MicroRNA-29a Mitigates Laminectomy-Induced Spinal Epidural Fibrosis and Gait Dysregulation by Repressing TGF-β1 and IL-6
Abstract
1. Introduction
2. Results
2.1. Mouse Model for Lumbar Laminectomy (LM) Procedures Characterization
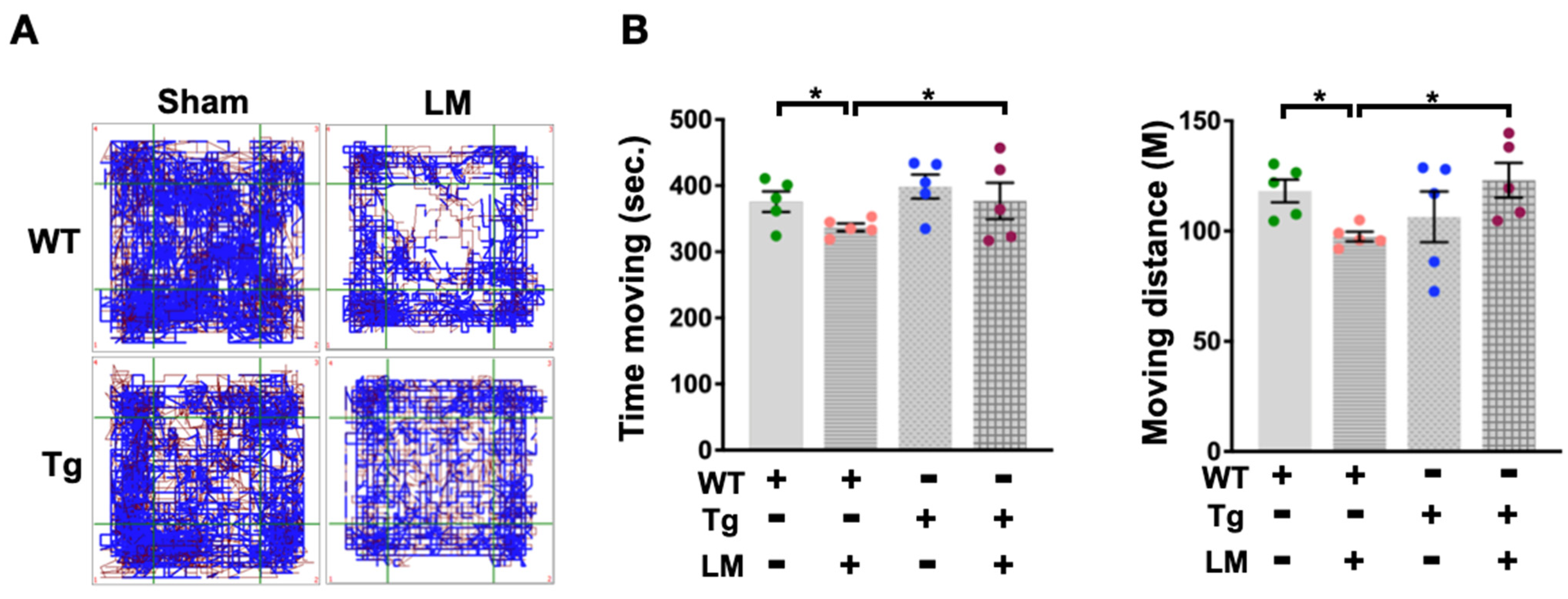
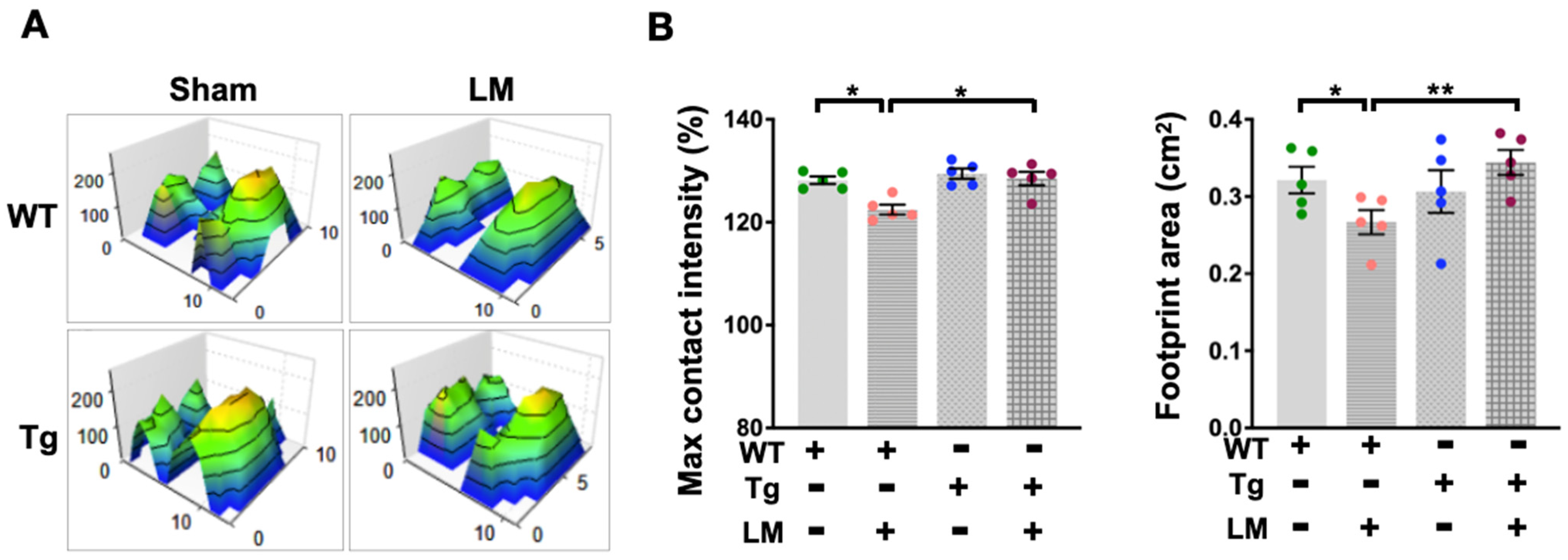
2.2. miR-29a Overexpression Improved LM-Induced Behavior Alteration
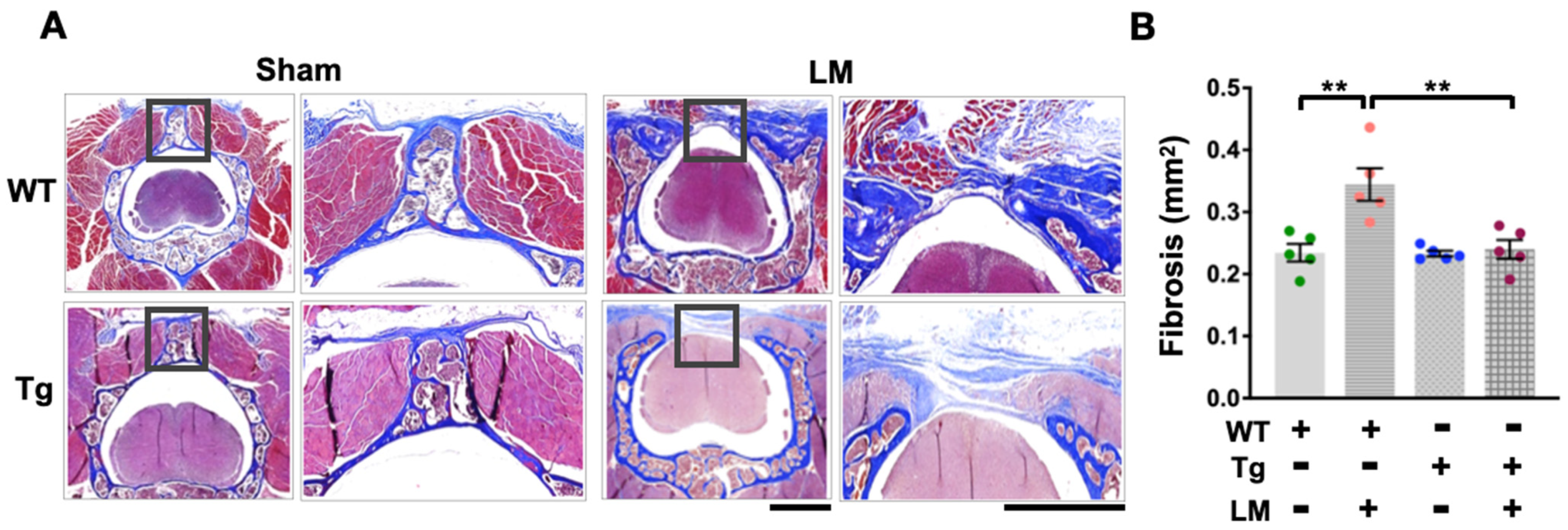
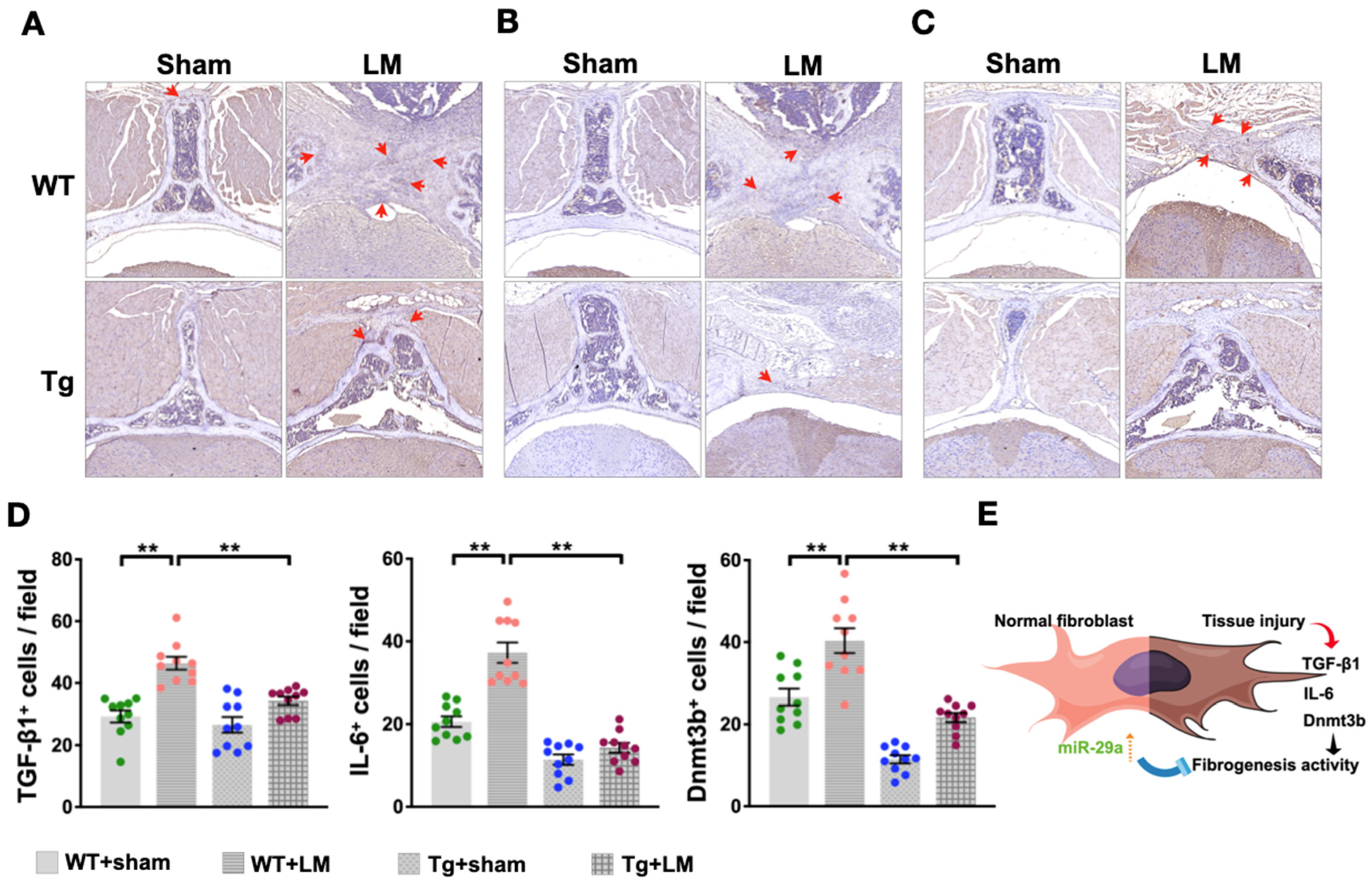
2.3. Overexpression of miR-29a Downregulates Fibrotic Matrix Formation and Expression of Related Genes in Lumbar Spinal Cord
3. Discussion
4. Materials and Methods
4.1. Transgenic Mice Overexpressing MiR-29a
4.2. Lumbar Laminectomy Model
4.3. Assessment of Gait Profile and Mobility Patterns
4.4. Lumbar Tissue for Fibrotic Activity Quantification and Immunohistochemical Analysis
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bozkurt, I.; Kazanci, A.; Gurcan, O.; Gurcay, A.G.; Arikok, A.T.; Bavbek, M. Spinal epidural fibrosis following hemostatic agent employment. Br. J. Neurosurg. 2023, 37, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Hussain, N.; Orhurhu, V.; D’Souza, R. Spinal Cord Burst Stimulation vs Placebo Stimulation for Patients with Chronic Radicular Pain After Lumbar Spine Surgery. JAMA 2023, 329, 845–846. [Google Scholar] [CrossRef]
- Taccola, G.; Barber, S.; Horner, P.J.; Bazo, H.A.C.; Sayenko, D. Complications of epidural spinal stimulation: Lessons from the past and alternatives for the future. Spinal Cord 2020, 58, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Hahn, B.S.; Park, J.Y. Incorporating New Technologies to Overcome the Limitations of Endoscopic Spine Surgery: Navigation, Robotics, and Visualization. World Neurosurg. 2021, 145, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; Han, Q.; Chen, H.; Zhang, A.; Liu, Y.; Gong, X.; Wang, Y.; Wang, J.; Wu, M. Artificial lamina after laminectomy: Progress, applications, and future perspectives. Front. Surg. 2023, 10, 1019410. [Google Scholar] [CrossRef]
- Lin, C.Y.; Liu, T.Y.; Chen, M.H.; Sun, J.S.; Chen, M.H. An injectable extracellular matrix for the reconstruction of epidural fat and the prevention of epidural fibrosis. Biomed. Mater. 2016, 11, 035010. [Google Scholar] [CrossRef]
