Preliminary Development of a Brainwave Model for K1 Kickboxers Using Quantitative Electroencephalography (QEEG) with Open Eyes
Abstract
1. Introduction
- Will the recognised brain activity of kickboxing athletes position itself within the reference standards for healthy people?
- What is the inter-group differentiation regarding the level of brain activity for individual frequency bands of the observed athletes compared to the control group?
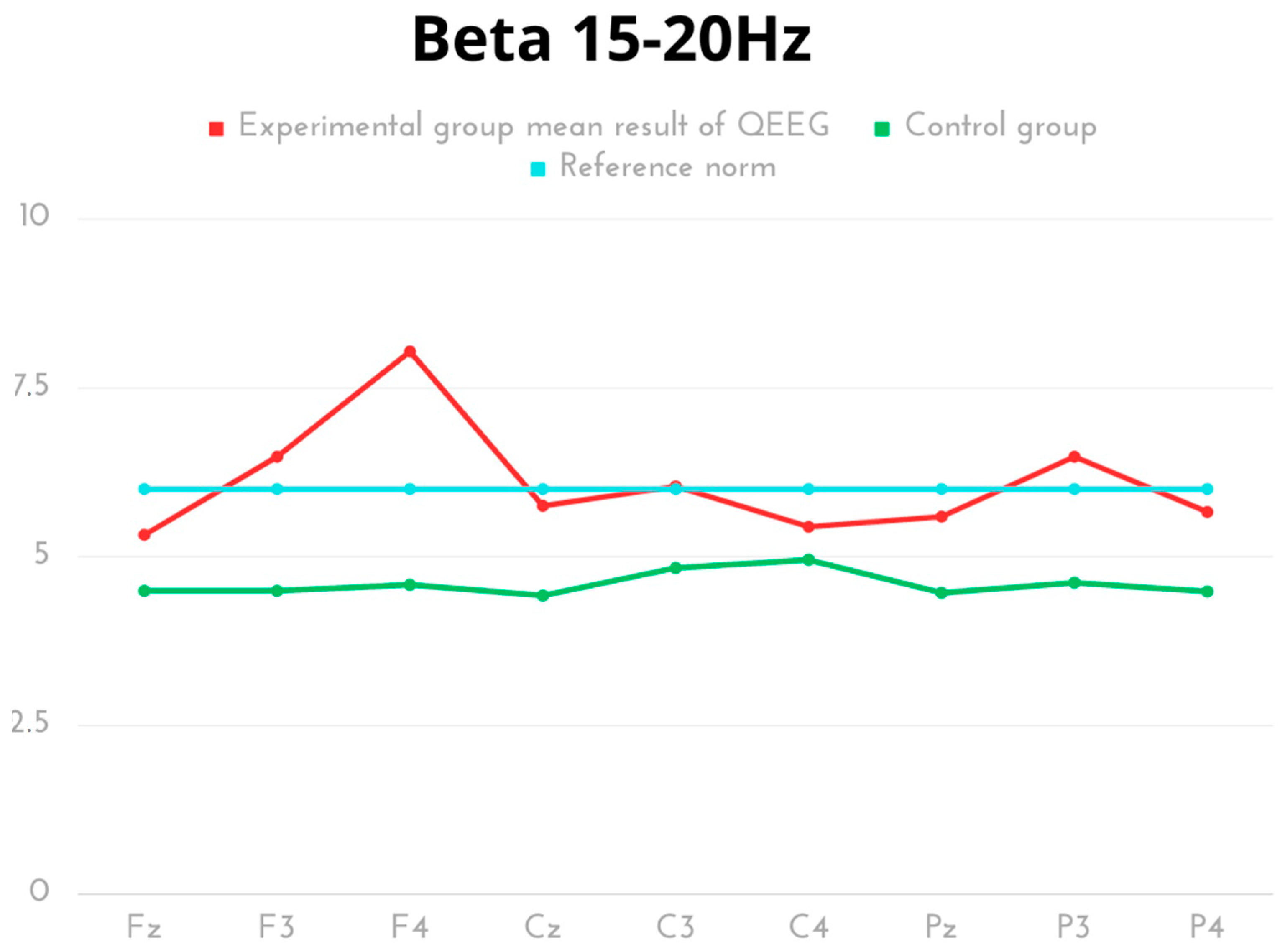
2. Results
3. Discussion
3.1. Conclusions/Summary
3.2. Limitation of Study
4. Materials and Methods
4.1. Study Design
4.2. Experimental Group
4.3. Control Group
4.4. QEEG Procedure
4.5. Methods of Statistical Analysis
5. Conclusions
- In addition to problems with concentration or over-stimulation of neural structures, high Delta waves, with elevated Alpha, Theta and Beta 2 waves can cause disorders in the limbic system and problems in the cerebral cortex (e.g., cortical–subcortical conflict). Further research is needed to determine the exact changes in function caused by various sports activities.
- High amplitudes beyond the normative scale were found in the Delta, Alpha, SMR, Beta 1 and Beta 2 frequencies in the frontal lobe frequency. Therefore, it can be concluded that athletes are accompanied by an accumulation of emotions that negatively affect planning, situational assessment and coordination.
- The results and comparative analyses between the studied groups demonstrated significant differences regarding the activity of brain waves in the majority of the measured areas, with higher results in the kickboxer group. This suggests that the environmental influence in the form of specialised kickboxing training has an impact on the increased activity of brain waves in specific areas.
- Based on the presented results, it is clear that more research is needed to better understand the impact of different types of sports activities on brain function. The results suggest that specialised kickboxing training can have a significant impact on brainwave activity, highlighting the need for a more targeted and personalised approach to monitoring and treating combat sports athletes. Further research should be conducted to determine changes occurring before and after a kickboxing match and to study its long-term effects.
