Inflammation: The Link between Neural and Vascular Impairment in the Diabetic Retina and Therapeutic Implications
Abstract
1. Introduction
2. Inflammation and Diabetic Retinopathy
2.1. Cells Involved in the Inflammatory Response That Occurs in the Diabetic Retina
2.2. Inflammatory Cytokines and Their Role in DR Development
3. Targeting Inflammation in DR
3.1. Anti-Inflammatory Treatments
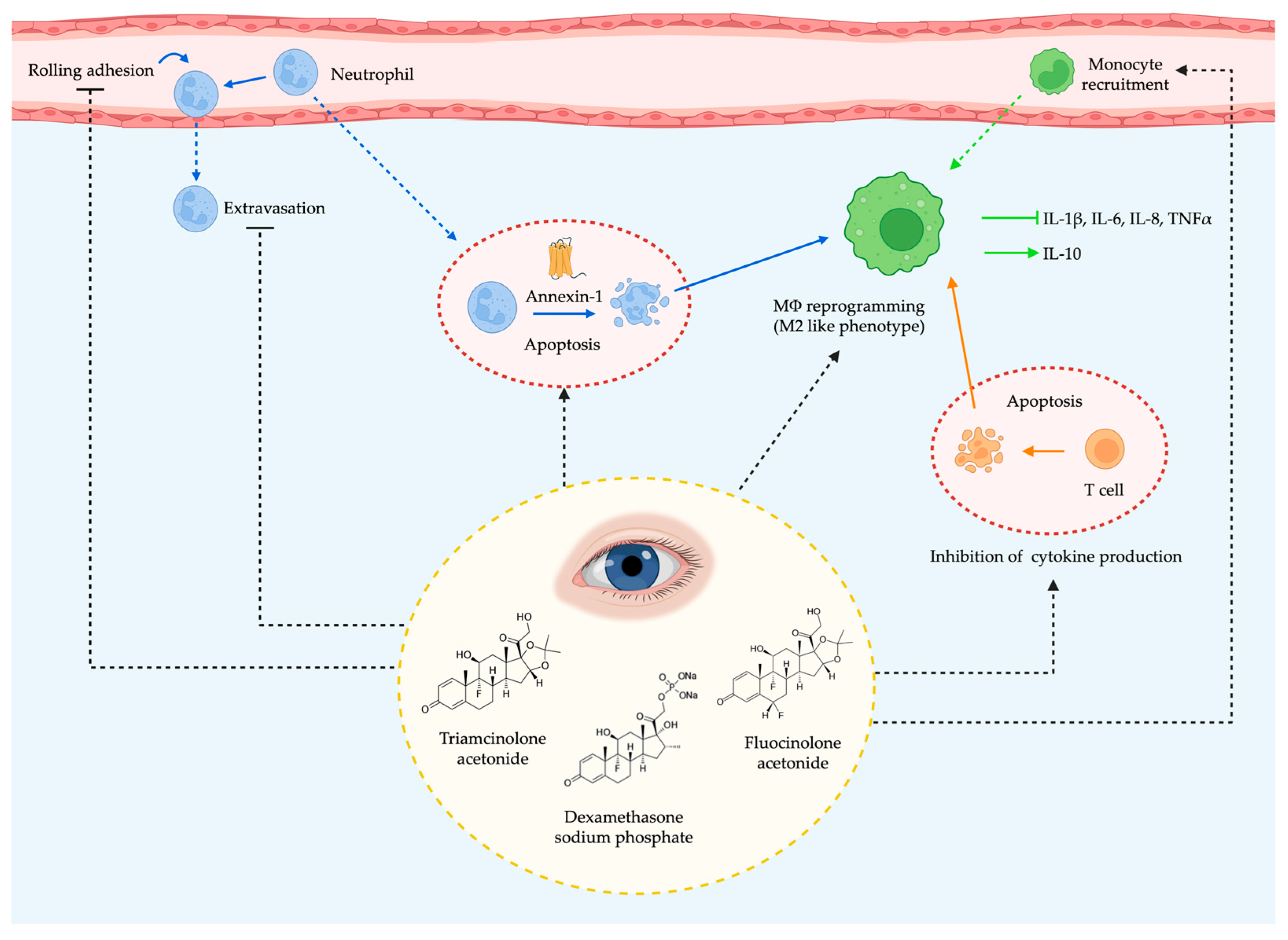
3.1.1. Corticosteroids
3.1.2. Nonsteroidal Anti-Inflammatory Drugs
3.1.3. TNF-α Blockade
3.1.4. Blocking Other Specific Inflammatory Mediators
3.1.5. Suppressors of Cytokine Signaling (SOCS) Proteins
3.2. Antioxidants
3.3. Blockers of the Renin–Angiotensin System
3.4. Neurotrophic Factors
3.5. Stem Cell-Based Treatments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Sapra, A.; Bhandari, P. Diabetes Mellitus. [Updated 26 June 2022]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island: FL, USA, 2022; Available online: https://www-ncbi-nlm-nih-gov.are.uab.cat/books/NBK551501/ (accessed on 12 February 2023).
- Goyal, R.; Jialal, I. Diabetes Mellitus Type 2. [Updated 19 June 2022]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022; Available online: https://www-ncbi-nlm-nih-gov.are.uab.cat/books/NBK513253/ (accessed on 25 January 2023).
- Deshpande, A.D.; Harris-Hayes, M.; Schootman, M. Epidemiology of Diabetes and Diabetes-Related Complications. Phys. Ther. 2008, 88, 1254–1264. [Google Scholar] [CrossRef] [PubMed]
- Harding, J.L.; Pavkov, M.E.; Magliano, D.J.; Shaw, J.E.; Gregg, E.W. Global trends in diabetes complications: A review of current evidence. Diabetologia. 2019, 62, 3–16. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation. Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2022; Available online: http://www.idf.org/diabtesatlas (accessed on 23 May 2022).