- Sun, K.; Li, X.; Scherer, P.E. Extracellular Matrix (ECM) and Fibrosis in Adipose Tissue: Overview and Perspectives. Compr. Physiol. 2023, 13, 4387–4407. [Google Scholar] [CrossRef]
- Yang, L.; Tang, J.; Chen, H.; Ge, D.; Sui, T.; Que, J.; Cao, X.; Ge, Y. Taurine Reduced Epidural Fibrosis in Rat Models after Laminectomy via Downregulating EGR1. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2016, 38, 2261–2271. [Google Scholar] [CrossRef]
- Qian, Z.Y.; Jiang, F.; Tang, J.; Ge, D.W.; Chen, H.T.; Zheng, S.N.; Cao, X.J.; Ge, Y.B.; Yang, L. Potential roles of lncRNA-Cox2 and EGR1 in regulating epidural fibrosis following laminectomy. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 7191–7199. [Google Scholar] [CrossRef]
- Ugwoke, C.K.; Cvetko, E.; Umek, N. Pathophysiological and Therapeutic Roles of Fascial Hyaluronan in Obesity-Related Myofascial Disease. Int. J. Mol. Sci. 2022, 23, 11843. [Google Scholar] [CrossRef]
- Geudeke, M.W.; Krediet, A.C.; Bilecen, S.; Huygen, F.; Rijsdijk, M. Effectiveness of Epiduroscopy for Patients with Failed Back Surgery Syndrome: A Systematic Review and Meta-analysis. Pain Pract. 2021, 21, 468–481. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Zeng, W.; Han, X.; Qian, T.; Sun, J.; Qi, F.; Liu, C.; Wang, Q.; Jin, H. Honokiol protects against epidural fibrosis by inhibiting fibroblast proliferation and extracellular matrix overproduction in rats post-laminectomy. Int. J. Mol. Med. 2020, 46, 2057–2068. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Kwon, W.K.; Bae, T.; Kim, S.; Lee, J.B.; Cho, T.H.; Park, J.Y.; Kim, K.; Hur, J.K.; Hur, J.W. CCN5 Reduces Ligamentum Flavum Hypertrophy by Modulating the TGF-β Pathway. J. Orthop. Res. 2019, 37, 2634–2644. [Google Scholar] [CrossRef] [PubMed]
- Urits, I.; Schwartz, R.H.; Brinkman, J.; Foster, L.; Miro, P.; Berger, A.A.; Kassem, H.; Kaye, A.D.; Manchikanti, L.; Viswanath, O. An Evidence Based Review of Epidurolysis for the Management of Epidural Adhesions. Psychopharmacol. Bull. 2020, 50, 74–90. [Google Scholar]
- Zhang, C.; Kong, X.; Ning, G.; Liang, Z.; Qu, T.; Chen, F.; Cao, D.; Wang, T.; Sharma, H.S.; Feng, S. All-trans retinoic acid prevents epidural fibrosis through NF-κB signaling pathway in post-laminectomy rats. Neuropharmacology 2014, 79, 275–281. [Google Scholar] [CrossRef]
