Blood T Helper Memory Cells: A Tool for Studying Skin Inflammation in HS?
Abstract
1. Introduction
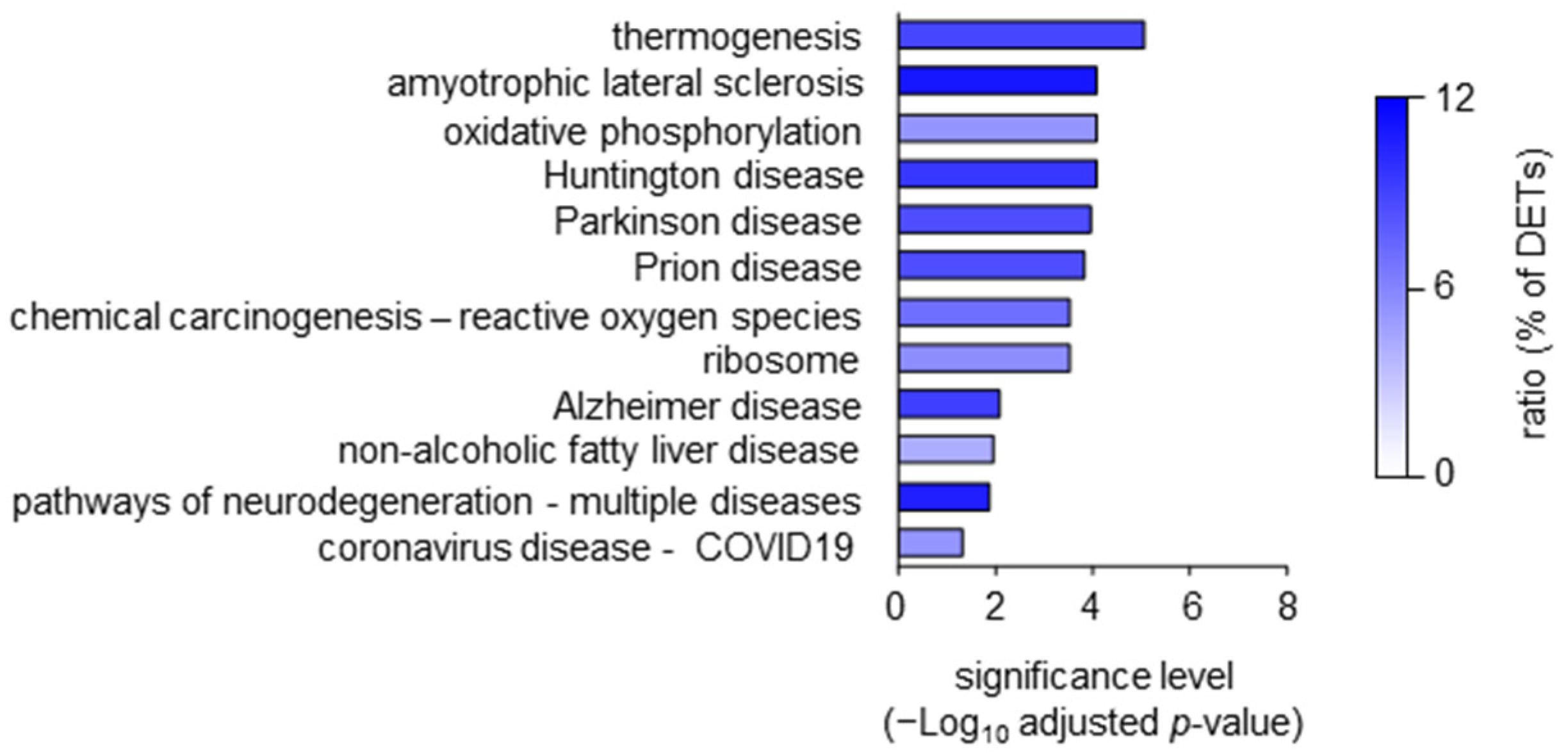
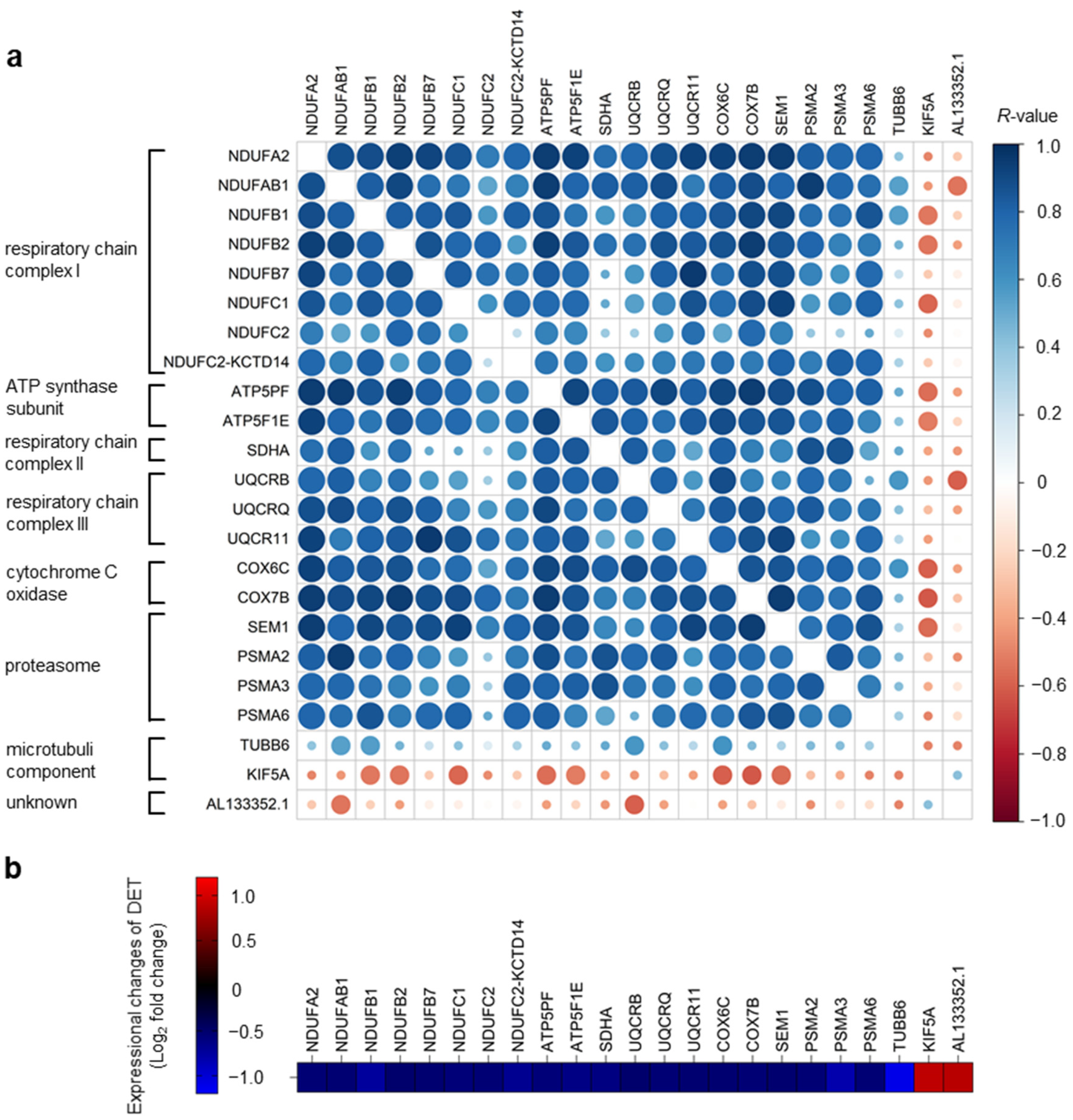
2. Results and Discussion
3. Materials and Methods
3.1. Patients and Healthy Donors
3.2. Cell Isolation
3.3. Flow Cytometry
3.4. Transcriptome Analysis
3.5. Gene Set Enrichment Analysis
3.6. Overrepresentation of Regulatory Target Gene Set Analysis
3.7. Further Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sabat, R.; Jemec, G.B.E.; Matusiak, L.; Kimball, A.B.; Prens, E.; Wolk, K. Hidradenitis suppurativa. Nat. Rev. Dis. Primers 2020, 6, 18. [Google Scholar] [CrossRef]
- Prens, L.M.; Bouwman, K.; Troelstra, L.D.; Prens, E.P.; Alizadeh, B.Z.; Horvath, B. New insights in hidradenitis suppurativa from a population-based Dutch cohort: Prevalence, smoking behaviour, socioeconomic status and comorbidities. Br. J. Dermatol. 2022, 186, 814–822. [Google Scholar] [CrossRef]
- Sabat, R.; Tsaousi, A.; Ghoreschi, K.; Wolk, K.; Schneider-Burrus, S. Sex-disaggregated population analysis in patients with hidradenitis suppurativa. Front. Med. 2022, 9, 1028943. [Google Scholar] [CrossRef] [PubMed]
- Fabbrocini, G.; Ruina, G.; Giovanardi, G.; Dini, V.; Raone, B.; Venturini, M.; Caro, R.D.C.; Veraldi, S.; Cannavò, S.P.; Merlo, G.; et al. Hidradenitis Suppurativa in a Large Cohort of Italian Patients: Evaluation of the Burden of Disease. Dermatology 2022, 238, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Patel, A.; Solanki, D.; Mansuri, U.; Singh, A.; Sharma, P.; Solanki, S. Hidradenitis Suppurativa in the United States: Insights From the National Inpatient Sample (2008–2017) on Contemporary Trends in Demographics, Hospitalization Rates, Chronic Comorbid Conditions, and Mortality. Cureus 2022, 14, e24755. [Google Scholar] [CrossRef] [PubMed]
- Schneider-Burrus, S.; Tsaousi, A.; Barbus, S.; Huss-Marp, J.; Witte, K.; Wolk, K.; Fritz, B.; Sabat, R. Features Associated with Quality of Life Impairment in Hidradenitis Suppurativa Patients. Front. Med. 2021, 8, 676241. [Google Scholar] [CrossRef] [PubMed]
- Montero-Vilchez, T.; Diaz-Calvillo, P.; Rodriguez-Pozo, J.A.; Cuenca-Barrales, C.; Martinez-Lopez, A.; Arias-Santiago, S.; Molina-Leyva, A. The Burden of Hidradenitis Suppurativa Signs and Symptoms in Quality of Life: Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 6709. [Google Scholar] [CrossRef] [PubMed]
- Kurek, A.; Peters, E.M.; Chanwangpong, A.; Sabat, R.; Sterry, W.; Schneider-Burrus, S. Profound disturbances of sexual health in patients with acne inversa. J. Am. Acad. Dermatol. 2012, 67, 422–428.e1. [Google Scholar] [CrossRef]
- Singh, R.; Kelly, K.A.; Senthilnathan, A.; Feldman, S.R.; Pichardo, R.O. Stigmatization, a social perception which may have a debilitating impact on hidradenitis suppurativa patients: An observational study. Arch. Dermatol. Res. 2022, 315, 1049–1052. [Google Scholar] [CrossRef]
