1. Introduction
Metastasis is the leading cause of increased mortality rates in cancer patients, accounting for 90% of cancer-related deaths [
1]. During this complex multistep invasion-metastasis cascade, cancer cells invade distant organs through the lymphatic system or blood circulation by intravasation into blood vessels and then attachment to endothelial cells (ECs) that line blood vessels, allowing cancer cells to extravasate at secondary metastatic sites and penetrate tissues or organs, and then start to proliferate [
2]. Thus, this process cannot be accomplished without neovascularization. In 1971, Judah Folkman discovered that tumor growth is angiogenesis-dependent and tumors cannot grow more than 1–2 mm
3 in diameter without vascularization [
3]. Angiogenesis plays a pivotal role in tumor metastasis development, as it aids in the formation of new blood vessels that supply tumor cells with oxygen and nutrients to sustain their growth and dissemination [
4]. In addition, angiogenesis is associated with normal physiological processes vital for wound healing, embryogenesis, inflammation, and immune responses and contributes to pathological processes, including diabetes, inflammatory disorders, and cancer [
5,
6]. Angiogenesis occurs through distinct biological steps, including endothelial cell proliferation, migration, and organization into vessels. This cascade of events is tightly regulated by maintaining a balance between endothelial cells’ stimulatory pro- and antiangiogenic factors. This balance is disturbed by the prevalence of angiogenic factors in cancer, such as the vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), and chemokines, which led to the concept of the ‘angiogenic switch’ [
7,
8,
9]. Therefore, targeting angiogenesis in cancer therapy has become an attractive but challenging approach that will require the design and development of antiangiogenic molecules.
Several studies have identified multiple angiogenic inhibitors that aim to prevent the growth of blood vessels; one of the angiogenic inhibitors that has been recently studied is the 16-kilodalton fragment of prolactin (16 kDa PRL) [
10,
11,
12]. Prolactin belongs to the family of polypeptide hormones that also comprise growth hormone and placental lactogen. Members of this family share many similarities in structural and biological features [
13]; thus, we are interested in testing a similar fragment from the growth hormone that has not been extensively studied. The 14-kilodalton human growth hormone (14 kDa hGH) N-terminal fragment is cleaved from its 22 kD full-length proangiogenic counterpart by thrombin and plasmin [
14,
15]. A high level of hGH was associated with an increase in cell proliferation and cancer promotion [
16]. Targeting the hGH receptor reduces tumor progression and epithelial-mesenchymal transition in human melanoma cells [
17]. However, the effect of 14k hGH-derived fragments on tumor melanoma or other types of cancers has not been investigated until now. This molecule belongs to the family of inhibins and attains antiangiogenic properties, unlike its native form; however, the role of this fragment in cancer remains not fully investigated [
18,
19]. In this study, we showed that 14 kDa hGH is capable of inhibiting tumor growth, angiogenesis, and metastasis and reducing angiogenesis in endothelial cells; we also showed that the antiangiogenic function of 14 kDa hGH is mediated by the plasminogen activator inhibitor-1 (PAI-1) molecule. Our data demonstrated, for the first time, the in vivo antitumoral, antiangiogenic, and antimetastatic effects of 14 kDa hGH. It was also suggested that the 14 kDa hGH fragment could be used as an antiangiogenic molecule to reduce primary cancer and block metastasis formation.
3. Discussion
The 14 kDa hGH fragment belongs to the family of vasoinhibins, in which the members of this family share similar structural and functional properties and exert various effects on endothelial cells, such as inhibiting vasodilation, vasopermeability, and angiogenesis [
20,
21]; therefore, we are interested in investigating the role of the 14 kDa human growth hormone that has not been extensively studied compared to the 16kDa fragment of prolactin [
11,
12,
22,
23,
24,
25,
26,
27], and to understand their underlying mechanisms of action.
In the present study, we demonstrated the antitumoral, antiangiogenic, and antimetastatic functions of 14 kDa hGH using complementary in vitro and in vivo models. In vitro, 14 kDa hGH instigated an inhibition of cellular proliferation, migration, and angiogenesis and induced cellular apoptosis in B16-F10 tumor cells. Furthermore, we confirmed the antiangiogenic functions of 14 kDa hGH on HBME cells, as indicated by a significant decrease in cell survival, cell migration, tube formation abilities, and a notable increase in cellular apoptosis. In concordance with previous studies demonstrating the role of 16kD PRL in reducing B16-F10, HCT116, and PC-3 tumor cell growth in vivo [
11,
28,
29], we have shown that 14 kDa hGH significantly reduces primary tumor growth and metastasis in C57BL/6 mice. The diminution in tumor growth was accompanied by a marked reduction in the number of tumor blood vessels and a change in vessel morphology determined by vessel immunostaining and quantification on tumor sections, suggesting that decreased blood vessel formation may contribute to reduced tumor growth after 14 kDa hGH release from the B16-F10 14 kDa hGH tumors. Similarly, the lungs of mice subcutaneously injected with B16-F10 14 kDa hGH showed almost no tumor nodules compared to their corresponding controls. This reduction in the number of tumor nodules could be attributed either to a direct effect of 14 kDa hGH on tumor cells or an indirect reduction in angiogenesis.