- Song, Z.; Wu, T.; Sun, J.; Wang, H.; Hua, F.; Nicolas, Y.S.M.; Kc, R.; Chen, K.; Jin, Z.; Liu, J.; et al. Metformin attenuates post-epidural fibrosis by inhibiting the TGF-β1/Smad3 and HMGB1/TLR4 signaling pathways. J. Cell. Mol. Med. 2021, 25, 3272–3283. [Google Scholar] [CrossRef]
- Le, L.T.; Swingler, T.E.; Crowe, N.; Vincent, T.L.; Barter, M.J.; Donell, S.T.; Delany, A.M.; Dalmay, T.; Young, D.A.; Clark, I.M. The microRNA-29 family in cartilage homeostasis and osteoarthritis. J. Mol. Med. 2016, 94, 583–596. [Google Scholar] [CrossRef]
- Han, X.; Wang, S.; Yong, Z.; Zhang, X.; Wang, X. miR-29b ameliorates atrial fibrosis in rats with atrial fibrillation by targeting TGFβRΙ and inhibiting the activation of Smad-2/3 pathway. J. Bioenerg. Biomembr. 2022, 54, 81–91. [Google Scholar] [CrossRef]
- Hussein, R.M.; Arafa, E.A.; Raheem, S.A.; Mohamed, W.R. Thymol protects against bleomycin-induced pulmonary fibrosis via abrogation of oxidative stress, inflammation, and modulation of miR-29a/TGF-β and PI3K/Akt signaling in mice. Life Sci. 2023, 314, 121256. [Google Scholar] [CrossRef]
- Liu, H.; Yan, L.; Li, X.; Li, D.; Wang, G.; Shen, N.N.; Li, J.J.; Wang, B. MicroRNA expression in osteoarthritis: A meta-analysis. Clin. Exp. Med. 2023. [Google Scholar] [CrossRef]
- Lin, C.L.; Lee, P.H.; Hsu, Y.C.; Lei, C.C.; Ko, J.Y.; Chuang, P.C.; Huang, Y.T.; Wang, S.Y.; Wu, S.L.; Chen, Y.S.; et al. MicroRNA-29a promotion of nephrin acetylation ameliorates hyperglycemia-induced podocyte dysfunction. J. Am. Soc. Nephrol. JASN 2014, 25, 1698–1709. [Google Scholar] [CrossRef] [PubMed]
- Szostak, J.; Gorący, A.; Durys, D.; Dec, P.; Modrzejewski, A.; Pawlik, A. The Role of MicroRNA in the Pathogenesis of Diabetic Nephropathy. Int. J. Mol. Sci. 2023, 24, 6214. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Elfimova, N.; Müller, M.; Bachurski, D.; Koitzsch, U.; Drebber, U.; Mahabir, E.; Hansen, H.P.; Friedman, S.L.; Klein, S.; et al. Autophagy-Related Activation of Hepatic Stellate Cells Reduces Cellular miR-29a by Promoting Its Vesicular Secretion. Cell. Mol. Gastroenterol. Hepatol. 2022, 13, 1701–1716. [Google Scholar] [CrossRef] [PubMed]
- Li, S.C.; Wang, F.S.; Yang, Y.L.; Tiao, M.M.; Chuang, J.H.; Huang, Y.H. Microarray Study of Pathway Analysis Expression Profile Associated with MicroRNA-29a with Regard to Murine Cholestatic Liver Injuries. Int. J. Mol. Sci. 2016, 17, 324. [Google Scholar] [CrossRef]
