Three Major Causes of Metabolic Retinal Degenerations and Three Ways to Avoid Them
Abstract
1. Introduction
2. Aims
3. Retinal Health: The Major Metabolic Threats to It and Ways to Avoid Damage
4. Aging
4.1. Progression of Retinal Aging–Common Features in Model Species and Humans
4.2. Cellular and Subcellular Changes
4.3. Molecular Considerations
4.4. Preventive and Therapeutic Measures during Aging
5. Diabetic Retinopathy
5.1. Risk Factors and Disease Progression
5.2. Cellular Stress Response: Oxidative Stress and Autophagy in DR
5.3. Immune Response: Neuroinflammation and Glial Cell Activation in DR
5.4. Therapeutic Approaches
5.4.1. Flavonoids, Polyphenols and Other Natural Antioxidants (Table S1)
5.4.2. Endogenous Retinal Factors
5.4.3. Synthetic Drugs
6. Glaucoma
6.1. Risk factors and Disease Progression
6.2. Cellular Mechanisms
6.3. Molecular Considerations
6.4. Therapeutic Approaches
6.4.1. Some Herbal Remedies
6.4.2. Synthetic Glaucoma Drugs
7. Combination Treatments for the “Big Three”
7.1. Device-Assisted Minimally Invasive Therapy plus Drug Application Approach
7.2. Anti-Angiogenic Injection Therapy and Drugs
7.3. Internal Neuroprotective Factors and Drugs
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AGEs | advanced glycation end products |
| AMD | age-related macular degeneration |
| AION | anterior ischemic optic neuropathy |
| AREDS | Age-Related Eye Disease Studies |
| BRB | blood–retinal barrier |
| BDNF | brain-derived neurotrofic factor |
| CNTF | ciliary neurotrophic factor |
| COX | cyclooxygenase |
| DR | diabetic retinopathy |
| DME | diabetic macular edema |
| ERG | electroretinogram |
| EPO | erythropoietin |
| GFAP | glial fibrillary acidic protein |
| GPX | gluthatione peroxidase |
| IL | interleukin |
| ICAM-1 | intercellular adhesion molecule 1 |
| IOP | intraocular pressure |
| NVU | neurovascularunit |
| NMDA | N-methyl-D-aspartate |
| NFκB | nuclear factor κB |
| PEDF | pigment epithelium-derived factor |
| PACAP | pituitary adenylate cyclase-activating polypeptide |
| PR | photoreceptor |
| POAG | primary open-angle glaucoma |
| ROS | reactive oxygen species |
| RGC | retinal ganglion cells |
| RPE | retinal pigment epithelium |
| ROP | retinopathy of prematurity |
| ROCK | Rho-associated coiled-coil containing protein kinase |
| PEX | serum of pseudoexfoliation syndrome |
| SST | somatostatin |
| SOD | superoxid dismutase |
| TM | trabecular meshwork |
| TNF | tumor necrosis factor |
| VEGF | vascular endothelial growth factor |
References
- Pollen, D.A. Explicit Neural Representations, Recursive Neural Networks and Conscious Visual Perception. Cereb. Cortex 2003, 13, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Victor, J.D. Analyzing Receptive Fields, Classification Images and Functional Images: Challenges with Opportunities for Synergy. Nat. Neurosci. 2005, 8, 1651–1656. [Google Scholar] [CrossRef] [PubMed]
- Dowling, J.E. The Retina: An Approachable Part of the Brain; Belknap Press: Cambridge, MA, USA, 2012; p. 355. [Google Scholar]
- Pacione, L.R.; Szego, M.J.; Ikeda, S.; Nishina, P.M.; McInnes, R.R. Progress toward Understanding the Genetic and Biochemical Mechanisms of Inherited Photoreceptor Degenerations. Annu. Rev. Neurosci. 2003, 26, 657–700. [Google Scholar] [CrossRef]
