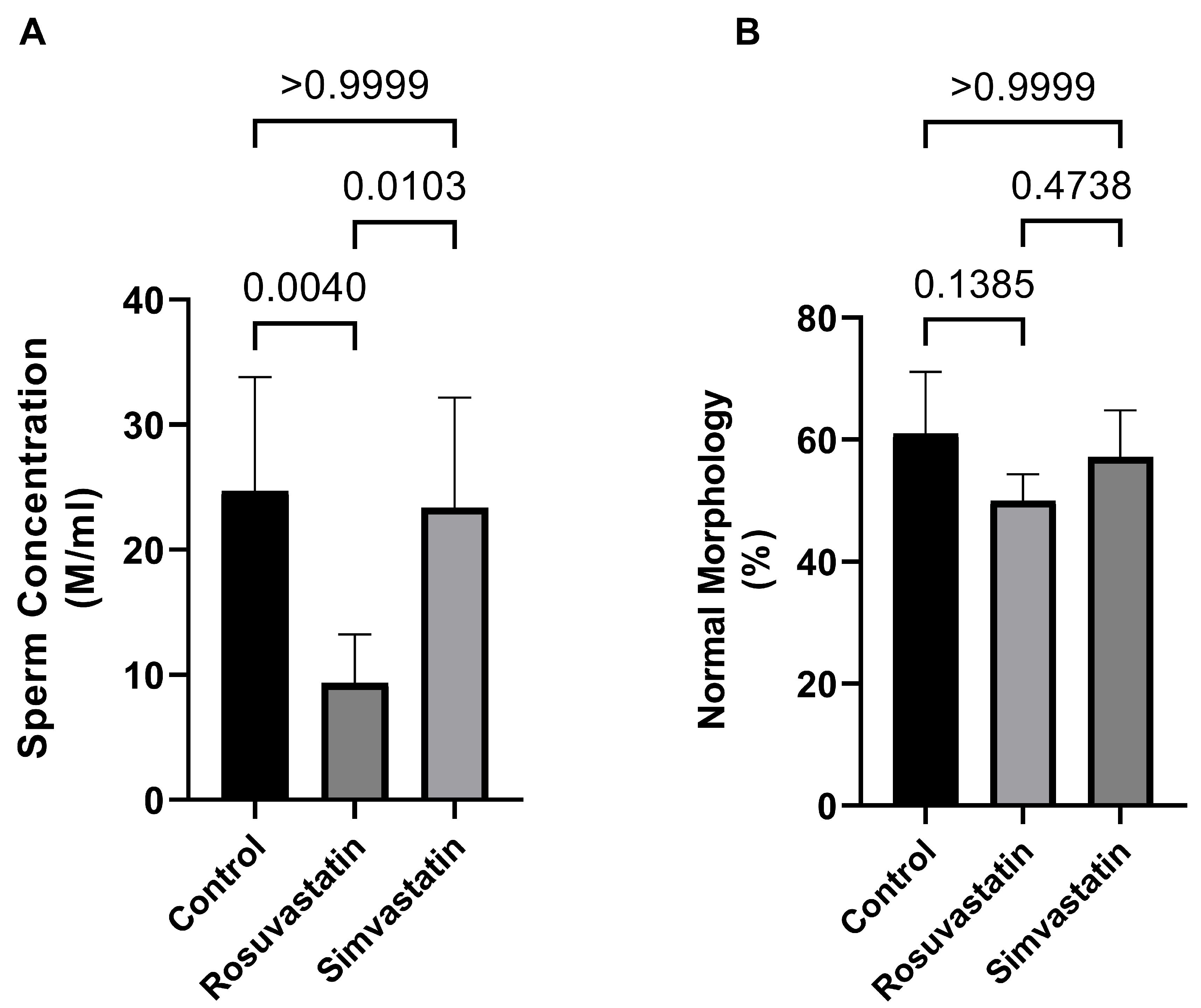
2.1. Rosuvastatin and Simvastatin on Male Reproductive Parameters
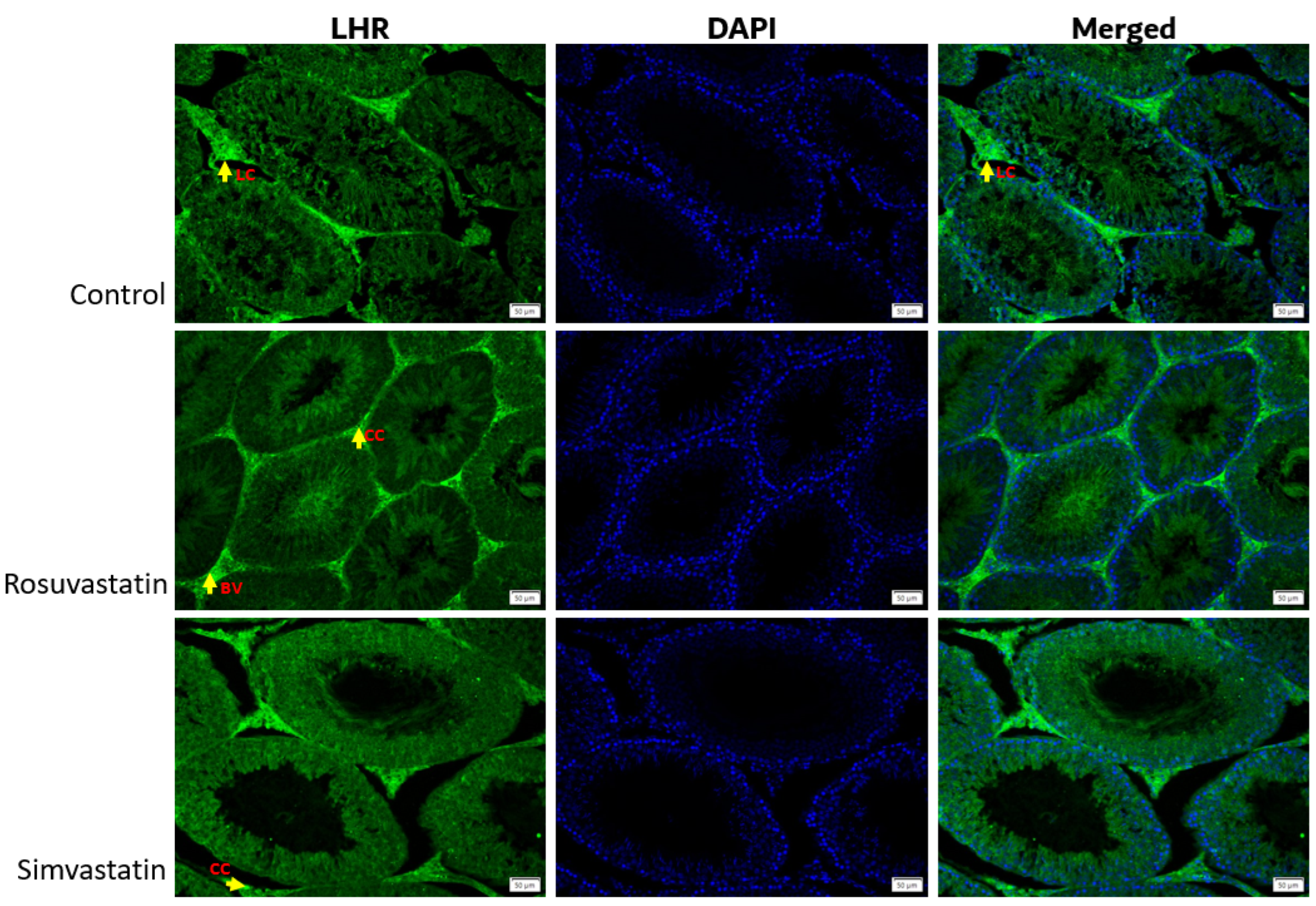
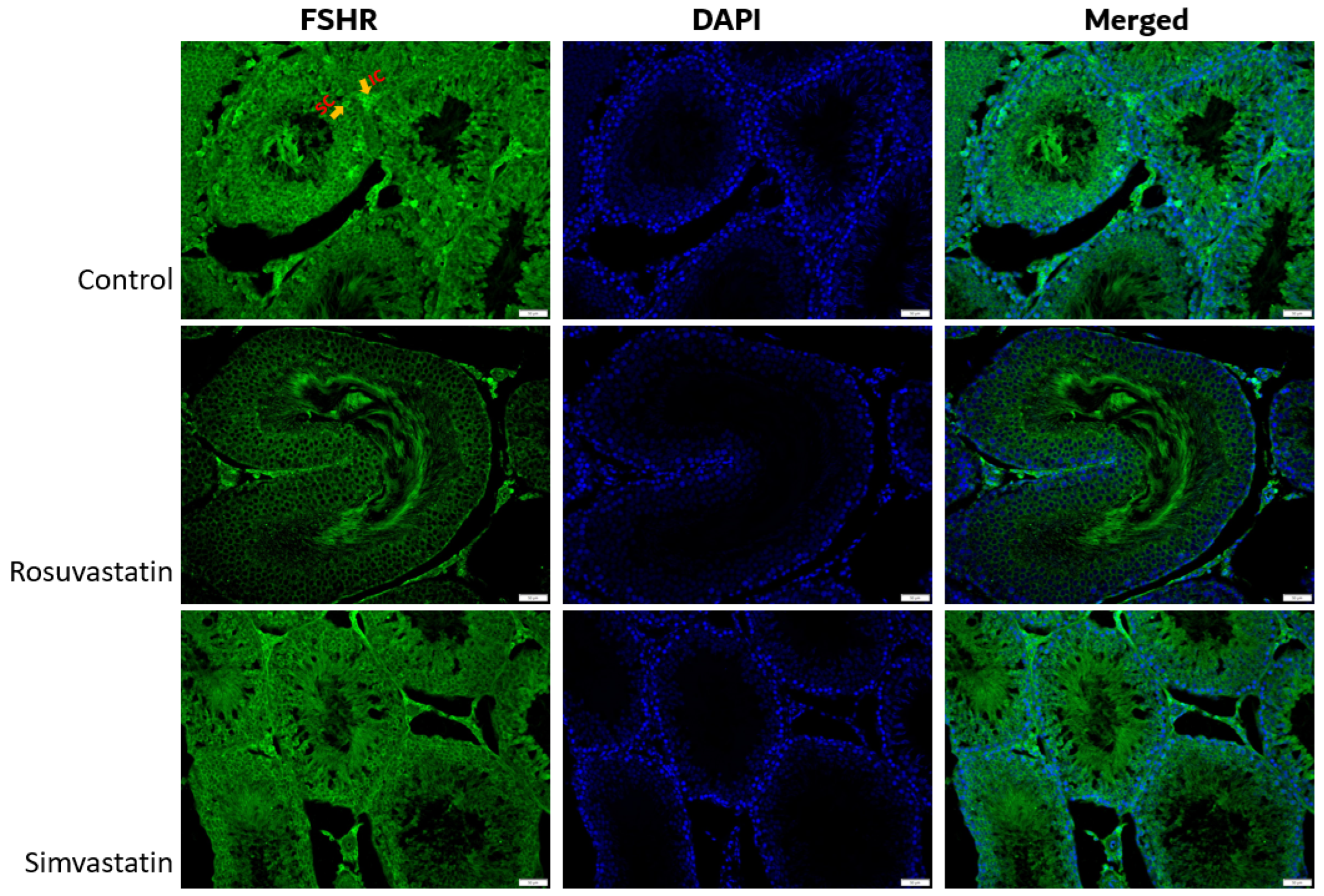
2.2. mRNA Expression of Solute Carrier Transporters (SLCO1B1, SLCO1B2 and SLCO1B3) and Gonadal Hormone Receptors (LHR, FSHR) in Rat Sertoli and Leydig Cells
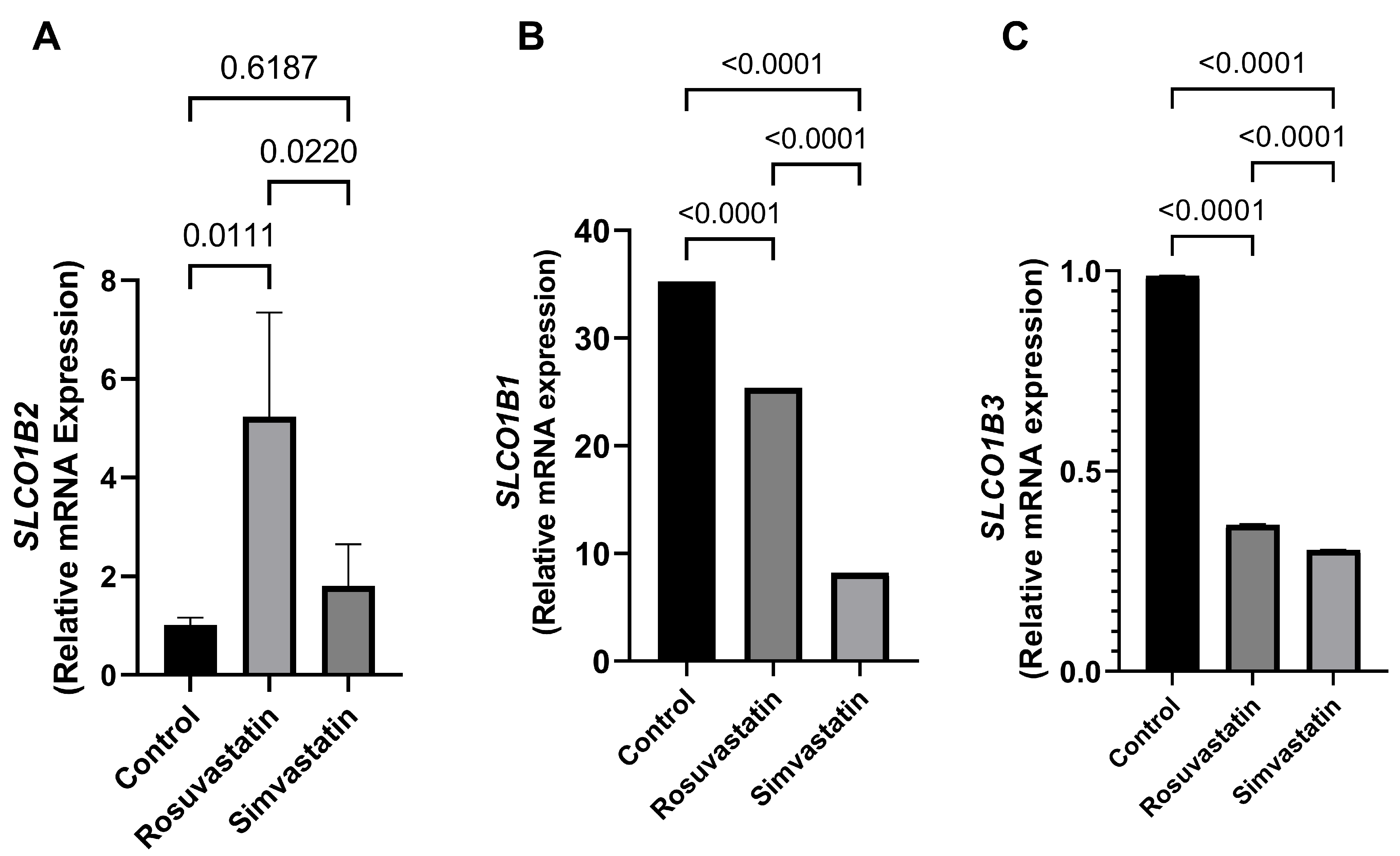
2.3. Rosuvastatin and Simvastatin Effect on Testis mRNA Expression of SLCO1B1, SLCO1B2 and SLCO1B3
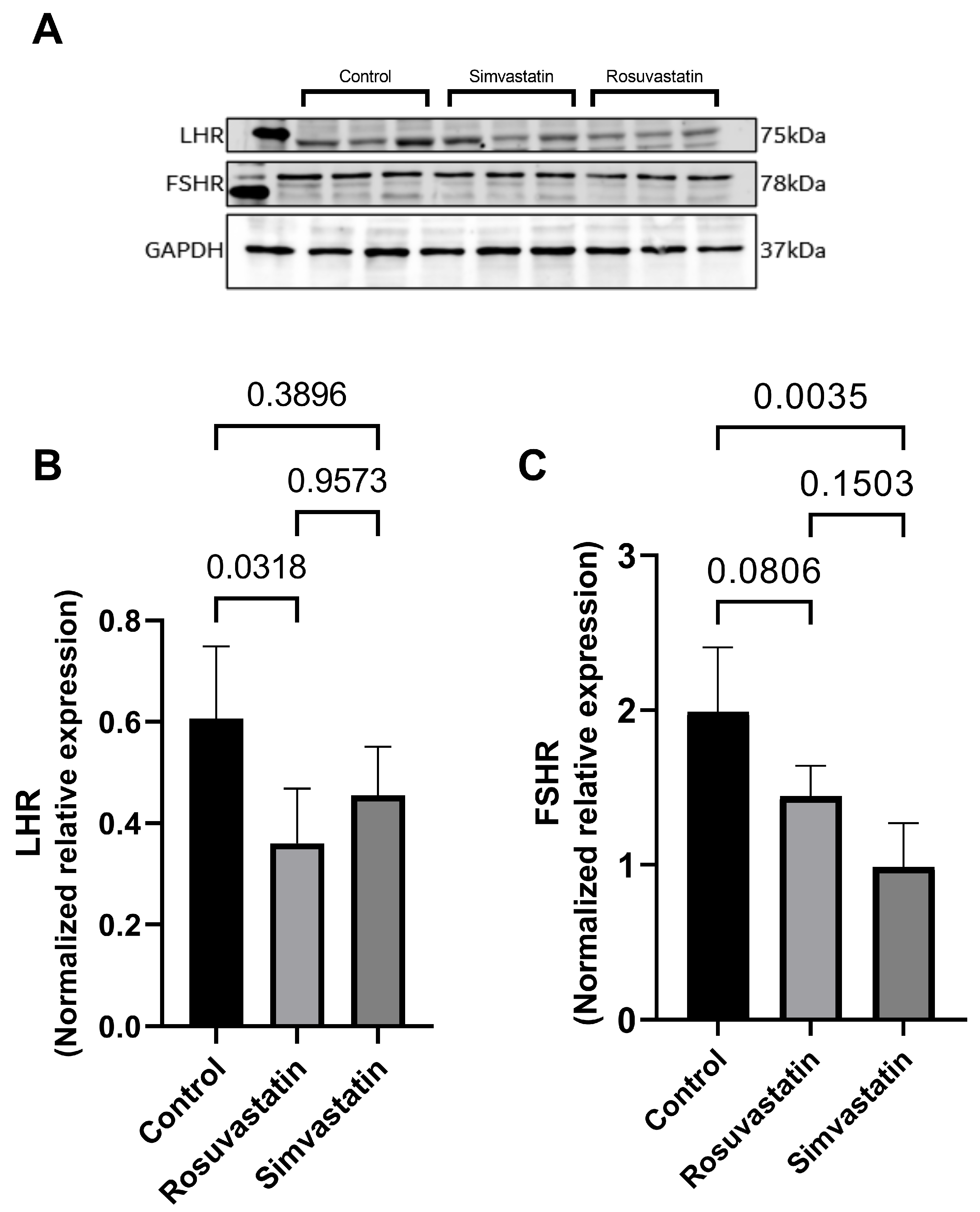
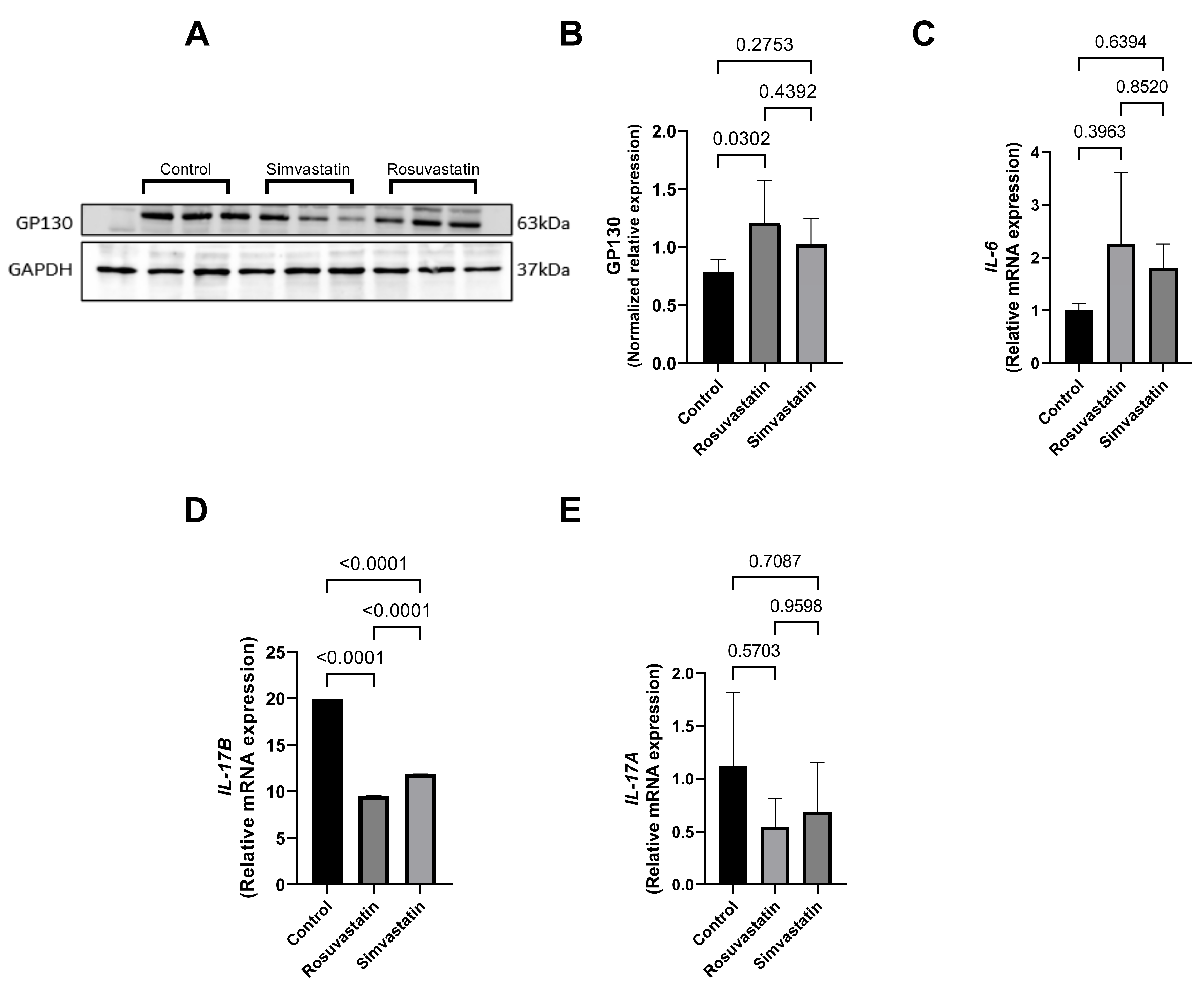
2.4. Rosuvastatin and Simvastatin Effect on Protein Expression of Gonadal Hormone Receptors
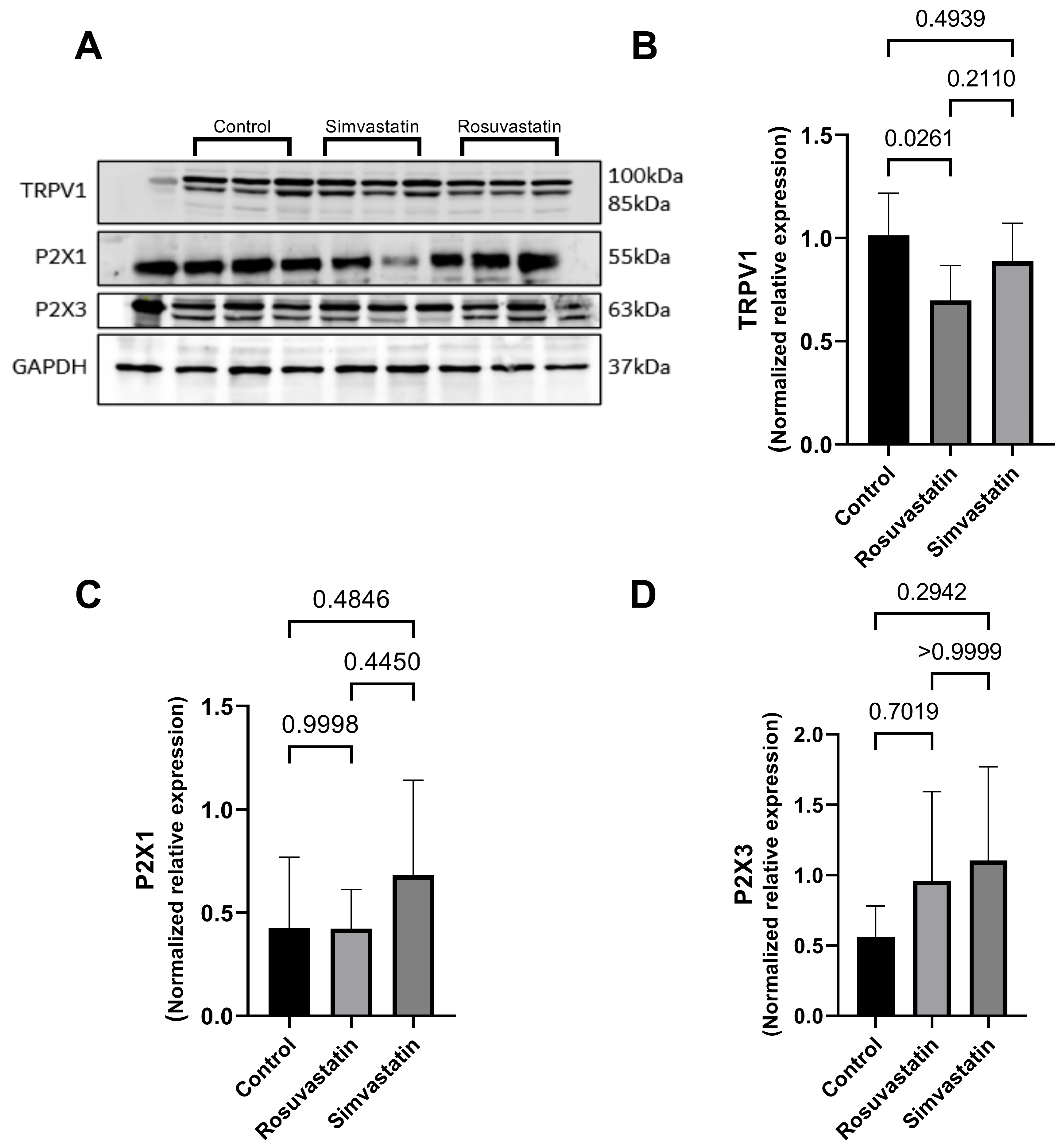
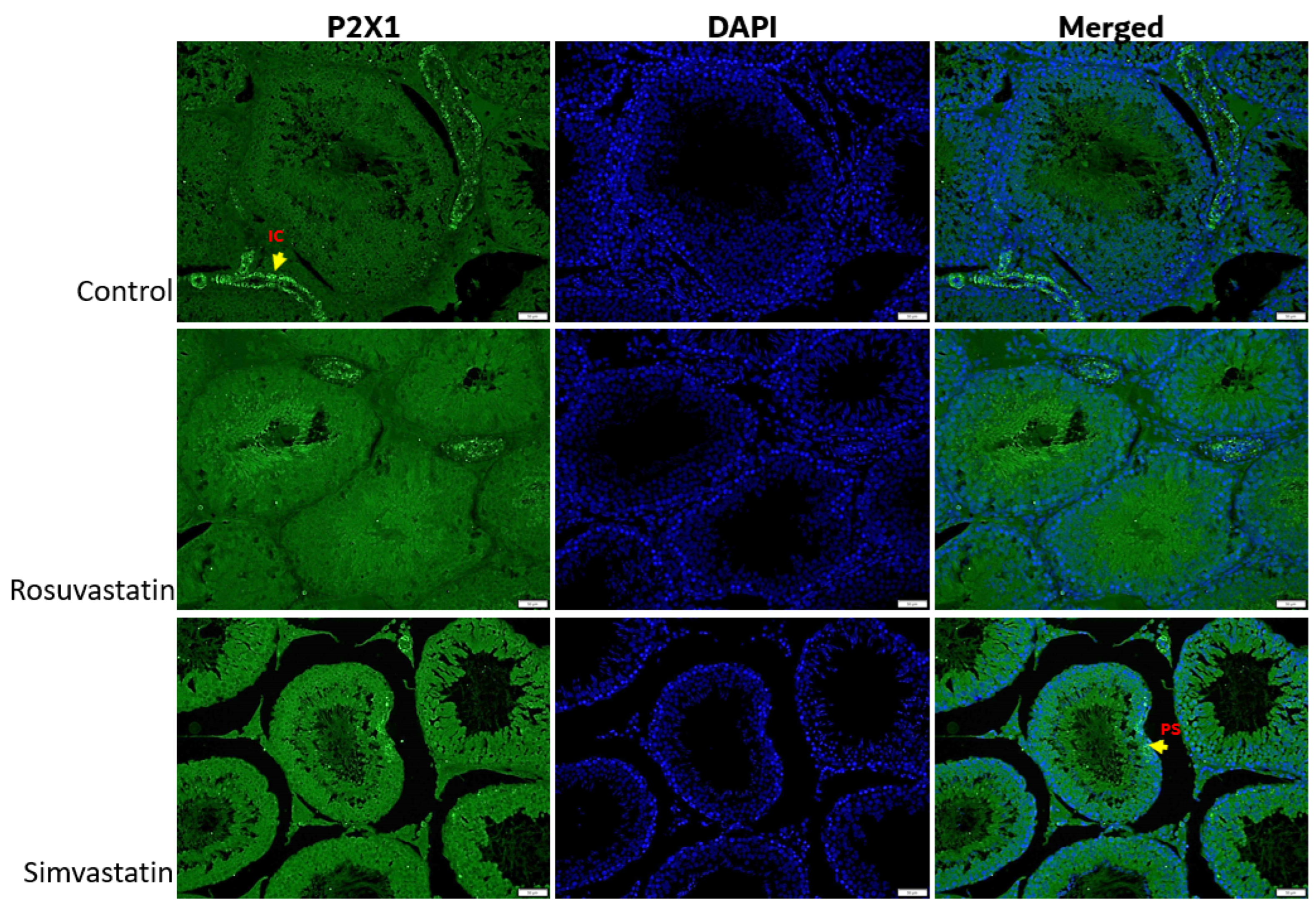
2.5. The Effect of Rosuvastatin and Simvastatin on Pain Receptors (TRPV1, P2X1, P2X3)
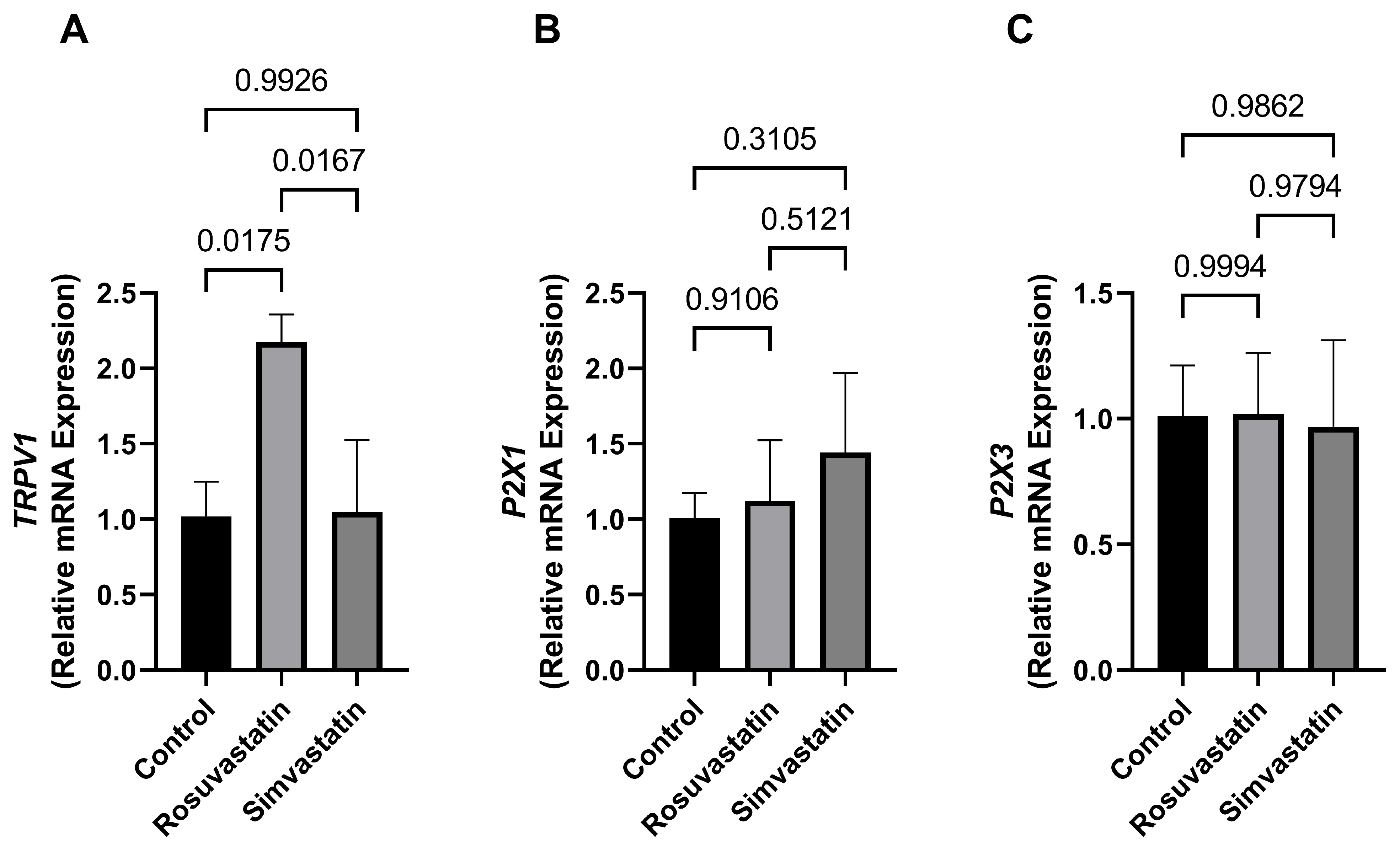
2.6. The Effect of Rosuvastatin and Simvastatin on Testicular mRNA Expression of TRPV1, P2X1, P2X3
2.7. The Effect of Rosuvastatin and Simvastatin on Testicular Protein Expression of TRPV1, P2X1, P2X3
2.8. Rosuvastatin and Simvastatin on the Testicular Protein and mRNA Expression of Inflammatory Cytokines
2.9. Qualitative Findings
3. Discussion
4. Materials and Methods
4.1. Ethics and Animal Care
4.2. Study Design
4.3. Sperm Retrieval for Concentration and Morphology
4.4. Isolation of Leydig Cells and Sertoli Cells
4.5. Isolation of Leydig Cells
4.6. Isolation of Sertoli Cells
4.7. Quantification of Genes
RNA Isolation and Reverse Transcriptase (RT)-PCR
4.8. Western Blot
4.9. Immunofluorescence
4.10. Statistics
5. Conclusions
Study Limitation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Navarese, E.P.; Buffon, A.; Andreotti, F.; Kozinski, M.; Welton, N.; Fabiszak, T.; Caputo, S.; Grzesk, G.; Kubica, A.; Swiatkiewicz, I.; et al. Meta-analysis of impact of different types and doses of statins on new-onset diabetes mellitus. Am. J. Cardiol. 2013, 111, 1123–1130. [Google Scholar] [CrossRef]
- Leite, G.A.A.; de lima Rosa, J.; Sanabria, M.; Cavariani, M.M.; Franci, J.A.A.; Pinheiro, P.F.F.; Kempinas, W.D.G. Delayed reproductive development in pubertal male rats exposed to the hypolipemiant agent rosuvastatin since prepuberty. Reprod. Toxicol. 2014, 44, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Leite, G.A.A.; de Barros, J.W.F.; de Martins, A.C.; Anselmo-Franci, J.A.; Barbosa, F.; Kempinas, W.D.G. Ascorbic acid supplementation ameliorates testicular hormonal signaling, sperm production and oxidative stress in male rats exposed to rosuvastatin during pre-puberty. J. Appl. Toxicol. 2019, 39, 305–321. [Google Scholar] [CrossRef]
