CNS-Related Effects Caused by Vanadium at Realistic Exposure Levels in Humans: A Comprehensive Overview Supplemented with Selected Animal Studies
Abstract
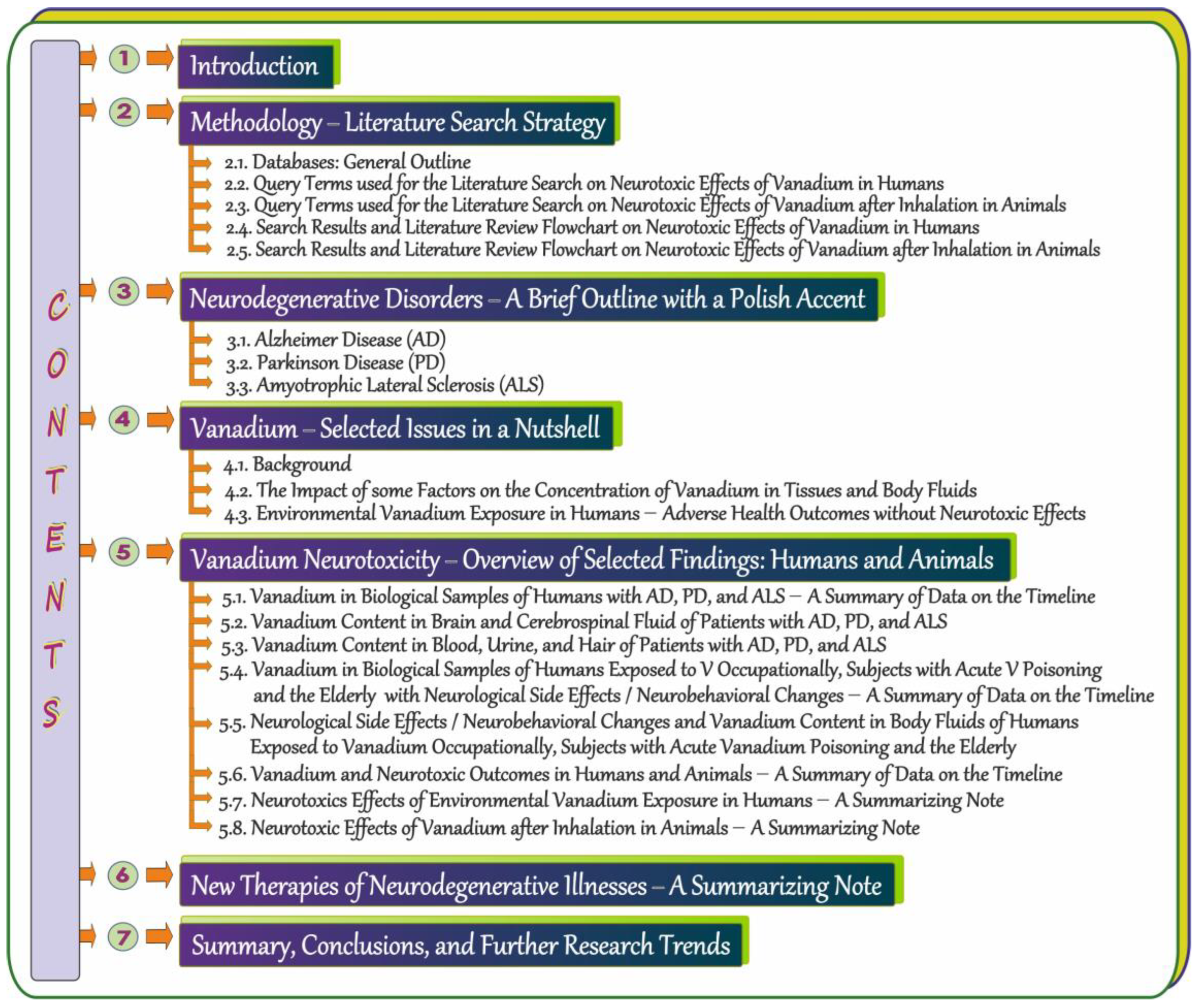
1. Introduction
2. Methodology—Literature Search Strategy
2.1. Databases: General Outline
2.2. Query Terms Used for the Literature Search on Neurotoxic Effects of Vanadium in Humans
2.3. Query Terms Used for the Literature Search on Neurotoxic Effects of Vanadium after Inhalation in Animals
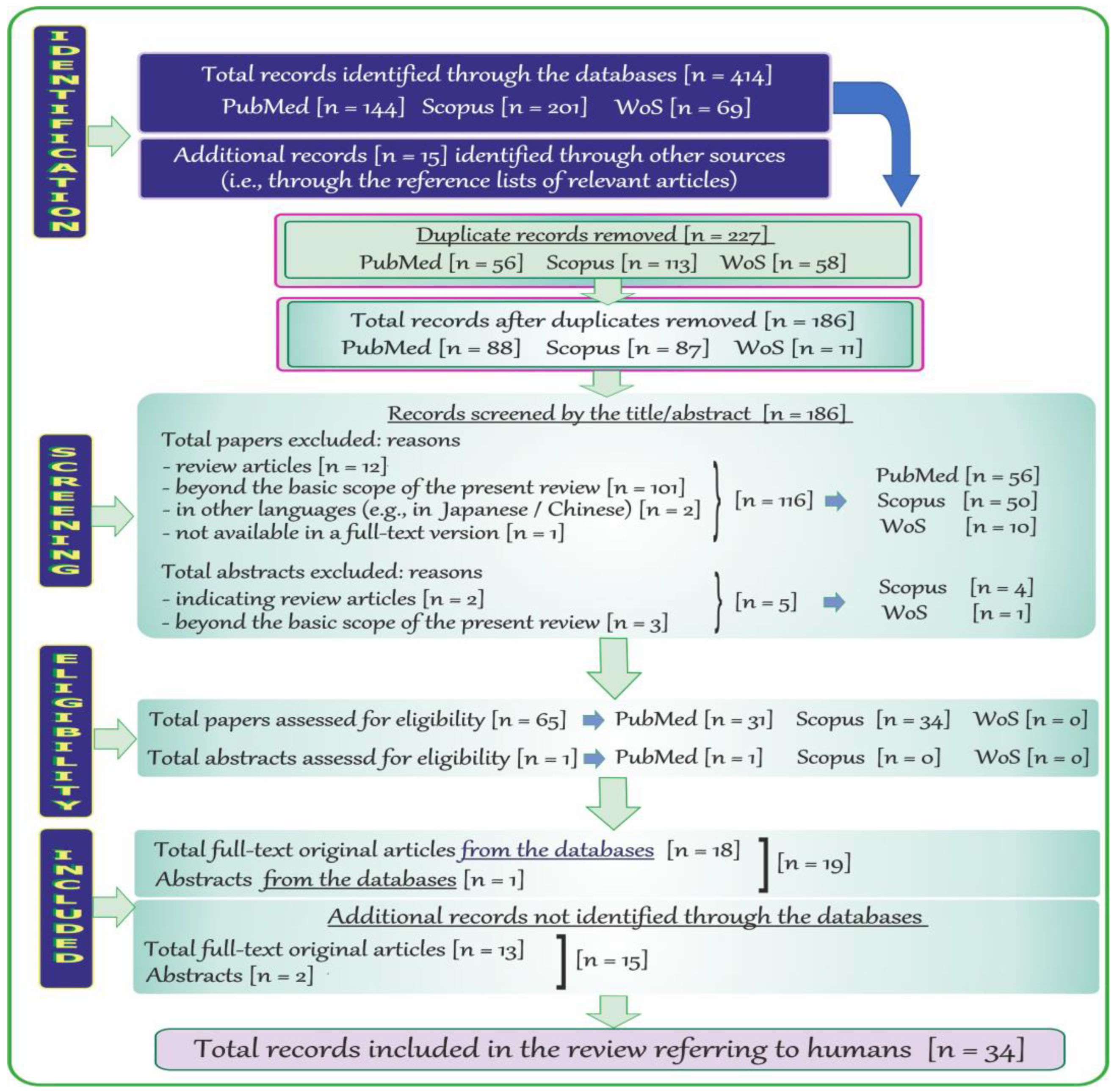
2.4. Search Results and Literature Review Flowchart on Neurotoxic Effects of Vanadium in Humans
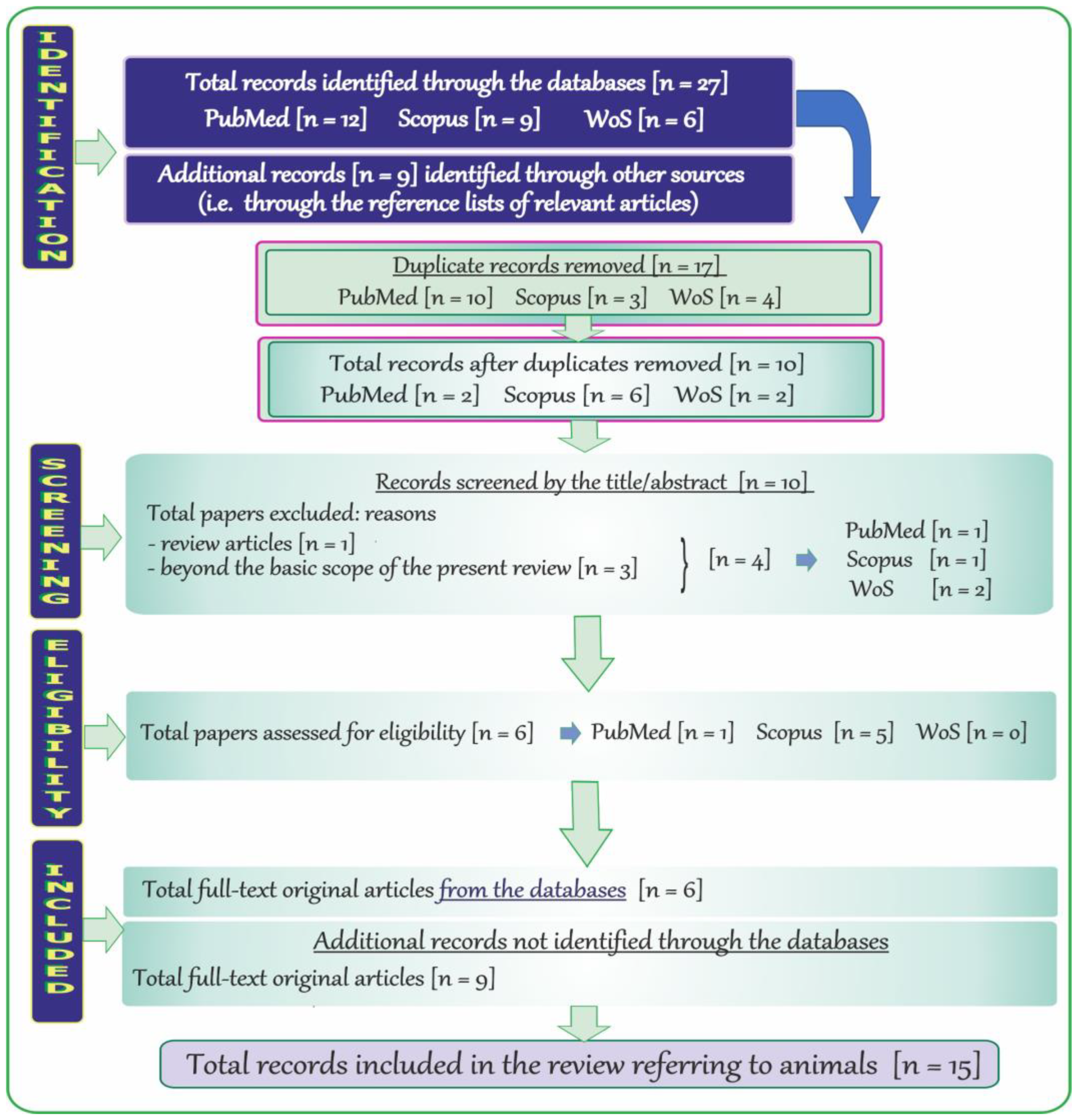
2.5. Search Results and Literature Review Flowchart on Neurotoxic Effects of Vanadium after Inhalation in Animals
3. Neurodegenerative Disorders—A Brief Outline with a Polish Accent
3.1. Alzheimer Disease (AD)
3.2. Parkinson Disease (PD)
3.3. Amyotrophic Lateral Sclerosis (ALS)
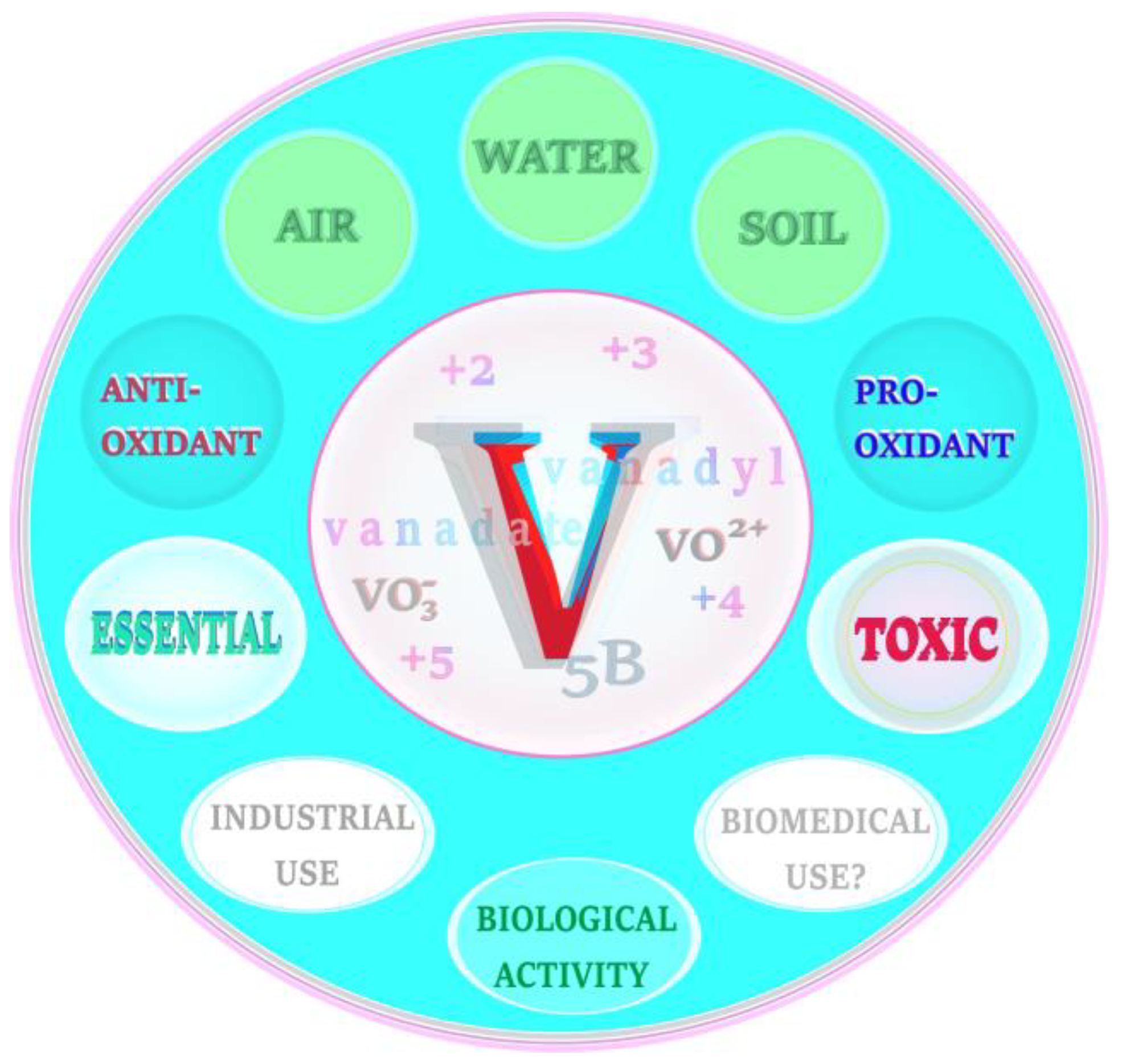
4. Vanadium—Selected Issues in a Nutshell
4.1. Background
4.2. The Impact of Certain Factors on the Concentration of Vanadium in Tissues and Body Fluids
4.3. Environmental Vanadium Exposure in Humans—Adverse Health Outcomes Excluding Neurotoxic Effects
5. Vanadium Neurotoxicity—Overview of Selected Findings: Humans and Animals
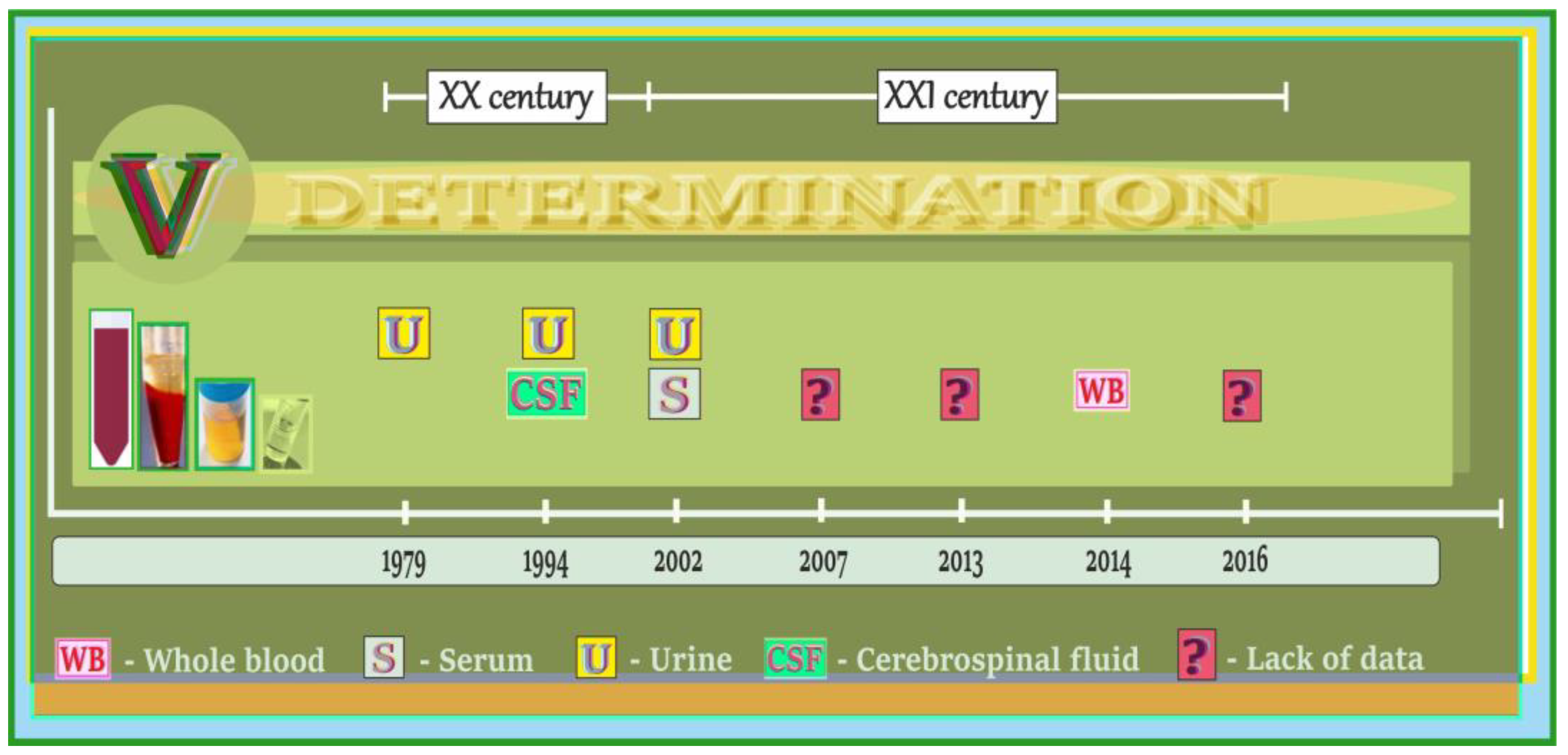
5.1. Vanadium in Biological Samples of Humans with AD, PD, and ALS—A Summary of Data on the Timeline
