Optimizing Fluid Management Guided by Volumetric Parameters in Patients with Sepsis and ARDS
Abstract
1. Introduction
2. Results
3. Discussion
3.1. Pulmonary and Extrapulmonary ARDS
3.2. Fluid Balance
3.3. Limitations
4. Materials and Methods
Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Levy, M.M.; Evans, L.E.; Rhodes, A. The Surviving Sepsis Campaign Bundle: 2018 update. Intensive Care Med. 2018, 44, 925–928. [Google Scholar] [CrossRef] [PubMed]
- Roger, C.; AzuRea Group; Zieleskiewicz, L.; Demattei, C.; Lakhal, K.; Piton, G.; Louart, B.; Constantin, J.-M.; Chabanne, R.; Faure, J.-S.; et al. Time course of fluid responsiveness in sepsis: The fluid challenge revisiting (FCREV) study. Crit. Care 2019, 23, 179. [Google Scholar] [CrossRef] [PubMed]
- Marik, P.E.; Byrne, L.; van Haren, F. Fluid resuscitation in sepsis: The great 30 mL per kg hoax. J. Thorac. Dis. 2020, 12, S37–S47. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, A.L.; Dechert, R.E.; Park, P.K.; Bartlett, R.H.; NIH NHLBI ARDS Network. Review of a large clinical series: Association of cumulative fluid balance on outcome in acute lung injury: A retrospective review of the ARDSnet tidal volume study cohort. J. Intensive Care Med. 2008, 24, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Boyd, J.H.; Forbes, J.; Nakada, T.-A.; Walley, K.R.; Russell, J.A. Fluid resuscitation in septic shock: A positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit. Care Med. 2011, 39, 259–265. [Google Scholar] [CrossRef]
- Malbrain, M.L.; Marik, P.E.; Witters, I.; Cordemans, C.; Kirkpatrick, A.W.; Roberts, D.J.; Van Regenmortel, N. Fluid overload, de-resuscitation, and outcomes in critically ill or injured patients: A systematic review with suggestions for clinical practice. Anaesthesiol. Intensive Ther. 2014, 46, 361–380. [Google Scholar] [CrossRef]
- Prowle, J.R.; Echeverri, J.E.; Ligabo, E.V.; Ronco, C.; Bellomo, R. Fluid balance and acute kidney injury. Nat. Rev. Nephrol. 2010, 6, 107–115. [Google Scholar] [CrossRef]
- Cordemans, C.; De Laet, I.; Van Regenmortel, N.; Schoonheydt, K.; Dits, H.; Martin, G.; Huber, W.; Malbrain, M.L. Aiming for a negative fluid balance in patients with acute lung injury and increased intra-abdominal pressure: A pilot study looking at the effects of PAL-treatment. Ann. Intensive Care 2012, 2, 15. [Google Scholar] [CrossRef]