- Leite, G.A.A.; Figueiredo, T.M.; Pacheco, T.L.; Sanabria, M.; e Silva, P.V.; Fernandes, F.H.; Kempinas, W.D.G. Vitamin C partially prevents reproductive damage in adult male rats exposed to rosuvastatin during prepuberty. Food Chem. Toxicol. 2017, 109, 272–283. [Google Scholar] [CrossRef] [PubMed]
- E Silva, P.V.; Borges, C.D.S.; Rosa, J.D.L.; Pacheco, T.L.; Figueiredo, T.M.; Leite, G.A.A.; Guerra, M.T.; Anselmo-Franci, J.A.; Klinefelter, G.R.; Kempinas, W.D.G. Effects of isolated or combined exposure to sibutramine and rosuvastatin on reproductive parameters of adult male rats. J. Appl. Toxicol. 2020, 40, 947–964. [Google Scholar] [CrossRef]
- Akdeniz, E.; Onger, M.E.; Bolat, M.S.; Firat, F.; Gur, M.; Cinar, O.; Bakirtas, M.; Acikgoz, A.; Erdemir, F. Effect of atorvastatin on spermatogenesis in rats: A stereological study. Trop. J. Pharm. Res. 2020, 19, 2609–2614. [Google Scholar] [CrossRef]
- Heeba, G.H.; Hamza, A.A. Rosuvastatin ameliorates diabetes-induced reproductive damage via suppression of oxidative stress, inflammatory and apoptotic pathways in male rats. Life Sci. 2015, 141, 13–19. [Google Scholar] [CrossRef]
- Pons-Rejraji, H.; Brugnon, F.; Sion, B.; Maqdasy, S.; Gouby, G.; Pereira, B.; Marceau, G.; Gremeau, A.S.; Drevet, J.; Grizard, G.; et al. Evaluation of atorvastatin efficacy and toxicity on spermatozoa, accessory glands and gonadal hormones of healthy men: A pilot prospective clinical trial. Reprod. Biol. Endocrinol. 2014, 12, 65. [Google Scholar] [CrossRef]
- Tada, Y.; Kitaya, K.; Hayashi, T.; Iwaki, Y.; Karita, M.; Taguchi, S.; Funabiki, M.; Nakamura, Y. Transient azoospermia following rosuvastatin medication for hypercholesterolemia. Clin. Exp. Obstet. Gynecol. 2015, 42, 545–546. [Google Scholar] [CrossRef]
- Bruckert, E.; Giral, P.; Heshmati, H.M.; Turpin, G. Men treated with hypolipidaemic drugs complain more frequently of erectile dysfunction. J. Clin. Pharm. Ther. 1996, 21, 89–94. [Google Scholar] [CrossRef]
- De Graaf, L.; Brouwers, A.H.P.M.; Diemont, W.L. Is decreased libido associated with the use of HMG-CoA-reductase inhibitors? Br. J. Clin. Pharmacol. 2004, 58, 326–328. [Google Scholar] [CrossRef] [PubMed]