Practical Implications
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Di Marino, S. A Complete Guide to Kickboxing; Enslow Publishing: New York, NY, USA, 2018. [Google Scholar]
- Rydzik, Ł.; Maciejczyk, M.; Czarny, W.; Kędra, A.; Ambroży, T. Physiological Responses and Bout Analysis in Elite Kickboxers during International K1 Competitions. Front. Physiol. 2021, 12, 737–741. [Google Scholar] [CrossRef] [PubMed]
- Ouergui, I.; Delleli, S.; Bouassida, A.; Bouhlel, E.; Chaabene, H.; Ardigò, L.P.; Franchini, E. Technical–tactical analysis of small combat games in male kickboxers: Effects of varied number of opponents and area size. BMC Sports Sci. Med. Rehabil. 2021, 13, 158. [Google Scholar] [CrossRef] [PubMed]
- Lukanova-Jakubowska, A.; Piechota, K.; Grzywacz, T.; Ambroży, T.; Rydzik, Ł.; Ozimek, M. The Impact of Four High-Altitude Training Camps on the Aerobic Capacity of a Short Track PyeongChang 2018 Olympian: A Case Study. Int. J. Environ. Res. Public Health 2022, 19, 3814. [Google Scholar] [CrossRef] [PubMed]
- Ambroży, T.; Rydzik, Ł.; Kędra, A.; Ambroży, D.; Niewczas, M.; Sobiło, E.; Czarny, W. The effectiveness of kickboxing techniques and its relation to fights won by knockout. Arch. Budo 2020, 16, 11–17. [Google Scholar]
- Grindon, L. Knockout: The Boxer and Boxing in American Cinema; University Press of Mississippi: Jackson, MS, USA, 2011. [Google Scholar]
- Grodin, L.; Nowinski, A.; Cantu, K. Punch counts, knockouts, and injury risk in professional boxing. Med. Sci. Sport. Exerc. 2006, 38, 1824–1830. [Google Scholar]
- Rydzik, Ł. Indices of technical and tactical training during kickboxing at different levels of competition in the K1 Formula. J. Kinesiol. Exerc. Sci. 2022, 32, 1–5. [Google Scholar] [CrossRef]
- Rydzik, Ł.; Ambroży, T. Physical Fitness and the Level of Technical and Tactical Training of Kickboxers. Int. J. Environ. Res. Public Health 2021, 18, 3088. [Google Scholar] [CrossRef] [PubMed]
- Jordan, B.D. Chronic traumatic brain injury associated with boxing. Semin. Neurol. 2000, 20, 179–185. [Google Scholar] [CrossRef]
- McAllister, T.; McCrea, M. Long-Term Cognitive and Neuropsychiatric Consequences of Repetitive Concussion and Head-Impact Exposure. J. Athl. Train. 2017, 52, 309–317. [Google Scholar] [CrossRef]
- Gardner, A.; Iverson, G.L.; McCrory, P. Chronic traumatic encephalopathy in sport: A systematic review. Br. J. Sports Med. 2014, 48, 84–90. [Google Scholar] [CrossRef]
- Johnson, B.; Bazarian, J. Clinical and pathological features of chronic traumatic encephalopathy in boxers: A meta-analysis. Neurosurg. Rev. 2016, 39, 479–485. [Google Scholar] [CrossRef]
- Costanza, A.; Weber, K.; Gandy, S.; Bouras, C.; Hof, P.R.; Giannakopoulos, P.; Canuto, A. Review: Contact sport-related chronic traumatic encephalopathy in the elderly: Clinical expression and structural substrates. Neuropathol. Appl. Neurobiol. 2011, 37, 570–584. [Google Scholar] [CrossRef] [PubMed]
- McKee, A.C.; Cantu, R.C.; Nowinski, C.J.; Hedley-Whyte, E.T.; Gavett, B.E.; Budson, A.E.; Santini, V.E.; Lee, H.-S.; Kubilus, C.A.; Stern, R.A. Chronic Traumatic Encephalopathy in Athletes: Progressive Tauopathy After Repetitive Head Injury. J. Neuropathol. Exp. Neurol. 2009, 68, 709–735. [Google Scholar] [CrossRef]
- Clay, M.B.; Glover, K.L.; Lowe, D.T. Epidemiology of concussion in sport: A literature review. J. Chiropr. Med. 2013, 12, 230–251. [Google Scholar] [CrossRef]
- Manley, G.; Gardner, A.J.; Schneider, K.J.; Guskiewicz, K.M.; Bailes, J.; Cantu, R.C.; Castellani, R.J.; Turner, M.; Jordan, B.D.; Randolph, C.; et al. A systematic review of potential long-term effects of sport-related concussion. Br. J. Sports Med. 2017, 51, 969–977. [Google Scholar] [CrossRef]
