Prenatal Fumonisin Exposure Impairs Bone Development via Disturbances in the OC/Leptin and RANKL/RANK/OPG Systems in Weaned Rat Offspring
Abstract
1. Introduction
2. Results
2.1. Body Weight and Morphometrical Bone Parameters
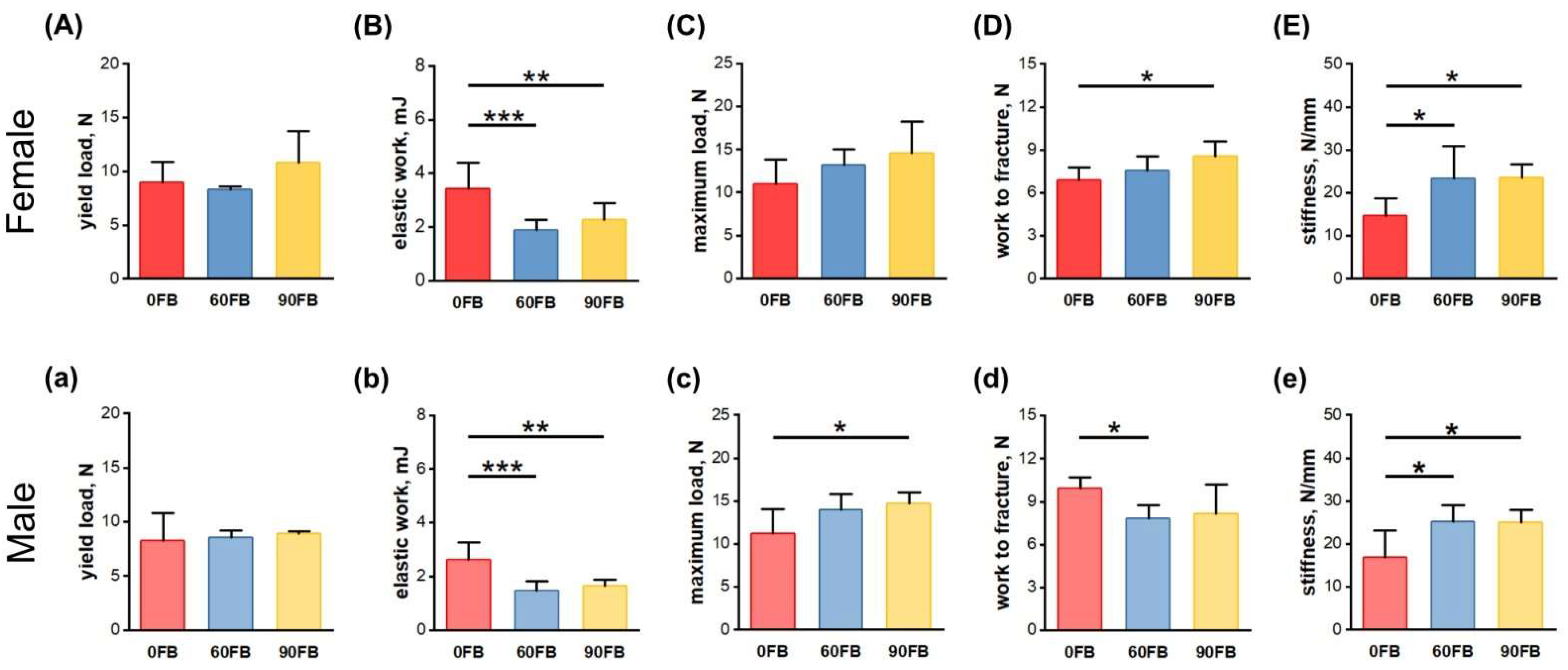
2.2. Mechanical Bone Parameters
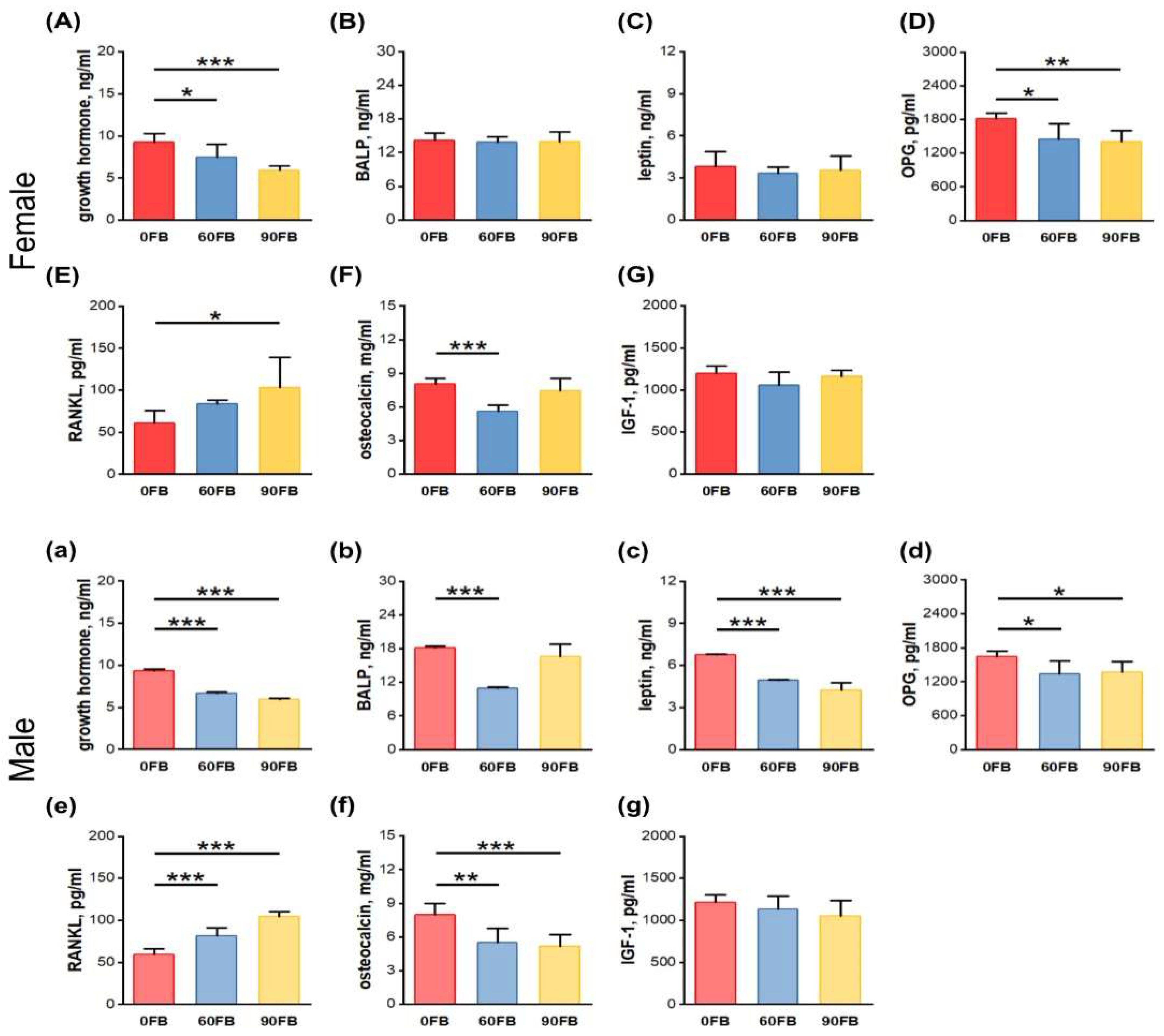
2.3. Hormonal Analysis
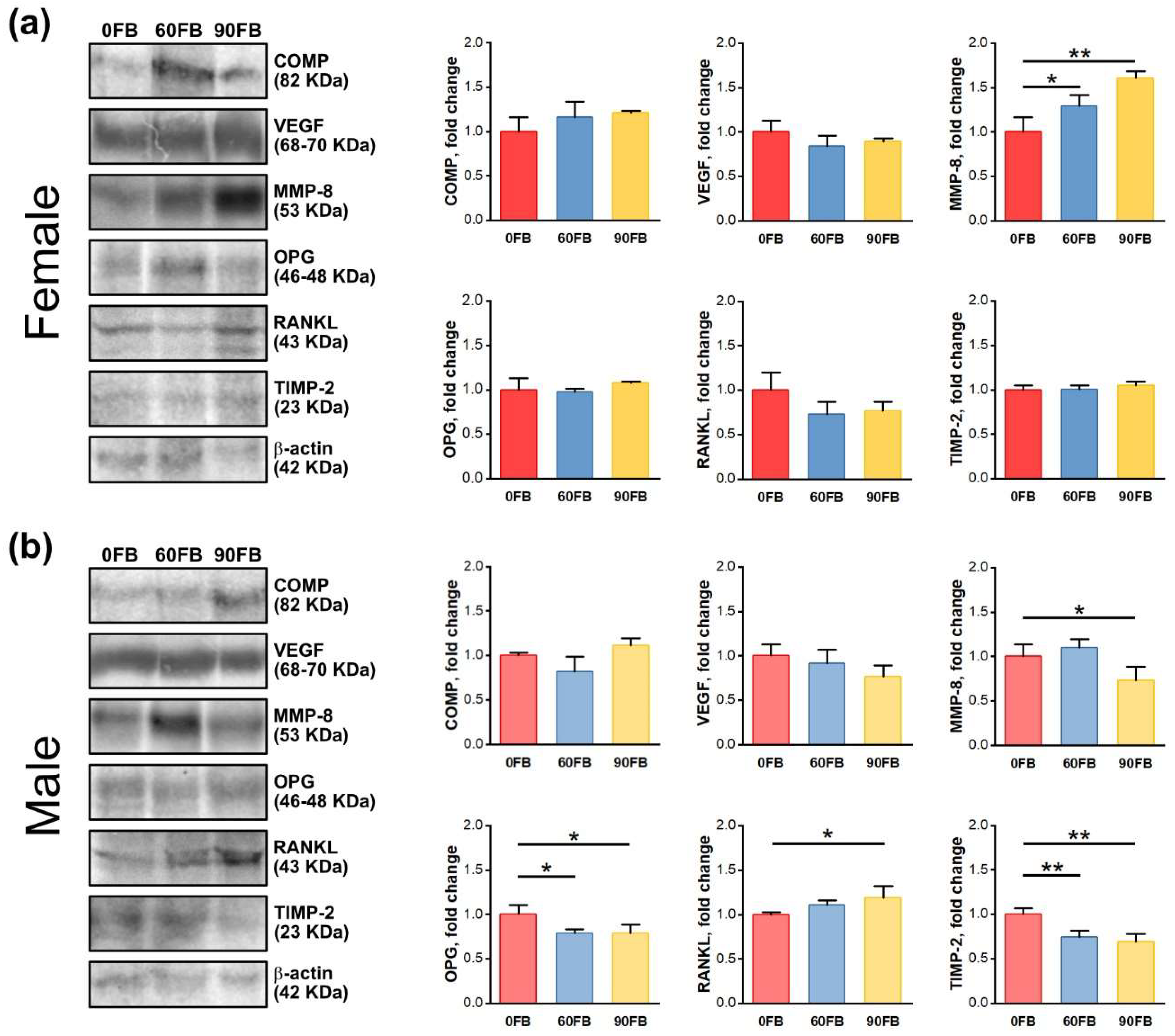
2.4. Protein Expression
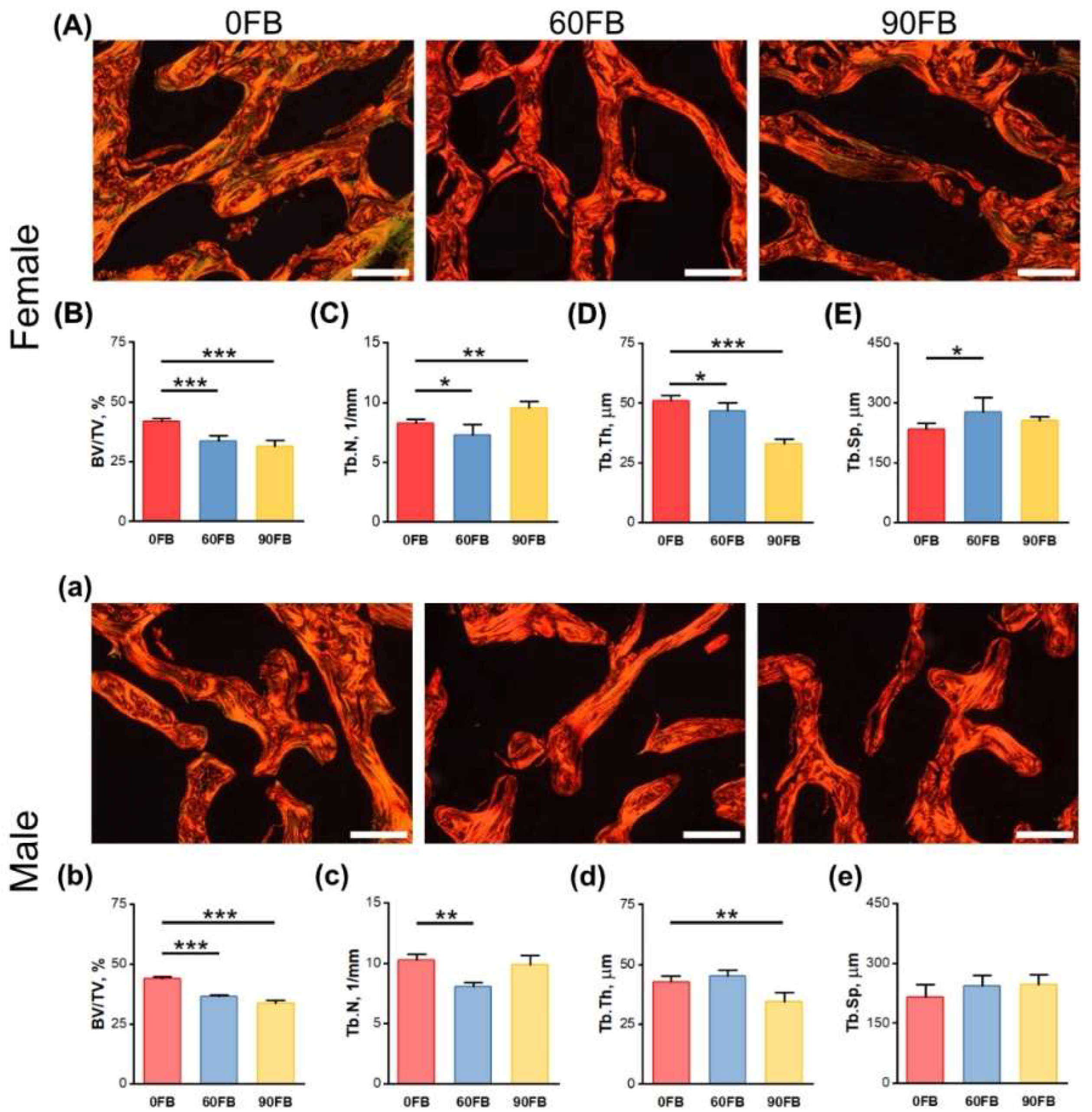
2.5. Trabecular Bone Morphology
3. Discussion
4. Materials and Methods
4.1. Fumonisins Preparation and Quantification
4.2. Animals and Experimental Design
4.3. Mechanical Testing
4.4. Trabecular Bone Morphology
4.5. Western Blot
4.6. Hormonal Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Śliwa, E.; Dobrowolski, P.; Tatara, M.R.; Piersiak, T.; Siwicki, A.; Rokita, E.; Pierzynowski, S.G. Alpha-ketoglutarate protects the liver of piglets exposed during prenatal life to chronic excess of dexamethasone from metabolic and structural changes. J. Anim. Physiol. Anim. Nutr. 2009, 93, 192–202. [Google Scholar] [CrossRef]
- Śliwa, E.; Kowalik, S.; Tatara, M.R.; Krupski, W.; Majcher, P.; Łuszczewska-Sierakowska, I.; Pierzynowski, S.G.; Studziński, T. Effect of alpha-ketoglutarate (AKG) given to pregnant sows on development of humerus and femur in newborns. Bull. Vet. Inst. Pulawy 2005, 49, 117–120. [Google Scholar]
- Tomaszewska, E.; Muszyński, S.; Świetlicka, I.; Wojtysiak, D.; Dobrowolski, P.; Arciszewski, M.B.; Donaldson, J.; Czech, A.; Hułas-Stasiak, M.; Kuc, D.; et al. Prenatal acrylamide exposure results in time-dependent changes in liver function and basal hematological, and oxidative parameters in weaned Wistar rats. Sci. Rep. 2022, 12, 14882. [Google Scholar] [CrossRef]
- Nesic, K.; Ivanovic, S.; Nesic, V. Fusarial toxins: Secondary metabolites of Fusarium fungi. Rev. Environ. Contam. Toxicol. 2014, 228, 101–120. [Google Scholar]
- Streit, E.; Naehrer, K.; Rodrigues, I.; Schatzmayr, G. Mycotoxin occurrence in feed and feed raw materials worldwide: Long-term analysis with special focus on Europe and Asia. J. Sci. Food Agric. 2013, 93, 2892–2899. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.A. Fumonisin toxicosis. In Clinical Veterinary Advisor: The Horse; Elsevier: Amsterdam, The Netherlands, 2012; pp. 214–215. [Google Scholar]
- Placinta, C.M.; D’Mello, J.P.F.; Macdonald, A.M.C. A review of worldwide contamination of cereal grains and animal feed with Fusarium mycotoxins. Anim. Feed Sci. Technol. 1999, 78, 21–37. [Google Scholar] [CrossRef]