- Lee, R.; Wong, T.Y.; Sabanayagam, C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis. 2015, 2, 17. [Google Scholar] [CrossRef] [PubMed]
- Simó, R.; Hernández, C. New Insights into Treating Early and Advanced Stage Diabetic Retinopathy. Int. J. Mol. Sci. 2022, 23, 8513. [Google Scholar] [CrossRef] [PubMed]
- Simó, R.; Stitt, A.W.; Gardner, T.W. Neurodegeneration in diabetic retinopathy: Does it really matter? Diabetologia 2018, 61, 1902–1912. [Google Scholar] [CrossRef]
- Du, Y.; Veenstra, A.; Palczewski, K.; Kern, T.S. Photoreceptor cells are major contributors to diabetes-induced oxidative stress and local inflammation in the retina. Proc. Natl. Acad. Sci. USA 2013, 110, 16586–16591. [Google Scholar] [CrossRef]
- Pahwa, R.; Goyal, A.; Jialal, I. Chronic Inflammation. [Updated 8 August 2022]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022; Available online: https://www-ncbi-nlm-nih-gov.are.uab.cat/books/NBK493173/ (accessed on 17 February 2023).
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef]
- InformedHealth.org [Internet]. Cologne, Germany: Institute for Quality and Efficiency in Health Care (IQWiG); 2006. What Is an inflammation? 23 November 2010 [Updated 22 February 2018]. Available online: https://www-ncbi-nlm-nih-gov.are.uab.cat/books/NBK279298/ (accessed on 17 February 2023).
- Hannoodee, S.; Nasuruddin, D.N. Acute Inflammatory Response. [Updated 14 November 2022]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island: FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK556083/ (accessed on 22 February 2023).
- Warsinske, H.C.; DiFazio, R.M.; Linderman, J.J.; Flynn, J.L.; Kirschner, D.E. Identifying mechanisms driving formation of granuloma-associated fibrosis during Mycobacterium tuberculosis infection. J. Theor. Biol. 2017, 429, 1–17. [Google Scholar] [CrossRef]
- Vujosevic, S.; Toma, C. Diabetic retinopathy: An inflammatory disease. Ann. Eye Sci. 2018, 10, 52–62. [Google Scholar] [CrossRef]
- Noda, K.; Nakao, S.; Ishida, S.; Ishibash, T. Leukocyte Adhesion Molecules in Diabetic Retinopathy. J. Ophthalmol. 2012, 2012, 279037. [Google Scholar] [CrossRef]
- Safi, S.Z.; Qvist, R.; Kumar, S.; Batumalaie, K.; Ismail, I.S. Molecular mechanisms of diabetic retinopathy, general preventive strategies, and novel therapeutic targets. Biomed Res. Int. 2014, 2014, 801269. [Google Scholar] [CrossRef]
- Rübsam, A.; Parikh, S.; Fort, P.E. Role of Inflammation in Diabetic Retinopathy. Int. J. Mol. Sci. 2018, 19, 942. [Google Scholar] [CrossRef]
- Hawkins, B.T.; Davis, T.P. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol. Rev. 2005, 57, 173–185. [Google Scholar] [CrossRef]
- Antonetti, D.A.; Klein, R.; Gardner, T.W. Diabetic retinopathy. N. Engl. J. Med. 2012, 366, 1227–1239. [Google Scholar] [CrossRef]
- Gardner, T.W.; Davila, J.R. The neurovascular unit and the pathophysiologic basis of diabetic retinopathy. Graefes Arch. Clin. Exp. Ophthalmol. 2017, 255, 1–6. [Google Scholar] [CrossRef]
- Duh, E.J.; Sun, J.K.; Stitt, A.W. Diabetic retinopathy: Current understanding, mechanisms, and treatment strategies. JCI Insight 2017, 2, e93751. [Google Scholar] [CrossRef]
- Díaz-Coránguez, M.; Ramos, C.; Antonetti, D.A. The inner blood-retinal barrier: Cellular basis and development. Vis. Res. 2017, 139, 123–137. [Google Scholar] [CrossRef]
- Klaassen, I.; Van Noorden, C.J.F.; Schlingemann, R.O. Molecular basis of the inner blood-retinal barrier and its breakdown in diabetic macular edema and other pathological conditions. Prog. Retin. Eye Res. 2013, 34, 19–48. [Google Scholar] [CrossRef]
- Cunha-Vaz, J.G. The blood-retinal barriers system. Basic concepts and clinical evaluation. Exp. Eye Res. 2004, 78, 715–721. [Google Scholar] [CrossRef]
- Antonetti, D.A.; Lieth, E.; Barber, A.J.; Gardner, T.W. Molecular mechanisms of vascular permeability in diabetic retinopathy. Semin. Ophthalmol. 1999, 14, 240–248. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, J.; Chen, L. The cells involved in the pathological process of diabetic retinopathy. Biomed Pharmacother. 2020, 132, 110818. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, F.S.; Allkabes, M.; Salsini, G.; Bonifazzi, C.; Perri, P. The importance of glial cells in the homeostasis of the retinal microenvironment and their pivotal role in the course of diabetic retinopathy. Life Sci. 2016, 162, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Coorey, N.J.; Shen, W.; Chung, S.H.; Zhu, L.; Gillies, M.C. The role of glia in retinal vascular disease. Clin. Exp. Optom. 2012, 95, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Arroba, A.I.; Valverde, Á.M. Modulation of microglia in the retina: New insights into diabetic retinopathy. Acta Diabetol. 2017, 54, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, X.; Elliott, M.H.; Zhu, M.; Le, Y.Z. Müller cell-derived VEGF is essential for diabetes-induced retinal inflammation and vascular leakage. Diabetes 2010, 59, 2297–2305. [Google Scholar] [CrossRef]