5.2. Vanadium Content in Brain and Cerebrospinal Fluid of Patients with AD, PD, and ALS
5.3. Vanadium Content in Blood, Urine, and Hair of Patients with AD, PD, and ALS
5.4. Vanadium in Biological Samples of Humans Exposed to Vanadium Occupationally, Subjects with Acute Vanadium Poisoning and the Elderly with Neurological Side Effects/Neurobehavioral Changes—A Summary of Data on the Timeline
5.5. Neurological Side Effects/Neurobehavioral Changes and Vanadium Content in Body Fluids of Humans Exposed to Vanadium Occupationally, Subjects with Acute Vanadium Poisoning and the Elderly
5.6. Vanadium and Neurotoxic Outcomes in Humans and Animals—A Summary of Data on the Timeline
5.7. Neurotoxic Effects of Environmental Vanadium Exposure in Humans—A Summarizing Note
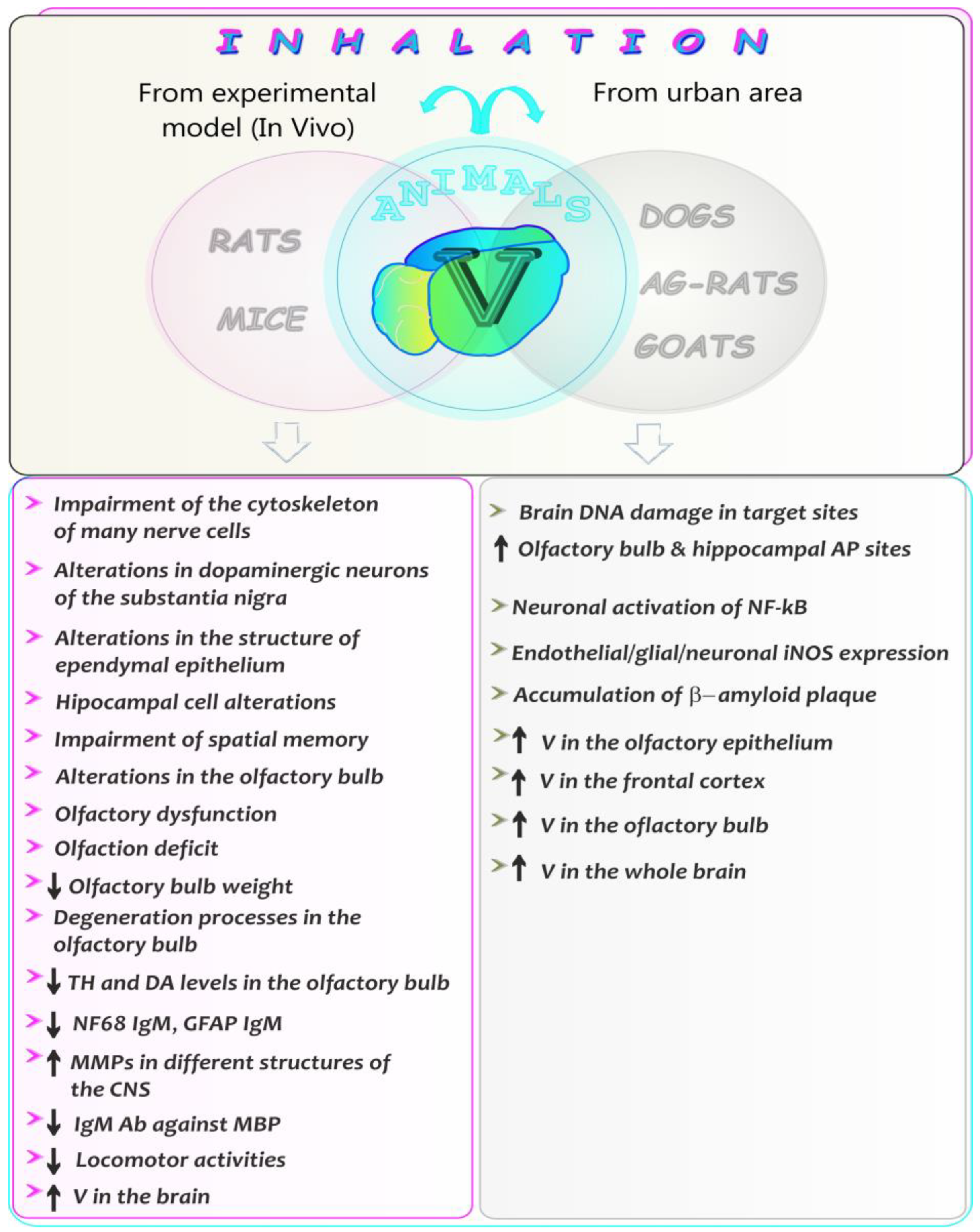
5.8. Neurotoxic Effects of Vanadium after Inhalation in Animals—A Summarizing Note
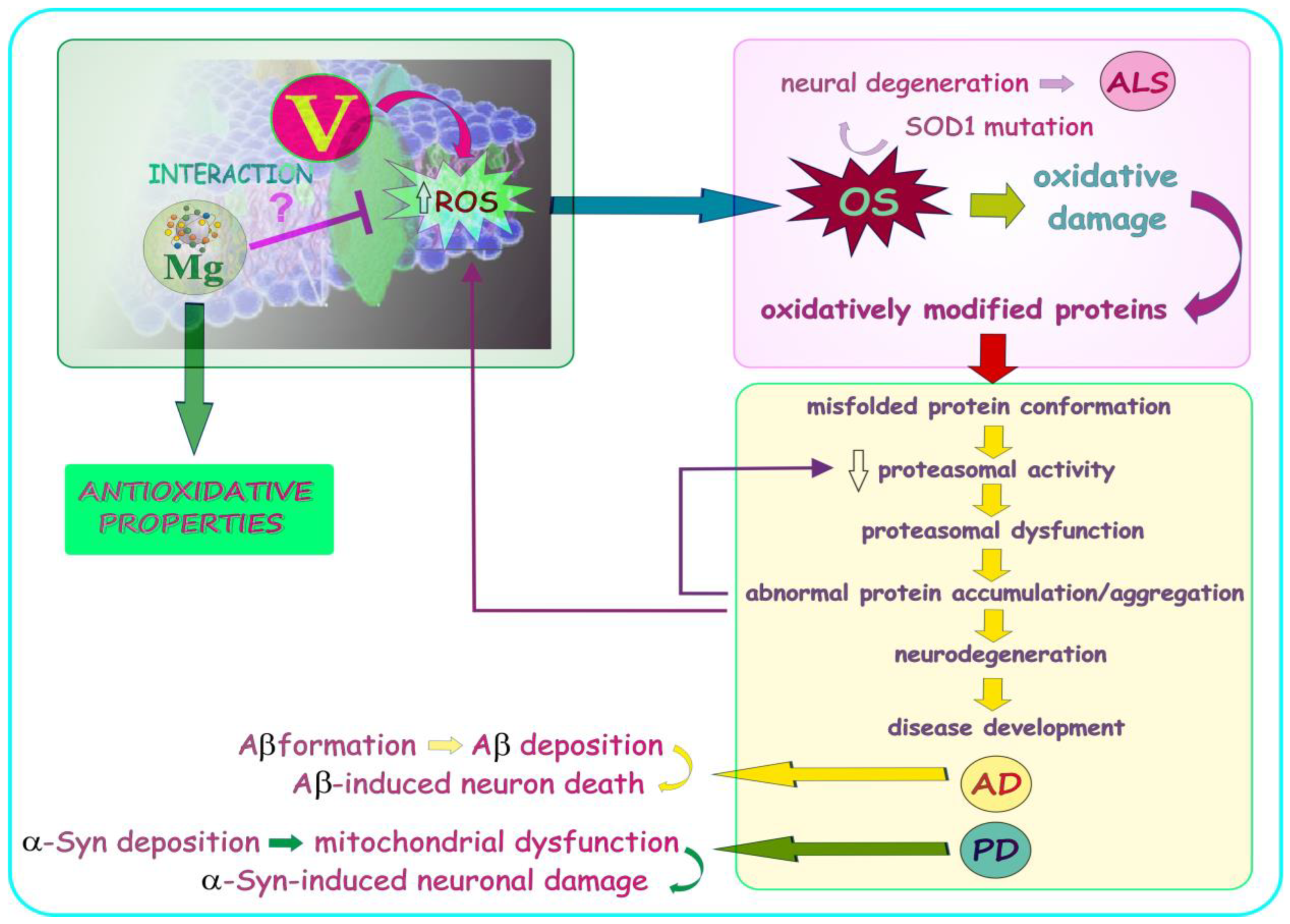
6. New Therapies of Neurodegenerative Illnesses—A Summarizing Note
7. Summary, Conclusions, and Further Research Trends
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- NIK o Opiece Nad Chorymi Na Alzheimera. 16 May 2017. Available online: https://www.nik.gov.pl/aktualnosci/nik-o-opiece-nad-chorymi-na-alzheimera.html (accessed on 14 April 2023).