- Stockmann-Juvala, H.; Savolainen, K. A review of the toxic effects and mechanisms of action of fumonisin B1. Hum. Exp. Toxicol. 2008, 27, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, H.; Shima, H.; Vesonder, R.F.; Tokuda, H.; Nishino, H.; Katoh, S.; Tamura, S.; Sugimura, T.; Nagao, M. Inhibition of protein serine/threonine phosphatases by fumonisin B1, a mycotoxin. Biochem. Biophys. Res. Commun. 1996, 220, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.P.; Kang, S.C. Endoplasmic reticulum stress-mediated autophagy activation attenuates fumonisin B1 induced hepatotoxicity in vitro and in vivo. Food Chem. Toxicol. 2017, 110, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fan, Y.; Xia, B.; Xiao, Q.; Wang, Q.; Sun, W.; Zhang, H.; He, C. The immunosuppressive characteristics of FB1 by inhibition of maturation and function of BMDCs. Int. Immunopharmacol. 2017, 47, 206–211. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Rudyk, H.; Dobrowolski, P.; Donaldson, J.; Świetlicka, I.; Puzio, I.; Kamiński, D.; Wiącek, D.; Kushnir, V.; Brezvyn, O.; et al. Changes in the intestinal histomorphometry, the expression of intestinal tight junction proteins, and the bone structure and liver of pre-laying hens following oral administration of fumonisins for 21 days. Toxins 2021, 13, 375. [Google Scholar] [CrossRef] [PubMed]
- Ülger, T.G.; Uçar, A.; Çakıroğlu, F.P.; Yilmaz, S. Genotoxic effects of mycotoxins. Toxicon. 2020, 185, 104–113. [Google Scholar] [CrossRef]
- Wei, T.; Zhu, W.; Pang, M.; Liu, Y.; Dong, J. Natural occurrence of fumonisins B1 and B2 in corn in four provinces of China. Food Addit. Contam. Part B 2013, 6, 270–274. [Google Scholar] [CrossRef]
- Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; Grasl-Kraupp, B.; et al. Risks for animal health related to the presence of fumonisins, their modified forms and hidden forms in feed. EFSA J. 2018, 16, 5242. [Google Scholar]
- Rudyk, H.; Tomaszewska, E.; Kotsyumbas, I.; Muszyński, S.; Tomczyk-Warunek, A.; Szymańczyk, S.; Dobrowolski, P.; Wiącek, D.; Kamiński, D.; Brezvyn, O. Bone homeostasis in experimental fumonisins intoxication of rats. Ann. Anim. Sci. 2019, 19, 403–419. [Google Scholar] [CrossRef]
- Riley, R.T.; Voss, K.A. Differential sensitivity of rat kidney and liver to fumonisin toxicity: Organ-specific differences in toxin accumulation and sphingoid base metabolism. Toxicol. Sci. 2006, 92, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Devreese, M.; De Backer, P.; Croubels, S. Overview of the most important mycotoxins for the pig and poultry husbandry. Vlaams Diergeneeskd. Tijdschr. 2013, 82, 171–180. [Google Scholar] [CrossRef]
- Gelderblom, W.; Marasas, W. Controversies in fumonisin mycotoxicology and risk assessment. Hum. Exp. Toxicol. 2012, 31, 215–235. [Google Scholar] [CrossRef] [PubMed]
- Coppock, R.W.; Jacobsen, B.J. Mycotoxins in animal and human patients. Toxicol. Ind. Health 2009, 25, 637–655. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Groopman, J.D.; Pestka, J.J. Public health impacts of foodborne mycotoxins. Annu. Rev. Food Sci. Technol. 2014, 5, 351–372. [Google Scholar] [CrossRef]