- Osborne, N.N.; Casson, R.J.; Wood, J.P.M.; Chidlow, G.; Graham, M.; Melena, J. Retinal Ischemia: Mechanisms of Damage and Potential Therapeutic Strategies. Prog. Retin. Eye Res. 2004, 23, 91–147. [Google Scholar] [CrossRef]
- Atlasz, T.; Szabadfi, K.; Kiss, P.; Babai, N.; Koszegi, Z.; Tamas, A.; Reglodi, D.; Gabriel, R. PACAP-Mediated Neuroprotection of Neurochemically Identified Cell Types in MSG-Induced Retinal Degeneration. J. Mol. Neurosci. 2008, 36, 97–104. [Google Scholar] [CrossRef]
- Hernández, C.; Simó, R. Neuroprotection in Diabetic Retinopathy. Curr. Diab. Rep. 2012, 12, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Pinazo-Durán, M.D.; Gallego-Pinazo, R.; García-Medina, J.J.; Zanón-Moreno, V.; Nucci, C.; Dolz-Marco, R.; Martínez-Castillo, S.; Galbis-Estrada, C.; Marco-Ramírez, C.; López-Gálvez, M.I.; et al. Oxidative Stress and Its Downstream Signaling in Aging Eyes. Clin. Interv. Aging 2014, 9, 637–652. [Google Scholar] [CrossRef]
- Masmoudi-Kouki, O.; Douiri, S.; Hamdi, Y.; Kaddour, H.; Bahdoudi, S.; Vaudry, D.; Basille, M.; Leprince, J.; Fournier, A.; Vaudry, H.; et al. Pituitary Adenylate Cyclase-Activating Polypeptide Protects Astroglial Cells against Oxidative Stress-Induced Apoptosis. J. Neurochem. 2011, 117, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Steinle, J.J.; Sharma, S.; Smith, C.P.; McFayden-Ketchum, L.S. Normal Aging Involves Modulation of Specific Inflammatory Markers in the Rat Retina and Choroid. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64, 325–331. [Google Scholar] [CrossRef]
- Xu, H.; Chen, M.; Forrester, J.V. Para-Inflammation in the Aging Retina. Prog. Retin. Eye Res. 2009, 28, 348–368. [Google Scholar] [CrossRef]
- Chen, M.; Muckersie, E.; Forrester, J.V.; Xu, H. Immune Activation in Retinal Aging: A Gene Expression Study. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5888–5896. [Google Scholar] [CrossRef]
- Hussain, R.M.; Shaukat, B.A.; Ciulla, L.M.; Berrocal, A.M.; Sridhar, J. Vascular Endothelial Growth Factor Antagonists: Promising Players in the Treatment of Neovascular Age-Related Macular Degeneration. Drug. Des. Devel. Ther. 2021, 15, 2653–2665. [Google Scholar] [CrossRef]
- Ientile, R.; Macaione, V.; Teletta, M.; Pedale, S.; Torre, V.; Macaione, S. Apoptosis and Necrosis Occurring in Excitotoxic Cell Death in Isolated Chick Embryo Retina. J. Neurochem. 2001, 79, 71–78. [Google Scholar] [CrossRef]
- Chen, Y.; Coorey, N.J.; Zhang, M.; Zeng, S.; Madigan, M.C.; Zhang, X.; Gillies, M.C.; Zhu, L.; Zhang, T. Metabolism Dysregulation in Retinal Diseases and Related Therapies. Antioxidants 2022, 11, 942. [Google Scholar] [CrossRef]
- Wang, J.; Li, M.; Geng, Z.; Khattak, S.; Ji, X.; Wu, D.; Dang, Y. Role of Oxidative Stress in Retinal Disease and the Early Intervention Strategies: A Review. Oxid. Med. Cell. Longev. 2022, 2022, 7836828. [Google Scholar] [CrossRef] [PubMed]
- Projected Change in Vision Loss 2020 to 2050—The International Agency for the Prevention of Blindness. Available online: https://www.iapb.org/learn/vision-atlas/magnitude-and-projections/projected-change/ (accessed on 27 January 2023).