- Silversides, J.A.; Ferguson, A.J.; McAuley, D.F.; Blackwood, B.; Marshall, J.C.; Fan, E. Fluid strategies and outcomes in patients with acute respiratory distress syndrome, systemic inflammatory response syndrome and sepsis: A protocol for a systematic review and meta-analysis. Syst. Rev. 2015, 4, 162. [Google Scholar] [CrossRef]
- Silversides, J.A.; Major, E.; Ferguson, A.J.; Mann, E.E.; McAuley, D.F.; Marshall, J.C.; Blackwood, B.; Fan, E. Conservative fluid management or deresuscitation for patients with sepsis or acute respiratory distress syndrome following the resuscitation phase of critical illness: A systematic review and meta-analysis. Intensive Care Med. 2016, 43, 155–170. [Google Scholar] [CrossRef]
- Silversides, J.A.; Fitzgerald, E.; Manickavasagam, U.S.; Lapinsky, S.E.; Nisenbaum, R.; Hemmings, N.; Nutt, C.; Trinder, T.J.; Pogson, D.G.; Fan, E.; et al. Deresuscitation of patients with iatrogenic fluid overload is associated with reduced mortality in critical illness. Crit. Care Med. 2018, 46, 1600–1607. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Corl, K.; Levy, M.M. Fluid Therapy and Acute Respiratory Distress Syndrome. Crit. Care Clin. 2021, 37, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Wiedermann, C.J. Phases of fluid management and the roles of human albumin solution in perioperative and critically ill patients. Curr. Med. Res. Opin. 2020, 36, 1961–1973. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.-L.; de Mendonca, A.; Cantraine, F.; Moreno, R.; Takala, J.; Suter, P.M.; Sprung, C.L.; Colardyn, F.; Blecher, S. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: Results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit. Care Med. 1998, 26, 1793–1800. [Google Scholar] [CrossRef]
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Wiedemann, H.P.; Wheeler, A.P.; Bernard, G.R.; Thompson, B.T.; Hayden, D.; Deboisblanc, B.; Connors, A.F.J.; Hite, R.D.; Harabin, A.L. Comparison of two fluid-management strategies in acute lung injury. N. Engl. J. Med. 2006, 354, 2564–2575. [Google Scholar] [CrossRef]
