Complex Formation of an RNA Aptamer with a Part of HIV-1 Tat through Induction of Base Triples in Living Human Cells Proven by In-Cell NMR
Abstract
1. Introduction
2. Results
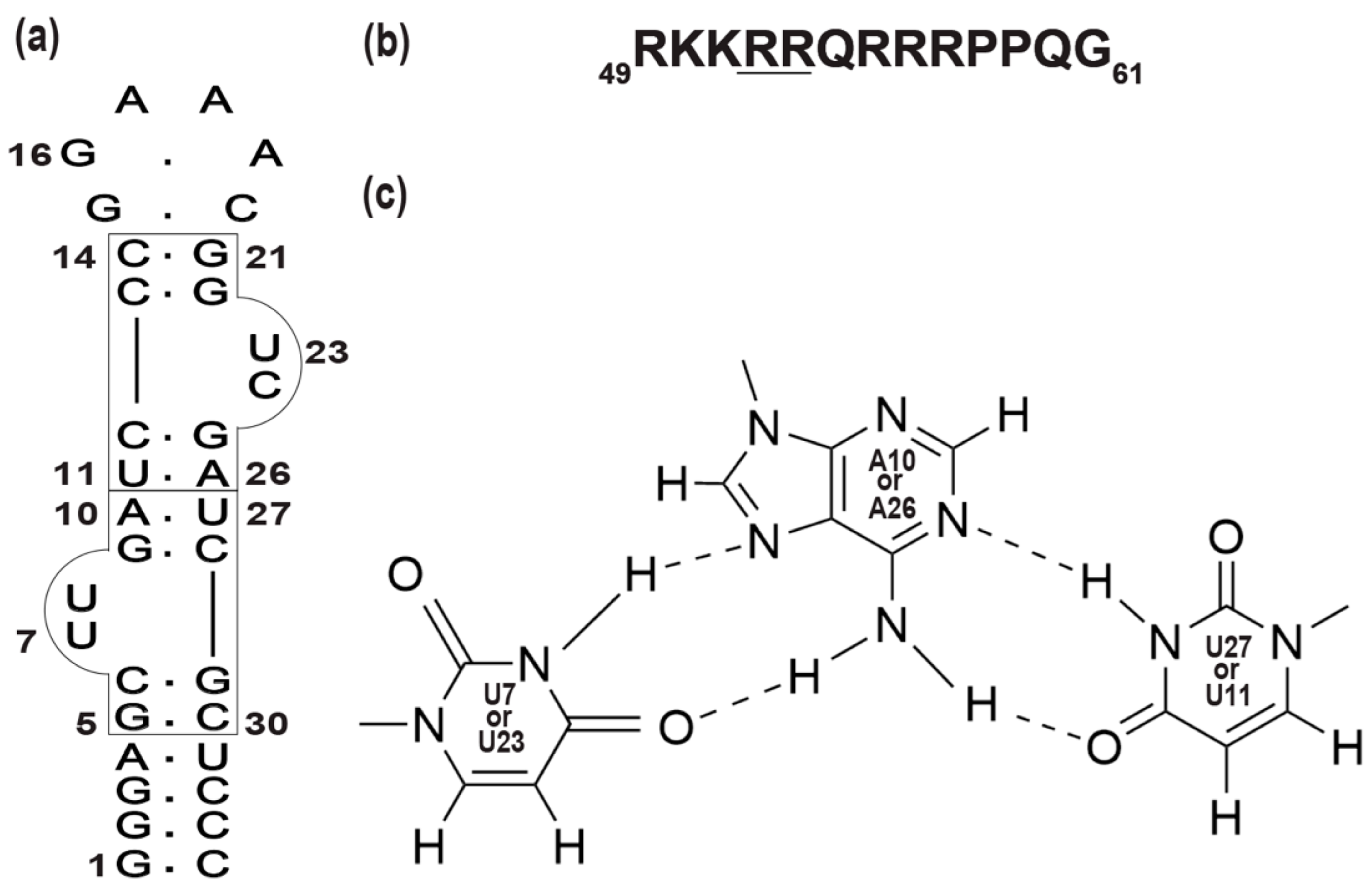
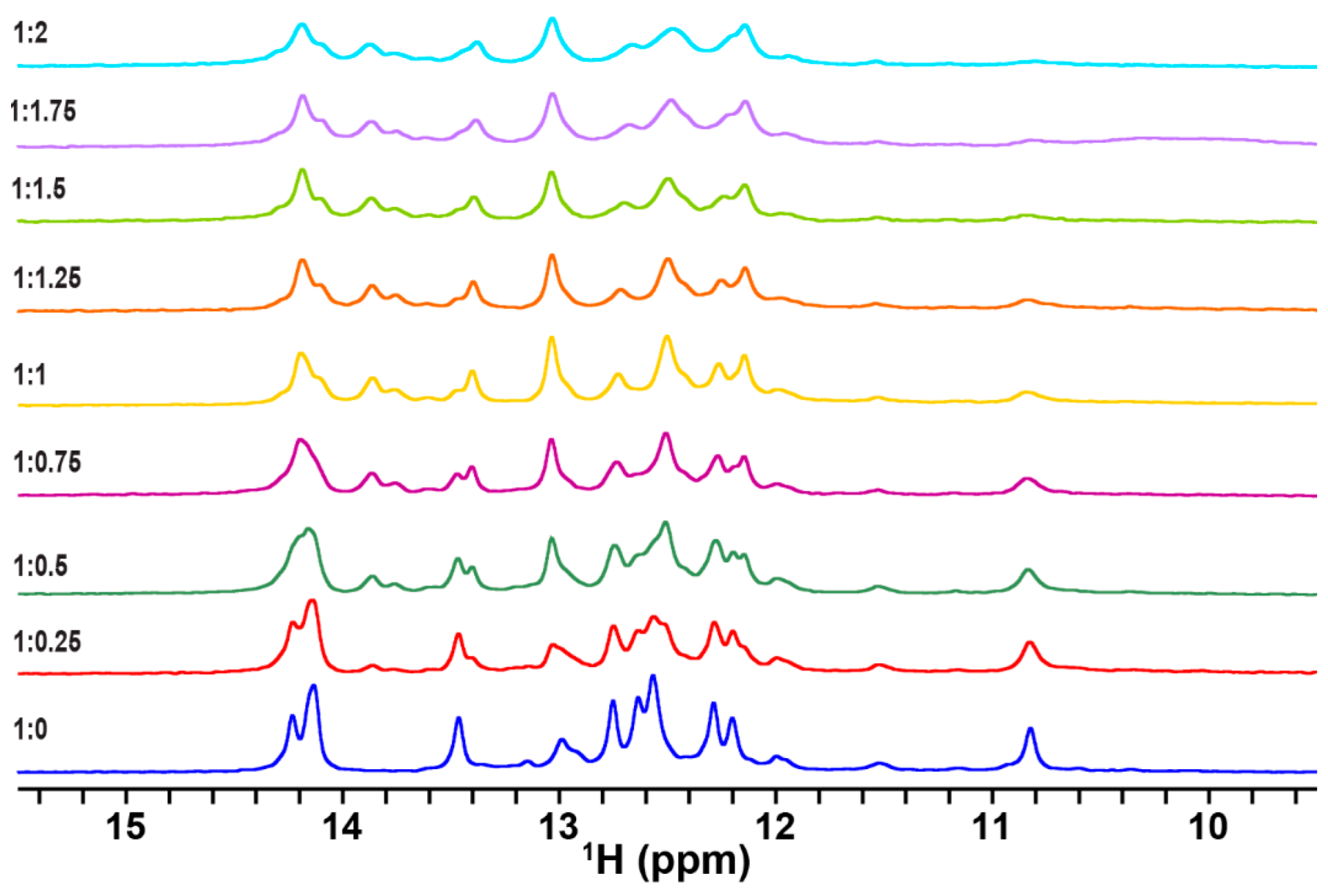
2.1. Complex Formation of TA with the RG Peptide, with the Explicit Evidence of U-A∗U Base Triples
2.2. Intermolecular NOEs between TA and the RG Peptide
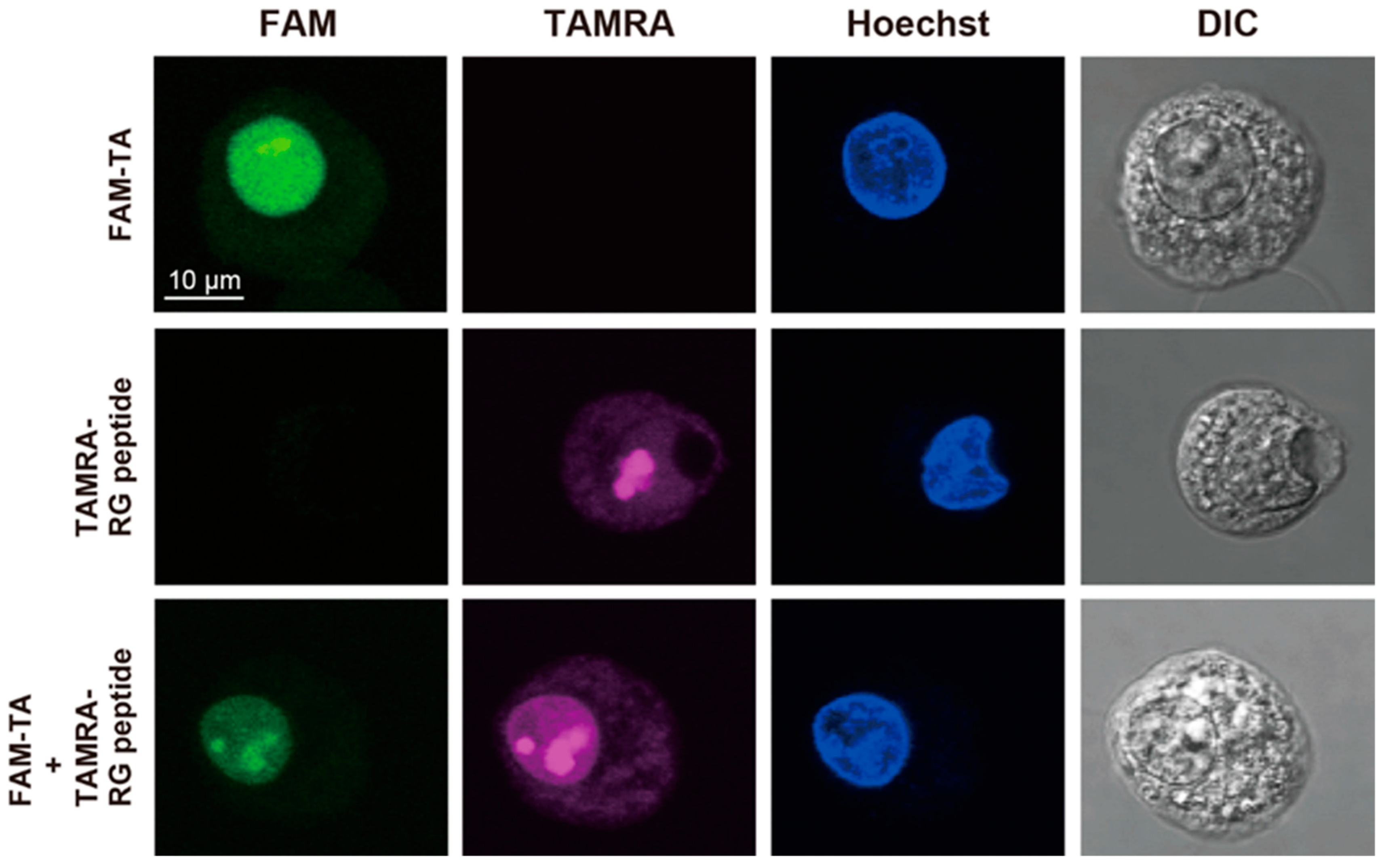
2.3. Confocal Fluorescence Microscopy Analysis
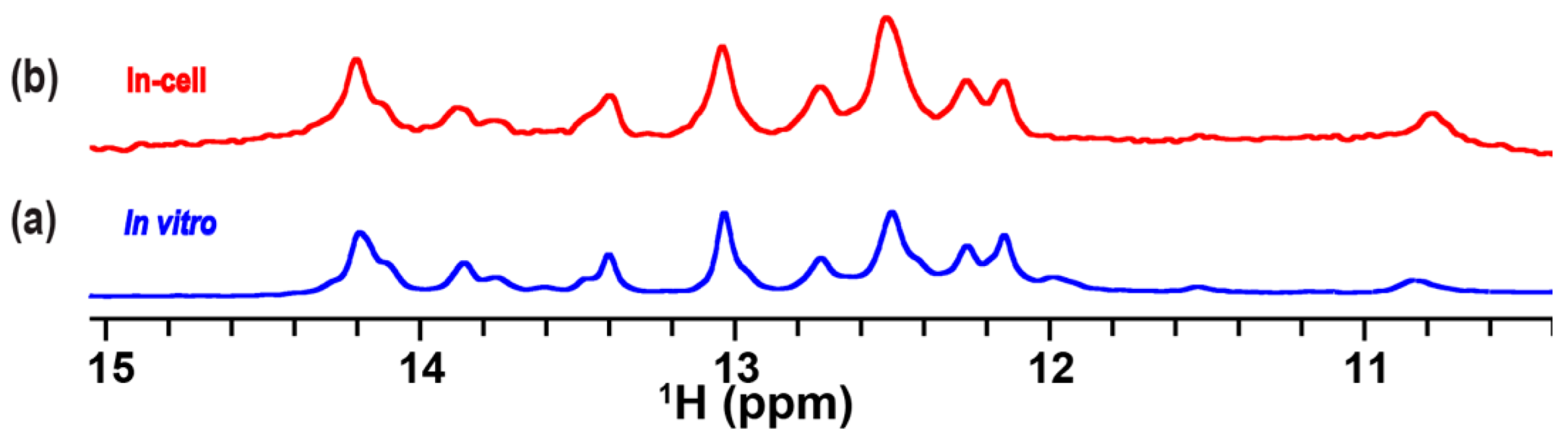
2.4. In-Cell NMR Spectrum of TA in Complex with the RG Peptide in Living Human Cells
3. Discussion
4. Materials and Methods
4.1. Preparation of RNA and RG Peptide
4.2. In Vitro NMR Measurements
4.3. In-Cell NMR Measurements Using a Bioreactor System
4.4. Confocal Fluorescence Microscopy Analysis
4.5. Temperature-Dependent UV Spectroscopy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nishida, N.; Ito, Y.; Shimada, I. In situ structural biology using in-cell NMR. Biochim. Biophys. Acta Gen. Subj. 2020, 1864, 129364. [Google Scholar] [CrossRef] [PubMed]
- Luchinat, E.; Cremonini, M.; Banci, L. Radio Signals from Live Cells: The Coming of Age of In-Cell Solution NMR. Chem. Rev. 2022, 122, 9267–9306. [Google Scholar] [CrossRef] [PubMed]
- Theillet, F.X. In-Cell Structural Biology by NMR: The Benefits of the Atomic Scale. Chem. Rev. 2022, 122, 9497–9570. [Google Scholar] [CrossRef] [PubMed]
