Performance and Mechanism of Functionalized Water Hyacinth Biochar for Adsorption and Removal of Benzotriazole and Lead in Water
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Materials
2.2. Adsorption Effect
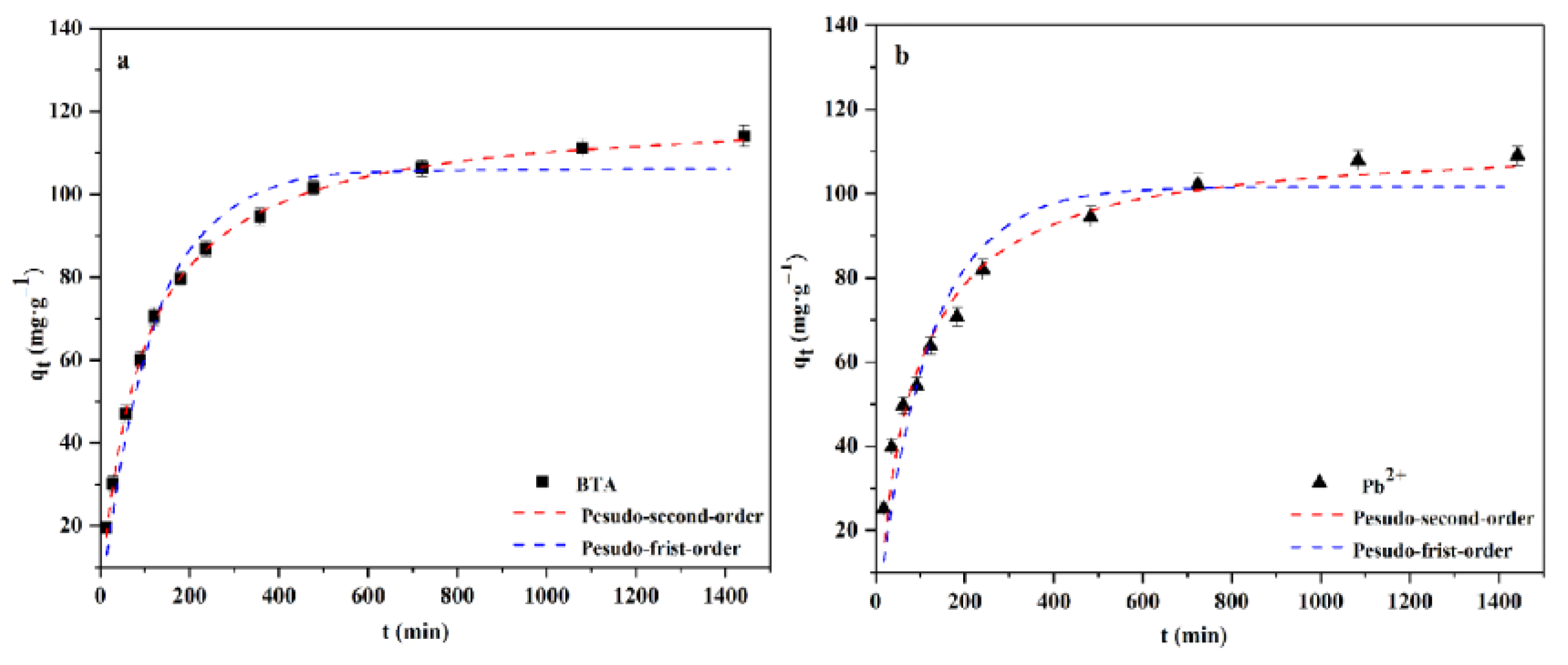
2.3. Adsorption Kinetics
2.4. Adsorption Isotherm
2.5. Adsorption Thermodynamics
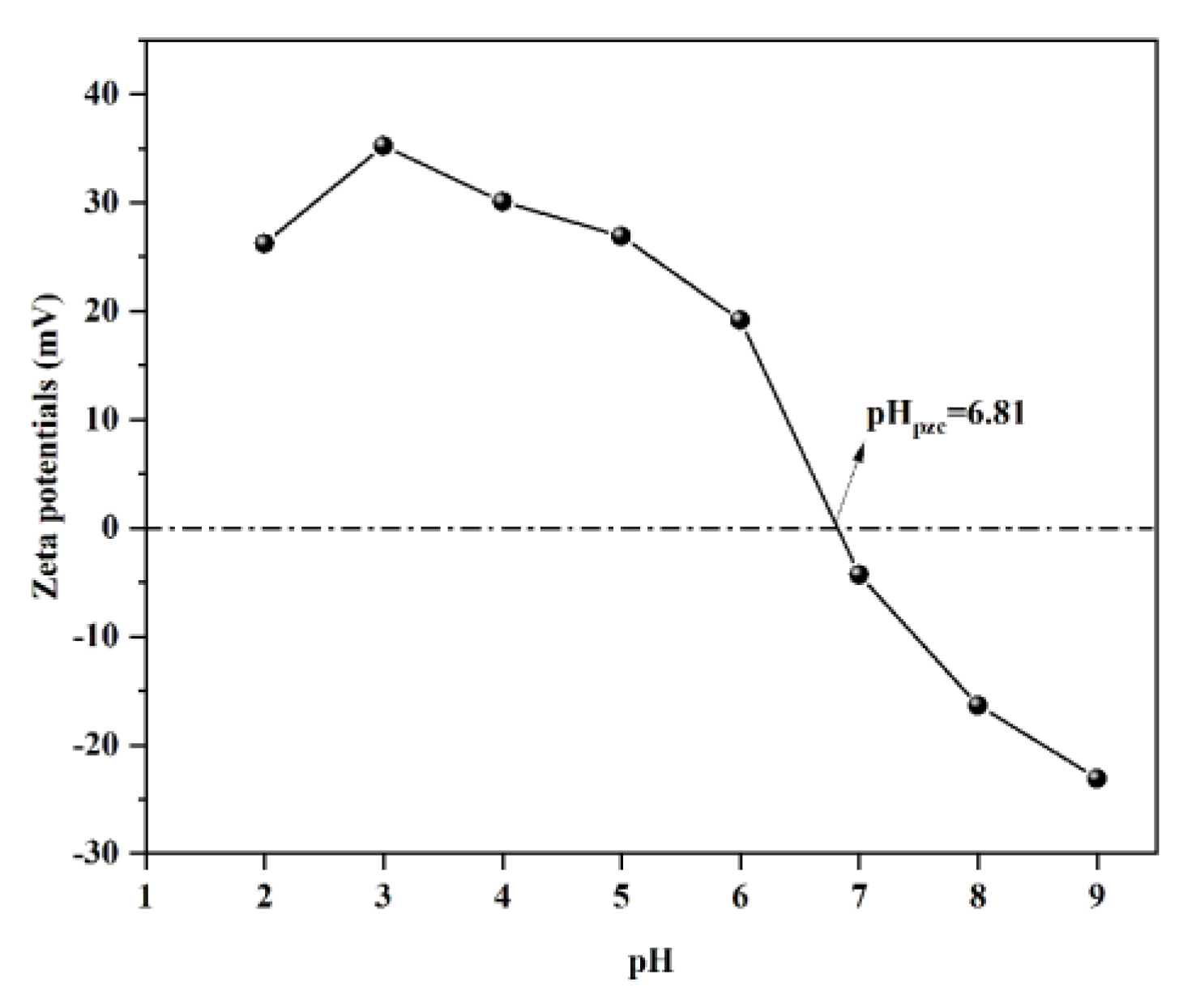
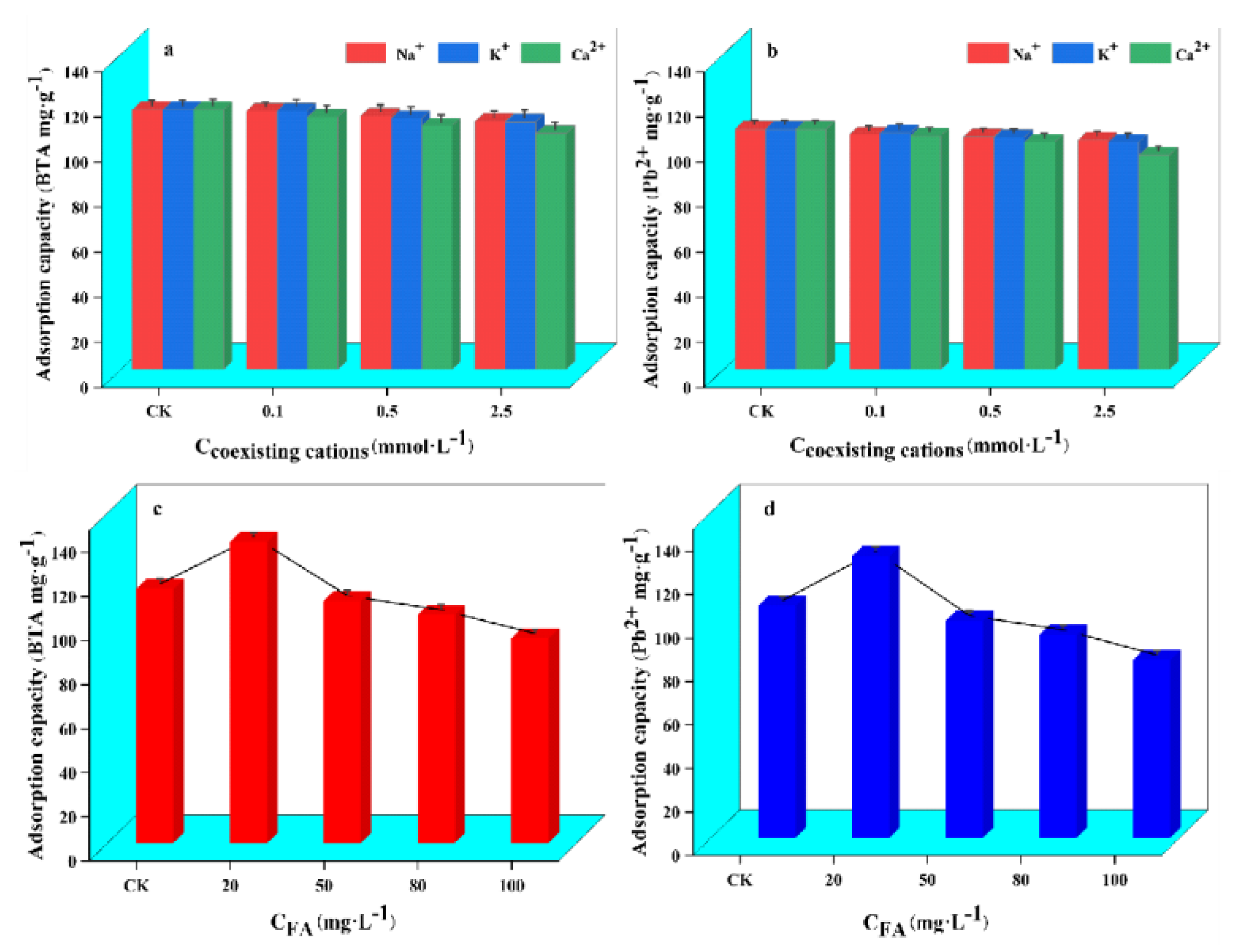
2.6. Effects of Coexisting Cations and FA on the Adsorption of WL on BTA and Pb2+
2.7. Competitive Adsorption of BTA and Pb2+
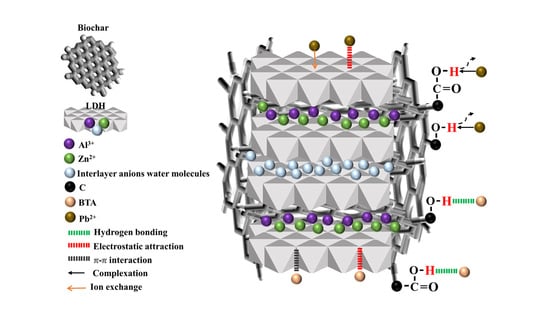
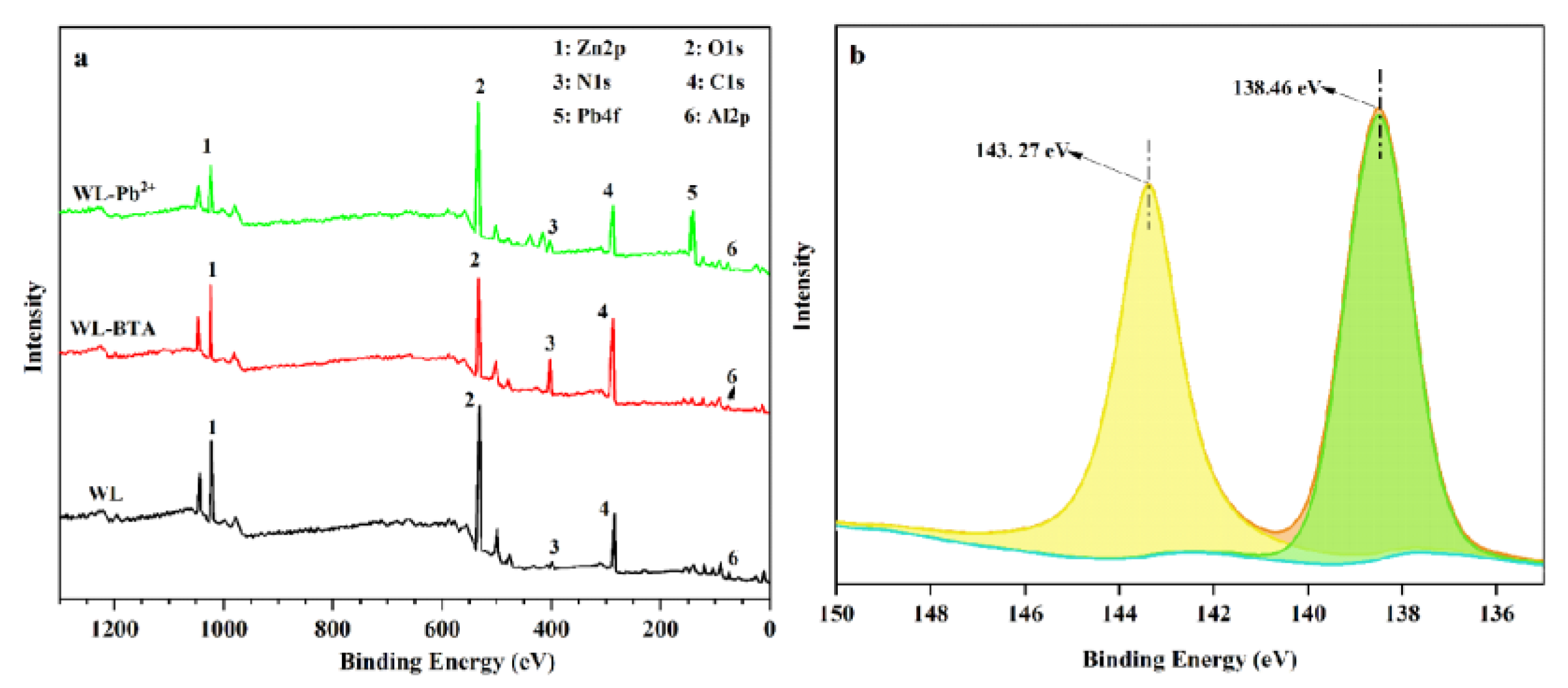
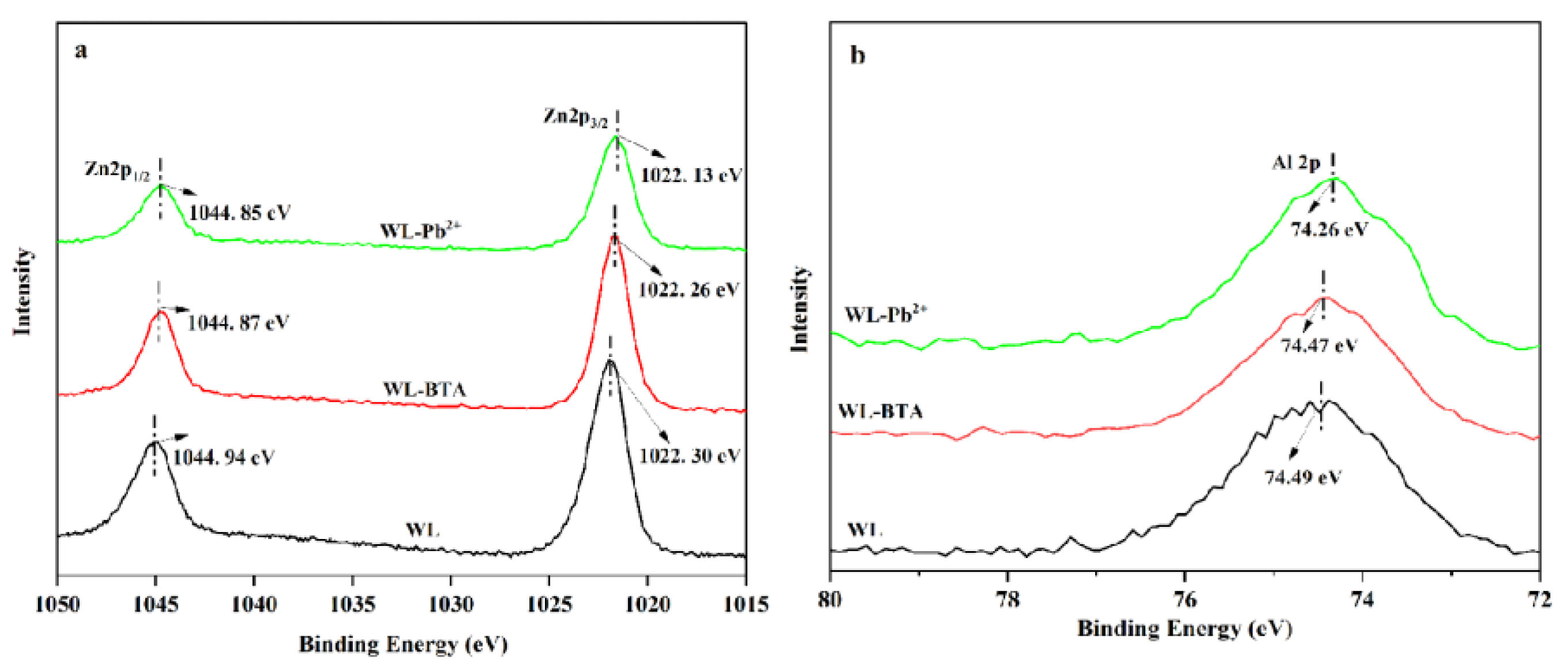
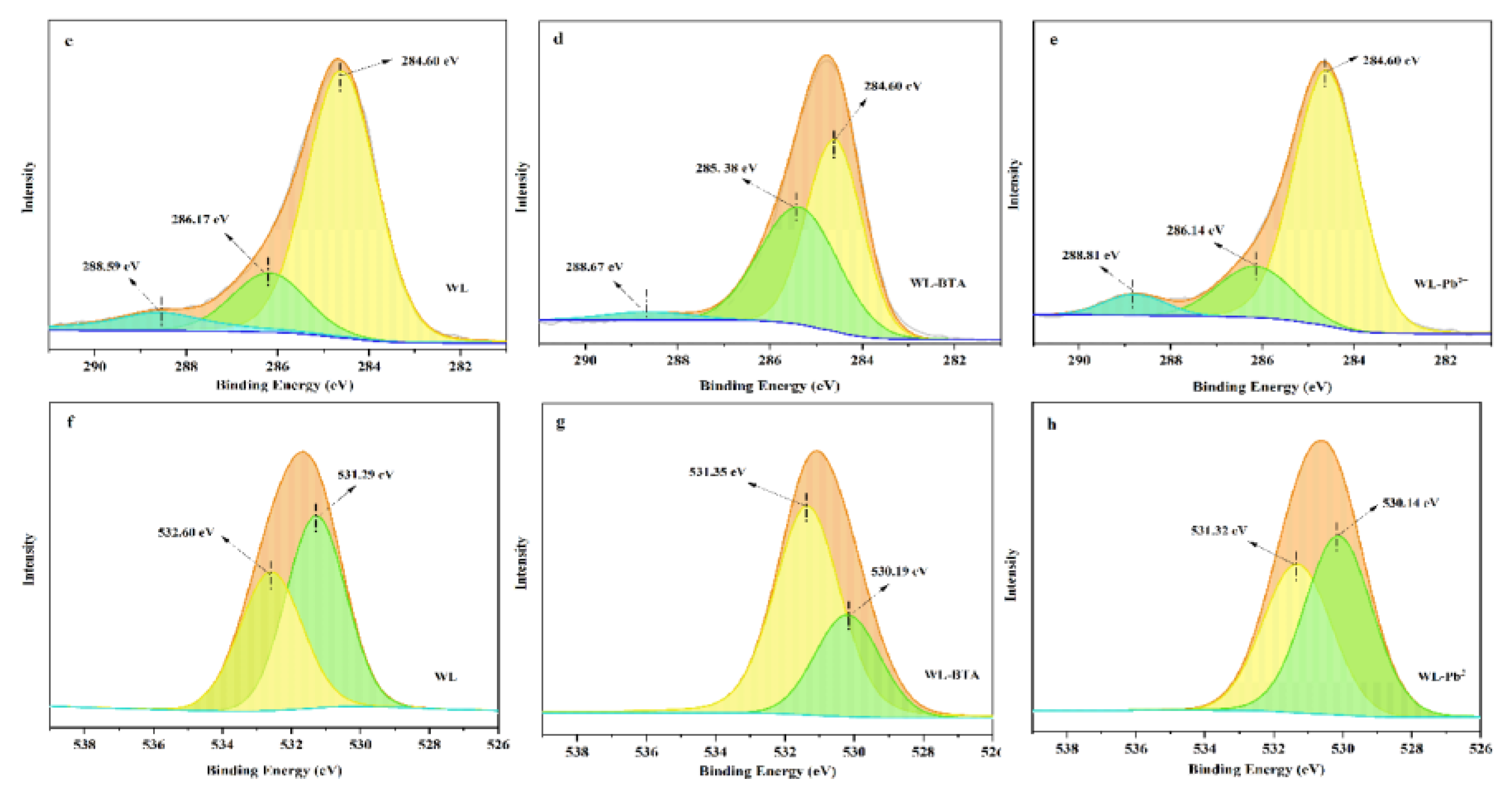
2.8. Adsorption Mechanism
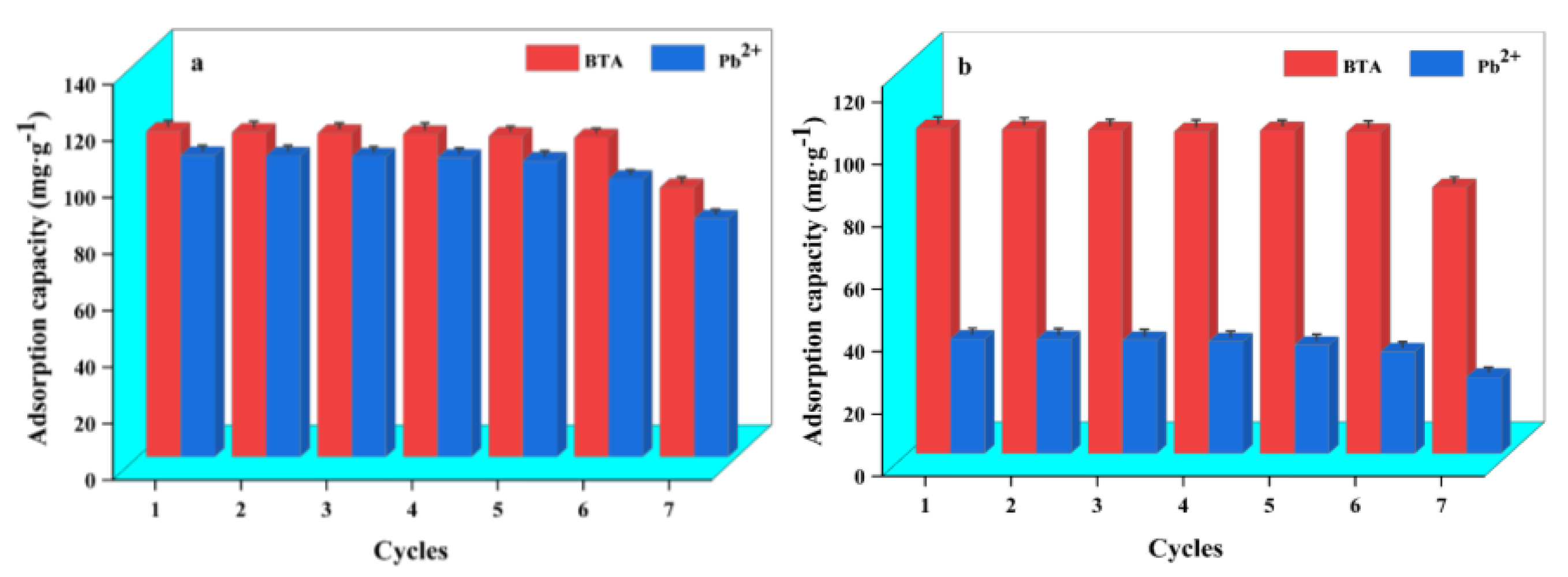
2.9. Recycling Performance of WL
3. Methods and Materials
3.1. Materials
3.2. Preparation of Adsorbent
3.3. Characterization of Samples
3.4. Adsorption Experiment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, J.; Wei, S.; Gu, H.; Rapole, S.B.; Wang, Q.; Luo, Z.; Haldolaarachchige, N.; Young, D.P.; Guo, Z. One-Pot Synthesis of Magnetic Graphene Nanocomposites Decorated with Core@Double-shell Nanoparticles for Fast Chromium Removal. Environ. Sci. Technol. 2012, 46, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wei, Z.; Zhang, X.; Chen, X.; Yue, D.; Yin, Q.; Xiao, L.; Yang, L. Biochar from Alternanthera philoxeroides could remove Pb(II) efficiently. Bioresour. Technol. 2014, 171, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.; Wilson, V.; Hou, A.; Meng, G. Source of lead pollution, its influence on public health and the countermeasures. Int. J. Health Anim. Sci. Food Saf. 2015, 2, 18–31. [Google Scholar]
- Ranđelović, M.; Purenović, M.; Zarubica, A.; Purenović, J.; Matović, B.; Momčilović, M. Synthesis of composite by application of mixed Fe, Mg (hydr)oxides coatings onto bentonite--a use for the removal of Pb(II) from water. J. Hazard. Mater. 2012, 199–200, 367–374. [Google Scholar] [CrossRef]
- Shi, Z.Q.; Liu, Y.S.; Xiong, Q.; Cai, W.W.; Ying, G.G. Occurrence, toxicity and transformation of six typical benzotriazoles in the environment: A review. Sci. Total Environ. 2019, 661, 407–421. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.J.; Ying, G.G.; Ma, Y.B.; Chen, Z.F.; Chen, F.; Liu, Y.S. Occurrence and dissipation of benzotriazoles and benzotriazole ultraviolet stabilizers in biosolid-amended soils. Environ. Toxicol. Chem. 2014, 33, 761–767. [Google Scholar] [CrossRef]
