Modulation of Gut Microbiome in Ecstasy/MDMA-Induced Behavioral and Biochemical Impairment in Rats and Potential of Post-Treatment with Anacyclus pyrethrum L. Aqueous Extract to Mitigate Adverse Effects
Abstract
1. Introduction
2. Results
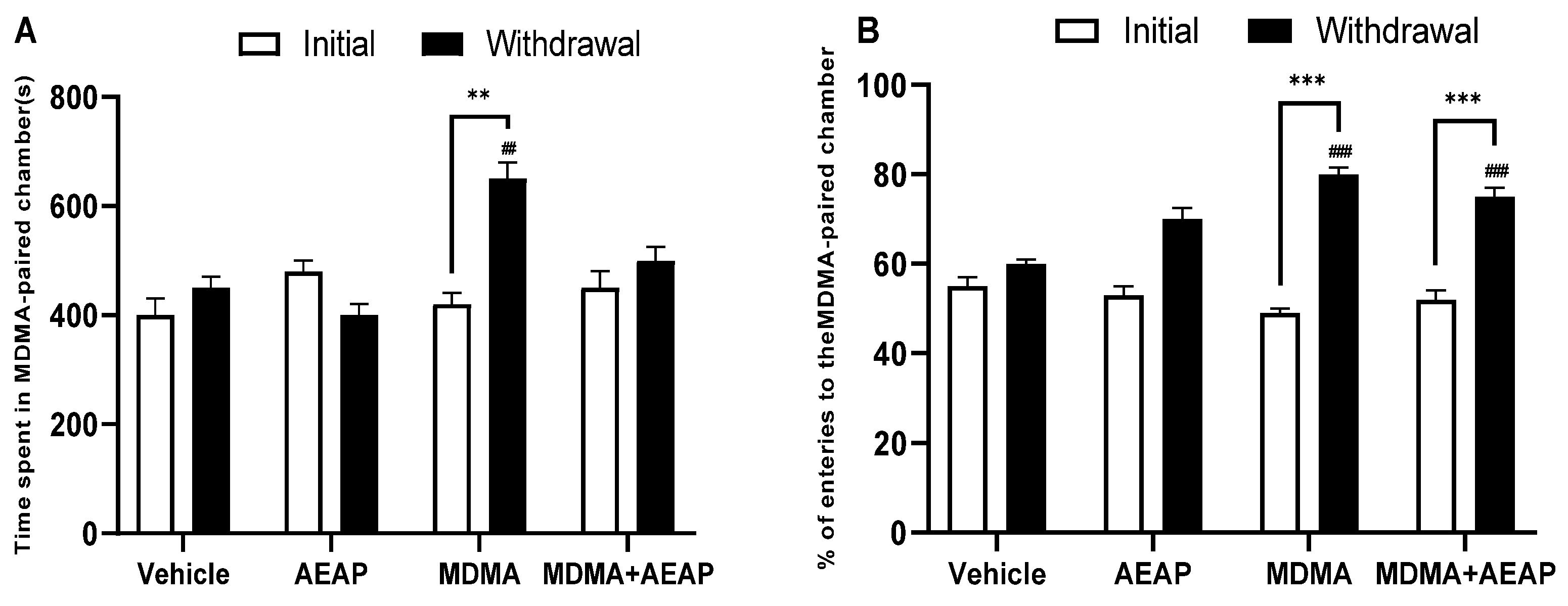
2.1. MDMA-Induced CPP
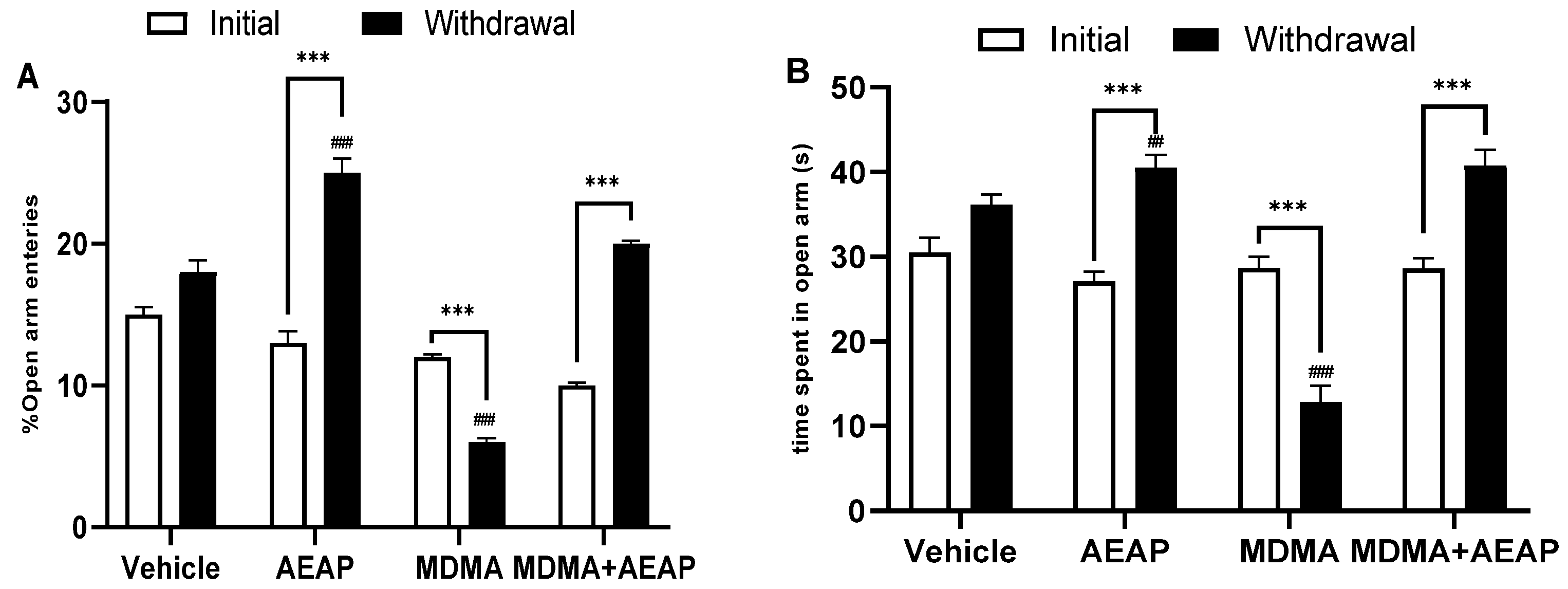
2.2. MDMA-Induced Behavioral Impairments and Potential of AEAP Post-Treatement to Mitigate Adverse Effects
2.3. Ameliorative Effects of AEAP on MDMA-Induced Stress
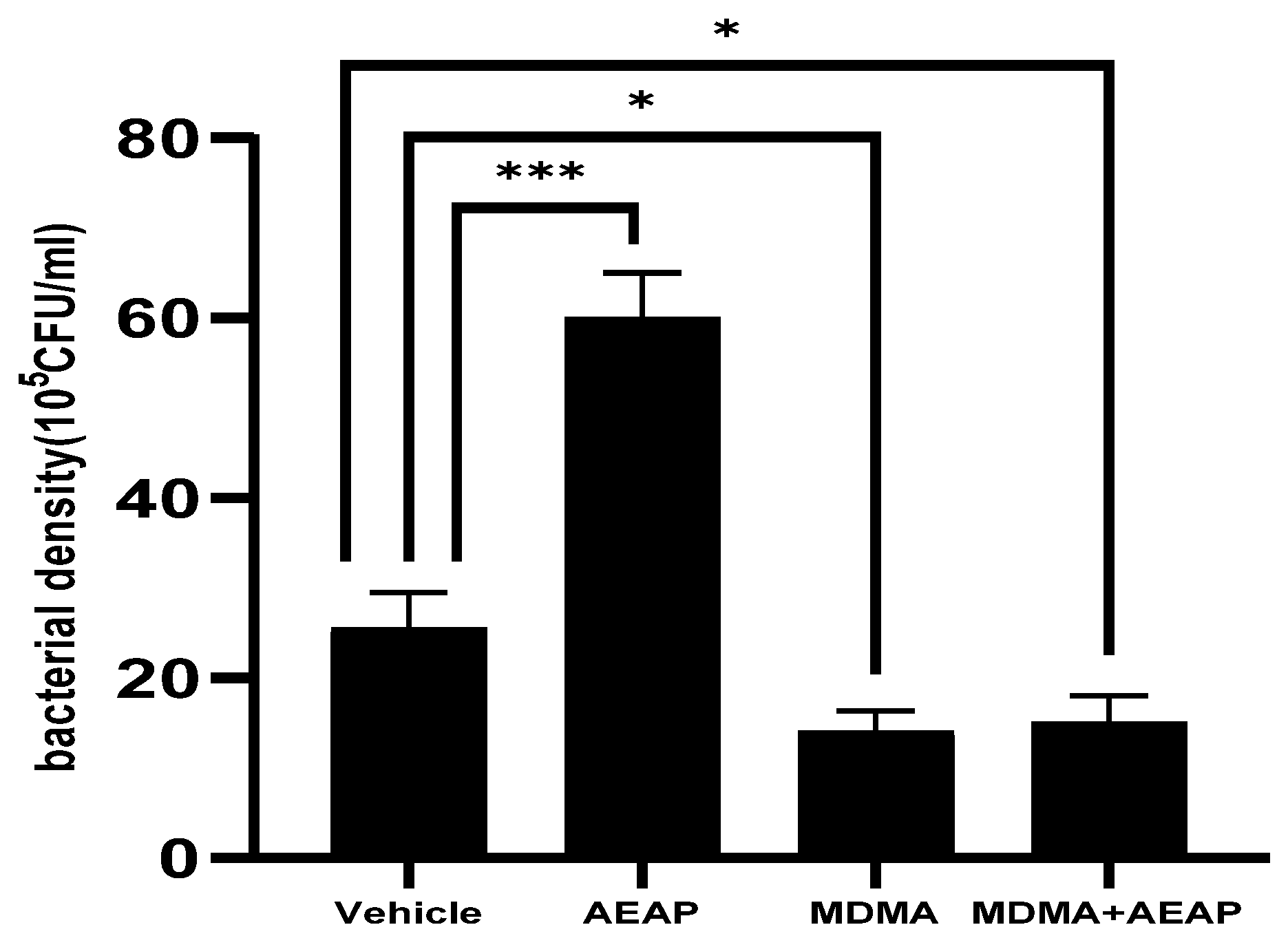
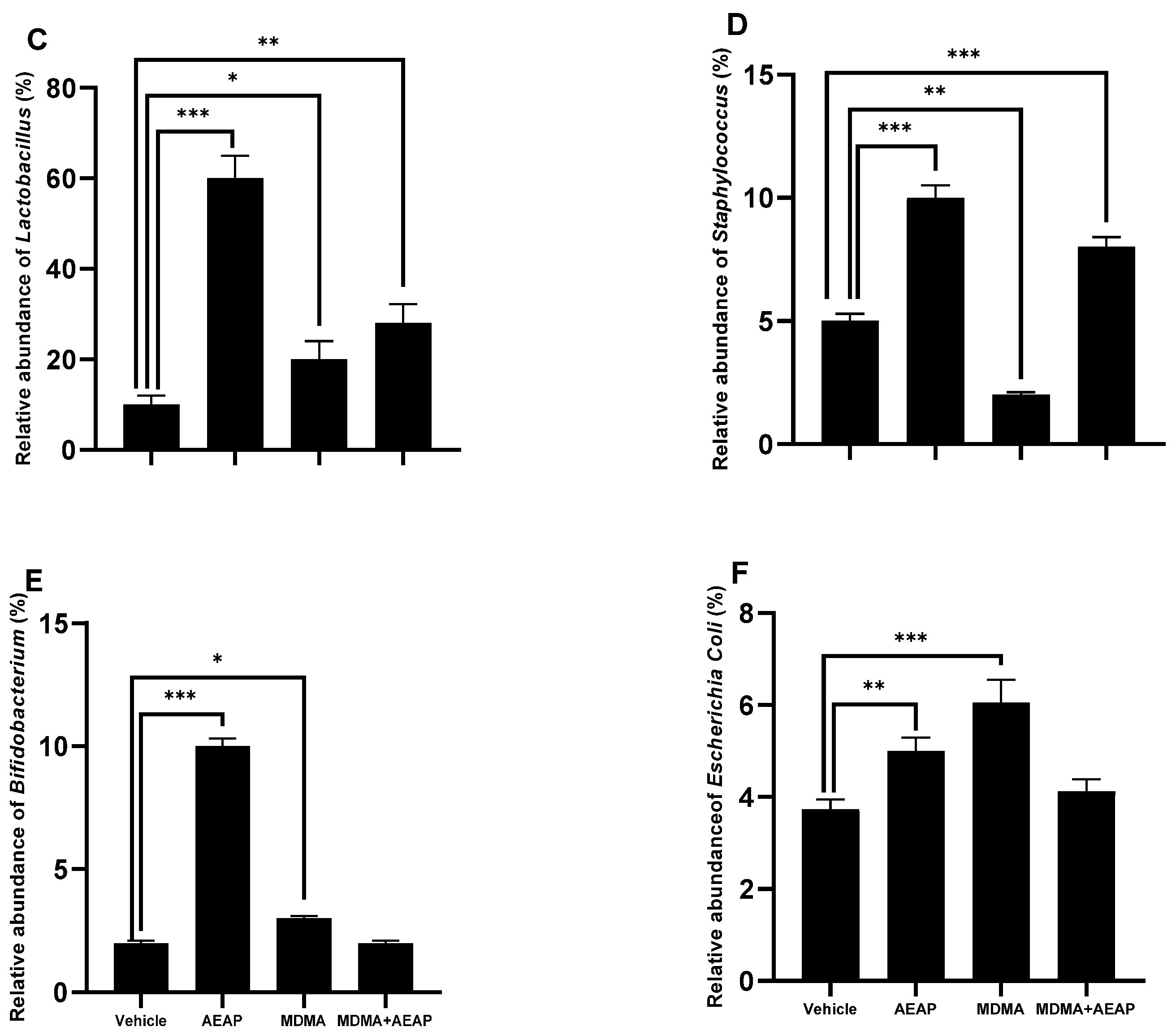
2.4. AEAP Mitigates MDMA-Induced Alterations in Gut Microbiota Composition in Rats
