Alterations in the Glycan Composition of Serum Glycoproteins in Attention-Deficit Hyperactivity Disorder
Abstract
1. Introduction
2. Results
2.1. Lectin-Based Glycoprotein Microarray Analysis
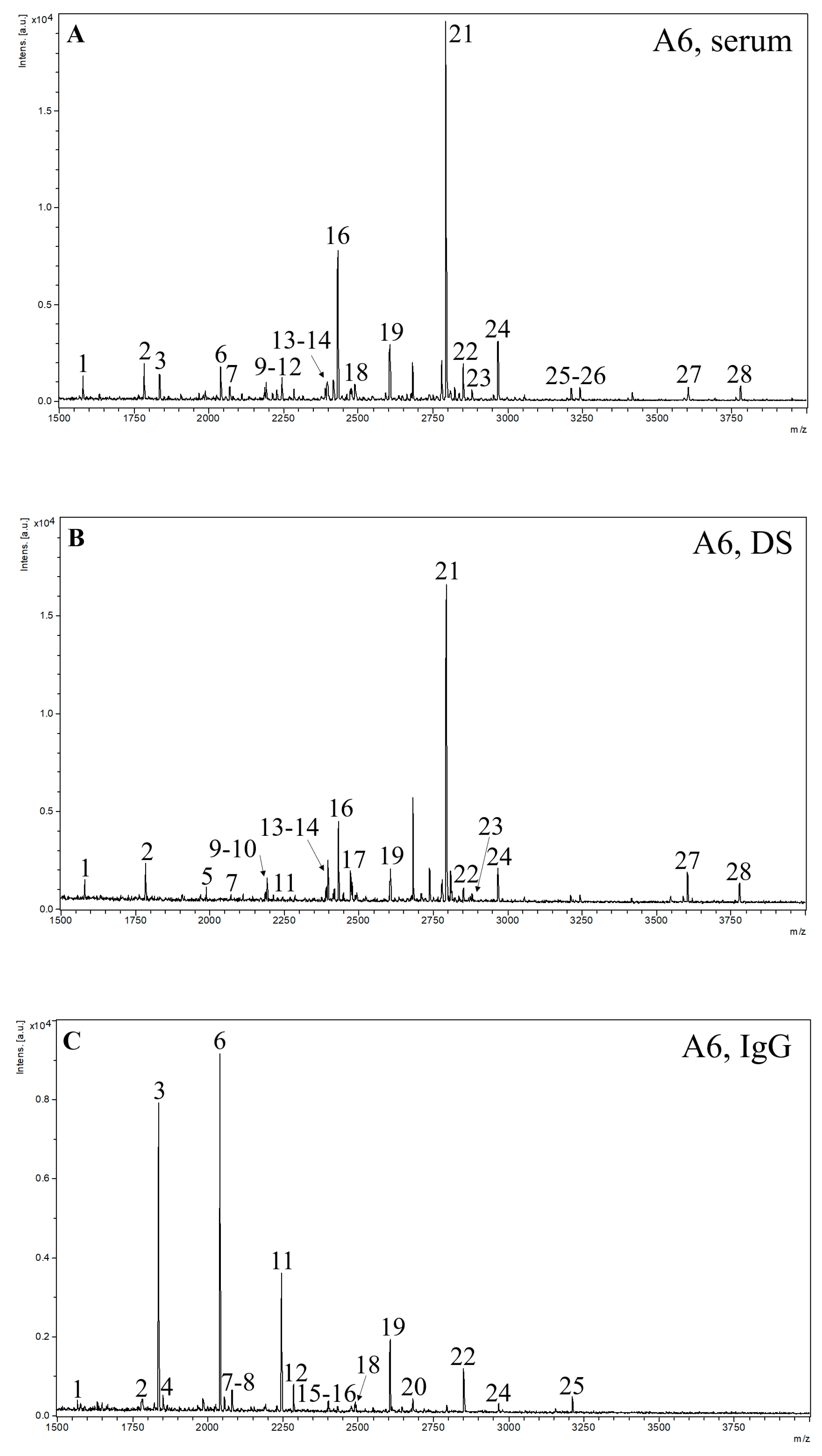
2.2. MALDI-TOF MS Analysis
3. Discussion
4. Materials and Methods
4.1. Samples
4.2. Sera Fragments
4.3. Sera Depletion
4.4. IgG Fraction
4.5. Protein Concentrations
4.6. Lectin-Based Glycoprotein Microarray
4.7. MALDI-TOF MS Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harris, J.C. New classification for neurodevelopmental disorders in DSM-5. Curr. Opin. Psychiatry 2014, 27, 95–97. [Google Scholar] [CrossRef]
- Santos, S.; Ferreira, H.; Martins, J.; Gonçalves, J.; Castelo-Branco, M. Male sex bias in early and late onset neurodevelopmental disorders: Shared aspects and differences in Autism Spectrum Disorder, Attention Deficit/hyperactivity Disorder, and Schizophrenia. Neurosci. Biobehav. Rev. 2022, 135, 104577. [Google Scholar] [CrossRef]
- Association, A.P. Neurodevelopmental Disorders: DSM-5® Selections; American Psychiatric Association Publishing: Washington DC, USA, 2015; ISBN 1615370137. [Google Scholar]
- Morris-Rosendahl, D.J.; Crocq, M.-A. Neurodevelopmental disorders—The history and future of a diagnostic concept. Dialogues Clin. Neurosci. 2020, 22, 65–72. [Google Scholar] [CrossRef]
- Wolraich, M.L.; Chan, E.; Froehlich, T.; Lynch, R.L.; Bax, A.; Redwine, S.T.; Ihyembe, D.; Hagan, J.F., Jr. ADHD Diagnosis and Treatment Guidelines: A Historical Perspective. Pediatrics 2019, 144, e20191682. [Google Scholar] [CrossRef]
- Carta, A.; Fucà, E.; Guerrera, S.; Napoli, E.; Valeri, G.; Vicari, S. Characterization of Clinical Manifestations in the Co-occurring Phenotype of Attention Deficit/Hyperactivity Disorder and Autism Spectrum Disorder. Front. Psychol. 2020, 11, 861. [Google Scholar] [CrossRef]
- Mowlem, F.D.; Rosenqvist, M.A.; Martin, J.; Lichtenstein, P.; Asherson, P.; Larsson, H. Sex differences in predicting ADHD clinical diagnosis and pharmacological treatment. Eur. Child Adolesc. Psychiatry. 2019, 28, 481–489. [Google Scholar] [CrossRef]
- Faheem, M.; Akram, W.; Akram, H.; Khan, M.A.; Siddiqui, F.A.; Majeed, I. Gender-based differences in prevalence and effects of ADHD in adults: A systematic review. Asian J. Psychiatr. 2022, 75, 103205. [Google Scholar] [CrossRef]
- García, T.; Rodríguez, C. Sustained Change in ADHD: Interventions and Variables That Lead to Durable Improvements in Symptoms and Quality of Life. Sustainability 2021, 13, 13721. [Google Scholar] [CrossRef]
- Saha, P.; Sarkar, D. Characterization and Classification of ADHD Subtypes: An Approach Based on the Nodal Distribution of Eigenvector Centrality and Classification Tree Model. Child Psychiatry Hum. Dev. 2022. in print. [Google Scholar] [CrossRef]