- Sabat, R.; Chanwangpong, A.; Schneider-Burrus, S.; Metternich, D.; Kokolakis, G.; Kurek, A.; Philipp, S.; Uribe, D.; Wolk, K.; Sterry, W. Increased prevalence of metabolic syndrome in patients with acne inversa. PLoS ONE 2012, 7, e31810. [Google Scholar] [CrossRef]
- Gau, S.Y.; Hsiao, Y.P.; Liao, W.C.; Ma, K.S.; Wu, M.C. Risk of liver dysfunction and non-alcoholic fatty liver diseases in people with hidradenitis suppurativa: A systematic review and meta-analysis of real-world evidences. Front. Immunol. 2022, 13, 959691. [Google Scholar] [CrossRef]
- Gonzalez-Lopez, M.A.; Hernandez, J.L.; Lacalle, M.; Mata, C.; Lopez-Escobar, M.; Lopez-Mejias, R.; Portilla, V.; Fuentevilla, P.; Corrales, A.; González-Vela, M.C.; et al. Increased prevalence of subclinical atherosclerosis in patients with hidradenitis suppurativa (HS). J. Am. Acad. Dermatol. 2016, 75, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.; Strunk, A.; Jemec, G.B.E.; Garg, A. Incidence of Myocardial Infarction and Cerebrovascular Accident in Patients with Hidradenitis Suppurativa. JAMA Dermatol. 2020, 156, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Schneider-Burrus, S.; Witte-Haendel, E.; Christou, D.; Rigoni, B.; Sabat, R.; Diederichs, G. High Prevalence of Back Pain and Axial Spondyloarthropathy in Patients with Hidradenitis Suppurativa. Dermatology 2016, 232, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Almuhanna, N.; Finstrad, A.; Alhusayen, R. Association between Hidradenitis Suppurativa and Inflammatory Arthritis: A Systematic Review and Meta-Analysis. Dermatology 2021, 237, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Schneeweiss, M.C.; Kirchgesner, J.; Wyss, R.; Jin, Y.; York, C.; Merola, J.F.; Mostaghimi, A.; Silverberg, J.I.; Schneeweiss, S.; Glynn, R.J. Occurrence of inflammatory bowel disease in patients with chronic inflammatory skin diseases: A cohort study: Classification: Epidemiology. Br. J. Dermatol. 2022, 187, 692–703. [Google Scholar] [CrossRef]
- Huilaja, L.; Tiri, H.; Jokelainen, J.; Timonen, M.; Tasanen, K. Patients with Hidradenitis Suppurativa Have a High Psychiatric Disease Burden: A Finnish Nationwide Registry Study. J. Investig. Dermatol. 2018, 138, 46–51. [Google Scholar] [CrossRef]
- Phan, K.; Huo, Y.R.; Smith, S.D. Hidradenitis suppurativa and psychiatric comorbidities, suicides and substance abuse: Systematic review and meta-analysis. Ann. Transl. Med. 2020, 8, 821. [Google Scholar] [CrossRef]
- Schneider-Burrus, S.; Kalus, S.; Fritz, B.; Wolk, K.; Gomis-Kleindienst, S.; Sabat, R. The impact of hidradenitis suppurativa on professional life. Br. J. Dermatol. 2023, 188, 122–130. [Google Scholar] [CrossRef]
- Scholl, L.; Schneider-Burrus, S.; Fritz, B.; Sabat, R.; Bechara, F.G. The impact of surgical interventions on the psychosocial well-being of patients with hidradenitis suppurativa. J. Dtsch. Dermatol. Ges. 2023, 21, 131–139. [Google Scholar] [CrossRef]