- Manthey, A.L.; Chiu, K.; So, K.F. Effects of Lycium Barbarum on the Visual System. Int. Rev. Neurobiol. 2017, 135, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.A.; Sood, G.C.; Raman, R. Ocular Neurofibromatosis. Indian. J. Ophthalmol. 1981, 29, 117–120. [Google Scholar] [CrossRef]
- Coles, R.S.; Laval, J. Retinal Detachments Occurring in Cataract Associated with Neurodermatitis. AMA Arch. Ophthalmol. 1952, 48, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Bair, B.; Dodd, J.; Heidelberg, K.; Krach, K. Cataracts in Atopic Dermatitis: A Case Presentation and Review of the Literature. Arch. Dermatol. 2011, 147, 585–588. [Google Scholar] [CrossRef] [PubMed]
- Langan, S.M.; Irvine, A.D.; Weidinger, S. Atopic Dermatitis. Lancet 2020, 396, 345–360. [Google Scholar] [CrossRef]
- Tian, C.; Duan, L.; Fu, C.; He, J.; Dai, J.; Zhu, G. Study on the Correlation Between Iris Characteristics and Schizophrenia. Neuropsychiatr. Dis. Treat. 2022, 18, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Sabel, B.A.; Wang, J.; Cárdenas-Morales, L.; Faiq, M.; Heim, C. Mental Stress as Consequence and Cause of Vision Loss: The Dawn of Psychosomatic Ophthalmology for Preventive and Personalized Medicine. EPMA J. 2018, 9, 133–160. [Google Scholar] [CrossRef]
- Edwards, M.J.M.; Hazi, A.; Crewther, S.G. Acute Psychosocial Stress Induces a Myopic Shift in Undergraduate Students. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2841. [Google Scholar]
- Tian, B.; Bilsbury, E.; Doherty, S.; Teebagy, S.; Wood, E.; Su, W.; Gao, G.; Lin, H. Ocular Drug Delivery: Advancements and Innovations. Pharmaceutics 2022, 14, 1931. [Google Scholar] [CrossRef] [PubMed]
- Nian, S.; Lo, A.C.Y.; Nian, S.; Lo, A.C.Y. Protecting the Aging Retina. Neuroprotection 2019, 5, 1–20. [Google Scholar] [CrossRef]
- Fisher, D.E.; Klein, B.E.K.; Wong, T.Y.; Rotter, J.I.; Li, X.; Shrager, S.; Burke, G.L.; Klein, R.; Cotch, M.F. Incidence of Age-Related Macular Degeneration in a Multi-Ethnic United States Population: The Multi-Ethnic Study of Atherosclerosis. Ophthalmology 2016, 123, 1297–1308. [Google Scholar] [CrossRef]
- Sivaprasad, S.; Gupta, B.; Crosby-Nwaobi, R.; Evans, J. Prevalence of Diabetic Retinopathy in Various Ethnic Groups: A Worldwide Perspective. Surv. Ophthalmol. 2012, 57, 347–370. [Google Scholar] [CrossRef]
- Jonas, J.B.; Aung, T.; Bourne, R.R.; Bron, A.M.; Ritch, R.; Panda-Jonas, S. Glaucoma. Lancet 2017, 390, 2183–2193. [Google Scholar] [CrossRef]
- H Gao, J.G.H. Aging of the Human Retina. Differential Loss of Neurons and Retinal Pigment Epithelial Cells. Investig. Ophthalmol. Vis. Sci. 1992, 33, 1–17. [Google Scholar]
- Curcio, C.A.; Drucker, D.N. Retinal Ganglion Cells in Alzheimer’s Disease and Aging. Ann. Neurol. 1993, 33, 248–257. [Google Scholar] [CrossRef]
- Panda-Jonas, S.; Jonas, J.B.; Jakobczyk-Zmija, M. Retinal Photoreceptor Density Decreases with Age. Ophthalmology 1995, 102, 1853–1859. [Google Scholar] [CrossRef]
