Serum IGFBP-1 Concentration as a Predictor of Outcome after Ischemic Stroke—A Prospective Observational Study
Abstract
1. Introduction
2. Results
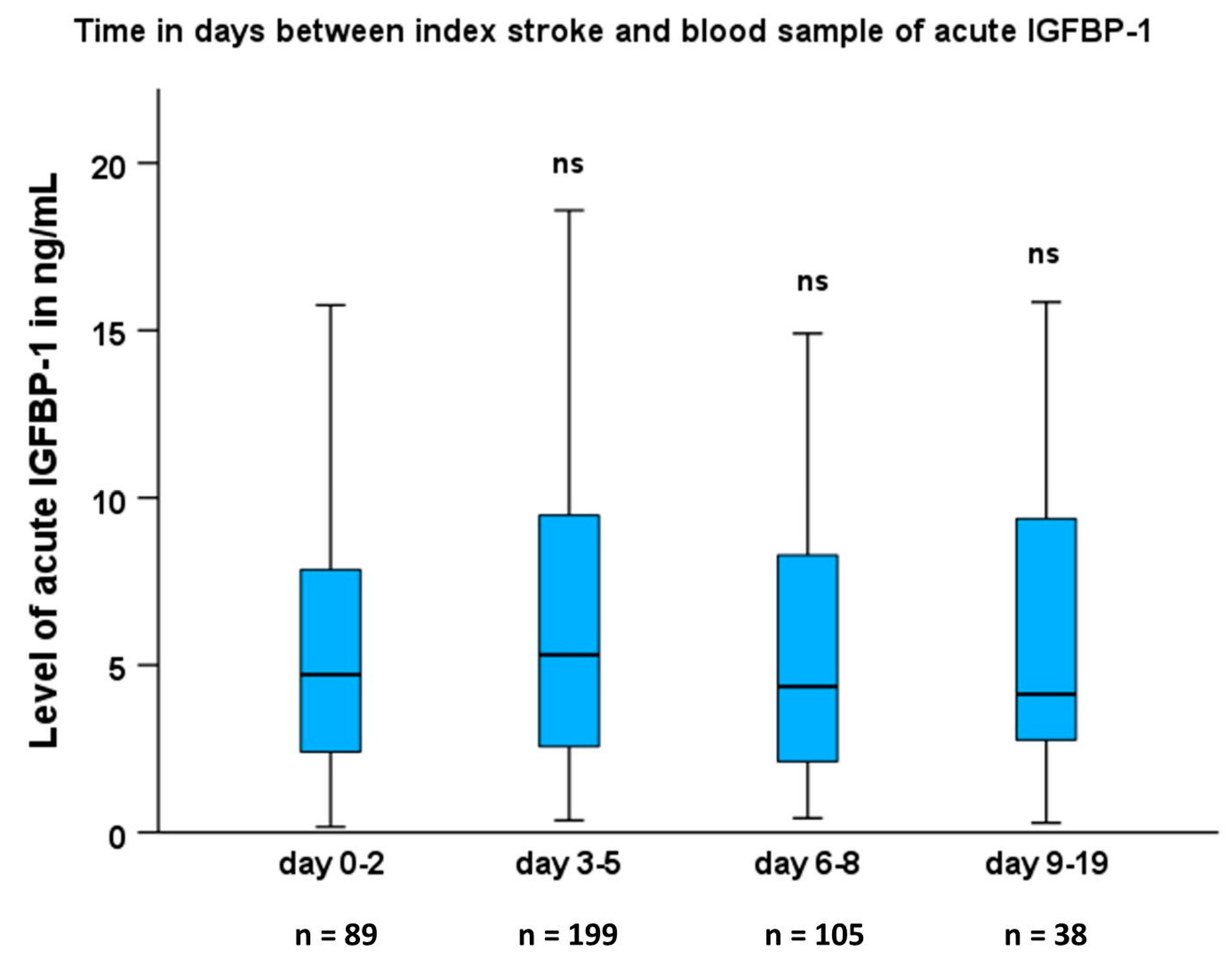
2.1. Baseline Characteristics and s-IGFBP-1 in Patients and Controls
2.2. S-IGFBP-1 in Stroke Etiology Subtypes
2.3. Bivariate Correlations
2.4. S-IGFBP-1 and Functional Outcome
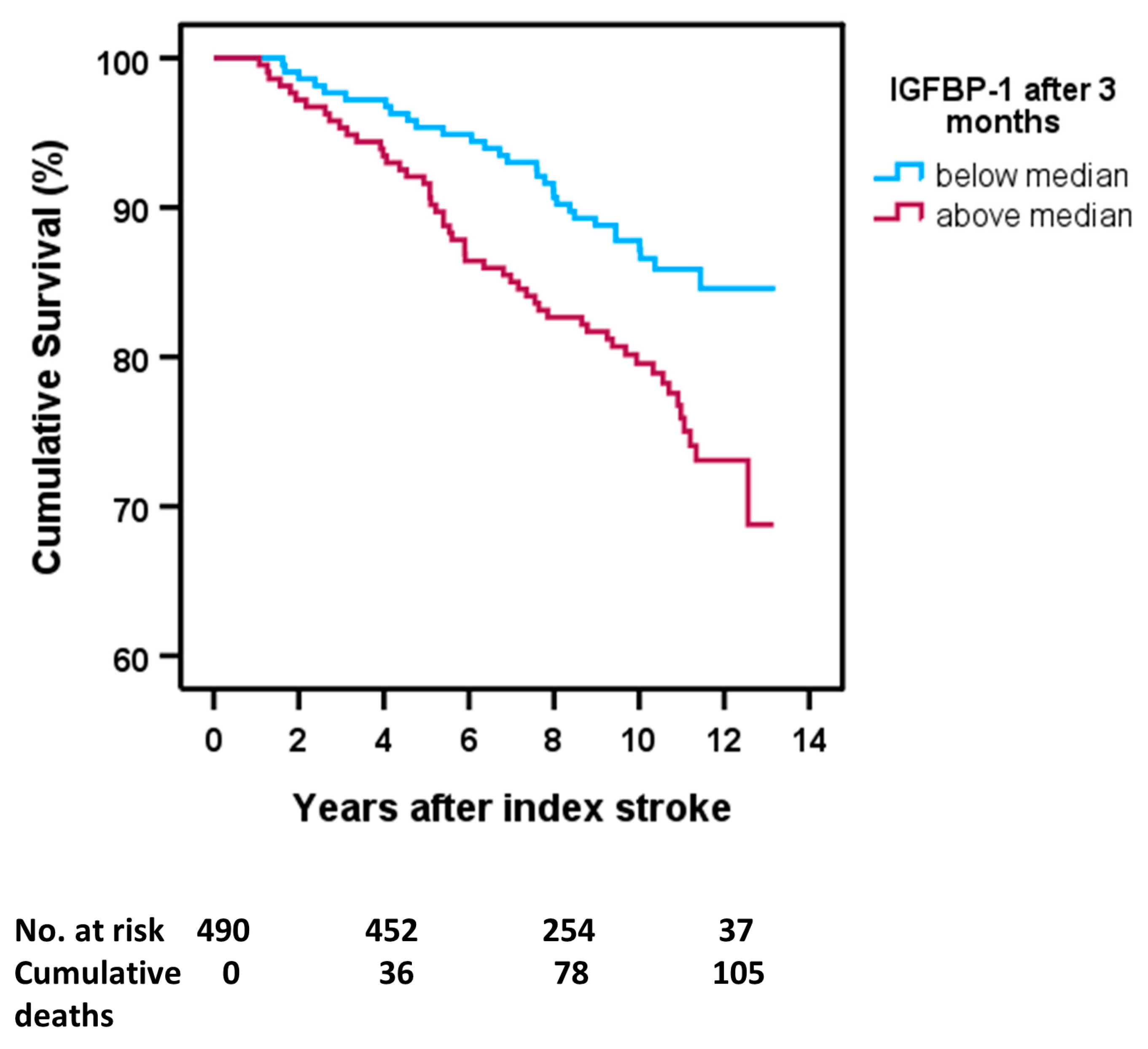
2.5. Poststroke Long-Term Mortality and s-IGFBP-1
2.6. Poststroke Long-Term Mortality and s-IGFBP-1 in Stroke Subtypes
3. Discussion
Strengths and Limitations
4. Materials and Methods
4.1. Patients and Controls
4.2. Classification of Stroke Etiology
4.3. Stroke Severity and Functional Outcome
4.4. Assessment of Covariates and Ethical Considerations
4.5. Biochemical Analysis
4.6. Statistical Evaluation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schneider, H.; Pagotto, U.; Stalla, G. Central effects of the somatotropic system. Eur. J. Endocrinol. 2003, 149, 377–392. [Google Scholar] [CrossRef] [PubMed]
- Russo, V.C.; Gluckman, P.D.; Feldman, E.; Werther, G.A. The Insulin-Like Growth Factor System and Its Pleiotropic Functions in Brain. Endocr. Rev. 2005, 26, 916–943. [Google Scholar] [CrossRef] [PubMed]
- Allard, J.B.; Duan, C. IGF-Binding Proteins: Why Do They Exist and Why Are There So Many? Front. Endocrinol. 2018, 9, 117. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Zhou, X.-X.; Hu, Y.; Li, G.; Wang, Y. The Roles of Insulin-Like Growth Factor Binding Protein Family in Development and Diseases. Adv. Ther. 2020, 38, 885–903. [Google Scholar] [CrossRef]
- Bondanelli, M.; Ambrosio, M.R.; Onofri, A.; Bergonzoni, A.; Lavezzi, S.; Zatelli, M.C.; Valle, D.; Basaglia, N.; degli Uberti, E. Predictive Value of Circulating Insulin-Like Growth Factor I Levels in Ischemic Stroke Outcome. J. Clin. Endocrinol. Metab. 2006, 91, 3928–3934. [Google Scholar] [CrossRef]
- Bondanelli, M.; Ambrosio, M.R.; Zatelli, M.C.; Basaglia, N.; Degli Uberti, E.C. Prevalence of hypopituitarism in patients with cerebrovascular diseases. J. Endocrinol. Investig. 2008, 31, 16–20. [Google Scholar]