- Shin, E.S.; Huang, Q.; Gurel, Z.; Sorenson, C.M.; Sheibani, N. High glucose alters retinal astrocytes phenotype through increased production of inflammatory cytokines and oxidative stress. PLoS ONE 2014, 9, e103148. [Google Scholar] [CrossRef]
- Chen, J.; Sun, Z.; Jin, M.; Tu, Y.; Wang, S.; Yang, X.; Chen, Q.; Zhang, x.; Han, Y.; Pi, R. Inhibition of AGEs/RAGE/Rho/ROCK pathway suppresses non-specific neuroinflammation by regulating BV2 microglial M1/M2 polarization through the NF-κB pathway. J. Neuroimmunol. 2017, 305, 108–114. [Google Scholar] [CrossRef]
- Subedi, L.; Lee, J.H.; Gaire, B.P.; Kim, S.Y. Sulforaphane Inhibits MGO-AGE-Mediated Neuroinflammation by Suppressing NF-κB, MAPK, and AGE-RAGE Signaling Pathways in Microglial Cells. Antioxidants 2020, 9, E792. [Google Scholar] [CrossRef]
- Zeng, H.Y.; Green, W.R.; Tso, M.O. Microglial activation in human diabetic retinopathy. Arch. Ophthalmol. 2008, 126, 227–232. [Google Scholar] [CrossRef]
- Altmann, C.; Schmidt, M.H.H. The Role of Microglia in Diabetic Retinopathy: Inflammation, Microvasculature Defects and Neurodegeneration. Int. J. Mol. Sci. 2018, 19, 110. [Google Scholar] [CrossRef]
- Tang, J.; Kern, T.S. Inflammation in diabetic retinopathy. Prog. Retin. Eye Res. 2011, 30, 343–358. [Google Scholar] [CrossRef]
- Tonade, D.; Liu, H.; Palczewski, K.; Kern, T.S. Photoreceptor cells produce inflammatory products that contribute to retinal vascular permeability in a mouse model of diabetes. Diabetologia 2017, 60, 2111–2120. [Google Scholar] [CrossRef]
- Xu, Y.; Cheng, Q.; Yang, B.; Yu, S.; Xu, F.; Lu, L.; Liang, X. Increased sCD200 Levels in Vitreous of Patients with Proliferative Diabetic Retinopathy and Its Correlation With VEGF and Proinflammatory Cytokines. Investig. Ophthalmol. Vis. Sci. 2015, 56, 6565–6572. [Google Scholar] [CrossRef]
- Kuo, C.Y.J.; Murphy, R.; Rupenthal, I.D.; Mugisho, O.O. Correlation between the progression of diabetic retinopathy and inflammasome biomarkers in vitreous and serum—A systematic review. BMC Ophthalmol. 2022, 22, 238. [Google Scholar] [CrossRef]
- Vujosevic, S.; Simó, R. Local and Systemic Inflammatory Biomarkers of Diabetic Retinopathy: An Integrative Approach. Investig. Ophthalmol. Vis. Sci. 2017, 58, BIO68–BIO75. [Google Scholar] [CrossRef]
- Vujosevic, S.; Micera, A.; Bini, S.; Berton, M.; Esposito, G.; Midena, E. Proteome analysis of retinal glia cells-related inflammatory cytokines in the aqueous humour of diabetic patients. Acta Ophthalmol. 2016, 94, 56–64. [Google Scholar] [CrossRef]
- El-Asrar, A.M.A.; Nawaz, M.I.; Kangave, D.; Abouammoh, M.; Mohammad, G. High-mobility group box-1 and endothelial cell angiogenic markers in the vitreous from patients with proliferative diabetic retinopathy. Mediat. Inflamm. 2012, 2012, 697489. [Google Scholar] [CrossRef]
- Taghavi, Y.; Hassanshahi, G.; Kounis, N.G.; Koniari, I.; Khorramdelazad, H. Monocyte chemoattractant protein-1 (MCP-1/CCL2) in diabetic retinopathy: Latest evidence and clinical considerations. J. Cell Commun. Signal. 2019, 13, 451–462. [Google Scholar] [CrossRef]
- El-Asrar, A.M.A.; Nawaz, M.I.; Kangave, D.; Geboes, K.; Ola, M.S.; Ahmad, S.; Al-Shabrawey, M. High-mobility group box-1 and biomarkers of inflammation in the vitreous from patients with proliferative diabetic retinopathy. Mol. Vis. 2011, 17, 1829–1838. [Google Scholar]
- Xie, Z.; Liang, H. Association between diabetic retinopathy in type 2 diabetes and the ICAM-1 rs5498 polymorphism: A meta-analysis of case-control studies. BMC Ophthalmol. 2018, 18, 297. [Google Scholar] [CrossRef]
- Aveleira, C.A.; Lin, C.M.; Abcouwer, S.F.; Ambrósio, A.F.; Antonetti, D.A. TNF-α signals through PKCζ/NF-κB to alter the tight junction complex and increase retinal endothelial cell permeability. Diabetes 2010, 59, 2872–2882. [Google Scholar] [CrossRef] [PubMed]
- Forbes, J.M.; Cooper, M.E. Mechanisms of diabetic complications. Physiol. Rev. 2013, 93, 137–188. [Google Scholar] [CrossRef] [PubMed]
- Murugeswari, P.; Shukla, D.; Rajendran, A.; Kim, R.; Namperumalsamy, P.; Muthukkaruppan, V. Proinflammatory cytokines and angiogenic and anti-angiogenic factors in vitreous of patients with proliferative diabetic retinopathy and eales’ disease. Retina 2008, 28, 817–824. [Google Scholar] [CrossRef] [PubMed]
