Post-Traumatic Stress Disorder Is Associated with Elevated Plasma Cholesterol in Female TT Homozygotes of LDLR rs5925
Abstract
1. Introduction
2. Results
2.1. LDLR rs5925 Genotype and Frequency of Alleles
2.2. Prevalence of PTSD in Subjects with Different LDLR rs5925 Genotypes
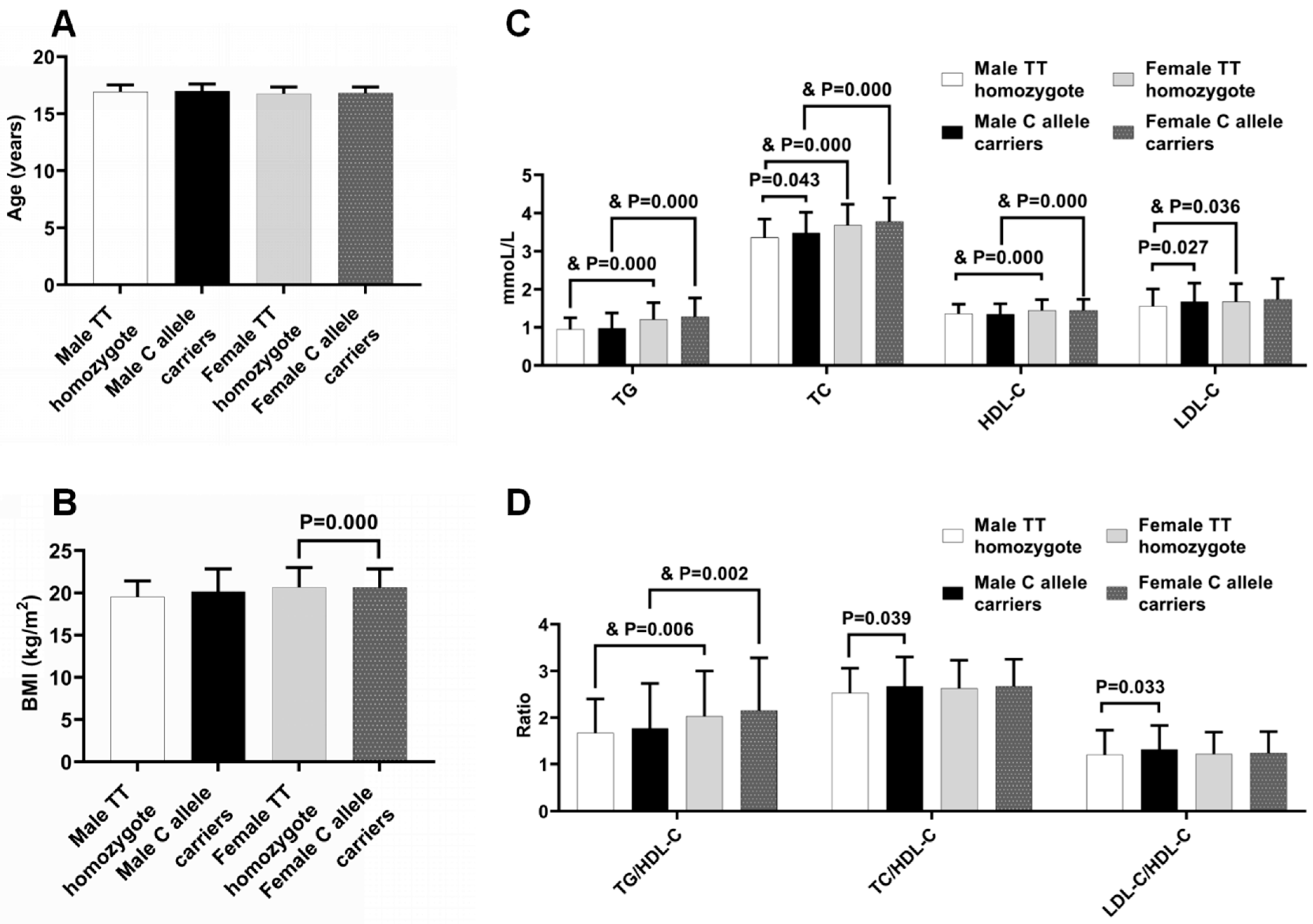
2.3. Anthropometric Characteristics and Plasma Lipid Profiles of the Subjects with Different Genotypes of LDLR rs5925
2.4. Anthropometric Characteristics and Plasma Lipid Profiles in the Subjects with Different Genotypes of LDLR rs5925 and with or without PTSD
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Questionnaires and Measurements
4.3. DNA Extraction and Genotyping
4.4. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
| PTSD | Post-traumatic stress disorder |
| LDLR | Low-density lipoprotein receptor |
| TC | Total cholesterol |
| LDL-C | Low-density lipoprotein cholesterol |
| HDL-C | High-density lipoprotein cholesterol |
| TG | Triglyceride |
| PCL-C | PTSD Checklist—Civilian Version |
| BMI | Body Mass Index |
References
- Britnell, S.R.; Jackson, A.D.; Brown, J.N.; Capehart, B.P. Aripiprazole for Post-traumatic Stress Disorder: A Systematic Review. Clin. Neuropharmacol. 2017, 40, 273–278. [Google Scholar] [CrossRef]
- Oppizzi, L.M.; Umberger, R. The Effect of Physical Activity on PTSD. Issues Ment. Health Nurs. 2018, 39, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Wang, Y.; Lu, L.; Lu, Y.; Xu, J. Post-traumatic growth among 5195 adolescents at 8.5 years after exposure to the Wenchuan earthquake: Roles of post-traumatic stress disorder and self-esteem. J. Health Psychol. 2021, 26, 2450–2459. [Google Scholar] [CrossRef] [PubMed]
- Burg, M.M.; Soufer, R. Post-traumatic Stress Disorder and Cardiovascular Disease. Curr. Cardiol. Rep. 2016, 18, 94. [Google Scholar] [CrossRef]
- Remch, M.; Laskaris, Z.; Flory, J.; Mora-McLaughlin, C.; Morabia, A. Post-Traumatic Stress Disorder and Cardiovascular Diseases: A Cohort Study of Men and Women Involved in Cleaning the Debris of the World Trade Center Complex. Circ. Cardiovasc. Qual. Outcomes 2018, 11, e004572. [Google Scholar] [CrossRef]
- Sagud, M.; Jaksic, N.; Vuksan-Cusa, B.; Loncar, M.; Loncar, I.; Peles, A.M.; Milicic, D.; Jakovljevic, M. Cardiovascular Disease Risk Factors in Patients with Posttraumatic Stress Disorder (PTSD): A Narrative Review. Psychiatr. Danub. 2017, 29, 421–430. [Google Scholar] [CrossRef]
- Jergovic, M.; Bendelja, K.; Savic Mlakar, A.; Vojvoda, V.; Aberle, N.; Jovanovic, T.; Rabatic, S.; Sabioncello, A.; Vidovic, A. Circulating levels of hormones, lipids, and immune mediators in post-traumatic stress disorder—A 3-month follow-up study. Front. Psychiatry 2015, 6, 49. [Google Scholar] [CrossRef]
- Karlovic, D.; Buljan, D.; Martinac, M.; Marcinko, D. Serum lipid concentrations in Croatian veterans with post-traumatic stress disorder, post-traumatic stress disorder comorbid with major depressive disorder, or major depressive disorder. J. Korean Med. Sci. 2004, 19, 431–436. [Google Scholar] [CrossRef]