- Silversides, J.A.; McAuley, D.F.; Blackwood, B.; Fan, E.; Ferguson, A.J.; Marshall, J.C. Fluid management and deresuscitation practices: A survey of critical care physicians. J. Intensive Care Soc. 2019, 21, 111–118. [Google Scholar] [CrossRef]
- Bissell, B.D.; Laine, M.E.; Bastin, M.L.T.; Flannery, A.H.; Kelly, A.; Riser, J.; Neyra, J.A.; Potter, J.; Morris, P.E. Impact of protocolized diuresis for deresuscitation in the intensive care unit. Crit. Care 2020, 24, 70. [Google Scholar] [CrossRef]
- Grams, M.E.; Estrella, M.M.; Coresh, J.; Brower, R.G.; Liu, K.D.; National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome Network. Fluid balance, diuretic use, and mortality in acute kidney injury. Clin. J. Am. Soc. Nephrol. 2011, 6, 966–973. [Google Scholar] [CrossRef]
- Martin, G.S.; Moss, M.; Wheeler, A.P.; Mealer, M.; Morris, J.A.; Bernard, G.R. A randomized, controlled trial of furosemide with or without albumin in hypoproteinemic patients with acute lung injury. Crit. Care Med. 2005, 33, 1681–1687. [Google Scholar] [CrossRef]
- Yoon, B.R.; Leem, A.Y.; Park, M.S.; Kim, Y.S.; Chung, K.S. Optimal timing of initiating continuous renal replacement therapy in septic shock patients with acute kidney injury. Sci. Rep. 2019, 9, 11981. [Google Scholar] [CrossRef]
- Silversides, J.A.; Perner, A.; Malbrain, M.L.N.G. Liberal versus restrictive fluid therapy in critically ill patients. Intensive Care Med. 2019, 45, 1440–1442. [Google Scholar] [CrossRef] [PubMed]
- Van Regenmortel, N.; Verbrugghe, W.; Roelant, E.; Van den Wyngaert, T.; Jorens, P.G. Maintenance fluid therapy and fluid creep impose more significant fluid, sodium, and chloride burdens than resuscitation fluids in critically ill patients: A retrospective study in a tertiary mixed ICU population. Intensive Care Med. 2018, 44, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Tagami, T.; Ong, M.E.H. Extravascular lung water measurements in acute respiratory distress syndrome: Why, how, and when? Curr. Opin. Crit. Care 2018, 24, 209–215. [Google Scholar] [CrossRef]
- Malbrain, M.L.N.G.; Van Regenmortel, N.; Saugel, B.; De Tavernier, B.; Van Gaal, P.-J.; Joannes-Boyau, O.; Teboul, J.-L.; Rice, T.W.; Mythen, M.; Monnet, X. Principles of fluid management and stewardship in septic shock: It is time to consider the four D’s and the four phases of fluid therapy. Ann. Intensive Care 2018, 8, 66. [Google Scholar] [CrossRef] [PubMed]