- Du, X.; Wang, X.; Geng, M. Alzheimer’s disease hypothesis and related therapies. Transl. Neurodegener. 2018, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Kivipelto, M.; Mangialasche, F.; Snyder, H.M.; Allegri, R.; Andrieu, S.; Arai, H.; Baker, L.; Belleville, S.; Brodaty, H.; Brucki, S.M.; et al. World-Wide FINGERS Network: A global approach to risk reduction and prevention of dementia. Alzheimer’s Dement. 2020, 16, 1078–1094. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef]
- Budrewicz, S. Choroba Parkinsona; Elsevier Essentials Parkinson, Edra Urban & Partner: Wroclaw, Poland, 2018. [Google Scholar]
- Elkouzi, A.; Vedam-Mai, V.; Eisinger, R.S.; Okun, M.S. Emerging therapies in Parkinson disease—Repurposed drugs and new approaches. Nat. Rev. Neurol. 2019, 15, 204–223. [Google Scholar] [CrossRef] [PubMed]
- Banach, M.; Boguski, A.; Liberski, P. Choroby Rdzenia Kręgowego; Medycyna Praktyczna: Gdańsk, Poland, 2007. [Google Scholar]
- Kiernan, M.C.; Vucic, S.; Cheah, B.C.; Turner, M.R.; Eisen, A.; Hardiman, O.; Burrell, J.R.; Zoing, M.C. Amyotrophic lateral sclerosis. Lancet 2011, 377, 942–955. [Google Scholar] [CrossRef] [PubMed]
- Kabata-Pendias, A.; Pendias, H. Biogeochemia Pierwiastków Śladowych; Wydanie 2; Wydawnictwo Naukowe PWN: Warsaw, Poland, 1999. [Google Scholar]
- Rehder, D. Bioinorganic Vanadium Chemistry; John Wiley and Sons: Chichester, UK, 2008; pp. 1–23. [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR). Public Health Statement for Vanadium; U.S. Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2012.
