Gonadotropins and Sex Steroid Hormones in Captive-Reared Small Yellow Croaker (Larimichthys polyactis) and Their Role in Female Reproductive Dysfunction
Abstract
1. Introduction
2. Results
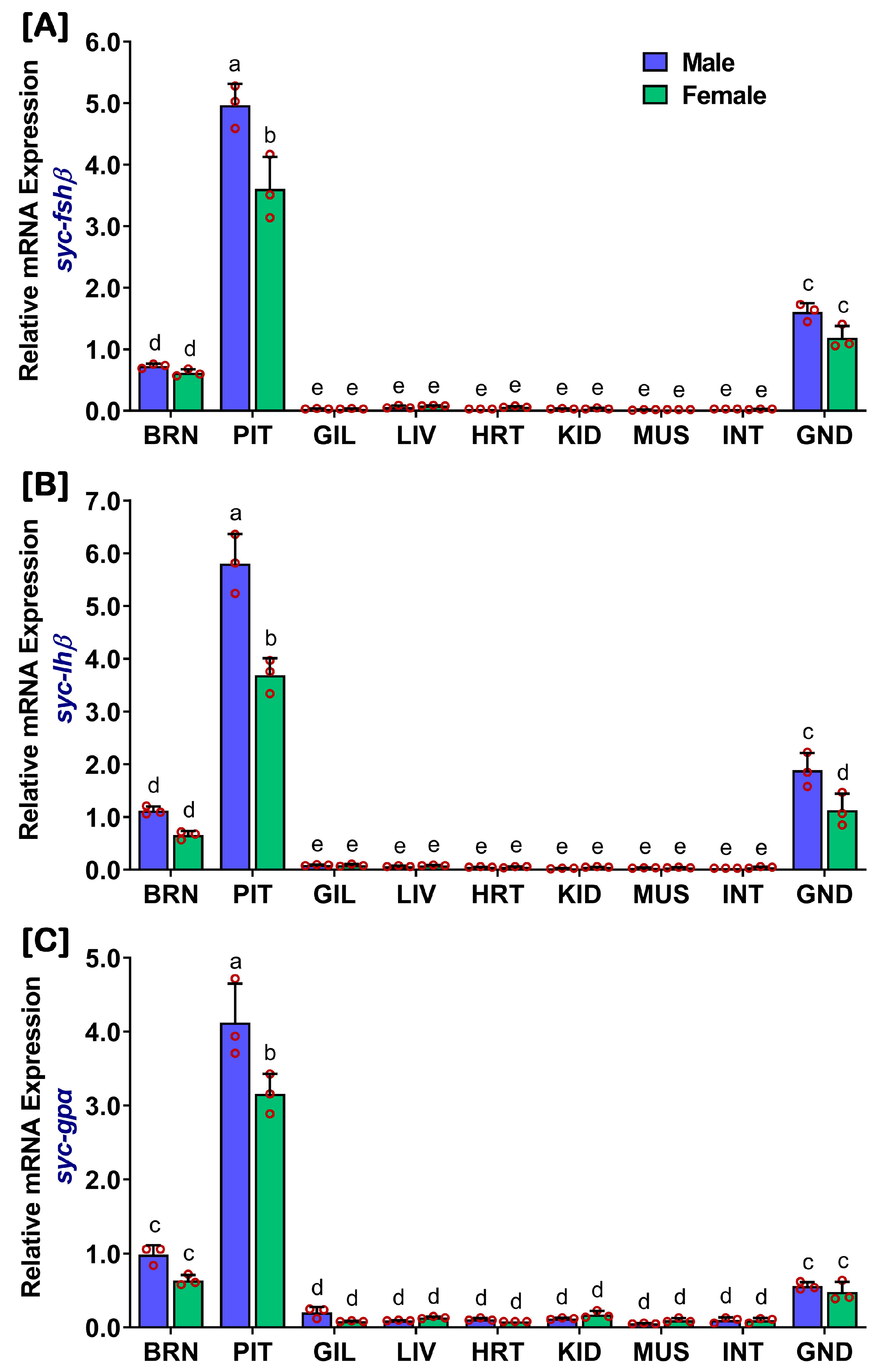
2.1. Distribution of fshβ, lhβ, and gpα in Different Organs of Captive-Reared Mature SYC
2.2. Gonadal Histology
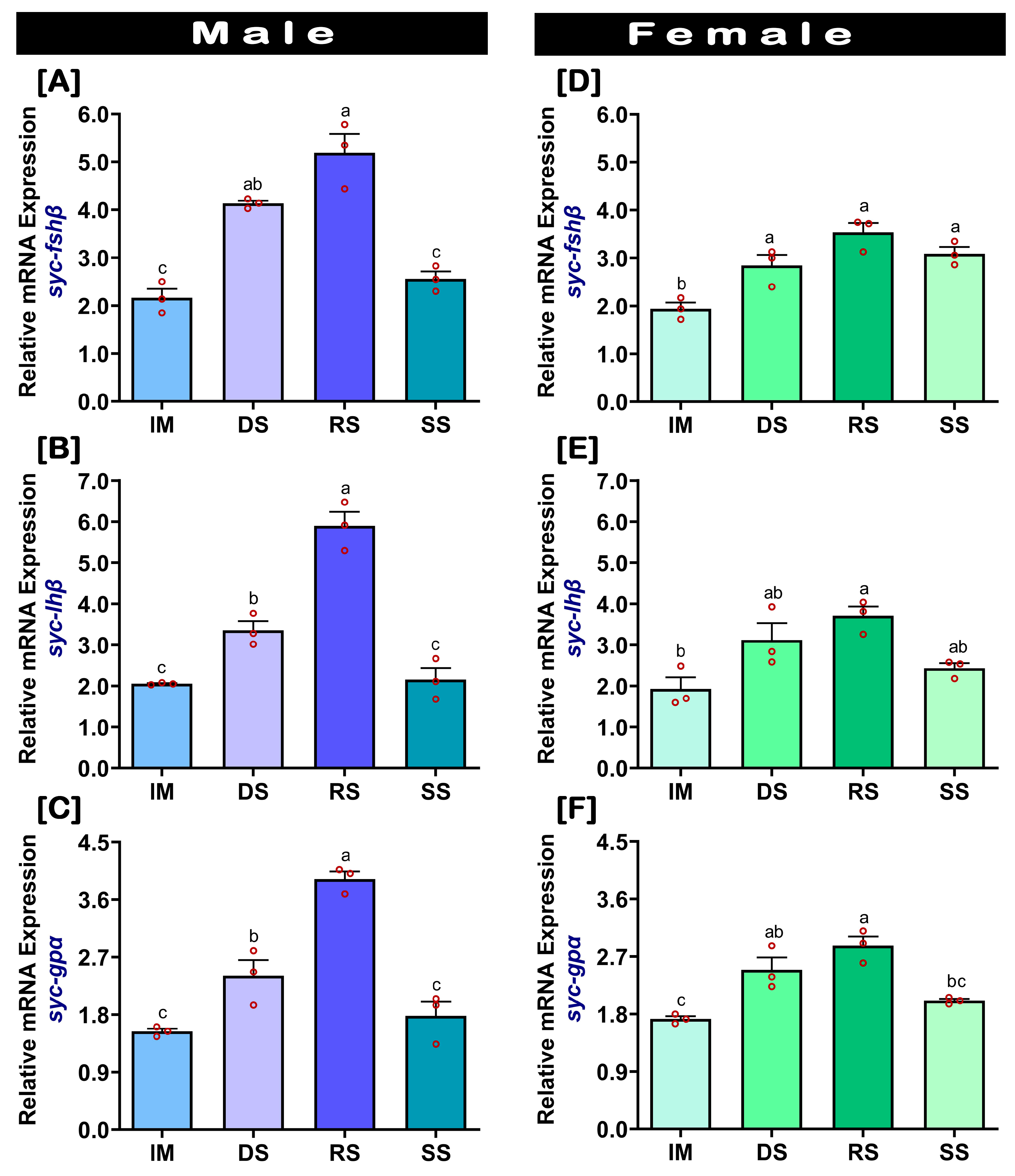
2.3. Changes in mRNA Expression Levels of fshβ, lhβ, and gpα in the Pituitary at Different Gonadal Developmental Stages
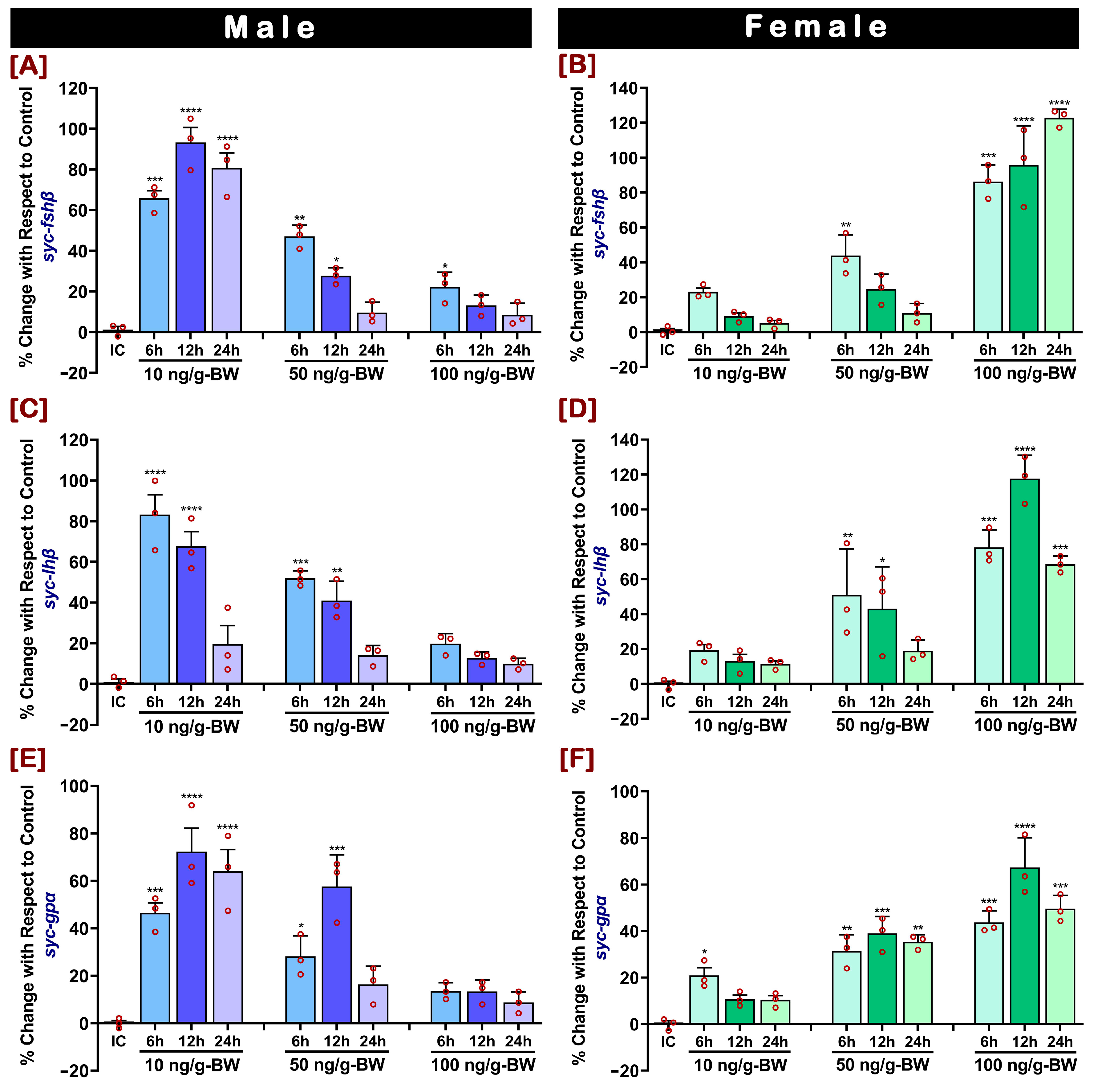