- Ocker, L.; Abu Rached, N.; Seifert, C.; Scheel, C.; Bechara, F.G. Current Medical and Surgical Treatment of Hidradenitis Suppurativa-A Comprehensive Review. J. Clin. Med. 2022, 11, 7240. [Google Scholar] [CrossRef] [PubMed]
- Witte, K.; Sabat, R.; Witte-Handel, E.; Ghoreschi, K.; Wolk, K. Phytotherapeuthics Affecting the IL-1/IL-17/G-CSF Axis: A Complementary Treatment Option for Hidradenitis Suppurativa? Int. J. Mol. Sci. 2022, 23, 9057. [Google Scholar] [CrossRef] [PubMed]
- Gamell, C.; Bankovacki, A.; Scalzo-Inguanti, K.; Sedgmen, B.; Alhamdoosh, M.; Gail, E.; Turkovic, L.; Millar, C.; Johnson, L.; Wahlsten, M.; et al. CSL324, a G-CSF receptor antagonist, blocks neutrophil migration markers that are upregulated in hidradenitis suppurativa. Br. J. Dermatol. 2023, 188, 636–648. [Google Scholar] [CrossRef] [PubMed]
- Swierczewska, Z.; Lewandowski, M.; Surowiecka, A.; Baranska-Rybak, W. Immunomodulatory Drugs in the Treatment of Hidradenitis Suppurativa-Possibilities and Limitations. Int. J. Mol. Sci. 2022, 23, 9716. [Google Scholar] [CrossRef] [PubMed]
- Shih, T.; De, D.; Daveluy, S.D.; Hogeling, M.; Lowes, M.A.; Sayed, C.; Shi, V.Y.; Hsiao, J.L. Real-World Considerations of Candidacy for Biologics in Hidradenitis Suppurativa. Am. J. Clin. Dermatol. 2022, 23, 749–753. [Google Scholar] [CrossRef]
- Dos Santos-Silva, C.A.; Tricarico, P.M.; Vilela, L.M.B.; Roldan-Filho, R.S.; Amador, V.C.; d’Adamo, A.P.; Rêgo, M.d.S.; Benko-Iseppon, A.M.; Crovella, S. Plant Antimicrobial Peptides as Potential Tool for Topic Treatment of Hidradenitis Suppurativa. Front. Microbiol. 2021, 12, 795217. [Google Scholar] [CrossRef]
- Wolk, K.; Join-Lambert, O.; Sabat, R. Aetiology and pathogenesis of hidradenitis suppurativa. Br. J. Dermatol. 2020, 183, 999–1010. [Google Scholar] [CrossRef]
- Abu Rached, N.; Gambichler, T.; Dietrich, J.W.; Ocker, L.; Seifert, C.; Stockfleth, E.; Bechara, F.G. The Role of Hormones in Hidradenitis Suppurativa: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 15250. [Google Scholar] [CrossRef]
- Wolk, K.; Warszawska, K.; Hoeflich, C.; Witte, E.; Schneider-Burrus, S.; Witte, K.; Kunz, S.; Buss, A.; Roewert, H.J.; Krause, M.; et al. Deficiency of IL-22 contributes to a chronic inflammatory disease: Pathogenetic mechanisms in acne inversa. J. Immunol. 2011, 186, 1228–1239. [Google Scholar] [CrossRef]
- Swierczewska, Z.; Lewandowski, M.; Surowiecka, A.; Baranska-Rybak, W. Microbiome in Hidradenitis Suppurativa—What We Know and Where We Are Heading. Int. J. Mol. Sci. 2022, 23, 11280. [Google Scholar] [CrossRef]
