Mechanical Properties of Graphene Networks under Compression: A Molecular Dynamics Simulation
Abstract
1. Introduction
2. Results and Discussion
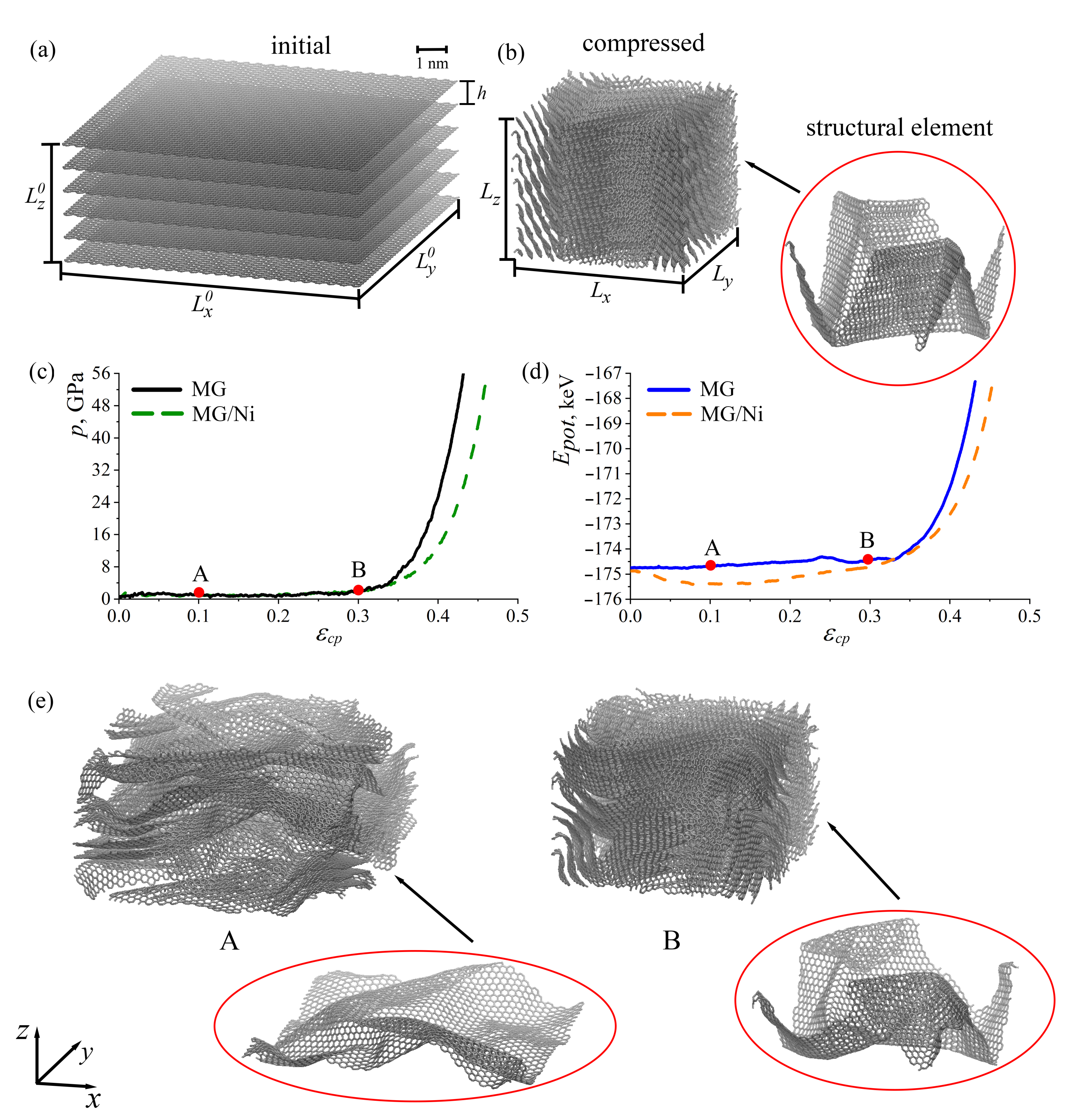