2.4. In Vivo Effect of GnRHa on mRNA Expression of fshβ, lhβ, and gpα in Pituitary
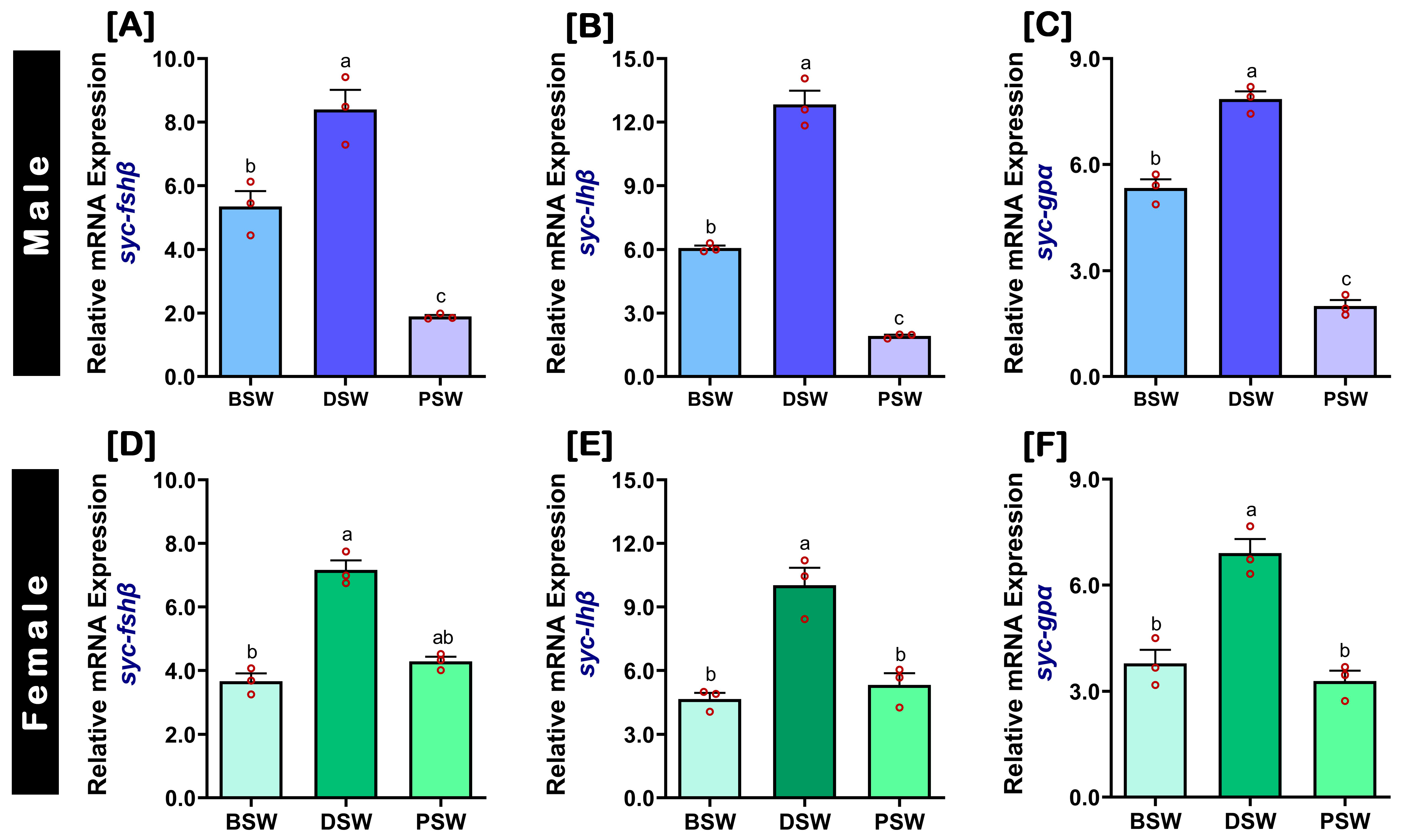
2.5. Changes in mRNA Expression Levels of fshβ, lhβ, and gpα in the Pituitary at Different Induced Spawning Events
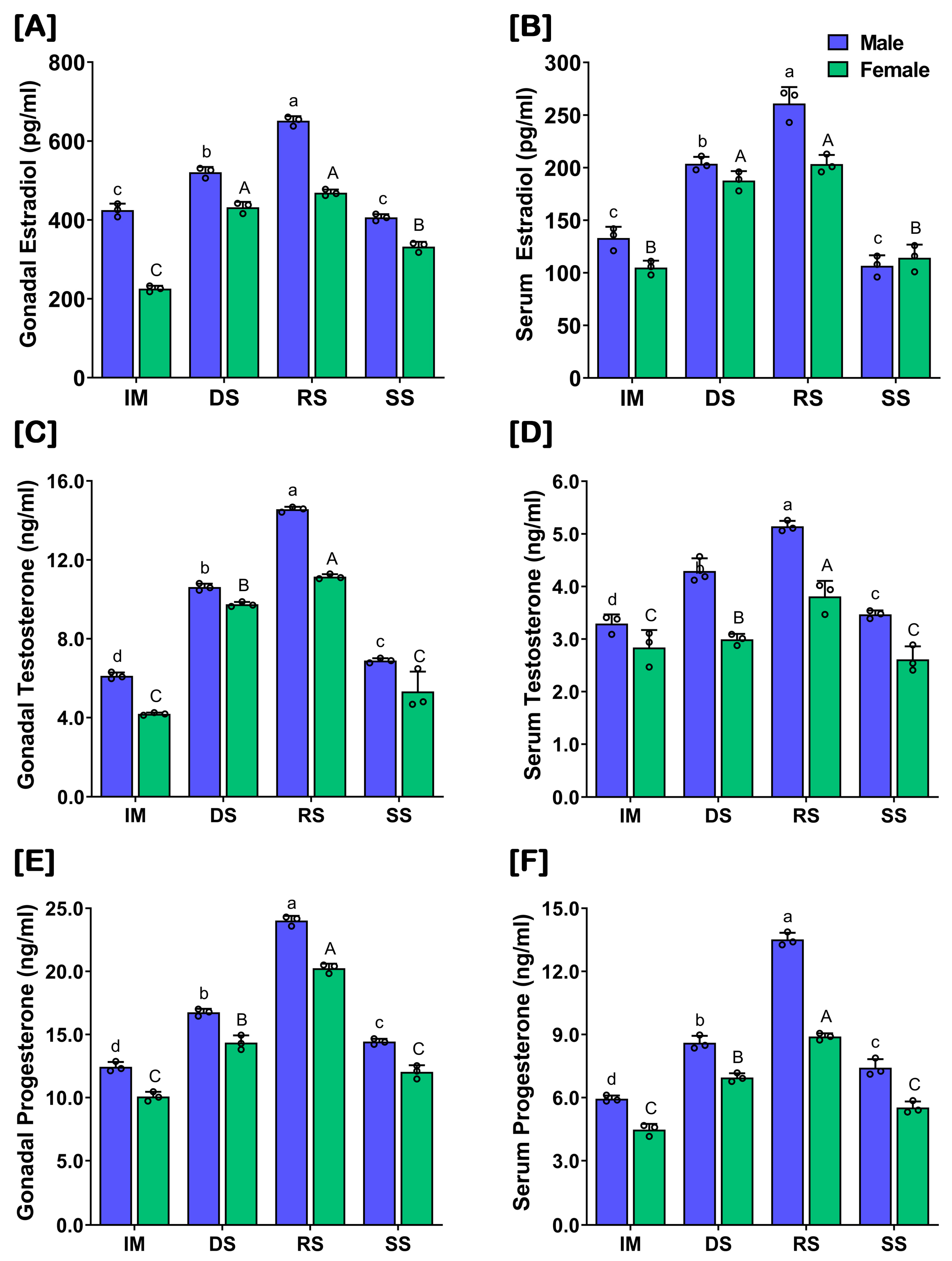
2.6. Changes in Gonadal and Serum Levels of Sex Steroids at Different Gonadal Developmental Stages of Captive-Reared SYC
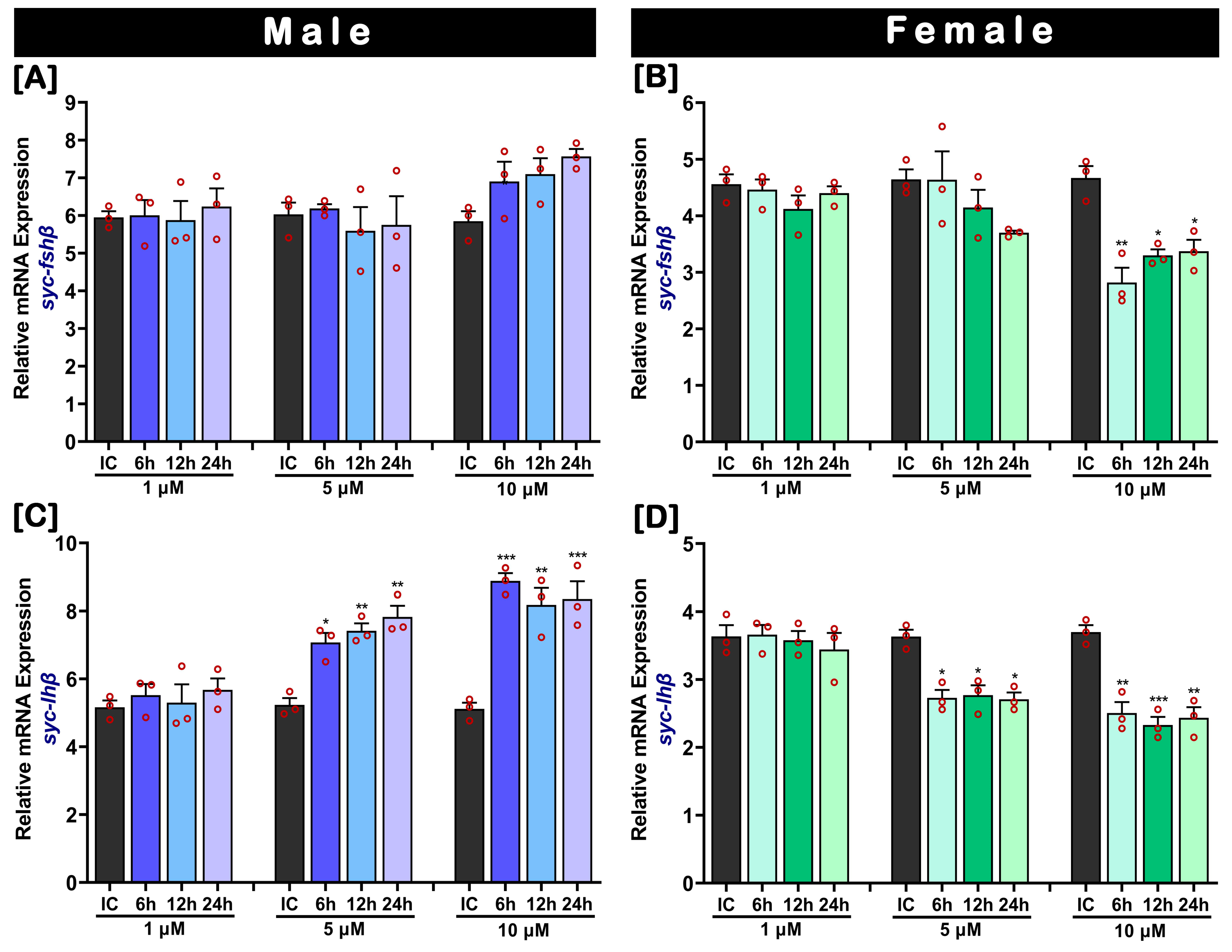
2.7. In Vitro Effect of 17β-Estradiol (E2) on fshβ and lhβ mRNA Levels in Cultured Pituitary
2.8. In Vitro Effect of 17α-Methyltestosterone (MT) on fshβ and lhβ Levels in Cultured Pituitary
3. Discussions
4. Materials and Methods
4.1. Experimental Fish and Husbandry
4.2. Sample Collection from Different Experimental Conditions
- Sampling of blood: Blood samples (1–2 mL) were drawn from the caudal vein of all sampled fish (10 fish at each developmental stage) using a 5 mL syringe and were maintained in heparinized tubes. Centrifugation was performed at 5000× g for 15 min at 4 °C to separate the plasma. Plasma was transferred to fresh tubes and preserved at −80 °C until the extraction of steroids.
- Sampling of the pituitary: The pituitaries of each sampled fish were collected carefully from underneath the brain by dissecting the head of the fish. Afterward, they were washed with 1× PBS and immediately frozen in liquid nitrogen. Frozen samples were kept at −80 °C until the total RNA was extracted.
- Sampling of gonads: Gonad samples were collected by dissecting the belly of the fish, washed with 1× PBS, flash frozen in liquid nitrogen, and stored at −80 °C until total RNA extraction. Furthermore, a portion of the gonad was fixed in 4% PFA for gonadal histology.