- Rastrelli, G.; Corona, G.; Maggi, M. Testosterone and sexual function in men. Maturitas 2018, 112, 46–52. [Google Scholar] [CrossRef]
- Rastrelli, G.; Corona, G.; Tarocchi, M.; Mannucci, E.; Maggi, M. How to define hypogonadism? Results from a population of men consulting for sexual dysfunction. J. Endocrinol. Investig. 2016, 39, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Smals, A.G.H.; Weusten, J.J.A.M.; Benraad, T.J. The HMG-CoA reductase inhibitor simvastatin suppresses human testicular testosterone synthesis in vitro by a selective inhibitory effect on 17-ketosteroid-oxidoreductase enzyme activity. J. Steroid Biochem. Mol. Biol. 1991, 38, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Linnebur, S.A.; Hiatt, W.H. Probable statin-induced testicular pain. Ann. Pharmacother. 2007, 41, 138–142. [Google Scholar] [CrossRef]
- Klinefelter, R.G.; Laskey, J.W.; Amann, R.P. Statin drugs markedly inhibit testosterone production by rat Leydig cells in vitro: Implications for men. Reprod. Toxicol. 2014, 45, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Rezvani, M.R.; Saadatjoo, S.A.; Sorouri, S.; Fard, M.H. Comparison of serum free testosterone, luteinizing hormone and follicle stimulating hormone levels in diabetics and non-diabetics men- a case-control study. J. Res. Health Sci. 2012, 12, 98–100. [Google Scholar] [CrossRef]
- Omolaoye, T.S.; Halabi, M.O.; Mubarak, M.; Cyril, A.C.; Duvuru, R.; Radhakrishnan, R.; Du Plessis, S.S. Statins and Male Fertility: Is There a Cause for Concern? Toxics 2022, 10, 627. [Google Scholar] [CrossRef]
- Jay, R.; Sturley, R.; Stirling, C.; McGarrigle, H.; Katz, M.; Reckless, J.; Betteridge, D. Effects of pravastatin and cholestyramine on gonadal and adrenal steroid production in familial hypercholesterolaemia. Br. J. Clin. Pharmacol. 1991, 32, 417–422. [Google Scholar] [CrossRef]
- Mohamad, N.V.; Wong, S.K.; Wan Hasan, W.N.; Jolly, J.J.; Nur-Farhana, M.F.; Ima-Nirwana, S.; Chin, K.Y. The relationship between circulating testosterone and inflammatory cytokines in men. Aging Male 2019, 22, 129–140. [Google Scholar] [CrossRef]
- Moore, C.; Gupta, R.; Jordt, S.E.; Chen, Y.; Liedtke, W.B. Regulation of Pain and Itch by TRP Channels. Neurosci. Bull. 2018, 34, 120–142. [Google Scholar] [CrossRef]
- Zhang, W.; Luo, H.-L.; Zhu, Z.-M. The role of P2X4 receptors in chronic pain: A potential pharmacological target. Biomed. Pharmacother. 2020, 129, 110447. [Google Scholar] [CrossRef]