- Yamaoki, Y.; Nagata, T.; Sakamoto, T.; Katahira, M. Recent progress of in-cell NMR of nucleic acids in living human cells. Biophys. Rev. 2020, 12, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Yamaoki, Y.; Kiyoishi, A.; Miyake, M.; Kano, F.; Murata, M.; Nagata, T.; Katahira, M. The first successful observation of in-cell NMR signals of DNA and RNA in living human cells. Phys. Chem. Chem. Phys. 2018, 20, 2982–2985. [Google Scholar] [CrossRef]
- Dzatko, S.; Krafcikova, M.; Hänsel-Hertsch, R.; Fessl, T.; Fiala, R.; Loja, T.; Krafcik, D.; Mergny, J.L.; Foldynova-Trantirkova, S.; Trantirek, L. Evaluation of the Stability of DNA i-Motifs in the Nuclei of Living Mammalian Cells. Angew. Chem. Int. Ed. 2018, 57, 2165–2169. [Google Scholar] [CrossRef]
- Cheng, M.; Qiu, D.; Tamon, L.; Ištvánková, E.; Víšková, P.; Amrane, S.; Guédin, A.; Chen, J.; Lacroix, L.; Ju, H.; et al. Thermal and pH Stabilities of i-DNA: Confronting in vitro Experiments with Models and In-Cell NMR Data. Angew. Chem. Int. Ed. 2021, 60, 10286–10294. [Google Scholar] [CrossRef]
- Bao, H.L.; Liu, H.S.; Xu, Y. Hybrid-type and two-tetrad antiparallel telomere DNA G-quadruplex structures in living human cells. Nucleic Acids Res. 2019, 47, 4940–4947. [Google Scholar] [CrossRef]
- Bao, H.L.; Xu, Y. Telomeric DNA-RNA-hybrid G-quadruplex exists in environmental conditions of HeLa cells. Chem. Commun. 2020, 56, 6547–6550. [Google Scholar] [CrossRef]
- Bao, H.L.; Masuzawa, T.; Oyoshi, T.; Xu, Y. Oligonucleotides DNA containing 8-trifluoromethyl-2′-deoxyguanosine for observing Z-DNA structure. Nucleic Acids Res. 2020, 48, 7041–7051. [Google Scholar] [CrossRef]
- Sakamoto, T.; Yamaoki, Y.; Nagata, T.; Katahira, M. Detection of parallel and antiparallel DNA triplex structures in living human cells using in-cell NMR. Chem. Commun. 2021, 57, 6364–6367. [Google Scholar] [CrossRef] [PubMed]