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Drugs Administration
4.3. Plant Material and Preparation of the Aqueous Extract
4.4. Conditioned Place Preference (CPP)
4.5. Behavioral Tests
4.5.1. Elevated Plus Maze (EPM)
4.5.2. Porsolt’s Forced Swim Test (FST) for Depression
4.5.3. Open Field Test (OFT)
4.6. Biochemical Analysis
4.7. Gut Microbiota Determination
4.7.1. MALDI-TOF MS Spectra Preparation for Mass Spectral Profiles
4.7.2. MALDI-TOF MS Data Acquisition and Processing
4.8. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UNODC World Drug Report 2022 Highlights Trends on Cannabis Post-Legalization, Environmental Impacts of Illicit Drugs, and Drug Use among Women and Youth. Available online: https://www.unodc.org/unodc/en/frontpage/2022/June/unodc-world-drug-report-2022-highlights-trends-on-cannabis-post-legalization--environmental-impacts-of-illicit-drugs--and-drug-use-among-women-and-youth.html (accessed on 18 March 2023).
- Volkow, N.D.; Boyle, M. Neuroscience of Addiction: Relevance to Prevention and Treatment. Am. J. Psychiatry 2018, 175, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Abuse NI on D. MDMA (Ecstasy/Molly) DrugFacts. National Institute on Drug Abuse. 2020. Available online: http://nida.nih.gov/publications/drugfacts/mdma-ecstasymolly (accessed on 8 April 2023).