- Pellman, E.J.; Lovell, M.R.; Viano, D.C.; Casson, I.R.; Tucker, A.M. Concussion in Professional Football: Neuropsychological Testing—Part 6. Neurosurgery 2004, 55, 1290–1305. [Google Scholar] [CrossRef] [PubMed]
- Register-Mihalik, J.K.; Guskiewicz, K.M.; Mihalik, J.P.; Schmidt, J.D.; Kerr, Z.Y.; McCrea, M.A. Reliable Change, Sensitivity, and Specificity of a Multidimensional Concussion Assessment Battery. J. Head Trauma Rehabil. 2013, 28, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Zasler, N.D. Sports concussion headache. Brain Inj. 2015, 29, 207–220. [Google Scholar] [CrossRef]
- Rau, R.; Raschka, C.; Brunner, K.; Banzer, W. Spectral analysis of electroencephalography changes after choking in judo (juji-jime). Med. Sci. Sport. Exerc. 1998, 30, 1356–1362. [Google Scholar] [CrossRef]
- Rodriguez, G.; Francione, S.; Gardella, M.; Marenco, S.; Nobili, F.; Novellone, G.; Reggiani, E.; Rosadini, G. Judo and choking: EEG and regional cerebral blood flow findings. J. Sports Med. Phys. Fitness 1991, 31, 605–610. [Google Scholar]
- Guterman, A.; Smith, R.W. Neurological Sequelae of Boxing. Sport. Med. 1987, 4, 194–210. [Google Scholar] [CrossRef] [PubMed]
- Tanriverdi, F.; Unluhizarci, K.; Coksevim, B.; Selcuklu, A.; Casanueva, F.F.; Kelestimur, F. Kickboxing sport as a new cause of traumatic brain injury-mediated hypopituitarism. Clin. Endocrinol. 2007, 66, 360–366. [Google Scholar] [CrossRef]
- Isaev, A.; Romanov, Y.; Erlikh, V. Integrative activity of the kickboxer’s body within modern sport training using biofeedback. Gazz. Medica Ital. Arch. Sci. Med. 2018, 177, 43–55. [Google Scholar] [CrossRef]
- Romanov, Y.N.; Isaev, A.P.; Shevtsov, A.V.; Romanova, L.A.; Cieslicka, M.; Muszkieta, R. Integrative assessment of kick boxers’ brain blood circulation and bio-electrical activity in conditions of correction technologies’ application. Phys. Educ. Stud. 2016, 20, 23–31. [Google Scholar] [CrossRef]
- Rydzik, Ł.; Wąsacz, W.; Ambroży, T.; Pałka, T.; Sobiło-Rydzik, E.; Kopańska, M. Comparison of Head Strike Incidence under K1 Rules of Kickboxing with and without Helmet Protection—A Pilot Study. Int. J. Environ. Res. Public Health 2023, 20, 4713. [Google Scholar] [CrossRef]
- Shoeibi, A.; Rezaei, M.; Ghassemi, N.; Namadchian, Z.; Zare, A.; Gorriz, J.M. Automatic Diagnosis of Schizophrenia in EEG Signals Using Functional Connectivity Features and CNN-LSTM Model. In Proceedings of the Artificial intelligence in Neuroscience: Affective Analysis and Health Applications: 9th International Work-Conference on the Interplay between Natural and Artificial Computation, IWINAC 2022, Puerto de la Cruz, Tenerife, Spain, 31 May–3 June 2022; pp. 63–73. [Google Scholar]
- Murashko, A.A.; Shmukler, A. EEG correlates of face recognition in patients with schizophrenia spectrum disorders: A systematic review. Clin. Neurophysiol. 2019, 130, 986–996. [Google Scholar] [CrossRef]
- Shoeibi, A.; Ghassemi, N.; Khodatars, M.; Moridian, P.; Alizadehsani, R.; Zare, A.; Khosravi, A.; Subasi, A.; Rajendra Acharya, U.; Gorriz, J.M. Detection of epileptic seizures on EEG signals using ANFIS classifier, autoencoders and fuzzy entropies. Biomed. Signal Process. Control 2022, 73, 103417. [Google Scholar] [CrossRef]
- Khodatars, M.; Shoeibi, A.; Sadeghi, D.; Ghaasemi, N.; Jafari, M.; Moridian, P.; Khadem, A.; Alizadehsani, R.; Zare, A.; Kong, Y.; et al. Deep learning for neuroimaging-based diagnosis and rehabilitation of Autism Spectrum Disorder: A review. Comput. Biol. Med. 2021, 139, 104949. [Google Scholar] [CrossRef]
- Kopańska, M.; Kuduk, B.; Łagowska, A.; Mytych, W.; Muchacka, R.; Banaś-Za̧bczyk, A. Quantitative electroencephalography interpretation of human brain activity after COVID-19 before and after Sudarshan Kriya Yoga. Front. Hum. Neurosci. 2022, 16, 988021. [Google Scholar] [CrossRef]