- Scott, P.M. Recent research on fumonisins: A review. Food Addit. Contam. Chem. Anal. Control Expo. Risk Assess. 2012, 29, 242–248. [Google Scholar] [CrossRef] [PubMed]
- European Commission (EC). Commission Regulation (EU) 2005/856 amending Regulation 2001/466/EC as regards Fusarium toxins. Off. J. Eur. Union 2005, 143, 3–8. [Google Scholar]
- European Commission (EC). Commission Recommendation 2006/576/EC on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding. Off. J. Eur. Union 2006, 229, 7–9. [Google Scholar]
- Food and Drug Administration (FDA). Guidance for industry: Fumonisin levels in human foods and animal feeds. Fed. Regist. 2001, 66, 56688–56689. [Google Scholar]
- Didwania, N.; Joshi, M. Mycotoxins: A critical review on occurrence and significance. Int. J. Pharm. Sci. 2013, 5, 1014–1019. [Google Scholar]
- Streit, E.; Schatzmayr, G.; Tassis, P.; Tzika, E.; Marin, D.; Taranu, I.; Tabuc, C.; Nicolau, A.; Aprodu, I.; Puel, O.; et al. Current situation of mycotoxin contamination and co-occurrence in animal feed—Focus on Europe. Toxins 2012, 4, 788–809. [Google Scholar] [CrossRef]
- Kamle, M.; Mahato, D.K.; Devi, S.; Lee, K.E.; Kang, S.G.; Kumar, P. Fumonisins: Impact on agriculture, food, and human health and their management strategies. Toxins 2019, 11, 328. [Google Scholar] [CrossRef]
- Rodrigues, I.; Handl, J.; Binder, E. Mycotoxin occurrence in commodities, feeds and feed ingredients sourced in the Middle East and Africa. Food Addit. Contam. Part B 2011, 4, 168–179. [Google Scholar] [CrossRef]
- Cheng, S.; Feng, X.; Liu, G.; Zhao, N.; Liu, J.; Zhang, Z.; Yang, N.; Zhou, L.; Pang, M.; Tang, B.; et al. Natural occurrence of mycotoxins in maize in north China. Toxins 2022, 14, 521. [Google Scholar] [CrossRef]
- Twaruzek, M.; Skrzydlewski, P.; Kosicki, R.; Grajewski, J. Mycotoxins survey in feed materials and feedingstuffs in years 2015–2020. Toxicon 2021, 202, 27–39. [Google Scholar] [CrossRef]
- Muller, S.; Dekant, W.; Mally, A. Fumonisin B1 and the kidney: Modes of action for renal tumor formation by fumonisin B1 in rodents. Food Chem. Toxicol. 2012, 50, 3833–3846. [Google Scholar] [CrossRef]
- Marin, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Mycotoxins: Occurrence, toxicology, and exposure assessment. Food Chem. Toxicol. 2013, 60, 218–237. [Google Scholar] [CrossRef]
- Jalilzadeh-Amin, G.; Dalir-Naghadeh, B.; Ahmadnejad-Asl-Gavgani, M.; Fallah, A.A.; Mousavi Khaneghah, A. Prevalence and concentration of mycotoxins in animal feed in the Middle East and North Africa (MENA): A systematic review and meta-analysis. Toxins 2023, 15, 214. [Google Scholar] [CrossRef]
