Dy4, Dy5, and Ho2 Complexes of an N3O2 Aminophenol Donor: A Dy5-µ3-Peroxide Single Molecule Magnet
Abstract
1. Introduction
2. Results and Discussion
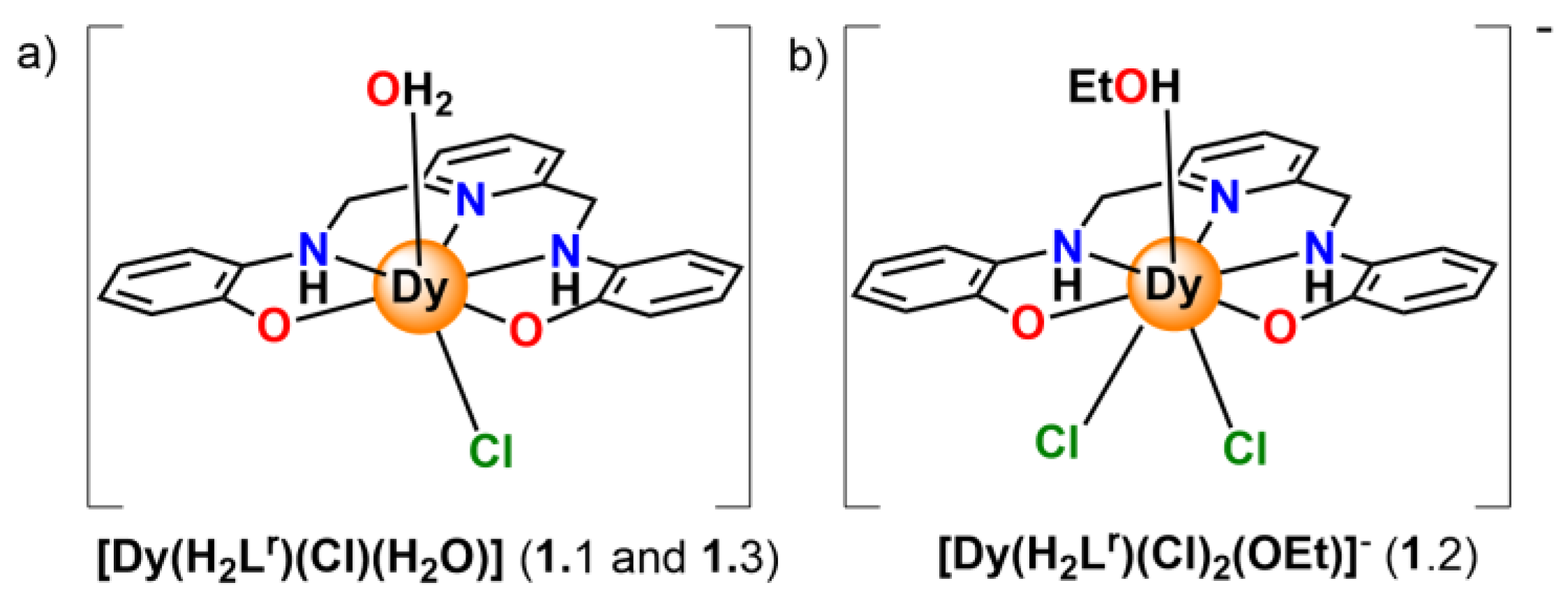
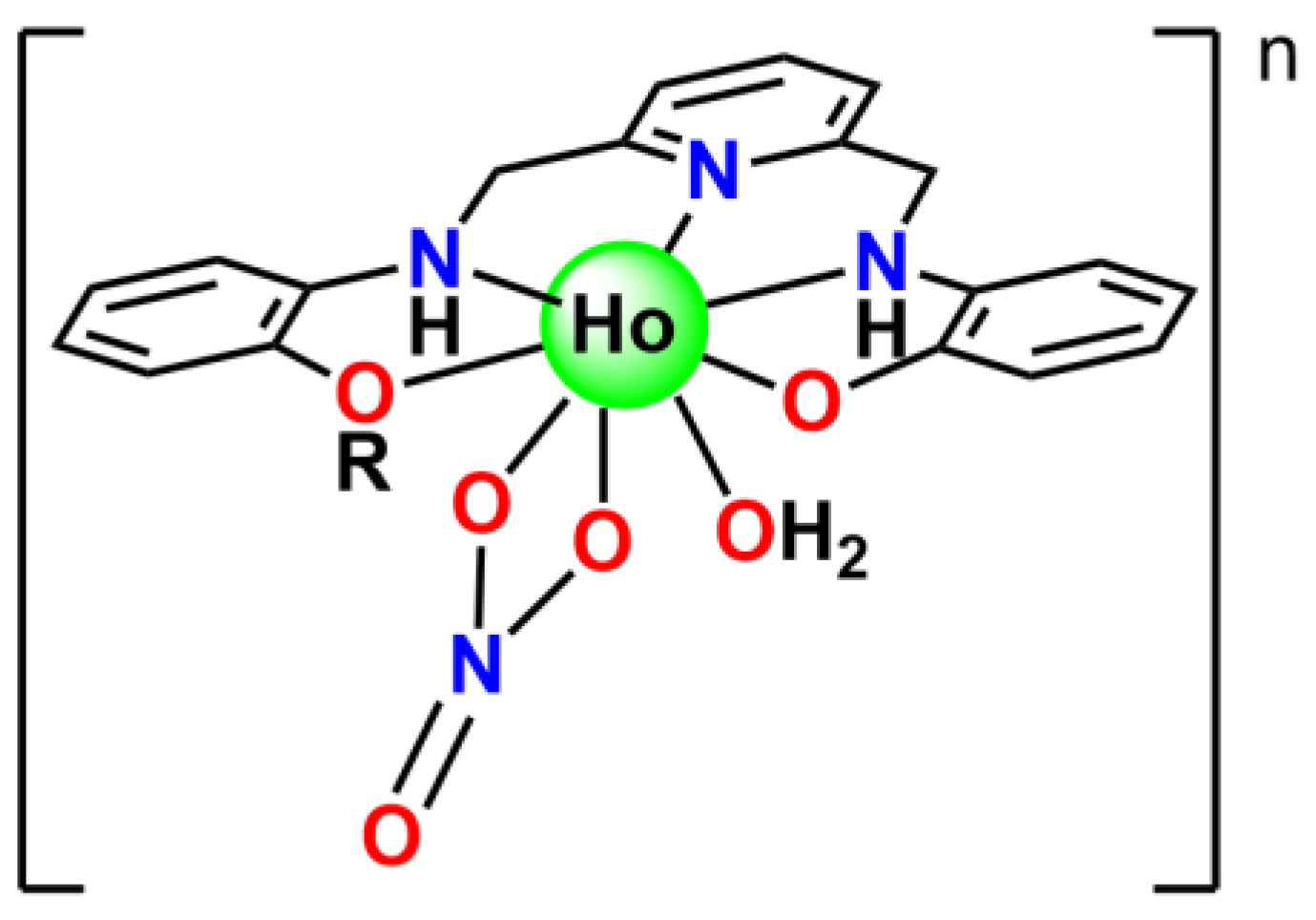
2.1. Synthesis
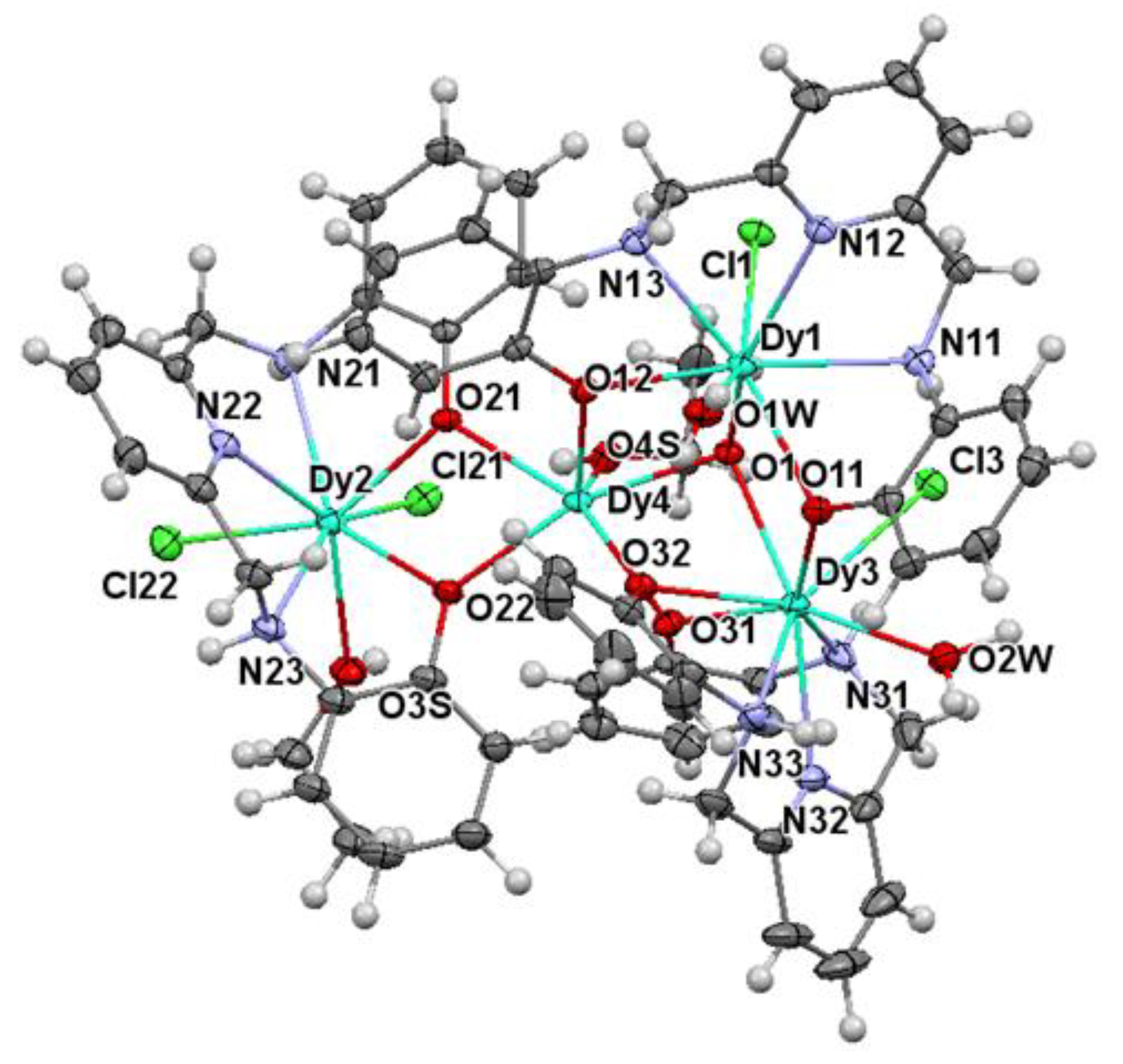
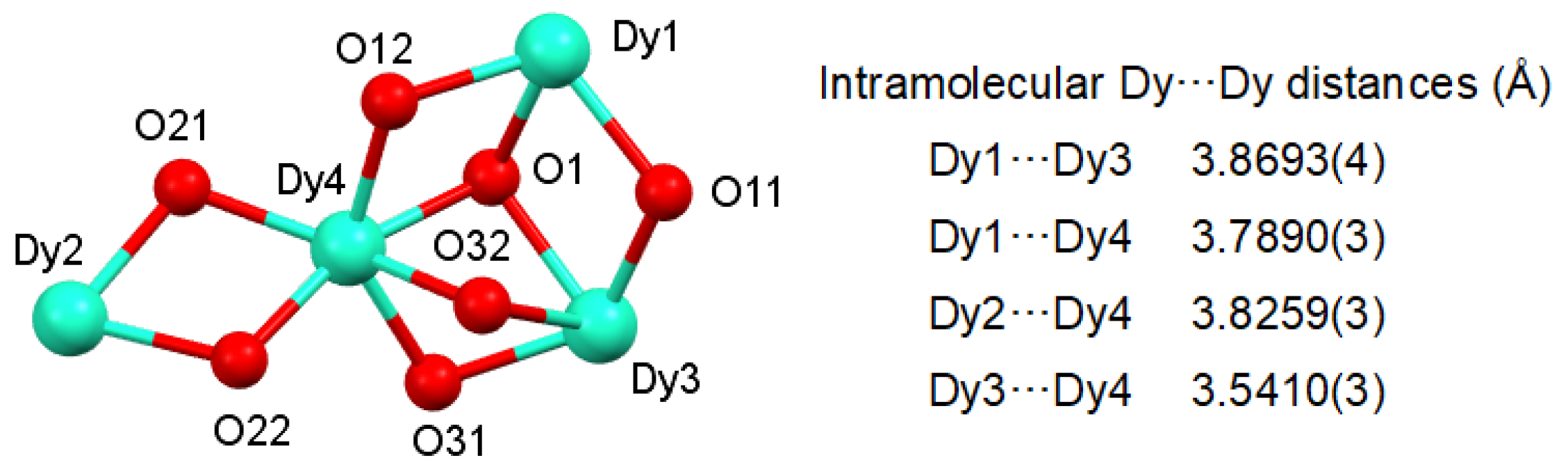
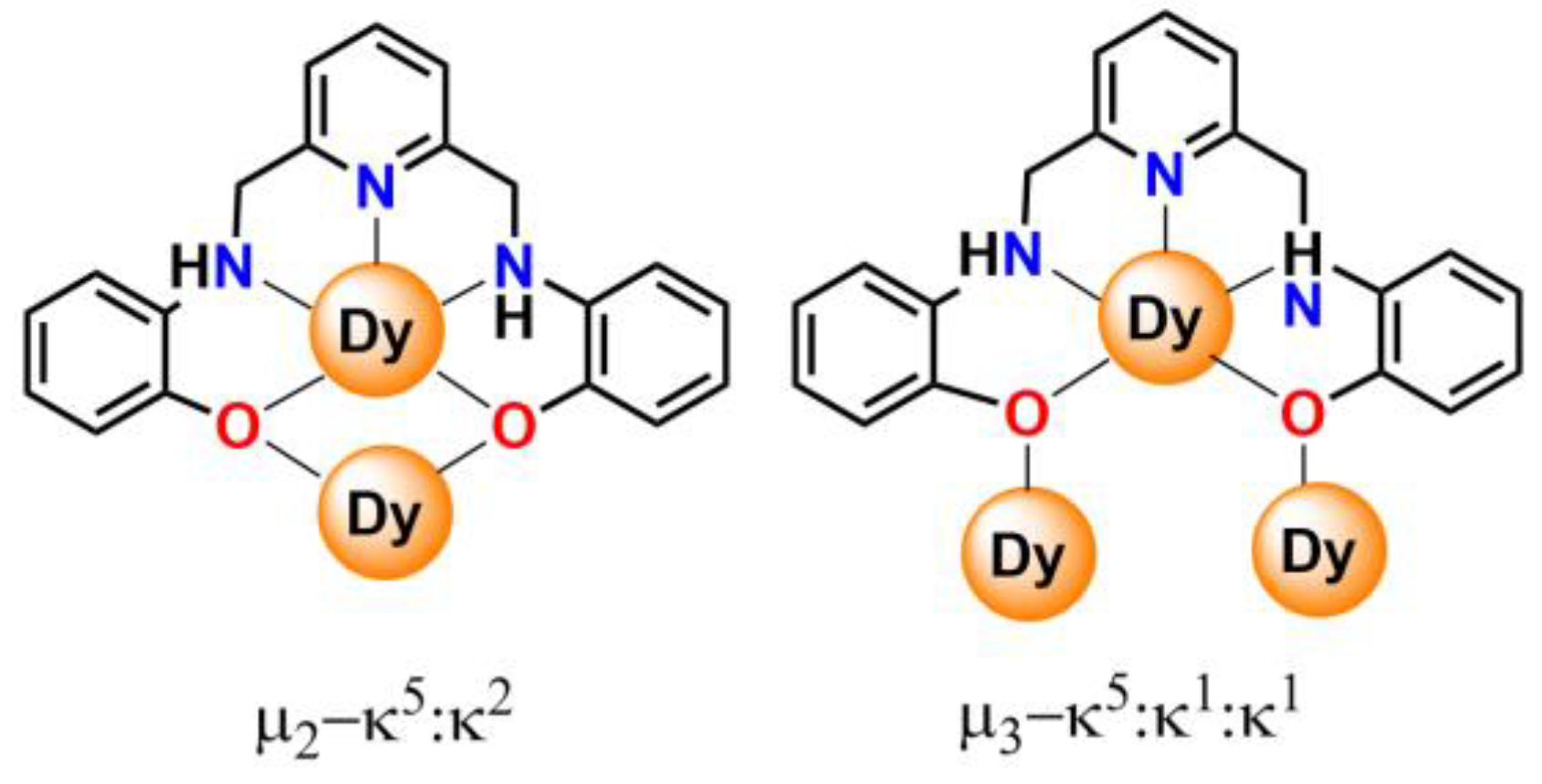
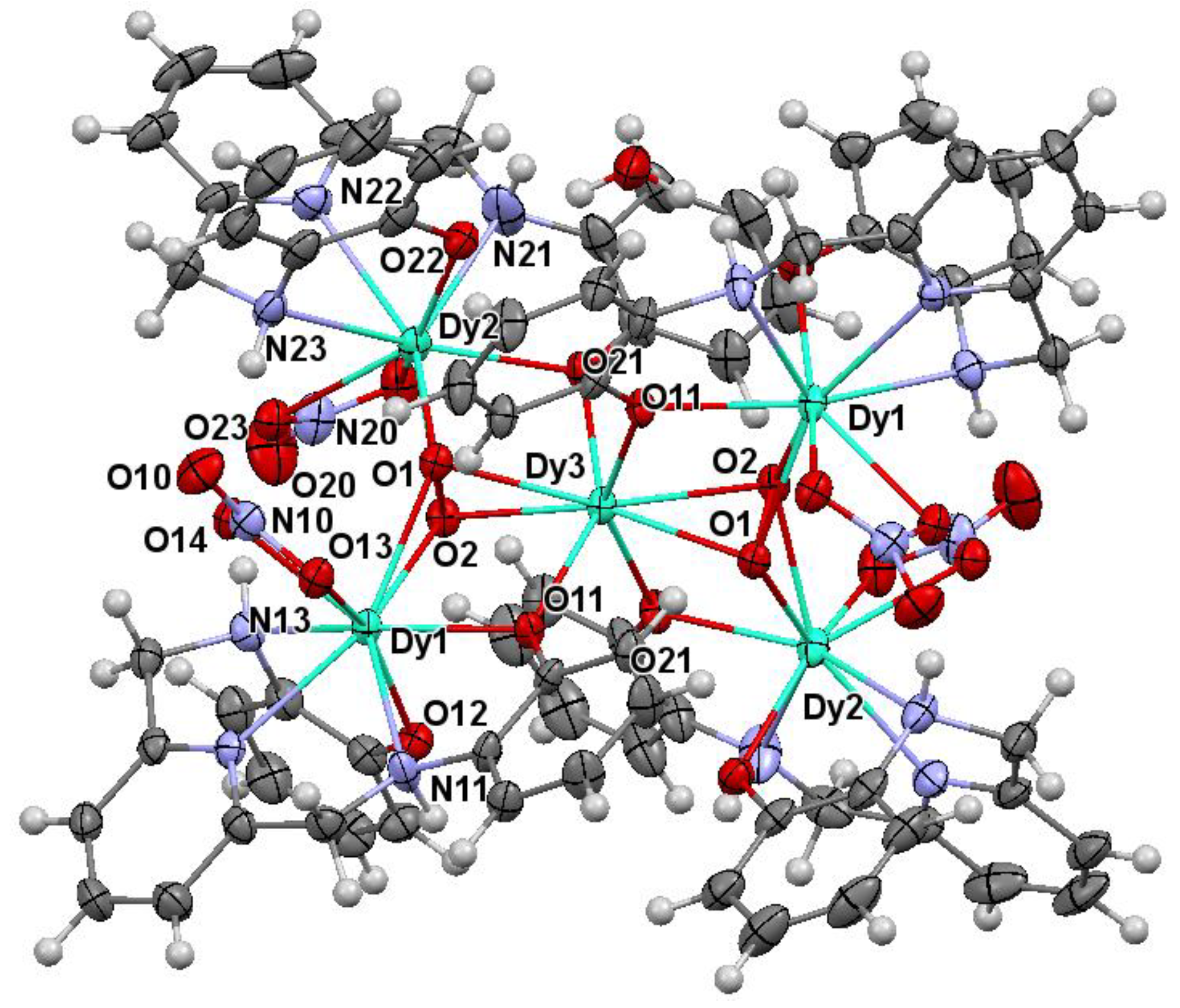
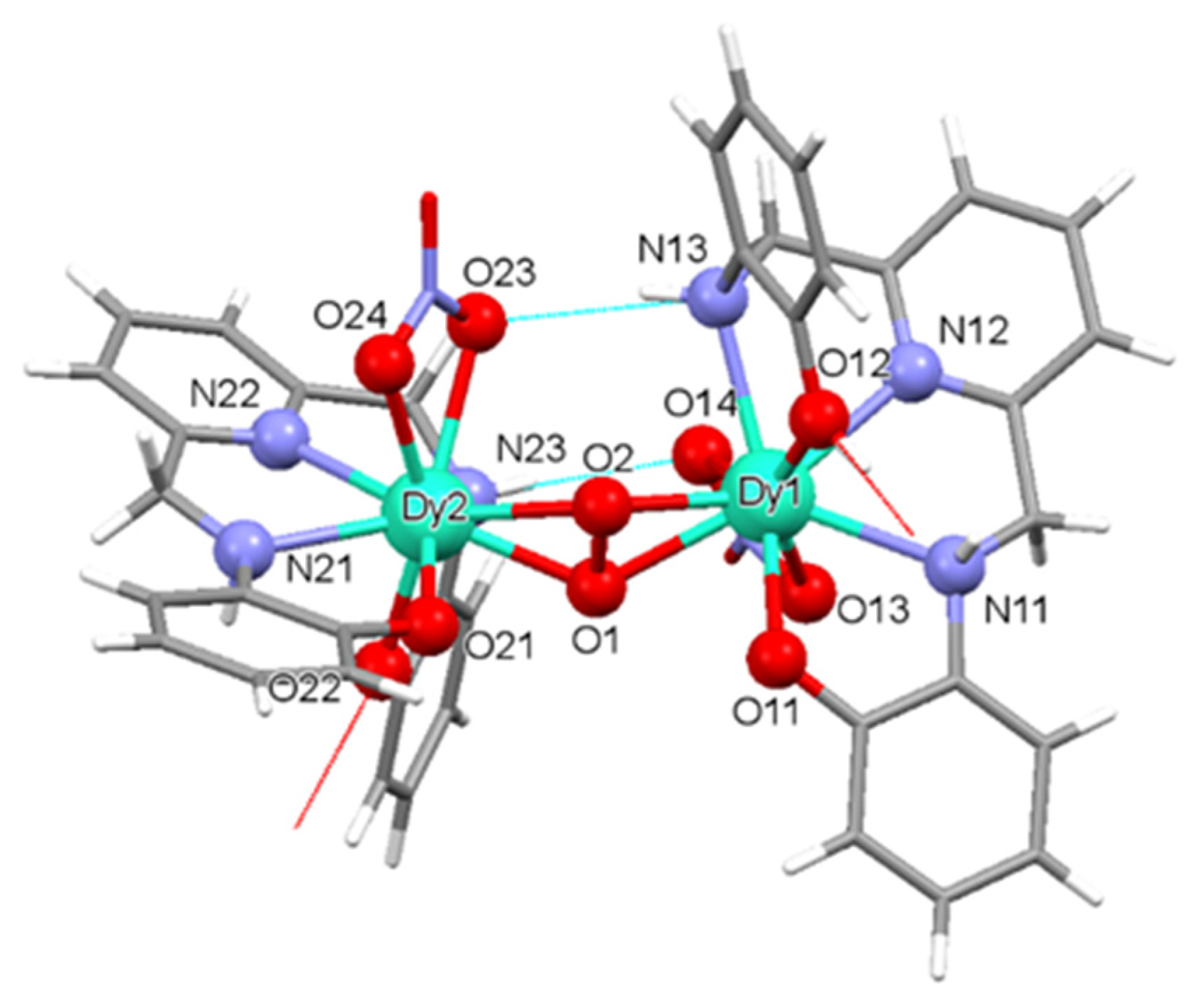
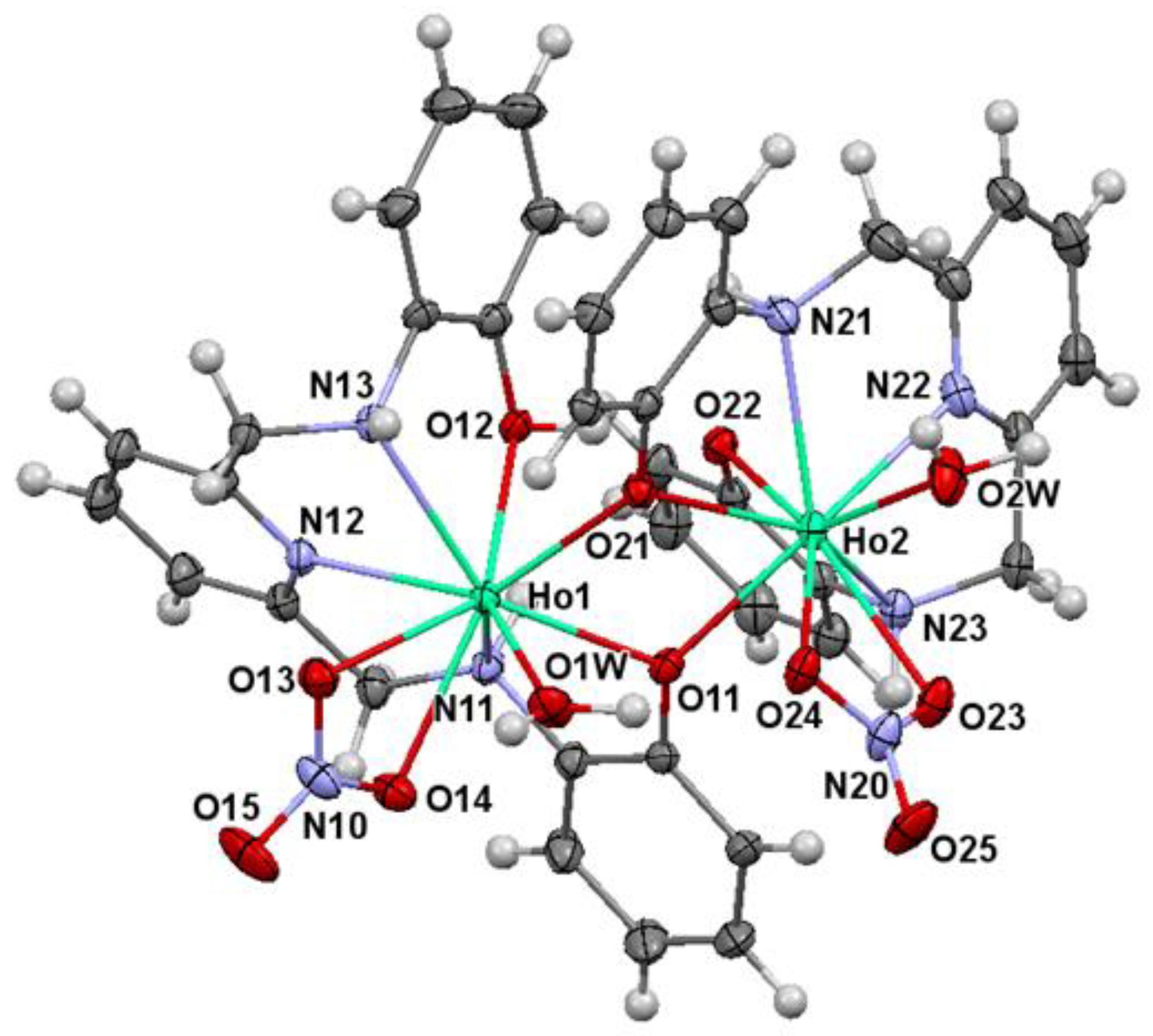
2.2. X-ray Diffraction Studies
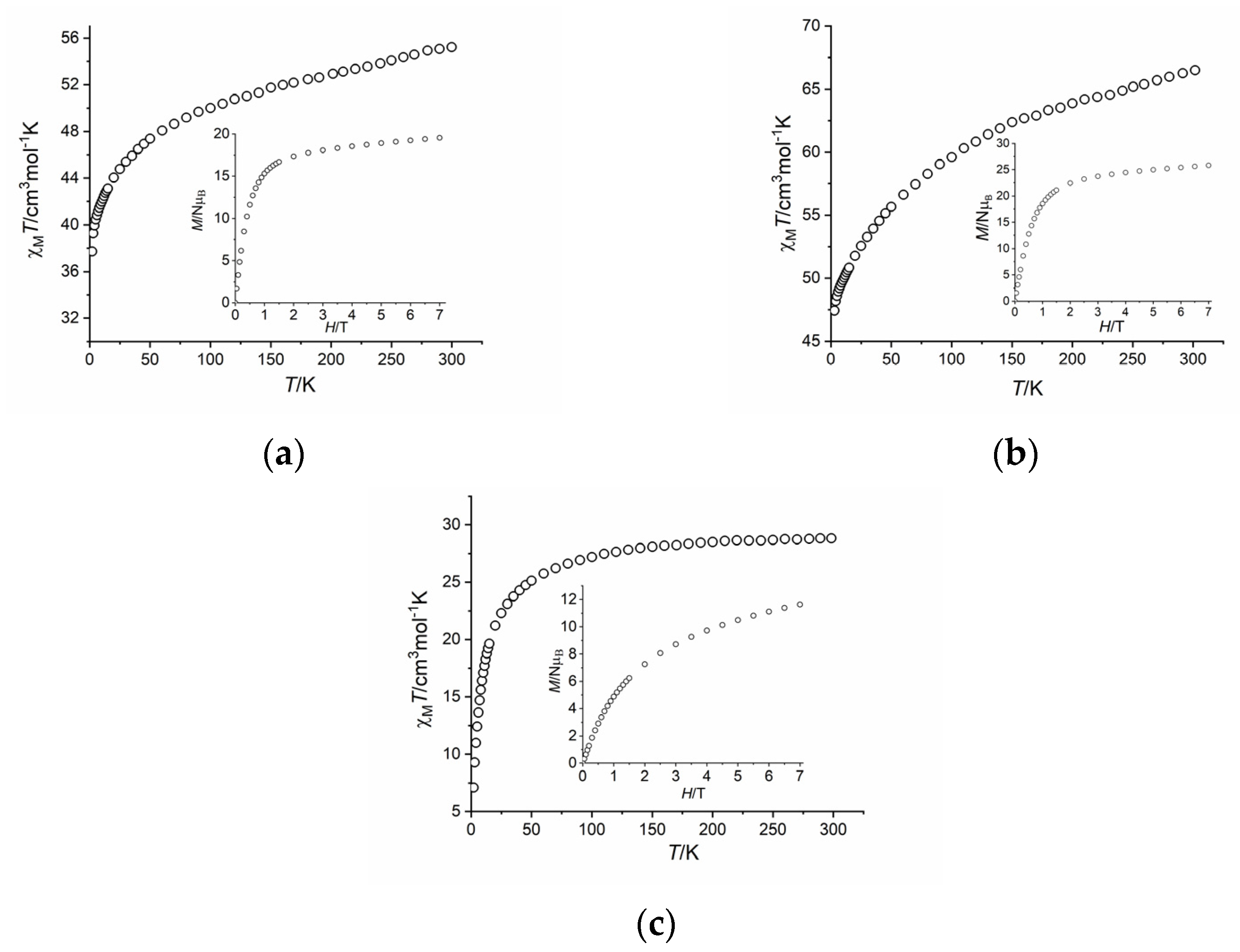
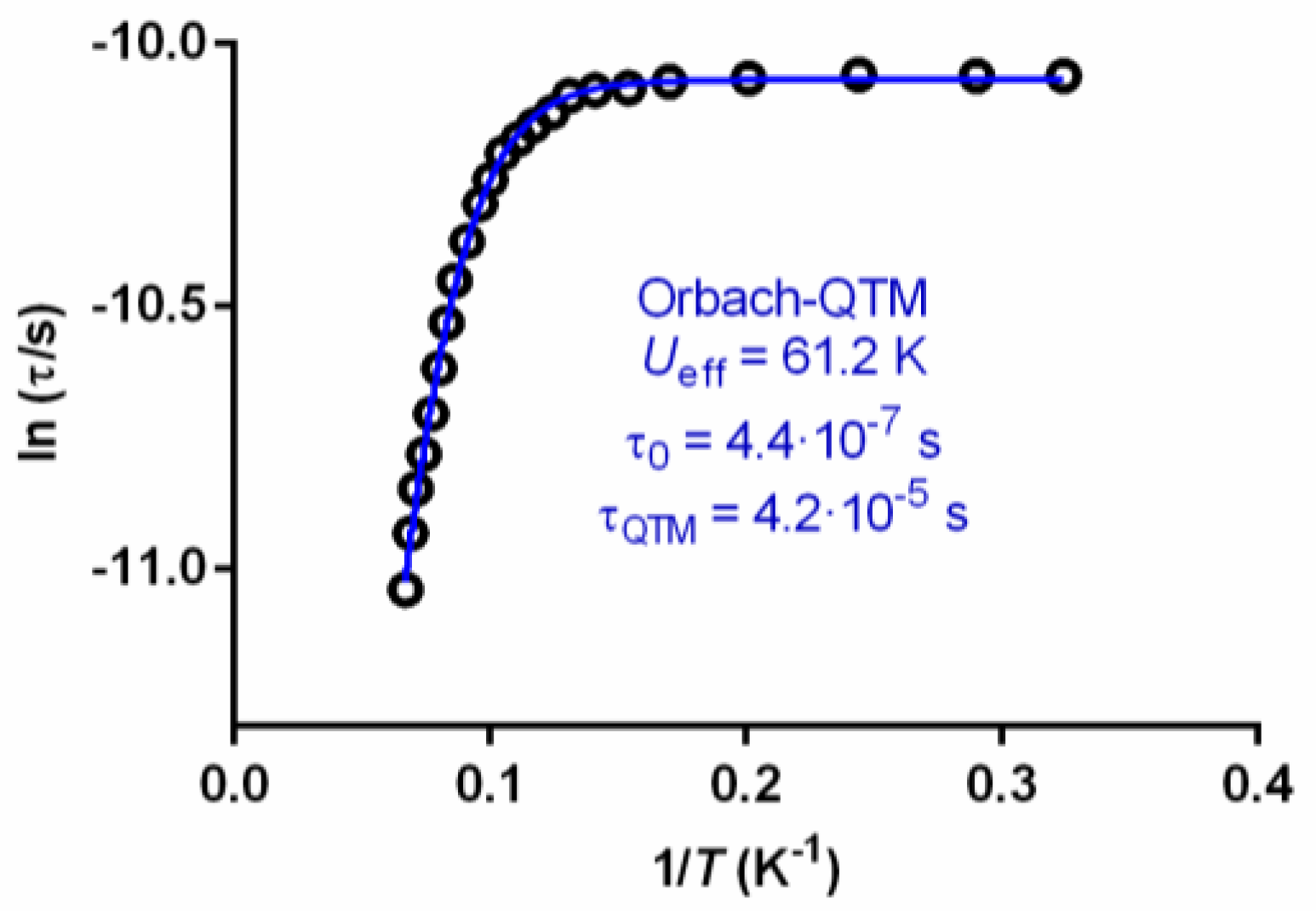
2.3. Magnetic Properties
3. Materials and Methods
3.1. Synthesis
3.2. Crystallographic Refinement and Structure Solution
3.3. Powder X-ray Diffraction Studies
3.4. Magnetic Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gatteschi, D.; Sessoli, R.; Villain, J. Molecular Nanomagnets; Oxford University Press: Oxford, UK, 2006. [Google Scholar] [CrossRef]
- Gaita-Ariño, A.; Luis, F.; Hill, S.; Coronado, E. Molecular spins for quantum computation. Nat. Chem. 2019, 11, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, N.; Sugita, M.; Ishikawa, T.; Koshihara, S.-y.; Kaizu, Y. Lanthanide double-decker complexes functioning as magnets at the single-molecular level. J. Am. Chem. Soc. 2003, 125, 8694–8695. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.-S.; Day, B.M.; Chen, Y.-C.; Tong, M.-L.; Mansikkamäki, A.; Layfield, R.A. Magnetic hysteresis up to 80 kelvin in a dysprosium metallocene single-molecule magnet. Science 2018, 362, 1400–1403. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Dong, Y.; Yin, B.; Ma, P.; Li, D. Improving the single-molecule magnet properties of two pentagonal bipyramidal Dy3+ compounds by the introduction of both electron-withdrawing and-donating groups. Dalton Trans. 2021, 50, 12607–12618. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhao, C.; Feng, T.; Liu, X.; Ying, X.; Li, X.-L.; Zhang, Y.-Q.; Tang, J. Air-stable chiral single-molecule magnets with record anisotropy barrier exceeding 1800 K. J. Am. Chem. Soc. 2021, 143, 10077–10082. [Google Scholar] [CrossRef]