- Deng, J.; Li, X.; Wei, X.; Liu, Y.; Liang, J.; Tang, N.; Song, B.; Chen, X.; Cheng, X. Sulfamic acid modified hydrochar derived from sawdust for removal of benzotriazole and Cu(II) from aqueous solution: Adsorption behavior and mechanism. Bioresour. Technol. 2019, 290, 121765. [Google Scholar] [CrossRef]
- Lee, S.; Kim, S.; Park, J.; Kim, H.J.; Lee, J.J.; Choi, G.; Choi, S.; Kim, S.; Kim, S.Y.; Choi, K.; et al. Synthetic musk compounds and benzotriazole ultraviolet stabilizers in breast milk: Occurrence, time-course variation and infant health risk. Environ. Res. 2015, 140, 466–473. [Google Scholar] [CrossRef]
- Li, Z.; Liang, X.; Liu, W.; Zhao, Y.; Yang, H.; Li, W.; Adamovsky, O.; Martyniuk, C.J. Elucidating mechanisms of immunotoxicity by benzotriazole ultraviolet stabilizers in zebrafish (Danio rerio): Implication of the AHR-IL17/IL22 immune pathway. Environ. Pollut. 2020, 262, 114291. [Google Scholar] [CrossRef]
- Tang, L.; Yu, J.; Pang, Y.; Zeng, G.; Deng, Y.; Wang, J.; Ren, X.; Ye, S.; Peng, B.; Feng, H. Sustainable efficient adsorbent: Alkali-acid modified magnetic biochar derived from sewage sludge for aqueous organic contaminant removal. Chem. Eng. J. 2018, 336, 160–169. [Google Scholar] [CrossRef]
- Sebakhy, K.O.; Vitale, G.; Pereira-Almao, P. Dispersed Ni-Doped Aegirine Nanocatalysts for the Selective Hydrogenation of Olefinic Molecules. ACS Appl. Nano Mater. 2018, 1, 6269–6280. [Google Scholar] [CrossRef]
- Abbas, H.; Manasrah, A.D.; Saad, A.A.; Sebakhy, K.O.; Bouhadda, Y. Adsorption of algerian asphaltenes onto synthesized maghemite iron oxide nanoparticles. Pet. Chem. 2021, 61, 67–75. [Google Scholar] [CrossRef]
- Xu, B.; Wu, F.; Zhao, X.; Liao, H. Benzotriazole removal from water by Zn–Al–O binary metal oxide adsorbent: Behavior, kinetics and mechanism. J. Hazard. Mater. 2010, 184, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Li, Y.; Meng, T.; Zhang, R.; Song, M.; Ren, J. Removal of sulfonamide antibiotics and human metabolite by biochar and biochar/H2O2 in synthetic urine. Water Res. 2018, 147, 91–100. [Google Scholar] [CrossRef]