- Kaushik, P. QEEG Characterizations During Hyperventilation, Writing and Reading Conditions: A Pre–Post Cognitive-Behavioral Intervention Study on Students with Learning Difficulty. Clin. EEG Neurosci. 2023, 155005942211471. [Google Scholar] [CrossRef]
- Haneef, Z.; Levin, H.S.; Frost, J.D.; Mizrahi, E.M. Electroencephalography and Quantitative Electroencephalography in Mild Traumatic Brain Injury. J. Neurotrauma 2013, 30, 653–656. [Google Scholar] [CrossRef] [PubMed]
- Rydzik, Ł.; Wąsacz, W.; Ambroży, T.; Javdaneh, N.; Brydak, K.; Kopańska, M. The Use of Neurofeedback in Sports Training: Systematic Review. Brain Sci. 2023, 13, 660. [Google Scholar] [CrossRef] [PubMed]
- Rydzik, Ł.; Pałka, T.; Sobiło-Rydzik, E.; Tota, Ł.; Ambroży, D.; Ambroży, T.; Ruzbarsky, P.; Czarny, W.; Kopańska, M. An Attempt to Develop a Model of Brain Waves Using Quantitative Electroencephalography with Closed Eyes in K1 Kickboxing Athletes—Initial Concept. Sensors 2023, 23, 4136. [Google Scholar] [CrossRef] [PubMed]
- Kopańska, M.; Ochojska, D.; Muchacka, R.; Dejnowicz-Velitchkov, A.; Banaś-Ząbczyk, A.; Szczygielski, J. Comparison of QEEG Findings before and after Onset of Post-COVID-19 Brain Fog Symptoms. Sensors 2022, 22, 6606. [Google Scholar] [CrossRef]
- Steriade, M.; McCarley, R. Brainstem Control of Wakefulness and Sleep; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Steriade, M.; McCormick, D.A.; Sejnowski, T.J. Thalamocortical Oscillations in the Sleeping and Aroused Brain. Science 1993, 262, 679–685. [Google Scholar] [CrossRef]
- Appelhans, B.M.; Luecken, L.J. Heart rate variability and pain: Associations of two interrelated homeostatic processes. Biol. Psychol. 2008, 77, 174–182. [Google Scholar] [CrossRef]
- Ulrich-Lai, Y.M.; Herman, J.P. Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 2009, 10, 397–409. [Google Scholar] [CrossRef]
- Filaire, E.; Sagnol, M.; Ferrand, C.; Maso; Lac, G. Psychophysiological stress in judo athletes during competitions. J. Sports Med. Phys. Fit. 2001, 41, 263–268. [Google Scholar]
- Kolayis, H. Using EEG biofeedback in karate: The relationship among anxiety, motivation and brain waves. Arch. Budo 2012, 8, 13–18. [Google Scholar] [CrossRef]
- Guskiewicz, K.M.; McCrea, M.; Marshall, S.W.; Cantu, R.C.; Randolph, C.; Barr, W.; Onate, J.A.; Kelly, J.P. Cumulative Effects Associated with Recurrent Concussion in Collegiate Football Players. JAMA 2003, 290, 2549. [Google Scholar] [CrossRef]
- Erlanger, D.M. Exposure to sub-concussive head injury in boxing and other sports. Brain Inj. 2015, 29, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, R.; Fiest, K.M.; McChesney, J.; Kwon, C.S.; Jette, N.; Frolkis, A.D.; Atta, C.; Mah, S.; Dhaliwal, H.; Reid, A.; et al. The international incidence of traumatic brain injury: A systematic review and meta-analysis. Can. J. Neurol. Sci. 2016, 43, 774–785. [Google Scholar] [CrossRef]
- Corsellis, J.A. Boxing and the brain. BMJ 1989, 298, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Bernick, C.; Banks, S. What boxing tells us about repetitive head trauma and the brain. Alzheimers. Res. Ther. 2013, 5, 23. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, P.; Novotny, M.; Valis, M.; Klimova, B. Head injury in mixed martial arts: A review of epidemiology, affected brain structures and risks of cognitive decline. Phys. Sportsmed. 2021, 49, 371–380. [Google Scholar] [CrossRef]
- Malhi, G.S.; Ivanovski, B.; Hadzi-Pavlovic, D.; Mitchell, P.B.; Vieta, E.; Sachdev, P. Neuropsychological deficits and functional impairment in bipolar depression, hypomania and euthymia. Bipolar Disord. 2007, 9, 114–125. [Google Scholar] [CrossRef]