- Rinehart, J.D.; Long, J.R. Exploiting single-ion anisotropy in the design of f-element single-molecule magnets. Chem. Sci. 2011, 2, 2078–2085. [Google Scholar] [CrossRef]
- Gould, C.A.; McClain, K.R.; Reta, D.; Kragskow, J.G.C.; Marchiori, D.A.; Lachman, E.; Choi, E.-S.; Analytis, J.G.; Britt, R.D.; Chilton, N.F.; et al. Ultrahard magnetism from mixed-valence dilanthanide complexes with metal-metal bonding. Science 2022, 375, 198–202. [Google Scholar] [CrossRef]
- Krylov, D.S.; Liu, F.; Avdoshenko, S.M.; Spree, L.; Weise, B.; Waske, A.; Wolter, A.U.B.; Büchner, B.; Popov, A.A. Record-high thermal barrier of the relaxation of magnetization in the nitride clusterfullerene Dy2ScN@C80-Ih. Chem. Commun. 2017, 53, 7901–7904. [Google Scholar] [CrossRef]
- Fondo, M.; Corredoira-Vázquez, J.; García-Deibe, A.M.; Sanmartín-Matalobos, J.; Gómez-Coca, S.; Ruiz, E.; Colacio, E. Slow magnetic relaxation in dinuclear dysprosium and holmium phenoxide bridged complexes: A Dy2 single molecule magnet with a high energy barrier. Inorg. Chem. Front. 2021, 8, 2532–2541. [Google Scholar] [CrossRef]
- Corredoira-Vázquez, J.; González-Barreira, C.; Fondo, M.; García-Deibe, A.M.; Sanmartín-Matalobos, J.; Gómez-Coca, S.; Ruiz, E.; Colacio, E. Dinuclear fluoride single-bridged lanthanoid complexes as molecule magnets: Unprecedented coupling constant in a fluoride-bridged gadolinium compound. Inorg. Chem. 2022, 61, 9946–9959. [Google Scholar] [CrossRef]