- McCann, M.; Higgins, K.; Perra, O.; McCartan, C.; McLaughlin, A. Adolescent ecstasy use and depression: Cause and effect, or two outcomes of home environment? Eur. J. Public Health 2014, 24, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Morton, J. Ecstasy: Pharmacology and neurotoxicity. Curr. Opin. Pharmacol. 2005, 5, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Lanteri, C.; Doucet, E.L.; Hernández Vallejo, S.J.; Godeheu, G.; Bobadilla, A.-C.; Salomon, L.; Lanfumey, L.; Tassin, J.-P. Repeated exposure to MDMA triggers long-term plasticity of noradrenergic and serotonergic neurons. Mol. Psychiatry 2014, 19, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Nestler, E.J. Is there a common molecular pathway for addiction? Nat. Neurosci. 2005, 8, 1445–1449. [Google Scholar] [CrossRef] [PubMed]
- Robledo, P.; Balerio, G.; Berrendero, F.; Maldonado, R. Study of the behavioural responses related to the potential addictive properties of MDMA in mice. Naunyn Schmiedebergs Arch. Pharmacol. 2004, 369, 338–349. [Google Scholar] [CrossRef]
- Xiao, H.-w.; Ge, C.; Feng, G.-x.; Li, Y.; Luo, D.; Dong, J.-l.; Li, H.; Wang, H.; Cui, M.; Fan, S.-J. Gut microbiota modulates alcohol withdrawal-induced anxiety in mice. Toxicol. Lett. 2018, 287, 23–30. [Google Scholar] [CrossRef]
- Lynch, S.V.; Pedersen, O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef]
- Baj, A.; Moro, E.; Bistoletti, M.; Orlandi, V.; Crema, F.; Giaroni, C. Glutamatergic Signaling Along The Microbiota-Gut-Brain Axis. Int. J. Mol. Sci. 2019, 20, 1482. [Google Scholar] [CrossRef]
- Cryan, J.F.; Dinan, T.G. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Chen, X.; Zheng, H. Exploring an adverse impact of smartphone overuse on academic performance via health issues: A stimulus-organism-response perspective. Behav. Inf. Technol. 2021, 40, 663–675. [Google Scholar] [CrossRef]
- Gicquelais, R.E.; Bohnert, A.S.; Thomas, L.; Foxman, B. Opioid agonist and antagonist use and the gut microbiota: Associations among people in addiction treatment. Sci. Rep. 2020, 10, 19471. [Google Scholar] [CrossRef] [PubMed]
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Tóth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Kundu, P.; et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014, 6, 263ra158. [Google Scholar] [CrossRef] [PubMed]
- Kiraly, D.D.; Walker, D.M.; Calipari, E.S.; Labonte, B.; Issler, O.; Pena, C.J.; Ribeiro, E.A.; Russo, S.J.; Nestler, E.J. Alterations of the Host Microbiome Affect Behavioral Responses to Cocaine. Sci. Rep. 2016, 6, 35455. [Google Scholar] [CrossRef] [PubMed]
- Scorza, C.; Piccini, C.; Martínez Busi, M.; Abin Carriquiry, J.A.; Zunino, P. Alterations in the Gut Microbiota of Rats Chronically Exposed to Volatilized Cocaine and Its Active Adulterants Caffeine and Phenacetin. Neurotox. Res. 2019, 35, 111–121. [Google Scholar] [CrossRef]
- Ning, T.; Gong, X.; Xie, L.; Ma, B. Gut Microbiota Analysis in Rats with Methamphetamine-Induced Conditioned Place Preference. Front. Microbiol. 2017, 8, 1620. [Google Scholar] [CrossRef]
- Kim, Y.K.; Shin, C. The Microbiota-Gut-Brain Axis in Neuropsychiatric Disorders: Pathophysiological Mechanisms and Novel Treatments. Curr. Neuropharmacol. 2018, 16, 559–573. [Google Scholar] [CrossRef]
- Dey, P. Gut microbiota in phytopharmacology: A comprehensive overview of concepts, reciprocal interactions, biotransformations and mode of actions. Pharmacol. Res. 2019, 147, 104367. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.F.; Krause, E.G.; Melhorn, S.J.; Sakai, R.R.; Benoit, S.C. Dominant rats are natural risk takers and display increased motivation for food reward. Neuroscience 2009, 162, 23–30. [Google Scholar] [CrossRef]
- Dhama, K.; Tiwari, R.; Chakraborty, S.; Saminathan, M.; Kumar, A.; Karthik, K.; Wani, M.Y.; Amarpal; Singh, S.V.; Rahal, A. Evidence based antibacterial potentials of medicinal plants and herbs countering bacterial pathogens especially in the era of emerging drug resistance: An integrated update. Int. J. Pharmacol. 2014, 10, 1–43. [Google Scholar] [CrossRef]
- Rosenblat, J.D.; McIntyre, R.S. Efficacy and tolerability of minocycline for depression: A systematic review and meta-analysis of clinical trials. J. Affect. Disord. 2018, 227, 219–225. [Google Scholar] [CrossRef]