- Olaithe, M.; Bucks, R.S.; Hillman, D.R.; Eastwood, P.R. Cognitive deficits in obstructive sleep apnea: Insights from a meta-review and comparison with deficits observed in COPD, insomnia, and sleep deprivation. Sleep Med. Rev. 2018, 38, 39–49. [Google Scholar] [CrossRef]
- Liu, H.; Huang, Z.; Deng, B.; Chang, Z.; Yang, X.; Guo, X.; Yuan, F.; Yang, Q.; Wang, L.; Zou, H.; et al. QEEG Signatures are Associated with Nonmotor Dysfunctions in Parkinson’s Disease and Atypical Parkinsonism: An Integrative Analysis. Aging Dis. 2023, 14, 204. [Google Scholar] [CrossRef]
- Larson-Prior, L.J.; Power, J.D.; Vincent, J.L.; Nolan, T.S.; Coalson, R.S.; Zempel, J.; Snyder, A.Z.; Schlaggar, B.L.; Raichle, M.E.; Petersen, S.E. Modulation of the brain’s functional network architecture in the transition from wake to sleep. Prog. Brain Res. 2011, 193, 277–294. [Google Scholar]
- Sämann, P.G.; Tully, C.; Spoormaker, V.I.; Wetter, T.C.; Holsboer, F.; Wehrle, R.; Czisch, M. Increased sleep pressure reduces resting state functional connectivity. Magn. Reson. Mater. Phys. Biol. Med. 2010, 23, 375–389. [Google Scholar] [CrossRef]
- Young, T.; Peppard, P.E.; Gottlieb, D.J. Epidemiology of obstructive sleep apnea: A population health perspective. Am. J. Respir. Crit. Care Med. 2002, 165, 1217–1239. [Google Scholar] [CrossRef] [PubMed]
- Halasz, P.; Terzano, M.; Parrino, L.; Bodizs, R. The nature of arousal in sleep. J. Sleep Res. 2004, 13, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Kropotov, J.D. Functional Neuromarkers for Psychiatry: Applications for Diagnosis and Treatment; Academic Pressa is an imprint of Elsevier: Amsterdam, The Netherlands, 2016; ISBN 978-0-12-801872-7. [Google Scholar]
- Del Percio, C.; Infarinato, F.; Marzano, N.; Iacoboni, M.; Aschieri, P.; Lizio, R.; Soricelli, A.; Limatola, C.; Rossini, P.M.; Babiloni, C. Reactivity of alpha rhythms to eyes opening is lower in athletes than non-athletes: A high-resolution EEG study. Int. J. Psychophysiol. 2011, 82, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Piepiora, P.; Szmajke, A.; Migasiewicz, J.; Witkowski, K. The karate culture and aggressiveness in kumite competitors. Ido Mov. Cult. J. Martial Arts Anthropol. 2016, 16, 41–47. [Google Scholar]
- Leończyk, W. Oyama Karate Styl Stulecia Leończyk; Słupski Klub Oyama Karate, Słupsk: Słupsk, Poland, 2014. [Google Scholar]
- Klimesch, W. EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Res. Rev. 1999, 29, 169–195. [Google Scholar] [CrossRef]
- Klimesch, W. Alpha-band oscillations, attention, and controlled access to stored information. Trends Cogn. Sci. 2012, 16, 606–617. [Google Scholar] [CrossRef]
- Engel, A.K.; Fries, P. Beta-band oscillations—Signalling the status quo? Curr. Opin. Neurobiol. 2010, 20, 156–165. [Google Scholar] [CrossRef]
- Sauseng, P.; Klimesch, W.; Gruber, W.R.; Birbaumer, N. Cross-frequency phase synchronization: A brain mechanism of memory matching and attention. Neuroimage 2008, 40, 308–317. [Google Scholar] [CrossRef]
- Fetz, E.E. Volitional control of neural activity: Implications for brain-computer interfaces. J. Physiol. 2007, 579, 571–579. [Google Scholar] [CrossRef]
- Jurewicz, K.; Paluch, K.; Kublik, E.; Rogala, J.; Mikicin, M.; Wróbel, A. EEG-neurofeedback training of beta band (12–22 Hz) affects alpha and beta frequencies—A controlled study of a healthy population. Neuropsychologia 2018, 108, 13–24. [Google Scholar] [CrossRef]
- Zamysłowski, S. Schemes of EEG electrode placement in humans. In Licensing Training for a Biofeedback Specialist and Therapist, 2nd ed.; Kubik, A., Ed.; Polish Society of Clinical Neurophysiology: Warszawa, Poland, 2015; pp. 47–51. [Google Scholar]