- Liang, J.; Fang, Y.; Luo, Y.; Zeng, G.; Deng, J.; Tan, X.; Tang, N.; Li, X.; He, X.; Feng, C.; et al. Magnetic nanoferromanganese oxides modified biochar derived from pine sawdust for adsorption of tetracycline hydrochloride. Environ. Sci. Pollut. Res. Int. 2019, 26, 5892–5903. [Google Scholar] [CrossRef]
- Vithanage, M.; Ashiq, A.; Ramanayaka, S.; Bhatnagar, A. Implications of layered double hydroxides assembled biochar composite in adsorptive removal of contaminants: Current status and future perspectives. Sci. Total Environ. 2020, 737, 139718. [Google Scholar] [CrossRef]
- Qiu, M.; Hu, B.; Chen, Z.; Yang, H.; Zhuang, L.; Wang, X. Challenges of organic pollutant photocatalysis by biochar-based catalysts. Biochar 2021, 3, 117–123. [Google Scholar] [CrossRef]
- Fan, G.; Li, F.; Evans, D.G.; Duan, X. Catalytic applications of layered double hydroxides: Recent advances and perspectives. Chem. Soc. Rev. 2014, 43, 7040–7066. [Google Scholar] [CrossRef]
- Jawad, A.; Peng, L.; Liao, Z.; Zhou, Z.; Shahzad, A.; Ifthikar, J.; Zhao, M.; Chen, Z.; Chen, Z. Selective removal of heavy metals by hydrotalcites as adsorbents in diverse wastewater: Different intercalated anions with different mechanisms. J. Clean. Prod. 2019, 211, 1112–1126. [Google Scholar] [CrossRef]
- Yang, Y.; Yan, X.; Hu, X.; Feng, R.; Zhou, M. In-situ growth of ZIF-8 on layered double hydroxide: Effect of Zn/Al molar ratios on their structural, morphological and adsorption properties. J. Colloid Interface Sci. 2017, 505, 206–212. [Google Scholar] [CrossRef]
- Mohapatra, L.; Parida, K. A Review on Recent Progress, Challenges and Perspective of Layered Double Hydroxides as Promising Photocatalysts. J. Mater. Chem. A 2016, 4, 10744–10766. [Google Scholar] [CrossRef]
- Liu, R.; Wang, H.; Han, L.; Hu, B.; Qiu, M. Reductive and adsorptive elimination of U(VI) ions in aqueous solution by SFeS@Biochar composites. Environ. Sci. Pollut. Res. Int. 2021, 28, 55176–55185. [Google Scholar] [CrossRef] [PubMed]
- Malik, A. Environmental challenge vis a vis opportunity: The case of water hyacinth. Environ. Int. 2007, 33, 122–138. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Xing, Y.; Ren, A.; Ma, D.; Li, Y.; Hu, S. Study on adsorption properties of water hyacinth-derived biochar for uranium (VI). J. Radioanal. Nucl. Chem. 2020, 324, 1317–1327. [Google Scholar] [CrossRef]