- González, A.; Gómez, E.; Cortés-Lozada, A.; Hernández, S.; Ramírez-Apan, T.; Nieto-Camacho, A. Heptacoordinate tin(IV) compounds derived from pyridine schiff bases: Synthesis, characterization, in vitro cytotoxicity, anti-inflammatory and antioxidant activity. Chem. Pharm. Bull. 2009, 57, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Fondo, M.; Corredoira-Vázquez, J.; García-Deibe, A.M.; Gómez-Coca, S.; Ruiz, E.; Sanmartín-Matalobos, J. Dysprosium-based complexes with a flat pentadentate donor: A magnetic and ab initio study. Dalton Trans. 2020, 49, 8389–8401. [Google Scholar] [CrossRef] [PubMed]
- Corredoira-Vázquez, J.; Oreiro-Martínez, P.; García-Deibe, A.M.; Sanmartín-Matalobos, J.; Fondo, M. A DyIII complex of a pentadentate Schiff base with field-induced single-ion magnet behaviour. Magnetochemistry 2023, 9, 62. [Google Scholar] [CrossRef]
- Spek, A. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Crystallogr. C Struct. Chem. 2015, 71, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Allen, F. The Cambridge Structural Database: A quarter of a million crystal structures and rising. Acta Crystallogr. Sect. B Struct. Sci. 2002, 58, 380–388. [Google Scholar] [CrossRef]
- Mustapha, A.; Reglinski, J.; Kennedy, A.R. The use of hydrogenated Schiff base ligands in the synthesis of multi-metallic compounds. Inorg. Chim. Acta 2009, 362, 1267–1274. [Google Scholar] [CrossRef]
- Coles, M.P.; Hitchcock, P.B.; Khvostov, A.V.; Lappert, M.F.; Li, Z.; Protchenko, A.V. Crystalline amidocerium(IV) oxides and a side-on bridging dioxygen complex. Dalton Trans. 2010, 39, 6780–6788. [Google Scholar] [CrossRef]
- Wang, G.-C.; Sung, H.H.Y.; Williams, I.D.; Leung, W.-H. Tetravalent titanium, zirconium and cerium oxo and peroxo complexes containing an imidodiphosphinate ligand. Inorg. Chem. 2012, 51, 3640–3647. [Google Scholar] [CrossRef]
- Sang, Y.-L.; Lin, X.-S.; Li, X.-C.; Liu, Y.-H.; Zhang, X.-H. Synthesis, crystal structure and antibacterial activity of a novel phenolato- and peroxo-bridged dinuclear cerium(IV) complex with tripodal Schiff bases. Inorg. Chem. Commun. 2015, 62, 115–118. [Google Scholar] [CrossRef]