- Collins, T.F.; Shackelford, M.E.; Sprando, R.L.; Black, T.N.; Láborde, J.B.; Hansen, D.K.; Eppley, R.M.; Trucksess, M.W.; Howard, P.C.; Bryant, M.A.; et al. Effects of fumonisin B1 in pregnant rats. Food Chem. Toxicol. 1998, 36, 397–408. [Google Scholar] [CrossRef]
- Collins, T.F.; Sprando, R.L.; Black, T.N.; Shackelford, M.E.; Laborde, J.B.; Hansen, D.K.; Eppley, R.M.; Trucksess, M.W.; Howard, P.C.; Bryant, M.A.; et al. Effects of fumonisin B1 in pregnant rats. Part 2. Food Chem. Toxicol. 1998, 36, 673–685. [Google Scholar] [CrossRef]
- Gelineau-van Waes, J.; Voss, K.A.; Stevens, V.L.; Speer, M.C.; Riley, R.T. Maternal fumonisin exposure as a risk factor for neural tube defects. Adv. Food Nutr. Res. 2009, 56, 145–181. [Google Scholar]
- Alshannaq, A.; Yu, J.-H. Occurrence, toxicity, and analysis of major mycotoxins in food. Int. J. Environ. Res. Public Health 2017, 14, 632. [Google Scholar] [CrossRef]
- Alvito, P.; Pereira-da-Silva, L. Mycotoxin exposure during the first 1000 days of life and its impact on children’s health: A clinical overview. Toxins 2022, 14, 189. [Google Scholar] [CrossRef]
- Tesfamariam, K.; Argaw, A.; Hanley-Cook, G.T.; Gebreyesus, S.H.; Kolsteren, P.; Belachew, T.; Van de Velde, M.; De Saeger, S.; De Boevre, M.; Lachat, C. Multiple mycotoxin exposure during pregnancy and risks of adverse birth outcomes: A prospective cohort study in rural Ethiopia. Environ. Int. 2022, 160, 107052. [Google Scholar] [CrossRef]
- Mesfin, A.; Lachat, C.; Gebreyesus, S.H.; Roro, M.; Tesfamariam, K.; Belachew, T.; De Boevre, M.; De Saeger, S. Mycotoxins exposure of lactating women and its relationship with dietary and pre/post-harvest practices in rural Ethiopia. Toxins 2023, 15, 285. [Google Scholar] [CrossRef]
- Pietsch, C. Risk assessment for mycotoxin contamination in fish feeds in Europe. Mycotoxin Res. 2020, 36, 41–62. [Google Scholar]
- Mamo, F.T.; Abate, B.A.; Tesfaye, K.; Nie, C.; Wang, G.; Liu, Y. Mycotoxins in Ethiopia: A review on prevalence, economic and health impacts. Toxins 2020, 12, 648. [Google Scholar] [CrossRef]
- Lee, H.J.; Ryu, D. Worldwide occurrence of mycotoxins in cereals and cereal-derived food products: Public health perspectives of their co-occurrence. Agric. Food Chem. 2017, 65, 7034–7051. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Rudyk, H.; Świetlicka, I.; Hułas-Stasiak, M.; Donaldson, J.; Arczewska, M.; Muszyński, S.; Dobrowolski, P.; Puzio, I.; Kushnir, V.; et al. The influence of prenatal fumonisin exposure on bone properties, as well as OPG and RANKL expression and immunolocalization, in newborn offspring is sex and dose dependent. Int. J. Mol. Sci. 2021, 22, 13234. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Rudyk, H.; Świetlicka, I.; Hułas-Stasiak, M.; Donaldson, J.; Arczewska, M.; Muszyński, S.; Dobrowolski, P.; Mielnik-Błaszczak, M.; Arciszewski, M.B.; et al. Trabecular bone parameters, TIMP-2, MMP-8, MMP-13, VEGF expression and immunolocalization in bone and cartilage in newborn offspring prenatally exposed to fumonisins. Int. J. Mol. Sci. 2021, 22, 12528. [Google Scholar] [CrossRef]