- Åberg, D.; Jood, K.; Blomstrand, C.; Jern, C.; Nilsson, M.; Isgaard, J.; Åberg, N.D. Serum IGF-I Levels Correlate to Improvement of Functional Outcome after Ischemic Stroke. J. Clin. Endocrinol. Metab. 2011, 96, E1055–E1064. [Google Scholar] [CrossRef]
- Mattlage, A.E.; Rippee, M.A.; Sandt, J.; Billinger, S.A. Decrease in Insulin-Like Growth Factor-1 and Insulin-Like Growth Factor-1 Ratio in the First Week of Stroke Is Related to Positive Outcomes. J. Stroke Cerebrovasc. Dis. 2016, 25, 1800–1806. [Google Scholar] [CrossRef]
- Åberg, N.D.; Åberg, D.; Jood, K.; Nilsson, M.; Blomstrand, C.; Kuhn, H.G.; Svensson, J.; Jern, C.; Isgaard, J. Altered levels of circulating insulin-like growth factor I (IGF-I) following ischemic stroke are associated with outcome—A prospective observational study. BMC Neurol. 2018, 18, 106. [Google Scholar] [CrossRef]
- Åberg, N.D.; Åberg, D.; Lagging, C.; Holmegaard, L.; Redfors, P.; Jood, K.; Nilsson, M.; Åberg, M.; Blomstrand, C.; Svensson, J.; et al. Association Between Levels of Serum Insulin-like Growth Factor I and Functional Recovery, Mortality, and Recurrent Stroke at a 7-year Follow-up. Exp. Clin. Endocrinol. Diabetes 2019, 128, 303–310. [Google Scholar] [CrossRef]
- Wheatcroft, S.B.; Kearney, M.T. IGF-dependent and IGF-independent actions of IGF-binding protein-1 and -2: Implications for metabolic homeostasis. Trends Endocrinol. Metab. 2009, 20, 153–162. [Google Scholar] [CrossRef]
- Hoeflich, A.; Russo, V.C. Physiology and pathophysiology of IGFBP-1 and IGFBP-2—Consensus and dissent on metabolic control and malignant potential. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 685–700. [Google Scholar] [CrossRef]
- Gibson, J.M.; Westwood, M.; Young, R.J.; White, A. Reduced insulin-like growth factor binding protein-1 (IGFBP-1) levels correlate with increased cardiovascular risk in non-insulin dependent diabetes mellitus (NIDDM). J. Clin. Endocrinol. Metab. 1996, 81, 860–863. [Google Scholar] [CrossRef]
- Lu, J.; Liu, K.; Schulz, N.; Karampelias, C.; Charbord, J.; Hilding, A.; Rautio, L.; Bertolino, P.; Östenson, C.; Brismar, K.; et al. IGFBP 1 increases β-cell regeneration by promoting α- to β-cell transdifferentiation. EMBO J. 2016, 35, 2026–2044. [Google Scholar] [CrossRef]