- Bélanger, S.A.; Andrews, D.; Gray, C.; Korczak, D. ADHD in children and youth: Part 1—Etiology, diagnosis, and comorbidity. Paediatr. Child Health 2018, 23, 447–453. [Google Scholar] [CrossRef]
- Ahmadi, A.; Kashefi, M.; Shahrokhi, H.; Nazari, M.A. Computer aided diagnosis system using deep convolutional neural networks for ADHD subtypes. Biomed. Signal Process. Control 2021, 63, 102227. [Google Scholar] [CrossRef]
- Dubreuil-Vall, L.; Ruffini, G.; Camprodon, J.A. Deep Learning Convolutional Neural Networks Discriminate Adult ADHD From Healthy Individuals on the Basis of Event-Related Spectral EEG. Front. Neurosci. 2020, 14, 251. [Google Scholar] [CrossRef]
- Sen, B.; Borle, N.C.; Greiner, R.; Brown, M.R.G. A general prediction model for the detection of ADHD and Autism using structural and functional MRI. PLoS ONE 2018, 13, e0194856. [Google Scholar] [CrossRef]
- Shi, L.; Liu, X.; Wu, K.; Sun, K.; Lin, C.; Li, Z.; Zhao, S.; Fan, X. Surface values, volumetric measurements and radiomics of structural MRI for the diagnosis and subtyping of attention-deficit/hyperactivity disorder. Eur. J. Neurosci. 2021, 54, 7654–7667. [Google Scholar] [CrossRef]
- Yoo, J.H.; Kim, J.I.; Kim, B.N.; Jeong, B. Exploring characteristic features of attention-deficit/hyperactivity disorder: Findings from multi-modal MRI and candidate genetic data. Brain Imaging Behav. 2020, 14, 2132–2147. [Google Scholar] [CrossRef]
- Wang, L.-J.; Kuo, H.-C.; Lee, S.-Y.; Huang, L.-H.; Lin, Y.; Lin, P.-H.; Li, S.-C. MicroRNAs serve as prediction and treatment-response biomarkers of attention-deficit/hyperactivity disorder and promote the differentiation of neuronal cells by repressing the apoptosis pathway. Transl. Psychiatry 2022, 12, 67. [Google Scholar] [CrossRef]
- Lambacher, G.; Pascale, E.; Pucci, M.; Mangiapelo, S.; D’Addario, C.; Adriani, W. Search for an epigenetic biomarker in ADHD diagnosis, based on the DAT1 gene 5’-UTR methylation: A new possible approach. Psychiatry. Res. 2020, 291, 113154. [Google Scholar] [CrossRef]
- Mooney, M.A.; Ryabinin, P.; Wilmot, B.; Bhatt, P.; Mill, J.; Nigg, J.T. Large epigenome-wide association study of childhood ADHD identifies peripheral DNA methylation associated with disease and polygenic risk burden. Transl. Psychiatry 2020, 10, 8. [Google Scholar] [CrossRef]
- Sahin, M.; Jones, S.R.; Sweeney, J.A.; Berry-Kravis, E.; Connors, B.W.; Ewen, J.B.; Hartman, A.L.; Levin, A.R.; Potter, W.Z.; Mamounas, L.A. Discovering translational biomarkers in neurodevelopmental disorders. Nat. Rev. Drug Discov. 2019, 18, 235–236. [Google Scholar] [CrossRef]