Since we previously demonstrated a major role of PAI-1 in mediating the antiangiogenic effects of 16kDa PRL [
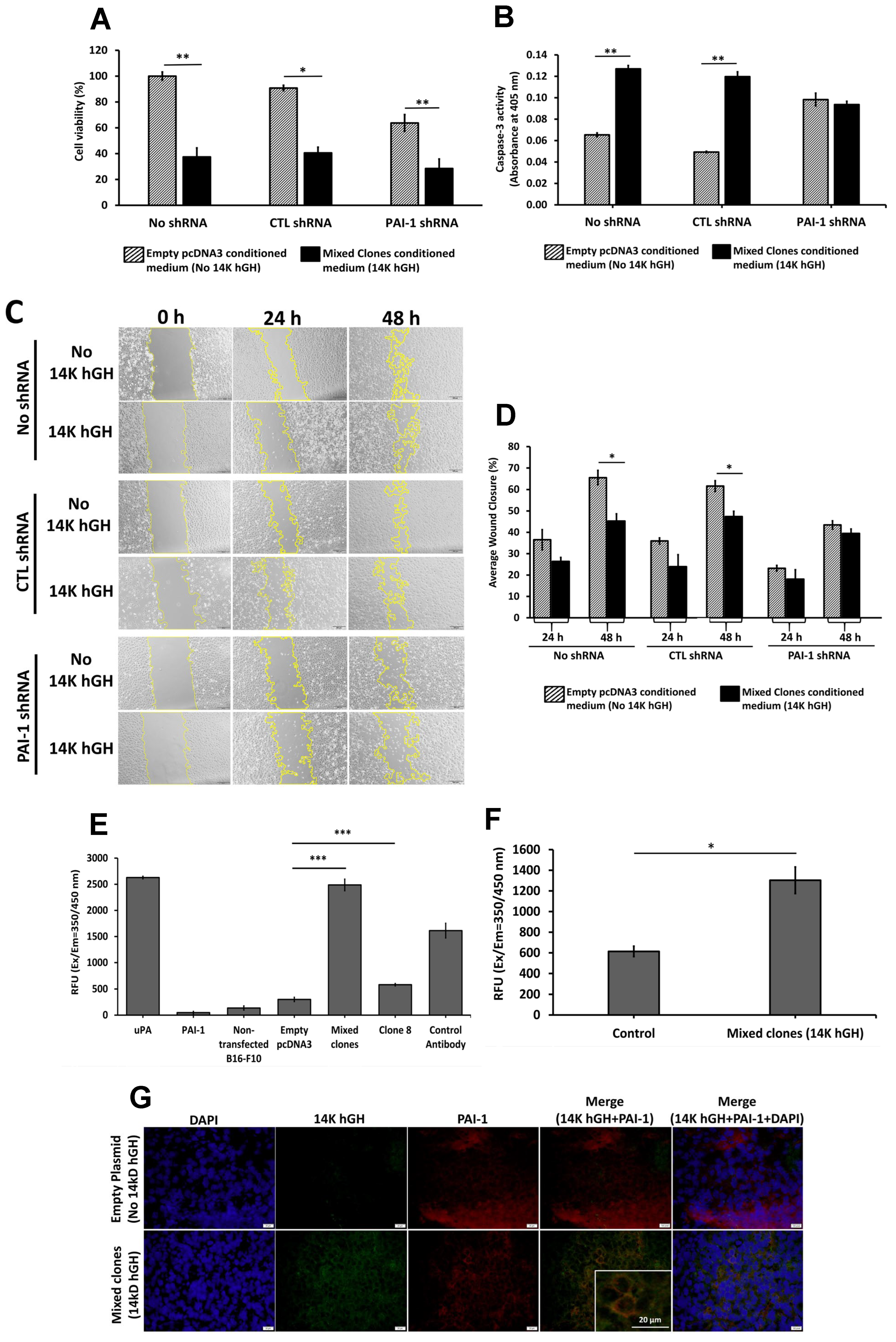
12], we treated HBME cells stably transfected with PAI-1 shRNA with conditioned media collected from B16-F10 14 kDa hGH transfected cells. The downregulation of PAI-1 expression in HBME cells abolished the antimigratory and proapoptotic effects of 14 kDa hGH and confirmed the key role of PAI-1 in mediating the antiangiogenic function of 14 kDa hGH. However, the reduction in PAI-1 expression did not affect the 14 kDa hGH antisurvival activity.
Further validation of the PAI-1/14 kDa hGH interaction hypothesis is the inhibitory effect of 14 kDa hGH-rich conditioned media on recombinant PAI-1 activity and the increase in uPA activity in 14 kDa hGH tumors compared to control tumors. Those data corroborate the previously described function of PAI-1 as a major fibrin scaffold stabilizer for endothelial cell growth and motility [
30]. The stable downregulation of PAI-1 expression in HBME cells was associated with a significant reduction in cellular proliferation and migration and an increase in cellular apoptosis, which confirms our previous study [
31].
Several antiangiogenic molecules were developed, such as anti-VEFG, with high potential for therapeutic use but with substantial side effects [
32]. The 14 kDa hGH is generated by proteolytic cleavage of the growth hormone. The same applies to angiostatin and endostatin, which are derived from plasminogen and collagen cleavage, respectively. Those molecules are strong antiangiogenic molecules with fewer side effects [
33]. Therefore, targeting cancer using 14 kDa hGH or its derived peptides will have fewer side effects since few physiological functions are associated with 14 kDa hGH. Subsequently, 14 kDa hGH will require more extensive studies to establish its efficiency and safety compared to other antitumoral and antiangiogenic molecules.
Contrary to the full-length growth hormone with various vital physiological functions in the organism, the generated 14 kDa hGH does not induce the same effects as its full-length counterpart, and its physiological effects are limited to vasopermeability, vasodilation, and angiogenesis. In our experiment, 14 kDa hGH was expressed by tumor cells to ensure local expression of the molecule. However, a systemic expression of 14 kDa GH would be possible using an adenovirus vector, as we previously performed using 16kDa PRL [
11]. Since we showed that 14 kDa hGH requires PAI-1 to mediate its antitumoral and antiangiogenic effects, the levels of PAI-1 expressed by tumor cells or in the tumor microenvironment will be a determinant factor for the efficiency of 14 kDa hGH. Therefore, testing 14 kDa hGH in additional tumor models will validate our finding.
It is well established that high levels of PAI-1 are correlated with poor prognosis in several types of tumors [
34], which could be explained by its proangiogenic functions [
12,
35]. Inhibiting PAI-1 with 14 kDa hGH could be one of the mechanisms by which 14 kDa hGH could mediate its antiangiogenic effects. We previously demonstrated that 16kDa PRL requires PAI-1 to induce its antiangiogenic and antitumoral effects and that PAI-1 is a binding partner for 16kDa PRL responsible for the internalization of the 16kD PRL into endothelial cells [
12]. Our data showed the colocalization of 14 kDa hGH and PAI-1 on the cell membrane of tumor cells expressing 14 kDa hGH. The colocalization in the tumor tissues could be preliminary evidence of a direct interaction between the two molecules. However, the affinity and dynamic interaction between 14 kDa GH and PAI-1 require additional experiments, and the mechanism regarding 14 kDa hGH internalization remains unclear and requires further investigation.