- Dong, C.; Zhang, W.-J.; Luo, H.-L. Association between P2X3 receptors and neuropathic pain: As a potential therapeutic target for therapy. Biomed. Pharmacother. 2022, 150, 113029. [Google Scholar] [CrossRef]
- Chen, L.K.; Chen, S.S. Risk factors for developing pain in normospermic patients with varicocoele. Int. J. Androl. 2012, 35, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Mizrak, S.C.; van Dissel-Emiliani, F.M.F. Transient receptor potential vanilloid receptor-1 confers heat resistance to male germ cells. Fertil. Steril. 2008, 90, 1290–1293. [Google Scholar] [CrossRef] [PubMed]
- Pilutin, A.; Misiakiewicz-Has, K.; Kolasa, A.; Baranowska-Bosiacka, I.; Marchlewicz, M.; Wiszniewska, B. The immunoexpression of androgen receptor, estrogen receptors α and β, vanilloid type 1 receptor and cytochrome p450 aromatase in rats testis chronically treated with letrozole, an aromatase inhibitor. Folia Histochem. Cytobiol. 2014, 52, 206–217. [Google Scholar] [CrossRef]
- Abbas, M.A. Modulation of TRPV1 channel function by natural products in the treatment of pain. Chem. Biol. Interact. 2020, 330, 109178. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Yang, P.; Mack, M.R.; Dryn, D.; Luo, J.; Gong, X.; Liu, S.; Oetjen, L.K.; Zholos, A.V.; Mei, Z.; et al. Sensory TRP channels contribute differentially to skin inflammation and persistent itch. Nat. Commun. 2017, 8, 980. [Google Scholar] [CrossRef]
- Caterina, M.J.; Julius, D. The vanilloid receptor: A molecular gateway to the pain pathway. Annu. Rev. Neurosci. 2001, 24, 487–517. [Google Scholar] [CrossRef]
- Su, K.H.; Lin, S.J.; Wei, J.; Lee, K.I.; Zhao, J.F.; Shyue, S.K.; Lee, T.S. The essential role of transient receptor potential vanilloid 1 in simvastatin-induced activation of endothelial nitric oxide synthase and angiogenesis. Acta Physiol. 2014, 212, 191–204. [Google Scholar] [CrossRef]
- Camargo, M.; Ibrahim, E.; Intasqui, P.; Belardin, L.B.; Antoniassi, M.P.; Lynne, C.M.; Brackett, N.L.; Bertolla, R.P. Seminal inflammasome activity in the adult varicocele. Hum. Fertil. 2021, 25, 548–556. [Google Scholar] [CrossRef]
- Leite, G.A.A.; Sanabria, M.; Cavariani, M.M.; Anselmo-Franci, J.A.; Pinheiro, P.F.F.; Domeniconi, R.F.; Kempinas, W.D.G. Lower sperm quality and testicular and epididymal structural impairment in adult rats exposed to rosuvastatin during prepuberty. J. Appl. Toxicol. 2018, 38, 914–929. [Google Scholar] [CrossRef]