- Wolk, K.; Wenzel, J.; Tsaousi, A.; Witte-Handel, E.; Babel, N.; Zelenak, C.; Volk, H.-D.; Sterry, W.; Schneider-Burrus, S.; Sabat, R. Lipocalin-2 is expressed by activated granulocytes and keratinocytes in affected skin and reflects disease activity in acne inversa/hidradenitis suppurativa. Br. J. Dermatol. 2017, 177, 1385–1393. [Google Scholar] [CrossRef] [PubMed]
- Di Caprio, R.; Balato, A.; Caiazzo, G.; Lembo, S.; Raimondo, A.; Fabbrocini, G.; Monfrecola, G. IL-36 cytokines are increased in acne and hidradenitis suppurativa. Arch. Dermatol. Res. 2017, 309, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Thomi, R.; Kakeda, M.; Yawalkar, N.; Schlapbach, C.; Hunger, R.E. Increased expression of the interleukin-36 cytokines in lesions of hidradenitis suppurativa. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 2091–2096. [Google Scholar] [CrossRef] [PubMed]
- Hessam, S.; Sand, M.; Gambichler, T.; Skrygan, M.; Ruddel, I.; Bechara, F.G. Interleukin-36 in hidradenitis suppurativa: Evidence for a distinctive proinflammatory role and a key factor in the development of an inflammatory loop. Br. J. Dermatol. 2018, 178, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Witte-Handel, E.; Wolk, K.; Tsaousi, A.; Irmer, M.L.; Mossner, R.; Shomroni, O.; Lingner, T.; Witte, K.; Kunkel, D.; Salinas, G.; et al. The IL-1 Pathway Is Hyperactive in Hidradenitis Suppurativa and Contributes to Skin Infiltration and Destruction. J. Investig. Dermatol. 2019, 139, 1294–1305. [Google Scholar] [CrossRef]
- Scala, E.; Di Caprio, R.; Cacciapuoti, S.; Caiazzo, G.; Fusco, A.; Tortorella, E.; Fabbrocini, G.; Balato, A. A new T helper 17 cytokine in hidradenitis suppurativa: Antimicrobial and proinflammatory role of interleukin-26. Br. J. Dermatol. 2019, 181, 1038–1045. [Google Scholar] [CrossRef]
- Wolk, K.; Brembach, T.C.; Simaite, D.; Bartnik, E.; Cucinotta, S.; Pokrywka, A.; Irmer, M.; Triebus, J.; Witte-Händel, E.; Salinas, G.; et al. Activity and components of the granulocyte colony-stimulating factor pathway in hidradenitis suppurativa. Br. J. Dermatol. 2021, 185, 164–176. [Google Scholar] [CrossRef]
- Sabat, R.; Simaite, D.; Gudjonsson, J.E.; Brembach, T.C.; Witte, K.; Krause, T.; Kokolakis, G.; Bartnik, E.; Nikolaou, C.; Rill, N.; et al. Neutrophilic granulocyte-derived B-cell activating factor supports B cells in skin lesions in hidradenitis suppurativa. J. Allergy Clin. Immunol. 2022, 151, 1015–1026. [Google Scholar] [CrossRef]
- Del Duca, E.; Morelli, P.; Bennardo, L.; Di Raimondo, C.; Nistico, S.P. Cytokine Pathways and Investigational Target Therapies in Hidradenitis Suppurativa. Int. J. Mol. Sci. 2020, 21, 8436. [Google Scholar] [CrossRef]
- Glatt, S.; Jemec, G.B.E.; Forman, S.; Sayed, C.; Schmieder, G.; Weisman, J.; Rolleri, R.; Seegobin, S.; Baeten, D.; Ionescu, L.; et al. Efficacy and Safety of Bimekizumab in Moderate to Severe Hidradenitis Suppurativa: A Phase 2, Double-blind, Placebo-Controlled Randomized Clinical Trial. JAMA Dermatol. 2021, 157, 1279–1288. [Google Scholar] [CrossRef]