- Kim, C.B.Y.; Tom, B.W.; Spear, P.D. Effects of Aging on the Densities, Numbers, and Sizes of Retinal Ganglion Cells in Rhesus Monkey. Neurobiol. Aging 1996, 17, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Liets, L.C.; Eliasieh, K.; Van Der List, D.A.; Chalupa, L.M. Dendrites of Rod Bipolar Cells Sprout in Normal Aging Retina. Proc. Natl. Acad. Sci. USA 2006, 103, 12156–12160. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, P.; Nag, T.C.; Wadhwa, S. Age-Related Decrease in Rod Bipolar Cell Density of the Human Retina: An Immunohistochemical Study. J. Biosci. 2007, 32, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Parikh, R.S.; Parikh, S.R.; Sekhar, G.C.; Prabakaran, S.; Babu, J.G.; Thomas, R. Normal Age-Related Decay of Retinal Nerve Fiber Layer Thickness. Ophthalmology 2007, 114, 921–926. [Google Scholar] [CrossRef]
- Freund, P.R.; Watson, J.; Gilmour, G.S.; Gaillard, F.; Sauvé, Y. Differential Changes in Retina Function with Normal Aging in Humans. Doc. Ophthalmol. 2011, 122, 177–190. [Google Scholar] [CrossRef]
- Samuel, M.A.; Zhang, Y.; Meister, M.; Sanes, J.R. Age-Related Alterations in Neurons of the Mouse Retina. J. Neurosci. 2011, 31, 16033–16044. [Google Scholar] [CrossRef]
- Cunea, A.; Powner, M.B.; Jeffery, G. Death by Color: Differential Cone Loss in the Aging Mouse Retina. Neurobiol. Aging 2014, 35, 2584–2591. [Google Scholar] [CrossRef]
- Cano, J.; Machado, A.; Reinoso-Suárez, F. Morphological Changes in the Retina of Ageing Rats. Arch. Gerontol. Geriatr. 1986, 5, 41–50. [Google Scholar] [CrossRef]
- Papazariri, P.; Podini, P.; Meldolesi, J.; Yamaguchi, T. Ageing Affects Cytosolic Ca2+ Binding Proteins and Synaptic Markers in the Retina but Not in Cerebral Cortex Neurons of the Rat. Neurosci. Lett. 1995, 186, 65–68. [Google Scholar] [CrossRef]
- Mansour, H.; Chamberlain, C.G.; Weible, M.W.; Hughes, S.; Chu, Y.; Chan-Ling, T. Aging-Related Changes in Astrocytes in the Rat Retina: Imbalance between Cell Proliferation and Cell Death Reduces Astrocyte Availability. Aging Cell 2008, 7, 526–540. [Google Scholar] [CrossRef] [PubMed]
- Nadal-Nicolás, F.M.; Vidal-Sanz, M.; Agudo-Barriuso, M. The Aging Rat Retina: From Function to Anatomy. Neurobiol. Aging 2018, 61, 146–168. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.E.I.; El-Shaarawy, E.A.A.; Youakim, M.F.; Shuaib, D.M.A.; Ahmed, M.M. Aging Changes in the Retina of Male Albino Rat: A Histological, Ultrastructural and Immunohistochemical Study. Folia Morphol. 2019, 78, 237–258. [Google Scholar] [CrossRef] [PubMed]
- Atlasz, T.; Szabadfi, K.; Kiss, P.; Tamas, A.; Toth, G.; Reglodi, D.; Gabriel, R. Evaluation of the Protective Effects of PACAP with Cell-Specific Markers in Ischemia-Induced Retinal Degeneration. Brain Res. Bull. 2010, 81, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Cuenca, N.; Pinilla, I.; Sauvé, Y.; Lund, R. Early Changes in Synaptic Connectivity Following Progressive Photoreceptor Degeneration in RCS Rats. Eur. J. Neurosci. 2005, 22, 1057–1072. [Google Scholar] [CrossRef]