- Shalaby, M.A.; El Zorba, H.Y.; Kamel, G.M. Effect of alpha-tocopherol and simvastatin on male fertility in hypercholesterolemic rats. Pharmacol. Res. 2004, 50, 137–142. [Google Scholar] [CrossRef]
- Al-Hilli, A.S.; Ali, E.S.; Ali, A.S.; Mhao, N.S.; Hadi, N.A.; Jamil, D.A.; Al-Aubaidy, H.A. Assessment of spermatozoal oxidative stress response to simvastatin in male infertility. Iraq Med. J. 2018, 2, 36–39. [Google Scholar]
- Purvis, K.; Tollefsrud, A.; Rui, H.; Haug, E.; Norseth, J.; Viksmoen, L.; Ose, L.; Lund, H. Short-term effects of treatment with simvastatin on testicular function in patients with heterozygous familial hypercholesterolaemia. Eur. J. Clin. Pharmacol. 1992, 42, 61–64. [Google Scholar] [CrossRef]
- Du Souich, P.; Roederer, G.; Dufour, R. Myotoxicity of statins: Mechanism of action. Pharmacol. Ther. 2017, 175, 1–16. [Google Scholar] [CrossRef]
- Hau, R.K.; Klein, R.R.; Wright, S.H.; Cherrington, N.J. Localization of Xenobiotic Transporters Expressed at the Human Blood-Testis Barrier. Drug Metab. Dispos. 2022, 50, 770–780. [Google Scholar] [CrossRef]
- Augustine, L.M.; Markelewicz, R.J.; Boekelheide, K.; Cherrington, N.J. Xenobiotic and endobiotic transporter mRNA expression in the blood-testis barrier. Drug Metab. Dispos. 2005, 33, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Hau, R.K.; Miller, S.R.; Wright, S.H.; Cherrington, N.J. Generation of a hTERT-Immortalized Human Sertoli Cell Model to Study Transporter Dynamics at the Blood-Testis Barrier. Pharmaceutics 2020, 12, 1005. [Google Scholar] [CrossRef] [PubMed]
- Schnabolk, G.W.; Gupta, B.; Mulgaonkar, A.; Kulkarni, M.; Sweet, D.H. Organic anion transporter 6 (Slc22a20) specificity and sertoli cell-specific expression provide new insight on potential endogenous roles. J. Pharmacol. Exp. Ther. 2010, 334, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Onogawa, T.; Asano, N.; Mizutamari, H.; Mikkaichi, T.; Tanemoto, M.; Abe, M.; Satoh, F.; Unno, M.; Nunoki, K.; et al. Identification and characterization of novel rat and human gonad-specific organic anion transporters. Mol. Endocrinol. 2003, 17, 1203–1215. [Google Scholar] [CrossRef] [PubMed]