- Kimball, A.B.; Loesche, C.; Prens, E.P.; Bechara, F.G.; Weisman, J.; Rozenberg, I.; Jarvis, P.; Peters, T.; Roth, L.; Wieczorek, G.; et al. IL-17A is a pertinent therapeutic target for moderate-to-severe hidradenitis suppurativa: Combined results from a pre-clinical and phase II proof-of-concept study. Exp. Dermatol. 2022, 31, 1522–1532. [Google Scholar] [CrossRef] [PubMed]
- Sabat, R.; Wolk, K.; Loyal, L.; Docke, W.D.; Ghoreschi, K. T cell pathology in skin inflammation. Semin. Immunopathol. 2019, 41, 359–377. [Google Scholar] [CrossRef] [PubMed]
- Schnell, A.; Littman, D.R.; Kuchroo, V.K. T(H)17 cell heterogeneity and its role in tissue inflammation. Nat. Immunol. 2023, 24, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Moran, B.; Sweeney, C.M.; Hughes, R.; Malara, A.; Kirthi, S.; Tobin, A.M.; Kirby, B.; Fletcher, J.M. Hidradenitis Suppurativa Is Characterized by Dysregulation of the Th17:Treg Cell Axis, Which Is Corrected by Anti-TNF Therapy. J. Investig. Dermatol. 2017, 137, 2389–2395. [Google Scholar] [CrossRef] [PubMed]
- Remedios, K.A.; Zirak, B.; Sandoval, P.M.; Lowe, M.M.; Boda, D.; Henley, E.; Bhattrai, S.; Scharschmidt, T.C.; Liao, W.; Naik, H.B.; et al. The TNFRSF members CD27 and OX40 coordinately limit T(H)17 differentiation in regulatory T cells. Sci. Immunol. 2018, 3, eaau2042. [Google Scholar] [CrossRef]
- Hessam, S.; Gambichler, T.; Hoxtermann, S.; Skrygan, M.; Sand, M.; Garcovich, S.; Meyer, T.; Stockfleth, E.; Bechara, F.G. Frequency of circulating subpopulations of T-regulatory cells in patients with hidradenitis suppurativa. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 834–838. [Google Scholar] [CrossRef]
- Kono, M. New insights into the metabolism of Th17 cells. Immunol. Med. 2022, 46, 15–24. [Google Scholar] [CrossRef]
- Spiljar, M.; Kuchroo, V.K. Metabolic regulation and function of T helper cells in neuroinflammation. Semin. Immunopathol. 2022, 44, 581–598. [Google Scholar] [CrossRef]
- Chakraborty, S.; Khamaru, P.; Bhattacharyya, A. Regulation of immune cell metabolism in health and disease: Special focus on T and B cell subsets. Cell Biol. Int. 2022, 46, 1729–1746. [Google Scholar] [CrossRef]
- Peng, Q.; Zhou, M.; Zuo, S.; Liu, Y.; Li, X.; Yang, Y.; He, Q.; Yu, X.; Zhou, J.; He, Z.; et al. Nuclear Factor Related to KappaB Binding Protein (NFRKB) Is a Telomere-Associated Protein and Involved in Liver Cancer Development. DNA Cell Biol. 2021, 40, 1298–1307. [Google Scholar] [CrossRef]
- Wang, S.; Bleeck, A.; Nadif Kasri, N.; Kleefstra, T.; van Rhijn, J.R.; Schubert, D. SETD1A Mediated H3K4 Methylation and Its Role in Neurodevelopmental and Neuropsychiatric Disorders. Front. Mol. Neurosci. 2021, 14, 772000. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Takata, A. The molecular pathology of schizophrenia: An overview of existing knowledge and new directions for future research. Mol. Psychiatry 2023. [Google Scholar] [CrossRef] [PubMed]