- El-Asrar, A.M. Role of inflammation in the pathogenesis of diabetic retinopathy. Middle East Afr. J. Ophthalmol. 2012, 19, 70–74. [Google Scholar] [CrossRef]
- Taub, D.D.; Anver, M.; Oppenheim, J.J.; Longo, D.L.; Murphy, W.J. T lymphocyte recruitment by interleukin-8 (IL-8). IL-8-induced degranulation of neutrophils releases potent chemoattractants for human T lymphocytes both in vitro and in vivo. J. Clin. Investig. 1996, 97, 1931–1941. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, H.; Al-Shabrawey, M.; Caldwell, R.W.; Caldwell, R.B. Inflammation and diabetic retinal microvascular complications. J. Cardiovasc. Dis. Res. 2011, 2, 96–103. [Google Scholar] [CrossRef]
- Hatch, H.M.; Zheng, D.; Jorgensen, M.L.; Petersen, B.E. SDF-1alpha/CXCR4: A mechanism for hepatic oval cell activation and bone marrow stem cell recruitment to the injured liver of rats. Cloning Stem Cells 2002, 4, 339–351. [Google Scholar] [CrossRef]
- Butler, J.M.; Guthrie, S.M.; Koc, M.; Afzal, A.; Caballero, S.; Brooks, H.L.; Mames, R.N.; Segal, M.S.; Grant, M.B.; Scott, E.W. SDF-1 is both necessary and sufficient to promote proliferative retinopathy. J. Clin. Investig. 2005, 115, 86–93. [Google Scholar] [CrossRef]
- Djuric, Z.; Sharei, V.; Rudofsky, G.; Morcos, M.; Li, H.; Hammes, H.P.; Nawroth, P.P.; Bierhaus, A.; Humpert, P.M.; Jonas, J.B. Association of homozygous SDF-1 3’A genotype with proliferative diabetic retinopathy. Acta Diabetol. 2010, 47, 79–82. [Google Scholar] [CrossRef]
- García-Ramírez, M.; Canals, F.; Hernández, C.; Colomé, N.; Ferrer, C.; Carrasco, E.; García-Arumí, J.; Simó, R. Proteomic analysis of human vitreous fluid by fluorescence-based difference gel electrophoresis (DIGE): A new strategy for identifying potential candidates in the pathogenesis of proliferative diabetic retinopathy. Diabetologia 2007, 50, 1294–1303. [Google Scholar] [CrossRef]
- Loukovaara, S.; Nurkkala, H.; Tamene, F.; Gucciardo, E.; Liu, X.; Repo, P.; Lehti, K.; Varjosalo, M. Quantitative Proteomics Analysis of Vitreous Humor from Diabetic Retinopathy Patients. J. Proteome Res. 2015, 14, 5131–5143. [Google Scholar] [CrossRef]
- Shitama, T.; Hayashi, H.; Noge, S.; Uchio, E.; Oshima, K.; Haniu, H.; Takemori, N.; Komori, N.; Matsumoto, H. Proteome Profiling of Vitreoretinal Diseases by Cluster Analysis. Proteom. Clin. Appl. 2008, 2, 1265–1280. [Google Scholar] [CrossRef]
- Hernández, C.; García-Ramírez, M.; Colomé, N.; Villarroel, M.; Corraliza, L.; García-Pacual, L.; Casado, J.; Canals, F.; Simó, R. New pathogenic candidates for diabetic macular edema detected by proteomic analysis. Diabetes Care 2010, 33, e92. [Google Scholar] [CrossRef]
- Hernández, C.; Garcia-Ramírez, M.; Simó, R. Overexpression of hemopexin in the diabetic eye: A new pathogenic candidate for diabetic macular edema. Diabetes Care 2013, 36, 2815–2821. [Google Scholar] [CrossRef]
- Gao, B.B.; Clermont, A.; Rook, S.; Fonda, S.J.; Srinivasan, V.J.; Wojtkowski, M.; Fujimoto, J.G.; Avey, R.L.; Arrigg, P.G.; Bursell, S.E.; et al. Extracellular carbonic anhydrase mediates hemorrhagic retinal and cerebral vascular permeability through prekallikrein activation. Nat. Med. 2007, 13, 181–188. [Google Scholar] [CrossRef]
- Wang, B.; Yang, A.; Zhao, Z.; He, C.; Liu, Y.; Colman, R.W.; Dai, J.; Wu, Y. The Plasma Kallikrein-Kininogen Pathway Is Critical in the Pathogenesis of Colitis in Mice. Front. Immunol. 2018, 9, 21. [Google Scholar] [CrossRef]
- Kempe, S.; Fois, G.; Brunner, C.; Hoffmann, T.K.; Hahn, J.; Greve, J. Bradykinin signaling regulates solute permeability and cellular junction organization in lymphatic endothelial cells. Microcirculation 2020, 27, e12592. [Google Scholar] [CrossRef]
- Abdulaal, M.; Haddad, N.M.N.; Sun, J.K.; Silva, P.S. The Role of Plasma Kallikrein-Kinin Pathway in the Development of Diabetic Retinopathy: Pathophysiology and Therapeutic Approaches. Semin. Ophthalmol. 2016, 31, 19–24. [Google Scholar] [CrossRef]
- Liu, J.; Feener, E.P. Plasma kallikrein-kinin system and diabetic retinopathy. Biol. Chem. 2013, 394, 319–328. [Google Scholar] [CrossRef]
- Zhang, J.; Gerhardinger, C.; Lorenzi, M. Early complement activation and decreased levels of glycosylphosphatidylinositol-anchored complement inhibitors in human and experimental diabetic retinopathy. Diabetes 2002, 51, 3499–3504. [Google Scholar] [CrossRef]
- Gerl, V.B.; Bohl, J.; Pitz, S.; Stoffelns, B.; Pfeiffer, N.; Bhakdi, S. Extensive deposits of complement C3d and C5b-9 in the choriocapillaris of eyes of patients with diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2002, 43, 1104–1108. [Google Scholar]