- Genet, S.; Kale, R.K.; Baquer, N.Z. Alterations in antioxidant enzymes and oxidative damage in experimental diabetic rat tissues: Effect of vanadate and fenugreek (Trigonella foenum graecum). Mol. Cell. Biochem. 2002, 236, 7–12. [Google Scholar] [CrossRef]
- Ramachandran, B.; Ravi, K.; Narayanan, V.; Kandaswamy, M.; Subramanian, S. Protective effect of macrocyclic binuclear oxovanadium complex on oxidative stress in pancreas of streptozotocin induced diabetic rats. Chem. Biol. Interact. 2004, 149, 9–21. [Google Scholar] [CrossRef]
- Tunali, S.; Yanardag, R. Protective effect of vanadyl sulfate on skin injury in streptozotocin-induced diabetic rats. Hum. Exp. Toxicol. 2013, 32, 1206–1212. [Google Scholar] [CrossRef]
- Xie, M.; Chen, D.; Zhang, F.; Willsky, G.R.; Crans, D.C.; Ding, W. Effects of vanadium (III, IV, V)-chlorodipicolinate on glycolysis and antioxidant status in the liver of STZ-induced diabetic rats. J. Inorg. Biochem. 2014, 136, 47–56. [Google Scholar] [CrossRef]
- Ścibior, A.; Pietrzyk, Ł.; Plewa, Z.; Skiba, A. Vanadium: Risks and possible benefits in the light of a comprehensive overview of its pharmacotoxicological mechanisms and multi-applications with a summary of further research trends. J. Trace Elem. Med. Biol. 2020, 61, 126508. [Google Scholar] [CrossRef]
- Ścibior, A.; Wnuk, E.; Gołębiowska, D. Wild animals in studies on vanadium bioaccumulation—Potential animal models of environmental vanadium contamination: A comprehensive overview with a Polish accent. Sci. Total Environ. 2021, 785, 147205. [Google Scholar] [CrossRef]
- Ścibior, A. Overview of research on vanadium-quercetin complexes with a historical outline. Antioxidants 2022, 11, 790. [Google Scholar] [CrossRef] [PubMed]
- Ścibior, A.; Kurus, J. Vanadium and oxidative stress markers-in vivo model: A review. Curr. Med. Chem. 2019, 26, 5456–5500. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.H.; Kim, J.E.; Rhie, S.J.; Yoon, S. The role of oxidative stress in neurodegenerative diseases. Exp. Neurobiol. 2015, 24, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Kučera, J.; Sabbioni, E. Baseline vanadium levels in human blood, serum, and urine. In Vanadium in the Environment. Part 2: Health Effects; Nriagu, J.O., Ed.; John Wiley and Sons: New York, NY, USA, 1998; Volume 31, pp. 75–91. [Google Scholar]
- Baierle, M.; Charão, M.F.; Gӧethel, G.; Barth, A.; Fracasso, R.; Bubols, G.; Sauer, E.; Campanharo, S.C.; Rocha, R.C.C.; Saint’Pierre, T.D.; et al. Are delta-aminolevulinate dehydratase inhibition and metal concentrations additional factors for the age-related cognitive decline? Int. J. Environ. Res. Public Health 2014, 11, 10851–10867. [Google Scholar] [CrossRef]
- Yang, B.; He, J.; Zhang, G.; Gua, J. Vanadium. Extraction, Manufacturing and Applications, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Nriagu, J.O. Vanadium in the Environment, Part 2: Health Effects; John Wiley and Sons: New York, NY, USA, 1998; pp. 1–424. [Google Scholar]
- Hu, J.; Xia, W.; Pan, X.; Zheng, T.; Zhang, B.; Zhou, A.; Buka, S.L.; Bassig, B.A.; Liu, W.; Wu, C.; et al. Association of adverse birth outcomes with prenatal exposure to vanadium: A population-based cohort study. Lancet Planet Health 2017, 1, e230–e241. [Google Scholar] [CrossRef]
- Hu, J.; Peng, Y.; Zheng, T.; Zhang, B.; Liu, W.; Wu, C.; Jiang, M.; Braun, J.M.; Liu, S.; Buka, S.L.; et al. Effects of trimester-specific exposure to vanadium on ultrasound measures of fetal growth and birth size: A longitudinal prospective prenatal cohort study. Lancet Planet Health 2018, 2, e427–e437. [Google Scholar] [CrossRef]
- Malandrino, P.; Russo, M.; Ronchi, A.; Minoia, C.; Cataldo, D.; Regalbuto, C.; Giordano, C.; Attard, M.; Squatrito, S.; Trimarchi, F.; et al. Increased thyroid cancer incidence in a basaltic volcanic area is associated with non-anthropogenic pollution and biocontamination. Endocrine 2016, 53, 471–479. [Google Scholar] [CrossRef]
- Minoia, C.; Pietra, R.; Sabbioni, E.; Ronchi, A.; Gatti, A.; Cavalleri, A.; Manzo, L. Trace element reference values in tissues from inhabitants of the European Community. III. The control of preanalytical factors in the biomonitoring of trace elements in biological fluids. Sci. Total Environ. 1992, 120, 63–79. [Google Scholar] [CrossRef]
- Lanocha-Arendarczyk, N.; Kosik-Bogacka, D.I.; Kalisinska, E.; Sokołowski, S.; Kolodziej, L.; Budis, H.; Safranow, K.; Kot, K.; Ciosek, Z.; Tomska, N.; et al. Influence of environmental factors and relationships between vanadium, chromium, and calcium in human bone. BioMed. Res. Inter. 2016, 2016, 8340425. [Google Scholar] [CrossRef]
- Sampath, V.P.; Singh, S.V.; Pelov, I.; Horesh, N.; Zannadeh, H.; Tirosh, O.; Erel, Y.; Lichtstein, D. Vanadium in bipolar disorders—Reviving an old hypothesis. Int. J. Mol. Sci. 2022, 23, 13901. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA) Panel on Opinion of the Scientific Panel on Dietetic products, Nutrition and Allergies on a request from the Commission related to the Tolerable Upper Intake Level of Vanadium. EFSA J. 2004, 33, 1–22. [CrossRef]
- Bernhard, D.; Rossmann, A.; Wick, G. Metals in cigarette smoke. Life 2005, 57, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Adachi, A.; Asai, K.; Koyama, Y.; Matsumoto, Y.; Okano, T. Determination of vanadium in cigarettes by atomic absorption spectrophotometry. Anal. Lett. 1998, 3, 1769–1776. [Google Scholar] [CrossRef]
- Toro-Román, V.; Bartolomé, I.; Siquier-Coll, J.; Alves, J.; Grijota, F.J.; Muñoz, D.; Maynar-Mariño, M. Serum vanadium concentrations in different sports modalities. J. Trace Elem. Med. Biol. 2021, 68, 126808. [Google Scholar] [CrossRef] [PubMed]
- Basu, R.; Harris, M.; Sie, L.; Malig, B.; Broadwin, R.; Green, R. Effects of fine particulate matter and its constituents on low birth weight among full-term infants in California. Environ. Res. 2014, 128, 42–51. [Google Scholar] [CrossRef]
- Bell, M.L.; Ebisu, K.; Peng, R.D.; Samet, J.M.; Dominici, F. Hospital admissions and chemical composition of fine particle air pollution. Am. J. Respir. Crit. Care Med. 2009, 179, 1115–1120. [Google Scholar] [CrossRef]
- Cakmak, S.; Dales, R.; Kauri, L.M.; Mahmud, M.; Van Ryswyk, K.; Vanos, J.; Liu, L.; Kumarathasan, P.; Thomson, E.; Vincent, R.; et al. Metal composition of fine particulate air pollution and acute changes in cardiorespiratory physiology. Environ. Poll. 2014, 189, 208–214. [Google Scholar] [CrossRef]
- Jiang, M.; Li, Y.; Zhang, B.; Zhou, A.; Zheng, T.; Qian, Z.; Du, X.; Zhou, Y.; Pan, X.; Hu, J.; et al. A nested case-control study of prenatal vanadium exposure and low brithweight. Hum. Reprod. 2016, 31, 2135–2141. [Google Scholar] [CrossRef]
- Jiang, S.; Zhou, S.; Liu, H.; Peng, C.; Zhang, X.; Zhou, H.; Wang, Z.; Lu, Q. Concentrations of vanadium in urine with hypertension prevalence and blood pressure levels. Ecotoxicol. Environ. Saf. 2021, 213, 112028. [Google Scholar] [CrossRef]
- Jin, S.; Xia, W.; Jiang, Y.; Sun, X.; Huang, S.; Zhang, B.; Zhou, A.; Zheng, T.; Xu, S.; Li, Y. Urinary vanadium concentration in relation to premature rupture of membranes: A birth cohort study. Chemosphere 2018, 210, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.M.; Hoepner, L.; Garfinkel, R.; Chillrud, S.; Reyes, A.; Quinn, J.W.; Perera, F.; Miller, R.L. Ambient metals, elemental carbon, and wheeze and cough in New York City children through 24 months of age. Am. J. Respir. Crit. Care Med. 2009, 180, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Pellegriti, G.; De Vathaire, F.; Scollo, C.; Attard, M.; Giordano, C.; Arena, S.; Dardanoni, G.; Frasca, F.; Malandrino, P.; Vermiglio, F.; et al. Papillary thyroid cancer indicence in the volcanic area of Sicily. J. Natl. Cancer Inst. 2009, 101, 1575–1583. [Google Scholar] [CrossRef]
- Seko, Y.; Watanabe, K.; Hasegawa, T. Vanadium in ground water from Mt. Fuji: Does it have health effect on habitants around the mountain? Chin. J. Geochem. 2006, 25 (Suppl. S1), 60–61. [Google Scholar] [CrossRef]
- Sitprija, V.; Eiam-Ong, S. Vanadium and metabolic problems. In Vanadium in the Environment. Part 2: Health Effects; Nriagu, J.O., Ed.; John Wiley and Sons: New York, NY, USA, 1998; Volume 31, pp. 91–120. [Google Scholar]
- Sørensen, M.; Schins, R.P.F.; Hertel, O.; Loft, S. Transition metals in personal samples of PM2.5 and oxidative stress in human volunteers. Cancer Epidemiol. Biomarkers Prev. 2005, 14, 1340–1343. [Google Scholar] [CrossRef]
- Blalock, T.L.; Hill, C.H. Studies on the role of iron in the reversal of vanadium toxicity in chicks. Biol. Trace Elem. Res. 1987, 14, 225–235. [Google Scholar] [CrossRef]
- Dai, L.; Bind, M.A.; Koutrakis, P.; Coull, B.A.; Sparrow, D.; Vokonas, P.S.; Schwarts, J.D. Fine particles, genetic pathways, and markers of inflammation and endothelial dysfunction: Analysis on particulate species and sources. J. Expo. Sci. Environ. Epidemiol. 2016, 26, 415–421. [Google Scholar] [CrossRef]
- Blankenberg, S.; Rupprecht, H.J.; Bickel, C.; Peetz, D.; Hafner, G.; Tiret, L.; Meyer, J. Circulating soluble adhesion molecules and death in patients with coronary artery disease. Circulation 2001, 104, 1336–1342. [Google Scholar] [CrossRef]
- Pradhan, A.D.; Rifai, N.; Ridker, P.M. Solube intercellular adhesion molecule-1, soluble vascular adhesion molecule-1, and the development of symptomatic peripheral artirial disease in men. Circulation 2002, 106, 820–825. [Google Scholar] [CrossRef]