- Meireles, M.; Moura, E.; Vieira-Coelho, M.A.; Santos-Buelga, C.; Gonzalez-Manzano, S.; Dueñas, M.; Mateus, N.; Faria, A.; Calhau, C. Flavonoids as dopaminergic neuromodulators. Mol. Nutr. Food Res. 2016, 60, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Kalim, M.D.; Bhattacharyya, D.; Banerjee, A.; Chattopadhyay, S. Oxidative DNA damage preventive activity and antioxidant potential of plants used in Unani system of medicine. BMC Complement. Altern. Med. 2010, 10, 77. [Google Scholar] [CrossRef] [PubMed]
- Bendjeddou, D.; Lalaoui, K.; Satta, D. Immunostimulating activity of the hot water-soluble polysaccharide extracts of Anacyclus pyrethrum, Alpinia galanga and Citrullus colocynthis. J. Ethnopharmacol. 2003, 88, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Rimbau, V.; Cerdan, C.; Vila, R.; Iglesias, J. Antiinflammatory activity of some extracts from plants used in the traditional medicine of north-African countries (II). Phytother. Res. PTR 1999, 13, 128–132. [Google Scholar] [CrossRef]
- Cameron, L.P.; Benson, C.J.; Dunlap, L.E.; Olson, D.E. Effects of N,N-Dimethyltryptamine on Rat Behaviors Relevant to Anxiety and Depression. ACS Chem. Neurosci. 2018, 9, 1582–1590. [Google Scholar] [CrossRef] [PubMed]
- Llorente-Berzal, A.; Puighermanal, E.; Burokas, A.; Ozaita, A.; Maldonado, R.; Marco, E.M.; Viveros, M.P. Sex-Dependent Psychoneuroendocrine Effects of THC and MDMA in an Animal Model of Adolescent Drug Consumption. PLoS ONE 2013, 8, e78386. [Google Scholar] [CrossRef]
- Rodríguez de Fonseca, F.; Ramos, J.A.; Bonnin, A.; Fernández-Ruiz, J.J. Presence of cannabinoid binding sites in the brain from early postnatal ages. Neuroreport 1993, 4, 135–138. [Google Scholar] [CrossRef]
- Åberg, M.; Wade, D.; Wall, E.; Izenwasser, S. Effect of MDMA (ecstasy) on activity and cocaine conditioned place preference in adult and adolescent rats. Neurotoxicol. Teratol. 2007, 29, 37–46. [Google Scholar] [CrossRef]
- Bilsky, E. CGS 10746B, a Novel Dopamine Release Inhibitor, Blocks the Establishment of Cocaine and MDMA Conditioned Place Preferences. Pharmacol. Biochem. Behav. 1998, 59, 215–220. [Google Scholar] [CrossRef]
- Ritz, M.C.; Kuhar, M.J. Psychostimulant drugs and a dopamine hypothesis regarding addiction: Update on recent research. Biochem. Soc. Symp. 1993, 59, 51–64. [Google Scholar] [PubMed]
- Ciudad-Roberts, A.; Camarasa, J.; Ciudad, C.J.; Pubill, D.; Escubedo, E. Alcohol enhances the psychostimulant and conditioning effects of mephedrone in adolescent mice; postulation of unique roles of D3 receptors and BDNF in place preference acquisition. Br. J. Pharmacol. 2015, 172, 4970–4984. [Google Scholar] [CrossRef] [PubMed]
- Kokare, D.M.; Dandekar, M.P.; Singru, P.S.; Gupta, G.L.; Subhedar, N.K. Involvement of α-MSH in the social isolation induced anxiety- and depression-like behaviors in rat. Neuropharmacology 2010, 58, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Yawalkar, R.; Changotra, H.; Gupta, G.L. Protective influences of N-acetylcysteine against alcohol abstinence-induced depression by regulating biochemical and GRIN2A, GRIN2B gene expression of NMDA receptor signaling pathway in rats. Neurochem. Int. 2018, 118, 73–81. [Google Scholar] [CrossRef]
- Thompson, V.B.; Heiman, J.; Chambers, J.B.; Benoit, S.C.; Buesing, W.R.; Norman, M.K.; Norman, A.B.; Lipton, J.W. Long-term behavioral consequences of prenatal MDMA exposure. Physiol. Behav. 2009, 96, 593–601. [Google Scholar] [CrossRef]
- Pahuja, M.; Mehla, J.; Reeta, K.H.; Joshi, S.; Gupta, Y.K. Root extract of Anacyclus pyrethrum ameliorates seizures, seizure-induced oxidative stress and cognitive impairment in experimental animals. Epilepsy Res. 2012, 98, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, N.D.; Purohit, S.S.; Sharma, S.S.; Kumar, T. A Handbook of Medicinal Plants: A Complete Source Book; Agrobios: Jodhpur, India, 2003. [Google Scholar]
- Manouze, H.; Bouchatta, O.; Gadhi, A.C.; Bennis, M.; Sokar, Z.; Ba-M’hamed, S. Anti-inflammatory, Antinociceptive, and Antioxidant Activities of Methanol and Aqueous Extracts of Anacyclus pyrethrum Roots. Front. Pharmacol. 2017, 8, 598. [Google Scholar] [CrossRef] [PubMed]
- Bellakhdar, J. Contribution à l’étude de la Pharmacopée Traditionnelle au Maroc: La Situation Actuelle, les Produits, les Sources du Savoir (Enquête Ethnopharmacologique de Terrain Réalisée de 1969 à 1992). Ph.D. Thesis, Université Paul Verlaine, Metz, France, 1997. Available online: https://hal.univ-lorraine.fr/tel-01752084 (accessed on 15 May 2023).
- Hmamouchi, M. Les Plantes Medicinales et Aromatiques Marocaines. 2001. Available online: https://scholar.google.com/scholar_lookup?title=Les+plantes+medicinales+et+aromatiques+marocaines&author=Hmamouchi%2C+M.&publication_year=2001 (accessed on 15 May 2023).