4.2.1. Different Organ Tissues from Captive-Reared Mature SYC of Both Sexes
4.2.2. Samples from Different Gonadal Developmental Stages of Captive-Reared SYC
4.2.3. Pituitary Samples from In Vivo Induction of GnRHa in SYC
4.2.4. Samples from Different Induced Spawning Events of Captive-Reared SYC
4.2.5. Pituitary Samples from In Vitro Cultures with E2 and MT
4.3. Gonadal Histology
4.3.1. Sample Preparation for Frozen Section
4.3.2. H&E Staining
4.4. Quantitative Real-Time PCR (qRT-PCR)
4.4.1. RNA Extraction and cDNA Syntheses
4.4.2. SYC GtHs Sequence Search and Primer Design
4.4.3. qRT-PCR Assay
4.5. Enzyme-Linked Immunosorbent Assay (ELISA)
4.5.1. Extraction of Steroid Hormones
- Plasma steroid extraction: First, 1 mL of the serum sample and an equal amount of diethyl ether were added to an Eppendorf and vortexed to mix. The top ether layer was carefully transferred to a new tube. This step was repeated twice, and the ether layers were combined in the tube. Then, the ether was evaporated to dryness from the sample under nitrogen. Then, the extracted steroid was resuspended in the supplied assay buffer and stored at −80 °C until use.
- Gonad tissue steroid extraction: First, gonad tissues were homogenized in 20 mM Tris–NaCl and sonicated. Then, the samples were centrifuged at 15,000× g for 15 min at 4 °C. Supernatant was collected and transferred to a new Eppendorf tube. Then, the steroids were extracted using diethyl ether, as described for the serum sample.
4.5.2. ELISA Protocol
4.5.3. ELISA Data Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Swanson, P.; Dickey, J.; Campbell, B. Biochemistry and physiology of fish gonadotropins. Fish Physiol. Biochem. 2003, 28, 53–59. [Google Scholar] [CrossRef]
- Zohar, Y.; Muñoz-Cueto, J.A.; Elizur, A.; Kah, O. Neuroendocrinology of reproduction in teleost fish. Gen. Comp. Endocrinol. 2010, 165, 438–455. [Google Scholar] [CrossRef] [PubMed]
- Sukhan, Z.P.; Kitano, H.; Selvaraj, S.; Yoneda, M.; Yamaguchi, A.; Matsuyama, M. Identification and distribution of three gonadotropin-releasing hormone (GnRH) isoforms in the brain of a clupeiform fish, Engraulis japonicus. Zool. Sci. 2013, 30, 1081–1091. [Google Scholar] [CrossRef]
- Levavi-Sivan, B.; Bogerd, J.; Mañanós, E.L.; Gómez, A.; Lareyre, J.J. Perspectives on fish gonadotropins and their receptors. Gen. Comp. Endocrinol. 2010, 165, 412–437. [Google Scholar] [CrossRef]
- Yaron, Z.; Gur, G.; Melamed, P.; Rosenfeld, H.; Elizur, A.; Levavi-Sivan, B. Regulation of fish gonadotropins. Int. Rev. Cytol. 2003, 225, 131–185. [Google Scholar] [CrossRef]
- Ando, H.; Urano, A. Molecular regulation of gonadotropin secretion by gonadotropin releasing hormone in salmonid fishes. Zool. Sci. 2005, 22, 379–389. [Google Scholar] [CrossRef]
- Omony, J.B.; Biran, J.; Kahwa, D.; Aizen, J.; Golan, M.; Nyatia, E.; Levavi-Sivan, B.; Rutaisire, J. Cloning of gonadotropin Gph-alpha, FSH-beta and LH-beta subunits and seasonal profiles of steroid hormones in wild-caught Nile perch, Lates niloticus. Gen. Comp. Endocrinol. 2022, 323, 114035. [Google Scholar] [CrossRef] [PubMed]
- Imanaga, Y.; Nyuji, M.; Amano, M.; Takahashi, A.; Kitano, H.; Yamaguchi, A.; Matsuyama, M. Characterization of gonadotropin-releasing hormone and gonadotropin in jack mackerel (Trachurus japonicus): Comparative gene expression analysis with respect to reproductive dysfunction in captive and wild fish. Aquaculture 2014, 428, 226–235. [Google Scholar] [CrossRef]
- Nyuji, M.; Selvaraj, S.; Kitano, H.; Ohga, H.; Yoneda, M.; Shimizu, A.; Kaneko, K.; Yamaguchi, A.; Matsuyama, M. Changes in the expression of pituitary gonadotropin subunits during reproductive cycle of multiple spawning female chub mackerel Scomber japonicus. Fish Physiol. Biochem. 2012, 38, 883–897. [Google Scholar] [CrossRef]
- Mateos, J.; Mañanos, E.; Carrillo, M.; Zanuy, S. Regulation of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) gene expression by gonadotropin-releasing hormone (GnRH) and sexual steroids in the Mediterranean Sea bass. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2002, 132, 75–86. [Google Scholar] [CrossRef]
- Gen, K.; Okuzawa, K.; Senthilkumaran, B.; Tanaka, H.; Moriyama, S.; Kagawa, H. Unique expression of gonadotropin-I and-II subunit genes in male and female red seabream (Pagrus major) during sexual maturation. Biol. Reprod. 2000, 63, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Nagahama, Y.; Yoshikuni, M.; Yamashita, M.; Tokumoto, T.; Katsu, Y. Regulation of oocyte growth and maturation in fish. Curr. Top. Dev. Biol. 1995, 30, 103–145. [Google Scholar] [CrossRef]
- Pankhurst, N.W. Gonadal steroids: Functions and patterns of change. In Fish Reproduction, 1st ed.; Rocha, M.J., Arukwe, A., Kapoor, B.G., Eds.; Taylor & Francis Group: New York, NY, USA, 2008; pp. 67–111. [Google Scholar]
- Saeed, S.S.; Imanpoor, M.; Aminian, F.B.; Gorgon, S. Study on sexual maturity and levels of gonad steroid hormones in female kutum Rutilus frisii kutum (Kamenskii, 1901) during spawning season from river Sefid-Rood of the southern Caspian Sea. J. Cell Anim. Biol. 2009, 3, 208–215. [Google Scholar]