- Karouach, F.; Ben Bakrim, W.; Ezzariai, A.; Sobeh, M.; Kibret, M.; Yasri, A.; Hafidi, M.; Kouisni, L. A Comprehensive Evaluation of the Existing Approaches for Controlling and Managing the Proliferation of Water Hyacinth (Eichhornia crassipes): Review. Front. Environ. Sci. 2022, 9, 767871. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, X.; Yin, D.; Peng, B.; Tan, C.; Liu, Y.; Tan, X.; Wu, S. Efficiency and mechanisms of Cd removal from aqueous solution by biochar derived from water hyacinth (Eichornia crassipes). J. Environ. Manag. 2015, 153, 68–73. [Google Scholar] [CrossRef]
- Coronado, E.; Martí-Gastaldo, C.; Navarro-Moratalla, E.; Ribera, A. Intercalation of [M(ox)3]3− (M=Cr, Rh) complexes into NiIIFeIII-LDH. Appl. Clay Sci. 2010, 48, 228–234. [Google Scholar] [CrossRef]
- Luo, X.; Huang, Z.; Lin, J.; Li, X.; Qiu, J.; Liu, J.; Mao, X. Hydrothermal carbonization of sewage sludge and in-situ preparation of hydrochar/MgAl-layered double hydroxides composites for adsorption of Pb(II). J. Clean. Prod. 2020, 258, 120991. [Google Scholar] [CrossRef]
- Silva, T.L.; Ronix, A.; Pezoti, O.; Souza, L.S.; Leandro, P.K.T.; Bedin, K.C.; Beltrame, K.K.; Cazetta, A.L.; Almeida, V.C. Mesoporous activated carbon from industrial laundry sewage sludge: Adsorption studies of reactive dye Remazol Brilliant Blue R. Chem. Eng. J. 2016, 303, 467–476. [Google Scholar] [CrossRef]
- Zhu, S.; Khan, M.; Wang, F.; Bano, Z.; Xia, M. Rapid removal of toxic metals Cu2+ and Pb2+ by amino trimethylene phosphonic acid intercalated layered double hydroxide: A combined experimental and DFT study. Chem. Eng. J. 2020, 392, 123711. [Google Scholar] [CrossRef]
- Wang, C.; Wang, H.; Gu, G. Ultrasound-assisted xanthation of cellulose from lignocellulosic biomass optimized by response surface methodology for Pb(II) sorption. Carbohydr. Polym. 2018, 182, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Amezquita-Garcia, H.J.; Razo-Flores, E.; Cervantes, F.J.; Rangel-Mendez, J.R. Activated carbon fibers as redox mediators for the increased reduction of nitroaromatics. Carbon 2013, 55, 276–284. [Google Scholar] [CrossRef]