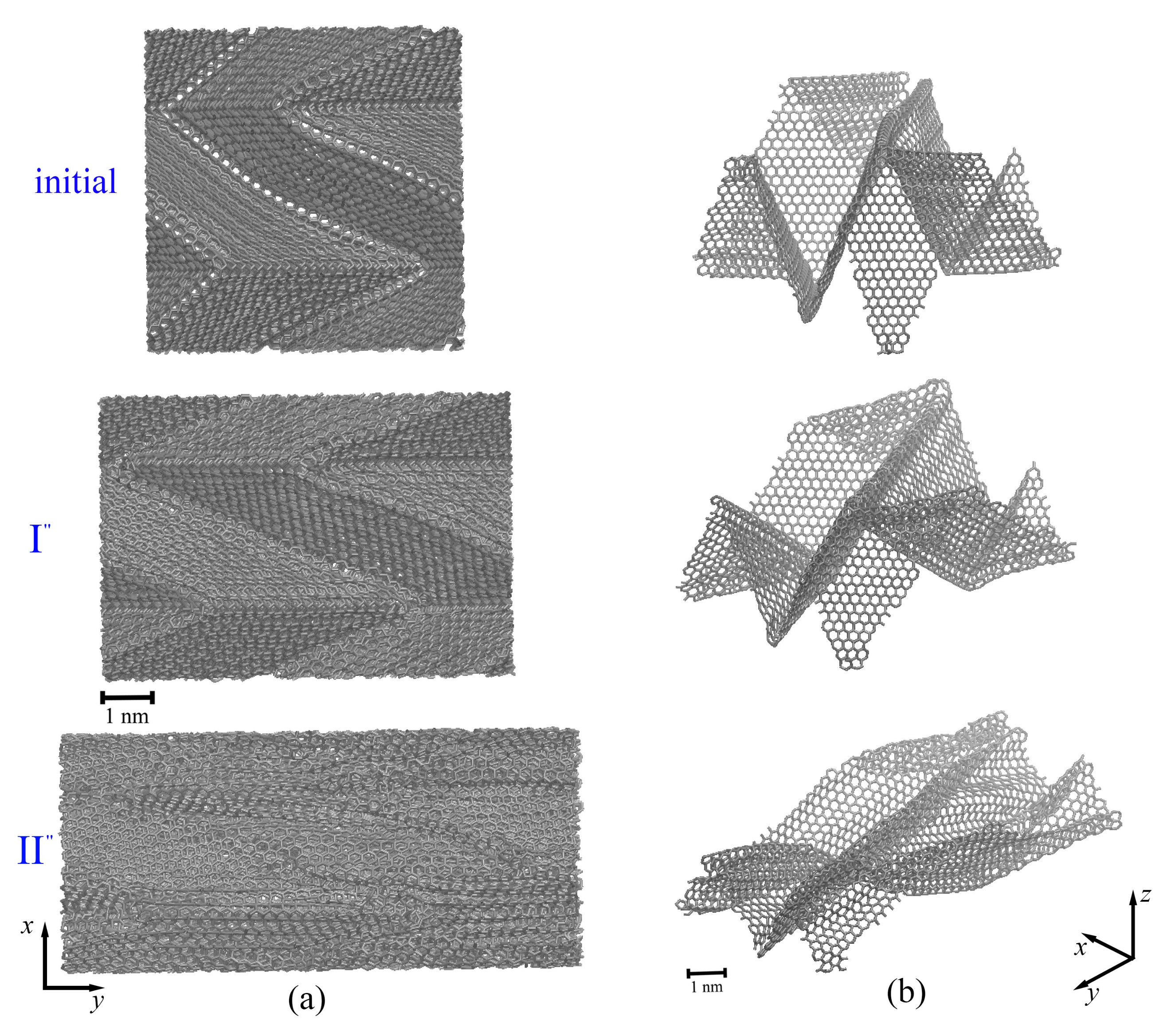
2.1. Multilayer Graphene under Compression
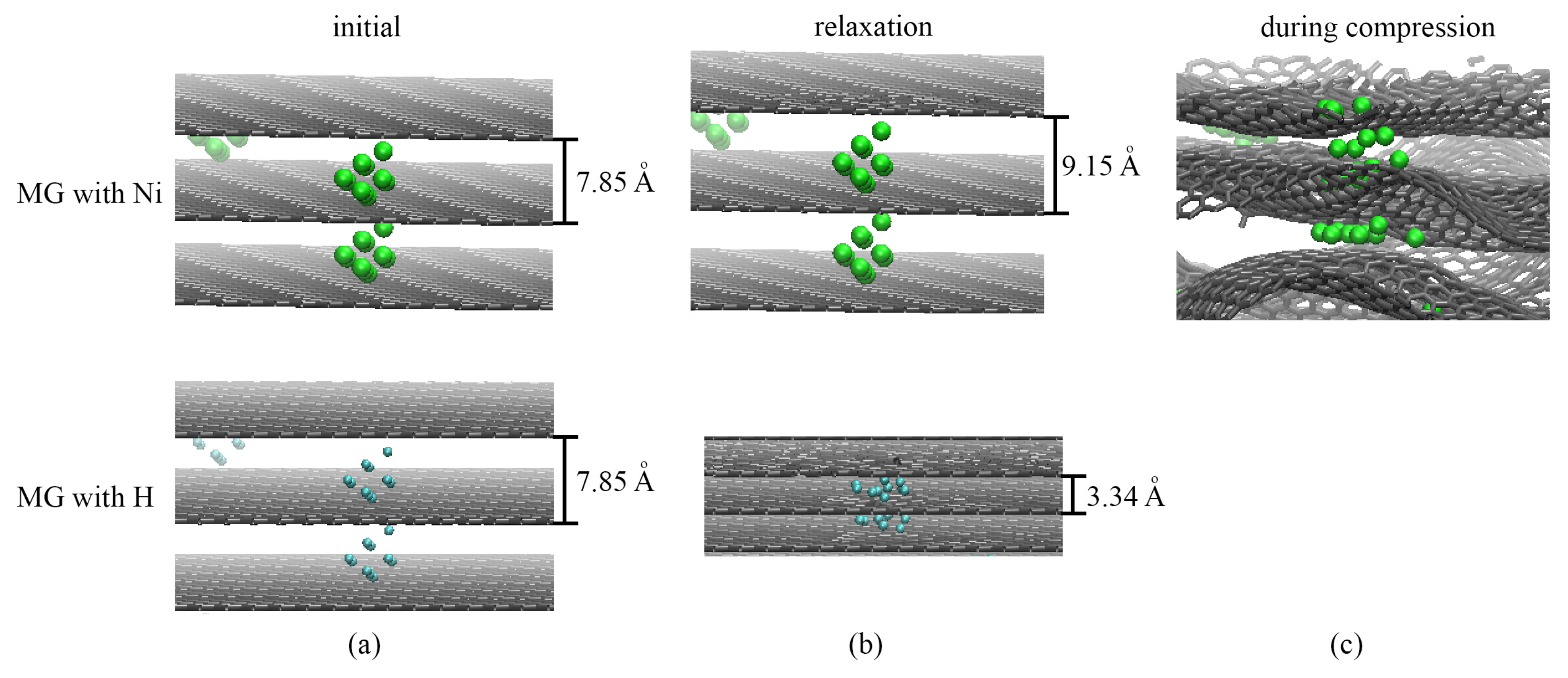
2.2. Multilayer Graphene with Ni and H Nanoclusters
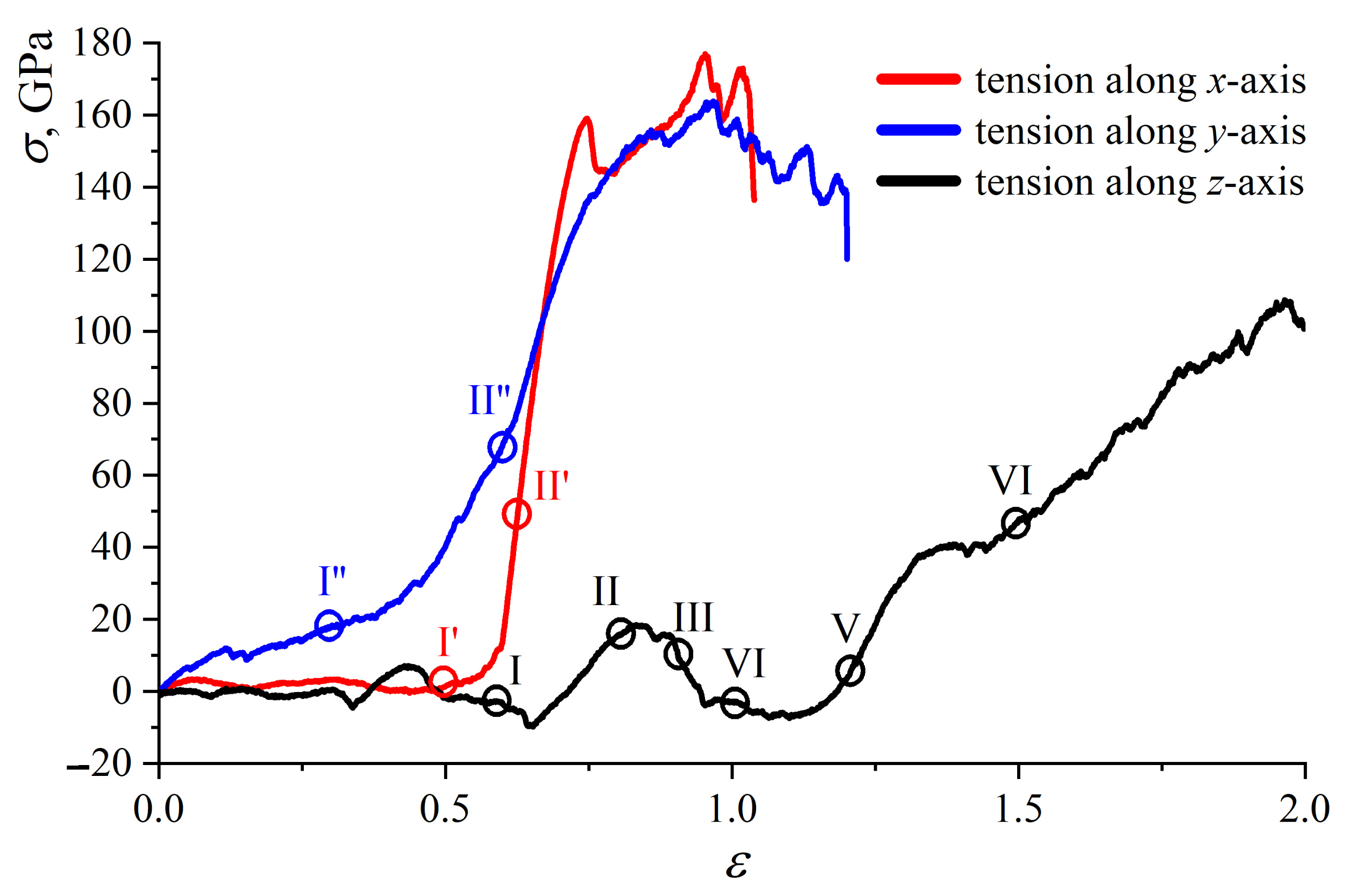