- Chen, J.; Rodopoulou, S.; de Hoogh, K.; Strak, M.; Jovanovic Andersen, Z.; Atkinson, R.; Bauwelinck, M.; Bellander, T.; Brandt, J.; Cesaroni, G.; et al. Long-term exposure to fine particle elemental components and natural and cause-specific mortality—A pooled analysis of eight European cohorts within the ELAPSE project. Environ. Health Perspect. 2021, 129, 47009. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, L.; Lee, M.; Liu, P.; Di, Q.; Zanobetti, A.; Schwartz, J.D. Long-term exposure to PM2.5 and mortality among older adults in the Southeastern US. Epidemiology 2017, 28, 207–2014. [Google Scholar] [CrossRef]
- Wolf, K.; Stafoggia, M.; Cesaroni, G.; Jovanovic Andersen, Z.; Beelen, R.; Galassi, C.; Hennig, F.; Migliore, E.; Penell, J.; Ricceri, F.; et al. Long-term exposure to particulate matter constituents and the incidence of coronary events in 11 European cohorts. Epidemiology 2015, 26, 565–574. [Google Scholar] [CrossRef]
- Hvidtfeldt, U.A.; Chen, J.; Jovanovic Andersen, Z.; Atkinson, R.; Bauwelinck, M.; Bellander, T.; Brandt, J.; Brunekreef, B.; Cesaroni, G.; Concin, H.; et al. Long-term exposure to fine particle elemental components and lung cancer incidence in the ELAPSE pooled cohort. Environ. Res. 2021, 193, 110568. [Google Scholar] [CrossRef]
- Eeftens, M.; Hoek, G.; Gruzieva, O.; Mölter, A.; Agius, R.; Beelen, R.; Brunekreef, B.; Custovic, A.; Cyrys, J.; Fuerte, E.; et al. Elemental composition of particulate matter and the association with lung function. Epidemiology 2014, 25, 648–657. [Google Scholar] [CrossRef]
- Alfaro-Moreno, E.; Ponce-de-León, S.; Osorino-Vargas, A.R.; García-Cuellar, C.; Martínez, L.; Rosas, I. Potential toxic effects associated to metals and endotoxin present in PM10: An ancillary study using multivariate analysis. Inhal. Toxicol. 2007, 19 (Suppl. S1), 49–53. [Google Scholar] [CrossRef]
- Campen, M.J.; Nolan, J.P.; Schladweiler, M.C.J.; Kodavanti, U.P.; Evansky, P.A.; Costa, D.L.; Watkinson, W.P. Cardiovascular and thermoregulatory effects of inhaled PM-associated transition metals: A potential interaction between nickel and vanadium sulfate. Toxicol. Sci. 2001, 64, 243–252. [Google Scholar] [CrossRef]
- Szabo, S.T.; Harry, G.J.; Hayden, K.M.; Szabo, D.T.; Birnbaum, L. Comparison of metal levels between postmortem brain and ventricular fluid in Alzheimer’s disease and nondemented elderly controls. Toxicol. Sci. 2016, 150, 292–300. [Google Scholar] [CrossRef]
- Gerhardsson, L.; Lundh, T.; Minthon, L.; Londos, E. Metal concentrations in plasma and cerebrospinal fluid in patients with Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2008, 25, 508–515. [Google Scholar] [CrossRef]
- Hershey, C.O.; Hershey, L.A.; Varnes, A.; Vibhakar, S.D.; Lavin, P.; Strain, W. Cerebrospinal fluid trace element content in dementia: Clinical, radiologic, and pathologic correlations. Neurology 1983, 33, 1350–1353. [Google Scholar] [CrossRef]
- Ward, N.I.; Mason, J.A. Neutron activation analysis techniques for identifying elemental status in Alzheimer’s disease. J. Radioanal. Nucl. Chem. 1987, 113, 515–526. [Google Scholar] [CrossRef]
- Gerhardsson, L.; Blennow, K.; Lundh, T.; Londos, E.; Minthon, L. Concentrations of metals, β-amyloid and tau-markers in cerebrospinal fluid in patients with Alzheimer’s disease. Dement. Geriatr. Cogn. Disor. 2009, 28, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.A.; Jain, J.C. Scavenger receptor class B type I expression and elemental analysis in cerebellum and parietal cortex regions of the Alzheimer’s disease brain. J. Neurol. Sci. 2002, 196, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Roos, P.M.; Vesterberg, O.; Syversen, T.; Flaten, T.P.; Nordberg, M. Metal concentration in cerebrospinal fluid and blood plasma from patients with amyotrophic lateral sclerosis. Biol. Trace Elem. Res. 2013, 151, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Gellein, K.; Garruto, R.M.; Syversen, T.; Sjøbakk, T.E.; Flaten, P. Concentrations of Cd, Co, Cu, Fe, Mn, Rb, V, and Zn in formalin-fixed brain tissue in amyotrophic lateral sclerosis and parkinsonism-dementia complex of guam determined by high-resolution ICP-MS. Biol. Trace Elem. Res. 2003, 96, 39–60. [Google Scholar] [CrossRef] [PubMed]
- Patti, F.; Fiore, M.; Chisari, C.G.; D’Amico, E.; Lo Fermo, S.; Toscano, S.; Copat, C.; Ferrante, M.; Zappia, M. CSF neurotoxic metals/metalloids levels in amyotrophic lateral sclerosis patients: Comparison between bulbar and spinal onset. Environ. Res. 2020, 188, 109820. [Google Scholar] [CrossRef]
- Shi, M.T. Determination of multiple chemical elements in CSF in Parkinson disease after intracerebral autotransplantation of the adrenal medulla. Zhonghua Wai Ke Za Zhi 1991, 29, 129–132. [Google Scholar]
- Bocca, B.; Alimonti, A.; Petrucci, F.; Violante, N.; Sancesario, G.; Forte, G.; Senofonte, O. Quantification of trace elements by sector field indutcively coupled plasma mass spectrometry in urine, serum, blood and cerebrospinal fluid of patients with Parkinson’s disease. Spectrochim. Acta Part B 2004, 59, 559–566. [Google Scholar] [CrossRef]
- Uitti, R.J.; Rajput, A.H.; Rozdilsky, B.; Bickis, M.; Wollin, T.; Yuen, W.K. Regional metal concentrations in Parkinson’s disease, other chronić neurological diseases, and control brains. Can. J. Neurol. Sci. 1989, 16, 310–314. [Google Scholar] [CrossRef]
- González-Dominguez, R.; Garcia-Barrera, T.; Gómez-Ariza, J.L. Characterization of metal profiles in serum during the progression of Alzheimer’s disease. Metallomics 2014, 6, 292–300. [Google Scholar] [CrossRef]
- Paglia, G.; Miedico, O.; Cristofano, A.; Vitale, M.; Angiolillo, A.; Chiaravalle, A.E.; Corso, G.; Di Costanzo, A. Distinctive pattern of serum elements during the progression of Alzheimer’s disease. Sci. Rep. 2016, 6, 22769. [Google Scholar] [CrossRef]
- Akanle, O.A.; Spyrou, N.M.; Damyanova, A.A.; Shaw, D.M.; Ali, L. Investigation of elemental models in senile dementia and depressives using neutron activation analysis. J. Radioanal. Nucl. Chem. 1987, 113, 405–416. [Google Scholar] [CrossRef]
- Lavanya, R.D.; Reddy, B.S.; Sattar, S.A.; Rao, A.D.P. Trace element imbalances in blood serum of Alzheimer’s disease patients. Spectre. Lett. 2021, 54, 458–471. [Google Scholar] [CrossRef]
- Guan, C.; Dang, R.; Cui, Y.; Liu, L.; Chen, X.; Wang, X.; Zhu, J.; Li, D.; Li, J.; Wang, D. Characterization of plasma metal profiles in Alzheimer’s disease using multivariate statistical analysis. PLoS ONE 2017, 12, e0178271. [Google Scholar] [CrossRef] [PubMed]
- Alimonti, A.; Ristori, G.; Giubilei, F.; Stazi, M.A.; Pino, A.; Visconti, A.; Brescianini, S.; Monti, M.S.; Forte, G.; Stanzione, P.; et al. Serum chemical elements and oxidative status in Alzheimer’s disease, Parkinson disease and multiple sclerosis. Neurotoxicology 2007, 28, 450–456. [Google Scholar] [CrossRef]
- Bocca, B.; Forte, G.; Petrucci, F.; Pino, A.; Marchione, F.; Bombi, G.; Senofonte, O.; Giubilei, F.; Alimonti, A. Monitoring of chemical elements and oxidative damage in patients affectd by Alzheimer’s disease. Ann. Ist. Super Sanita 2005, 41, 197–203. [Google Scholar]
- Forte, G.; Alimonti, A.; Pino, A.; Stanzione, P.; Brescianini, S.; Brusa, L.; Sancesario, G.; Violante, N.; Bocca, B. Metals and oxidative stress in patients with Parkinson’s disease. Ann. Ist. Super Sanita 2005, 41, 189–195. [Google Scholar]
- Royce-Nagel, G.; Cudkowicz, M.; Myers, D.; Nicholson, K.; Shui, A.; Schoenfeld, D.; Huang, X.; Brown, R.H. Vanadium, aluminium, magnesium, and manganese are not elevated in hair samples in amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. 2010, 11, 492–493. [Google Scholar] [CrossRef]
- Barth, A.; Schaffer, A.W.; Konnaris, C.; Blauensteiner, R.; Winker, R.; Osterode, W.; Rüdiger, H.W. Neurobehavioral effects of vanadium. J. Toxicol. Environ. Health Part A 2002, 65, 677–683. [Google Scholar] [CrossRef]
- Usutani, S.; Nishiyama, K.; Sato, I.; Matsuura, K.; Sawada, Y.; Kawabata, T.; Hosokawa, Y.; Izumi, S. Results of the special physical examination of workers in a vanadium plant. Jpn. J. Ind. Health 1979, 21, 21–28. [Google Scholar] [CrossRef]
- Li, H.; Zhou, D.; Zhang, Q.; Feng, C.; Zheng, W.; He, K.; Lan, Y. Vanadium exposure-induced neurobehavioral alterations among Chinese workers. Neurotoxicology 2013, 36, 49–54. [Google Scholar] [CrossRef]
- Zhou, D.; Feng, C.; Lan, Y.J.; Wang, Z.; Huang, S.; Wang, M.; Zhu, T. Paired-control study on the effect of vanadium on neurobehavioral functions. Sichuan Da Xue Xue Bao Yi Xue Ban 2007, 38, 468–470. [Google Scholar]
- Zhu, C.W.; Liu, Y.X.; Huang, C.J.; Gao, W.; Hu, G.L.; Li, J.; Zhang, O.; Lan, Y.J. Effect of vanadium exposure on neurobehavioral function in workers. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 2016, 34, 103–106. [Google Scholar] [CrossRef]
- Schlake, H.P.; Bertram, H.P.; Husstedt, I.W.; Schuierer, G. Acute systemic vanadate poisoning presenting as cerebrovascular ischemia with prolonged reversible neurological deficits (PRIND). Clin. Neurol. Neurosurg. 1994, 96, 92–95. [Google Scholar] [CrossRef]
- World Health Organization (WHO). International Atomic Energy Agency & Food and Agriculture Organization of the United Nations, 1996. Trace Elements in Human Nutrition and Health. World Health Organization. Available online: https://apps.who.int/iris/handle/10665/37931 (accessed on 17 January 2023).