- Yamaoki, Y.; Nagata, T.; Kondo, K.; Sakamoto, T.; Takami, S.; Katahira, M. Shedding light on the base-pair opening dynamics of nucleic acids in living human cells. Nat. Commun. 2022, 13, 7143. [Google Scholar] [CrossRef] [PubMed]
- Krafcikova, M.; Dzatko, S.; Caron, C.; Granzhan, A.; Fiala, R.; Loja, T.; Teulade-Fichou, M.P.; Fessl, T.; Hänsel-Hertsch, R.; Mergny, J.L.; et al. Monitoring DNA-Ligand Interactions in Living Human Cells Using NMR Spectroscopy. J. Am. Chem. Soc. 2019, 141, 13281–13285. [Google Scholar] [CrossRef] [PubMed]
- Krafčík, D.; Ištvánková, E.; Džatko, Š.; Víšková, P.; Foldynová-Trantírková, S.; Trantírek, L. Towards Profiling of the G-Quadruplex Targeting Drugs in the Living Human Cells Using NMR Spectroscopy. Int. J. Mol. Sci. 2021, 22, 6042. [Google Scholar] [CrossRef] [PubMed]
- Broft, P.; Dzatko, S.; Krafcikova, M.; Wacker, A.; Hänsel-Hertsch, R.; Dötsch, V.; Trantirek, L.; Schwalbe, H. In-Cell NMR Spectroscopy of Functional Riboswitch Aptamers in Eukaryotic Cells. Angew. Chem. Int. Ed. 2021, 60, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Eladl, O.; Yamaoki, Y.; Kondo, K.; Nagata, T.; Katahira, M. Detection of interaction between RNA aptamer and its target compound in living human cells using 2D in-cell NMR. Chem. Commun. 2022, 59, 102–105. [Google Scholar] [CrossRef]
- Schlagnitweit, J.; Friebe Sandoz, S.; Jaworski, A.; Guzzetti, I.; Aussenac, F.; Carbajo, R.J.; Chiarparin, E.; Pell, A.J.; Petzold, K. Observing an Antisense Drug Complex in Intact Human Cells by in-Cell NMR Spectroscopy. ChemBioChem 2019, 20, 2474–2478. [Google Scholar] [CrossRef]