In conclusion, we demonstrated for the first time that 14 kDa hGH attains antitumoral, antimetastatic, and antiangiogenic functions and could be used as a new therapeutic molecule. We also provide preliminary evidence for the involvement of PAI-1 in its antiangiogenic functions. Further studies are needed to deeply understand its mechanism of interaction with PAI-1 in the pathology of cancer and other pathologies related to high levels of PAI-1.
4. Materials and Methods
4.1. Plasmid Construction
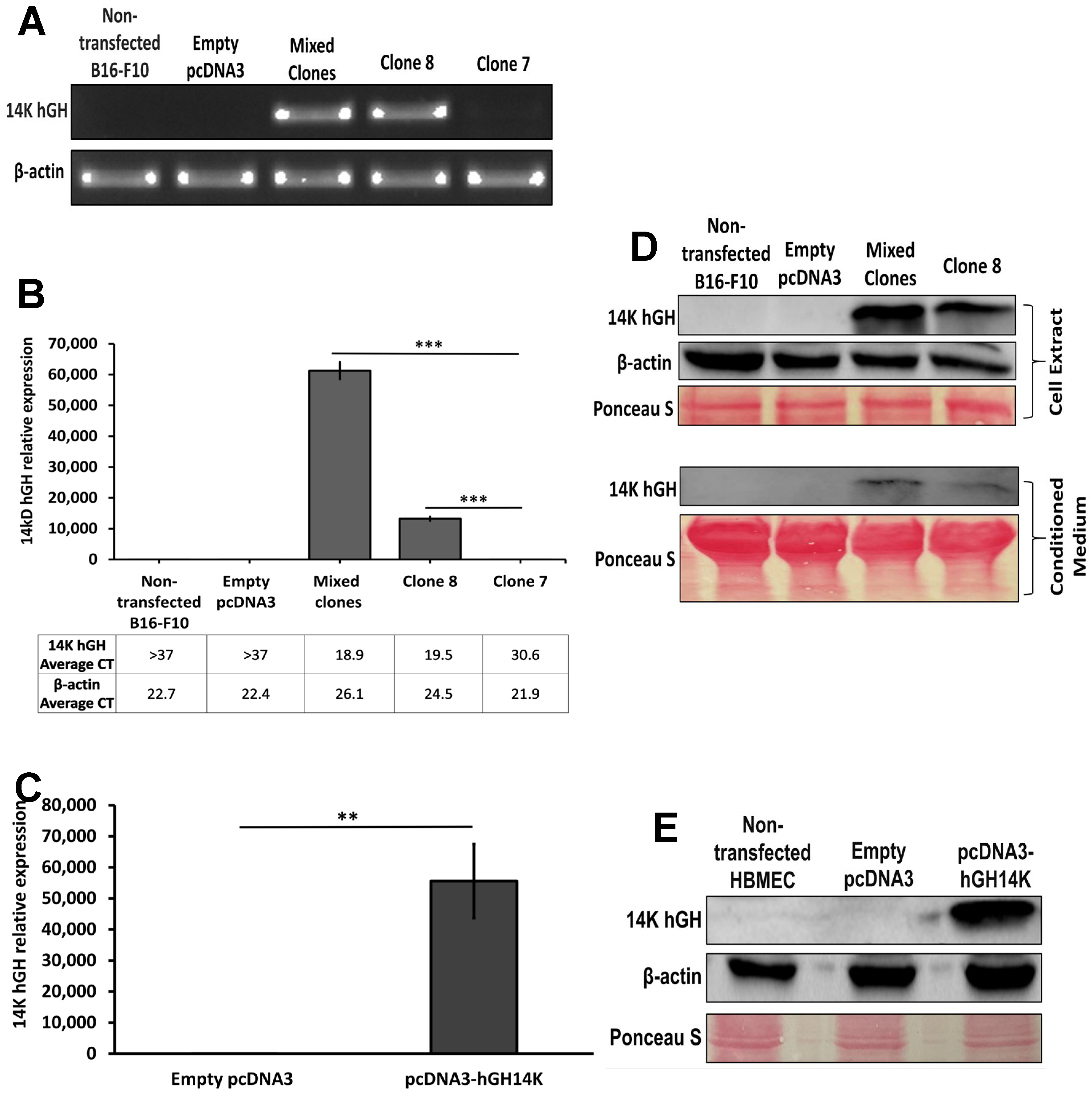
The 14 kDa hGH cDNA sequence was digested from the expression vector Pshuttle2-hGH14K by KpnI and NdeI restriction enzymes (Promega, Madison, WI, USA) and subcloned into KpnI and NdeI restriction sites in the pcDNA3 vector. The newly constructed expression vector contains an ampicillin and neomycin resistance gene cassette, allowing for the selection and maintenance of the transfected clones. Subsequently, the pcDNA3 expression vector was expanded using DHa5-competent E. coli and extracted using the maxiprep plasmid extraction kit (Sigma-Aldrich, MO, USA) according to the manufacturer’s protocol. Sanger sequencing was performed using the BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s protocol to verify the incorporation of 14 kDa hGH insert in the correct orientation within the pcDNA3 plasmid vector. An empty pcDNA3 plasmid vector was used as a negative control.
4.2. Cell Culture
B16-F10 mouse melanoma cells were acquired from the American Type Culture Collection (CRL-6475, Rockville, MD) and cultured in Dulbecco’s modified eagle medium-high glucose containing 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (all from Sigma-Aldrich, saint louis, MO, USA). Human brain microvascular endothelial cells (HBMECs) were surgically isolated from resected brain tissues of children with seizure disorders, as previously described [
36]. The cells were maintained in RPMI-1640 medium with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin (Pen/Strep) (all from Sigma-Aldrich, saint louis, MO, USA). Both cell lines were incubated in a 5% CO
2 humidified chamber at 37 °C.