- Iurilli, M.L.; Zhou, B.; Bennett, J.E.; Carrillo-Larco, R.M.; Sophiea, M.K.; Rodriguez-Martinez, A.; Bixby, H.; Solomon, B.D.; Taddei, C.; Danaei, G.; et al. Heterogeneous contributions of change in population distribution of body mass index to change in obesity and underweight. Elife 2021, 10, e60060. [Google Scholar] [CrossRef]
- Gudmundsson, S.; Runarsson, T.P.; Sigurdsson, S.; Eiriksdottir, G.; Johnsen, K. Reliability of quantitative EEG features. Clin. Neurophysiol. 2007, 118, 2162–2171. [Google Scholar] [CrossRef] [PubMed]
- Marzbani, H.; Marateb, H.; Mansourian, M. Methodological Note: Neurofeedback: A Comprehensive Review on System Design, Methodology and Clinical Applications. Basic Clin. Neurosci. J. 2016, 7, 143–158. [Google Scholar] [CrossRef] [PubMed]
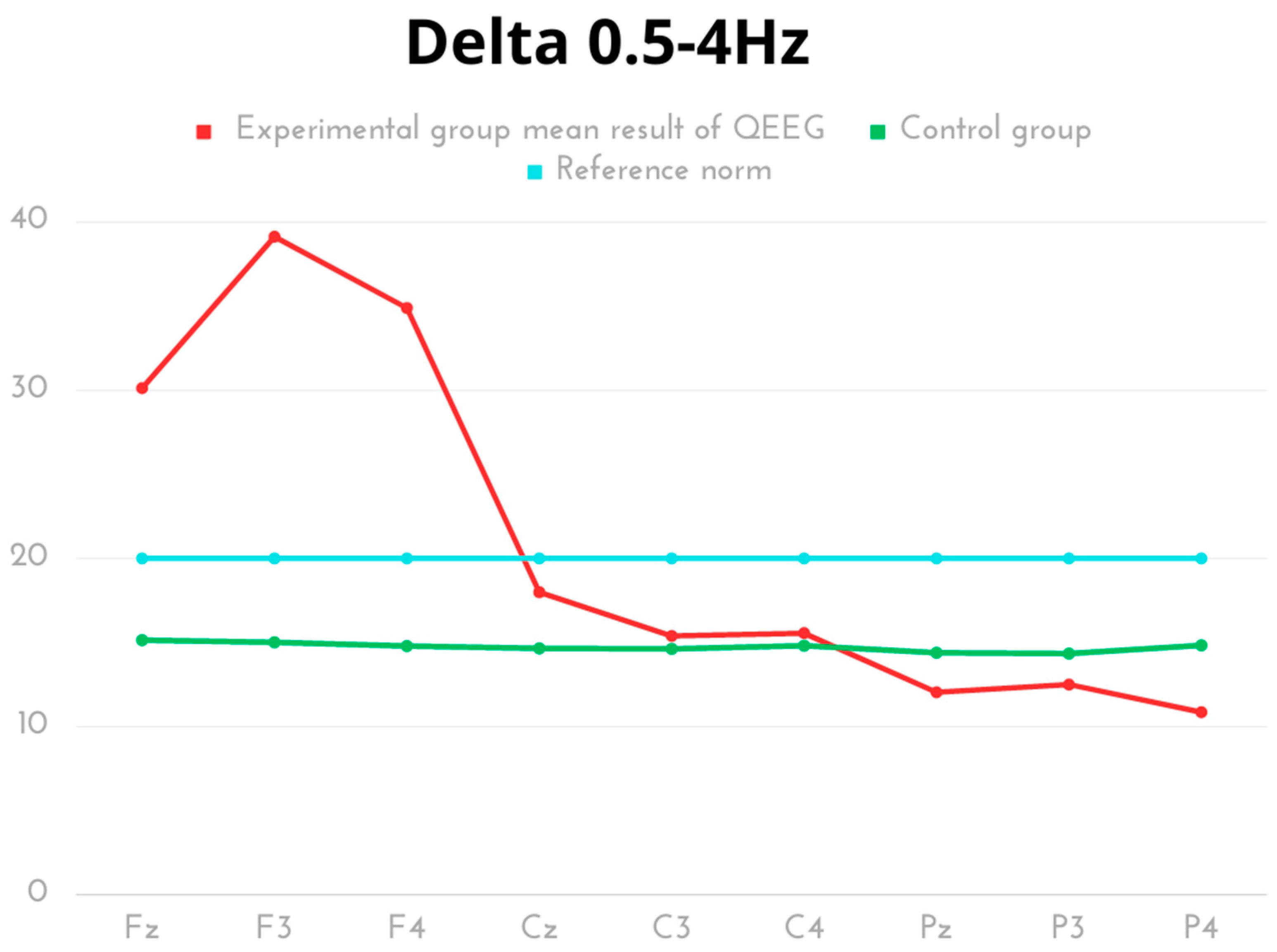
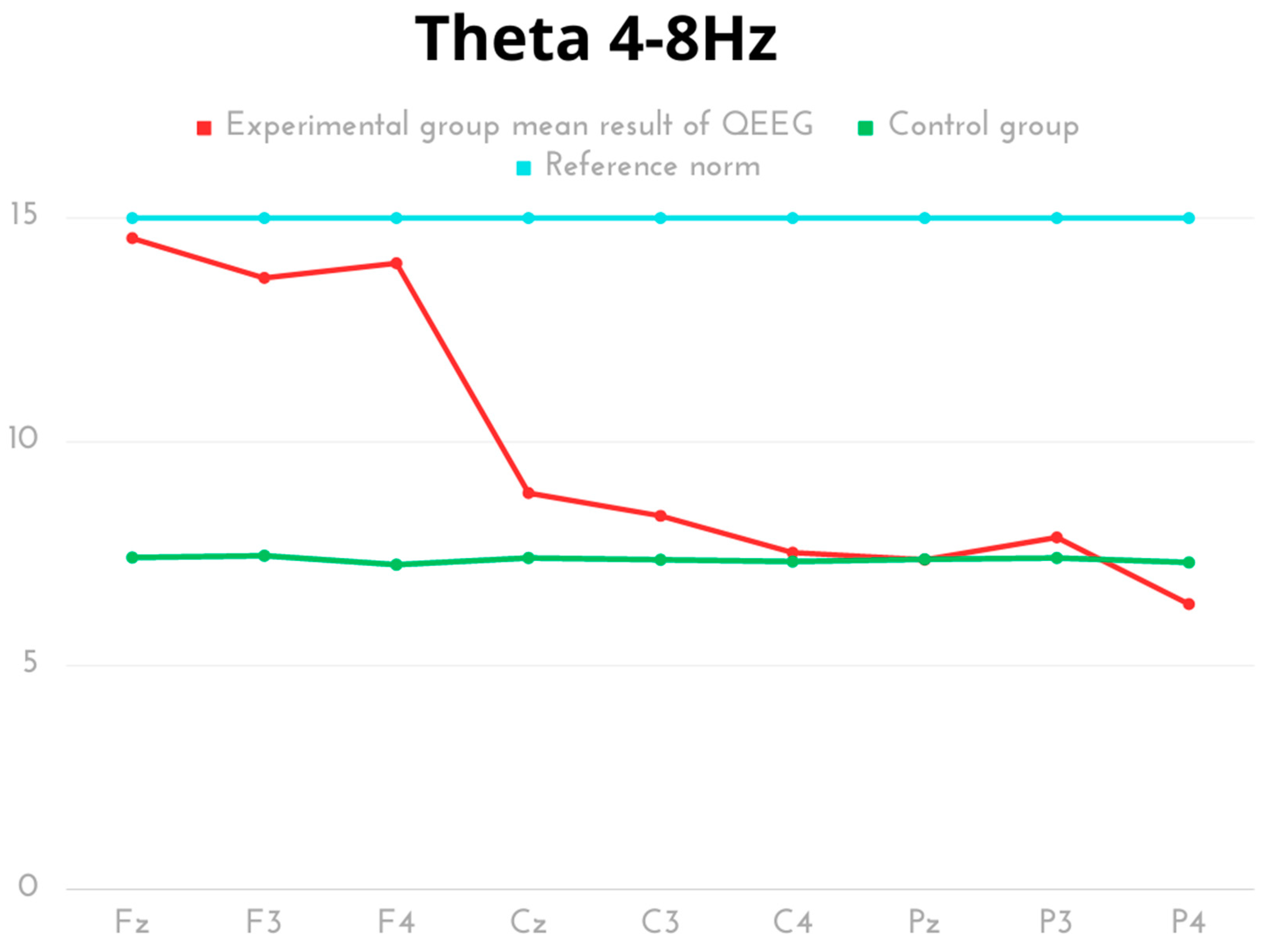
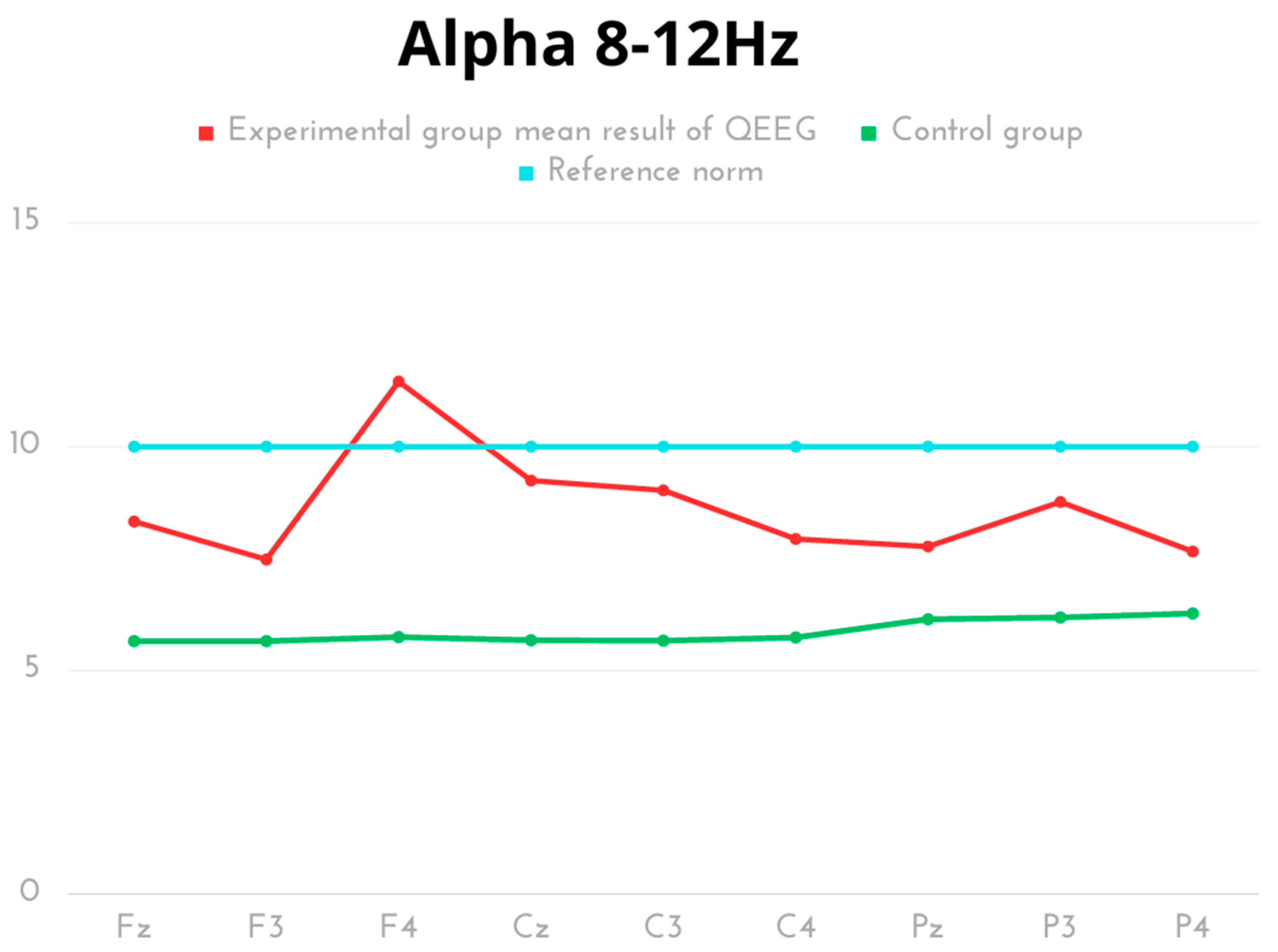
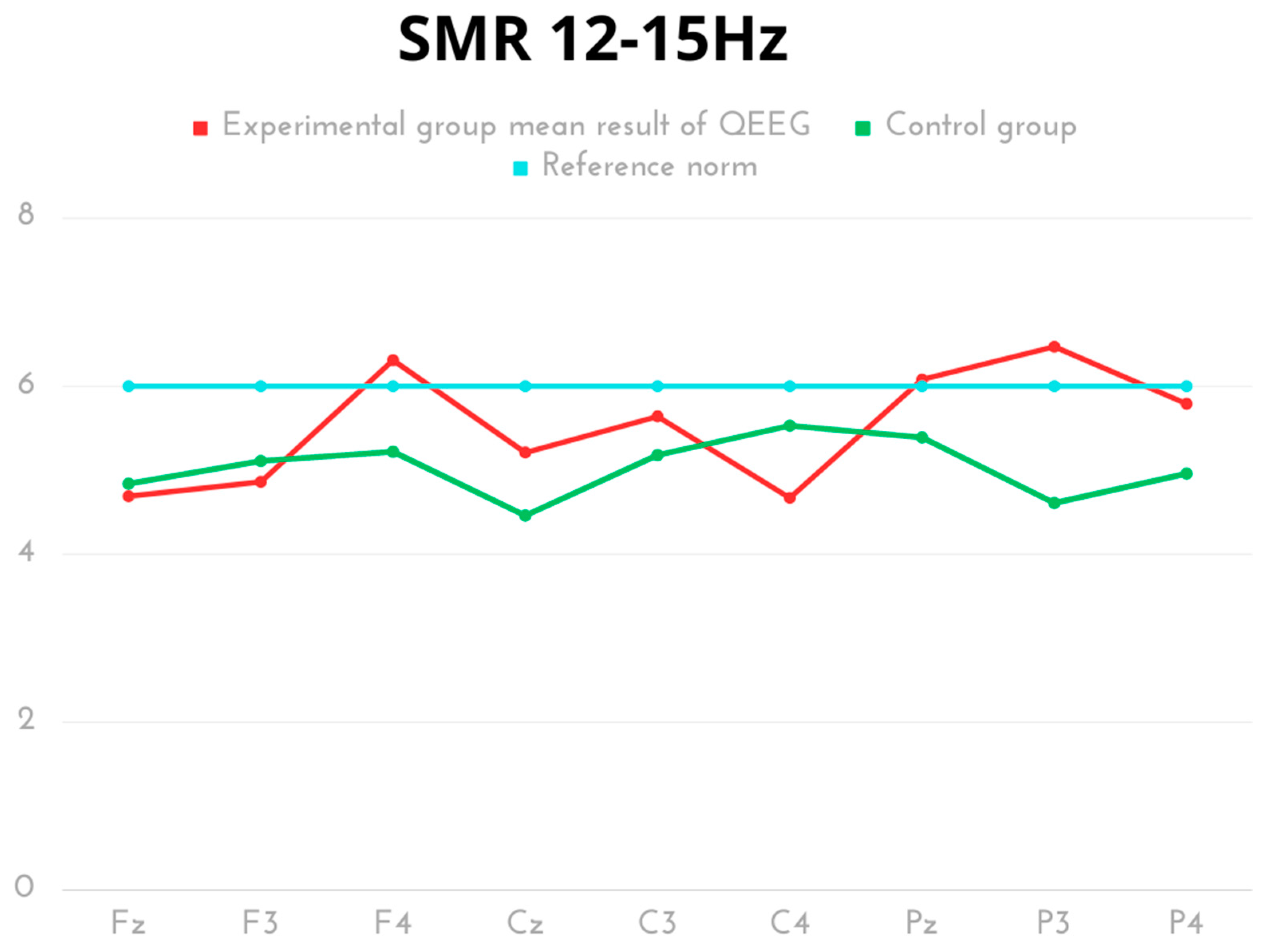

| Delta 0.5–4 Hz | Mean | SD | Min | Max | Q1 | Q3 | p | Cohen’s d |
|---|---|---|---|---|---|---|---|---|
| Experimental Fz | 30.12 | 6.19 | 21.37 | 40.65 | 25.60 | 34.09 | p< 0.001 | 4.23 |
| Control Fz | 15.14 | 0.89 | 14.30 | 16.98 | 14.56 | 15.43 | ||
| Experimental F3 | 39.13 | 6.16 | 33.53 | 50.56 | 34.89 | 43.22 | p< 0.001 | 7.03 |
| Control F3 | 15.01 | 0.70 | 14.23 | 15.98 | 14.43 | 15.56 | ||
| Experimental F4 | 34.89 | 3.81 | 29.59 | 38.74 | 30.62 | 38.64 | p< 0.001 | 8.41 |
| Control F4 | 14.79 | 0.97 | 13.28 | 15.92 | 14.63 | 15.69 | ||
| Experimental Cz | 17.99 | 3.88 | 13.02 | 24.40 | 15.36 | 20.36 | p< 0.001 | 1.52 |
| Control Cz | 14.65 | 0.52 | 14.21 | 15.89 | 14.43 | 14.56 | ||
| Experimental C3 | 15.39 | 3.02 | 12.85 | 20.63 | 13.32 | 18.21 | p = 0.288 | 0.44 |
| Control C3 | 14.62 | 0.46 | 14.23 | 15.90 | 14.43 | 14.56 | ||
| Experimental C4 | 15.55 | 4.10 | 11.63 | 23.24 | 12.05 | 17.72 | p = 0.460 | 0.29 |
| Control C4 | 14.81 | 1.01 | 13.25 | 15.98 | 14.63 | 15.88 | ||
| Experimental Pz | 12.04 | 1.61 | 8.79 | 14.4 | 10.87 | 12.82 | p< 0.001 | 2.04 |
| Control Pz | 14.39 | 0.69 | 13.43 | 15.12 | 13.63 | 14.96 | ||
| Experimental P3 | 12.50 | 1.56 | 10.04 | 14.40 | 11.46 | 14.26 | p< 0.001 | 1.66 |
| Control P3 | 14.34 | 0.66 | 13.39 | 15.29 | 13.63 | 14.76 | ||
| Experimental P4 | 10.85 | 1.67 | 8.66 | 12.82 | 8.79 | 12.47 | p< 0.001 | 3.10 |
| Control P4 | 14.83 | 0.90 | 13.48 | 15.95 | 14.63 | 15.73 |
| Theta 4–8 Hz | Mean | SD | Min | Max | Q1 | Q3 | p | Cohen’s d |
|---|---|---|---|---|---|---|---|---|
| Experimental Fz | 14.55 | 5.30 | 9.19 | 27.55 | 11.77 | 14.91 | p < 0.001 | 2.62 |
| Control Fz | 7.42 | 0.14 | 7.18 | 7.62 | 7.27 | 7.54 | ||
| Experimental F3 | 13.66 | 4.08 | 9.88 | 22.19 | 11.78 | 13.70 | p < 0.001 | 2.97 |
| Control F3 | 7.46 | 0.10 | 7.21 | 7.54 | 7.42 | 7.54 | ||
| Experimental F4 | 13.99 | 3.87 | 9.90 | 22.37 | 11.91 | 14.72 | p < 0.001 | 3.18 |
| Control F4 | 7.26 | 0.36 | 6.54 | 7.89 | 6.98 | 7.47 | ||
| Experimental Cz | 8.86 | 1.70 | 6.69 | 11.67 | 7.38 | 10.10 | p = 0.001 | 1.57 |
| Control Cz | 7.41 | 0.15 | 7.08 | 7.58 | 7.29 | 7.54 | ||
| Experimental C3 | 8.35 | 1.67 | 6.59 | 11.10 | 6.77 | 9.07 | p = 0.018 | 1.05 |
| Control C3 | 7.37 | 0.19 | 7.06 | 7.59 | 7.19 | 7.54 | ||
| Experimental C4 | 7.53 | 1.78 | 5.78 | 11.11 | 6.58 | 8.08 | p = 0.628 | 0.18 |
| Control C4 | 7.33 | 0.43 | 6.54 | 7.83 | 6.95 | 7.65 | ||
| Experimental Pz | 7.37 | 1.63 | 5.14 | 10.07 | 6.19 | 8.16 | p = 0.976 | 0.01 |
| Control Pz | 7.38 | 0.18 | 7.08 | 7.61 | 7.23 | 7.54 | ||
| Experimental P3 | 7.87 | 1.65 | 6.01 | 10.07 | 6.54 | 9.84 | p = 0.241 | 0.51 |
| Control P3 | 7.41 | 0.15 | 7.04 | 7.54 | 7.33 | 7.54 | ||
| Experimental P4 | 6.38 | 1.33 | 5.08 | 8.15 | 5.11 | 7.87 | p = 0.051 | 1.04 |
| Control P4 | 7.31 | 0.46 | 6.54 | 7.89 | 6.86 | 7.65 |
| Alpha 8–12 Hz | Mean | SD | Min | Max | Q1 | Q3 | p | Cohen’s d |
|---|---|---|---|---|---|---|---|---|