- Marik, P.; Bellomo, R. A rational approach to fluid therapy in sepsis. BJA Br. J. Anaesth. 2016, 3, 339–349. [Google Scholar] [CrossRef]
- Michard, F.; Fernandez-Mondejar, E.; Kirov, M.Y.; Malbrain, M.; Tagami, T. A new and simple definition for acute lung injury. Crit. Care Med. 2014, 40, 1004–1006. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Cui, N.; Su, L.; Long, Y.; Wang, X.; Zhou, X.; Chai, W.; Liu, D. Prognostic value of extravascular lung water and its potential role in guiding fluid therapy in septic shock after initial resuscitation. Crit. Care 2016, 33, 106–113. [Google Scholar] [CrossRef]
- Hamzaoui, O.; Shi, R.; Carelli, S.; Sztrymf, B.; Prat, D.; Jacobs, F.; Monnet, X.; Gouëzel, C.; Teboul, J.-L. Changes in pulse pressure variation to assess preload responsiveness in mechanically ventilated patients with spontaneous breathing activity: An observational study. Br. J. Anaesth. 2021, 4, 532–538. [Google Scholar] [CrossRef]
- Teboul, J.-L.; Monnet, X.; Chemla, D.; Michard, F. Arterial pulse pressure variation with mechanical ventilation. Am. J. Respir. Crit. Care Med. 2019, 1, 22–31. [Google Scholar] [CrossRef]
- Kaneko, T.; Kawamura, Y.; Maekawa, T.; Tagami, T.; Nakamura, T.; Saito, N.; Kitazawa, Y.; Ishikura, H.; Sugita, M.; Okuchi, K.; et al. Global end-diastolic volume is an important contributor to increased extravascular lung water in patients with acute lung injury and acute respiratory distress syndrome: A multicenter observational study. J. Intensive Care 2014, 1, 25. [Google Scholar] [CrossRef]
- Rocco, P.R.; Pelosi, P. Pulmonary and extrapulmonary acute respiratory distress syndrome: Myth or reality? Curr. Opin. Crit. Care 2008, 14, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.-M.; Jung, H.; Koh, Y.; Lee, J.S.; Shim, T.-S.; Lee, S.-D.; Kim, W.-S.; Kim, D.S.; Kim, W.-D. Effect of alveolar recruitment maneuver in early acute respiratory distress syndrome according to antiderecruitment strategy, etiological category of diffuse lung injury, and body position of the patient. Crit. Care Med. 2003, 31, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Pelosi, P.; Suter, P.M.; Pedoto, A.; Vercesi, P.; Lissoni, A. Acute respiratory distress syndrome caused by pulmonary and extrapulmonary disease. Different syndromes? Am. J. Respir. Crit. Care Med. 1998, 158, 3–11. [Google Scholar] [CrossRef]