4.3. Cell Transfection
B16-F10 and HBME cells were seeded at a density of 8 × 10
5 cells/60 mm
2 culture plate. After reaching 80% confluency, 11 µg of the control pcDNA3 or the pcDNA3-14 kDa hGH was used to transfect B16-F10 cells or HBME cells using Lipofectamine™ 3000 according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA, USA). Stably transfected B16-F10 cells were then selected using 800 µg/mL of geneticin (G418; InvivoGen, Carlsbad, CA, USA). Individual clones were picked out, grown to confluence, and expanded for further analyses; the remaining resistant cells were allowed to grow and amalgamate to form the mixed clones. The HBME cells were used 48 h after transient transfection with the control pcDNA3 plasmid or the pcDNA3-14 kDa hGH plasmid. Short-hairpin RNA was used to suppress the secretion of PAI-1 in HBME cells using the pSIH-H1 cloning and expression Lentivectors Kit (System Biosciences, Palo Alto, CA, USA) following the manufacturer’s protocol. Human PAI-1 cDNA (GenBank accession number X12701) (5′-AAGGACGAGATCAGCACCACA-3′) or scrambled sequence as previously described [
31] were used to transfect HBME cells with Lipofectamine™ 3000 according to the manufacturer’s protocols (Invitrogen, Carlsbad, CA, USA). Copepod green fluorescent protein (copGFP) was used as a reporter for the transfected/transduced cells. The cells with higher shRNA expression levels were selected after two copGFP cell sortings.
4.4. Reverse Transcription and Real-Time PCR
Total RNA was extracted from B16-F10 and HBME cells using RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocols. Then, 500 ng of total RNA were used to synthesize cDNA using SuperScript™ First-Strand Synthesis System (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions. Next, cDNA was used for reverse transcription or qPCR using REDTaq ® ReadyMix™ PCR Reaction Mix (Sigma-Aldrich, Saint Louis, MO, USA) or 5x HOT FIREPol® EvaGreen® qPCR Supermix (Solis BioDyne, Tartu, Estonia), respectively. The following primers were used: mouse β-actin (Internal control), forward: 5’-AGCTTCTTTGCAGCTCCTTC-3’, reverse: 5’-CCACCATCACACCCTGGT-3’; 14 kDa hGH, forward: 5’-CAGTGCCTTCCCAACCATTC-3’, reverse: 5’-GGAGCAGCTCTAGGTTGGAT-3’. The relative expression of the 14 kDa hGH gene was calculated according to the method. The reverse transcription and qPCR were performed in three separate wells for each condition.
4.5. Protein Extraction and Western Blotting
Transfected B16-F10 and HBME cells were harvested and lysed using an ice-cold lysis buffer containing 150 mM NaCl, 50 mM Tris-HCl, 5 mM EDTA, 1% Triton X-100, 10% glycerol, and 1x Protease and Phosphatase Inhibitor Cocktail (Thermo Fisher Scientific, Waltham, MA, USA). Cell lysates and B16-F10 conditioned media were centrifuged at 18,000× g for 15 min at 4 °C. Soluble protein concentrations in cell lysates were then quantified using Micro BCA™ Protein Assay Kit (Thermo Fisher Scientific) following the manufacturer’s protocols. Denatured protein samples were resolved on 12% SDS-PAGE gel and then transferred onto a nitrocellulose membrane (Bio-Rad, Hercules, CA, USA). Equal protein loading was subsequently confirmed using Ponceau S staining solution. Thereafter, the membranes were blocked overnight at 4 °C with 5% skimmed milk in Tris-Buffered Saline Buffer with Tween® 20 (TBST), followed by incubation for 2 h with primary antibodies: rabbit polyclonal anti-14 kDa hGH antibody (1:1000 dilution; in-house developed; a gift from Dr. Jean Closset, University of Liege, Belgium) and rabbit monoclonal anti-β-actin antibody (1:1000 dilution; 4970; Cell Signaling Technologies, Danvers, MA, USA). Subsequently, the membranes were incubated with HRP-conjugated goat antirabbit IgG secondary antibody (1:1000 dilution; 7074; Cell Signaling Technologies, Danvers, MA, USA) for 1 h at room temperature. The immunoblots were visualized on ChemiDoc Imaging Systems (Bio-Rad, Hercules, CA, USA) using Clarity™ Western ECL Substrate (Bio-Rad, Hercules, CA, USA).