- Paul, M.; Shirase, S.; Morimoto, Y.; Mathey, L.; Murugesapandian, B.; Tanaka, S.; Itoh, S.; Tsurugi, H.; Mashima, K. Cerium-complex-caralyzed oxidation of arylmethanols under atmosphetic pressure of dioxygen and its mechanism through a side-on µ-peroxo dicerium(IV) complex. Chem. Eur. J. 2016, 22, 4008–4014. [Google Scholar] [CrossRef]
- Shirase, S.; Shinohara, K.; Tsurugi, H.; Mashima, K. Oxidation of alcohols to carbonyl compounds catalyzed by oxo-bridged dinuclear cerium complexes with pentadentate Schiff-base ligands under a dioxygen atmosphere. ACS Catal. 2018, 8, 6939−6947. [Google Scholar] [CrossRef]
- Deacon, G.B.; Forsyth, C.M.; Freckmann, D.; Junk, P.C.; Konstas, K.; Luu, J.; Meyer, G.; Werner, D. Adventiously obtained rare-earth peroxide complexes and their structural characterisation. Aust. J. Chem. 2014, 67, 1860–1865. [Google Scholar] [CrossRef]
- Roitershtein, D.M.; Vinogradov, A.A.; Lyssenko, K.A.; Nifant’ev, I.E. Self-assembly of heteroleptic tetranuclear carboxylate complexes of yttrium and lanthanides during hydrolysis and oxidation of rare earth homoleptic carboxylates. Inorg. Chem. Commun. 2017, 84, 225–228. [Google Scholar] [CrossRef]
- Miller, J.T.; Ren, Y.; Li, S.; Tan, K.; McCandless, G.; Jacob, C.; Wu, Z.; Chu, C.-W.-; Lv, B.; Biewer, M.C.; et al. Peroxide-templated assembly of a trimetal neodymium complex single-molecule magnet. Inorg. Chem. 2020, 59, 10379–10383. [Google Scholar] [CrossRef] [PubMed]
- Neumüller, B.; Weller, F.; Gröb, T.; Dehnicke, K. Lanthanoid peroxo complexes with µ3-ɳ2:ɳ2:ɳ2-(O22-) coordination. Crystal structures of [Ln4(O2)2Cl8(Py)10]∙Py mit Ln = Sm, Eu, Gd. Z. Anorg. Allg. Chem. 2002, 628, 2365–2371. [Google Scholar] [CrossRef]
- Xémard, M.; Goudy, V.; Braun, A.; Tricoire, M.; Cordier, M.; Ricard, L.; Castro, L.; Louyriac, E.; Kefalidis, C.E.; Clavaguéra, C.; et al. Reductive disproportionation of CO2 with bulky divalent samarium complexes. Orgametallics 2017, 36, 4660–4668. [Google Scholar] [CrossRef]