- Zięba-Palus, J.; Wesełucha-Birczyńska, A.; Trzcińska, B.; Kowalski, R.; Moskal, P. Analysis of degraded papers by infrared and Raman spectroscopy for forensic purposes. J. Mol. Struct. 2017, 1140, 154–162. [Google Scholar] [CrossRef]
- Lu, Y.; Xie, Q.; Tang, L.; Yu, J.; Wang, J.; Yang, Z.; Fan, C.; Zhang, S. The reduction of nitrobenzene by extracellular electron transfer facilitated by Fe-bearing biochar derived from sewage sludge. J. Hazard. Mater. 2021, 403, 123682. [Google Scholar] [CrossRef]
- Yao, W.; Wang, X.; Liang, Y.; Yu, S.; Gu, P.; Sun, Y.; Xu, C.; Chen, J.; Hayat, T.; Alsaedi, A.; et al. Synthesis of novel flower-like layered double oxides/carbon dots nanocomposites for U(VI) and 241Am(III) efficient removal: Batch and EXAFS studies. Chem. Eng. J. 2018, 332, 775–786. [Google Scholar] [CrossRef]
- Hou, T.; Yan, L.; Li, J.; Yang, Y.; Shan, L.; Meng, X.; Li, X.; Zhao, Y. Adsorption performance and mechanistic study of heavy metals by facile synthesized magnetic layered double oxide/carbon composite from spent adsorbent. Chem. Eng. J. 2020, 384, 123331. [Google Scholar] [CrossRef]
- Qiu, M.; Wang, M.; Zhao, Q.; Hu, B.; Zhu, Y. XANES and EXAFS investigation of uranium incorporation on nZVI in the presence of phosphate. Chemosphere 2018, 201, 764–771. [Google Scholar] [CrossRef]
- Hu, B.; Wang, H.; Liu, R.; Qiu, M. Highly efficient U(VI) capture by amidoxime/carbon nitride composites: Evidence of EXAFS and modeling. Chemosphere 2021, 274, 129743. [Google Scholar] [CrossRef]
- Deng, J.; Liu, Y.; Liu, S.; Zeng, G.; Tan, X.; Huang, B.; Tang, X.; Wang, S.; Hua, Q.; Yan, Z. Competitive adsorption of Pb(II), Cd(II) and Cu(II) onto chitosan-pyromellitic dianhydride modified biochar. J. Colloid Interface Sci. 2017, 506, 355–364. [Google Scholar] [CrossRef]
- Iram, M.; Guo, C.; Guan, Y.; Ishfaq, A.; Liu, H. Adsorption and magnetic removal of neutral red dye from aqueous solution using Fe3O4 hollow nanospheres. J. Hazard. Mater. 2010, 181, 1039–1050. [Google Scholar] [CrossRef]
- Gong, J.; Liu, T.; Wang, X.; Hu, X.; Zhang, L. Efficient removal of heavy metal ions from aqueous systems with the assembly of anisotropic layered double hydroxide nanocrystals@carbon nanosphere. Environ. Sci. Technol. 2011, 45, 6181–6187. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, D.; Kim, J.; Ji, M.K.; Han, Y.S.; Park, Y.T.; Yun, H.S.; Choi, J. As(III) and As(V) removal from the aqueous phase via adsorption onto acid mine drainage sludge (AMDS) alginate beads and goethite alginate beads. J. Hazard. Mater. 