- Jha, P.; Bora, P.S.; Bora, N.S. The role of complement system in ocular diseases including uveitis and macular degeneration. Mol. Immunol. 2007, 44, 3901–3908. [Google Scholar] [CrossRef] [PubMed]
- Lundh von Leithner, P.; Kam, J.H.; Bainbridge, J.; Catchpole, I.; Gough, G.; Coffey, P. Complement factor h is critical in the maintenance of retinal perfusion. Am. J. Pathol. 2009, 175, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Alexandrov, P.N.; Pogue, A.; Bhattacharjee, S.; Lukiw, W.J. Retinal amyloid peptides and complement factor H in transgenic models of Alzheimer’s disease. Neuroreport 2011, 22, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, M.M.; Li, Y.B.; Liu, G.D.; Teng, Y.; Liu, X.M. Association of CFH and CFB gene polymorphisms with retinopathy in type 2 diabetic patients. Mediat. Inflamm. 2013, 2013, 748435. [Google Scholar] [CrossRef]
- Iyer, S.S.R.; Lagrew, M.K.; Tillit, S.M.; Roohipourmoallai, R.; Korntner, S. The Vitreous Ecosystem in Diabetic Retinopathy: Insight into the Patho-Mechanisms of Disease. Int. J. Mol. Sci. 2021, 22, 7142. [Google Scholar] [CrossRef]
- Bajic, G.; Degn, S.E.; Thiel, S.; Andersen, G.R. Complement activation, regulation, and molecular basis for complement-related diseases. EMBO J. 2015, 34, 2735–2757. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, J.H.; Yu, Y.S.; Min, B.H.; Kim, K.-W. Protective effect of clusterin on blood-retinal barrier breakdown in diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1659–1665. [Google Scholar] [CrossRef]
- Gerhardinger, C.; Costa, M.B.; Coulombe, M.C.; Toth, I.; Hoehn, T.; Grosu, P. Expression of acute-phase response proteins in retinal Müller cells in diabetes. Investig. Ophthalmol. Vis. Sci. 2005, 46, 349–357. [Google Scholar] [CrossRef]
- Simó, R.; Hernández, C. European Consortium for the Early Treatment of Diabetic Retinopathy (EUROCONDOR). Neurodegeneration in the diabetic eye: New insights and therapeutic perspectives. Trends Endocrinol. Metab. 2014, 25, 23–33. [Google Scholar] [CrossRef]
- Sundstrom, J.M.; Hernández, C.; Weber, S.R.; Zhao, Y.; Dunklebarger, M.; Tiberti, N.; Laremore, T.; Simó-Servat, O.; García-Ramírez, M.; Barber, A.J.; et al. Proteomic analyisis in early diabetic retinopathy reveals mediators of neurodegenerative brain diseases. Investig. Ophthalmol. Vis. Sci. 2018, 59, 2264–2274. [Google Scholar] [CrossRef]
- Nian, S.; Lo, A.C.Y.; Mi, Y.; Ren, K.; Yang, D. Neurovascular unit in diabetic retinopathy: Pathophysiological roles and potential therapeutical targets. Eye Vis. 2021, 8, 15. [Google Scholar] [CrossRef]
- Barnes, P.J. How corticosteroids control inflammation: Quintiles Prize Lecture 2005. Br. J. Pharmacol. 2006, 148, 245–254. [Google Scholar] [CrossRef]
- Semeraro, F.; Morescalchi, F.; Cancarini, A.; Russo, A.; Rezzola, S.; Costagliola, C. Diabetic retinopathy, a vascular and inflammatory disease: Therapeutic implications. Diabetes Metab. 2019, 45, 517–527. [Google Scholar] [CrossRef]
- Busillo, J.M.; Cidlowski, J.A. The five Rs of glucocorticoid action during inflammation: Ready, reinforce, repress, resolve, and restore. Trends Endocrinol. Metab. 2013, 24, 109–119. [Google Scholar] [CrossRef]
- Oakley, R.H.; Cidlowski, J.A. The biology of the glucocorticoid receptor: New signaling mechanisms in health and disease. J. Allergy Clin. Immunol. 2013, 132, 1033–1044. [Google Scholar] [CrossRef]
- Cunningham, M.A.; Edelman, J.L.; Kaushal, S. Intravitreal steroids for macular edema: The past, the present, and the future. Surv. Ophthalmol. 2008, 53, 139–149. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, H.; Rojas, M.; Caldwell, R.W.; Caldwell, R.B. Anti-inflammatory therapy for diabetic retinopathy. Immunotherapy 2011, 3, 609–628. [Google Scholar] [CrossRef]
- Sun, J.K.; Jampol, L.M. The Diabetic Retinopathy Clinical Research Network (DRCR.net) and Its Contributions to the Treatment of Diabetic Retinopathy. Ophthalmic Res. 2019, 62, 225–230. [Google Scholar] [CrossRef]
- Dugel, P.U.; Bandello, F.; Loewenstein, A. Dexamethasone intravitreal implant in the treatment of diabetic macular edema. Clin. Ophthalmol. 2015, 9, 1321–1335. [Google Scholar] [CrossRef]
- Lynch, S.K.; Lee, K.; Chen, Z.; Folk, J.C.; Schmidt-Erfurth, U.; Gerendas, B.S.; Wahle, A.; Wykoff, C.C.; Abràmoff, M.D. Intravitreal Fluocinolone Acetonide May Decelerate Diabetic Retinal Neurodegeneration. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2134–2139. [Google Scholar] [CrossRef] [PubMed]
- Kaštelan, S.; Tomić, M.; Gverović Antunica, A.; Salopek Rabatić, J.; Ljubić, S. Inflammation and pharmacological treatment in diabetic retinopathy. Mediat. Inflamm. 2013, 2013, 213130. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.G.; Scott, I.U.; Stewart, M.W.; Flynn, H.W., Jr. Update on corticosteroids for diabetic macular edema. Clin. Ophthalmol. 2016, 10, 1723–1730. [Google Scholar] [CrossRef] [PubMed]