- DiLoreto, D.A.; Luo, C.; Calkins, D.J.; Del Cerro, M. An Ultrastructural Study of the Pathology of the Retinal Pigment Epithelium, Bruch’s Membrane, and the Choriocapillaris in the Aged Fischer 344 Rat. Curr. Eye Res. 2006, 31, 749–763. [Google Scholar] [CrossRef]
- Harman, A.M.; MacDonald, A.; Meyer, P.; Ahmat, A. Numbers of Neurons in the Retinal Ganglion Cell Layer of the Rat Do Not Change throughout Life. Gerontology 2003, 49, 350–355. [Google Scholar] [CrossRef]
- Morrison, J.C.; Cork, L.C.; Dunkelberger, G.R.; Brown, A.; Quigley, H.A. Aging Changes of the Rhesus Monkey Optic Nerve. Investig. Ophthalmol. Vis. Sci. 1990, 31, 1623–1627. [Google Scholar]
- Cavallotti, C.; Artico, M.; Pescosolido, N.; Tranquilli Leali, F.M.; Feher, J. Age-Related Changes in the Human Retina. Can. J. Ophthalmol. 2004, 39, 61–68. [Google Scholar] [CrossRef]
- Esquiva, G.; Lax, P.; Pérez-Santonja, J.J.; García-Fernández, J.M.; Cuenca, N. Loss of Melanopsin-Expressing Ganglion Cell Subtypes and Dendritic Degeneration in the Aging Human Retina. Front. Aging Neurosci. 2017, 9, 79. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.L.; Robison, W.G. Evidence of Cell Loss from the Rat Retina during Senescence. Exp. Eye Res. 1986, 42, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Danias, J.; Lee, K.C.; Zamora, M.F.; Chen, B.; Shen, F.; Filippopoulos, T.; Su, Y.; Goldblum, D.; Podos, S.M.; Mittag, T. Quantitative Analysis of Retinal Ganglion Cell (RGC) Loss in Aging DBA/2NNia Glaucomatous Mice: Comparison with RGC Loss in Aging C57/BL6 Mice. Investig. Ophthalmol. Vis. Sci. 2003, 44, 5151–5162. [Google Scholar] [CrossRef]
- Feng, L.; Sun, Z.; Han, H.; Zhou, Y.; Zhang, M. No Age-Related Cell Loss in Three Retinal Nuclear Layers of the Long-Evans Rat. Vis. Neurosci. 2007, 24, 799–803. [Google Scholar] [CrossRef] [PubMed]
- Weisse, I.; Loosen, H.; Peil, H. Age-Related Retinal Changes--Comparison between Albino and Pigmented Rats. Lens Eye Toxic. Res. 1990, 7, 717–739. [Google Scholar]
- Terzibasi, E.; Calamusa, M.; Novelli, E.; Domenici, L.; Strettoi, E.; Cellerino, A. Age-Dependent Remodelling of Retinal Circuitry. Neurobiol. Aging 2009, 30, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Eliasieh, K.; Liets, L.C.; Chalupa, L.M. Cellular Reorganization in the Human Retina during Normal Aging. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2824–2830. [Google Scholar] [CrossRef] [PubMed]
- Bonnel, S.; Mohand-Said, S.; Sahel, J.-A.A. The Aging of the Retina. Exp. Gerontol. 2003, 38, 825–831. [Google Scholar] [CrossRef]
- Mohand-Said, S.; Deudon-Combe, A.; Hicks, D.; Simonutti, M.; Forster, V.; Fintz, A.C.; Léveillard, T.; Dreyfus, H.; Sahel, J.A. Normal Retina Releases a Diffusible Factor Stimulating Cone Survival in the Retinal Degeneration Mouse. Proc. Natl. Acad. Sci. USA 1998, 95, 8357–8362. [Google Scholar] [CrossRef]