- Wang, Y.; Han, X.; Zang, T.; Kang, P.; Jiang, W.; Niu, N. miR-29b enhances the proliferation and migration of bone marrow mesenchymal stem cells in rats with castration-induced osteoporosis through the PI3K/AKT and TGF-β/Smad signaling pathways. Exp. Ther. Med. 2020, 20, 3185–3195. [Google Scholar] [CrossRef]
- Wu, R.W.; Lian, W.S.; Chen, Y.S.; Kuo, C.W.; Ke, H.C.; Hsieh, C.K.; Wang, S.Y.; Ko, J.Y.; Wang, F.S. MicroRNA-29a Counteracts Glucocorticoid Induction of Bone Loss through Repressing TNFSF13b Modulation of Osteoclastogenesis. Int. J. Mol. Sci. 2019, 20, 5141. [Google Scholar] [CrossRef]
- Ahmed, R.U.; Alam, M.; Zheng, Y.P. Experimental spinal cord injury and behavioral tests in laboratory rats. Heliyon 2019, 5, e01324. [Google Scholar] [CrossRef]
- Muhammad, S.; Roeske, S.; Chaudhry, S.R.; Kinfe, T.M. Burst or High-Frequency (10 kHz) Spinal Cord Stimulation in Failed Back Surgery Syndrome Patients with Predominant Back Pain: One Year Comparative Data. Neuromodulation 2017, 20, 661–667. [Google Scholar] [CrossRef]
- Alizadeh, R.; Sharifzadeh, S.R. Pathogenesis, etiology and treatment of failed back surgery syndrome. Neurochirurgie 2022, 68, 426–431. [Google Scholar] [CrossRef]
- Cho, J.H.; Lee, J.H.; Song, K.S.; Hong, J.Y.; Joo, Y.S.; Lee, D.H.; Hwang, C.J.; Lee, C.S. Treatment Outcomes for Patients with Failed Back Surgery. Pain Physician 2017, 20, E29–E43. [Google Scholar] [CrossRef]
- Mohi Eldin, M.M.; Abdel Razek, N.M. Epidural Fibrosis after Lumbar Disc Surgery: Prevention and Outcome Evaluation. Asian Spine J. 2015, 9, 370–385. [Google Scholar] [CrossRef] [PubMed]
- Traeger, A.C.; Gilbert, S.E.; Harris, I.A.; Maher, C.G. Spinal cord stimulation for low back pain. Cochrane Database Syst. Rev. 2023, 3, CD014789. [Google Scholar] [CrossRef]
- Caldo, D.; Bologna, S.; Conte, L.; Amin, M.S.; Anselma, L.; Basile, V.; Hossain, M.M.; Mazzei, A.; Heritier, P.; Ferracini, R.; et al. Machine learning algorithms distinguish discrete digital emotional fingerprints for web pages related to back pain. Sci. Rep. 2023, 13, 4654. [Google Scholar] [CrossRef]
- Karjalainen, T.V.; Silagy, M.; O’Bryan, E.; Johnston, R.V.; Cyril, S.; Buchbinder, R. Autologous blood and platelet-rich plasma injection therapy for lateral elbow pain. Cochrane Database Syst. Rev. 2021, 9, Cd010951. [Google Scholar] [CrossRef]