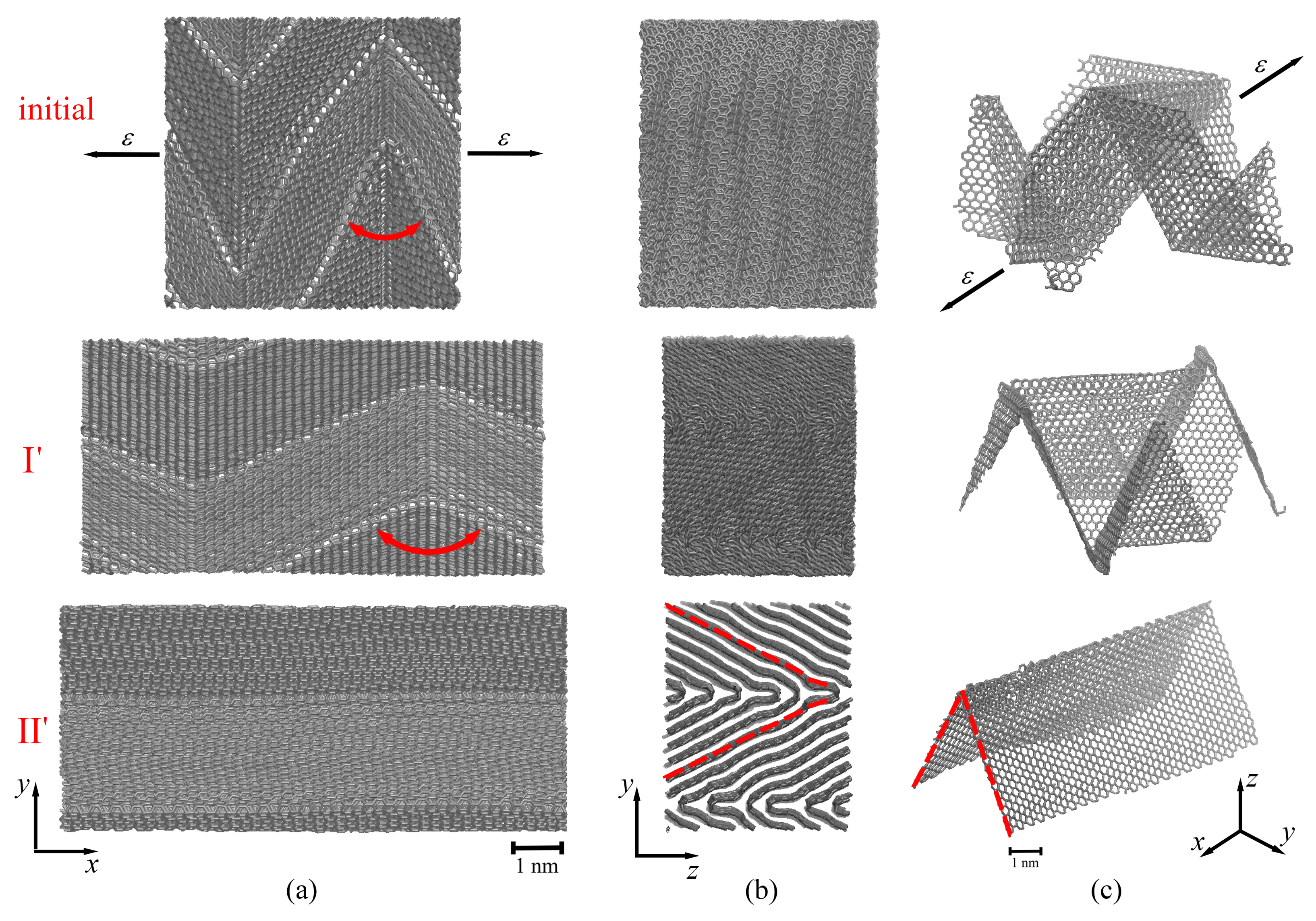
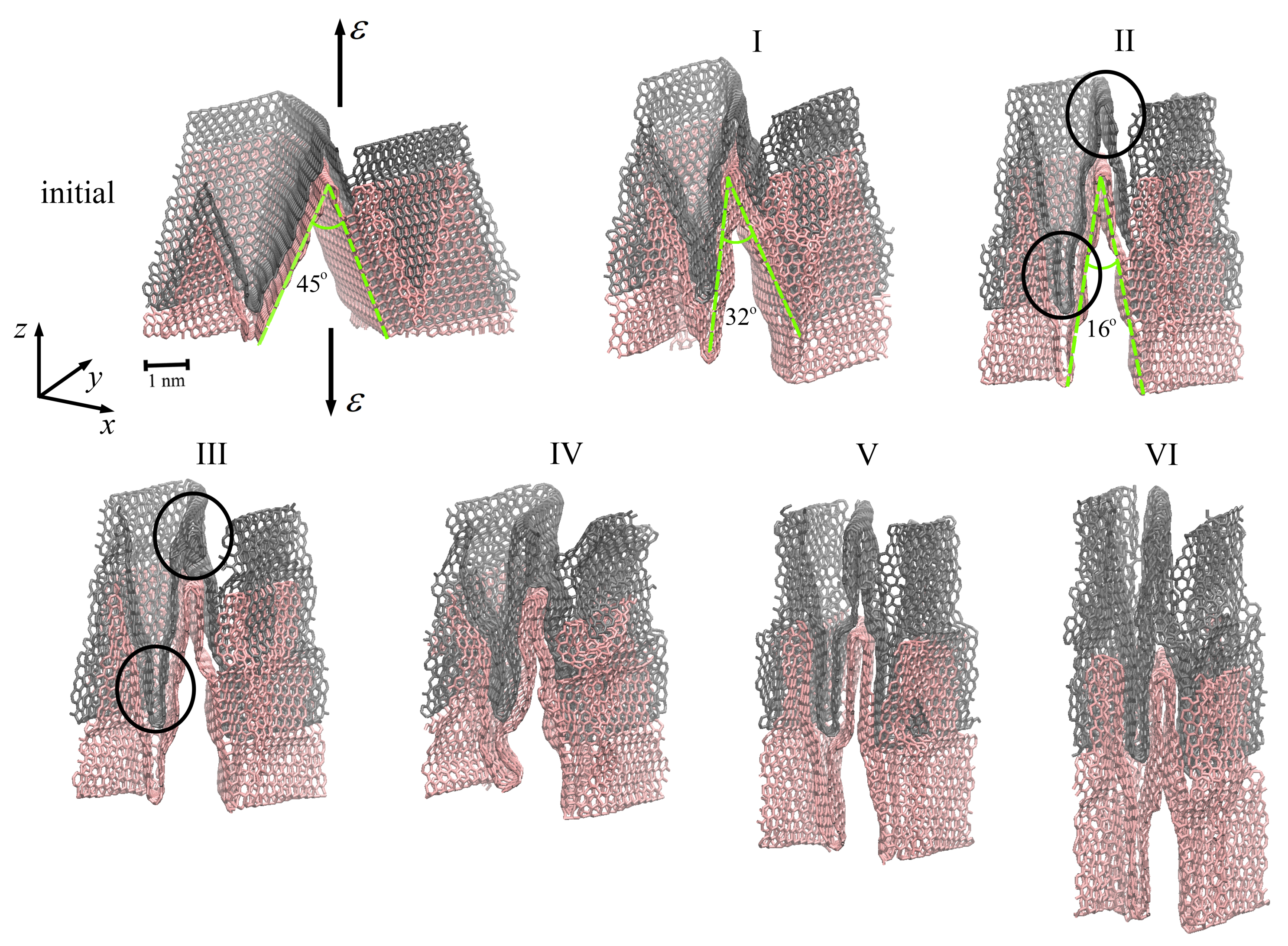
2.3. Mechanical Behavior of Multilayer Graphene under Uniaxial Tension
3. Methods and Materials
4. Conclusions
- (i)
- Multilayer graphene shows good mechanical properties under compressive and tensile strain, which can be explained by the strong interaction induced by the crumpling of graphene layers and entangled microstructure.