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef] [PubMed]
- Elazzouzi, H.; Fadili, K.; Cherrat, A.; Amalich, S.; Zekri, N.; Zerkani, H.; Tagnaout, I.; Hano, C.; Lorenzo, J.M.; Zair, T. Phytochemistry, Biological and Pharmacological Activities of the Anacyclus pyrethrum (L.) Lag: A Systematic Review. Plants 2022, 11, 2578. [Google Scholar] [CrossRef]
- de la Torre, R.; Farré, M.; Roset, P.N.; Lopez, C.H.; Mas, M.; Ortuño, J.; Menoyo, E.; Pizarro, N.; Segura, J.; CAMÍ, J. Pharmacology of MDMA in humans. Ann. N. Y. Acad. Sci. 2000, 914, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.S.; Baggott, M.; Mendelson, J.H.; Mendelson, J.E.; Jones, R.T. Subjective and hormonal effects of 3,4-methylenedioxymethamphetamine (MDMA) in humans. Psychopharmacology 2002, 162, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Dumont, G.J.H.; Verkes, R.J. A review of acute effects of 3,4-methylenedioxymethamphetamine in healthy volunteers. J. Psychopharmacol. 2006, 20, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Bora, F.; Yılmaz, F.; Bora, T. Ecstasy (MDMA) and its effects on kidneys and their treatment: A review. Iran. J. Basic Med. Sci. 2016, 19, 1151–1158. [Google Scholar] [PubMed]
- Parrott, A.C.; Montgomery, C.; Wetherell, M.A.; Downey, L.A.; Stough, C.; Scholey, A.B. MDMA, cortisol, and heightened stress in recreational ecstasy users. Behav. Pharmacol. 2014, 25, 458–472. [Google Scholar] [CrossRef]
- Osimo, E.F.; Pillinger, T.; Rodriguez, I.M.; Khandaker, G.M.; Pariante, C.M.; Howes, O.D. Inflammatory markers in depression: A meta-analysis of mean differences and variability in 5166 patients and 5083 controls. Brain Behav. Immun. 2020, 87, 901–909. [Google Scholar] [CrossRef]
- Koob, G.F.; Le Moal, M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology 2001, 24, 97–129. [Google Scholar] [CrossRef]
- Russell, J.T.; Zhou, Y.; Weinstock, G.M.; Bubier, J.A. The Gut Microbiome and Substance Use Disorder. Front. Neurosci. 2021, 15, 725500. [Google Scholar] [CrossRef] [PubMed]
- Prom-Wormley, E.C.; Ebejer, J.; Dick, D.M.; Bowers, M.S. The genetic epidemiology of substance use disorder: A review. Drug Alcohol Depend. 2017, 180, 241–259. [Google Scholar] [CrossRef]
- Strandwitz, P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018, 1693, 128–133. [Google Scholar] [CrossRef]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Finegold, S.M.; Dowd, S.E.; Gontcharova, V.; Liu, C.; Henley, K.E.; Wolcott, R.D.; Youn, E.; Summanen, P.H.; Granpeesheh, D.; Dixon, D.; et al. Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe 2010, 16, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Carding, S.; Verbeke, K.; Vipond, D.T.; Corfe, B.M.; Owen, L.J. Dysbiosis of the gut microbiota in disease. Microb. Ecol. Health Dis. 2015, 26, 26191. [Google Scholar] [CrossRef] [PubMed]
- Neut, C.; Bulois, P.; Desreumaux, P.; Membré, J.M.; Lederman, E.; Gambiez, L.; Cortot, A.; Quandalle, P.; van Kruiningen, H.; Colombel, J.-F. Changes in the bacterial flora of the neoterminal ileum after ileocolonic resection for Crohn’s disease. Am. J. Gastroenterol. 2002, 97, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.; Knight, R.; Gordon, J.I. The human microbiome project: Exploring the microbial part of ourselves in a changing world. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef]
- Kootte, R.S.; Vrieze, A.; Holleman, F.; Dallinga-Thie, G.M.; Zoetendal, E.G.; de Vos, W.M.; Groen, A.K.; Hoekstra, J.B.L.; Stroes, E.S.; Nieuwdorp, M. The therapeutic potential of manipulating gut microbiota in obesity and type 2 diabetes mellitus. Diabetes Obes. Metab. 2012, 14, 112–120. [Google Scholar] [CrossRef]
- Larsen, N.; Vogensen, F.K.; Van Den Berg, F.W.J.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Al-Soud, W.A.; Sørensen, S.J.; Hansen, L.H.; Jakobsen, M. Gut Microbiota in Human Adults with Type 2 Diabetes Differs from Non-Diabetic Adults. PLoS ONE 2010, 5, e9085. [Google Scholar] [CrossRef]
- Oteiza, P.I.; Fraga, C.G.; Mills, D.A.; Taft, D.H. Flavonoids and the gastrointestinal tract: Local and systemic effects. Mol. Aspects Med. 2018, 61, 41–49. [Google Scholar] [CrossRef]
- Qin, C.; Hu, J.; Wan, Y.; Cai, M.; Wang, Z.; Peng, Z.; Liao, Y.; Li, D.; Yao, P.; Liu, L.; et al. Narrative review on potential role of gut microbiota in certain substance addiction. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 106, 110093. [Google Scholar] [CrossRef] [PubMed]
- Vuong, H.E.; Yano, J.M.; Fung, T.C.; Hsiao, E.Y. The Microbiome and Host Behavior. Annu. Rev. Neurosci. 2017, 40, 21–49. [Google Scholar] [CrossRef] [PubMed]
- Belujon, P.; Grace, A.A. Dopamine System Dysregulation in Major Depressive Disorders. Int. J. Neuropsychopharmacol. 2017, 20, 1036–1046. [Google Scholar] [CrossRef] [PubMed]
- Luqman, A.; Nega, M.; Nguyen, M.T.; Ebner, P.; Götz, F. SadA-Expressing Staphylococci in the Human Gut Show Increased Cell Adherence and Internalization. Cell Rep. 2018, 22, 535–545. [Google Scholar] [CrossRef]