- Zaher, H.; Zu Schwabedissen, H.E.M.; Tirana, R.G.; Cox, M.L.; Obert, L.A.; Agrawal, N.; Palandra, J.; Stock, J.L.; Kim, R.B.; Ware, J.A. Targeted disruption of murine organic anion-transporting polypeptide 1b2 (oatp1 b2/slco1b2) significantly alters disposition of prototypical drug substrates pravastatin and rifampin. Mol. Pharmacol. 2008, 74, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, R.; Balasubramanian, K. Effects of gamma radiation on luteinizing hormone (LH) receptor expression, signal transduction and steroidogenesis in cultured rat Leydig cells. Int. J. Radiat. Biol. 2005, 81, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Narayan, P. Genetic Models for the Study of Luteinizing Hormone Receptor Function. Front. Endocrinol. 2015, 6, 152. [Google Scholar] [CrossRef] [PubMed]
- Derkach, K.V.; Bakhtyukov, A.A.; Morina, I.Y.; Romanova, I.V.; Bayunova, L.V.; Shpakov, A.O. Comparative Study of the Restoring Effect of Metformin, Gonadotropin, and Allosteric Agonist of Luteinizing Hormone Receptor on Spermatogenesis in Male Rats with Streptozotocin-Induced Type 2 Diabetes Mellitus. Bull. Exp. Biol. Med. 2022, 172, 435–440. [Google Scholar] [CrossRef]
- Kero, J.; Poutanen, M.; Zhang, F.; Rahman, N.; Mcnicol, A.M.; Nilson, J.H.; Keri, R.A.; Huhtaniemi, I.T. Elevated luteinizing hormone induces expression of its receptor and promotes steroidogenesis in the adrenal cortex. J. Clin. Investig. 2000, 105, 633–641. [Google Scholar] [CrossRef]
- Azzarito, C.; Boiardi, L.; Zini, M.; Agosti, A.; Dotti, C.; Biagi, R.; Portioli, I. Long-term therapy with high-dose simvastatin does not affect adrenocortical and gonadal hormones in hypercholesterolemic patients. Metabolism 1992, 41, 148–153. [Google Scholar] [CrossRef]
- Kelly, D.M.; Sellers, D.J.; Woodroofe, M.N.; Jones, T.H.; Channer, K.S. Effect of testosterone on inflammatory markers in the development of early atherogenesis in the testicular-feminized mouse model. Endocr. Res. 2013, 38, 125–138. [Google Scholar] [CrossRef]
- Demirtaş Şahin, T.; Yazir, Y.; Utkan, T.; Gacar, G.; Furat Rençber, S.; Gocmez, S.S. TNF-α antagonism with etanercept enhances penile NOS expression, cavernosal reactivity, and testosterone levels in aged rats. Can. J. Physiol. Pharmacol. 2018, 96, 200–207. [Google Scholar] [CrossRef]
- Bobjer, J.; Katrinaki, M.; Tsatsanis, C.; Lundberg Giwercman, Y.; Giwercman, A. Negative Association between Testosterone Concentration and Inflammatory Markers in Young Men: A Nested Cross-Sectional Study. PLoS ONE 2013, 8, 61466. [Google Scholar] [CrossRef]