2015, 292, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, H.; Wu, L.; Lan, H.; Qu, J. Preparation of amino-Fe(III) functionalized mesoporous silica for synergistic adsorption of tetracycline and copper. Chemosphere 2015, 138, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.; Wang, J. Ear-like poly (acrylic acid)-activated carbon nanocomposite: A highly efficient adsorbent for removal of Cd(II) from aqueous solutions. Chemosphere 2017, 169, 443–449. [Google Scholar] [CrossRef]
- Yu, S.; Zhai, L.; Wang, Y.; Liu, X.; Xu, L.; Cheng, L. Synthesis of magnetic chrysotile nanotubes for adsorption of Pb(II), Cd(II) and Cr(III) ions from aqueous solution. J. Environ. Chem. Eng. 2015, 3, 752–762. [Google Scholar] [CrossRef]
- Yang, G.-X.; Jiang, H. Amino modification of biochar for enhanced adsorption of copper ions from synthetic wastewater. Water Res. 2014, 48, 396–405. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, J.; Zhao, L.; Zhang, S.; Huang, Y.; Wu, X.; Wang, X. Synthesis of amidoxime-functionalized Fe3O4@SiO2 core–shell magnetic microspheres for highly efficient sorption of U(VI). Chem. Eng. J. 2014, 235, 275–283. [Google Scholar] [CrossRef]
- Xu, P.; Zeng, G.M.; Huang, D.L.; Lai, C.; Zhao, M.H.; Wei, Z.; Li, N.J.; Huang, C.; Xie, G.X. Adsorption of Pb(II) by iron oxide nanoparticles immobilized Phanerochaete chrysosporium: Equilibrium, kinetic, thermodynamic and mechanisms analysis. Chem. Eng. J. 2012, 203, 423–431. [Google Scholar] [CrossRef]
- Ngambia, A.; Ifthikar, J.; Shahib, I.I.; Jawad, A.; Shahzad, A.; Zhao, M.; Wang, J.; Chen, Z.; Chen, Z. Adsorptive purification of heavy metal contaminated wastewater with sewage sludge derived carbon-supported Mg(II) composite. Sci. Total Environ. 2019, 691, 306–321. [Google Scholar] [CrossRef]
- Li, B.; Lv, J.Q.; Guo, J.Z.; Fu, S.Y.; Guo, M.; Yang, P. The polyaminocarboxylated modified hydrochar for efficient capturing methylene blue and Cu(II) from water. Bioresour. Technol. 2019, 275, 360–367. [Google Scholar] [CrossRef]