- Garcia, C.; Pelosi, P.; Rocco, P.R. Pulmonary and extrapulmonary acute respiratory distress syndrome: Are they different? Rev. Bras. Ter. Intensive 2008, 20, 178–183. [Google Scholar] [CrossRef]
- Pelosi, P.; Caironi, P.; Gattinoni, L. Pulmonary and extrapulmonary forms of acute respiratory distress syndrome. Semin. Respir. Crit. Care Med. 2001, 22, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Morisawa, K.; PiCCO Pulmonary Edema Study Group; Fujitani, S.; Taira, Y.; Kushimoto, S.; Kitazawa, Y.; Okuchi, K.; Ishikura, H.; Sakamoto, T.; Tagami, T.; et al. Difference in pulmonary permeability between indirect and direct acute respiratory distress syndrome assessed by the transpulmonary thermodilution technique: A prospective, observational, multi-institutional study. J. Intensive Care 2014, 2, 24. [Google Scholar] [CrossRef] [PubMed]
- Menezes, S.L.S.; Bozza, P.T.; Neto, H.C.C.F.; Laranjeira, A.P.; Negri, E.M.; Capelozzi, V.L.; Zin, W.A.; Rocco, P.R.M. Pulmonary and extrapulmonary acute lung injury: Inflammatory and ultrastructural analyses. J. Appl. Physiol. 2005, 98, 1777–1783. [Google Scholar] [CrossRef]
- Coppola, S.; Froio, S.; Marino, A.; Brioni, M.; Cesana, B.M.; Cressoni, M.; Gattinoni, L.; Chiumello, D. Respiratory mechanics, lung recruitability, and gas exchange in pulmonary and extrapulmonary acute respiratory distress syndrome. Crit. Care Med. 2019, 6, 792–799. [Google Scholar] [CrossRef]
- Sirvent, J.M.; Ferri, C.; Baró, A.; Murcia, C.; Lorencio, C. Fluid balance in sepsis and sepsis shock as a determining factor of mortality. Am. J. Emerg. Med. 2015, 2, 186–189. [Google Scholar] [CrossRef]
- Kirov, M.; Kuzkov, V.; Saugel, B. (Eds.) Advanced Hemodynamic Monitoring: Basics and New Horizons; Springer: Cham, Switzerland, 2021; 298p, ISBN 978-3-030-71751-3/978-3-030-71752-0. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Ferguson, N.D.; Fan, E.; Camporota, L.; Antonelli, M.; Anzueto, A.; Beale, R.; Brochard, L.; Brower, R.; Esteban, A.; Gattinoni, L.; et al. The Berlin definition of ARDS: An expanded rationale, justification, and supplementary material. Intensive Care Med. 2012, 10, 1573–1582. [Google Scholar] [CrossRef] [PubMed]