- (ii)
- One of the main deformation mechanisms is the slippage of graphene layers during tension.
- (iii)
- Two competitive mechanisms can be distinguished both for compression and tension: the crumpling of graphene layers, which increases the stresses, and the sliding of graphene layers through the surface-to-surface connection, which lowers the deformation stresses.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, B.; Zhou, K. Recent progress on graphene-analogous 2D nanomaterials: Properties, modeling and applications. Prog. Mater. Sci. 2019, 100, 99–169. [Google Scholar] [CrossRef]
- Khadem, A.H.; Hasan, T.U.; Rahman, A.M.; Smriti, S.A.; Alimuzzaman, S. Fabrication, properties, and performance of graphene-based textile fabrics for supercapacitor applications: A review. J. Energy Storage 2022, 56, 105988. [Google Scholar] [CrossRef]
- Zhang, T.; Gao, X.; Li, J.; Xiao, L.; Gao, H.; Zhao, F.; Ma, H. Progress on the application of graphene-based composites toward energetic materials: A review. Def. Technol. 2023, in press. [Google Scholar] [CrossRef]
- Kamboj, S.; Thakur, A. Applications of Graphene-Based Composites—A Review. Mater. Today Proc. 2023, in press. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Tung, V.C.; Allen, M.J.; Yang, Y.; Kaner, R.B. High-throughput solution processing of large-scale graphene. Nat. Nanotechnol. 2008, 4, 25–29. [Google Scholar] [CrossRef]
- Charrier, A.; Coati, A.; Argunova, T.; Thibaudau, F.; Garreau, Y.; Pinchaux, R.; Forbeaux, I.; Debever, J.M.; Sauvage-Simkin, M.; Themlin, J.M. Solid-state decomposition of silicon carbide for growing ultra-thin heteroepitaxial graphite films. J. Appl. Phys. 2002, 92, 2479–2484. [Google Scholar] [CrossRef]
- Ono, R.; Hosoda, M.; Okuzawa, M.; Tagawa, M.; Oshima, C.; Otani, S. Electronic States of Monolayer Graphene on Pt (755) and TiC (755). TANSO 2000, 2000, 400–404. [Google Scholar] [CrossRef]
- Tanaka, T.; Itoh, A.; Yamashita, K.; Rokuta, E.; Oshima, C. Heteroepitaxial System of h-BN/Monolayer Graphene on Ni(111). Surf. Rev. Lett. 2003, 10, 697–703. [Google Scholar] [CrossRef]
- Coraux, J.; N‘Diaye, A.T.; Busse, C.; Michely, T. Structural Coherency of Graphene on Ir(111). Nano Lett. 2008, 8, 565–570. [Google Scholar] [CrossRef]
- Yang, X.; Dou, X.; Rouhanipour, A.; Zhi, L.; Räder, H.J.; Müllen, K. Two-Dimensional Graphene Nanoribbons. J. Am. Chem. Soc. 2008, 130, 4216–4217. [Google Scholar] [CrossRef]
- Bae, S.; Kim, H.; Lee, Y.; Xu, X.; Park, J.S.; Zheng, Y.; Balakrishnan, J.; Lei, T.; Kim, H.R.; Song, Y.I.; et al. Roll-to-roll production of 30-inch graphene films for transparent electrodes. Nat. Nanotechnol. 2010, 5, 574–578. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef]
- Bahri, M.; Gebre, S.H.; Elaguech, M.A.; Dajan, F.T.; Sendeku, M.G.; Tlili, C.; Wang, D. Recent advances in chemical vapour deposition techniques for graphene-based nanoarchitectures: From synthesis to contemporary applications. Coord. Chem. Rev. 2023, 475, 214910. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, Q.; Yu, Y.; Chen, W.; Hu, H.; Li, H. Naturally Dried Graphene Aerogels with Superelasticity and Tunable Poisson’s Ratio. Adv. Mater. 2016, 28, 9223–9230. [Google Scholar] [CrossRef]
- Liu, H.; Cao, Z.; Luo, H.; Shi, J.; Yao, G. Performance of closed-cell aluminum foams subjected to impact loading. Mater. Sci. Eng. A 2013, 570, 27–31. [Google Scholar] [CrossRef]
- Baimova, J.A.; Rysaeva, L.K.; Liu, B.; Dmitriev, S.V.; Zhou, K. From flat graphene to bulk carbon nanostructures. Phys. Status Solidi B 2015, 252, 1502–1507. [Google Scholar] [CrossRef]
- Baimova, J.A.; Liu, B.; Dmitriev, S.V.; Zhou, K. Mechanical properties of crumpled graphene under hydrostatic and uniaxial compression. J. Phys. D Appl. Phys. 2015, 48, 095302. [Google Scholar] [CrossRef]
- Dimitrakakis, G.K.; Tylianakis, E.; Froudakis, G.E. Pillared Graphene: A New 3-D Network Nanostructure for Enhanced Hydrogen Storage. Nano Lett. 2008, 8, 3166–3170. [Google Scholar] [CrossRef]