4.6. Cell Viability Assay
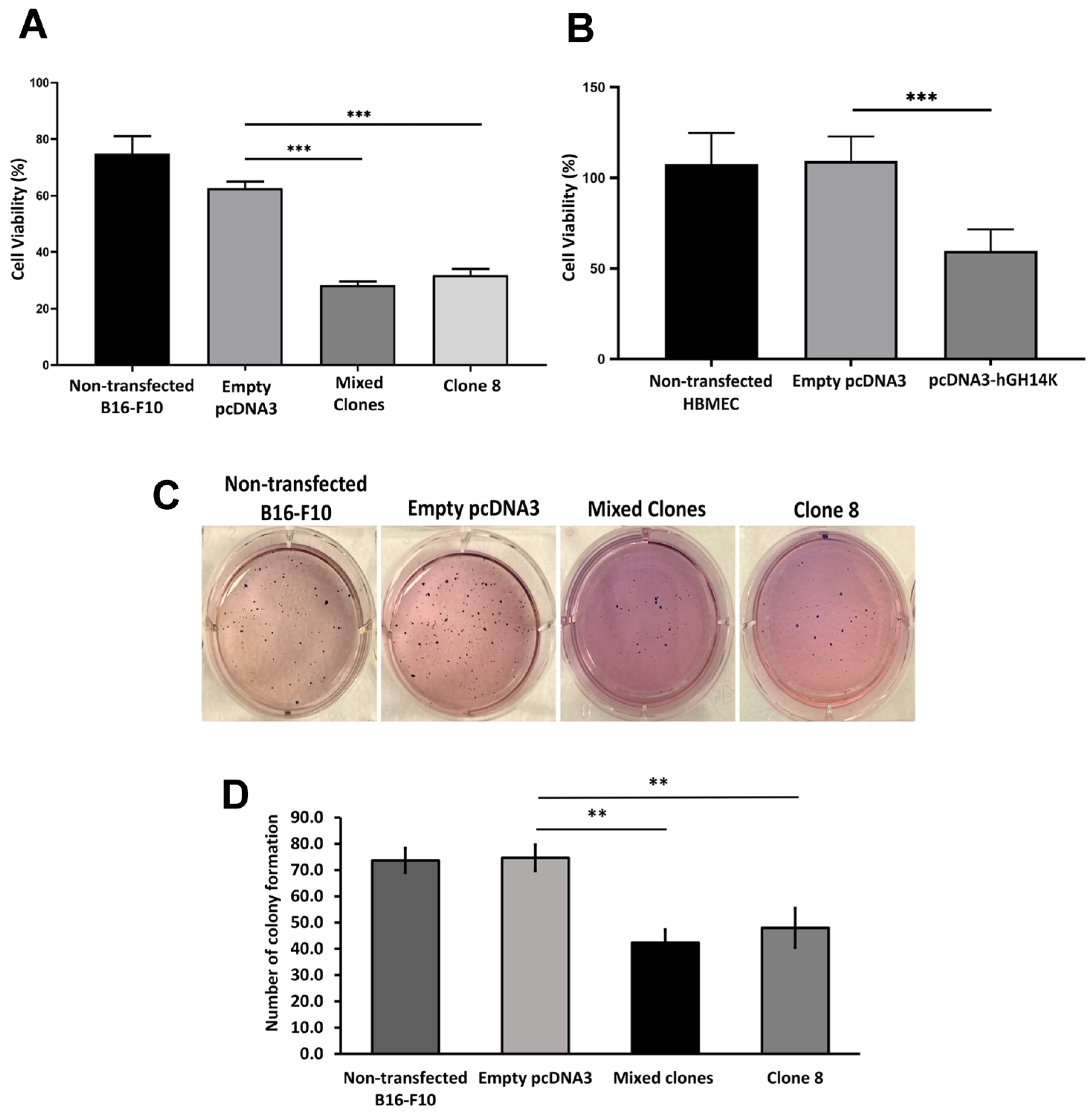
Cell proliferation was quantified using the MTT assay [bromide-soluble tetrazolium dye, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium] (Sigma-Aldrich, MO, USA). Transfected B16-F10 and HBME cells with the control pcDNA3 plasmid or the pcDNA3-14 kDa hGH plasmid (3 × 103 cells/well) were seeded in triplicates into a 96-well plate. In addition, HBME cells transfected with control plasmid or PAI-1 shRNA plasmid were seeded at a density of 5 × 103 cells/well and treated with B16-F10 empty pcDNA3 (control) or B16-F10 mixed clones (14 kDa hGH) conditioned media for 48 h. After 48 h of incubation, the MTT reagent (5 mg/mL) was loaded onto the cells and incubated for 3 h in a humid chamber with 5% CO2 at 37 °C. After 3 h, the produced formazan crystals were solubilized using dimethyl sulfoxide (DMSO). The color absorbance was measured at 570 nm wavelength using a microplate reader. Cell viability was normalized to the nontransfected cells used as controls.
4.7. Cell Apoptosis
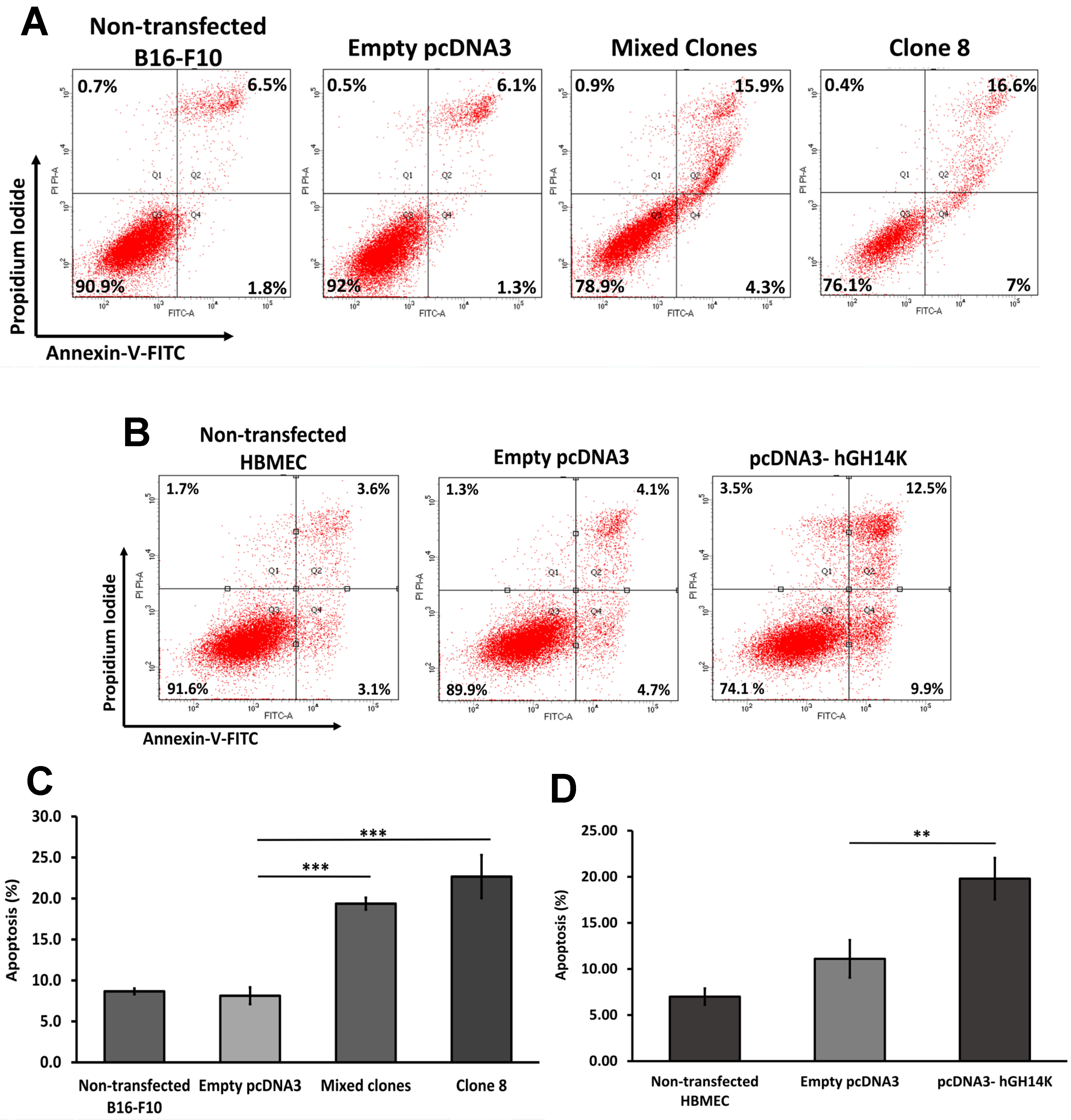
The degree of apoptosis in B16-F10 and HBME cells was evaluated using the Annexin V-FITC Apoptosis Detection Kit (Thermo Fisher Scientific, MA, USA) according to the manufacturer’s protocol. Briefly, floating and attached cells were collected 48 h postincubation, washed with PBS, suspended in Annexin-V binding buffer, and stained with Propidium Iodide and Annexin V-FITC. Stained cells were then analyzed using the BD FACSAria III cell sorter at 475/650 nm excitation/emission spectra (BD Biosciences, Franklin Lakes, NJ, USA). The results were analyzed using FlowJo software (BD Bioscience, NJ, USA). The experiment was performed in triplicates for each condition and compared to cells transfected with empty pcDNA3 plasmid using a 2-tailed student’s t-test.