- Edelman, J.L. Differentiating intraocular glucocorticoids. Ophthalmologica 2010, 224 (Suppl. S1), 25–30. [Google Scholar] [CrossRef] [PubMed]
- Sarao, V.; Veritti, D.; Boscia, F.; Lanzetta, P. Intravitreal steroids for the treatment of retinal diseases. Sci. World J. 2014, 2014, 989501. [Google Scholar] [CrossRef]
- Blankenship, G.W. Evaluation of a single intravitreal injection of dexamethasone phosphate in vitrectomy surgery for diabetic retinopathy complications. Graefes Arch. Clin. Exp. Ophthalmol. 1991, 229, 62–65. [Google Scholar] [CrossRef]
- Gonzalez-Cortes, J.H.; Martinez-Pacheco, V.A.; Gonzalez-Cantu, J.E.; Bilgic, A.; de Ribot, F.M.; Sudhalkar, A.; Mohamed-Hamsho, J.; Kodjikian, L.; Mathis, T. Current Treatments and Innovations in Diabetic Retinopathy and Diabetic Macular Edema. Pharmaceutics 2022, 15, 122. [Google Scholar] [CrossRef]
- Cáceres-del-Carpio, J.; Costa, R.D.; Haider, A.; Narayanan, R.; Kuppermann, B.D. Corticosteroids: Triamcinolone, Dexamethasone and Fluocinolone. Dev. Ophthalmol. 2016, 55, 221–231. [Google Scholar]
- Kirk, J.; Fraser-Bell, S. Steroids for Diabetic Macular Oedema—A Brief Review of the Data. Eur. Ophth. 2019, 13, 44–48. [Google Scholar] [CrossRef]
- Ohira, A.; Hara, K.; Jóhannesson, G.; Tanito, M.; Ásgrímsdóttir, G.M.; Lund, S.H.; Loftsson, T.; Stefánsson, E. Topical dexamethasone γ-cyclodextrin nanoparticle eye drops increase visual acuity and decrease macular thickness in diabetic macular oedema. Acta Ophthalmol. 2015, 93, 610–615. [Google Scholar] [CrossRef]
- Schoenberger, S.D.; Kim, S.J. Nonsteroidal anti-inflammatory drugs for retinal disease. Int. J. Inflam. 2013, 2013, 281981. [Google Scholar] [CrossRef]
- Kern, T.S.; Miller, C.M.; Du, Y.; Zheng, L.; Mohr, S.; Ball, S.L.; Kim, M.; Jamison, J.A.; Bingaman, D.P. Topical administration of nepafenac inhibits diabetes-induced retinal microvascular disease and underlying abnormalities of retinal metabolism and physiology. Diabetes 2007, 56, 373–379. [Google Scholar] [CrossRef]
- Huang, H.; Gandhi, J.K.; Zhong, X.; Wei, Y.; Gong, J.; Duh, E.J.; Vinores, S.A. TNFalpha is required for late BRB breakdown in diabetic retinopathy, and its inhibition prevents leukostasis and protects vessels and neurons from apoptosis. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1336–1344. [Google Scholar] [CrossRef]
- Chu, C.J.; Johnston, R.L.; Buscombe, C.; Sallam, A.B.; Mohamed, Q.; Yang, Y.C. United Kingdom Pseudophakic Macular Edema Study Group. Risk Factors and Incidence of Macular Edema after Cataract Surgery: A Database Study of 81984 Eyes. Ophthalmology 2016, 123, 316–323. [Google Scholar] [CrossRef]
- Chen, X.Y.; Song, W.J.; Cai, H.Y.; Zhao, L. Macular edema after cataract surgery in diabetic eyes evaluated by optical coherence tomography. Int. J. Ophthalmol. 2016, 9, 81–85. [Google Scholar]
- Singh, R.P.; Staurenghi, G.; Pollack, A.; Adewale, A.; Walker, T.M.; Sager, D.; Lehmann, R. Efficacy of nepafenac ophthalmic suspension 0.1% in improving clinical outcomes following cataract surgery in patients with diabetes: An analysis of two randomized studies. Clin. Ophthalmol. 2017, 11, 1021–1029. [Google Scholar] [CrossRef]
- Singh, R.P.; Lehmann, R.; Martel, J.; Jong, K.; Pollack, A.; Tsorbatzoglou, A.; Staurenghi, G.; Cervantes-Coste Cervantes, G.; Alpern, L.; Modi, S.; et al. Nepafenac 0.3% after Cataract Surgery in Patients with Diabetic Retinopathy: Results of 2 Randomized Phase 3 Studies. Ophthalmology 2017, 124, 776–785. [Google Scholar] [CrossRef]
- Huang, H.; Li, W.; He, J.; Barnabie, P.; Shealy, D.; Vinores, S.A. Blockade of Tumor Necrosis Factor Alpha Prevents Complications of Diabetic Retinopathy. J. Clin. Exp. Ophthalmol. 2014, 5, 384. [Google Scholar]
- Ye, Q.; Lin, Y.N.; Xie, M.S.; Yao, Y.H.; Tang, S.M.; Huang, Y.; Wang, X.H.; Zhu, Y.H. Effects of etanercept on the apoptosis of ganglion cells and expression of Fas, TNF-α, caspase-8 in the retina of diabetic rats. Int. J. Ophthalmol. 2019, 12, 1083–1088. [Google Scholar] [CrossRef]
- Guo, J.; Yu, Q.; Yang, G.; Wei, W.; Tang, P.; Zheng, Y.; Zhang, F.; Liu, Q.; Wang, Y. Neuroprotective effects of etanercept on diabetic retinopathy via regulation of the TNF-α/NF-κB signaling pathway. Trop. J. Pharm. Res. 2022, 21, 2077–2083. [Google Scholar] [CrossRef]