- Anger, W.K. Reconsideration of the WHO NCTB strategy and test selection. Neurotoxicology 2014, 45, 224–231. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Serrano-Sierra, A.; Torres-Jardón, R.; Zhu, H.; Yuan, Y.; Smith, D.; Delgado-Chávez, R.; Cross, J.V.; Medina-Cortina, H.; Kavanaugh, M.; et al. The impact of environmental metals in young urbanites’ brains. Exp. Toxicol. Pathol. 2013, 65, 503–511. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Vojdani, A.; Blaurock-Bush, E.; Busch, Y.; Friedle, A.; Franco-Lira, M.; Sarathi-Mukherjee, P.; Martínez-Aguirre, X.; Park, S.B.; Torres-Jardón, R.; et al. Air pollution and children: Neural and tight junction antibodies and combustion metals, the role of barier breakdown and brain immunity in neurodegeneration. J. Alzheimer’s Dis. 2015, 43, 1039–1058. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Avila-Ramírez, J.; Calderón-Garcidueñas, A.; González-Heredia, T.; Acuña-Ayala, H.; Chao, C.; Thompson, C.; Ruiz-Ramos, R.; Cortéz-González, V.; Martínez- Martínez, L.; et al. Cerebrospinal fluid biomarkers in highly exposed PM2.5 urbanites: The risk of Alzheimer’s and Parkinson’s diseases in young Mexico City residents. J. Alzheimer’s Dis. 2016, 52, 597–613. [Google Scholar] [CrossRef]
- Yu, Z.; Peters, S.; van Boxmeer, L.; Downward, G.S.; Hoek, G.; Kioumourtzoglou, M.A.; Weisskopf, M.G.; Hansen, J.; van den Berg, L.H.; Vermeulen, R.C.H. Long-term exposure to ultrafine particles and particulate matter constituents and the risk of amyotrophic lateral sclerosis. Environ. Health Perspect. 2021, 129, 097702. [Google Scholar] [CrossRef]
- Johnson, F.O.; Atchison, W. The role of environmental mercury, lead, and pesticide exposure in development of amyotrophic lateral sclerosis. Neurotoxicology 2009, 30, 761–765. [Google Scholar] [CrossRef]
- Willis, A.W.; Evanoff, B.A.; Lian, M.; Galarza, A.; Wegrzyn, A.; Schootman, M.; Racette, B.A. Metal emissions and urban incydent Parkinson disease: A community health study of medicare beneficiaries by using geographic information systems. Am. J. Epidemiol. 2010, 172, 1357–1363. [Google Scholar] [CrossRef]
- Elonheimo, H.M.; Andersen, H.R.; Katsonouri, A.; Tolonen, H. Environmental substances associated with Alzheimer’s disease-a scoping review. Int. J. Environ. Res. Public Health 2021, 18, 11839. [Google Scholar] [CrossRef]
- Barceloux, D.G. Vanadium. J. Toxicol. Clin. Toxicol. 1999, 37, 265–278. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). Cobalt in hard metals and cobalt sulfate, gallium arsenide, indium phosphide and vanadium pentoxide. IARC Monogr. Eval. Carcinog. Risks Hum. 2006, 86, 119–237. [Google Scholar]
- De Hoogh, K.; Wang, M.; Adam, M.; Badaloni, C.; Beelen, R.; Birk, M.; Cesaroni, G.; Cirach, M.; Declercq, C.; Dedele, A.; et al. Development of land use regression models for particle composition in twenty study areas in Europe. Environ. Sci. Technol. 2013, 47, 5778–5786. [Google Scholar] [CrossRef]
- Bosco, M.L.; Varrica, D.; Dongarria, G. Case study: Inorganic pollutants associated with particulate matter from an area near a petrochemical plant. Environ. Res. 2005, 99, 18–30. [Google Scholar] [CrossRef]
- Moreno, T.; Querol, X.; Alastuey, A.; de la Rosa, J.; Sánchez de la Campa, A.M.; Mingullón, M.; Pandolfi, M.; González-Castanedo, Y.; Monfort, E.; Gibbons, W. Variations in vanadium, nickel and lanthanoid element concentrations in urban air. Sci. Total Environ. 2010, 408, 4569–4579. [Google Scholar] [CrossRef]
- U.S. Department of Health and Hunam Services Public Health Service Agency for Toxic Substances and Disease Registry. Toxicological Profile for Vanadium (TPV); U.S. Department of Health and Hunam Services Public Health Service Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2012.
- Dye, J.A.; Adler, K.B.; Richards, J.H.; Dreher, K.L. Role of soluble metals in oil fly ash-induced airway epithelial injury and cytokine gene expression. Am. J. Physiol. 1999, 277, L498–L510. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Toxicological review of vanadium pentoxide (V2O5) (CAS No. 1314-62-1). In Support of Summary Information on the Integrated Risk Information System (IRIS); U.S. Environmental Protection Agency Publ.: Washington, DC, USA, 2011; 210p. [Google Scholar]
- Korbecki, J.; Baranowska-Bosiacka, I.; Gutowska, I.; Chlubek, D. Vanadium compounds as pro-inflammatory agents: Effects on cyclooxygenases. Int. J. Mol. Sci. 2015, 16, 12648–12668. [Google Scholar] [CrossRef]
- Todorich, B.; Olopade, J.O.; Surguladze, N.; Zhang, X.; Neely, E.; Connor, J.R. The mechanism of vanadium-mediated developmental hypomyelination is related to destruction of oligodendrocyte progenitors through a relationship with ferritin and iron. Neurotox. Res. 2011, 19, 361–373. [Google Scholar] [CrossRef]
- Soazo, M.; Garcia, G.B. Vanadium exposure through lactation produces behavioral alterations and CNS myelin deficyt in neonatal rats. Neurotoxicol. Teratol. 2007, 29, 503–510. [Google Scholar] [CrossRef]
- Azeez, I.A.; Olopade, F.; Laperchia, C.; Andriolo, A.; Scambi, I.; Onwuka, S.K.; Bentivoglio, M.; Olopade, J.O. Regional myelin and axon damage and neuroinflammation in the adult mouse brain after long-term postnatal vanadium exposure. J. Neuropathol. Exp. Neurol. 2016, 75, 843–854. [Google Scholar] [CrossRef]
- Usende, I.L.; Olopade, J.O.; Azeez, I.A.; Andrioli, A.; Bankole, M.O.; Olopade, F.E.; Nafady, A.A.; Bentivoglio, M. Neuroecotoxicology: Effects of environmental heavy metal exposure on the brain of African giant rats and the contribution of vanadium to the neuropathology. IBRO Neurosci. Rep. 2022, 13, 215–234. [Google Scholar] [CrossRef]
- Nickel, M.; Gu, C. Regulation of central nervous system myelination in higher brain functions. Neural Plast. 2018, 2018, 6436453. [Google Scholar] [CrossRef]
- Kazana, W.; Zabłocka, A. Brain-derived neurotrophic factor as a potential therapeutic tool in the treatment of nervous system disorders. Postępy Hig. Med. Doświadczalnej 2020, 74, 517–531. [Google Scholar] [CrossRef]
- Miranda, M.; Morici, J.F.; Zanoni, M.B.; Bekinschtein, P. Brain-derived neurotrophic factor: A key molecule for memory in the healthy and the pathological brain. Front. Cell. Neurosci. 2019, 13, 363. [Google Scholar] [CrossRef]
- Wang, D.C.; Lin, Y.Y.; Lin, H.T. Recovery of motor coordination after exercise is correlated to enhancement of brain-derived neurotrophic factor in lactational vanadium-exposed rats. Neurosci. Lett. 2015, 600, 232–237. [Google Scholar] [CrossRef]
- González-Maciel, A.; Reynoso-Robles, R.; Torres-Jardón, R.; Mukherjee, P.S.; Calderón-Garcidueñas, L.G. Combustion-derived nanoparticles in key brain target cells and organelles in young urbanites: Culprit hidden in plain sight in Alzheimer’s disease development. J. Alzheimers Dis. 2017, 59, 189–208. [Google Scholar] [CrossRef]
- Cole-Hunter, T.; Zhang, J.; So, R.; Samoli, E.; Liu, S.; Chen, J.; Strak, M.; Wolf, K.; Weinmayr, G.; Rodopolou, S.; et al. Long-term air pollution exposure and Parkinson’s disease mortality in a large pooled European cohort: An ELAPSE study. Environ. Int. 2023, 171, 107667. [Google Scholar] [CrossRef]
- Montiel-Flores, E.; Mejía-García, O.A.; Ordoñez-Librado, J.L.; Gutierrez-Valdez, A.L.; Espinosa-Villanueva, J.E.; Dorado-Martínez, C.; Reynoso-Erazo, L.; Tron-Alvarez, R.; Rodríguez-Lara, V.; Avila-Costa, M.R. Alzheimer-like cell death after vanadium pentoxide inhalation. Heliyon 2021, 7, e07856. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Maronpot, R.R.; Torres-Jardon, R.; Henríquez-Roldán, C.; Schoonhoven, R.; Acuña-Ayala, H.; Villarreal-Calderón, A.; Nakamura, J.; Fernando, R.; Reed, W.; et al. DNA damage in nasal and brain tissues of canines exposed to air pollutants is asscociated with evidence of chronić brain inflammation and neurodegeneration. Toxicol. Pathol. 2003, 31, 524–538. [Google Scholar] [CrossRef]
- Sánchez, G.I.; López, I.; Mussali, P.; Bizarro, N.P.; Nińo, G.; Saldivar, L.; Espejel, G.; Avila, M.; Morales, D.; Colin, L.; et al. Vanadium concentrations in lung, liver, kidney, testes and brain after the inhalation of 0.02 M of V2O5. An experimental model in mice. In Proceedings of the 42nd Annual Meeting of the Society of Toxicology, Salt Lake City, UT, USA, 9–13 March 2003. [Google Scholar]