- Kurek, A.; Johanne Peters, E.M.; Sabat, R.; Sterry, W.; Schneider-Burrus, S. Depression is a frequent co-morbidity in patients with acne inversa. J. Dtsch. Dermatol. Ges. 2013, 11, 743–749. [Google Scholar] [CrossRef]
- Caccavale, S.; Tancredi, V.; Boccellino, M.P.; Babino, G.; Fulgione, E.; Argenziano, G. Hidradenitis Suppurativa Burdens on Mental Health: A Literature Review of Associated Psychiatric Disorders and Their Pathogenesis. Life 2023, 13, 189. [Google Scholar] [CrossRef]
- Hertzberg, L.; Maggio, N.; Muler, I.; Yitzhaky, A.; Majer, M.; Haroutunian, V.; Zuk, O.; Katsel, P.; Domany, E.; Weiser, M. Comprehensive Gene Expression Analysis Detects Global Reduction of Proteasome Subunits in Schizophrenia. Schizophr. Bull. 2021, 47, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Katz Shroitman, N.; Yitzhaky, A.; Ben Shachar, D.; Gurwitz, D.; Hertzberg, L. Meta-analysis of brain samples of individuals with schizophrenia detects down-regulation of multiple ATP synthase encoding genes in both females and males. J. Psychiatr. Res. 2023, 158, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Liu, Y.; Pu, J.; Gui, S.; Zhong, X.; Chen, X.; Chen, X.; Wang, H.; Cheng, K.; Zhao, L.; et al. Transcriptomics Analysis Reveals Shared Pathways in Peripheral Blood Mononuclear Cells and Brain Tissues of Patients with Schizophrenia. Front. Psychiatry 2021, 12, 716722. [Google Scholar] [CrossRef] [PubMed]
- Amrutha, K.; Mishra, A.; Singh, S. Implications of intracellular protein degradation pathways in Parkinson’s disease and therapeutics. J. Neurosci. Res. 2022, 100, 1834–1844. [Google Scholar]
- Tecalco-Cruz, A.C.; Pedraza-Chaverri, J.; Briones-Herrera, A.; Cruz-Ramos, E.; Lopez-Canovas, L.; Zepeda-Cervantes, J. Protein degradation-associated mechanisms that are affected in Alzheimer s disease. Mol. Cell. Biochem. 2022, 477, 915–925. [Google Scholar] [CrossRef]
- Hommen, F.; Bilican, S.; Vilchez, D. Protein clearance strategies for disease intervention. J. Neural Transm. 2022, 129, 141–172. [Google Scholar] [CrossRef]
- Dilliott, A.A.; Andary, C.M.; Stoltz, M.; Petropavlovskiy, A.A.; Farhan, S.M.K.; Duennwald, M.L. DnaJC7 in Amyotrophic Lateral Sclerosis. Int. J. Mol. Sci. 2022, 23, 4076. [Google Scholar] [CrossRef] [PubMed]
- Benn, J.A.; Mukadam, A.S.; McEwan, W.A. Targeted protein degradation using intracellular antibodies and its application to neurodegenerative disease. Semin. Cell Dev. Biol. 2022, 126, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Sasso, J.M.; Tenchov, R.; Wang, D.; Johnson, L.S.; Wang, X.; Zhou, Q.A. Molecular Glues: The Adhesive Connecting Targeted Protein Degradation to the Clinic. Biochemistry 2022, 62, 601–623. [Google Scholar] [CrossRef]
- George, D.E.; Tepe, J.J. Advances in Proteasome Enhancement by Small Molecules. Biomolecules 2021, 11, 1789. [Google Scholar] [CrossRef] [PubMed]