- Kras, K.; Rudyk, H.; Muszyński, S.; Tomaszewska, E.; Dobrowolski, P.; Kushnir, V.; Muzyka, V.; Brezvyn, O.; Arciszewski, M.B.; Kotsyumbas, I. Morphology and chemical coding of rat duodenal enteric neurons following prenatal exposure to fumonisins. Animals 2022, 12, 1055. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Rudyk, H.; Wojtysiak, D.; Donaldson, J.; Muszyński, S.; Arciszewski, M.B.; Lisova, N.; Brezvyn, O.; Puzio, I.; Abramowicz, B.; et al. Basal blood morphology, serum biochemistry, and the liver and muscle structure of weaned Wistar rats prenatally exposed to fumonisins. Animals 2022, 12, 2353. [Google Scholar] [CrossRef]
- Kyei, N.N.A.; Boakye, D.; Gabrysch, S. Maternal mycotoxin exposure and adverse pregnancy outcomes: A systematic review. Mycotoxin Res. 2020, 36, 243–255. [Google Scholar] [CrossRef]
- Gelineau-Van Waes, J. Fumonisins. In Reproductive and Developmental Toxicology, 3rd ed.; Gupta, R.C., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 955–981. [Google Scholar]
- Kyei, N.N.A.; Waid, J.L.; Ali, N.; Cramer, B.; Humpf, H.U.; Gabrysch, S. Maternal exposure to multiple mycotoxins and adverse pregnancy outcomes: A prospective cohort study in rural Bangladesh. Arch. Toxicol. 2023; in press. [Google Scholar] [CrossRef]
- Voss, K.A.; Riley, R.T.; Gelineau-van Waes, J. Fumonisin B1 induced neural tube defects were not increased in LM/Bc mice fed folate-deficient diet. Mol. Nutr. Food Res. 2014, 58, 1190–1198. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Muszyński, S.; Dobrowolski, P.; Kostro, K.; Taszkun, I.; Żmuda, A.; Blicharski, T.; Kędzia, P. Bentonite diminishes DON-induced changes in bone development in mink dams. J. Vet. Res. 2016, 60, 349–355. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Dobrowolski, P.; Muszyński, S.; Kostro, K.; Taszkun, I.; Żmuda, A.; Blicharski, T.; Hułas-Stasiak, M. DON-induced changes in bone homeostasis in mink dams. J. Vet. Res. 2017, 61, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Parfitt, A.M. What is the normal rate of bone remodeling? Bone 2004, 35, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Delaisse, J.M.; Andersen, T.L.; Kristensen, H.B.; Jensen, P.R.; Andreasen, C.M.; Soe, K. Re-thinking the bone remodeling cycle mechanism and the origin of bone loss. Bone 2020, 141, 115628. [Google Scholar] [CrossRef]
- Zoch, M.L.; Clemens, T.L.; Riddle, R.C. New insights into the biology of osteocalcin. Bone 2016, 82, 42–49. [Google Scholar] [CrossRef]