- Malkin, C.J.; Pugh, P.J.; Jones, R.D.; Kapoor, D.; Channer, K.S.; Jones, T.H. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J. Clin. Endocrinol. Metab. 2004, 89, 3313–3318. [Google Scholar] [CrossRef]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef]
- Ma, L.; Zhong, J.; Zhao, Z.; Luo, Z.; Ma, S.; Sun, J.; He, H.; Zhu, T.; Liu, D.; Zhu, Z.; et al. Activation of TRPV1 reduces vascular lipid accumulation and attenuates atherosclerosis. Cardiovasc. Res. 2011, 92, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Amorim, M.A.; Jentsch Matias de Oliveira, J.R.; Souza Oliveira, V.H.; Cabrini, D.A.; Otuki, M.F.; André, E. Role of nitric oxide, bradykinin B2 receptor, and TRPV1 in the airway alterations caused by simvastatin in rats. Eur. J. Pharmacol. 2021, 912, 174591. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.; Wang, S.; Shao, F.; Fang, J.; Xu, Y.; Wang, W.; Sun, H.; Liu, X.; Du, J.; Fang, J. Electroacupuncture stimulation alleviates CFA-induced inflammatory pain via suppressing P2X3 expression. Int. J. Mol. Sci. 2019, 20, 3248. [Google Scholar] [CrossRef]
- Xia, L.P.; Luo, H.; Ma, Q.; Xie, Y.K.; Li, W.; Hu, H.; Xu, Z.Z. GPR151 in nociceptors modulates neuropathic pain via regulating P2X3 function and microglial activation. Brain 2021, 144, 3405–3420. [Google Scholar] [CrossRef] [PubMed]
- Maget, M. Guide D’étude Directe des Comportements Culturels; Roosevelt: Paris, France, 1953; Volume 8, ISBN 978-0-309-15400-0. [Google Scholar]
- Chang, Y.F.; Lee-Chang, J.S.; Panneerdoss, S.; MacLean, J.A.; Rao, M.K. Isolation of Sertoli, Leydig, and spermatogenic cells from the mouse testis. Biotechniques 2011, 51, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Auzanneau, C.; Norez, C.; Noël, S.; Jougla, C.; Becq, F.; Vandebrouck, C. Pharmacological profile of inhibition of the chloride channels activated by extracellular acid in cultured rat Sertoli cells. Reprod. Nutr. Dev. 2006, 46, 241–255. [Google Scholar] [CrossRef]
- Omolaoye, T.; Windvogel, S.; du Plessis, S. Testicular oxidative stress and apoptosis status in streptozotocin-induced diabetic rats after treatment with rooibos (Aspalathus linearis), honeybush (Cyclopia intermedia), and sutherlandia (Lessertia frutescens) infusions. Asian Pac. J. Reprod. 2021, 10, 11–20. [Google Scholar] [CrossRef]
- Omolaoye, T.; Plessis, S. Du Descriptive histomorphological evaluation of the testis and caudal epididymis following treatment with rooibos (Aspalathus linearis), honeybush (Cyclopia intermedia) and sutherlandia (Lessertia frutescens) in healthy and streptozotocin-induced diabetic rats. Asian Pac. J. Reprod. 2021, 10, 176. [Google Scholar]