- Xie, Q.; Qu, S.; Zhang, Y.; Zhao, P. Nitrogen-enriched graphene-like carbon architecture with tunable porosity derived from coffee ground as high performance anodes for lithium ion batteries. Appl. Surf. Sci. 2021, 537, 148092. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.; Huang, L.; Li, C.; Hong, J.D.; Shi, G. Highly Compressible Macroporous Graphene Monoliths via an Improved Hydrothermal Process. Adv. Mater. 2014, 26, 4789–4793. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Jung, G.S.; Kang, M.J.; Buehler, M.J. The mechanics and design of a lightweight three-dimensional graphene assembly. Sci. Adv. 2017, 3, e1601536. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Wang, R.; Wang, Y.; Cao, Y.; Shen, Y.; Huang, Y.; Chen, Y. Recent advances in graphene aerogels as absorption-dominated electromagnetic interference shielding materials. Carbon 2023, 205, 112–137. [Google Scholar] [CrossRef]
- Worsley, M.A.; Pauzauskie, P.J.; Olson, T.Y.; Biener, J.; Satcher, J.H.; Baumann, T.F. Synthesis of Graphene Aerogel with High Electrical Conductivity. J. Am. Chem. Soc. 2010, 132, 14067–14069. [Google Scholar] [CrossRef] [PubMed]
- Zu, G.; Kanamori, K.; Nakanishi, K.; Lu, X.; Yu, K.; Huang, J.; Sugimura, H. Superelastic Multifunctional Aminosilane-Crosslinked Graphene Aerogels for High Thermal Insulation, Three-Component Separation, and Strain/Pressure-Sensing Arrays. ACS Appl. Mater. Interfaces 2019, 11, 43533–43542. [Google Scholar] [CrossRef]
- Wan, Y.J.; Zhu, P.L.; Yu, S.H.; Sun, R.; Wong, C.P.; Liao, W.H. Ultralight, super-elastic and volume-preserving cellulose fiber/graphene aerogel for high-performance electromagnetic interference shielding. Carbon 2017, 115, 629–639. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, R.; Gu, J.; Liu, H.; Liu, C.; Luo, C.; Kong, J.; Shao, Q.; Wang, N.; Guo, Z.; et al. Ultralight, highly compressible and fire-retardant graphene aerogel with self-adjustable electromagnetic wave absorption. Carbon 2018, 139, 1126–1135. [Google Scholar] [CrossRef]
- Xie, J.; Niu, L.; Qiao, Y.; Chen, P.; Rittel, D. Impact energy absorption behavior of graphene aerogels prepared by different drying methods. Mater. Des. 2022, 221, 110912. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, J.; Sang, X.; Liu, C.; Luo, T.; Peng, L.; Han, B.; Tan, X.; Ma, X.; Wang, D.; et al. Cellular graphene aerogel combines ultralow weight and high mechanical strength: A highly efficient reactor for catalytic hydrogenation. Sci. Rep. 2016, 6, srep25830. [Google Scholar] [CrossRef]
- Li, L.; Gao, G.; Zheng, J.; Shi, X.; Liu, Z. Three-dimensional graphene aerogel supported Ir nanocomposite as a highly efficient catalyst for chemoselective cinnamaldehyde hydrogenation. Diam. Relat. Mater. 2019, 91, 272–282. [Google Scholar] [CrossRef]
- Apkadirova, N.; Krylova, K.; Baimova, J. Effect of external pressure on the hydrogen storage capacity of a graphene flake: Molecular dynamics. Lett. Mater. 2022, 12, 445–450. [Google Scholar] [CrossRef]
- Nassar, G.; Daou, E.; Najjar, R.; Bassil, M.; Habchi, R. A review on the current research on graphene-based aerogels and their applications. Carbon Trends 2021, 4, 100065. [Google Scholar] [CrossRef]
- Lobzenko, I.P.; Chechin, G.M.; Dmitriev, S.V. Comparison of Classical Molecular Dynamics and Ab Initio Modeling of Discrete Breathers in Graphene. Solid State Phenom. 2016, 258, 81–84. [Google Scholar] [CrossRef]
- Dmitriev, S.; Kashchenko, M.; Baimova, J.; Babicheva, R.; Gunderov, D.; Pushin, V. Molecular dynamics simulation of the effect of dislocations on the martensitic transformations in a two-dimensional model. Lett. Mater. 2017, 7, 442–446. [Google Scholar] [CrossRef]
- Rozhkov, M.; Abramenko, N.; Kolesnikova, A.; Romanov, A. Zero misorientation interfaces in graphene. Lett. Mater. 2020, 10, 551–557. [Google Scholar] [CrossRef]
- Shi, J.; Yu, W.; Hu, C.; Duan, H.; Ji, J.; Kang, Y.; Cai, K. Effects of Tearing Conditions on the Crack Propagation in a Monolayer Graphene Sheet. Int. J. Mol. Sci. 2022, 23, 6471. [Google Scholar] [CrossRef]
- Poletaev, G.; Bebikhov, Y.; Semenov, A.; Rakitin, R. Interaction of an edge dislocation with a 〈110〉 tilt boundary in nickel: Molecular dynamics simulation. Lett. Mater. 2022, 12, 303–308. [Google Scholar] [CrossRef]
- Pedrielli, A.; Dapor, M.; Gkagkas, K.; Taioli, S.; Pugno, N.M. Mechanical Properties of Twisted Carbon Nanotube Bundles with Carbon Linkers from Molecular Dynamics Simulations. Int. J. Mol. Sci. 2023, 24, 2473. [Google Scholar] [CrossRef]