- Reinehr, T.; Roth, C.L. A new link between skeleton, obesity and insulin resistance: Relationships between osteocalcin, leptin and insulin resistance in obese children before and after weight loss. Int. J. Obes. 2010, 34, 852–858. [Google Scholar] [CrossRef]
- Kalra, S.P.; Dube, M.G.; Iwaniec, U.T. Leptin increases osteoblast-specific osteocalcin release through a hypothalamic relay. Peptides 2009, 30, 967–973. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Udagawa, N.; Takahashi, N. Action of RANKL and OPG for osteoclastogenesis. Crit. Rev. Eukaryot. Gene Expr. 2009, 19, 61–72. [Google Scholar] [CrossRef]
- Ducy, P.; Desbois, C.; Boyce, B.; Pinero, G.; Story, B.; Dunstan, C.; Smith, E.; Bonadio, J.; Goldstein, S.; Gundberg, C.; et al. Increased bone formation in osteocalcin-deficient mice. Nature 1996, 382, 448–452. [Google Scholar] [CrossRef]
- Lee, N.K.; Sowa, H.; Hinoi, E.; Ferron, M.; Ahn, J.D.; Confavreux, C.; Dacquin, R.; Mee, P.J.; McKee, M.D.; Jung, D.Y.; et al. Endocrine regulation of energy metabolism by the skeleton. Cell 2007, 130, 456–469. [Google Scholar] [CrossRef]
- Bilotta, F.L.; Arcidiacono, B.; Messineo, S.; Greco, M.; Chiefari, E.; Britti, D.; Nakanishi, T.; Foti, D.P.; Brunetti, A. Insulin and osteocalcin: Further evidence for a mutual cross-talk. Endocrine 2018, 59, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Guedes, J.; Esteves, J.; Morais, M.; Zorn, T.; Furuya, D. Osteocalcin improves insulin resistance and inflammation in obese mice: Participation of white adipose tissue and bone. Bone 2018, 115, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Ueno, N.; Inui, A.; Kalra, P.S.; Kalra, S.P. Leptin transgene expression in the hypothalamus enforces euglycemia in diabetic, insulin-deficient nonobese Akita mice and leptin-deficient obese ob/ob mice. Peptides 2006, 27, 2332–2342. [Google Scholar] [CrossRef]
- Liu, X.; Liang, Y.; Xia, N.; Liu, W.; Yang, Q.; Wang, C. Decrease in leptin mediates rat bone metabolism impairments during high-fat diet-induced catch-up growth by modulating the OPG/RANKL balance. 3 Biotech. 2021, 11, 103. [Google Scholar] [CrossRef] [PubMed]
- Boyce, B.F.; Xing, L. The RANKL/RANK/OPG pathway. Curr. Osteoporos. Rep. 2007, 5, 98–104. [Google Scholar] [CrossRef]
- Wada, T.; Nakashima, T.; Hiroshi, N.; Penninger, J.M. RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol. Med. 2006, 12, 17–25. [Google Scholar] [CrossRef]
- Schett, G.; Hayer, S.; Zwerina, J.; Redlich, K.; Smolen, J.S. Mechanisms of disease: The link between RANKL and arthritic bone disease. Nat. Clin. Pract. Rheumatol. 2005, 1, 47–54. [Google Scholar] [CrossRef]