| Experimental Fz | 8.32 | 2.87 | 6.31 | 14.34 | 6.42 | 8.62 | p < 0.001 | 1.65 |
| Control Fz | 5.64 | 0.38 | 5.05 | 6.18 | 5.43 | 5.93 | ||
| Experimental F3 | 7.47 | 1.99 | 5.45 | 11.56 | 6.51 | 7.58 | p < 0.001 | 1.54 |
| Control F3 | 5.64 | 0.38 | 5.02 | 6.13 | 5.43 | 5.93 | ||
| Experimental F4 | 11.46 | 11.13 | 4.73 | 35.37 | 5.58 | 10.12 | p = 0.036 | 1.00 |
| Control F4 | 5.73 | 0.33 | 5.29 | 6.17 | 5.39 | 6.03 | ||
| Experimental Cz | 9.24 | 3.95 | 5.58 | 16.82 | 5.66 | 10.70 | p < 0.001 | 1.66 |
| Control Cz | 5.66 | 0.37 | 5.08 | 6.21 | 5.43 | 5.93 | ||
| Experimental C3 | 9.02 | 4.10 | 5.34 | 17.37 | 5.71 | 8.97 | p = 0.001 | 1.52 |
| Control C3 | 5.65 | 0.33 | 5.11 | 6.16 | 5.43 | 5.93 | ||
| Experimental C4 | 7.93 | 3.98 | 4.57 | 16.18 | 4.88 | 7.84 | p = 0.025 | 1.03 |
| Control C4 | 5.72 | 0.31 | 5.29 | 6.17 | 5.40 | 5.97 | ||
| Experimental Pz | 7.76 | 2.53 | 4.68 | 12.07 | 5.62 | 9.64 | p = 0.010 | 1.20 |
| Control Pz | 6.13 | 0.18 | 5.93 | 6.43 | 5.95 | 6.24 | ||
| Experimental P3 | 8.76 | 2.99 | 4.68 | 13.09 | 6.18 | 11.69 | p < 0.001 | 1.51 |
| Control P3 | 6.17 | 0.45 | 5.56 | 6.79 | 5.79 | 6.65 | ||
| Experimental P4 | 7.65 | 2.72 | 4.60 | 11.98 | 4.75 | 9.94 | p = 0.038 | 0.90 |
| Control P4 | 6.26 | 0.36 | 5.79 | 6.83 | 5.99 | 6.61 |
| SMR 12–15 Hz | Mean | SD | Min | Max | Q1 | Q3 | p | Cohen’s d |
|---|---|---|---|---|---|---|---|---|
| Experimental Fz | 4.69 | 0.81 | 3.74 | 6.14 | 4.00 | 5.08 | p = 0.606 | 0.17 |
| Control Fz | 4.84 | 0.93 | 3.73 | 5.92 | 3.73 | 5.78 | ||
| Experimental F3 | 4.86 | 0.87 | 3.67 | 6.11 | 3.83 | 5.31 | p = 0.297 | 0.38 |
| Control F3 | 5.11 | 0.46 | 4.44 | 5.75 | 4.98 | 5.57 | ||
| Experimental F4 | 6.31 | 4.37 | 3.12 | 15.64 | 3.58 | 5.25 | p = 0.300 | 0.44 |
| Control F4 | 5.22 | 0.57 | 4.58 | 5.91 | 4.73 | 5.82 | ||
| Experimental Cz | 5.21 | 1.12 | 3.68 | 7.02 | 4.16 | 5.87 | p = 0.043 | 0.69 |
| Control Cz | 4.46 | 1.04 | 3.12 | 5.73 | 3.92 | 5.73 | ||
| Experimental C3 | 5.64 | 1.29 | 3.79 | 7.58 | 4.40 | 6.34 | p = 0.165 | 0.51 |
| Control C3 | 5.18 | 0.51 | 4.52 | 5.93 | 4.98 | 5.70 | ||
| Experimental C4 | 4.67 | 1.22 | 3.00 | 6.60 | 3.48 | 5.41 | p = 0.009 | 1.02 |
| Control C4 | 5.53 | 0.47 | 4.55 | 5.94 | 5.47 | 5.87 | ||
| Experimental Pz | 6.08 | 1.84 | 3.52 | 9.18 | 4.55 | 7.28 | p = 0.129 | 0.61 |
| Control Pz | 5.39 | 0.42 | 4.61 | 5.92 | 5.23 | 5.81 | ||
| Experimental P3 | 6.47 | 1.81 | 4.39 | 8.80 | 4.45 | 8.47 | p < 0.001 | 1.62 |
| Control P3 | 4.61 | 0.48 | 3.93 | 5.01 | 4.06 | 4.98 | ||
| Experimental P4 | 5.79 | 2.06 | 3.52 | 9.18 | 3.58 | 7.38 | p = 0.116 | 0.59 |
| Control P4 | 4.96 | 0.77 | 3.92 | 5.73 | 4.09 | 5.73 |
| Beta 15–20 Hz | Mean | SD | Min | Max | Q1 | Q3 | p | Cohen’s d |
|---|---|---|---|---|---|---|---|---|
| Experimental Fz | 5.32 | 0.67 | 4.50 | 6.59 | 5.03 | 5.63 | p < 0.001 | 1.71 |
| Control Fz | 4.49 | 0.30 | 4.01 | 4.82 | 4.46 | 4.82 | ||
| Experimental F3 | 6.48 | 1.60 | 4.98 | 9.77 | 5.60 | 6.77 | p < 0.001 | 2.09 |
| Control F3 | 4.49 | 0.30 | 4.01 | 4.82 | 4.46 | 4.82 | ||
| Experimental F4 | 8.04 | 5.25 | 4.09 | 19.30 | 5.58 | 6.69 | p = 0.008 | 1.27 |
| Control F4 | 4.58 | 0.18 | 4.39 | 4.82 | 4.44 | 4.82 | ||
| Experimental Cz | 5.75 | 0.94 | 4.810 | 7.59 | 5.20 | 6.19 | p < 0.001 | 2.40 |
| Control Cz | 4.42 | 0.17 | 4.12 | 4.56 | 4.39 | 4.56 | ||
| Experimental C3 | 6.04 | 1.06 | 4.80 | 7.83 | 5.38 | 6.93 | p < 0.001 | 1.41 |
| Control C3 | 4.83 | 0.66 | 4.32 | 5.98 | 4.32 | 4.87 | ||
| Experimental C4 | 5.44 | 0.92 | 4.21 | 6.88 | 4.82 | 6.27 | p = 0.073 | 0.62 |
| Control C4 | 4.95 | 0.65 | 4.36 | 5.82 | 4.41 | 5.82 | ||
| Experimental Pz | 5.59 | 1.056 | 4.21 | 7.51 | 4.53 | 6.33 | p < 0.001 | 1.66 |
| Control Pz | 4.46 | 0.30 | 4.01 | 4.78 | 4.33 | 4.78 | ||