- Barrett, E.; Ross, R.P.; O’Toole, P.W.; Fitzgerald, G.F.; Stanton, C. γ-Aminobutyric acid production by culturable bacteria from the human intestine. J. Appl. Microbiol. 2012, 113, 411–417. [Google Scholar] [CrossRef]
- Sittipo, P.; Choi, J.; Lee, S.; Lee, Y.K. The function of gut microbiota in immune-related neurological disorders: A review. J. Neuroinflamm. 2022, 19, 154. [Google Scholar] [CrossRef]
- Takanaga, H.; Ohtsuki, S.; Hosoya, K.I.; Terasaki, T. GAT2/BGT-1 as a system responsible for the transport of gamma-aminobutyric acid at the mouse blood-brain barrier. J. Cereb. Blood Flow Metab. 2001, 21, 1232–1239. [Google Scholar] [CrossRef]
- Frost, G.; Sleeth, M.L.; Sahuri-Arisoylu, M.; Lizarbe, B.; Cerdan, S.; Brody, L.; Anastasovska, J.; Ghourab, S.; Hankir, M.; Zhang, S.; et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat. Commun. 2014, 5, 3611. [Google Scholar] [CrossRef]
- Janik, R.; Thomason, L.A.M.; Stanisz, A.M.; Forsythe, P.; Bienenstock, J.; Stanisz, G.J. Magnetic resonance spectroscopy reveals oral Lactobacillus promotion of increases in brain GABA, N-acetyl aspartate and glutamate. NeuroImage 2016, 125, 988–995. [Google Scholar] [CrossRef]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef]
- Laroute, V.; Beaufrand, C.; Gomes, P.; Nouaille, S.; Tondereau, V.; Daveran-Mingot, M.L.; Theodorou, V.; Eutamene, H.; Mercier-Bonin, M.; Cocaign-Bousquet, M. Lactococcus lactis NCDO2118 exerts visceral antinociceptive properties in rat via GABA production in the gastro-intestinal tract. eLife 2022, 11, e77100. [Google Scholar] [CrossRef]
- Pokusaeva, K.; Johnson, C.; Luk, B.; Uribe, G.; Fu, Y.; Oezguen, N.; Matsunami, R.K.; Lugo, M.; Major, A.; Mori-Akiyama, Y.; et al. GABA-producing Bifidobacterium dentium modulates visceral sensitivity in the intestine. Neurogastroenterol. Motil. 2017, 29, e12904. [Google Scholar] [CrossRef] [PubMed]
- Freestone, P.P.; Williams, P.H.; Haigh, R.D.; Maggs, A.F.; Neal, C.P.; Lyte, M. Growth stimulation of intestinal commensal Escherichia coli by catecholamines: A possible contributory factor in trauma-induced sepsis. Shock 2002, 18, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Burokas, A.; Arboleya, S.; Moloney, R.D.; Peterson, V.L.; Murphy, K.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Targeting the Microbiota-Gut-Brain Axis: Prebiotics Have Anxiolytic and Antidepressant-like Effects and Reverse the Impact of Chronic Stress in Mice. Biol. Psychiatry 2017, 82, 472–487. [Google Scholar] [CrossRef] [PubMed]
- Kiyatkin, E.A.; Sharma, H.S. Breakdown of Blood-Brain and Blood-Spinal Cord Barriers During Acute Methamphetamine Intoxication: Role of Brain Temperature. CNS Neurol. Disord. Drug Targets 2016, 15, 1129–1138. [Google Scholar] [CrossRef]
- Hoogland, I.C.M.; Houbolt, C.; van Westerloo, D.J.; van Gool, W.A.; van de Beek, D. Systemic inflammation and microglial activation: Systematic review of animal experiments. J. Neuroinflamm. 2015, 12, 114. [Google Scholar] [CrossRef]
- Eskandarzadeh, S.; Effatpanah, M.; Khosravi-Darani, K.; Askari, R.; Hosseini, A.F.; Reisian, M.; Jazayeri, S. Efficacy of a multispecies probiotic as adjunctive therapy in generalized anxiety disorder: A double blind, randomized, placebo-controlled trial. Nutr. Neurosci. 2021, 24, 102–108. [Google Scholar] [CrossRef]
- Thumann, T.A.; Pferschy-Wenzig, E.M.; Moissl-Eichinger, C.; Bauer, R. The role of gut microbiota for the activity of medicinal plants traditionally used in the European Union for gastrointestinal disorders. J. Ethnopharmacol. 2019, 245, 112153. [Google Scholar] [CrossRef]
- Dinan, T.G.; Stanton, C.; Cryan, J.F. Psychobiotics: A novel class of psychotropic. Biol. Psychiatry 2013, 74, 720–726. [Google Scholar] [CrossRef]
- Lyte, M. Probiotics function mechanistically as delivery vehicles for neuroactive compounds: Microbial endocrinology in the design and use of probiotics. BioEssays News Rev. Mol. Cell. Dev. Biol. 2011, 33, 574–581. [Google Scholar] [CrossRef]
- Mahmood, H.K.; Barkat, M.Q.; Zeeshan, U.; Kamran, Q. Phytochemical and antioxidant screening of Anacylus pyrethrum, Apium graveolens, Boerhaavia diffusa, Cinnamomum cassia Blume, Cuscumis melo Linn, Cuscumis sativus Linn, Daucus sativus, Foeniculum vulgare, Trachyspermum ammii and their effect on various human ailments. Matrix Sci. Pharma 2018, 2, 06–14. [Google Scholar] [CrossRef]
- Su, L.; Mao, C.; Wang, X.; Li, L.; Tong, H.; Mao, J.; Ji, D.; Lu, T.; Hao, M.; Huang, Z.; et al. The Anti-colitis Effect of Schisandra chinensis Polysaccharide Is Associated with the Regulation of the Composition and Metabolism of Gut Microbiota. Front. Cell. Infect. Microbiol. 2020, 10, 519479. [Google Scholar] [CrossRef]
- Chen, P.; Hei, M.; Kong, L.; Liu, Y.; Yang, Y.; Mu, H.; Zhang, X.; Zhao, S.; Duan, J. One water-soluble polysaccharide from Ginkgo biloba leaves with antidepressant activities via modulation of the gut microbiome. Food Funct. 2019, 10, 8161–8171. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Cheng, L.; Zeng, X.; Zhang, X.; Wu, Z.; Weng, P. The modulatory effect of plant polysaccharides on gut flora and the implication for neurodegenerative diseases from the perspective of the microbiota-gut-brain axis. Int. J. Biol. Macromol. 2020, 164, 1484–1492. [Google Scholar] [CrossRef] [PubMed]