- Meyer, N.J.; Gattinoni, L.; Calfee, C.S. Acute respiratory distress syndrome. Lancet 2021, 398, 622–637. [Google Scholar] [CrossRef] [PubMed]
- Khromacheva, N.O.; Fot, E.V.; Kuzkov, V.V.; Kirov, M.Y. Goal-directed dehydration therapy in sepsis and acute respiratory distress syndrome guided by volumetric hemodynamic monitoring. Messenger Anesthesiol. Resusc. 2019, 16, 6–15. [Google Scholar] [CrossRef]


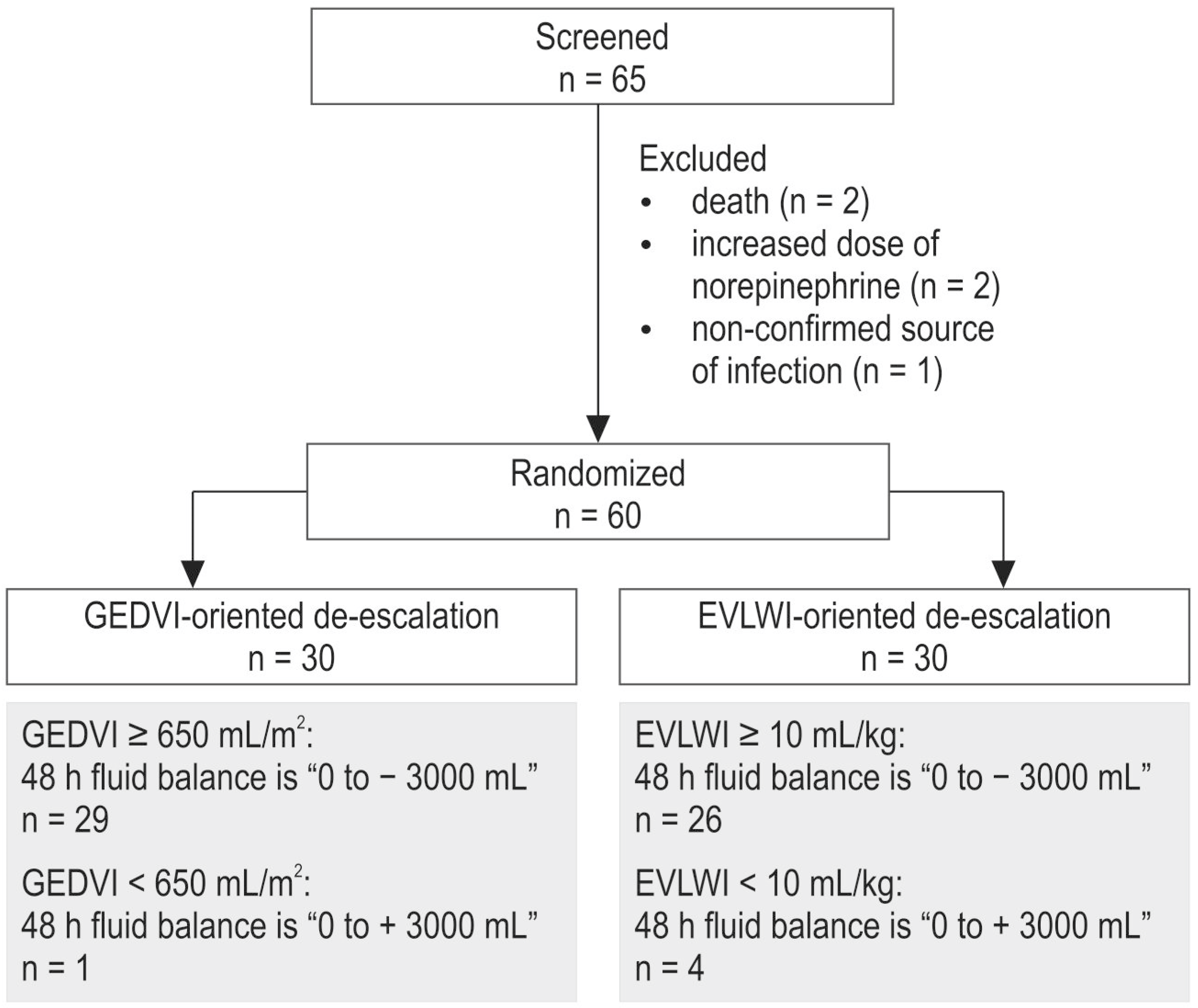
| Characteristics | Group | p | |
|---|---|---|---|
| EVLWI (n = 30) | GEDVI (n = 30) | ||
| Age, years | 54 (24–79) | 58 (30–86) | 0.4 |
| Sex, male/female | 16/14 | 20/10 | 0.3 |
| Origin of sepsis, n (%): | |||
| pneumonia | 19 (63.3) | 13 (43.3) | 0.2 |
| necrotizing pancreatitis | 5 (16.7) | 7 (23.3) | 0.7 |
| peritonitis | 2 (6.7) | 9 (30.0) | 0.04 |
| mediastinitis | 2 (6.7) | – | 0.5 |
| pyelonephritis | 1 (3.3) | 1(3.3) | 1.0 |
| soft tissue infection | 1 (3.3) | – | 1.0 |
| Direct ARDS, n (%) | 19 (63.3) | 13 (43.3) | 0.2 |
| Indirect ARDS, n (%) | 11 (36.7) | 17 (56.7) | 0.2 |
| ARDS severity, n (%): | |||
| mild | 14 (46.6) | 17 (56.6) | 0.6 |
| moderate | 14 (46.6) | 12 (40.6) | 0.8 |
| severe | 2 (6.8) | 1 (2.8) | 1.0 |
| Components of organ failure, n (%): | |||
| septic shock | 10 (33) | 11 (37) | 1.0 |
| DIC | 17 (57) | 19 (63) | 0.8 |
| acute liver failure | 8 (27) | 11 (37) | 0.6 |
| acute renal failure | 22 (73) | 18 (60) | 0.4 |
| SOFA score, baseline, pt. | 8 (3–14) | 8 (4–14) | 0.5 |
| SOFA score, 48 h, pt. | 6 (1–14) † | 7 (1–16) † | 0.1 |
| Incidence of norepinephrine administration, baseline, n (%) | 19 (63) | 21 (70) | 0.6 |
| Incidence of norepinephrine administration, 48 h, n (%) | 9 (30) † | 12 (40) † | 0.4 |
| Average dose of furosemide within 48 h, mg | 120 (120–320) | 120 (120–180) | 0.8 |
| Incidence of CVVH, n (%) | 12 (40) | 11 (37) | 0.6 |
| Fluid balance, baseline, mL | 690(−212 to +1512) | 430(−75 to +1725) | 0.9 |
| Fluid balance, 48 h, mL | −2210(−3020 to −1573) † | −2298(−2982 to −1616) † | 0.9 |
| Time in ICU before randomization, days | 2 (1–4) | 3 (1–4) | 0.3 |
| Duration of mechanical ventilation, days | 9 (4–16) | 7 (4–17) | 0.7 |
| Duration of ICU stay, days | 16 (9–22) | 12 (8–20) | 0.4 |