- Salgado, G.F.; Cazenave, C.; Kerkour, A.; Mergny, J.L. G-quadruplex DNA and ligand interaction in living cells using NMR spectroscopy. Chem. Sci. 2015, 6, 3314–3320. [Google Scholar] [CrossRef]
- Sharp, P.A.; Marciniak, R.A. HIV TAR: An RNA enhancer? Cell 1989, 59, 229–230. [Google Scholar] [CrossRef]
- Cordingley, M.G.; LaFemina, R.L.; Callahan, P.L.; Condra, J.H.; Sardana, V.V.; Graham, D.J.; Nguyen, T.M.; LeGrow, K.; Gotlib, L.; Schlabach, A.J. Sequence-specific interaction of Tat protein and Tat peptides with the transactivation-responsive sequence element of human immunodeficiency virus type 1 in vitro. Proc. Natl. Acad. Sci. USA 1990, 87, 8985–8989. [Google Scholar] [CrossRef]
- Rosen, C.A.; Pavlakis, G.N. Tat and Rev: Positive regulators of HIV gene expression. AIDS 1990, 4, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Matsugami, A.; Kobayashi, S.; Ouhashi, K.; Uesugi, S.; Yamamoto, R.; Taira, K.; Nishikawa, S.; Kumar, P.K.; Katahira, M. Structural basis of the highly efficient trapping of the HIV Tat protein by an RNA aptamer. Structure 2003, 11, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, R.; Katahira, M.; Nishikawa, S.; Baba, T.; Taira, K.; Kumar, P.K. A novel RNA motif that binds efficiently and specifically to the Tat protein of HIV and inhibits the trans-activation by Tat of transcription in vitro and in vivo. Genes Cells 2000, 5, 371–388. [Google Scholar] [CrossRef]