| Experimental P3 | 6.48 | 1.04 | 4.91 | 7.96 | 5.89 | 7.44 | p < 0.001 | 2.85 |
| Control P3 | 4.61 | 0.27 | 4.38 | 5.01 | 4.38 | 4.85 | ||
| Experimental P4 | 5.66 | 1.10 | 4.43 | 7.51 | 4.44 | 6.35 | p < 0.001 | 1.66 |
| Control P4 | 4.48 | 0.32 | 4.08 | 4.88 | 4.28 | 4.88 |
| Beta 2 20–35 Hz | Mean | SD | Min | Max | Q1 | Q3 | p | Cohen’s d |
|---|---|---|---|---|---|---|---|---|
| Experimental Fz | 6.53 | 0.59 | 5.66 | 7.96 | 6.16 | 6.89 | p < 0.001 | 2.15 |
| Control Fz | 4.97 | 0.86 | 4.12 | 5.96 | 4.12 | 5.89 | ||
| Experimental F3 | 8.89 | 2.72 | 6.06 | 14.67 | 7.31 | 9.41 | p < 0.001 | 2.82 |
| Control F3 | 4.72 | 0.24 | 4.51 | 5.12 | 4.51 | 4.74 | ||
| Experimental F4 | 9.90 | 3.29 | 5.21 | 16.17 | 7.08 | 12.56 | p < 0.001 | 2.61 |
| Control F4 | 5.00 | 0.47 | 4.34 | 5.51 | 4.58 | 5.51 | ||
| Experimental Cz | 7.03 | 0.76 | 6.18 | 9.25 | 6.25 | 7.49 | p < 0.001 | 2.43 |
| Control Cz | 5.00 | 0.91 | 4.07 | 5.96 | 4.07 | 5.89 | ||
| Experimental C3 | 7.34 | 1.02 | 6.14 | 9.82 | 6.40 | 7.64 | p < 0.001 | 3.35 |
| Control C3 | 4.76 | 0.52 | 4.32 | 5.69 | 4.46 | 4.65 | ||
| Experimental C4 | 7.57 | 0.96 | 6.38 | 9.28 | 6.69 | 7.89 | p < 0.001 | 1.90 |
| Control C4 | 5.79 | 0.91 | 4.82 | 6.91 | 4.95 | 6.91 | ||
| Experimental Pz | 7.29 | 0.83 | 6.18 | 9.28 | 6.69 | 7.73 | p < 0.001 | 2.72 |
| Control Pz | 4.92 | 0.91 | 4.01 | 5.89 | 4.01 | 5.88 | ||
| Experimental P3 | 8.02 | 0.79 | 7.08 | 9.29 | 7.10 | 8.46 | p < 0.001 | 3.08 |
| Control P3 | 5.28 | 0.99 | 4.17 | 6.51 | 4.34 | 6.51 | ||
| Experimental P4 | 7.54 | 1.09 | 5.85 | 8.83 | 7.14 | 8.60 | p < 0.001 | 3.00 |
| Control P4 | 4.99 | 0.61 | 4.22 | 5.54 | 4.32 | 5.54 |
| Parameter | Mean | SD | Min | Max | Q1 | Q3 |
|---|---|---|---|---|---|---|
| Body mass [kg] | 81.16 | 8.43 | 69.60 | 91.80 | 73.55 | 89.30 |
| Body height [cm] | 178.50 | 4.87 | 170.0 | 185.00 | 175.50 | 182.00 |
| BMI | 25.30 | 2.33 | 21.50 | 28.40 | 23.55 | 27.05 |
| BF [%] | 16.80 | 5.07 | 7.10 | 24.60 | 13.50 | 21.30 |
| FFM [kg] | 13.94 | 5.30 | 5.20 | 22.00 | 9.85 | 19.40 |
| LMB [kg] | 67.21 | 4.51 | 59.10 | 74.60 | 64.60 | 70.20 |
| TBW [kg] | 46.71 | 3.18 | 42.80 | 52.40 | 44.00 | 49.40 |
| SMM [kg] | 63.87 | 4.32 | 56.10 | 70.90 | 61.35 | 66.75 |
| BMD [kg] | 3.34 | 0.19 | 3.00 | 3.70 | 3.25 | 3.45 |
| Metabolic age [years] | 22.91 | 8.72 | 13.00 | 38.00 | 15.50 | 32.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rydzik, Ł.; Ambroży, T.; Pałka, T.; Wąsacz, W.; Spieszny, M.; Perliński, J.; Król, P.; Kopańska, M. Preliminary Development of a Brainwave Model for K1 Kickboxers Using Quantitative Electroencephalography (QEEG) with Open Eyes. Int. J. Mol. Sci. 2023, 24, 8882. https://doi.org/10.3390/ijms24108882
Rydzik Ł, Ambroży T, Pałka T, Wąsacz W, Spieszny M, Perliński J, Król P, Kopańska M. Preliminary Development of a Brainwave Model for K1 Kickboxers Using Quantitative Electroencephalography (QEEG) with Open Eyes. International Journal of Molecular Sciences. 2023; 24(10):8882. https://doi.org/10.3390/ijms24108882
Chicago/Turabian StyleRydzik, Łukasz, Tadeusz Ambroży, Tomasz Pałka, Wojciech Wąsacz, Michał Spieszny, Jacek Perliński, Paweł Król, and Marta Kopańska. 2023. "Preliminary Development of a Brainwave Model for K1 Kickboxers Using Quantitative Electroencephalography (QEEG) with Open Eyes" International Journal of Molecular Sciences 24, no. 10: 8882. https://doi.org/10.3390/ijms24108882
APA StyleRydzik, Ł., Ambroży, T., Pałka, T., Wąsacz, W., Spieszny, M., Perliński, J., Król, P., & Kopańska, M. (2023). Preliminary Development of a Brainwave Model for K1 Kickboxers Using Quantitative Electroencephalography (QEEG) with Open Eyes. International Journal of Molecular Sciences, 24(10), 8882. https://doi.org/10.3390/ijms24108882