- van Velzen, N.J.C.; Harder, S. Deca-arylsamarocene: An unusually inert Sm(II) sandwich complex. Organometallics 2018, 37, 2263–2271. [Google Scholar] [CrossRef]
- Gee, W.J.; MacLellan, J.G.; Forsyth, C.M.; Moubaraki, B.; Murray, K.S.; Andrews, P.C.; Junk, P.C. Caging peroxide: Anion-templated synthesis and characterization of a rare-earth cluster. Inorg. Chem. 2012, 51, 8661–8663. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, J.; Shen, S.; Liu, Z.; Tang, J. Coupling Dy3 triangles into hexanuclear dysprosium(III) clusters: Syntheses, structures and magnetic properties. Polyhedron 2018, 150, 40–46. [Google Scholar] [CrossRef]
- Niemeyer, M. Synthesis and structural characterization of several ytterbium bis(trimethylsilyl)amides including base-free [Yb{N(SiMe3)2}2(µ-Cl)]2-A coordinatively unsaturated complex with additional agostic Yb∙(H3C-Si) interactions. Z. Anorg. Allg. Chem. 2002, 628, 647–657. [Google Scholar] [CrossRef]
- Patrioniak, V.; Kubichi, M.; Mondry, A.; Lisowski, J.; Radecka-Paryzek, W. Pentaaza macrocyclic ytterbium(III) complex and solvent controlled supramolecular self-assembly of its dimeric µ-ɳ2:ɳ2 peroxo-bridged derivatives. Dalton Trans. 2004, 3295–3304. [Google Scholar] [CrossRef] [PubMed]
- Radecka-Paryzek, W.; Patroniak, V.; Kubicki, M. First example of template synthesis of pentaaza macrocyclic ytterbium(III) complex and solvent-controlled supramolecular self-assembly of its dimeric µ-ɳ2: ɳ2 peroxo-bridged derivative. Inorg. Chem. Commun. 2004, 7, 455–458. [Google Scholar] [CrossRef]
- Guo, Z.; Blair, V.L.; Deacon, G.B.; Junk, P.C. Widely contrasting outcomes from the use of tris(pentafluorophenyl)bismuth or pentafluorophenylsilver as oxidants in the reactions of lanthanoid metals with N,N’- diarylformamidines. Dalton Trans. 2020, 49, 13588–13600. [Google Scholar] [CrossRef] [PubMed]