4.8. Soft Agar Colony Formation Assay
Anchorage-independent soft agar colony formation assay was implemented as previously described [
37]. Briefly, B16-F10 cells (5 × 10
3) were mixed with a complete growth medium and 0.6% noble agar and then placed on top of a 1% noble agar layer in each well of a 6-well plate. The plate was incubated at room temperature in a culture hood for 30 min, allowing the cell/agar mixture to solidify. Cells were maintained for 21 days in a humidified chamber at 37 °C, with fresh medium replacement twice weekly. Colonies were stained with 0.5% crystal violet in 25% methanol, photographed, and counted in triplicate wells. Cells transfected with empty pcDNA3 plasmid were considered as a control condition.
4.9. Cell Migration
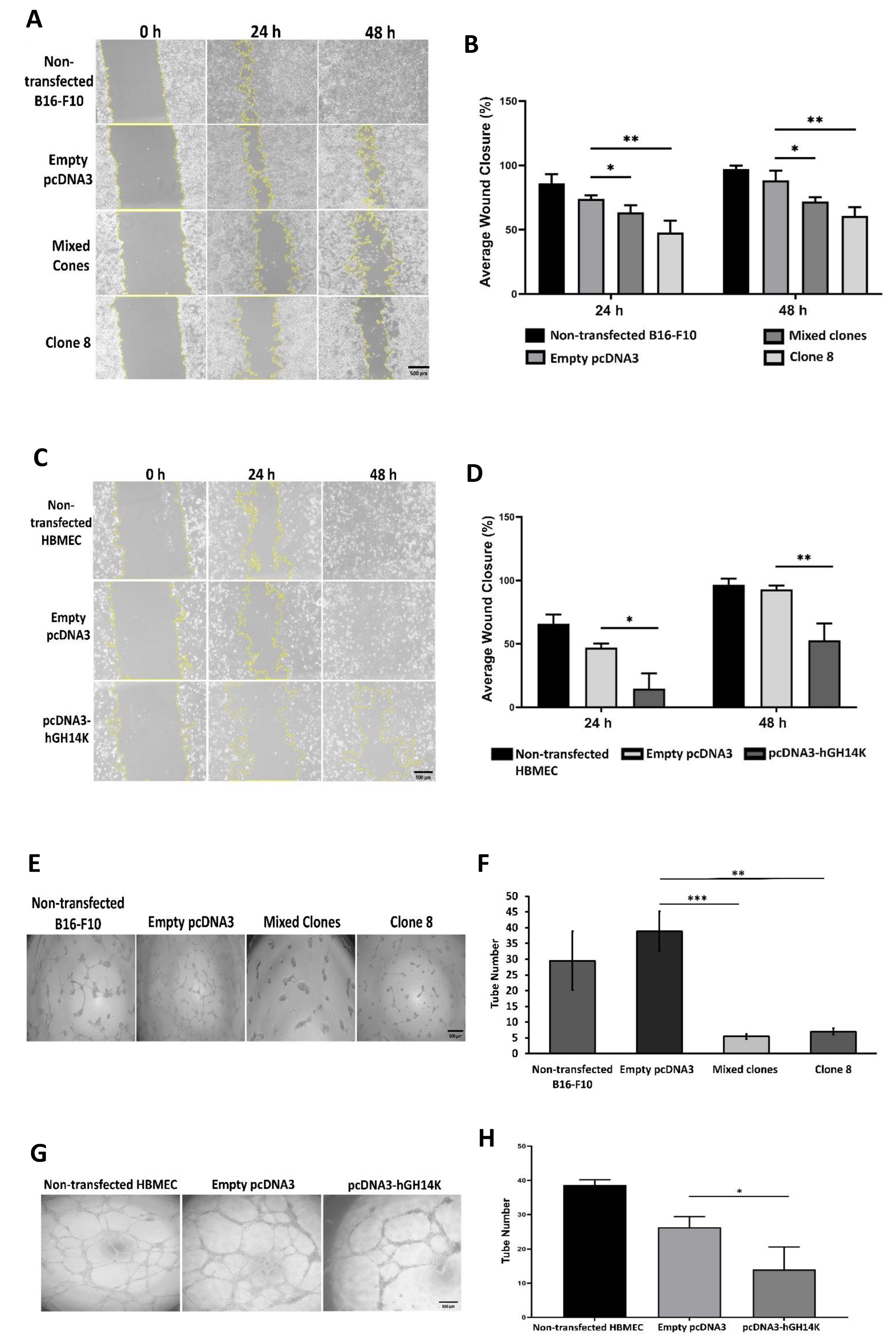
Stably transfected B16-F10 clones, transiently transfected HBME cells, and stably transfected HBME cells with control plasmid or PAI-1 shRNA plasmid were then treated with B16-F10 empty pcDNA3 (control) or B16-F10 mixed clones (14 kDa hGH) conditioned media for 48 h and cultured in 6-well plates at 2.5 × 105 cells/well seeding density. At 90% confluency, a linear wound was created by vertically scratching the confluent cell monolayer using a sterile 200 µL micropipette tip. Images were then captured at fixed positions along the wound at 0, 24, and 48 h using Olympus cellSens imaging software (Olympus, Tokyo, Japan). Pictures were successively analyzed for percent wound closure using ImageJ software. The average value for each condition is the mean of three measurements taken from three separate wells.