- Yeap, B.B.; Chubb, S.A.P.; McCaul, K.A.; Ho, K.K.Y.; Hankey, G.J.; Norman, P.E.; Flicker, L. Associations of IGF1 and IGFBPs 1 and 3 with all-cause and cardiovascular mortality in older men: The Health In Men Study. Eur. J. Endocrinol. 2011, 164, 715–723. [Google Scholar] [CrossRef]
- Nolte, A.A.; Movin, M.; Lundin, H.; Salminen, H. IGFBP-1 predicts all-cause mortality in elderly women independently of IGF-I. Growth Horm. IGF Res. 2015, 25, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-L.; Chen, J.-W.; Ting, C.-T.; Lin, S.-J.; Wang, P.H. Changes of the Insulin-Like Growth Factor I System during Acute Myocardial Infarction: Implications on Left Ventricular Remodeling1. J. Clin. Endocrinol. Metab. 1999, 84, 1575–1581. [Google Scholar] [CrossRef]
- Janszky, I.; Hallqvist, J.; Ljung, R.; Hammar, N. Insulin-like growth factor binding protein-1 is a long-term predictor of heart failure in survivors of a first acute myocardial infarction and population controls. Int. J. Cardiol. 2010, 138, 50–55. [Google Scholar] [CrossRef]
- Ritsinger, V.; Brismar, K.; Mellbin, L.; Näsman, P.; Rydén, L.; Söderberg, S.; Norhammar, A. Elevated levels of insulin-like growth factor-binding protein 1 predict outcome after acute myocardial infarction: A long-term follow-up of the glucose tolerance in patients with acute myocardial infarction (GAMI) cohort. Diabetes Vasc. Dis. Res. 2018, 15, 387–395. [Google Scholar] [CrossRef]
- Wallander, M.; Norhammar, A.; Malmberg, K.; Öhrvik, J.; Rydén, L.; Brismar, K. IGF Binding Protein 1 Predicts Cardiovascular Morbidity and Mortality in Patients With Acute Myocardial Infarction and Type 2 Diabetes. Diabetes Care 2007, 30, 2343–2348. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Yang, S.; Zhu, L.; Li, Y.; Chen, Y.; Jin, Y.; Zhao, X.; Zhao, H.; Chen, X.; Zhao, Y.; et al. Association study of IGFBP1 and IGFBP3 polymorphisms with hypertension and cardio-cerebral vascular diseases in a Chinese Han population. Oncotarget 2017, 8, 77836–77845. [Google Scholar] [CrossRef]