- Pineau, G.; Villemonteix, T.; Slama, H.; Kavec, M.; Balériaux, D.; Metens, T.; Baijot, S.; Mary, A.; Ramoz, N.; Gorwood, P.; et al. Dopamine transporter genotype modulates brain activity during a working memory task in children with ADHD. Res. Dev. Disabil. 2019, 92, 103430. [Google Scholar] [CrossRef]
- Shang, C.-Y.; Lin, H.-Y.; Gau, S.S.-F. Effects of the dopamine transporter gene on striatal functional connectivity in youths with attention-deficit/hyperactivity disorder. Psychol. Med. 2021, 51, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-J.; Chou, W.-J.; Tsai, C.-S.; Lee, M.-J.; Lee, S.-Y.; Hsu, C.-W.; Hsueh, P.-C.; Wu, C.-C. Novel plasma metabolite markers of attention-deficit/hyperactivity disorder identified using high-performance chemical isotope labelling-based liquid chromatography-mass spectrometry. World J. Biol. Psychiatry 2021, 22, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Liu, X.; Wang, Y.; Liu, Y.; Ma, J.; Sun, H.; Li, J.; Tang, X.; Guo, Z.; Sun, W.; et al. Urinary Metabolomic Study in a Healthy Children Population and Metabolic Biomarker Discovery of Attention-Deficit/Hyperactivity Disorder (ADHD). Front. Psychiatry 2022, 13, 819498. [Google Scholar] [CrossRef] [PubMed]
- Endreffy, I.; Bjørklund, G.; Urbina, M.A.; Chirumbolo, S.; Doşa, M.D.; Dicső, F. High Levels of Glycosaminoglycans in the Urines of Children with Attention-Deficit/Hyperactivity Disorder (ADHD). J. Mol. Neurosci. 2020, 70, 1018–1025. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-J.; Wu, C.-C.; Chou, W.-J.; Lee, M.-J.; Chou, M.-C.; Lee, S.-Y.; Wang, L.-J. Mitochondrial-associated protein biomarkers in patients with attention-deficit/hyperactivity disorder. Mitochondrion 2019, 49, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Krahel, A.; Paszynska, E.; Slopien, A.; Gawriolek, M.; Otulakowska-Skrzynska, J.; Rzatowski, S.; Hernik, A.; Hanć, T.; Bryl, E.; Szczesniewska, P.; et al. Stress/Immune Biomarkers in Saliva among Children with ADHD Status. Int. J. Environ. Res. Public Health 2021, 18, 769. [Google Scholar] [CrossRef]
- Pivac, N.; Knežević, A.; Gornik, O.; Pučić, M.; Igl, W.; Peeters, H.; Crepel, A.; Steyaert, J.; Novokmet, M.; Redžić, I.; et al. Human Plasma Glycome in Attention-Deficit Hyperactivity Disorder and Autism Spectrum Disorders. Mol. Cell Proteomics 2011, 10, M110.004200. [Google Scholar] [CrossRef]
- Robajac, D.; Masnikosa, R.; Nemčovič, M.; Križáková, M.; Belická Kluková, Ľ.; Baráth, P.; Katrlík, J.; Nedić, O. Glycoanalysis of the placental membrane glycoproteins throughout placental development. Mech. Ageing Dev. 2019, 183, 111151. [Google Scholar] [CrossRef]