- Sang, H.M.; Lam, H.S.; Hy, L.H.K.; Ky, P.X.; Minh-Thu, P. Changes in plasma and ovarian steroid hormone level in wild female blue tang fish Paracanthurus hepatus during a reproductive cycle. Animals 2019, 9, 889. [Google Scholar] [CrossRef]
- Alvarado, M.; Serrano, E.; Sánchez, J.C.; Valladares, L. Changes in plasma steroid hormones and gonadal histology associated with sexual maturation in wild southern hake (Merluccius australis). Lat. Am. J. Aquat. Res. 2015, 43, 632–640. [Google Scholar] [CrossRef]
- Kavadias, S.; Castritsi-Catharios, J.; Dessypris, A.; Miliou, H. Seasonal variation in steroid hormones and blood parameters in cage-farmed European seabass (Dicentrarchus labrax L.). J. Appl. Ichthyol. 2004, 20, 58–63. [Google Scholar] [CrossRef]
- Heidari, B.; Roozati, S.A.; Yavari, L. Changes in plasma levels of steroid hormones during oocyte development of Caspian Kutum (Rutilus frisii kutum, Kamensky, 1901). Anim. Reprod. 2010, 7, 373–381. [Google Scholar]
- Lee, W.K.; Yang, S.W. Relationship between ovarian development and serum levels of gonadal steroid hormones, and induction of oocyte maturation and ovulation in the cultured female Korean spotted sea bass Lateolabrax maculatus (Jeom-nong-eo). Aquaculture 2002, 207, 169–183. [Google Scholar] [CrossRef]
- Cornish, D. Seasonal steroid hormone profiles in plasma and gonads of the tilapia, Oreochromis mossambicus. Water SA 1998, 24, 257–263. [Google Scholar]
- Zohar, Y.; Mylonas, C.C. Endocrine manipulations of spawning in cultured fish: From hormones to genes. Aquaculture 2001, 197, 99–136. [Google Scholar] [CrossRef]
- de Jesus, L.W.O.; Bogerd, J.; Vieceli, F.M.; Branco, G.S.; Camargo, M.P.; Cassel, M.; Moreira, R.G.; Yan, C.Y.I.; Borella, M.I. Gonadotropin subunits of the characiform Astyanax altiparanae: Molecular characterization, spatiotemporal expression and their possible role on female reproductive dysfunction in captivity. Gen. Comp. Endocrinol. 2017, 246, 150–163. [Google Scholar] [CrossRef]
- Akhtar, M.S.; Ciji, A.; Sarma, D.; Rajesh, M.; Kamalam, B.S.; Sharma, P.; Singh, A.K. Reproductive dysfunction in females of endangered golden mahseer (Tor putitora) in captivity. Anim. Reprod. Sci. 2017, 182, 95–103. [Google Scholar] [CrossRef]
- Moreira, R.G.; Honji, R.M.; Melo, R.G.; Narcizo Ade, M.; Amaral, J.S.; Araújo Rde, C.; Hilsdorf, A.W. The involvement of gonadotropins and gonadal steroids in the ovulatory dysfunction of the potamodromous Salminus hilarii (Teleostei: Characidae) in captivity. Fish Physiol. Biochem. 2015, 41, 1435–1447. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, J.M.; Adam Luckenbach, J.; Swanson, P. Molecular characterization and quantification of sablefish (Anoplopoma fimbria) gonadotropins and their receptors: Reproductive dysfunction in female captive broodstock. Gen. Comp. Endocrinol. 2013, 193, 37–47. [Google Scholar] [CrossRef]
- Choi, M.-J.; Kim, D.-H. Assessment and Management of Small Yellow Croaker (Larimichthys polyactis) Stocks in South Korea. Sustainability 2020, 12, 8257. [Google Scholar] [CrossRef]
- Song, D.; Xiong, Y.; Jiang, T.; Yang, J.; Zhong, X.; Tang, J.; Kang, Z. Evaluation of Spawning- and Natal-Site Fidelity of Larimichthys polyactis in the Southern Yellow Sea Using Otolith Microchemistry. Front. Mar. Sci. 2021, 8, 820492. [Google Scholar] [CrossRef]
- Cheng, Q.; Chen, W.; Ma, L. Genetic diversity and population structure of small yellow croaker (Larimichthys polyactis) in the Yellow and East China seas based on microsatellites. Aquat. Living Resour. 2019, 32, 16. [Google Scholar] [CrossRef]
- Park, J.M. Historical consideration of Production and use of a croaker. Korean J. Agric. Hist. 2010, 9, 197–222. Available online: https://www.kci.go.kr/kciportal/ci/sereArticleSearch/ciSereArtiView.kci?sereArticleSearchBean.artiId=ART001460670 (accessed on 18 March 2023). [CrossRef]
- Kim, S.; Lim, J.; Lee, K.; Park, S. Effect of twine thickness on size-selectivity of driftnet for the yellow croaker Larimichthys polyactis in southwestern Sea of Korea. Chin. J. Ocean. Limnol. 2016, 34, 1199–1208. [Google Scholar] [CrossRef]
- Lou, B.; Zhan, W.; Chen, R.Y.; Liu, F.; Wang, L.G.; Xu, D.D.; Mao, G.M. Studies on techniques of the artificial breeding of Larimichthys polyactis. J. Zhejiang Univ. 2016, 35, 361–365. [Google Scholar]
- Sukhan, Z.P.; Cho, Y.; Hossen, S.; Yang, S.-W.; Hwang, N.-Y.; Lee, W.K.; Kho, K.H. Functional Characterization of Three GnRH Isoforms in Small Yellow Croaker Larimichthys polyactis Maintained in Captivity: Special Emphasis on Reproductive Dysfunction. Biology 2022, 11, 1200. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Liu, Y.; Chu, T.; Lou, B.; Zhan, W.; Chen, R. Interspecific hybridization and genetic characterization of Larimichthys polyactis (♀) and L. crocea (♂). Aquacult. Int. 2019, 27, 663–674. [Google Scholar] [CrossRef]
- Kim, H.W.; Kim, J.H.; Park, J.W.; Baek, H.J.; Kim, D.J. Effects of photoperiod and water temperature on male sex steroid levels in cultured small yellow croaker (Larimichthys polyactis). J. Life Sci. 2021, 31, 314–320. [Google Scholar] [CrossRef]
- Lim, H.K.; Le, M.H.; An, C.M.; Kim, S.Y.; Park, M.S.; Chang, Y.J. Reproductive cycle of yellow croaker Larimichthys polyactis in southern waters off Korea. Fish. Sci. 2010, 76, 971–980. [Google Scholar] [CrossRef]