- Yao, Y.; Zhu, H.; Zhu, L.; Fang, Z.; Fan, Y.; Liu, C.; Tian, Y.; Chen, Y.; Tang, W.; Ren, Z.; et al. A comprehensive contribution of genetic variations of the insulin-like growth factor 1 signalling pathway to stroke susceptibility. Atherosclerosis 2020, 296, 59–65. [Google Scholar] [CrossRef]
- Kaplan, R.C.; McGinn, A.P.; Pollak, M.N.; Kuller, L.H.; Strickler, H.D.; Rohan, T.E.; Cappola, A.R.; Xue, X.; Psaty, B.M. Association of Total Insulin-Like Growth Factor-I, Insulin-Like Growth Factor Binding Protein-1 (IGFBP-1), and IGFBP-3 Levels with Incident Coronary Events and Ischemic Stroke. J. Clin. Endocrinol. Metab. 2007, 92, 1319–1325. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, R.C.; Strizich, G.; Aneke-Nash, C.; Dominguez-Islas, C.; Bůžková, P.; Strickler, H.; Rohan, T.; Pollak, M.; Kuller, L.; Kizer, J.R.; et al. Insulinlike Growth Factor Binding Protein-1 and Ghrelin Predict Health Outcomes Among Older Adults: Cardiovascular Health Study Cohort. J. Clin. Endocrinol. Metab. 2016, 102, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Jood, K.; Ladenvall, C.; Rosengren, A.; Blomstrand, C.; Jern, C. Family History in Ischemic Stroke Before 70 Years of Age. Stroke 2005, 36, 1383–1387. [Google Scholar] [CrossRef]
- Peduzzi, P.; Concato, J.; Kemper, E.; Holford, T.R.; Feinstein, A.R. A simulation study of the number of events per variable in logistic regression analysis. J. Clin. Epidemiol. 1996, 49, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, J.; Hayward, K.; Kwakkel, G.; Ward, N.; Wolf, S.L.; Borschmann, K.; Krakauer, J.W.; Boyd, L.A.; Carmichael, S.T.; Corbett, D.; et al. Agreed definitions and a shared vision for new standards in stroke recovery research: The Stroke Recovery and Rehabilitation Roundtable taskforce. Int. J. Stroke 2017, 12, 444–450. [Google Scholar] [CrossRef]
- Kaplan, R.C.; Bùžková, P.; Cappola, A.R.; Strickler, H.D.; McGinn, A.P.; Mercer, L.D.; Arnold, A.M.; Pollak, M.N.; Newman, A.B. Decline in Circulating Insulin-Like Growth Factors and Mortality in Older Adults: Cardiovascular Health Study All-Stars Study. J. Clin. Endocrinol. Metab. 2012, 97, 1970–1976. [Google Scholar] [CrossRef]