| Duration of hospitalization, days | 24 (16–46) | 24 (15–32) | 0.4 |
| 28-day mortality, n (%) | 10 (33.3) | 14 (46.7) | 0.3 |
| Characteristics | Group | Stages | ||
|---|---|---|---|---|
| Baseline | 24 h | 48 h | ||
| MAP, mm Hg | EVLWI | 82 (71–94) | 77 (66–89) | 80 (73–90) |
| GEDVI | 80 (73–85) | 80 (72–84) | 83 (76–96) | |
| HR, bpm | EVLWI | 100 (80–111) | 89 (79–104) | 94 (78–104) |
| GEDVI | 92 (79–110) | 89 (78–109) | 93 (84–107) | |
| CI, L/min/m2 | EVLWI | 3.6 (3.0–4.2) | 3.4 (2.9–4.4) | 3.7 (3.1–4.4) |
| GEDVI | 3.7 (3.1–4.2) | 3.8 (3.3–4.3) | 3.9 (3.5–4.5) | |
| CVP, mm Hg | EVLWI | 10 (8–12) | 7 (5–10) | 8 (5–10) |
| GEDVI | 9 (7–13) | 9 (7–11) | 10 (8–12) | |
| GEDVI, mL/m2 | EVLWI | 816 (642–951) | 768 (636–956) | 751 (602–1005) |
| GEDVI | 776 (701–902) | 763 (708–903) | 778 (702–908) | |
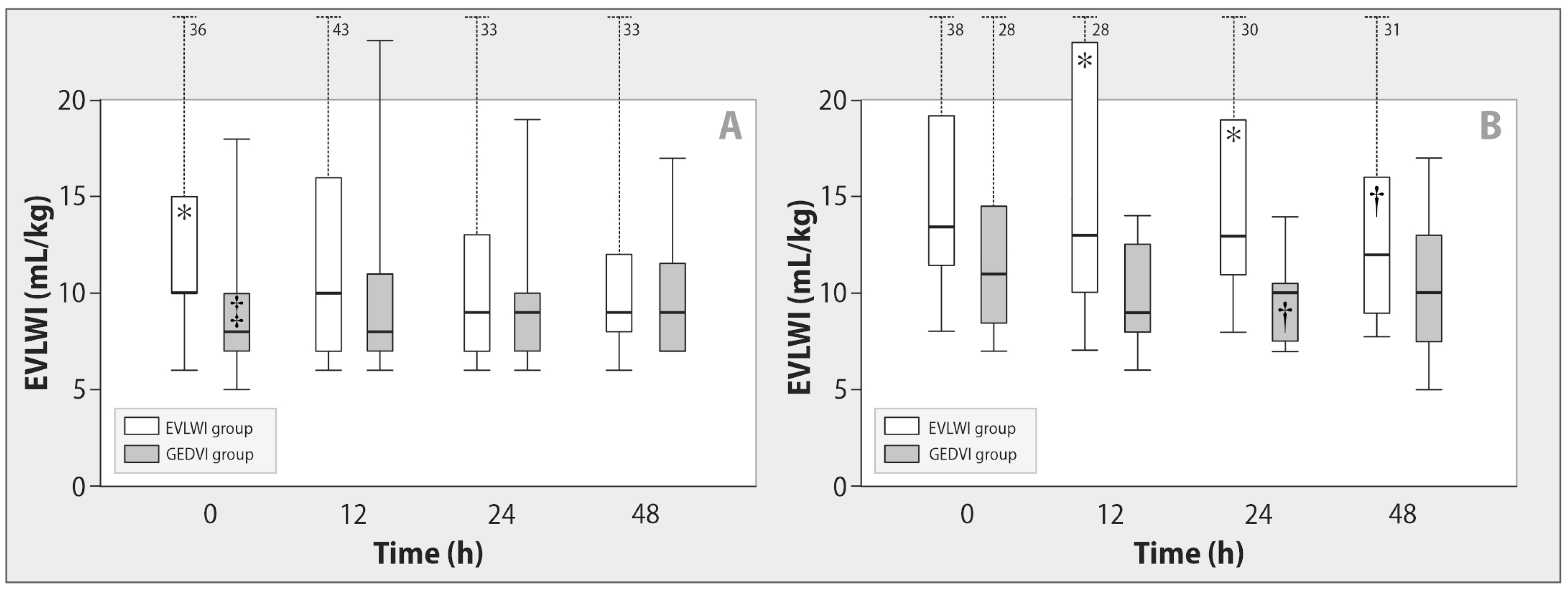
| EVLWI, mL/kg | EVLWI | 13 (10–18) | 12 (9–15) | 11 (8–16) † |
| GEDVI | 9 (7–11) * | 9 (7–12) * | 9 (7–12)* | |
| PVPI | EVLWI | 2.7 (2.2–3.2) | 2.6 (2.1–3.2) | 2.5 (2.0–3.0) † |
| GEDVI | 1.8 (1.5–2.1) * | 1.6 (1.4–1.8) * | 1.8 (1.6–2.0) * | |
| PPV, % | EVLWI | 10 (6–17) | 16 (9–19) | 15 (8–18) |
| GEDVI | 8 (5–13) | 10 (8–15) | 13 (7–16) † | |
| SVV, % | EVLWI | 10 (6–20) | 16 (9–19) | 15 (11–20) † |
| GEDVI | 9 (6–13) | 11 (8-17) | 13 (9–19) | |
| BE, mmol/L | EVLWI | −3.1 (−7.3 to −0.8) | −0.8 (−3.5 to +2.8) † | −0.8 (−2.1 to +1.5) † |
| GEDVI | −4.1 (−6.3 to −0.7) | −1.3 (−3.4 to +0.7) † | −0.4 (−2.8 to +1.6) † | |
| Lactate, mmol/L | EVLWI | 1.7 (1.1–3.0) | 2.0 (1.5–2.8) | 1.7 (1.2–2.6) |
| GEDVI | 1.8 (1.2–2.9) | 2.0 (1.6–2.3) | 1.9 (1.4–3.0) | |
| Albumin, g/L | EVLWI | 28 (25–33) | 28 (23–31) | 29 (23–32) |
| GEDVI | 26 (22–29) | 27 (23–29) | 24 (18–32) | |
| CRP, mg/L | EVLWI | 203 (114–370) | 218 (116–378) | 230 (124–361) |
| GEDVI | 194 (164–410) | 191 (153–381) | 198 (120–398) | |
| Potassium, mmol/L | EVLWI | 4.0 (3.6–4.4) | 3.5 (3.3–4.0) †,* | 4.0 (3.6–4.4) |
| GEDVI | 4.2 (3.8–4.5) | 4.3 (3.7–4.6) | 4.2 (3.8–4.6) | |
| Sodium, mmol/L | EVLWI | 141 (137–145) | 144 (139–146) † | 143 (139–151) † |
| GEDVI | 141 (137–147) | 143 (138–150) † | 144 (138–149) | |
| Creatinine, mcmol/L | EVLWI | 126 (98–168) | 119 (98–158) | 107 (93–142) † |