- Mohand-Said, S.; Hicks, D.; Dreyfus, H.; Sahel, J.A. Selective Transplantation of Rods Delays Cone Loss in a Retinitis Pigmentosa Model. Arch. Ophthalmol. 2000, 118, 807–811. [Google Scholar] [CrossRef]
- Mohand-Said, S.; Hicks, D.; Léveillard, T.; Picaud, S.; Porto, F.; Sahel, J.A. Rod-Cone Interactions: Developmental and Clinical Significance. Prog. Retin. Eye Res. 2001, 20, 451–467. [Google Scholar] [CrossRef]
- Fintz, A.C.; Audo, I.; Hicks, D.; Mohand-Said, S.; Léveillard, T.; Sahel, J. Partial Characterization of Retina-Derived Cone Neuroprotection in Two Culture Models of Photoreceptor Degeneration. Investig. Ophthalmol. Vis. Sci. 2003, 44, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Feeney-Burns, L.; Burns, R.P.; Gao, C.L. Age-Related Macular Changes in Humans over 90 Years Old. Am. J. Ophthalmol. 1990, 109, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Lin, N.; Sheedlo, H.J.; Turner, J.E. Muller and RPE Cell Response to Photoreceptor Cell Degeneration in Aging Fischer Rats. Exp. Eye Res. 1996, 63, 9–18. [Google Scholar] [CrossRef]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global Prevalence of Age-Related Macular Degeneration and Disease Burden Projection for 2020 and 2040: A Systematic Review and Meta-Analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.J.; Mirza, R.G.; Gill, M.K. Age-Related Macular Degeneration. Med. Clin. N. Am. 2021, 105, 473–491. [Google Scholar] [CrossRef] [PubMed]
- Age-Related Macular Degeneration: Diagnosis and Management. Br. Med. Bulletin. 2018, 85, 127–149.
- Takahashi, K.; Ishibashi, T.; Ogur, Y.; Yuzawa, M.; Working Group for Establishing Diagnostic Criteria for Age-Related Macular Degeneration. Classification and Diagnostic Criteria of Age-Related Macular Degeneration. Available online: https://pubmed.ncbi.nlm.nih.gov/19157028/ (accessed on 27 January 2023).
- Seddon, J.M.; Sharma, S.; Adelman, R.A. Evaluation of the Clinical Age-Related Maculopathy Staging System. Ophthalmology 2006, 113, 260–266. [Google Scholar] [CrossRef]
- The Age-Related Eye Disease Study System for Classifying Age-Related Macular Degeneration from Stereoscopic Color Fundus Photographs: The Age-Related Eye Disease Study Report Number 6. Am. J. Ophthalmol. 2001, 132, 668–681. [CrossRef] [PubMed]
- Wassell, J.; Davies, S.; Bardsley, W.; Boulton, M. The Photoreactivity of the Retinal Age Pigment Lipofuscin. J. Biol. Chem. 1999, 274, 23828–23832. [Google Scholar] [CrossRef]
- Boulton, M.; Dontsov, A.; Jarvis-Evans, J.; Ostrovsky, M.; Svistunenko, D. Lipofuscin Is a Photoinducible Free Radical Generator. J. Photochem. Photobiol. B 1993, 19, 201–204. [Google Scholar] [CrossRef]