- Hamm-Faber, T.E.; Gültuna, I.; van Gorp, E.J.; Aukes, H. High-Dose Spinal Cord Stimulation for Treatment of Chronic Low Back Pain and Leg Pain in Patients with FBSS, 12-Month Results: A Prospective Pilot Study. Neuromodulation 2020, 23, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Parisien, M.; Lima, L.V.; Dagostino, C.; El-Hachem, N.; Drury, G.L.; Grant, A.V.; Huising, J.; Verma, V.; Meloto, C.B.; Silva, J.R.; et al. Acute inflammatory response via neutrophil activation protects against the development of chronic pain. Sci. Transl. Med. 2022, 14, eabj9954. [Google Scholar] [CrossRef]
- Wang, B.B.; Xie, H.; Wu, T.; Xie, N.; Wu, J.; Gu, Y.; Tang, F.; Liu, J. Controlled-release mitomycin C-polylactic acid film prevents epidural scar hyperplasia after laminectomy by inducing fibroblast autophagy and regulating the expression of miRNAs. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 2526–2537. [Google Scholar] [PubMed]
- Yang, L.; Gao, Q.; Lv, F.; Guo, M.; Zhao, D. miR-519d-3p promotes TGFβ/Smad mediated postoperative epidural scar formation via suppression of BAMBI. Mol. Med. Rep. 2019, 20, 3901–3909. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Hu, X.; Liu, S.; Zou, Y.; Wang, Z.; Chu, Y. The clinical significance and function of miR-146 in the promotion of epidural fibrosis. Genet. Mol. Biol. 2021, 44, e20200447. [Google Scholar] [CrossRef]
- Ko, J.Y.; Lian, W.S.; Tsai, T.C.; Chen, Y.S.; Hsieh, C.K.; Kuo, C.W.; Wang, F.S. MicroRNA-29a Mitigates Subacromial Bursa Fibrosis in Rotator Cuff Lesion with Shoulder Stiffness. Int. J. Mol. Sci. 2019, 20, 5742. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.L.; Wang, F.S.; Lin, H.Y.; Huang, Y.H. Exogenous Therapeutics of Microrna-29a Attenuates Development of Hepatic Fibrosis in Cholestatic Animal Model through Regulation of Phosphoinositide 3-Kinase p85 Alpha. Int. J. Mol. Sci. 2020, 21, 3636. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Huo, Z.; Wu, L.; Liu, F.; Liang, J.; He, X.; Yang, D. The Role of MiR-29 in the Mechanism of Fibrosis. Mini Rev. Med. Chem. 2023. [Google Scholar] [CrossRef]