- Avila-Costa, M.R.; Montiel Flores, E.; Colin-Barenque, L.; Ordoñez, J.L.; Gutiérrez, A.L.; Niño-Cabrera, H.G.; Mussali-Galante, P.; Fortoul, T.I. Nigrostriatal modifications after vanadium inhalation: An immunocytochemical and cytological approach. Neurochem. Res. 2004, 29, 1365–1369. [Google Scholar] [CrossRef]
- Avila-Costa, M.R.; Colín-Barenque, L.; Zepeda-Rodríguez, A.; Antuna, S.B.; Saldivar, L.; Espejel-Maya, G.; Mussali-Galante, P.; del Carmen Avila-Casado, M.; Reyes-Olivera, A.; Anaya-Martinez, V.; et al. Ependymal epithelium disruption after vanadium pentoxide inhalation. A mice experimental model. Neurosci. Lett. 2005, 381, 21–25. [Google Scholar] [CrossRef]
- Avila-Costa, M.R.; Fortoul, T.I.; Niño-Cabrera, G.; Colín-Barenque, L.; Bizarro-Nevares, P.; Gutiérrez-Valdez, A.L.; Ordóñez-Librado, J.L.; Rodríguez-Lara, V.; Mussali-Galante, P.; Díaz-Bech, P.; et al. Hippocampal cell alterations induced by the inhalation of vanadium pentoxide (V2O5) promote memory deterioration. Neurotoxicology 2006, 27, 1007–1012. [Google Scholar] [CrossRef]
- Colín-Barenque, L.; Martínez-Hernández, M.G.; Baiza-Gutman, L.A.; Avila-Costa, M.R.; Ordóñez-Librado, J.L.; Bizarro-Nevares, P.; Rodriguez-Lara, V.; Piñón-Zarate, G.; Rojas-Lemus, M.; Mussali-Galante, P.; et al. Matrix metalloproteinases 2 and 9 in central nervous system and their modification after vanadium inhalation. J. Appl. Toxicol. 2008, 28, 718–723. [Google Scholar] [CrossRef]
- Igado, O.O.; Olopade, J.O.; Onwuka, S.K.; Chukwudi, A.C.; Daramola, O.A.; Ajufo, U.E. Evidence of environmental pollution in caprine brains ontained from a relatively unindustrialized area in Nigeria. Afr. J. Biomed. Res. 2008, 11, 305–309. [Google Scholar] [CrossRef]
- Ngwa, H.A.; Kanthasamy, A.; Jin, H.; Anantharam, V.; Kanthasamy, A.G. Vanadium exposure induces olfactory dysfunction in an animal model of metal neurotoxicity. Neurotoxicology 2014, 43, 73–81. [Google Scholar] [CrossRef]
- Colín-Barenque, L.; Pedraza-Chaverri, J.; Medina-Campos, O.; Jimenez-Martínez, R.; Bizarro-Nevares, P.; González-Villalva, A.; Rojas-Lemus, M.; Fortoul, T.I. Functional and morphological olfactory bulb modifications in mice after vanadium inhalation. Toxicol Pathol. 2015, 43, 282–291. [Google Scholar] [CrossRef]
- DeWitt, J.; Buck, B.; Goossens, D.; Hu, Q.; Chow, R.; David, W.; Young, S.; Teng, Y.; Leetham-Spencer, M.; Murphy, L.; et al. Health effects following subacute exposure to geogenic dusts from arsenic-rich sediment at the Nellis Dunes Recreation Area, Las vegas, NV. Toxicol. Appl. Pharmacol. 2016, 304, 79–89. [Google Scholar] [CrossRef]
- DeWitt, J.C.; Buck, B.J.; Goossens, D.; Teng, Y.; Pollard, J.; McLaurin, B.T.; Gerads, R.; Keil, D.E. Health effects following subacute exposure to geogenic dust collected from active drainage surfaces (Nellis Dunes Recreation Area, Las Vegas, NV). Toxicol. Rep. 2017, 4, 19–31. [Google Scholar] [CrossRef]
- Keil, D.; Buck, B.; Goossens, D.; Teng, Y.; Leetham, M.; Murphy, L.; Pollard, J.; Eggers, M.; McLaurin, B.; Gerads, R.; et al. Immunotoxicological and neurotoxicological profile of health effects following subacute exposure to geogenic dust from sand dunes at the Nellis Dune Recreation Area, Las Vegas, NV. Toxicol. Appl. Pharmacol. 2016, 291, 1–12. [Google Scholar] [CrossRef]
- Usende, I.L.; Emikpe, B.O.; Olopade, J.O. Heavy metal pollutants in selected organs of African giant rats from three agro-ecological zones of Nigeria: Evidence for their role as an environmental specimen bank. Environ. Sci. Pollut. Res. 2017, 24, 22570–22578. [Google Scholar] [CrossRef]
- Colín-Barenque, L.; Bizarro-Nevares, P.; González Villalva, A.; Pedraza-Chaverri, J.; Medina-Campos, O.N.; Jimenez-Martínez, R.; Rodríguez-Rangel, D.S.; Reséndiz, S.; Fortoul, T.I. Neuroprotective effect of carnosine in the olfactory bulb after vanadium inhalation in a mouse model. Int. J. Exp. Path. 2018, 99, 180–188. [Google Scholar] [CrossRef]
- Hara, Y.; Fillit, H.M. The dawn of a new era of Alzheimer’s research and drug development. J. Prev. Alzheimers Dis. 2022, 9, 385–386. [Google Scholar] [CrossRef]
- Sherzai, D.; Sherzai, A. Preventing Alzheimer’s: Our Most Urgent Health Care Priority. Am. J. Lifestyle Med. 2019, 13, 451–461. [Google Scholar] [CrossRef]
- Pagano, G.; Taylor, K.; Anzures-Cabrera, J.; Marchesi, M.; Simuni, T.; Marek, K.; Postuma, B.R.; Pavese, N.; Stocchi, F.; Azulay, J.P.; et al. Trial of prasinezumab in early-stage Parkinson’s Disease. N. Engl. J. Med. 2022, 387, 421–432. [Google Scholar] [CrossRef]
- Choroba Parkinsona. 5 December 2021. Available online: https://www.mp.pl/neurologia/choroba-parkinsona/232939,choroba-parkinsona (accessed on 14 April 2023).
- Dobrakowski, P.P.; Machowska-Majchrzak, A.K.; Labuz-Roszak, B.; Majchrzak, K.G.; Kluczewska, E.; Pierzchała, K.B. MR-guided focused ultrasound: A new generation treatment of Parkinson’s disease, essential tremor and neuropathic pain. Interv. Neuroradiol. 2014, 20, 275–282. [Google Scholar] [CrossRef]
- Khamaysa, M.; Pradat, P.F. Status of ALS treatment, insights into therapeutic challenges and dilemmas. J. Pers. Med. 2022, 12, 1601. [Google Scholar] [CrossRef]
- Szymczyk, H. Magnez—Pierwiastek niezbędny do prawidłowego funkcjonowania organizmu. Farm. Współ. 2016, 9, 217–223. [Google Scholar]
- Ścibior, A.; Gołębiowska, D.; Niedźwiecka, I. Magnesium can protect against vanadium-induced lipid peroxidation in the hepatic tissue. Oxid. Med. Cell. Longev. 2013, 2013, 802734. [Google Scholar] [CrossRef]
- Chui, D.; Chen, Z.; Yu, J.; Zhang, H.; Wang, W.; Song, Y.; Yang, H.; Zhou, L. Magnesium in the Central Nervous System; Vink, R., Nechifor, M., Eds.; University of Adelaide Press: Adelaide, AU, USA, 2011. Available online: https://www.ncbi.nlm.nih.gov/books/NBK507256/ (accessed on 17 January 2023).
- Ścibior, A.; Gołębiowska, D.; Adamczyk, A.; Niedźwiecka, I.; Fornal, E. The renal effects of vanadate exposure: Potential biomarkers and oxidative stress as a mechanism of functional renal disorders—Preliminary studies. BioMed Res. Int. 2014, 2014, 740105. [Google Scholar] [CrossRef]
- Bi, M.; Du, X.; Jiao, Q.; Chen, X.; Jiang, H. Expanding the role of proteasome homeostasis in Parkinson’s disease: Beyond protein breakdown. Cell Death Dis. 2021, 12, 154. [Google Scholar] [CrossRef]
- Tomaru, U.; Ito, T.; Ohmura, Y.; Higashikawa, K.; Miyajima, S.; Tomatsu, R.; Higashi, T.; Ishizu, A.; Kuge, Y.; Yoshioka, M.; et al. Decreased proteasomal function induces neuronal loss and memory impairment. Am. J. Pathol. 2021, 191, 144–156. [Google Scholar] [CrossRef]





| Patients | Number | Age (Mean/Median/Ranges) | Concentration (Mean/Median) * | Biological Fluids/ Tissues | Unit | Ref. |
|---|---|---|---|---|---|---|
| AD cases | ||||||
| Control | n = 10 | 88 | 3.0 | FC | ng/g | [57] |
| AD | n = 14 | 78 | 5.0 | FC | ng/g | |
| Control | n = 10 | 88 | <LOQ | VF | ng/mL | [57] |
| AD | n = 14 | 78 | <LOQ | VF | ng/mL | |
| Control | n = 54 | 73 (60–94) | 3.2 | CSF | µg/L | [58] |
| AD | n = 173 | 75 (52–86) | 2.9 ↓ | CSF | µg/L | |
| Control | n = 36 | 38.5/70.4 | ND | CSF | n/a | [59] |
| AD | n = 33 | ~76 | ND | CSF | n/a | |
| Control † | n = 30 | 61 (52–69) | 0.0352 | Hippocampus | µg/g dw | [60] |
| AD † | n = 28 | 63.5 (57–72) | 0.0978 | Hippocampus | µg/g dw | |
| Control ‡ | n = 30 | ~63 (55–72) | 0.0274 | Hippocampus | µg/g dw | |
| AD ‡ | n = 30 | 64.5 (61–75) | 0.0162 ↓ | Hippocampus | µg/g dw | |
| Control † | n = 30 | 0.0308 | CC | µg/g dw | ||
| AD † | n = 28 | 61 (52–69) | 0.0141 ↓ | CC | µg/g dw | |
| Control ‡ | n = 30 | 63.5 (57–72) | 0.0272 | CC | µg/g dw | |
| AD ‡ | n = 30 | ~63 (55–72) | 0.0133 ↓ | CC | µg/g dw | |
| Control †,‡ | n = 60 | 64.5 (61–75) | (0.0117–0.0506) | Hippocampus and CC | µg/g dw | |
| AD †,‡ | n = 58 | (0.0086–0.0209) | Hippocampus and CC | µg/g dw | ||
| Control | n = 54 | 73 | 3.2 (2.3–5.1) | CSF | µg/L | [61] |
| AD | n = 174 | 74 | 2.9 (<2.2–5.2) ↓ | CSF | µg/L | |
| Control | n = 4 (nos = 8) | age-matched | n/a f | C and PC | ppm | [62] |
| AD | n = 4 (nos = 8) | 78–88 | n/a f | C and PC | ppm | |
| ALS cases | ||||||
| Control | n = 10 | 45 (26–77) | 0.09 (0.03–0.19) | CSF | µg/L | [63] |
| ALS | n = 17 | 64 (47–85) | 0.14 ↑ (0.06–0.77) | CSF | µg/L | |