- Wolk, K.; Mitsui, H.; Witte, K.; Gellrich, S.; Gulati, N.; Humme, D.; Witte, E.; Gonsior, M.; Beyer, M.; Kadin, M.E.; et al. Deficient cutaneous antibacterial competence in cutaneous T-cell lymphomas: Role of Th2-mediated biased Th17 function. Clin. Cancer Res. 2014, 20, 5507–5516. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef]
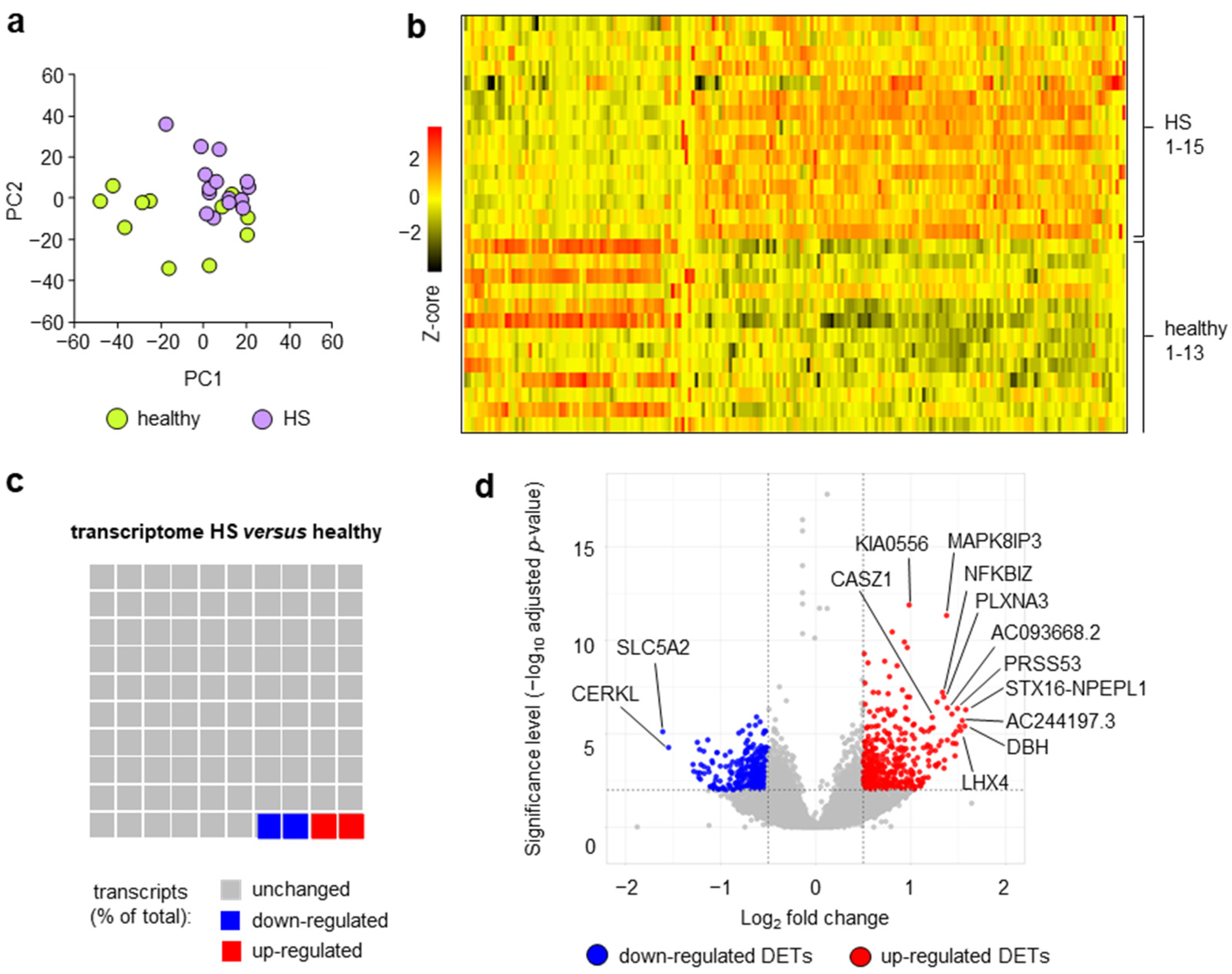
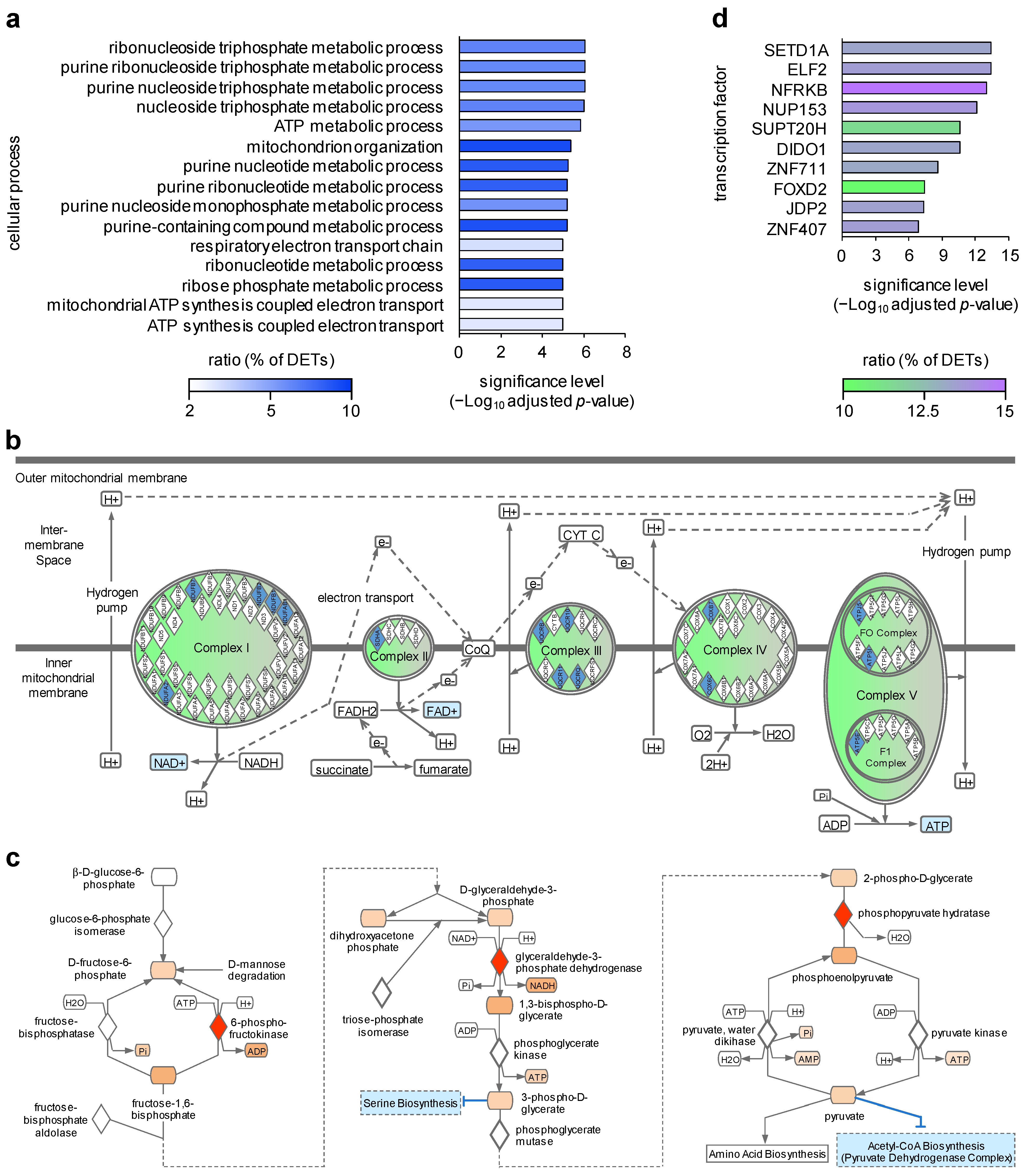
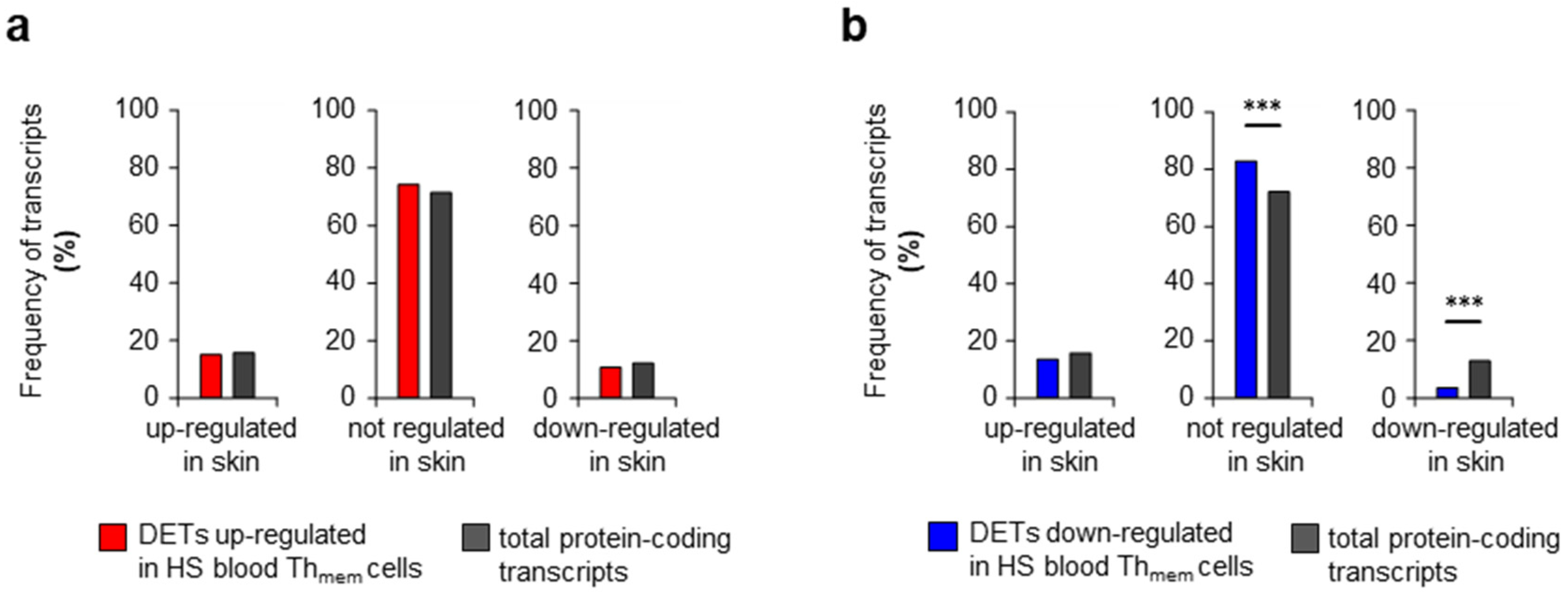
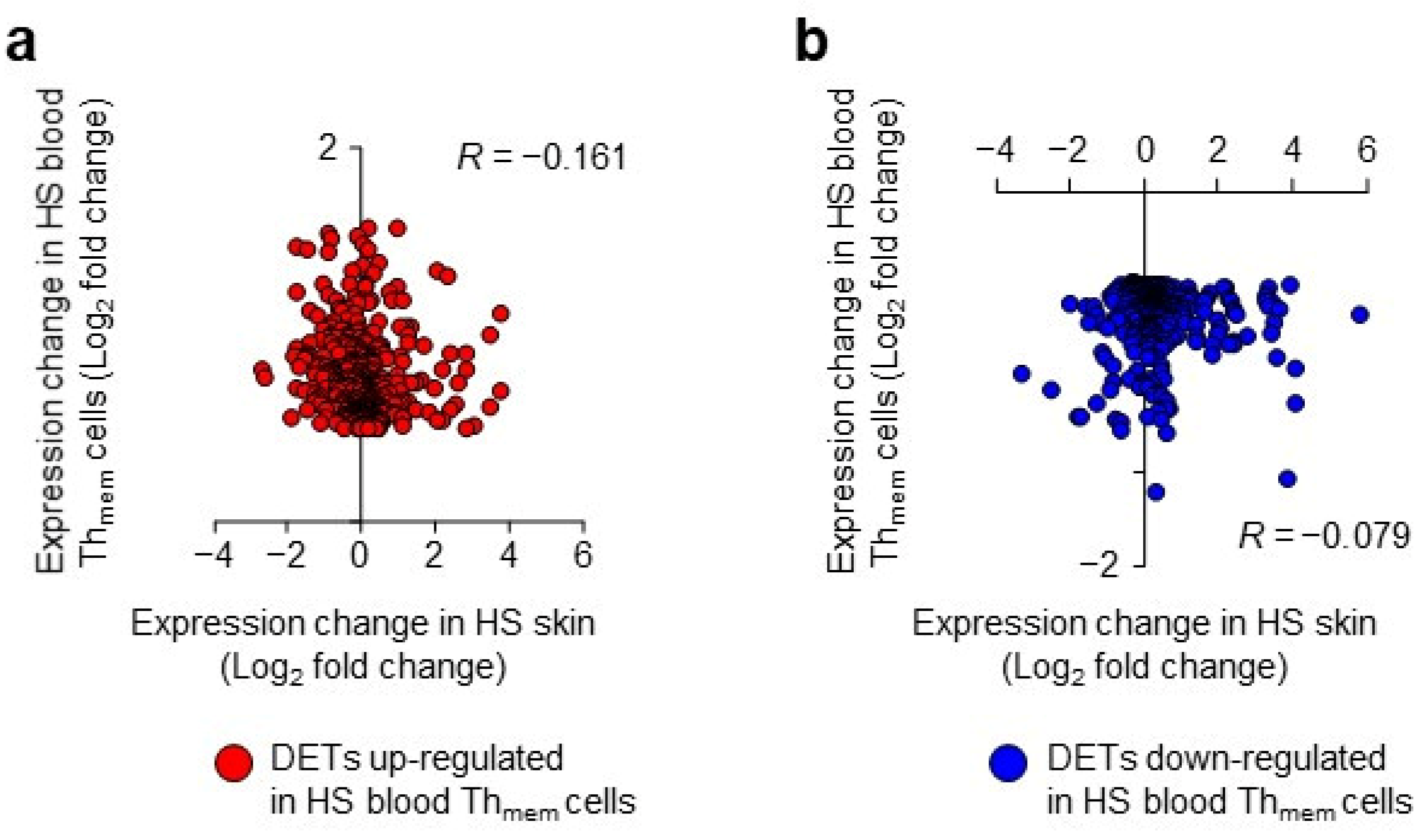
| HS | Healthy | |
|---|---|---|
| (n = 15) | (n = 13) | |
| Age, in years; mean ± SEM | 37.6 ± 3.1 | 37.5 ± 2.6 |
| Gender distribution; n (%) | ||
| Females: | 9 (60.0) | 8 (61.5) |
| Males: | 6 (40.0) | 5 (38.5) |
| Smoking habit; n (%) | ||
| Smokers: | 10 (66.6) | 7 (53.8) |
| Son-smokers: | 5 (33.3) | 6 (46.2) |
| Disease duration, in years; mean ± SEM | 15.2 ± 3.0 | n.a. |
| Positive family history for HS; n (%) | 5 (33.3) | n.a. |
| Hurley score; mean ± SEM | 1.4 ± 0.2 | n.a. |
| Sartorius score; mean ± SEM | 48.3 ± 11.8 | n.a. |
| Ensembl ID | Gene Name | Gene Description | Log2 Fold Change | p |
|---|---|---|---|---|
| ENSG00000254995 | STX16-NPEPL1 | syntaxin 16 | 1.58 | 5.17 × 10−7 |
| ENSG00000123454 | DBH | dopamine beta-hydroxylase | 1.57 | 3.88 × 10−6 |
| ENSG00000241489 | AC244197.3 | novel protein | 1.54 | 1.95 × 10−6 |
| ENSG00000121454 | LHX4 | LIM homeobox 4 | 1.53 | 6.64 × 10−6 |
| ENSG00000009724 | MASP2 | MBL associated serine protease 2 | 1.51 | 3.99 × 10−6 |
| ENSG00000151006 | PRSS53 | serine protease 53 | 1.49 | 4.40 × 10−7 |
| ENSG00000284946 | AC068831.7 | novel protein | 1.48 | 7.54 × 10−6 |
| ENSG00000167701 | GPT | glutamic-pyruvic transaminase | 1.48 | 3.36 × 10−5 |
| ENSG00000198838 | RYR3 | ryanodine receptor 3 | 1.47 | 1.52 × 10−4 |
| ENSG00000255423 | EBLN2 | endogenous Bornavirus-like nucleoprotein 2 | 1.45 | 1.10 × 10−5 |
| ENSG00000146373 | RNF217 | ring finger protein 217 | 1.44 | 3.20 × 10−5 |
| ENSG00000187726 | DNAJB13 | DnaJ homolog subfamily B member 13 | 1.43 | 8.80 × 10−7 |
| ENSG00000225987 | PBX2 | PBX homeobox 2 | 1.38 | 2.17 × 10−5 |
| ENSG00000284981 | AC093668.2 | novel protein | 1.38 | 4.10 × 10−7 |
| ENSG00000138834 | MAPK8IP3 | mitogen-activated protein kinase 8 interacting protein 3 | 1.38 | 4.69 × 10−12 |
| ENSG00000239732 | TLR9 | Toll-like receptor 9 | 1.35 | 2.54 × 10−4 |
| ENSG00000111886 | GABRR2 | gamma-aminobutyric acid type A receptor subunit rho2 | 1.35 | 6.89 × 10−4 |
| ENSG00000130827 | PLXNA3 | plexin A3 | 1.35 | 1.07 × 10−7 |
| ENSG00000144802 | NFKBIZ | NFKB inhibitor zeta | 1.33 | 6.00 × 10−8 |
| ENSG00000283199 | C13orf46 | uncharacterized protein | 1.32 | 2.45 × 10−5 |
| Ensembl ID | Gene Name | Gene Description | Log2 Fold Change | p |
|---|---|---|---|---|