- Pferschy-Wenzig, E.M.; Pausan, M.R.; Ardjomand-Woelkart, K.; Röck, S.; Ammar, R.M.; Kelber, O.; Moissl-Eichinger, C.; Bauer, R. Medicinal Plants and Their Impact on the Gut Microbiome in Mental Health: A Systematic Review. Nutrients 2022, 14, 2111. [Google Scholar] [CrossRef]
- Agrawal, A.; Mohan, M.; Kasture, S.; Foddis, C.; Frau, M.A.; Loi, M.C.; Maxia, A. Antidepressant activity of Ceratonia siliqua L. fruit extract, a source of polyphenols. Nat. Prod. Res. 2011, 25, 450–456. [Google Scholar] [CrossRef]
- Davinelli, S.; Maes, M.; Corbi, G.; Zarrelli, A.; Willcox, D.C.; Scapagnini, G. Dietary phytochemicals and neuro-inflammaging: From mechanistic insights to translational challenges. Immun. Ageing 2016, 13, 16. [Google Scholar] [CrossRef]
- Rieder, R.; Wisniewski, P.J.; Alderman, B.L.; Campbell, S.C. Microbes and mental health: A review. Brain Behav. Immun. 2017, 66, 9–17. [Google Scholar] [CrossRef]
- Sarris, J. Herbal medicines in the treatment of psychiatric disorders: 10-year updated review. Phytother. Res. PTR 2018, 32, 1147–1162. [Google Scholar] [CrossRef]
- Howes, M.R.; Perry, N.S.L.; Vásquez-Londoño, C.; Perry, E.K. Role of phytochemicals as nutraceuticals for cognitive functions affected in ageing. Br. J. Pharmacol. 2020, 177, 1294–1315. [Google Scholar] [CrossRef]
- Frederick, D.L.; Ali, S.F.; Slikker, W.; Gillam, M.P.; Allen, R.R.; Paule, M.G. Behavioral and neurochemical effects of chronic methylenedioxymethamphetamine (MDMA) treatment in rhesus monkeys. Neurotoxicol. Teratol. 1995, 17, 531–543. [Google Scholar] [CrossRef]
- Bezza, K.; Laadraoui, J.; Gabbas, Z.E.; Laaradia, M.A.; Oufquir, S.; Aboufatima, R.; Gharrassi, I.; Chait, A. Effects of Anacyclus pyrethrum on affective behaviors and memory during withdrawal from cigarette smoke exposure in rats. Pharmacogn. Mag. 2020, 16, 123. [Google Scholar] [CrossRef]
- OECD. Guidelines for the Testing of Chemicals. Acute Oral Toxicity—Acute Toxic Cl Method Test No-423; Organisation for Economic Co-operation and Development (OECD): Paris, France, 2001. [Google Scholar]
- Muñoz-Cuevas, F.J.; Athilingam, J.; Piscopo, D.; Wilbrecht, L. Cocaine-induced structural plasticity in frontal cortex correlates with conditioned place preference. Nat. Neurosci. 2013, 16, 1367–1369. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, R.J.; Dalvi, A. Anxiety, defense and the elevated plus-maze. Neurosci. Biobehav. Rev. 1997, 21, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Porsolt, R.D.; Anton, G.; Blavet, N.; Jalfre, M. Behavioural despair in rats: A new model sensitive to antidepressant treatments. Eur. J. Pharmacol. 1978, 47, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Gould, T.D.; Dao, D.T.; Kovacsics, C.E. The Open Field Test. In Mood and Anxiety Related Phenotypes in Mice: Characterization Using Behavioral Tests; Humana Press: Totowa, NJ, USA, 2009; pp. 1–20. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baslam, A.; Aitbaba, A.; Lamrani Hanchi, A.; Tazart, Z.; Aboufatima, R.; Soraa, N.; Ait-El-Mokhtar, M.; Boussaa, S.; Baslam, M.; Chait, A. Modulation of Gut Microbiome in Ecstasy/MDMA-Induced Behavioral and Biochemical Impairment in Rats and Potential of Post-Treatment with Anacyclus pyrethrum L. Aqueous Extract to Mitigate Adverse Effects. Int. J. Mol. Sci. 2023, 24, 9086. https://doi.org/10.3390/ijms24109086
Baslam A, Aitbaba A, Lamrani Hanchi A, Tazart Z, Aboufatima R, Soraa N, Ait-El-Mokhtar M, Boussaa S, Baslam M, Chait A. Modulation of Gut Microbiome in Ecstasy/MDMA-Induced Behavioral and Biochemical Impairment in Rats and Potential of Post-Treatment with Anacyclus pyrethrum L. Aqueous Extract to Mitigate Adverse Effects. International Journal of Molecular Sciences. 2023; 24(10):9086. https://doi.org/10.3390/ijms24109086
Chicago/Turabian StyleBaslam, Abdelmounaim, Abdelfatah Aitbaba, Asmae Lamrani Hanchi, Zakaria Tazart, Rachida Aboufatima, Nabila Soraa, Mohamed Ait-El-Mokhtar, Samia Boussaa, Marouane Baslam, and Abderrahman Chait. 2023. "Modulation of Gut Microbiome in Ecstasy/MDMA-Induced Behavioral and Biochemical Impairment in Rats and Potential of Post-Treatment with Anacyclus pyrethrum L. Aqueous Extract to Mitigate Adverse Effects" International Journal of Molecular Sciences 24, no. 10: 9086. https://doi.org/10.3390/ijms24109086
APA StyleBaslam, A., Aitbaba, A., Lamrani Hanchi, A., Tazart, Z., Aboufatima, R., Soraa, N., Ait-El-Mokhtar, M., Boussaa, S., Baslam, M., & Chait, A. (2023). Modulation of Gut Microbiome in Ecstasy/MDMA-Induced Behavioral and Biochemical Impairment in Rats and Potential of Post-Treatment with Anacyclus pyrethrum L. Aqueous Extract to Mitigate Adverse Effects. International Journal of Molecular Sciences, 24(10), 9086. https://doi.org/10.3390/ijms24109086