- Fan, Z.; Gong, F.; Nguyen, S.T.; Duong, H.M. Advanced multifunctional graphene aerogel—Poly(methyl methacrylate) composites: Experiments and modeling. Carbon 2015, 81, 396–404. [Google Scholar] [CrossRef]
- Lei, J.; Liu, Z. The structural and mechanical properties of graphene aerogels based on Schwarz-surface-like graphene models. Carbon 2018, 130, 741–748. [Google Scholar] [CrossRef]
- Bao, Q.; Yang, Z.; Lu, Z.; He, X. Effects of graphene thickness and length distribution on the mechanical properties of graphene networks: A coarse-grained molecular dynamics simulation. Appl. Surf. Sci. 2021, 570, 151023. [Google Scholar] [CrossRef]
- Moreno-Castilla, C.; Maldonado-Hódar, F. Carbon aerogels for catalysis applications: An overview. Carbon 2005, 43, 455–465. [Google Scholar] [CrossRef]
- Zhang, X.; Sui, Z.; Xu, B.; Yue, S.; Luo, Y.; Zhan, W.; Liu, B. Mechanically strong and highly conductive graphene aerogel and its use as electrodes for electrochemical power sources. J. Mater. Chem. 2011, 21, 6494. [Google Scholar] [CrossRef]
- Hu, H.; Zhao, Z.; Wan, W.; Gogotsi, Y.; Qiu, J. Ultralight and Highly Compressible Graphene Aerogels. Adv. Mater. 2013, 25, 2219–2223. [Google Scholar] [CrossRef]
- Zhu, C.; Han, T.Y.J.; Duoss, E.B.; Golobic, A.M.; Kuntz, J.D.; Spadaccini, C.M.; Worsley, M.A. Highly compressible 3D periodic graphene aerogel microlattices. Nat. Commun. 2015, 6, 6962. [Google Scholar] [CrossRef]
- Wu, Y.; Yi, N.; Huang, L.; Zhang, T.; Fang, S.; Chang, H.; Li, N.; Oh, J.; Lee, J.A.; Kozlov, M.; et al. Three-dimensionally bonded spongy graphene material with super compressive elasticity and near-zero Poisson’s ratio. Nat. Commun. 2015, 6, 6141. [Google Scholar] [CrossRef]
- Tang, D.M.; Ren, C.L.; Zhang, L.; Tao, Y.; Zhang, P.; Lv, W.; Jia, X.L.; Jiang, X.; Zhou, G.; Ohmura, T.; et al. Size Effects on the Mechanical Properties of Nanoporous Graphene Networks. Adv. Funct. Mater. 2019, 29, 1900311. [Google Scholar] [CrossRef]
- Qiu, L.; Huang, B.; He, Z.; Wang, Y.; Tian, Z.; Liu, J.Z.; Wang, K.; Song, J.; Gengenbach, T.R.; Li, D. Extremely Low Density and Super-Compressible Graphene Cellular Materials. Adv. Mater. 2017, 29, 1701553. [Google Scholar] [CrossRef]
- Lobkovsky, A.; Gentges, S.; Li, H.; Morse, D.; Witten, T.A. Scaling Properties of Stretching Ridges in a Crumpled Elastic Sheet. Science 1995, 270, 1482–1485. [Google Scholar] [CrossRef]
- Tallinen, T.; Åström, J.A.; Timonen, J. The effect of plasticity in crumpling of thin sheets. Nat. Mater. 2008, 8, 25–29. [Google Scholar] [CrossRef]
- Matan, K.; Williams, R.B.; Witten, T.A.; Nagel, S.R. Crumpling a Thin Sheet. Phys. Rev. Lett. 2002, 88, 076101. [Google Scholar] [CrossRef] [PubMed]
- Baimova, J.A.; Liu, B.; Dmitriev, S.V.; Srikanth, N.; Zhou, K. Mechanical properties of bulk carbon nanostructures: Effect of loading and temperature. Phys. Chem. Chem. Phys. 2014, 16, 1950. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Chen, Y. Three-dimensional graphene networks: Synthesis, properties and applications. Natl. Sci. Rev. 2014, 2, 40–53. [Google Scholar] [CrossRef]
- Nieto, A.; Boesl, B.; Agarwal, A. Multi-scale intrinsic deformation mechanisms of 3D graphene foam. Carbon 2015, 85, 299–308. [Google Scholar] [CrossRef]
- Pan, D.; Wang, C.; Wang, T.C.; Yao, Y. Graphene Foam: Uniaxial Tension Behavior and Fracture Mode Based on a Mesoscopic Model. ACS Nano 2017, 11, 8988–8997. [Google Scholar] [CrossRef]
- Pedrielli, A.; Taioli, S.; Garberoglio, G.; Pugno, N.M. Mechanical and thermal properties of graphene random nanofoams via Molecular Dynamics simulations. Carbon 2018, 132, 766–775. [Google Scholar] [CrossRef]
- Xu, Z.; Gao, C. Graphene chiral liquid crystals and macroscopic assembled fibres. Nat. Commun. 2011, 2, 571. [Google Scholar] [CrossRef]
- Parvin, N.; Kumar, V.; Joo, S.W.; Park, S.S.; Mandal, T.K. Recent Advances in the Characterized Identification of Mono-to-Multi-Layer Graphene and Its Biomedical Applications: A Review. Electronics 2022, 11, 3345. [Google Scholar] [CrossRef]
- Dai, X.; Mitchell, I.; Kim, S.; An, H.; Ding, F. Multilayer graphene sunk growth on Cu(111) surface. Carbon 2022, 199, 233–240. [Google Scholar] [CrossRef]
- Yoon, S.M.; Choi, W.M.; Baik, H.; Shin, H.J.; Song, I.; Kwon, M.S.; Bae, J.J.; Kim, H.; Lee, Y.H.; Choi, J.Y. Synthesis of Multilayer Graphene Balls by Carbon Segregation from Nickel Nanoparticles. ACS Nano 2012, 6, 6803–6811. [Google Scholar] [CrossRef]