| ENSG00000140675 | SLC5A2 | solute carrier family 5 member 2 | −1.61 | 7.66 × 10−6 |
| ENSG00000188452 | CERKL | ceramide kinase-like | −1.55 | 5.38 × 10−5 |
| ENSG00000104213 | PDGFRL | Platelet-derived growth factor receptor-like | −1.29 | 4.36 × 10−4 |
| ENSG00000163806 | SPDYA | speedy/RINGO cell cycle regulator family member A | −1.28 | 1.01 × 10−3 |
| ENSG00000257529 | RPL36A-HNRNPH2 | RPL36A-HNRNPH2 readthrough | −1.25 | 2.84 × 10−5 |
| ENSG00000156050 | FAM161B | family with sequence similarity 161, member B | −1.24 | 1.19 × 10−3 |
| ENSG00000096150 | RPS18 | ribosomal protein S18 | −1.22 | 6.93 × 10−5 |
| ENSG00000164142 | FAM160A1 | FHF complex subunit HOOK interacting protein 1A | −1.22 | 1.22 × 10−3 |
| ENSG00000168350 | DEGS2 | delta 4-desaturase | −1.21 | 2.69 × 10−3 |
| ENSG00000168000 | BSCL2 | BSCL2 lipid droplet biogenesis associated, seipin | −1.21 | 4.43 × 10−4 |
| ENSG00000162613 | FUBP1 | far upstream element binding protein 1 | −1.18 | 8.48 × 10−5 |
| ENSG00000253304 | TMEM200B | transmembrane protein 200B | −1.18 | 5.61 × 10−4 |
| ENSG00000108556 | CHRNE | cholinergic receptor nicotinic epsilon subunit | −1.17 | 1.31 × 10−3 |
| ENSG00000137731 | FXYD2 | FXYD domain containing ion transport regulator 2 | −1.14 | 2.11 × 10−5 |
| ENSG00000227507 | LTB | lymphotoxin beta | −1.13 | 6.89 × 10−4 |
| ENSG00000255508 | AP002990.1 | uncharacterized protein | −1.12 | 1.09 × 10−3 |
| ENSG00000267179 | AC008770.2 | uncharacterized protein | −1.11 | 6.45 × 10−3 |
| ENSG00000176014 | TUBB6 | tubulin beta 6 class V | −1.10 | 6.82 × 10−3 |
| ENSG00000167565 | SERTAD3 | SERTA domain containing 3 | −1.09 | 2.76 × 10−4 |
| ENSG00000226492 | CUTA | acetylcholinesterase-associated protein | −1.08 | 3.55 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Witte, K.; Schneider-Burrus, S.; Salinas, G.; Mössner, R.; Ghoreschi, K.; Wolk, K.; Sabat, R. Blood T Helper Memory Cells: A Tool for Studying Skin Inflammation in HS? Int. J. Mol. Sci. 2023, 24, 8854. https://doi.org/10.3390/ijms24108854
Witte K, Schneider-Burrus S, Salinas G, Mössner R, Ghoreschi K, Wolk K, Sabat R. Blood T Helper Memory Cells: A Tool for Studying Skin Inflammation in HS? International Journal of Molecular Sciences. 2023; 24(10):8854. https://doi.org/10.3390/ijms24108854
Chicago/Turabian StyleWitte, Katrin, Sylke Schneider-Burrus, Gabriela Salinas, Rotraut Mössner, Kamran Ghoreschi, Kerstin Wolk, and Robert Sabat. 2023. "Blood T Helper Memory Cells: A Tool for Studying Skin Inflammation in HS?" International Journal of Molecular Sciences 24, no. 10: 8854. https://doi.org/10.3390/ijms24108854
APA StyleWitte, K., Schneider-Burrus, S., Salinas, G., Mössner, R., Ghoreschi, K., Wolk, K., & Sabat, R. (2023). Blood T Helper Memory Cells: A Tool for Studying Skin Inflammation in HS? International Journal of Molecular Sciences, 24(10), 8854. https://doi.org/10.3390/ijms24108854