- Kang, D.Y.; Jo, K.C.; Lee, J.H.; Kang, H.W.; Kim, H.C.; Kim, G.H. Annual reproductive cycle of wild female yellow croaker, Larimichthys polyactis. J. Aquacul. 2006, 19, 188–196. [Google Scholar]
- So, W.K.; Kwok, H.F.; Ge, W. Zebrafish gonadotropins and their receptors: II. Cloning and characterization of zebrafish follicle-stimulating hormone and luteinizing hormone subunits—Their spatial-temporal expression patterns and receptor specificity. Biol. Reprod. 2005, 72, 1382–1396. [Google Scholar] [CrossRef]
- Shi, B.; Liu, X.; Xu, Y.; Wang, S. Molecular Characterization of three gonadotropin subunits and their expression patterns during ovarian maturation in Cynoglossus semilaevis. Int. J. Mol. Sci. 2015, 16, 2767–2793. [Google Scholar] [CrossRef]
- Shi, B.; Liu, X.; Xu, Y.; Sun, Z.; Chen, S.; Zang, K. Molecular and transcriptional characterization of GTHs and mPRα during ovarian maturation in rock bream Oplegnathus fasciatus. J. Exp. Zool. Ecol. Genet. Physiol. 2015, 323, 430–444. [Google Scholar] [CrossRef]
- Wong, T.T.; Zohar, Y. Novel expression of gonadotropin subunit genes in oocytes of the gilthead seabream (Sparus aurata). Endocrinology 2004, 145, 5210–5220. [Google Scholar] [CrossRef]
- Mittelholzer, C.; Andersson, E.; Taranger, G.L.; Karlsen, Ø.; Norberg, B. Quantification of gonadotropin subunits GPα, FSHβ, and LHβ mRNA expression from Atlantic cod (Gadus morhua) throughout a reproductive cycle. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2009, 153, 288–295. [Google Scholar] [CrossRef]
- Pandolfi, M.; Pozzi, A.G.; Cánepa, M.; Vissio, P.G.; Shimizu, A.; Maggese, M.C.; Lobo, G. Presence of β-follicle-stimulating hormone and β-luteinizing hormone transcripts in the brain of Cichlasoma dimerus (Perciformes: Cichlidae), effect of brain-derived gonadotropins on pituitary hormone release. Neuroendocrinology 2009, 89, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Pakarainen, T.; Ahtiainen, P.; Zhang, F.P.; Rulli, S.; Poutanen, M.; Huhtaniemi, I. Extragonadal LH/hCG action—Not yet time to rewrite textbooks. Mol. Cell. Endocrinol. 2007, 269, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Crim, L.W.; Evans, D.M. Seasonal levels of pituitary and plasma gonadotropin in male and female Atlantic salmon parr. Can. J. Zool. 1978, 56, 1550–1555. [Google Scholar] [CrossRef]
- Yoshida, D.; Nagae, M.; Ito, F.; Soyano, K. Molecular cloning of cDNAs encoding pituitary glycoprotein hormone α, FSH β and LH β subunits in ayu, Plecoglossus altivelis. Zool. Sci. 2001, 18, 929–936. [Google Scholar] [CrossRef]
- Mylonas, C.C.; Fostier, A.; Zanuy, S. Broodstock management and hormonal manipulations of fish reproduction. Gen. Comp. Endocrinol. 2010, 165, 516–534. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.P.; Cui, X.F.; Wang, Y.R.; Li, Z.Y.; Tian, C.X.; Jiang, D.N.; Zhu, C.H.; Zhang, Y.; Li, S.S.; Li, G.L. Identification, functional characterization, and estrogen regulation on gonadotropin-releasing hormone in the spotted scat, Scatophagus argus. Fish Physiol. Biochem. 2020, 46, 1743–1757. [Google Scholar] [CrossRef]
- Choi, C.Y.; Min, B.H.; Chang, Y.J.; Park, I.S.; Cho, S.H.; An, K.W. Effects of gonadotropin-releasing hormone analogue (GnRHa) on expression of the gonadotropin subunit gene and on synthesis of the sex steroids in black porgy, Acanthopagrus schlegeli. J. Aquacult. 2005, 18, 293–298. [Google Scholar]
- Forniés, M.A.; Carrillo, M.; Mañanós, E.; Sorbera, L.A.; Zohar, Y.; Zanuy, S. Relative potency of the forms of GnRH and their analogs on LH release in sea bass. J. Fish biol. 2003, 63, 73–89. [Google Scholar] [CrossRef]
- Ren, X.; Huang, Y.; Li, X.; Li, Z.; Yang, H.; He, R.; Zhong, H.; Li, G.; Chen, H. Identification and functional characterization of gonadotropin-releasing hormone in pompano (Trachinotus ovatus). Gen. Comp. Endocrinol. 2022, 316, 113958. [Google Scholar] [CrossRef]
- Hassin, S.; Gothilf, Y.; Blaise, O.; Zohar, Y. Gonadotropin-I and -II subunit gene expression of male striped bass (Morone saxatilis) after gonadotropin-releasing hormone analogue injection: Quantitation using an optimized ribonuclease protection assay. Biol. Reprod. 1999, 58, 1233–1240. [Google Scholar] [CrossRef]
- An, K.W.; Nelson, E.R.; Habibi, H.R.; Choi, C.Y. Molecular characterization and expression of three GnRH forms mRNA during gonad sex-change process, and effect of GnRHa on GTH subunits mRNA in the protandrous black porgy (Acanthopagrus schlegeli). Gen. Comp. Endocrinol. 2008, 159, 38–45. [Google Scholar] [CrossRef]
- Chi, M.L.; Ni, M.; Li, J.F.; He, F.; Qian, K.; Zhang, P.; Chai, S.H.; Wen, H.S. Molecular cloning and characterization of gonadotropin subunits (GTHα, FSHβ and LHβ) and their regulation by hCG and GnRHa in Japanese sea bass (Lateolabrax japonicas) in vivo. Fish Physiol. Biochem. 2015, 41, 587–601. [Google Scholar] [CrossRef]
- Khakoo, Z.; Bhatia, A.; Gedamu, L.; Habibi, H.R. Functional specificity for salmon gonadotropin-releasing hormone (GnRH) and chicken GnRH-II coupled to the gonadotropin release and subunit messenger ribonucleic acid level in the goldfish pituitary. Endocrinology 1994, 134, 838–847. [Google Scholar] [CrossRef]