- Ai, L.; Tian, T.; Jiang, J. Ultrathin Graphene Layers Encapsulating Nickel Nanoparticles Derived Metal–Organic Frameworks for Highly Efficient Electrocatalytic Hydrogen and Oxygen Evolution Reactions. ACS Sustain. Chem. Eng. 2017, 5, 4771–4777. [Google Scholar] [CrossRef]
- Ji, Z.; Wang, Y.; Yu, Q.; Shen, X.; Li, N.; Ma, H.; Yang, J.; Wang, J. One-step thermal synthesis of nickel nanoparticles modified graphene sheets for enzymeless glucose detection. J. Colloid Interface Sci. 2017, 506, 678–684. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, J.; Yeom, M.S.; Shin, J.W.; Kim, H.; Cui, Y.; Kysar, J.W.; Hone, J.; Jung, Y.; Jeon, S.; et al. Strengthening effect of single-atomic-layer graphene in metal–graphene nanolayered composites. Nat. Commun. 2013, 4, 2114. [Google Scholar] [CrossRef]
- Kuang, D.; Xu, L.; Liu, L.; Hu, W.; Wu, Y. Graphene–nickel composites. Appl. Surf. Sci. 2013, 273, 484–490. [Google Scholar] [CrossRef]
- Safina, L.L.; Baimova, J.A. Molecular dynamics simulation of fabrication of Ni-graphene composite: Temperature effect. Micro Nano Lett. 2020, 15, 176–180. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, M.; Du, J.; Zhang, W.; Zhou, S.; Ren, W.; Zhou, Q.; Shi, L. Construction of graphene network in Ni matrix composites: A molecular dynamics study of densification process. Carbon 2022, 191, 55–66. [Google Scholar] [CrossRef]
- Li, X.; Cai, W.; Colombo, L.; Ruoff, R.S. Evolution of Graphene Growth on Ni and Cu by Carbon Isotope Labeling. Nano Lett. 2009, 9, 4268–4272. [Google Scholar] [CrossRef]
- Chae, S.J.; Günes, F.; Kim, K.K.; Kim, E.S.; Han, G.H.; Kim, S.M.; Shin, H.J.; Yoon, S.M.; Choi, J.Y.; Park, M.H.; et al. Synthesis of Large-Area Graphene Layers on Poly-Nickel Substrate by Chemical Vapor Deposition: Wrinkle Formation. Adv. Mater. 2009, 21, 2328–2333. [Google Scholar] [CrossRef]
- Reina, A.; Thiele, S.; Jia, X.; Bhaviripudi, S.; Dresselhaus, M.S.; Schaefer, J.A.; Kong, J. Growth of large-area single- and Bi-layer graphene by controlled carbon precipitation on polycrystalline Ni surfaces. Nano Res. 2009, 2, 509–516. [Google Scholar] [CrossRef]
- Stuart, S.J.; Tutein, A.B.; Harrison, J.A. A reactive potential for hydrocarbons with intermolecular interactions. J. Chem. Phys. 2000, 112, 6472–6486. [Google Scholar] [CrossRef]
- Girifalco, L.A.; Weizer, V.G. Application of the Morse Potential Function to Cubic Metals. Phys. Rev. 1959, 114, 687–690. [Google Scholar] [CrossRef]
- Katin, K.P.; Prudkovskiy, V.S.; Maslov, M.M. Molecular dynamics simulation of nickel-coated graphene bending. Micro Nano Lett. 2018, 13, 160–164. [Google Scholar] [CrossRef]
- Galashev, A.Y.; Katin, K.P.; Maslov, M.M. Morse parameters for the interaction of metals with graphene and silicene. Phys. Lett. A 2019, 383, 252–258. [Google Scholar] [CrossRef]
- Safina, L.R.; Krylova, K.A.; Baimova, J.A. Molecular dynamics study of the mechanical properties and deformation behavior of graphene/metal composites. Mater. Today Phys. 2022, 28, 100851. [Google Scholar] [CrossRef]
- Zhou, K.; Liu, B. Application of molecular dynamics simulation in mechanical problems. In Molecular Dynamics Simulation; Elsevier: Amsterdam, The Netherlands, 2022; pp. 129–181. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polyakova, P.V.; Baimova, J.A. Mechanical Properties of Graphene Networks under Compression: A Molecular Dynamics Simulation. Int. J. Mol. Sci. 2023, 24, 6691. https://doi.org/10.3390/ijms24076691
Polyakova PV, Baimova JA. Mechanical Properties of Graphene Networks under Compression: A Molecular Dynamics Simulation. International Journal of Molecular Sciences. 2023; 24(7):6691. https://doi.org/10.3390/ijms24076691
Chicago/Turabian StylePolyakova, Polina V., and Julia A. Baimova. 2023. "Mechanical Properties of Graphene Networks under Compression: A Molecular Dynamics Simulation" International Journal of Molecular Sciences 24, no. 7: 6691. https://doi.org/10.3390/ijms24076691
APA StylePolyakova, P. V., & Baimova, J. A. (2023). Mechanical Properties of Graphene Networks under Compression: A Molecular Dynamics Simulation. International Journal of Molecular Sciences, 24(7), 6691. https://doi.org/10.3390/ijms24076691