- Pedersen, A.; Stanne, T.M.; Nilsson, S.; Klasson, S.; Rosengren, L.; Holmegaard, L.; Jood, K.; Blennow, K.; Zetterberg, H.; Jern, C. Circulating neurofilament light in ischemic stroke: Temporal profile and outcome prediction. J. Neurol. 2019, 266, 2796–2806. [Google Scholar] [CrossRef]
- Åberg, D.; Åberg, N.D.; Jood, K.; Holmegaard, L.; Redfors, P.; Blomstrand, C.; Isgaard, J.; Jern, C.; Svensson, J. Homeostasis model assessment of insulin resistance and outcome of ischemic stroke in non-diabetic patients—A prospective observational study. BMC Neurol. 2019, 19, 177. [Google Scholar] [CrossRef]
- Hallström, B.; Jönsson, A.-C.; Nerbrand, C.; Petersen, B.; Norrving, B.; Lindgren, A. Lund Stroke Register: Hospitalization pattern and yield of different screening methods for first-ever stroke. Acta Neurol. Scand. 2006, 115, 49–54. [Google Scholar] [CrossRef]
- Adams, H.P., Jr.; Bendixen, B.H.; Kappelle, L.J.; Biller, J.; Love, B.B.; Gordon, D.L.; Marsh, E.E. 3rd. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993, 24, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Ali, K.; Cheek, E.; Sills, S.; Crome, P.; Roffe, C. Development of a Conversion Factor to Facilitate Comparison of National Institute of Health Stroke Scale Scores with Scandinavian Stroke Scale Scores. Cerebrovasc. Dis. 2007, 24, 509–515. [Google Scholar] [CrossRef]
- Gray, L.J.; Ali, M.; Lyden, P.D.; Bath, P.M. Interconversion of the National Institutes of Health Stroke Scale and Scandinavian Stroke Scale in Acute Stroke. J. Stroke Cerebrovasc. Dis. 2009, 18, 466–468. [Google Scholar] [CrossRef]
- Lemmens, R.; Buysschaert, I.; Geelen, V.; Fernandez-Cadenas, I.; Montaner, J.; Schmidt, H.; Schmidt, R.; Attia, J.; Maguire, J.; Levi, C.; et al. The Association of the 4q25 Susceptibility Variant for Atrial Fibrillation With Stroke Is Limited to Stroke of Cardioembolic Etiology. Stroke 2010, 41, 1850–1857. [Google Scholar] [CrossRef] [PubMed]
- The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997, 20, 1183–1197. [Google Scholar] [CrossRef] [PubMed]
| Controls (n = 471) | Missing Values (n) | Patients (n = 470) | Missing Values (n) | LAA (n = 55) | SAO (n = 96) | Cardioembolic Stroke (n = 77) | Cryptogenic Stroke (n = 136) | |