- Lian, W.S.; Ko, J.Y.; Chen, Y.S.; Ke, H.J.; Hsieh, C.K.; Kuo, C.W.; Wang, S.Y.; Huang, B.W.; Tseng, J.G.; Wang, F.S. MicroRNA-29a represses osteoclast formation and protects against osteoporosis by regulating PCAF-mediated RANKL and CXCL12. Cell Death Dis. 2019, 10, 705. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.L.; Huang, Y.H.; Wang, F.S.; Tsai, M.C.; Chen, C.H.; Lian, W.S. MicroRNA-29a Compromises Hepatic Adiposis and Gut Dysbiosis in High Fat Diet-Fed Mice via Downregulating Inflammation. Mol. Nutr. Food Res. 2023, e2200348. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.; Wang, F.S.; Yang, Y.L.; Huang, Y.H. MicroRNA-29a Suppresses CD36 to Ameliorate High Fat Diet-Induced Steatohepatitis and Liver Fibrosis in Mice. Cells 2019, 8, 1298. [Google Scholar] [CrossRef]
- Ko, J.Y.; Lee, M.S.; Lian, W.S.; Weng, W.T.; Sun, Y.C.; Chen, Y.S.; Wang, F.S. MicroRNA-29a Counteracts Synovitis in Knee Osteoarthritis Pathogenesis by Targeting VEGF. Sci. Rep. 2017, 7, 3584. [Google Scholar] [CrossRef]
- Yang, Y.L.; Kuo, H.C.; Wang, F.S.; Huang, Y.H. MicroRNA-29a Disrupts DNMT3b to Ameliorate Diet-Induced Non-Alcoholic Steatohepatitis in Mice. Int. J. Mol. Sci. 2019, 20, 1499. [Google Scholar] [CrossRef]
- Lian, W.S.; Wu, R.W.; Chen, Y.S.; Ko, J.Y.; Wang, S.Y.; Jahr, H.; Wang, F.S. MicroRNA-29a Mitigates Osteoblast Senescence and Counteracts Bone Loss through Oxidation Resistance-1 Control of FoxO3 Methylation. Antioxidants 2021, 10, 1248. [Google Scholar] [CrossRef]
- Huang, Y.H.; Lian, W.S.; Wang, F.S.; Wang, P.W.; Lin, H.Y.; Tsai, M.C.; Yang, Y.L. MiR-29a Curbs Hepatocellular Carcinoma Incidence via Targeting of HIF-1α and ANGPT2. Int. J. Mol. Sci. 2022, 23, 1636. [Google Scholar] [CrossRef]
- Lian, W.S.; Wu, R.W.; Chen, Y.S.; Ko, J.Y.; Wang, S.Y.; Jahr, H.; Wang, F.S. MicroRNA-29a in Osteoblasts Represses High-Fat Diet-Mediated Osteoporosis and Body Adiposis through Targeting Leptin. Int. J. Mol. Sci. 2021, 22, 9135. [Google Scholar] [CrossRef]
- Zhou, R.; Ren, S.; Li, C.; Zhang, X.; Zhang, W. miR-29a is a potential protective factor for fibrogenesis in gluteal muscle contracture. Physiol. Res. 2020, 69, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Antar, S.A.; Ashour, N.A.; Marawan, M.E.; Al-Karmalawy, A.A. Fibrosis: Types, Effects, Markers, Mechanisms for Disease Progression, and Its Relation with Oxidative Stress, Immunity, and Inflammation. Int. J. Mol. Sci. 2023, 24, 4004. [Google Scholar] [CrossRef] [PubMed]