| GEDVI | 123 (79–179) | 136 (88–175) | 133 (85–161) | |
| Urea, mmol/L | EVLWI | 9 (8–12) | 10 (8–12) | 8 (6–12) |
| GEDVI | 12 (8–19) | 13 (9–19)* | 13 (9–17)* | |
| Characteristics | Group | Stages | ||
|---|---|---|---|---|
| Baseline | 24 h | 48 h | ||
| PaO2/FiO2,mm Hg | EVLWI | 195 (133–253) | 241 (168–310)† | 254 (159–319) † |
| GEDVI | 217 (185–272) | 258(215–341)† | 248 (194–330) † | |
| PaCO2, mm Hg | EVLWI | 37 (32–46) | 36 (31–39) | 37 (33–42) |
| GEDVI | 36 (31–42) | 36 (32–40) | 38 (33–40) | |
| Tidal volume, mL | EVLWI | 495 (443–607) | 620 (555–664) † | 582 (509–634) |
| GEDVI | 541 (478–648) | 507 (436–617) * | 506 (444–637) | |
| Minute volume of ventilation, L | EVLWI | 9.9 (8.1–12.2) | 10.7 (8.7–12.5) | 12.1 (9.8–13.9) † |
| GEDVI | 10.4 (8.2–13.7) | 9.6 (7.9–11.2) † | 9.9 (8.6–12.4) | |
| PEEP, cm H2O | EVLWI | 8 (8–10) | 8 (7–11) | 8 (6–10) |
| GEDVI | 8 (7–10) | 8 (6–10) | 8 (6–10) | |
| Mean airway pressure, cm H2O | EVLWI | 13 (12–15) | 13 (11–16) | 13 (10–16) |
| GEDVI | 14 (12–16) | 14 (11–15) | 14 (11–16) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fot, E.V.; Khromacheva, N.O.; Ushakov, A.A.; Smetkin, A.A.; Kuzkov, V.V.; Kirov, M.Y. Optimizing Fluid Management Guided by Volumetric Parameters in Patients with Sepsis and ARDS. Int. J. Mol. Sci. 2023, 24, 8768. https://doi.org/10.3390/ijms24108768
Fot EV, Khromacheva NO, Ushakov AA, Smetkin AA, Kuzkov VV, Kirov MY. Optimizing Fluid Management Guided by Volumetric Parameters in Patients with Sepsis and ARDS. International Journal of Molecular Sciences. 2023; 24(10):8768. https://doi.org/10.3390/ijms24108768
Chicago/Turabian StyleFot, Evgeniia V., Natalia O. Khromacheva, Aleksei A. Ushakov, Aleksei A. Smetkin, Vsevolod V. Kuzkov, and Mikhail Y. Kirov. 2023. "Optimizing Fluid Management Guided by Volumetric Parameters in Patients with Sepsis and ARDS" International Journal of Molecular Sciences 24, no. 10: 8768. https://doi.org/10.3390/ijms24108768
APA StyleFot, E. V., Khromacheva, N. O., Ushakov, A. A., Smetkin, A. A., Kuzkov, V. V., & Kirov, M. Y. (2023). Optimizing Fluid Management Guided by Volumetric Parameters in Patients with Sepsis and ARDS. International Journal of Molecular Sciences, 24(10), 8768. https://doi.org/10.3390/ijms24108768