- Jendricko, T.; Vidovic, A.; Grubisic-Ilic, M.; Romic, Z.; Kovacic, Z.; Kozaric-Kovacic, D. Homocysteine and serum lipids concentration in male war veterans with posttraumatic stress disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2009, 33, 134–140. [Google Scholar] [CrossRef]
- Vries, G.J.; Mocking, R.; Assies, J.; Schene, A.; Olff, M. Plasma lipoproteins in posttraumatic stress disorder patients compared to healthy controls and their associations with the HPA- and HPT-axis. Psychoneuroendocrinology 2017, 86, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.S.; Goldstein, J.L. Receptor-mediated control of cholesterol metabolism. Science 1976, 191, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Herz, J.; Hamann, U.; Rogne, S.; Myklebost, O.; Gausepohl, H.; Stanley, K.K. Surface location and high affinity for calcium of a 500-kd liver membrane protein closely related to the LDL-receptor suggest a physiological role as lipoprotein receptor. EMBO J. 1988, 7, 4119–4127. [Google Scholar] [CrossRef] [PubMed]
- Henderson, R.; O’Kane, M.; McGilligan, V.; Watterson, S. The genetics and screening of familial hypercholesterolaemia. J. Biomed. Sci. 2016, 23, 39. [Google Scholar] [CrossRef] [PubMed]
- Marais, A.D. Familial hypercholesterolaemia. Clin. Biochem. Rev. 2004, 25, 49–68. [Google Scholar] [PubMed]
- Santos, P.C.; Pereira, A.C. Type of LDLR mutation and the pharmacogenetics of familial hypercholesterolemia treatment. Pharmacogenomics 2015, 16, 1743–1750. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.P.; Zhan, S.Y.; Li, L.M.; Hu, Y.H.; Cao, W.H.; Wu, T.; Li, J.; Guo, X.X. Association between AvaII exon 13 polymorphism at the LDL receptor gene different and serum lipid levels in normotensives and essential hypertensives in Shanghai. Zhonghua Liu Xing Bing. Xue Za Zhi 2003, 24, 542–546. [Google Scholar] [PubMed]
- Long, X.J.; Yin, R.X.; Li, K.L.; Liu, W.Y.; Zhang, L.; Cao, X.L.; Miao, L.; Wu, D.F.; Htet Aung, L.H.; Hu, X.J. Low density lipoprotein receptor gene Ava II polymorphism and serum lipid levels in the Guangxi Bai Ku Yao and Han populations. Lipids Health Dis. 2011, 10, 34. [Google Scholar] [CrossRef]
- Rojas, C.; Ramirez, H.; Salazar, L.A.; Kalergis, A.M.; Galvez, A.S.; Escobar-Vera, J. Characterization of LDLR rs5925 and PCSK9 rs505151 genetic variants frequencies in healthy subjects from northern Chile: Influence on plasma lipid levels. J. Clin. Lab. Anal. 2019, 33, e23001. [Google Scholar] [CrossRef]
- Alsabbagh, Y.A.; Ahmed, S.A.; Salama, H.E.; Abd-Elmawla, M.A.; Elgendy, H.L. Role of low-density lipoprotein receptor rs5925 (1959C>T) gene polymorphism in pathogenesis of dyslipidemia among Egyptian lupus nephritis patients. Arch. Rheumatol. 2022, 37, 584–592. [Google Scholar] [CrossRef]
- Nicchio, I.G.; Cirelli, T.; Nepomuceno, R.; Hidalgo, M.A.R.; Rossa, C., Jr.; Cirelli, J.A.; Orrico, S.R.P.; Barros, S.P.; Theodoro, L.H.; Scarel-Caminaga, R.M. Polymorphisms in Genes of Lipid Metabolism Are Associated with Type 2 Diabetes Mellitus and Periodontitis, as Comorbidities, and with the Subjects’ Periodontal, Glycemic, and Lipid Profiles. J. Diabetes Res. 2021, 2021, 1049307. [Google Scholar] [CrossRef]