- Patroniak, V.; Kubicki, M.; Radecka-Paryzek, W. The first example of µ-ɳ2:ɳ2 peroxo-bridged macrocyclic lanthanide complex. The crystal structure of [Lu2{Me2pyo [16]trieneN5}2(µ-ɳ2:ɳ2 -O2)Cl2] (ClO4)2 dioxane solvate. J. Incl. Phenom. Macrocycl. Chem. 2004, 49, 121–125. [Google Scholar] [CrossRef]
- Wang, X.-T.; Dong, H.-M.; Wang, X.-G.; Yang, E.-C.; Zhao, X.-J. Two oxime-based {LnIII3NiII3} clusters with triangular {Ln3(µ3-O2)}7+ core: Solvothermal syntheses, crystal structures, and magnetic properties. Z. Anorg. Chem. 2016, 642, 1166–1172. [Google Scholar] [CrossRef]
- Liu, C.-M.; Zhang, D.-Q.; Hao, X.; Zhu, D.-B. Assembly of chiral 3d-4f wheel-like cluster complexes with achiral ligands: Single-molecule magnet behaviour and magnetocaloric effect. Inorg. Chem. Front. 2020, 7, 3340–3351. [Google Scholar] [CrossRef]
- Ke, H.; Lu, X.; Wei, W.; Wang, W.; Xie, G.; Chen, S. Unusual undenuclear heterobimetallic Zn4Ln7 (Ln = Gd, Dy) nano-sized clusters encapsulating two peroxide anions through spontaneous intake of dioxygen. Dalton Trans. 2017, 46, 8138–8145. [Google Scholar] [CrossRef] [PubMed]
- Bradley, D.; Ghotraf, J.S.G.; Hart, A.L.; Hursthouse, M.B.; Raithby, P.R. The peroxo -group as a doubly bidentate bridging ligand in five-co-ordinate lanthanide complexes. J. Chem. Soc. Chem. Commun. 1974, 40. [Google Scholar] [CrossRef]
- Pook, N.-P.; Adam, A. Synthesis, crystal structure, and vibrational spectra of five novel peroxidocerates(IV) and the occurrence of a new complex unit in K8[Ce2(O2)3(NTA)2]2·20H2O. Z. Anorg. Allg. Chem. 2014, 640, 2931–2938. [Google Scholar] [CrossRef]
- Wang, G.-C.; So, Y.-M.; Wong, K.-L.; Au-Yeung, K.-C.; Sung, H.H.-Y.; Williams, I.D.; Leung., W.-H. Synthesis, structure, and reactivity of a tetranuclear cerium(IV) oxo cluster supported by the Kläui tripodal ligand [Co(ɳ5-C5H5){P(O)(OEt)2}3]−. Chem. Eur. J. 2015, 21, 16126–16135. [Google Scholar] [CrossRef]