|---|---|---|---|---|---|---|---|---|
| Age (years) | 56.8 ± 0.46 | (0) | 56.8 ± 0.46 | (0) | 58.3 ± 1.00 | 59.2 ± 0.70 * | 58.4 ± 1.22 | 54.5 ± 0.91 * |
| Male sex, % | 64 | (0) | 64 | (0) | 70 | 64 | 66 | 60 |
| Hypertension, % | 40 | (1) | 62 *** | (6) | 58 * | 73 *** | 54 * | 40 *** |
| Diabetes mellitus, % | 6 | (2) | 20 *** | (0) | 36 *** | 26 *** | 19 *** | 13 ** |
| Current smoker, % | 18 | (0) | 40 *** | (3) | 56 *** | 45 *** | 39 *** | 39 *** |
| hsCRP, mg/L | 3.01 ± 0.26 a | (1) | 10.7± 1.04 *** | (19) | 12.5 ± 2.66 *** | 4.46 ± 0.48 * | 17.6 ± 3.46 *** | 7.80 ± 1.55 *** |
| LDL, ng/nL | 3.32 ± 0.04 a | (3) | 3.34 ± 0.05 | (81) | 3.56 ± 0.16 | 3.50 ± 0.11 | 3.01 ± 0.13 ** | 3.32 ± 0.08 |
| HOMA-IR | 2.06 ± 0.12 a | (5) | 4.84 ± 0.26 *** | (42) | 6.20 ± 0.90 *** | 5.13 ± 0.77 *** | 4.58 ± 0.57 *** | 3.70 ± 0.25 *** |
| BMI, kg/m2 | 26.6 ± 0.19 a | (1) | 26.6 ± 0.20 | (12) | 26.9 ± 0.66 | 27.1 ± 0.45 | 26.5 ± 0.52 | 26.4 ± 0.33 |
| Atrial fibrillation, % | 1 | (38) | 11 *** | (52) | 2 | 2 | 54 *** | 2 |
| Oral anticoagulation (OAC), % | NA | NA | 11 | (3) | 13 | 2 | 29 | 2 |
| NIHSS score baseline | NA | NA | 5.41 ± 0.27 | (1) | 6.70 ± 0.86 | 3.23 ± 0.27 | 6.82 ± 0.83 | 5.25 ± 0.47 |
| mRS score 3 months | NA | NA | 1.82 ± 0.06 | (29) | 2.20 ± 0.16 | 1.31 ± 0.10 | 2.11 ± 0.16 | 1.75 ± 0.09 |
| mRS score 2 years | NA | NA | 1.89 ± 0.06 | (4) | 2.43 ± 0.25 | 1.42 ± 0.10 | 2.22 ± 0.18 | 1.70 ± 0.10 |
| mRS score 7 years | NA | NA | 2.52 ± 0.10 | (95) | 3.46 ± 0.35 | 1.84 ± 0.20 | 3.30 ± 0.17 | 2.14 ± 0.17 |
| s-IGFBP-1, acute (ng/mL), geometric mean | 5.04 ± 0.31 a | (0) | 4.66 ± 0.37 | (0) | 4.43 ± 1.54 | 5.82 ± 0.64 | 5.32 ± 1.00 | 4.38 ± 0.71 |
| s-IGFBP-1, 3 months (ng/mL), geometric mean | NA | NA | 6.07 ± 0.48 ** | (40) | 5.15 ± 1.31 | 7.02 ± 1.02 ** | 7.16 ± 1.38 * | 5.59 ± 0.96 |
| Parameter | Correlation vs. Acute IGFBP-1 | Correlation vs. 3-Month IGFBP-1 | ||
|---|---|---|---|---|
| (r) | (p) | (r) | (p) | |
| Age | 0.17 | <0.001 | 0.19 | <0.001 |
| HOMA-IR | −0.33 | <0.001 | −0.26 | <0.001 |
| BMI | −0.25 | <0.001 | −0.28 | <0.001 |
| NIHSS | −0.18 | <0.001 | −0.04 | 0.46 |
| Current smoking | 0.11 | 0.016 | 0.10 | 0.035 |