| Control | n = 5 | ~56 (43–66) | 0.20 | GM | µg/g dw | [64] |
| ALS | n = 8 | ~53 (34–66) | 0.14 | GM | µg/g dw | |
| Control | n = 5 | ~56 (43–66) | 0.14 | WM | µg/g dw | [64] |
| ALS | n = 8 | ~53 (34–66) | 0.08 | WM | µg/g dw | |
| Control | nos = 3–8 | - | 0.16 | FL (GM) | µg/g dw | [64] |
| ALS | - | 0.15 | FL (GM) | µg/g dw | ||
| Control | nos = 3–8 | - | 0.23 | PL (GM) | µg/g dw | [64] |
| ALS | - | 0.13 | PL (GM) | µg/g dw | ||
| Control | nos = 3–8 | - | 0.21 | OL (GM) | µg/g dw | [64] |
| ALS | - | 0.09 | OL (GM) | µg/g dw | ||
| Control | nos = 3–8 | - | 0.10 | TL (GM) | µg/g dw | [64] |
| ALS | - | 0.18 | TL (GM) | µg/g dw | ||
| Control | nos = 3–8 | - | 0.21 | C (GM) | µg/g dw | [64] |
| ALS | - | 0.15 | C (GM) | µg/g dw | ||
| Control | nos = 3–8 | - | 0.26 | BG (GM) | µg/g dw | [64] |
| ALS | - | 0.16 | BG (GM) | µg/g dw | ||
| Control | nos = 3–8 | - | 0.12 | FL (WM) | µg/g dw | [64] |
| ALS | - | 0.09 | FL (WM) | µg/g dw | ||
| Control | nos = 3–8 | - | 0.06 | PL (WM) | µg/g dw | [64] |
| ALS | - | 0.07 | PL (WM) | µg/g dw | ||
| Control | nos = 3–8 | - | 0.22 | OL (WM) | µg/g dw | [64] |
| ALS | - | 0.08 | OL (WM) | µg/g dw | ||
| Control | nos = 3–8 | - | 0.19 | TL (WM) | µg/g dw | [64] |
| ALS | - | 0.08 | TL (WM) | µg/g dw | ||
| Control | nos = 3–8 | - | 0.20 | C (WM) | µg/g dw | [64] |
| ALS | - | 0.09 | C (WM) | µg/g dw | ||
| ALS ** | n = 31 | 65 (59–70) | 0.30 | CSF | µg/L | [65] |
| ALS ## | n = 6 | 70 (64–82) | 0.35 | CSF | µg/L | |
| PD cases | ||||||
| Control | n = 5 | ~56 (43–66) | 0.20 | GM | µg/g dw | [64] |
| PDC | n = 4 | ~58 (51–65) | 0.21 | GM | µg/g dw | |
| Control | n = 5 | ~56 (43–66) | 0.14 | WM | µg/g dw | [64] |
| PDC | n = 4 | ~58 (51–65) | 0.10 | WM | µg/g dw | |
| Control | nos = 3–8 | - | 0.16 | FL (GM) | µg/g dw | [64] |
| PDC | - | 0.04 | FL (GM) | µg/g dw | ||
| Control | nos = 3–8 | - | 0.23 | PL (GM) | µg/g dw | [64] |
| PDC | - | 0.33 | PL (GM) | µg/g dw | ||
| Control | nos = 3–8 | - | 0.21 | OL (GM) | µg/g dw | [64] |
| PDC | - | 0.14 | OL (GM) | µg/g dw | ||
| Control | nos = 3–8 | - | 0.10 | TL (GM) | µg/g dw | [64] |
| PDC | - | 0.25 | TL (GM) | µg/g dw | ||
| Control | nos = 3–8 | - | 0.21 | C (GM) | µg/g dw | [64] |
| PDC | - | 0.15 | C (GM) | µg/g dw | ||
| Control | nos = 3–8 | - | 0.26 | BG (GM) | µg/g dw | [64] |
| PDC | - | 0.16 | BG (GM) | µg/g dw | ||
| Control | nos = 3–8 | - | 0.12 | FL (WM) | µg/g dw | [64] |
| PDC | - | 0.08 | FL (WM) | µg/g dw | ||
| Control | nos = 3–8 | - | 0.06 | PL (WM) | µg/g dw | [64] |
| PDC | - | 0.13 | PL (WM) | µg/g dw | ||
| Control | nos = 3–8 | - | 0.22 | OL (WM) | µg/g dw | [64] |
| PDC | - | 0.10 | OL (WM) | µg/g dw | ||
| Control | nos = 3–8 | - | 0.19 | TL (WM) | µg/g dw | [64] |
| PDC | - | 0.10 | TL (WM) | µg/g dw | ||
| Control | nos = 3–8 | - | 0.20 | C (WM) | µg/g dw | [64] |
| PDC | - | 0.09 | C (WM) | µg/g dw | ||
| PD | n = 13 | n/a | 0.0372 | CSF | µmol/L | [66] |
| 1.0541 ↑ (1 wk) | CSF | µmol/L | ||||
| 0.2061 ↑ (2 wk) | CSF | µmol/L | ||||
| 0.4691 ↑ (4 wk) | CSF | µmol/L | ||||
| 0.2944 ↑ (6 wk) | CSF | µmol/L | ||||
| 0.1276 ↑ (8 wk) | CSF | µmol/L | ||||
| Control | n = 13 | ~64 | 0.12 | CSF | ng/mL | [67] |
| PD | n = 26 | ~65 | 0.07 ↓ | CSF | ng/mL | |
| Control | n = 12 | 70 | n/a | CN | µg/g dw | [68] |
| PD | n = 9 | 73 | n/a # | CN | µg/g dw | |
| Patients | Number | Age (Mean/Median/Ranges) | Concentration (Mean/Median) * | Biological Fluids/Other | Unit | Ref. |
|---|---|---|---|---|---|---|
| AD cases | ||||||
| Control | n = 54 | 73 (60–94) | <2.2 | plasma | µg/L | [58] |
| AD | n = 173 | 75 (52–86) | <2.2 | plasma | µg/L | |
| Control | n = 30 | 74 | 0.0613 | serum | µg/L | [69] |
| AD | n = 30 | ~80 | 0.0673 | serum | µg/L | |
| Control | n = 40 | 65 (57–87) | 0.04 (0.03–0.09) | serum | µg/L | [70] |
| AD | n = 34 | 72 (54–84) | 0.08 ↑ (0.03–0.53) | serum | µg/L | |
| Control | n = 17 | ~74 | 0.17 | hair | µg/g | [71] |
| AD | n = 9 | ~83 | 0.06 | hair | µg/g | |
| Control | n = 18 | age-matching | 39.9 | serum | ng/g | [72] |
| AD | n = 30 | 60 (45–84) | 76.3 ↑ | serum | ng/g | |
| Control | n = 161 | >60 | n/a # | plasma | n/a | [73] |
| AD | n = 92 | >60 | n/a # ↑ | plasma | n/a | |
| Control | n = 124 | 44.8 (20–84) | 0.06 (0.05–0.08) | serum | µg/L | [74] |
| AD | n = 53 | 74.5 (58–86) | 0.04 (0.03–0.07) | serum | µg/L | |
| Control | n = 44 | >45 | 0.06 | serum | ng/mL | [75] |
| AD | n = 60 | 74.6 | 0.05 | serum | ng/mL | |
| Control | n = 44 | >45 | 0.09 | WB | ng/mL | [75] |
| AD | n = 60 | 74.6 | 0.13 | WB | ng/mL | |
| PD cases | ||||||
| Control | n = 124 | 44.8 (20–84) | 0.06 (0.05–0.08) | serum | µg/L | [74] |
| PD | n = 71 | 65.5 (41–81) | 0.11 (0.06–0.16) | serum | µg/L | |
| Control | n = 44 | ~52 | 0.06 | serum | ng/mL | [76] |
| PD | n = 71 | 65.5 | 0.11 ↑ | serum | ng/mL | |
| Control | n = 13 | ~64 | 0.07 | WB | ng/mL | [67] |
| PD | n = 26 | ~65 | 0.22 ↑ | WB | ng/mL | |
| Control | n = 13 | ~64 | 0.15 | serum | ng/mL | [67] |
| PD | n = 26 | ~65 | 0.17 | serum | ng/mL | |
| Control | n = 13 | ~64 | 0.10 | urine | ng/mL | [67] |
| PD | n = 26 | ~65 | 0.20 ↑ | urine | ng/mL | |
| ALS cases | ||||||
| Control | n = 9 | 45 (26–77) | 0.07 | plasma | µg/L | [63] |
| ALS | n = 15 | 64 (47–85) | 0.07 | plasma | µg/L | |
| Control | n = 100 | 51 | 0.08 | hair | µg/g | [77] |
| ALS | n = 100 | 55 | 0.05 | hair | µg/g | |
| Item | Number | Age (Mean/Ranges) | Concentration (Mean/Range) * | Body Fluids | Unit | Findings | Ref. |
|---|---|---|---|---|---|---|---|
| Occupationally exposed | |||||||
| WEV | n = 49 | 41.4 (20.1–57.1) | 7.5 (2.18–46.4) | serum | µg/L | ↓ attention | [78] |
| C: 0.8 (0.3–3.1) | ↓ visuospatial abilities | ||||||
| n = 49 | 41.4 (20.1–57.1) | 14.4 (2.12–95.4) | urine | µg/L | |||
| C: 0.4 (0.07–1.4) | |||||||
| WEV | n = 19 (2) | ~34 | 15.5 (3.0–35.2) | urine | µg/L | tremor of finger | [79] |
| C: 6.0 | |||||||
| WEV | n = 463 | 39.5 (20–60) | n/a | n/a | n/a | ↑ anger-hostility | [80] |
| ↑ depression-dejection | |||||||
| ↑ fatigue-inertia | |||||||
| ↓ vigor-activity | |||||||
| ↓ auditory/visual memory | |||||||
| ↓ perception/motion speed | |||||||
| ↓ coordination | |||||||
| WEV | n = 106 | n/a | n/a | n/a | n/a | ↙ vision-memory | [81] |
| ↙ motor speed and accuracy | |||||||
| mood disorders (↗ negative moods) | |||||||
| WEV | n = 128 | n/a | n/a | n/a | n/a | ↙ hearing and visual memory | [82] |
| ↙ movement, velocity, accuracy | |||||||
| ↙ coordination | |||||||
| Acute poisoning cases | |||||||
| Woman | n = 1 | 22 | 61.5 †/12.3 †† <20 | urine CSF | µg/L µg/L | right-sided brachiofacial paresis right hemihypesthesia right sensomotor hemiparesis mixed amnestic and sensorimotor aphasia | [83] |
| Elderly subjects | |||||||
| Male/female | n = 50 | ≥60 | 24.44 (6.0–63.0) | WB | µg/L | ↓ general cognitive function | [22] |
| Y: 22.8 (6.0–49.0) | WB | µg/L | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ścibior, A.; Llopis, J.; Dobrakowski, P.P.; Męcik-Kronenberg, T. CNS-Related Effects Caused by Vanadium at Realistic Exposure Levels in Humans: A Comprehensive Overview Supplemented with Selected Animal Studies. Int. J. Mol. Sci. 2023, 24, 9004. https://doi.org/10.3390/ijms24109004
Ścibior A, Llopis J, Dobrakowski PP, Męcik-Kronenberg T. CNS-Related Effects Caused by Vanadium at Realistic Exposure Levels in Humans: A Comprehensive Overview Supplemented with Selected Animal Studies. International Journal of Molecular Sciences. 2023; 24(10):9004. https://doi.org/10.3390/ijms24109004
Chicago/Turabian StyleŚcibior, Agnieszka, Juan Llopis, Paweł Piotr Dobrakowski, and Tomasz Męcik-Kronenberg. 2023. "CNS-Related Effects Caused by Vanadium at Realistic Exposure Levels in Humans: A Comprehensive Overview Supplemented with Selected Animal Studies" International Journal of Molecular Sciences 24, no. 10: 9004. https://doi.org/10.3390/ijms24109004
APA StyleŚcibior, A., Llopis, J., Dobrakowski, P. P., & Męcik-Kronenberg, T. (2023). CNS-Related Effects Caused by Vanadium at Realistic Exposure Levels in Humans: A Comprehensive Overview Supplemented with Selected Animal Studies. International Journal of Molecular Sciences, 24(10), 9004. https://doi.org/10.3390/ijms24109004