- Harada, K.; Aoyama, S.; Matsugami, A.; Kumar, P.K.; Katahira, M.; Kato, N.; Ohkanda, J. RNA-directed amino acid coupling as a model reaction for primitive coded translation. ChemBioChem 2014, 15, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Draper, D.E. A guide to ions and RNA structure. RNA 2004, 10, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Kuipers, B.J.; Gruppen, H. Prediction of molar extinction coefficients of proteins and peptides using UV absorption of the constituent amino acids at 214 nm to enable quantitative reverse phase high-performance liquid chromatography-mass spectrometry analysis. J. Agric. Food Chem. 2007, 55, 5445–5451. [Google Scholar] [CrossRef]
- Schanda, P.; Kupce, E.; Brutscher, B. SOFAST-HMQC experiments for recording two-dimensional heteronuclear correlation spectra of proteins within a few seconds. J. Biomol. NMR 2005, 33, 199–211. [Google Scholar] [CrossRef]
- Lee, W.; Rahimi, M.; Lee, Y.; Chiu, A. POKY: A software suite for multidimensional NMR and 3D structure calculation of biomolecules. Bioinformatics 2021, 37, 3041–3042. [Google Scholar] [CrossRef]
- Theillet, F.X.; Binolfi, A.; Bekei, B.; Martorana, A.; Rose, H.M.; Stuiver, M.; Verzini, S.; Lorenz, D.; van Rossum, M.; Goldfarb, D.; et al. Structural disorder of monomeric α-synuclein persists in mammalian cells. Nature 2016, 530, 45–50. [Google Scholar] [CrossRef]
- Kubo, S.; Nishida, N.; Udagawa, Y.; Takarada, O.; Ogino, S.; Shimada, I. A gel-encapsulated bioreactor system for NMR studies of protein-protein interactions in living mammalian cells. Angew. Chem. Int. Ed. 2013, 52, 1208–1211. [Google Scholar] [CrossRef]
- Breindel, L.; DeMott, C.; Burz, D.S.; Shekhtman, A. Real-Time In-Cell Nuclear Magnetic Resonance: Ribosome-Targeted Antibiotics Modulate Quinary Protein Interactions. Biochemistry 2018, 57, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Puglisi, J.D.; Tinoco, I., Jr. [22] absorbance melting curves of RNA. Methods Enzymol. 1989, 180, 304–325. [Google Scholar] [PubMed]