- Zhao, Y.; Geng, J.; Wang, X.; Gu, X.; Gao, S. Tetracycline adsorption on kaolinite: pH, metal cations and humic acid effects. Ecotoxicology 2011, 20, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Zuo, X.; Liu, Z.; Chen, M. Effect of H2O2 concentrations on copper removal using the modified hydrothermal biochar. Bioresour. Technol. 2016, 207, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.F.; Liu, Y.G.; Gu, Y.L.; Xu, Y.; Zeng, G.M.; Hu, X.J.; Liu, S.B.; Wang, X.; Liu, S.M.; Li, J. Biochar-based nano-composites for the decontamination of wastewater: A review. Bioresour. Technol. 2016, 212, 318–333. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Choi, C.L.; Seo, Y.J.; Yeo, S.K.; Choi, J.; Komarneni, S.; Lee, J.H. Reactions of Cu2+ and Pb2+ with Mg/Al layered double hydroxide. Appl. Clay Sci. 2007, 37, 143–148. [Google Scholar] [CrossRef]
- Lyu, F.; Yu, H.; Hou, T.; Yan, L.; Zhang, X.; Du, B. Efficient and fast removal of Pb2+ and Cd2+ from an aqueous solution using a chitosan/Mg-Al-layered double hydroxide nanocomposite. J. Colloid Interface Sci. 2019, 539, 184–193. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, X.; Yuan, B.; Fu, M.L. A facile foaming-polymerization strategy to prepare 3D MnO2 modified biochar-based porous hydrogels for efficient removal of Cd(II) and Pb(II). Chemosphere 2020, 239, 124745. [Google Scholar] [CrossRef]
- Chen, S.; Huang, Y.; Han, X.; Wu, Z.; Lai, C.; Wang, J.; Deng, Q.; Zeng, Z.; Deng, S. Simultaneous and efficient removal of Cr(VI) and methyl orange on LDHs decorated porous carbons. Chem. Eng. J. 2018, 352, 306–315. [Google Scholar] [CrossRef]
- Olanrewaju, J.; Newalkar, B.L.; Mancino, C.; Komarneni, S. Simplified synthesis of nitrate form of layered double hydroxide. Mater. Lett. 2000, 45, 307–310. [Google Scholar] [CrossRef]
- Xu, S.; Chen, J.; Peng, H.; Leng, S.; Li, H.; Qu, W.; Hu, Y.; Li, H.; Jiang, S.; Zhou, W.; et al. Effect of biomass type and pyrolysis temperature on nitrogen in biochar, and the comparison with hydrochar. Fuel 2021, 291, 120128. [Google Scholar] [CrossRef]
- Jiang, J.-Q.; Yang, C.-X.; Yan, X.-P. Zeolitic Imidazolate Framework-8 for Fast Adsorption and Removal of Benzotriazoles from Aqueous Solution. ACS Appl. Mater. Interfaces 2013, 5, 9837–9842. [Google Scholar] [CrossRef]
- Andrew Lin, K.-Y.; Lee, W.-D. Self-assembled magnetic graphene supported ZIF-67 as a recoverable and efficient adsorbent for benzotriazole. Chem. Eng. J. 2016, 284, 1017–1027. [Google Scholar] [CrossRef]