- Mullins, R.F.; Russell, S.R.; Anderson, D.H.; Hageman, G.S. Drusen Associated with Aging and Age-Related Macular Degeneration Contain Proteins Common to Extracellular Deposits Associated with Atherosclerosis, Elastosis, Amyloidosis, and Dense Deposit Disease. FASEB J. 2000, 14, 835–846. [Google Scholar] [CrossRef]
- Curcio, C.A.; Millican, C.L.; Bailey, T.; Kruth, H.S. Accumulation of Cholesterol with Age in Human Bruch’s Membrane. Investig. Ophthalmol. Vis. Sci. 2001, 42, 265–274. [Google Scholar]
- Gresh, J.; Goletz, P.W.; Crouch, R.K.; Rohrer, B. Structure-Function Analysis of Rods and Cones in Juvenile, Adult, and Aged C57bl/6 and Balb/c Mice. Vis. Neurosci. 2003, 20, 211–220. [Google Scholar] [CrossRef]
- Williams, G.A.; Jacobs, G.H. Cone-Based Vision in the Aging Mouse. Vis. Res. 2007, 47, 2037–2046. [Google Scholar] [CrossRef] [PubMed]
- Kolesnikov, A.V.; Fan, J.; Crouch, R.K.; Kefalov, V.J. Age-Related Deterioration of Rod Vision in Mice. J. Neurosci. 2010, 30, 11222–11231. [Google Scholar] [CrossRef] [PubMed]
- Birch, D.G.; Hood, D.C.; Locke, K.G.; Hoffman, D.R.; Tzekov, R.T. Quantitative Electroretinogram Measures of Phototransduction in Cone and Rod Photoreceptors: Normal Aging, Progression with Disease, and Test-Retest Variability. Arch. Ophthalmol. 2002, 120, 1045–1051. [Google Scholar] [CrossRef]
- Chen, M.; Luo, C.; Zhao, J.; Devarajan, G.; Xu, H. Immune Regulation in the Aging Retina. Prog. Retin. Eye Res. 2019, 69, 159–172. [Google Scholar] [CrossRef]
- Payne, A.J.; Kaja, S.; Naumchuk, Y.; Kunjukunju, N.; Koulen, P. Antioxidant Drug Therapy Approaches for Neuroprotection in Chronic Diseases of the Retina. Int. J. Mol. Sci. 2014, 15, 1865–1886. [Google Scholar] [CrossRef] [PubMed]
- Barron, M.J.; Johnson, M.A.; Andrews, R.M.; Clarke, M.P.; Griffiths, P.G.; Bristow, E.; He, L.; Durham, S.; Turnbull, D.M. Mitochondrial Abnormalities in Ageing Macular Photoreceptors. Investig. Ophthalmol. Vis. Sci. 2001, 42, 3016–3022. [Google Scholar]
- Louie, J.L.; Kapphahn, R.J.; Ferrington, D.A. Proteasome Function and Protein Oxidation in the Aged Retina. Exp. Eye Res. 2002, 75, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Crabb, J.W.; Miyagi, M.; Gu, X.; Shadrach, K.; West, K.A.; Sakaguchi, H.; Kamei, M.; Hasan, A.; Yan, L.; Rayborn, M.E.; et al. Drusen Proteome Analysis: An Approach to the Etiology of Age-Related Macular Degeneration. Proc. Natl. Acad. Sci. USA 2002, 99, 14682–14687. [Google Scholar] [CrossRef] [PubMed]
- Milne, R.; Brownstein, S. Advanced Glycation End Products and Diabetic Retinopathy. Amino Acids 2013, 44, 1397–1407. [Google Scholar] [CrossRef]
- Adornetto, A.; Rombolà, L.; Morrone, L.A.; Nucci, C.; Corasaniti, M.T.; Bagetta, G.; Russo, R. Natural Products: Evidence for Neuroprotection to Be Exploited in Glaucoma. Nutrients 2020, 12, 3158. [Google Scholar] [CrossRef] [PubMed]
- Anand, A.; Modgil, S.; Sharma, V.L.; Shri, R.; Kaushik, S. Preserving Neural Retina through Re-Emerging Herbal Interventions. J. Cell. Biochem. 2014, 115, 1659–1668. [Google Scholar] [CrossRef] [PubMed]