| hsCRP | 0.034 | 0.49 | 0.022 | 0.65 |
| Hypertension | 0.001 | 0.99 | −0.50 | 0.30 |
| LDL | −0.10 | 0.032 | 0.034 | 0.50 |
| Diabetes | 0.13 | 0.006 | 0.13 | 0.007 |
| Total Stroke Population | Acute IGFBP-1 | 3 Months IGFBP-1 | ||||
|---|---|---|---|---|---|---|
| 2 years mRS | All/mRS ≥ 3 (n) | All/mRS ≥ 3 (n) | ||||
| Crude | 1.10 (0.62–1.97) | 447/91 | 0.74 | 3.11 (1.57–6.17) ** | 428/78 | 0.001 |
| Multivariate model A | 0.93 (0.51–1.70) | 0.81 | 2.76 (1.37–5.59) ** | 0.005 | ||
| Multivariate model B | 0.75 (0.39–1.45) | 0.39 | 2.38 (1.10–5.15) * | 0.028 | ||
| Multivariate model C | 1.59 (0.74–3.40) | 0.23 | 3.41 (1.37–8.52) ** | 0.009 | ||
| 7 years mRS (including mRS 6) | ||||||
| Crude | 2.17 (1.24–3.79) ** | 360/128 | 0.007 | 5.22 (2.69–10.1) *** | 347/117 | <0.001 |
| Multivariate model A | 1.97 (1.11–3.48) * | 0.02 | 4.73 (2.43–9.22) *** | <0.001 | ||
| Multivariate model B | 1.41 (0.75–2.63) | 0.28 | 3.68 (1.79–7.59) *** | <0.001 | ||
| Multivariate model C | 2.88 (1.40–5.92) ** | 0.004 | 5.69 (2.53–12.8) *** | <0.001 | ||
| 7 years mRS (excluding mRS 6 = dead) | ||||||
| Crude | 1.67 (0.82–3.41) | 300/68 | 0.16 | 4.24 (1.91–9.39) *** | 297/67 | <0.001 |
| Multivariate model A | 1.42 (0.67–2.97) | 0.36 | 3.86 (1.71–8.69) ** | 0.001 | ||
| Multivariate model B | 1.34 (0.61–2.98) | 0.47 | 3.63 (1.49–8.81) ** | 0.004 | ||
| Multivariate model C | 3.57 (1.36–9.40) * | 0.01 | 5.12 (1.82–14.4) ** | 0.002 |
| Total Stroke Population | Acute IGFBP-1 | 3 Months IGFBP-1 | ||
|---|---|---|---|---|
| Hazard Ratio | p | Hazard Ratio | p | |
| Crude | 2.09 (1.22–3.56) *** | 0.007 | 3.26 (1.82–5.84) *** | <0.001 |
| Multivariate model A | 1.81 (1.04–3.16) * | 0.037 | 3.10 (1.68–5.73) *** | <0.001 |
| Multivariate model B | 1.28 (0.72–2.26) | 0.404 | 1.99 (1.07–3.71) * | 0.031 |
| Multivariate model C | 1.38 (0.78–2.47) | 0.27 | 2.00 (1.07–3.73) * | 0.030 |
| (A) Per Log Increase of Acute s-IGFBP-1 | ||||
|---|---|---|---|---|
| Group of Ischemic Stroke (TOAST) | Crude | Age and sex adjusted | ||
| Hazard Ratio | p | Hazard Ratio | p | |
| large vessel disease | 5.77 (2.00–16.6) ** | 0.001 | 4.86 (1.61–14.7) ** | 0.005 |