- Robajac, D.; Križáková, M.; Šunderić, M.; Miljuš, G.; Gemeiner, P.; Nedić, O.; Katrlík, J. Lectin-Based Protein Microarray for the Glycan Analysis of Colorectal Cancer Biomarkers: The Insulin-Like Growth Factor System. In Glycan Microarrays: Methods and Protocols; Kilcoyne, M., Gerlach, J.Q., Eds.; Springer: New York, NY, USA, 2022; pp. 207–222. ISBN 978-1-0716-2147-9. [Google Scholar]
- Kobayashi, Y.; Tateno, H.; Dohra, H.; Moriwaki, K.; Miyoshi, E.; Hirabayashi, J.; Kawagishi, H. A novel core fucose-specific lectin from the mushroom Pholiota squarrosa. J. Biol. Chem. 2012, 287, 33973–33982. [Google Scholar] [CrossRef]
- Tateno, H.; Nakamura-Tsuruta, S.; Hirabayashi, J. Comparative analysis of core-fucose-binding lectins from Lens culinaris and Pisum sativum using frontal affinity chromatography. Glycobiology 2009, 19, 527–536. [Google Scholar] [CrossRef]
- Olausson, J.; Tibell, L.; Jonsson, B.H.; Påhlsson, P. Detection of a high affinity binding site in recombinant Aleuria aurantia lectin. Glycoconj. J. 2008, 25, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Pažitná, L.; Nemčovič, M.; Pakanová, Z.; Baráth, P.; Aliev, T.; Dolgikh, D.; Argentova, V.; Katrlík, J. Influence of media composition on recombinant monoclonal IgA1 glycosylation analysed by lectin-based protein microarray and MALDI-MS. J. Biotechnol. 2020, 314–315, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Hashimoto, H.; Okayasu, N.; Kameyama, A.; Onogi, H.; Nakagawasai, O.; Nakazawa, T.; Kurosawa, T.; Hao, Y.; Isaji, T.; et al. Alpha1,6-fucosyltransferase-deficient mice exhibit multiple behavioral abnormalities associated with a schizophrenia-like phenotype: Importance of the balance between the dopamine and serotonin systems. J. Biol. Chem. 2011, 286, 18434–18443. [Google Scholar] [CrossRef] [PubMed]
- Ng, B.G.; Xu, G.; Chandy, N.; Steyermark, J.; Shinde, D.N.; Radtke, K.; Raymond, K.; Lebrilla, C.B.; AlAsmari, A.; Suchy, S.F.; et al. Biallelic Mutations in FUT8 Cause a Congenital Disorder of Glycosylation with Defective Fucosylation. Am. J. Hum. Genet. 2018, 102, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liang, S.; Wang, L.; Han, P.; Jiang, X.; Wang, J.; Hao, Y.; Wu, L. Sialic acid and anti-ganglioside antibody levels in children with autism spectrum disorders. Brain Res. 2018, 1678, 273–277. [Google Scholar] [CrossRef]
- Yang, X.; Zou, M.; Pang, X.; Liang, S.; Sun, C.; Wang, J.; Fan, L.; Xia, W.; Wu, L. The association between NCAM1 levels and behavioral phenotypes in children with autism spectrum disorder. Behav. Brain Res. 2019, 359, 234–238. [Google Scholar] [CrossRef]
- Yang, X.; Li, L.; Chai, X.; Liu, J. The association between ST8SIA2 gene and behavioral phenotypes in children with autism spectrum disorder. Front. Behav. Neurosci. 2022, 16, 929878. [Google Scholar] [CrossRef]