- Sasano, Y.; Zhu, J.X.; Tsubota, M.; Takahashi, I.; Onodera, K.; Mizoguchi, I.; Kagayama, M. Gene expression of MMP8 and MMP13 during embryonic development of bone and cartilage in the rat mandible and hind limb. J. Histochem. Cytochem. 2002, 50, 325–332. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, Y.; Zhao, X.; Meng, C.; Ma, L.; Kong, Y. MicroRNA-411 inhibited matrix metalloproteinase 13 expression in human chondrocytes. Am. J. Transl. Res. 2015, 7, 2000–2006. [Google Scholar]
- Posey, K.L.; Coustry, F.; Hecht, J.T. Cartilage oligomeric matrix protein: COMPopathies and beyond. Matrix Biol. 2018, 71–72, 161–173. [Google Scholar] [CrossRef]
- Stetler-Stevenson, W.G.; Seo, D.W. TIMP-2: An endogenous inhibitor of angiogenesis. Trends Mol. Med. 2005, 11, 97–103. [Google Scholar] [CrossRef]
- Hu, K.; Olsen, B.T. The roles of vascular endothelial growth factor in bone repair and regeneration. Bone 2016, 91, 30–38. [Google Scholar] [CrossRef]
- Seedor, J.G.; Quartuccio, H.A.; Thompson, D.D. The biophosphanate alendronate (MK-217) inhibit bone loss due to ovariectomy in rats. J. Bone Miner. Res. 1991, 1, 339–346. [Google Scholar]
- Hage, M.; Kamenický, P.; Chanson, P. Growth Hormone response to oral glucose load: From normal to pathological conditions. Neuroendocrinology 2019, 108, 244–255. [Google Scholar] [CrossRef]
- Masuda, A.; Shibasaki, T.; Nakahara, M.; Imaki, T.; Kiyosawa, Y.; Jibiki, K.; Demura, H.; Shizume, K.; Ling, N. The effect of glucose on growth hormone (GH)-releasing hormone-mediated GH secretion in man. J. Clin. Endocrinol. Metab. 1985, 60, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Vimalraj, S. Alkaline phosphatase: Structure, expression and its function in bone mineralization. Gene 2020, 754, 144855. [Google Scholar] [CrossRef] [PubMed]
- Persico, M.; Sessa, R.; Cesaro, E.; Dini, I.; Costanzo, P.; Ritieni, A.; Fattorusso, C.; Grosso, M. A multidisciplinary approach disclosing unexplored Aflatoxin B1 roles in severe impairment of vitamin D mechanisms of action. Cell Biol. Toxicol. 2022, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewska, E.; Dobrowolski, P.; Siwicki, A.K. Maternal treatment with dexamethasone at minimal therapeutic doses inhibits neonatal bone development in a gender-dependent manner. Livest. Sci. 2012, 146, 175–182. [Google Scholar] [CrossRef]
- Śliwa, E. 2-Oxoglutaric acid administration diminishes fundectomy-induced osteopenia in pigs. J. Anim. Physiol. Anim. Nutr. 2010, 94, e86–e95. [Google Scholar] [CrossRef]
- Floss, J.L.; Casteel, S.W.; Johnson, G.C.; Rottinghaus, G.E.; Krause, G.F. Development toxicity of fumonisin in Syrian hamsters. Mycopathologia 1994, 128, 33–38. [Google Scholar] [CrossRef]
- Lebepe-Mazur, S.; Bal, H.; Hopmans, E.; Murphy, P.; Hendrich, S. Fumonisin B1 is fetotoxic in rats. Vet. Hum. Toxicol. 1995, 37, 126–130. [Google Scholar]