| small vessel disease | 1.69 (0.29–9.85) | 0.56 | 1.60 (0.26–9.76) | 0.61 |
| cardioembolic stroke | 1.91 (0.50–7.31) | 0.33 | 1.14 (0.41–3.18) | 0.80 |
| cryptogenic stroke | 18.0 (0.61–529) | 0.098 | 1.49 (0.34–6.48) | 0.60 |
| (B) Per Log Increase of 3 Months s-IGFBP-1 | ||||
| Group of ischemic stroke (TOAST) | Crude | Age and sex adjusted | ||
| Hazard Ratio | p | Hazard Ratio | p | |
| large vessel disease | 2.71 (0.77–9.60) | 0.12 | 1.90 (0.49–7.34) | 0.35 |
| small vessel disease | 1.48 (0.27–8.25) | 0.65 | 1.34 (0.22–8.28) | 0.75 |
| cardioembolic stroke | 3.80 (1.32–10.9) * | 0.013 | 4.76 (1.42–16.0) * | 0.012 |
| cryptogenic stroke | 5.29 (1.27–22.0) * | 0.022 | 5.53 (1.12–27.3) * | 0.036 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Åberg, D.; Gadd, G.; Jood, K.; Redfors, P.; Stanne, T.M.; Isgaard, J.; Blennow, K.; Zetterberg, H.; Jern, C.; Åberg, N.D.; et al. Serum IGFBP-1 Concentration as a Predictor of Outcome after Ischemic Stroke—A Prospective Observational Study. Int. J. Mol. Sci. 2023, 24, 9120. https://doi.org/10.3390/ijms24119120
Åberg D, Gadd G, Jood K, Redfors P, Stanne TM, Isgaard J, Blennow K, Zetterberg H, Jern C, Åberg ND, et al. Serum IGFBP-1 Concentration as a Predictor of Outcome after Ischemic Stroke—A Prospective Observational Study. International Journal of Molecular Sciences. 2023; 24(11):9120. https://doi.org/10.3390/ijms24119120
Chicago/Turabian StyleÅberg, Daniel, Gustaf Gadd, Katarina Jood, Petra Redfors, Tara M. Stanne, Jörgen Isgaard, Kaj Blennow, Henrik Zetterberg, Christina Jern, N. David Åberg, and et al. 2023. "Serum IGFBP-1 Concentration as a Predictor of Outcome after Ischemic Stroke—A Prospective Observational Study" International Journal of Molecular Sciences 24, no. 11: 9120. https://doi.org/10.3390/ijms24119120
APA StyleÅberg, D., Gadd, G., Jood, K., Redfors, P., Stanne, T. M., Isgaard, J., Blennow, K., Zetterberg, H., Jern, C., Åberg, N. D., & Svensson, J. (2023). Serum IGFBP-1 Concentration as a Predictor of Outcome after Ischemic Stroke—A Prospective Observational Study. International Journal of Molecular Sciences, 24(11), 9120. https://doi.org/10.3390/ijms24119120