- Sfikakis, P.P.; Markomichelakis, N.; Theodossiadis, G.P.; Grigoropoulos, V.; Katsilambros, N.; Theodossiadis, P.G. Regression of sight-threatening macular edema in type 2 diabetes following treatment with the anti-tumor necrosis factor monoclonal antibody infliximab. Diabetes Care. 2005, 28, 445–447. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Hernandez-Bogantes, E.; Roca, J.A.; Arevalo, J.F.; Barraza, K.; Lasave, A.F. Intravitreal tumor necrosis factor inhibitors in the treatment of refractory diabetic macular edema: A pilot study from the Pan-American Collaborative Retina Study Group. Retina 2011, 31, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Mirshahi, A.; Hoehn, R.; Lorenz, K.; Kramann, C.; Baatz, H. Anti-tumor necrosis factor alpha for retinal diseases: Current knowledge and future concepts. J. Ophthalmic Vis. Res. 2012, 7, 39–44. [Google Scholar] [PubMed]
- Stahel, M.; Becker, M.; Graf, N.; Michels, S. Systemic interleukin 1beta inhibition in proliferative diabetic retinopathy: A prospective open-label study using canakinumab. Retina 2016, 36, 385–391. [Google Scholar] [CrossRef]
- Rao, V.R.; Prescott, E.; Shelke, B.B.; Trivedi, R.; Thomas, P.; Struble, C.; Gadek, T.; O’Neill, C.A.; Kompella, U.B. Delivery of SAR 1118 to retina via ophthalmic drops and its effectiveness in reduction of retinal leukostasis and vascular leakiness in rat streptozotocin (STZ) model of diabetic retinopathy (DR). Investig. Ophthalmol. Vis. Sci. 2010, 51, 5198–51204. [Google Scholar] [CrossRef]
- Hernández, C.; Bogdanov, P.; Gómez-Guerrero, C.; Sampedro, J.; Solà-Adell, C.; Espejo, C.; García-Ramírez, M.; Prieto, I.; Egido, J.; Simó, R. SOCS1-Derived Peptide Administered by Eye Drops Prevents Retinal Neuroinflammation and Vascular Leakage in Experimental Diabetes. Int. J. Mol. Sci. 2019, 20, 3615. [Google Scholar] [CrossRef]
- Kang, Q.; Yang, C. Oxidative stress and diabetic retinopathy: Molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020, 37, 101799. [Google Scholar] [CrossRef]
- Garcia-Medina, J.J.; Rubio-Velazquez, E.; Foulquie-Moreno, E.; Casaroli-Marano, R.P.; Pinazo-Duran, M.D.; Zanon-Moreno, V.; Del-Rio-Vellosillo, M. Update on the Effects of Antioxidants on Diabetic Retinopathy: In Vitro Experiments, Animal Studies and Clinical Trials. Antioxidants 2020, 9, 561. [Google Scholar] [CrossRef]
- Rahimi, Z.; Moradi, M.; Nasri, H. A systematic review of the role of renin angiotensin aldosterone system genes in diabetes mellitus, diabetic retinopathy and diabetic neuropathy. J. Res. Med. Sci. 2014, 19, 1090–1098. [Google Scholar]
- Zhang, J.Z.; Xi, X.; Gao, L.; Kern, T.S. Captopril inhibits capillary degeneration in the early stages of diabetic retinopathy. Curr. Eye Res. 2007, 32, 883–889. [Google Scholar] [CrossRef]
- Wang, B.; Wang, F.; Zhang, Y.; Zhao, S.H.; Zhao, W.J.; Yan, S.L.; Wang, Y.G. Effects of RAS inhibitors on diabetic retinopathy: A systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015, 3, 263–274. [Google Scholar] [CrossRef]
- Wright, A.D.; Dodson, P.M. Diabetic retinopathy and blockade of the renin-angiotensin system: New data from the DIRECT study programme. Eye 2010, 24, 1–6. [Google Scholar] [CrossRef]
- Stem, M.S.; Gardner, T.W. Neurodegeneration in the pathogenesis of diabetic retinopathy: Molecular mechanisms and therapeutic implications. Curr. Med. Chem. 2013, 20, 3241–3250. [Google Scholar] [CrossRef]
- Hernández, C.; Arroba, A.I.; Bogdanov, P.; Ramos, H.; Simó-Servat, O.; Simó, R.; Valverde, A.M. Effect of Topical Administration of Somatostatin on Retinal Inflammation and Neurodegeneration in an Experimental Model of Diabetes. J. Clin. Med. 2020, 9, 2579. [Google Scholar] [CrossRef]
- Zheng, B.; Li, T.; Chen, H.; Xu, X.; Zheng, Z. Correlation between ficolin-3 and vascular endothelial growth factor-to-pigment epithelium-derived factor ratio in the vitreous of eyes with proliferative diabetic retinopathy. Am. J. Ophthalmol. 2011, 152, 1039–1043. [Google Scholar] [CrossRef]
- Reiter, C.E.; Wu, X.; Sandirasegarane, L.; Nakamura, M.; Gilbert, K.A.; Singh, R.S.; Fort, P.E.; Antonetti, D.A.; Gardner, T.W. Diabetes reduces basal retinal insulin receptor signaling: Reversal with systemic and local insulin. Diabetes 2006, 55, 1148–1156. [Google Scholar] [CrossRef]
- Hernández, C.; Bogdanov, P.; Corraliza, L.; García-Ramírez, M.; Solà-Adell, C.; Arranz, J.A.; Arroba, A.I.; Valverde, A.M.; Simó, R. Topical Administration of GLP-1 Receptor Agonists Prevents Retinal Neurodegeneration in Experimental Diabetes. Diabetes 2016, 65, 172–187. [Google Scholar] [CrossRef]
- Hebsgaard, J.B.; Pyke, C.; Yildirim, E.; Knudsen, L.B.; Heegaard, S.; Kvist, P.H. Glucagon-like peptide-1 receptor expression in the human eye. Diabetes Obes. Metab. 2018, 20, 2304–2308. [Google Scholar] [CrossRef]