- Huang, Q.; Chen, Y.; Yu, H.; Yan, L.; Zhang, J.; Wang, B.; Du, B.; Xing, L. Magnetic graphene oxide/MgAl-layered double hydroxide nanocomposite: One-pot solvothermal synthesis, adsorption performance and mechanisms for Pb2+, Cd2+, and Cu2+. Chem. Eng. J. 2018, 341, 1–9. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, F.; Li, H.; Li, Y.; Liu, Y.; Chen, Y.; Li, M.; Li, L.; Jiang, H.; Chen, L. Simple hydrothermal synthesis of magnetic MnFe2O4-sludge biochar composites for removal of aqueous Pb2+. J. Anal. Appl. Pyrolysis 2021, 156, 105173. [Google Scholar] [CrossRef]




| Pollutants | Pseudo-First-Order | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|---|
| K1 (min−1) | qe (mg·g−1) | R2 | K2 (g·(mg·min)−1) | qe (mg·g−1) | R2 | |
| BTA | 0.0087 | 107.16 | 0.9711 | 0.0003 | 115.32 | 0.9989 |
| Pb2+ | 0.0091 | 102.35 | 0.8962 | 0.0001 | 109.02 | 0.9674 |
| Pollutants | T (°C) | Freundlich | Langmuir | ||||
|---|---|---|---|---|---|---|---|
| KF (L·mg−1) | 1/n | R2 | KL (L·mg−1) | qm (mg·g−1) | R2 | ||
| BTA | 25 | 38.91 | 0.323 | 0.8941 | 0.0352 | 248.44 | 0.9884 |
| Pb2+ | 25 | 35.76 | 0.324 | 0.9318 | 0.3321 | 227.13 | 0.9785 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bian, P.; Shao, Q. Performance and Mechanism of Functionalized Water Hyacinth Biochar for Adsorption and Removal of Benzotriazole and Lead in Water. Int. J. Mol. Sci. 2023, 24, 8936. https://doi.org/10.3390/ijms24108936
Bian P, Shao Q. Performance and Mechanism of Functionalized Water Hyacinth Biochar for Adsorption and Removal of Benzotriazole and Lead in Water. International Journal of Molecular Sciences. 2023; 24(10):8936. https://doi.org/10.3390/ijms24108936
Chicago/Turabian StyleBian, Pengyang, and Qinqin Shao. 2023. "Performance and Mechanism of Functionalized Water Hyacinth Biochar for Adsorption and Removal of Benzotriazole and Lead in Water" International Journal of Molecular Sciences 24, no. 10: 8936. https://doi.org/10.3390/ijms24108936
APA StyleBian, P., & Shao, Q. (2023). Performance and Mechanism of Functionalized Water Hyacinth Biochar for Adsorption and Removal of Benzotriazole and Lead in Water. International Journal of Molecular Sciences, 24(10), 8936. https://doi.org/10.3390/ijms24108936