- De Bem, A.; Engel, D.; de Oliveira, J.; Moreira, E.L.; Neis, V.B.; Santos, D.B.; Lopes, J.B.; Rodrigues, A.L.; Brocardo, P. Hypercholesterolemia as a risk factor for depressive disorder? Free. Radic. Biol. Med. 2014, 75, S28. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lin, J.; Kong, L.N.; Shen, Y.L.; Chen, Y.L.; Guo, Q.W.; Zhang, J.C.; Yang, M.; Fang, D.Z. Effects of earthquake and related environmental factors on relationship of posttraumatic stress disorder with LDLR rs5925. Sci. Total Environ. 2020, 714, 136811. [Google Scholar] [CrossRef] [PubMed]
- Millan, J.; Pinto, X.; Munoz, A.; Zuniga, M.; Rubies-Prat, J.; Pallardo, L.F.; Masana, L.; Mangas, A.; Hernandez-Mijares, A.; Gonzalez-Santos, P.; et al. Lipoprotein ratios: Physiological significance and clinical usefulness in cardiovascular prevention. Vasc. Health Risk Manag. 2009, 5, 757–765. [Google Scholar] [PubMed]
- Maia, D.B.; Marmar, C.R.; Mendlowicz, M.V.; Metzler, T.; Nobrega, A.; Peres, M.C.; Coutinho, E.S.; Volchan, E.; Figueira, I. Abnormal serum lipid profile in Brazilian police officers with post-traumatic stress disorder. J. Affect. Disord. 2008, 107, 259–263. [Google Scholar] [CrossRef]
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017, 38, 2459–2472. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.I.; Lin, L.C.; Tien, H.C.; Que, J.; Ting, W.C.; Chen, P.C.; Wu, H.M.; Ho, C.H.; Wang, J.J.; Wang, R.H.; et al. Hyperlipidemia and statins use for the risk of new-onset anxiety/depression in patients with head and neck cancer: A population-based study. PLoS ONE 2017, 12, e0174574. [Google Scholar] [CrossRef]
- Engel, D.F.; de Oliveira, J.; Lopes, J.B.; Santos, D.B.; Moreira, E.L.G.; Farina, M.; Rodrigues, A.L.S.; de Souza Brocardo, P.; de Bem, A.F. Is there an association between hypercholesterolemia and depression? Behavioral evidence from the LDLr(-/-) mouse experimental model. Behav. Brain Res. 2016, 311, 31–38. [Google Scholar] [CrossRef]
- Fan, F.; Zhang, Y.; Yang, Y.; Mo, L.; Liu, X. Symptoms of posttraumatic stress disorder, depression, and anxiety among adolescents following the 2008 Wenchuan earthquake in China. J. Trauma. Stress. 2011, 24, 44–53. [Google Scholar] [CrossRef]
- Silwal, S.; Dybdahl, R.; Chudal, R.; Sourander, A.; Lien, L. Psychiatric symptoms experienced by adolescents in Nepal following the 2015 earthquakes. J. Affect. Disord. 2018, 234, 239–246. [Google Scholar] [CrossRef]
- Maslovaric, G.; Zaccagnino, M.; Mezzaluna, C.; Perilli, S.; Trivellato, D.; Longo, V.; Civilotti, C. The Effectiveness of Eye Movement Desensitization and Reprocessing Integrative Group Protocol with Adolescent Survivors of the Central Italy Earthquake. Front. Psychol. 2017, 8, 1826. [Google Scholar] [CrossRef]
- China, N.C.C.O. Chinese Cardiovascular Health and Disease Report. 2020; Science Press: Beijing, China, 2022; p. 231. [Google Scholar]