4.10. Matrigel Angiogenic Assay
Matrigel (BD Biosciences, NJ, USA) was added at 50 µL/well of a 96-well plate and incubated for 30 min in a humidified incubator at 37 °C allowing the Matrigel to polymerize. B16-F10 and HBME cells (2 × 10⁴) were suspended with appropriate growth media and loaded on top of the Matrigel-coated wells in triplicates for each condition. Cells were incubated overnight at 37 °C, and pictures were acquired from five independent fields per well using Olympus cellSens imaging software (Olympus, Tokyo, Japan) to quantify the number of tubes in triplicate wells. The average value for each condition is the mean of three tube numbers taken from three separate wells. Cells transfected with empty pcDNA3 plasmid were considered as a control condition.
4.11. Mice
Female C57BL/6 mice (six- to eight-week-old) were obtained and maintained in the Animal Facility of the Sharjah Institute for Medical Research at the University of Sharjah (SIMR, Sharjah, UAE). All experimental mouse procedures were performed in accordance with the standard Public Health Service and international standard protocols and approved by the Animal Care and Use Committee of the University of Sharjah, Sharjah, UAE.
4.12. In Vivo Tumorigenicity
The B16-F10 syngeneic tumor model was used for in vivo tumorigenicity assay. Groups of 14 mice were randomly divided and allocated into the control group (cells transfected with empty pcDNA3 plasmid, n = 7) and the mixed clone groups (cells transfected with a pcDNA3-14 kDa hGH plasmid, n = 7). For the experimental procedure, mice were anesthetized with isoflurane (Wellona Pharma, Gujarat, India) by inhalation. B16-F10 cells (1 × 106 cells in 100 µL PBS) were injected subcutaneously into the right dorsal flank of mice using a 1 mL syringe with a 30-gauge needle. Tumor sizes (length and width) were measured with a vernier caliper every seven days after tumors became palpable, and tumor volume was calculated using the following formula: length × width2 × 0.5. Mice had free access to food and water and were monitored for signs of illness or infection. Mice were sacrificed via cervical dislocation 21 days postinoculation, and their tumors were surgically resected and weighed. Tumor specimens were cut into two halves; one half was frozen in cryomolds immersed in polyfreeze tissue freezing medium (Sigma-Aldrich, MO, USA) and stored at −80 °C for tissue sectioning and immunohistological analyses, and the other half was used for protein extraction using the Dounce Homogenizer for further analyses.
4.13. Immunohistological Analysis of Tumor Vascularization and Colocalization of 14 kDa hGH and PAI-1
Seven tumors were retrieved from C57BL/6 mice from each group: control (empty pcDNA3) and mixed clones (14 kDa hGH) to prepare the tumor tissue sections, perform immunostaining, and determine the average number of vessels. Tumor vascularity, angiogenesis, and immunofluorescence colocalization were assessed on the frozen tumor tissue sections. Tumor vascularity, angiogenesis, and immunofluorescence colocalization were assessed on frozen tumor tissue sections. Cryosections (6-µm thick) were rehydrated and blocked with 5% bovine serum albumin (BSA), followed by incubation with primary antibodies: rabbit polyclonal anticollagen IV antibody (1:100 dilution; in-house developed; University of Liege, Belgium), rabbit polyclonal anti-14 kDa hGH antibody (1:100 dilution; in-house developed; a gift from Dr. Jean Closset, University of Liege, Belgium), and mouse monoclonal anti-PAI-1 antibody (1:100 dilution; MA-33H1F7; in-house developed; a gift from Professor Paul Declerck, University of KU Leuven, Belgium) for 1 h at room temperature. After three washes with TBS for 10 min, the slides were incubated with secondary antibodies: Cy3 goat antirabbit antibody (1:100 dilution; 111-165-144 for collagen IV; Jackson ImmunoResearch, West Grove, PA, USA), FITC donkey antirabbit antibody (1:100 dilution; for 14 kDa hGH; Vector Laboratories, CA, USA), Texas Red Horse antimouse IgG antibody (1:100 dilution; TI20001.5 for PAI-1; Vector Laboratories, CA, USA), and DAPI for 1 h at room temperature. Following incubation, the slides were washed, mounted with Fluoroshield™ Histology Mounting Medium (Sigma-Aldrich, MO, USA), and viewed under a fluorescence microscope. Eight to ten fields were examined per section, and the number of vessels/mm2 was quantified using Olympus cellSens imaging software (Olympus, Tokyo, Japan). A 2-tailed student’s t-test was used to determine the statistical difference between the two groups.