- Kumar, P.; Flores-González, J.; Prakash-Sahu, P.; Ahmed, N.; Acharya, J.; Kumar, V.; Cador, O.; Pointillart, F.; Chandrasekhar, V. Magnetocaloric effect and slow magnetic relaxation in peroxide-assisted tetranuclear lanthanide assemblies. Inorg. Chem. Front. 2022, 9, 5072–5092, Metais: Gd, Tb, Dy e Er. [Google Scholar] [CrossRef]
- Llunell, M.; Casanova, D.; Cirera, J.; Alemany, P.; Alvarez, S. SHAPE: Program for the Stereochemical Analysis of Molecular Fragments by Means of Continuous Shape Measures and Associated Tools; University of Barcelona: Barcelona, Spain, 2010. [Google Scholar]
- Liu, J.-L.; Chen, Y.-C.; Tong, M.-L. Symmetry strategies for high performance lanthanide-based single-molecule magnets. Chem. Soc. Rev. 2018, 47, 2431–2453. [Google Scholar] [CrossRef] [PubMed]
- Amjad, A.; Madalan, A.M.; Andruh, M.; Caneschi, A.; Sorace, L. Slow relaxation of magnetization in an isostructural series of zinc-lanthanide complexes: An integrated EPR and AC susceptibility study. Chem. Eur. J. 2016, 22, 12849–12858. [Google Scholar] [CrossRef] [PubMed]
- Fondo, M.; Corredoira-Vázquez, J.; Herrera-Lanzós, A.; García-Deibe, A.M.; Sanmartín-Matalobos, J.; Herrera, J.M.; Colacio, E.; Nuñez, C. Improving the SMM and luminescence properties of lanthanide complexes with LnO9 cores in the presence of ZnII: An emissive Zn2Dy single ion magnet. Dalton Trans. 2017, 46, 17000–17009. [Google Scholar] [CrossRef]
- Fondo, M.; Corredoira-Vazquez, J.; Garcia-Deibe, A.M.; Sanmartin-Matalobos, J.; Herrera, J.M.; Colacio, E. Designing ligands to isolate ZnLn and Zn2Ln complexes: Field-induced single-ion magnet behavior of the ZnDy, Zn2Dy, and Zn2Er analogues. Inorg. Chem. 2017, 56, 5646–5656. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SADABS, Area-Detector Absorption Correction; Siemens Industrial Automation, Inc.: Madison, WI, USA, 2007. [Google Scholar]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]