- Huff, W.E. Discrepancies between bone ash and toe ash during aflatoxicosis. Poult. Sci. 1980, 59, 2213–2215. [Google Scholar] [CrossRef]
- Rudyk, H.; Tomaszewska, E.; Arciszewski, M.B.; Muszyński, S.; Tomczyk-Warunek, A.; Dobrowolski, P.; Donaldson, J.; Brezvyn, O.; Kotsyumbas, I. Histomorphometrical changes in intestine structure and innervation following experimental fumonisins intoxication in male Wistar rats. Pol. J. Vet. Sci. 2020, 23, 77–88. [Google Scholar] [PubMed]
- Morgan, E.F.; Unnikrisnan, G.U.; Hussein, A.I. Bone mechanical properties in healthy and diseased states. Annu. Rev. Biomed. Eng. 2018, 20, 119–143. [Google Scholar] [CrossRef] [PubMed]
- Lattouf, R.; Younes, R.; Lutomski, D.; Naaman, N.; Godeau, G.; Senni, K.; Changotade, S. Picrosirius red staining: A useful tool to appraise collagen networks in normal and pathological tissues. J. Histochem. Cytochem. 2014, 62, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Dempster, D.W.; Compston, J.E.; Drezner, M.K.; Glorieux, F.H.; Kanis, J.A.; Malluche, H.; Meunier, P.J.; Ott, S.M.; Recker, R.R.; Parfitt, A.M. Standardized nomenclature, symbols, and units for bone histomorphometry: A 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 2013, 28, 2–17. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomaszewska, E.; Rudyk, H.; Muszyński, S.; Hułas-Stasiak, M.; Leszczyński, N.; Mielnik-Błaszczak, M.; Donaldson, J.; Dobrowolski, P. Prenatal Fumonisin Exposure Impairs Bone Development via Disturbances in the OC/Leptin and RANKL/RANK/OPG Systems in Weaned Rat Offspring. Int. J. Mol. Sci. 2023, 24, 8743. https://doi.org/10.3390/ijms24108743
Tomaszewska E, Rudyk H, Muszyński S, Hułas-Stasiak M, Leszczyński N, Mielnik-Błaszczak M, Donaldson J, Dobrowolski P. Prenatal Fumonisin Exposure Impairs Bone Development via Disturbances in the OC/Leptin and RANKL/RANK/OPG Systems in Weaned Rat Offspring. International Journal of Molecular Sciences. 2023; 24(10):8743. https://doi.org/10.3390/ijms24108743
Chicago/Turabian StyleTomaszewska, Ewa, Halyna Rudyk, Siemowit Muszyński, Monika Hułas-Stasiak, Norbert Leszczyński, Maria Mielnik-Błaszczak, Janine Donaldson, and Piotr Dobrowolski. 2023. "Prenatal Fumonisin Exposure Impairs Bone Development via Disturbances in the OC/Leptin and RANKL/RANK/OPG Systems in Weaned Rat Offspring" International Journal of Molecular Sciences 24, no. 10: 8743. https://doi.org/10.3390/ijms24108743
APA StyleTomaszewska, E., Rudyk, H., Muszyński, S., Hułas-Stasiak, M., Leszczyński, N., Mielnik-Błaszczak, M., Donaldson, J., & Dobrowolski, P. (2023). Prenatal Fumonisin Exposure Impairs Bone Development via Disturbances in the OC/Leptin and RANKL/RANK/OPG Systems in Weaned Rat Offspring. International Journal of Molecular Sciences, 24(10), 8743. https://doi.org/10.3390/ijms24108743