- Demirci, E.; Guler, Y.; Ozmen, S.; Canpolat, M.; Kumandas, S. Levels of Salivary Sialic Acid in Children with Autism Spectrum Disorder; Could It Be Related to Stereotypes and Hyperactivity? Clin. Psychopharmacol. Neurosci. 2019, 17, 415–422. [Google Scholar] [CrossRef]
- Schnaar, R.L.; Gerardy-Schahn, R.; Hildebrandt, H. Sialic Acids in the Brain: Gangliosides and Polysialic Acid in Nervous System Development, Stability, Disease, and Regeneration. Physiol. Rev. 2014, 94, 461–518. [Google Scholar] [CrossRef]
- Liu, F.; Simpson, A.B.; D’Costa, E.; Bunn, F.S.; van Leeuwen, S.S. Sialic acid, the secret gift for the brain. Crit. Rev. Food Sci. Nutr. 2022. in print. [Google Scholar] [CrossRef]
- Barone, R.; Sturiale, L.; Fiumara, A.; Palmigiano, A.; Bua, R.O.; Rizzo, R.; Zappia, M.; Garozzo, D. CSF N-glycan profile reveals sialylation deficiency in a patient with GM2 gangliosidosis presenting as childhood disintegrative disorder. Autism Res. 2016, 9, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Kang, P.; Mechref, Y.; Novotny, M.V. High-throughput solid-phase permethylation of glycans prior to mass spectrometry. Rapid Commun. Mass Spectrom. 2008, 22, 721–734. [Google Scholar] [CrossRef] [PubMed]
- Wada, Y.; Azadi, P.; Costello, C.E.; Dell, A.; Dwek, R.A.; Geyer, H.; Geyer, R.; Kakehi, K.; Karlsson, N.G.; Kato, K.; et al. Comparison of the methods for profiling glycoprotein glycans—HUPO Human Disease Glycomics/Proteome Initiative multi-institutional study. Glycobiol. 2007, 17, 411–422. [Google Scholar] [CrossRef] [PubMed]


| Lectin | SNA | ConA | RCA | LCA | AAL | PHA-E | PhoSL | WGA | MAL-II | PHA-L | MAL-I | ||
| Sugar Specificity | α2-6 SA | Man | Gal, GalNAc | Man (with Core Fuc) | Fuc | 2/3 Ant. Bisecting CNG | Core Fuc | GlcNAc, SA | α2-3 SA | 3/4 Ant CNG | α2-3 SA | ||
| Relative signal intensity (%) | Serum | ADHD | 40.95 | 24.01 | 17.36 | 3.59 | 5.54 ** | 2.23 | 3.30 | 1.74 * | 0.99 | 0.29 | n.a. |
| Controls | 41.52 | 24.14 | 16.89 | 3.55 | 4.73 ** | 2.53 | 3.27 | 1.90 * | 1.10 | 0.38 | n.a. | ||
| Depleted serum | ADHD | 30.95 | 23.34 * | 26.02 * | 2.91 | 4.85 | 4.18 ** | 1.34 | 4.85 | 1.09 ** | 0.19 | 0.26 | |
| Controls | 30.15 | 24.22 * | 24.81 * | 3.24 | 4.38 | 4.93 ** | 1.31 | 5.08 | 1.34 ** | 0.20 | 0.34 | ||
| IgG | ADHD | 16.69 * | 21.52 | 13.37 | 22.57 | 10.80 * | 11.28 | 1.66 | 1.21 | 0.07 | 0.78 | 0.06 | |
| Controls | 22.37 * | 20.07 | 13.57 | 20.92 | 9.05 * | 10.21 | 1.84 | 1.06 | 0.10 | 0.72 | 0.08 | ||
| Structure Nr. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | ||
| Structure Notation | H5N2 | H6N2 | H3N4F1 | H4N4 | H7N2 | H4N4F1 | H5N4 | H3N5F1 | H5N3SA1 | H8N2 | H5N4F1 | H4N5F1 | H6N3SA1 | H9N2 | ||
| Relative signal intensity (%) | Serum | ADHD | 4.22 | 6.02 | 2.95 * | - | - | 5.03 * | 1.41 | - | 1.18 | 2.99 | 3.36 | 1.51 | 1.40 | 4.37 |