- Rau, M.J.; Hall, K.B. 2-Aminopurine fluorescence as a probe of local RNA structure and dynamics and global folding. Methods Enzymol. 2015, 558, 99–124. [Google Scholar] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eladl, O.; Yamaoki, Y.; Kondo, K.; Nagata, T.; Katahira, M. Complex Formation of an RNA Aptamer with a Part of HIV-1 Tat through Induction of Base Triples in Living Human Cells Proven by In-Cell NMR. Int. J. Mol. Sci. 2023, 24, 9069. https://doi.org/10.3390/ijms24109069
Eladl O, Yamaoki Y, Kondo K, Nagata T, Katahira M. Complex Formation of an RNA Aptamer with a Part of HIV-1 Tat through Induction of Base Triples in Living Human Cells Proven by In-Cell NMR. International Journal of Molecular Sciences. 2023; 24(10):9069. https://doi.org/10.3390/ijms24109069
Chicago/Turabian StyleEladl, Omar, Yudai Yamaoki, Keiko Kondo, Takashi Nagata, and Masato Katahira. 2023. "Complex Formation of an RNA Aptamer with a Part of HIV-1 Tat through Induction of Base Triples in Living Human Cells Proven by In-Cell NMR" International Journal of Molecular Sciences 24, no. 10: 9069. https://doi.org/10.3390/ijms24109069
APA StyleEladl, O., Yamaoki, Y., Kondo, K., Nagata, T., & Katahira, M. (2023). Complex Formation of an RNA Aptamer with a Part of HIV-1 Tat through Induction of Base Triples in Living Human Cells Proven by In-Cell NMR. International Journal of Molecular Sciences, 24(10), 9069. https://doi.org/10.3390/ijms24109069