- Villar, V.H.; Subotički, T.; Đikić, D.; Mitrović-Ajtić, O.; Simon, F.; Santibanez, J.F. Transforming Growth Factor-β1 in Cancer Immunology: Opportunities for Immunotherapy. Adv. Exp. Med. Biol. 2023, 1408, 309–328. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.T.; Meng, X.M. TGF-β/Smad and Renal Fibrosis. Adv. Exp. Med. Biol. 2019, 1165, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, Y.; Chen, S.; Zhou, J.; Li, J.; Cheng, Y. Epigenetics-based therapeutics for myocardial fibrosis. Life Sci. 2021, 271, 119186. [Google Scholar] [CrossRef] [PubMed]
- Alpoim-Moreira, J.; Szóstek-Mioduchowska, A.; Słyszewska, M.; Rebordão, M.R.; Skarzynski, D.J.; Ferreira-Dias, G. 5-Aza-2′-Deoxycytidine (5-Aza-dC, Decitabine) Inhibits Collagen Type I and III Expression in TGF-β1-Treated Equine Endometrial Fibroblasts. Animals 2023, 13, 1212. [Google Scholar] [CrossRef]
- Li, J.; Cen, B.; Chen, S.; He, Y. MicroRNA-29b inhibits TGF-β1-induced fibrosis via regulation of the TGF-β1/Smad pathway in primary human endometrial stromal cells. Mol. Med. Rep. 2016, 13, 4229–4237. [Google Scholar] [CrossRef]
- Yuan, R.; Dai, X.; Li, Y.; Li, C.; Liu, L. Exosomes from miR-29a-modified adipose-derived mesenchymal stem cells reduce excessive scar formation by inhibiting TGF-β2/Smad3 signaling. Mol. Med. Rep. 2021, 24, 758. [Google Scholar] [CrossRef]
- Francés, R.; Mata-Garrido, J.; de la Fuente, R.; Carcelén, M.; Lafarga, M.; Berciano, M.T.; García, R.; Hurlé, M.A.; Tramullas, M. Identification of Epigenetic Interactions between MicroRNA-30c-5p and DNA Methyltransferases in Neuropathic Pain. Int. J. Mol. Sci. 2022, 23, 13994. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, J.; Xiang, Z.; Wu, J.; Huang, Y.; Yang, J. Myocarsdial-derived miR-29a-regulated DNMTs: A novel therapeutic target for myocardial fibrosis. Int. J. Cardiol. 2022, 358, 76. [Google Scholar] [CrossRef]
- Zhang, G.Y.; Wu, L.C.; Liao, T.; Chen, G.C.; Chen, Y.H.; Zhao, Y.X.; Chen, S.Y.; Wang, A.Y.; Lin, K.; Lin, D.M.; et al. A novel regulatory function for miR-29a in keloid fibrogenesis. Clin. Exp. Dermatol. 2016, 41, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Papalia, G.F.; Russo, F.; Vadalà, G.; Pascarella, G.; De Salvatore, S.; Ambrosio, L.; Di Martino, S.; Sammartini, D.; Sammartini, E.; Carassiti, M.; et al. Non-Invasive Treatments for Failed Back Surgery Syndrome: A Systematic Review. Global Spine J. 2023, 13, 1153–1162. [Google Scholar] [CrossRef]