| Genes | Forward | Reverse | Amplicon Size |
|---|---|---|---|
| TRPV1 | TCCAAGGCACTTGCTCCATT | TGGAGGTGGCTTGCAGTTAG | 181 |
| P2X1 | CGGATGGTGCTGGTACGAAA | CTTCACGGACACACTGCTGA | 147 |
| FSHR | CTGTACATCAACCCGGAGGC | TGAAGGAGTTCCTGGCAACG | 172 |
| LHR | GCCTCGCCAGACTATCTCT | TTGAGGAGGTTGTCAAAGGC | 148 |
| P2X3 | AGGACATAAAGAGGTGCCGC | GGCCTTGTCTAGATCGCACA | 164 |
| IL-6 | CACTTCACAAGTCGGAGGCT | TCTGACAGTGCATCATCGCT | 114 |
| SLCO1B2 | GAGCTCAGGGTAGACGACCA | TGTAGCTGAAGGAGAGGGCT | 197 |
| IL-17A | AGCTGATCAGGACGAGCGAC | GGGATGAGTACCGCTGCCTT | 110 |
| GAPDH | CCGCATCTTCTTGTGCAGTG | CGATACGGCCAAATCCGTTC | 79 |
| GAPDH-H | AGGTCGGAGTCAACGGATTT | TGACAAGCTTCCCGTTCTCA | 192 |
| SLCO1B1-H | AAAGGGTGGACTTGTTGCAG | GTGACAGAGCTGCCAAGAAC | 210 |
| SLCO1B3-H | CGTTGTTCCCACCTCCTAGA | TGAATCCATTGCAGCGTCTT | 196 |
| IL-17B-H | ATTCTCTCGGCGGCATCTG | CCTTCCTCTTGCTTTTGGGG | 158 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omolaoye, T.S.; Cyril, A.C.; Radhakrishnan, R.; Rawat, S.S.; Karuvantevida, N.; du Plessis, S.S. The Effect of Statins on Male Reproductive Parameters: A Mechanism Involving Dysregulation of Gonadal Hormone Receptors and TRPV1. Int. J. Mol. Sci. 2023, 24, 9221. https://doi.org/10.3390/ijms24119221
Omolaoye TS, Cyril AC, Radhakrishnan R, Rawat SS, Karuvantevida N, du Plessis SS. The Effect of Statins on Male Reproductive Parameters: A Mechanism Involving Dysregulation of Gonadal Hormone Receptors and TRPV1. International Journal of Molecular Sciences. 2023; 24(11):9221. https://doi.org/10.3390/ijms24119221
Chicago/Turabian StyleOmolaoye, Temidayo S., Asha C. Cyril, Rajan Radhakrishnan, Surendra Singh Rawat, Noushad Karuvantevida, and Stefan S. du Plessis. 2023. "The Effect of Statins on Male Reproductive Parameters: A Mechanism Involving Dysregulation of Gonadal Hormone Receptors and TRPV1" International Journal of Molecular Sciences 24, no. 11: 9221. https://doi.org/10.3390/ijms24119221
APA StyleOmolaoye, T. S., Cyril, A. C., Radhakrishnan, R., Rawat, S. S., Karuvantevida, N., & du Plessis, S. S. (2023). The Effect of Statins on Male Reproductive Parameters: A Mechanism Involving Dysregulation of Gonadal Hormone Receptors and TRPV1. International Journal of Molecular Sciences, 24(11), 9221. https://doi.org/10.3390/ijms24119221