| Controls | 3.37 | 5.88 | 1.88 * | - | - | 3.23 * | 1.40 | - | 1.16 | 3.12 | 2.64 | 1.30 | 1.51 | 3.32 | ||
| Depleted serum | ADHD | 6.12 | 7.40 | - | - | 2.78 | - | 2.34 | - | 2.15 | 4.19 | 2.26 | - | 2.45 | 6.57 | |
| Controls | 7.41 | 8.23 | - | - | 3.04 | - | 2.32 | - | 2.35 | 4.32 | 2.29 | - | 2.61 | 5.75 | ||
| IgG | ADHD | 0.72 | 1.22 | 21.36 | 0.95 | - | 33.45 | 1.03 | 3.56 | - | - | 17.09 | 6.12 | - | - | |
| Controls | 0.73 | 1.09 | 18.87 | 0.75 | - | 30.55 | 0.70 | 3.64 | - | - | 17.51 | 6.62 | - | - | ||
| Structure Nr. | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | ||
| Structure Notation | H4N4F1SA1 | H5N4SA1 | H4N5SA1 | H5N5F1 | H5N4F1SA1 | H5N5SA1 | H5N4SA2 | H5N5F1SA1 | H6N5SA1 | H5N4F1SA2 | H5N5F1SA2 | H6N5SA2 | H6N5SA3 | H6N5F1SA3 | ||
| Relative signal intensity (%) | Serum | ADHD | - | 14.74 | - | 1.37 | 5.71 * | - | 31.08 | 3.24 | 0.84 | 4.98 | 1.37 | 0.72 | 0.87 | 0.64 |
| Controls | - | 15.35 | - | 1.60 | 6.66 * | - | 33.81 | 4.70 | 0.81 | 4.57 | 1.27 | 0.91 | 0.98 | 0.52 | ||
| Depleted serum | ADHD | - | 11.06 | 4.53 | - | 4.50 | - | 29.92 | 2.27 | 1.74 | 4.98 * | - | - | 2.54 | 2.19 | |
| Controls | - | 11.89 | 2.49 | - | 4.74 | - | 30.46 | 2.66 | 1.72 | 4.02 * | - | - | 2.10 | 1.59 | ||
| IgG | ADHD | 0.92 | 0.77 | - | 1.92 | 5.60 ** | 0.78 | - | 3.12 * | - | 0.61 | 0.77 | - | - | - | |
| Controls | 0.99 | 0.85 | - | 2.22 | 8.33 ** | 0.62 | - | 4.92 * | - | 0.74 | 0.87 | - | - | - | ||
| Nr. | m/z | Structure Notation | Structure | Nr. | m/z | Structure Notation | Structure |
|---|---|---|---|---|---|---|---|
| 1 | 1579.8 | H5N2 |  | 15 | 2401.2 | H4N4F1SA1 | 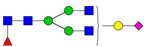 |
| 2 | 1783.9 | H6N2 |  | 16 | 2431.2 | H5N4SA1 | 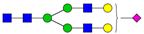 |
| 3 | 1835.9 | H3N4F1 | 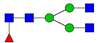 | 17 | 2472.2 | H4N5SA1 | 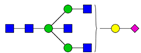 |
| 4 | 1865.9 | H4N4 | 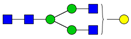 | 18 | 2489.3 | H5N5F1 | 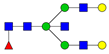 |
| 5 | 1988 | H7N2 | 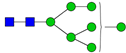 | 19 | 2605.3 | H5N4F1SA1 | 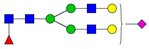 |
| 6 | 2040 | H4N4F1 |  | 20 | 2676.3 | H5N5SA1 |  |
| 7 | 2070 | H5N4 | 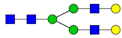 | 21 | 2792.4 | H5N4SA2 |  |
| 8 | 2081.1 | H3N5F1 | 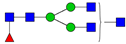 | 22 | 2850.4 | H5N5F1SA1 | 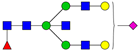 |
| 9 | 2186.1 | H5N3SA1 | 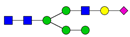 | 23 | 2880.4 | H6N5SA1 | 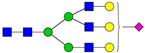 |
| 10 | 2192.1 | H8N2 | 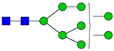 | 24 | 2966.5 | H5N4F1SA2 | 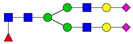 |