- Bertram, L.; Hsiao, M.; McQueen, M.B.; Parkinson, M.; Mullin, K.; Blacker, D.; Tanzi, R.E. The LDLR locus in Alzheimer’s disease: A family-based study and meta-analysis of case-control data. Neurobiol. Aging 2007, 28, 18.e1–18.e4. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.I.; Kamboh, M.I.; Aston, C.E.; Ferrell, R.E.; Hamman, R.F. Role of common genetic polymorphisms in the LDL receptor gene in affecting plasma cholesterol levels in the general population. Arter. Thromb. 1994, 14, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Rios-Gonzalez, B.E.; Ibarra-Cortes, B.; Ramirez-Lopez, G.; Sanchez-Corona, J.; Magana-Torres, M.T. Association of polymorphisms of genes involved in lipid metabolism with blood pressure and lipid values in mexican hypertensive individuals. Dis. Mrk. 2014, 2014, 150358. [Google Scholar] [CrossRef]
- Humphries, S.; Coviello, D.A.; Masturzo, P.; Balestreri, R.; Orecchini, G.; Bertolini, S. Variation in the low density lipoprotein receptor gene is associated with differences in plasma low density lipoprotein cholesterol levels in young and old normal individuals from Italy. Arter. Thromb. 1991, 11, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Hansen, P.S.; Defesche, J.C.; Kastelein, J.J.; Gerdes, L.U.; Fraza, L.; Gerdes, C.; Tato, F.; Jensen, H.K.; Jensen, L.G.; Klausen, I.C.; et al. Phenotypic variation in patients heterozygous for familial defective apolipoprotein B (FDB) in three European countries. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Lagos, J.; Zambrano, T.; Rosales, A.; Salazar, L.A. APOE polymorphisms contribute to reduced atorvastatin response in Chilean Amerindian subjects. Int. J. Mol. Sci. 2015, 16, 7890–7899. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.D.; Hsiao, K.M.; Wang, T.C.; Lee, T.H.; Kuo, Y.W.; Huang, Y.C.; Hsu, H.L.; Lin, Y.H.; Wu, C.Y.; Huang, Y.C.; et al. Mutual effect of rs688 and rs5925 in regulating low-density lipoprotein receptor splicing. DNA Cell Biol. 2014, 33, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Avanci, J.Q.; Serpeloni, F.; de Oliveira, T.P.; de Assis, S.G. Posttraumatic stress disorder among adolescents in Brazil: A cross-sectional study. BMC Psychiatry 2021, 21, 75. [Google Scholar] [CrossRef]
- Zhang, F.; Rao, S.; Cao, H.; Zhang, X.; Wang, Q.; Xu, Y.; Sun, J.; Wang, C.; Chen, J.; Xu, X.; et al. Genetic evidence suggests posttraumatic stress disorder as a subtype of major depressive disorder. J. Clin. Invest. 2022, 132, e145942. [Google Scholar] [CrossRef]
- Jeromin, A.; Lasseter, H.C.; Provost, A.C.; Daskalakis, N.P.; Etkin, A.; Gehrman, P.; Lancashire, L.; Marx, B.P.; McGlinchey, R.; Haas, M. Driving Progress in Posttraumatic Stress Disorder Biomarkers. Biol. Psychiatry 2020, 87, e13–e14. [Google Scholar] [CrossRef]
- Al Jowf, G.I.; Ahmed, Z.T.; Reijnders, R.A.; de Nijs, L.; Eijssen, L.M.T. To Predict, Prevent, and Manage Post-Traumatic Stress Disorder (PTSD): A Review of Pathophysiology, Treatment, and Biomarkers. Int. J. Mol. Sci. 2023, 24, 5238. [Google Scholar] [CrossRef] [PubMed]