- Yoshida, Y.; Yamagishi, S.; Matsui, T.; Jinnouchi, Y.; Fukami, K.; Imaizumi, T.; Yamakawa, R. Protective role of pigment epithelium-derived factor (PEDF) in early phase of experimental diabetic retinopathy. Diabetes Metab. Res. Rev. 2009, 25, 678–686. [Google Scholar] [CrossRef]
- Liu, Y.; Leo, L.F.; McGregor, C.; Grivitishvili, A.; Barnstable, C.J.; Tombran-Tink, J. Pigment epithelium-derived factor (PEDF) peptide eye drops reduce inflammation, cell death and vascular leakage in diabetic retinopathy in Ins2(Akita) mice. Mol. Med. 2012, 18, 1387–1401. [Google Scholar] [CrossRef]
- Zerbini, G.; Maestroni, S.; Leocani, L.; Mosca, A.; Godi, M.; Paleari, R.; Belvedere, A.; Gabellini, D.; Tirassa, P.; Castoldi, V.; et al. Topical nerve growth factor prevents neurodegenerative and vascular stages of diabetic retinopathy. Front. Pharmacol. 2022, 13, 1015522. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Kang, S.S.; Kim, J.Y.; Tchah, H. Nerve Growth Factor Attenuates Apoptosis and Inflammation in the Diabetic Cornea. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6767–6775. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Chang, Z.P.; Ren, R.T.; Wei, S.H.; Zhou, H.F.; Chen, X.F.; Hou, B.K.; Jin, X.; Zhang, M.N. Protective Effects of Adeno-associated Virus Mediated Brain-derived Neurotrophic Factor Expression on Retinal Ganglion Cells in Diabetic Rats. Cell Mol. Neurobiol. 2012, 32, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Simó, R.; Hernández, C.; Porta, M.; Bandello, F.; Grauslund, J.; Harding, S.P.; Aldington, S.J.; Egan, C.; Frydkjaer-Olsen, U.; García-Arumí, J.; et al. European Consortium for the Early Treatment of Diabetic Retinopathy (EUROCONDOR). Effects of Topically Administered Neuroprotective Drugs in Early Stages of Diabetic Retinopathy: Results of the EUROCONDOR Clinical Trial. Diabetes 2019, 68, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Ramos, H.; Bogdanov, P.; Sampedro, J.; Huerta, J.; Simó, R.; Hernández, C. Beneficial Effects of Glucagon-Like Peptide-1 (GLP-1) in Diabetes-Induced Retinal Abnormalities: Involvement of Oxidative Stress. Antioxidants 2020, 9, 846. [Google Scholar] [CrossRef] [PubMed]
- Ramos, H.; Bogdanov, P.; Huerta, J.; Deàs-Just, A.; Hernández, C.; Simó, R. Antioxidant Effects of DPP-4 Inhibitors in Early Stages of Experimental Diabetic Retinopathy. Antioxidants 2022, 11, 1418. [Google Scholar] [CrossRef]
- Sampedro, J.; Bogdanov, P.; Ramos, H.; Solà-Adell, C.; Turch, M.; Valeri, M.; Simó-Servat, O.; Lagunas, C.; Simó, R.; Hernández, C. New Insights into the Mechanisms of Action of Topical Administration of GLP-1 in an Experimental Model of Diabetic Retinopathy. J. Clin. Med. 2019, 8, 339. [Google Scholar] [CrossRef]
- Hernández, C.; Bogdanov, P.; Solà-Adell, C.; Sampedro, J.; Valeri, M.; Genís, X.; Simó-Servat, O.; García-Ramírez, M.; Simó, R. Topical administration of DPP-IV inhibitors prevents retinal neurodegeneration in experimental diabetes. Diabetologia 2017, 60, 2285–2298. [Google Scholar] [CrossRef]
- Cervia, D.; Catalani, E.; Casini, G. Neuroprotective Peptides in Retinal Disease. J. Clin. Med. 2019, 8, 1146. [Google Scholar] [CrossRef]
- Öner, A. Stem Cell Treatment in Retinal Diseases: Recent Developments. Turk. J. Ophthalmol. 2018, 48, 33–38. [Google Scholar] [CrossRef]
- Mannino, G.; Russo, C.; Longo, A.; Anfuso, C.D.; Lupo, G.; Lo Furno, D.; Giuffrida, R.; Giurdanella, G. Potential therapeutic applications of mesenchymal stem cells for the treatment of eye diseases. World J. Stem Cells. 2021, 13, 632–644. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos, H.; Hernández, C.; Simó, R.; Simó-Servat, O. Inflammation: The Link between Neural and Vascular Impairment in the Diabetic Retina and Therapeutic Implications. Int. J. Mol. Sci. 2023, 24, 8796. https://doi.org/10.3390/ijms24108796
Ramos H, Hernández C, Simó R, Simó-Servat O. Inflammation: The Link between Neural and Vascular Impairment in the Diabetic Retina and Therapeutic Implications. International Journal of Molecular Sciences. 2023; 24(10):8796. https://doi.org/10.3390/ijms24108796
Chicago/Turabian StyleRamos, Hugo, Cristina Hernández, Rafael Simó, and Olga Simó-Servat. 2023. "Inflammation: The Link between Neural and Vascular Impairment in the Diabetic Retina and Therapeutic Implications" International Journal of Molecular Sciences 24, no. 10: 8796. https://doi.org/10.3390/ijms24108796
APA StyleRamos, H., Hernández, C., Simó, R., & Simó-Servat, O. (2023). Inflammation: The Link between Neural and Vascular Impairment in the Diabetic Retina and Therapeutic Implications. International Journal of Molecular Sciences, 24(10), 8796. https://doi.org/10.3390/ijms24108796