| Metal Complex a | Ueff (K)/Hdc (Oe) | Ref. |
|---|---|---|
| [Zn4Dy7(L1)8(O2)2(OH)4(Cl)4(H2O)4]Cl | 19.5/0 | [38] |
| [Zn3Dy3(L2)3(O2)(PyCO2)3](OH)2(ClO4)2 | 126.5/1000 | [37] |
| [Zn3Tb3(L2)3(O2)(PyCO2)3](OH)2(ClO4)2 | 14.4/1400 | [37] |
| [Cu3Dy3(L2)3(O2)(PyCO2)3](OH)2(ClO4)2 | 25.6/1400 | [37] |
| [Cu3Tb3(L2)3(O2)(PyCO2)3](OH)2(ClO4)2 | 35.1/0 | [37] |
| [Dy3Ni3(H2O)3(mpko)9(O2)(NO3)3](ClO4) | ≈4.1/0 | [36] |
| [Dy4(L3H2)2(η1-Piv)(η2-Piv)(O2)2(H2O)2] | 23/1000 | [42] |
| [Dy5(H2L)2(H2.5L)2(NO3)4(µ3-O2)2] | 61.2/0 | [This work] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corredoira-Vázquez, J.; Oreiro-Martínez, P.; Nieto-Pastoriza, D.; García-Deibe, A.M.; Sanmartín-Matalobos, J.; Fondo, M. Dy4, Dy5, and Ho2 Complexes of an N3O2 Aminophenol Donor: A Dy5-µ3-Peroxide Single Molecule Magnet. Int. J. Mol. Sci. 2023, 24, 9061. https://doi.org/10.3390/ijms24109061
Corredoira-Vázquez J, Oreiro-Martínez P, Nieto-Pastoriza D, García-Deibe AM, Sanmartín-Matalobos J, Fondo M. Dy4, Dy5, and Ho2 Complexes of an N3O2 Aminophenol Donor: A Dy5-µ3-Peroxide Single Molecule Magnet. International Journal of Molecular Sciences. 2023; 24(10):9061. https://doi.org/10.3390/ijms24109061
Chicago/Turabian StyleCorredoira-Vázquez, Julio, Paula Oreiro-Martínez, Daniel Nieto-Pastoriza, Ana M. García-Deibe, Jesús Sanmartín-Matalobos, and Matilde Fondo. 2023. "Dy4, Dy5, and Ho2 Complexes of an N3O2 Aminophenol Donor: A Dy5-µ3-Peroxide Single Molecule Magnet" International Journal of Molecular Sciences 24, no. 10: 9061. https://doi.org/10.3390/ijms24109061
APA StyleCorredoira-Vázquez, J., Oreiro-Martínez, P., Nieto-Pastoriza, D., García-Deibe, A. M., Sanmartín-Matalobos, J., & Fondo, M. (2023). Dy4, Dy5, and Ho2 Complexes of an N3O2 Aminophenol Donor: A Dy5-µ3-Peroxide Single Molecule Magnet. International Journal of Molecular Sciences, 24(10), 9061. https://doi.org/10.3390/ijms24109061