| 11 | 2244.1 | H5N4F1 | 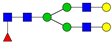 | 25 | 3211.6 | H5N5F1SA2 |  |
| 12 | 2285.2 | H4N5F1 |  | 26 | 3241.6 | H6N5SA2 | 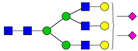 |
| 13 | 2390.2 | H6N3SA1 | 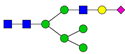 | 27 | 3602.8 | H6N5SA3 |  |
| 14 | 2396.2 | H9N2 | 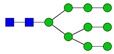 | 28 | 3776.9 | H6N5F1SA3 | 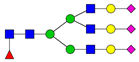 |
| Lectin | Origin | Sugar Target |
|---|---|---|
| SNA | Sambucus nigra | α2-6 linked sialic acid |
| ConA | Canavalia ensiformis | Manα1-6Man, Manα1-3Man, Manα1-2Man, high mannose |
| RCA | Ricinus communis | Galβ1-4GlcNAc, GalNAc, Gal |
| LCA | Lens culinaris | αMan in N-glycans with core fucose, αMan in N-glycans |
| AAL | Aleuria aurantia | α1-3, α1-2, α1-4, α1-6 linked fucose |
| PHA-E | Phaseolus vulgaris (erythroagglutinin) | di-/triantennary complex type N-glycans with bisecting GlcNAc |
| PhoSL | Pholiota squarrosa | α1-6 linked fucose (core fucose) |
| WGA | Triticum vulgaris | GlcNAc, sialic acid |
| MAL-II | Maackia amurensis (hemagglutinin) | α2-3 linked sialic acid in O-glycans |
| PHA-L | Phaseolus vulgaris (leukoagglutinin) | tri/tetra-antennary complex type N-glycans |
| MAL-I | Maackia amurensis (leukoagglutinin) | α2-3 linked sialic acid in N-glycans |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kianičková, K.; Pažitná, L.; Kundalia, P.H.; Pakanová, Z.; Nemčovič, M.; Baráth, P.; Katrlíková, E.; Šuba, J.; Trebatická, J.; Katrlík, J. Alterations in the Glycan Composition of Serum Glycoproteins in Attention-Deficit Hyperactivity Disorder. Int. J. Mol. Sci. 2023, 24, 8745. https://doi.org/10.3390/ijms24108745
Kianičková K, Pažitná L, Kundalia PH, Pakanová Z, Nemčovič M, Baráth P, Katrlíková E, Šuba J, Trebatická J, Katrlík J. Alterations in the Glycan Composition of Serum Glycoproteins in Attention-Deficit Hyperactivity Disorder. International Journal of Molecular Sciences. 2023; 24(10):8745. https://doi.org/10.3390/ijms24108745
Chicago/Turabian StyleKianičková, Kristína, Lucia Pažitná, Paras H. Kundalia, Zuzana Pakanová, Marek Nemčovič, Peter Baráth, Eva Katrlíková, Ján Šuba, Jana Trebatická, and Jaroslav Katrlík. 2023. "Alterations in the Glycan Composition of Serum Glycoproteins in Attention-Deficit Hyperactivity Disorder" International Journal of Molecular Sciences 24, no. 10: 8745. https://doi.org/10.3390/ijms24108745
APA StyleKianičková, K., Pažitná, L., Kundalia, P. H., Pakanová, Z., Nemčovič, M., Baráth, P., Katrlíková, E., Šuba, J., Trebatická, J., & Katrlík, J. (2023). Alterations in the Glycan Composition of Serum Glycoproteins in Attention-Deficit Hyperactivity Disorder. International Journal of Molecular Sciences, 24(10), 8745. https://doi.org/10.3390/ijms24108745