- Dickey, J.T.; Swanson, P. Effects of salmon gonadotropin-releasing hormone on follicle stimulating hormone secretion and subunit gene expression in coho salmon (Oncorhynchus kisutch). Gen. Comp. Endocrinol. 2000, 118, 436–449. [Google Scholar] [CrossRef] [PubMed]
- Klausen, C.; Chang, J.P.; Habibi, H.R. Time- and dose-related effects of gonadotropin-releasing hormone on growth hormone and gonadotropin subunit gene expression in the goldfish pituitary. Can. J. Physiol. Pharmacol. 2002, 80, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, J.M.; Cal, R.; García-López, A.; Chereguini, O.; Kight, K.; Olmedo, M.; Sarasquete, C.; Mylonas, C.C.; Peleteiro, J.B.; Zohar, Y.; et al. Effects of in vivo treatment with the dopamine antagonist pimozide and gonadotropin-releasing hormone agonist (GnRHa) on the reproductive axis of Senegalese sole (Solea senegalensis). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2011, 158, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Shahjahan, M.; Hamabata, T.; Motohashi, E.; Doi, H.; Ando, H. Differential expression of three types of gonadotropin-releasing hormone genes during the spawning season in grass puffer, Takifugu niphobles. Gen. Comp. Endocrinol. 2010, 167, 153–163. [Google Scholar] [CrossRef]
- Bauer, C.M.; Fudickar, A.M.; Anderson-Buckingham, S.; Abolins-Abols, M.; Atwell, J.W.; Ketterson, E.D.; Greives, T.J. Seasonally sympatric but allochronic: Differential expression of hypothalamic genes in a songbird during gonadal development. Proc. Royal Soc. B Biol. Sci. 2018, 285, 20181735. [Google Scholar] [CrossRef]
- Schulz, R.W.; Miura, T. Spermatogenesis and its endocrine regulation. Fish Physiol. Biochem. 2002, 26, 43–56. [Google Scholar] [CrossRef]
- Golan, M.; Biran, J.; Levavi-Sivan, B. A novel model for development, organization, and function of gonadotropes in fish pituitary. Front. Endocrinol. 2014, 5, 182. [Google Scholar] [CrossRef]
- Sukhan, Z.P.; Hossen, S.; Cho, Y.; Lee, W.K.; Kho, K.H. Hdh-Tektin-4 regulates motility of fresh and cryopreserved sperm in Pacific abalone, Haliotis discus hannai. Front. Cell. Dev. Biol. 2022, 10, 870743. [Google Scholar] [CrossRef] [PubMed]
- Sukhan, Z.P.; Cho, Y.; Sharker, M.R.; Hossen, S.; Rha, S.J.; Kho, K.H. Effective accumulative temperature affects gonadal maturation by controlling expression of GnRH, GnRH receptor, serotonin receptor and APGWamide gene in Pacific abalone, Haliotis discus hannai during broodstock conditioning in hatcheries. J. Therm. Biol. 2021, 100, 103037. [Google Scholar] [CrossRef] [PubMed]


| Gonadotropin Subunits | |||
|---|---|---|---|
| fshβ | lhβ | gpα | |
| Sequence Information | |||
| NCBI nucleotide accession number | MT239441 | MW192797 | MW192796 |
| NCBI protein accession number | QNG62443 | QOW40925 | QOW40924 |
| ORF (bp) | 348 | 444 | 399 |
| Amino acid sequence (aa) | 115 | 147 | 132 |
| Amino acid sequence identity (%) | |||
| Large yellow croaker | 95.01 | 95.24 | 93.18 |
| Largemouth bass | 75.65 | 84.25 | 85.61 |
| Skipjack tuna | 72.55 | 92.11 | 90.32 |
| European seabass | 71.30 | 85.03 | 84.68 |
| Red seabream | 66.09 | 81.51 | 88.03 |
| Chub mackerel | 66.09 | 80.95 | 84.62 |
| Pike perch | 60.00 | 80.88 | 73.48 |
| Name | Nucleotide Sequence (5′–3′) | GC Percentage | Tm (°C) | Length (bp) | Accession No. |
|---|---|---|---|---|---|
| SYC-FSHβ-Fw | GGCAACACCGAGTTCATCG | 58 | 59.5 | 199 | MT239441 |
| SYC-FSHβ-Rv | CGTTGCATGCGCTACACTC | 58 | 59.5 | ||
| SYC-LHβ-Fw | CATCACCAAGGACCCTGTC | 58 | 59.5 | 198 | MW192797 |
| SYC-LHβ-Rv | CTCTCGAAGGTGCTGTCAG | 58 | 59.5 | ||
| SYC-GPα-Fw | TGCACACTGAGCAAGAACAG | 50 | 58.4 | 178 | MW192796 |
| SYC-GPα-Rv | CCTCTATCTCGTAGCTGTGC | 55 | 60.5 | ||
| SYC-β-Actin-Fw | GCTGTCTTCCCATCCATCG | 58 | 59.5 | 193 | MT330378 |
| SYC-β-Actin-Rv | CGTTGTAGAAGGTGTGATGC | 50 | 58.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kho, K.H.; Sukhan, Z.P.; Yang, S.-W.; Hwang, N.-Y.; Lee, W.-K. Gonadotropins and Sex Steroid Hormones in Captive-Reared Small Yellow Croaker (Larimichthys polyactis) and Their Role in Female Reproductive Dysfunction. Int. J. Mol. Sci. 2023, 24, 8919. https://doi.org/10.3390/ijms24108919
Kho KH, Sukhan ZP, Yang S-W, Hwang N-Y, Lee W-K. Gonadotropins and Sex Steroid Hormones in Captive-Reared Small Yellow Croaker (Larimichthys polyactis) and Their Role in Female Reproductive Dysfunction. International Journal of Molecular Sciences. 2023; 24(10):8919. https://doi.org/10.3390/ijms24108919
Chicago/Turabian StyleKho, Kang Hee, Zahid Parvez Sukhan, Seok-Woo Yang, Nam-Yong Hwang, and Won-Kyo Lee. 2023. "Gonadotropins and Sex Steroid Hormones in Captive-Reared Small Yellow Croaker (Larimichthys polyactis) and Their Role in Female Reproductive Dysfunction" International Journal of Molecular Sciences 24, no. 10: 8919. https://doi.org/10.3390/ijms24108919
APA StyleKho, K. H., Sukhan, Z. P., Yang, S.-W., Hwang, N.-Y., & Lee, W.-K. (2023). Gonadotropins and Sex Steroid Hormones in Captive-Reared Small Yellow Croaker (Larimichthys polyactis) and Their Role in Female Reproductive Dysfunction. International Journal of Molecular Sciences, 24(10), 8919. https://doi.org/10.3390/ijms24108919