- Sebaaly, A.; Lahoud, M.J.; Rizkallah, M.; Kreichati, G.; Kharrat, K. Etiology, Evaluation, and Treatment of Failed Back Surgery Syndrome. Asian Spine J. 2018, 12, 574–585. [Google Scholar] [CrossRef] [PubMed]
- Mo, W.Y.; Cao, S.Q. MiR-29a-3p: A potential biomarker and therapeutic target in colorectal cancer. Clin. Transl. Oncol. 2023, 25, 563–577. [Google Scholar] [CrossRef]
- Liu, X.; Lv, X.; Liu, Z.; Zhang, M.; Leng, Y. MircoRNA-29a in Astrocyte-derived Extracellular Vesicles Suppresses Brain Ischemia Reperfusion Injury via TP53INP1 and the NF-κB/NLRP3 Axis. Cell. Mol. Neurobiol. 2022, 42, 1487–1500. [Google Scholar] [CrossRef]
- Chen, K.S.; McGinley, L.M.; Kashlan, O.N.; Hayes, J.M.; Bruno, E.S.; Chang, J.S.; Mendelson, F.E.; Tabbey, M.A.; Johe, K.; Sakowski, S.A.; et al. Targeted intraspinal injections to assess therapies in rodent models of neurological disorders. Nat. Protoc. 2019, 14, 331–349. [Google Scholar] [CrossRef]
- Wu, R.W.; Lian, W.S.; Kuo, C.W.; Chen, Y.S.; Ko, J.Y.; Wang, F.S. S100 Calcium Binding Protein A9 Represses Angiogenic Activity and Aggravates Osteonecrosis of the Femoral Head. Int. J. Mol. Sci. 2019, 20, 5786. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, I.-T.; Lin, Y.-H.; Lian, W.-S.; Wang, F.-S.; Wu, R.-W. MicroRNA-29a Mitigates Laminectomy-Induced Spinal Epidural Fibrosis and Gait Dysregulation by Repressing TGF-β1 and IL-6. Int. J. Mol. Sci. 2023, 24, 9158. https://doi.org/10.3390/ijms24119158
Lin I-T, Lin Y-H, Lian W-S, Wang F-S, Wu R-W. MicroRNA-29a Mitigates Laminectomy-Induced Spinal Epidural Fibrosis and Gait Dysregulation by Repressing TGF-β1 and IL-6. International Journal of Molecular Sciences. 2023; 24(11):9158. https://doi.org/10.3390/ijms24119158
Chicago/Turabian StyleLin, I-Ting, Yu-Han Lin, Wei-Shiung Lian, Feng-Sheng Wang, and Re-Wen Wu. 2023. "MicroRNA-29a Mitigates Laminectomy-Induced Spinal Epidural Fibrosis and Gait Dysregulation by Repressing TGF-β1 and IL-6" International Journal of Molecular Sciences 24, no. 11: 9158. https://doi.org/10.3390/ijms24119158
APA StyleLin, I.-T., Lin, Y.-H., Lian, W.-S., Wang, F.-S., & Wu, R.-W. (2023). MicroRNA-29a Mitigates Laminectomy-Induced Spinal Epidural Fibrosis and Gait Dysregulation by Repressing TGF-β1 and IL-6. International Journal of Molecular Sciences, 24(11), 9158. https://doi.org/10.3390/ijms24119158