4.14. In Vivo Pulmonary Metastasis Model
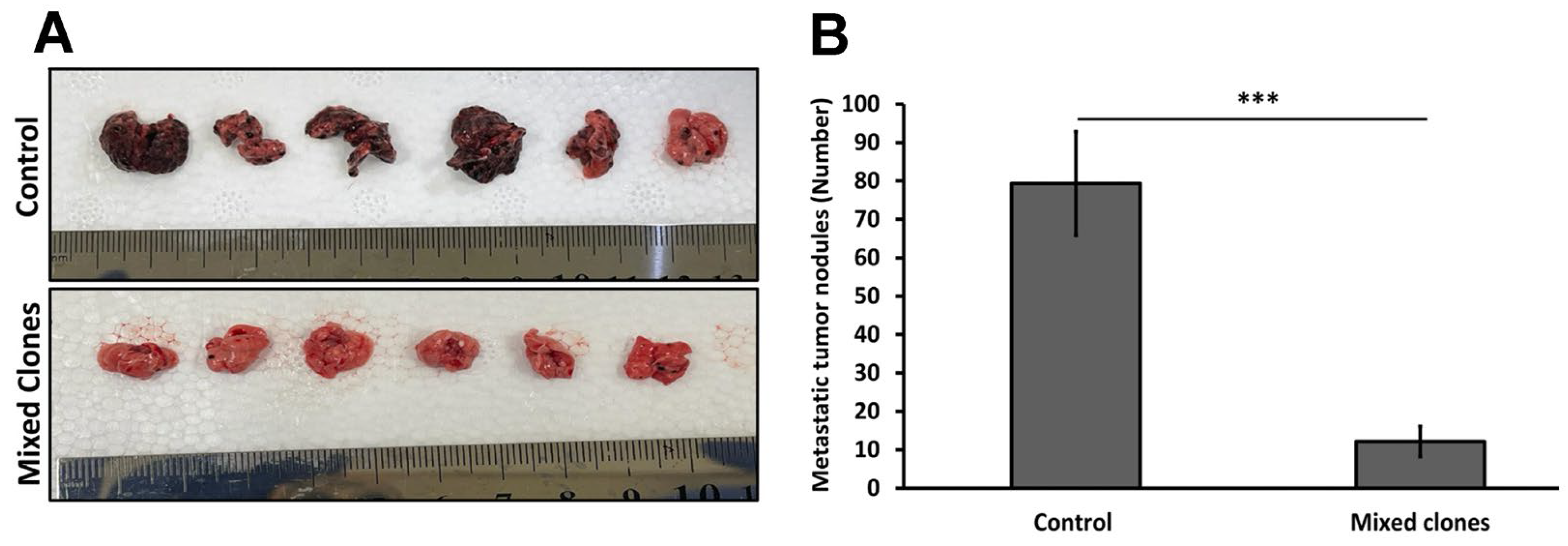
The B16-F10 syngeneic tumor model was used for the in vivo pulmonary metastasis assay. Additionally, 5 × 105 cells in 100 µL PBS of B16-F10 control cells (transfected with empty pcDNA3 plasmid) and B16-F10 mixed clone cells (transfected with the pcDNA3-14 kDa hGH plasmid) were inoculated intravenously into the lateral tail vein of C57BL/6 mice (n = 6/group). Mice had free access to food and water and were monitored for signs of illness or infection. Nineteen days after inoculation, mice were sacrificed, lungs were harvested, and the average number of metastatic colonies on the lung surface of each group (n = 6) was counted and reported as a mean value. A 2-tailed student’s t-test was used to determine the statistical difference between the two groups.
4.15. Urokinase Activity Assay
Conditioned media from stably transfected B16-F10 with empty pcDNA3 or pcDNA3-14 kDa hGH were collected, lyophilized, and reconstituted in proportional quantities of PBS. In addition, tumors retrieved from C57BL/6 mice implanted with B16-F10 cells stably transfected with empty pcDNA3 vector (control, n = 3) or 14 kDa hGH (mixed clones, n = 3) were homogenized using Dounce Homogenizer, and total proteins were extracted using RIPA lysis buffer (Sigma-Aldrich, MO, USA) and quantified using Micro BCA™ Protein Assay Kit (Thermo Fisher Scientific, MA, USA) following the manufacturer’s protocols. The urokinase activity assay kit (Abcam, Cambridge, UK) was used to assess the amount of urokinase-type plasminogen activator (uPA) in 48 µL of the conditioned media, and 48 µL of tissue lysates at 1 mg/mL following the manufacturer’s protocols. In a 96-well white plate, triplicates of each condition medium were incubated or not with 2.2 µg/mL uPA, 20 µg/mL PAI-1, and 300 µg/mL anti-PAI-1, and the fluorescence was measured at Ex/Em = 350/450 nm using GloMax Discover microplate reader (Promega, Madison, WI, USA) after 1 h of incubation at room temperature.
4.16. Caspase-3 Activity Assay
The activity of caspase-3 was measured using the Caspase-3 Colorimetric Assay (Abcam, Cambridge, UK) according to the manufacturer’s instructions. Briefly, HBME cells transfected with control plasmid or PAI-1 shRNA plasmid were cultured in a 60-mm culture dish at 8 × 105 cells seed density, then treated with B16-F10 empty pcDNA3 (control) or B16-F10 mixed clones (14 kDa hGH) conditioned media for 48 h. After 48 h of treatment, cells were collected and lysed in 50 µL of the supplied lysis buffer. Then, 100 µg of cell lysate proteins were suspended in 50 µL of reaction buffer containing DEVD-p-NA substrate and 10 mM dithiothreitol (DTT) and incubated at 37 °C for 2 h. The reactions were measured at 405 nm using the GloMax Discover microplate reader (Promega, Madison, WI, USA).
4.17. Statistical Analysis
In vitro experiments were performed in triplicates of an independent assay and repeated at least two to three times; data were represented as mean ± standard deviation (SD). For in vivo experiments, data were represented as means ± standard error (SEM). Analyses for statistical significance were determined using a 2-tailed student’s t-test. p values < 0.05 were considered significant.