- Richter, D.; Berger, K. Post-traumatic stress disorder following patient assaults among staff members of mental health hospitals: A prospective longitudinal study. BMC Psychiatry 2006, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Gargano, L.M.; Locke, S.; Brackbill, R.M. Parent Physical and Mental Health Comorbidity and Adolescent Behavior. Int. J. Emerg. Ment. Health 2017, 19, 358. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, S.E.; Pineda, O.; Chaves, D.Z.; Zhong, Q.Y.; Gelaye, B.; Simon, G.E.; Rondon, M.B.; Williams, M.A. Childhood physical and sexual abuse experiences associated with post-traumatic stress disorder among pregnant women. Ann. Epidemiol. 2017, 27, 716–723.e1. [Google Scholar] [CrossRef]
- Andrykowski, M.A.; Cordova, M.J.; Studts, J.L.; Miller, T.W. Posttraumatic stress disorder after treatment for breast cancer: Prevalence of diagnosis and use of the PTSD Checklist-Civilian Version (PCL-C) as a screening instrument. J. Consult. Clin. Psychol. 1998, 66, 586–590. [Google Scholar] [CrossRef]

| Total (n = 709) n (%) | Hardy–Weinberg p | Males (n = 312) n (%) | Females (n = 397) n (%) | p | |
|---|---|---|---|---|---|
| Genotype frequencies | |||||
| TT | 392 (55.29) | 0.33 | 177 (56.73) | 215 (54.16) | 0.63 |
| CT | 277 (39.07) | 116 (37.18) | 161 (40.55) | ||
| CC | 40 (5.64) | 19 (6.09) | 21 (5.29) | ||
| Allele frequencies | |||||
| T | 1061 (74.82) | 470 (75.32) | 591 (74.43) | 0.71 | |
| C | 357 (25.18) | 154 (24.68) | 203 (25.57) | ||
| PTSD | TT Homozygote | C Allele Carriers | ||||||
|---|---|---|---|---|---|---|---|---|
| Males n (%) | Females n (%) | χ2, p | Males n (%) | Females n (%) | χ2, p | χ2, p | χ2, p | |
| With | 6 (3.39) | 11 (5.12) | χ2 = 0.697, | 12 (8.89) | 46 (25.27) | χ2 = 13.921, | χ2 = 4.260, | χ2 = 32.573, |
| Without | 171 (96.61) | 204 (94.88) | p = 0.403 | 123 (91.11) | 136 (74.73) | p = 0.000 | * p = 0.039 | # p = 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Jia, K.; Guo, Q.; Liu, J.; Cai, J.; Shen, Y.; Su, G.; Chen, X.; Lin, J.; Fang, D. Post-Traumatic Stress Disorder Is Associated with Elevated Plasma Cholesterol in Female TT Homozygotes of LDLR rs5925. Int. J. Mol. Sci. 2023, 24, 9016. https://doi.org/10.3390/ijms24109016
Wang J, Jia K, Guo Q, Liu J, Cai J, Shen Y, Su G, Chen X, Lin J, Fang D. Post-Traumatic Stress Disorder Is Associated with Elevated Plasma Cholesterol in Female TT Homozygotes of LDLR rs5925. International Journal of Molecular Sciences. 2023; 24(10):9016. https://doi.org/10.3390/ijms24109016
Chicago/Turabian StyleWang, Jinhua, Kexin Jia, Qiwei Guo, Junyi Liu, Jiajing Cai, Yilin Shen, Guoming Su, Xu Chen, Jia Lin, and Dingzhi Fang. 2023. "Post-Traumatic Stress Disorder Is Associated with Elevated Plasma Cholesterol in Female TT Homozygotes of LDLR rs5925" International Journal of Molecular Sciences 24, no. 10: 9016. https://doi.org/10.3390/ijms24109016
APA StyleWang, J., Jia, K., Guo, Q., Liu, J., Cai, J., Shen, Y., Su, G., Chen, X., Lin, J., & Fang, D. (2023). Post-Traumatic Stress Disorder Is Associated with Elevated Plasma Cholesterol in Female TT Homozygotes of LDLR rs5925. International Journal of Molecular Sciences, 24(10), 9016. https://doi.org/10.3390/ijms24109016