- Tsang, J.K.W.; Wolf, S.A.; Pompoes, I.M.; Joussen, A.M.; Lam, W.C.; Yang, D.; Lo, A.C.Y. Potential Effects of Nutraceuticals in Retinopathy of Prematurity. Life 2021, 11, 79. [Google Scholar] [CrossRef]
- Pokkalath, A.S.; Sawant, A.; Sawarkar, S.P. Herbal Medicine for Ocular Diseases: An Age Old Therapy and Its Future Perspective. J. Drug Deliv. Sci. Technol. 2022, 68, 102979. [Google Scholar] [CrossRef]
- Nebbioso, M.; Franzone, F.; Greco, A.; Gharbiya, M.; Bonfiglio, V.; Polimeni, A. Recent Advances and Disputes About Curcumin in Retinal Diseases. Clin. Ophthalmol. 2021, 15, 2553–2571. [Google Scholar] [CrossRef]
- Radomska-Leśniewska, D.M.; Osiecka-Iwan, A.; Hyc, A.; Góźdź, A.; Dąbrowska, A.M.; Skopiński, P. Therapeutic Potential of Curcumin in Eye Diseases. Cent. J. Immunol. 2019, 44, 181–189. [Google Scholar] [CrossRef]
- Behl, T.; Kotwani, A. Chinese Herbal Drugs for the Treatment of Diabetic Retinopathy. J. Pharm. Pharmacol. 2017, 69, 223–235. [Google Scholar] [CrossRef]
- Heitmar, R.; Brown, J.; Kyrou, I. Saffron (Crocus sativus L.) in Ocular Diseases: A Narrative Review of the Existing Evidence from Clinical Studies. Nutrients 2019, 11, 649. [Google Scholar] [CrossRef]
- Camelo, S.; Latil, M.; Veillet, S.; Dilda, P.J.; Lafont, R. Beyond AREDS Formulations, What Is Next for Intermediate Age-Related Macular Degeneration (IAMD) Treatment? Potential Benefits of Antioxidant and Anti-Inflammatory Apocarotenoids as Neuroprotectors. Oxid. Med. Cell. Longev. 2020, 2020, 4984927. [Google Scholar] [CrossRef] [PubMed]
- Karhanová, M.; Eliášová, M.; Kuběna, T.; Pešková, H.; Mlčák, P.; Fryšák, Z.; Marešová, K.; Zapletalová, J. [ProVens® in the Therapy of Glaucoma and Ocular Hypertension]—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/26782917/ (accessed on 27 January 2023).
- Schönlau, F.; Rohdewald, P. Pycnogenol for Diabetic Retinopathy. A Review. Int. Ophthalmol. 2001, 24, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Alok, S.; Jain, S.K.; Verma, A.; Kumar, M.; Mahor, A.; Sabharwal, M. Herbal Antioxidant in Clinical Practice: A Review. Asian Pac. J. Trop. Biomed. 2014, 4, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.X.; Lin, F.J.; Li, H.; Li, H.B.; Wu, D.T.; Geng, F.; Ma, W.; Wang, Y.; Miao, B.H.; Gan, R.Y. Recent Advances in Bioactive Compounds, Health Functions, and Safety Concerns of Onion (Allium Cepa L.). Front. Nutr. 2021, 8, 669805. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.J.; Uddin, T.M.; Matin Zidan, B.M.R.; Mitra, S.; Das, R.; Nainu, F.; Dhama, K.; Roy, A.; Hossain, M.J.; Khusro, A.; et al. Allium Cepa: A Treasure of Bioactive Phytochemicals with Prospective Health Benefits. Evid. Based Complement. Alternat. Med. 2022, 2022, 4586318. [Google Scholar] [CrossRef]
- Silveira, M.A.D.; De Jong, D.; Berretta, A.A.; Galvão, E.B.d.S.; Ribeiro, J.C.; Cerqueira-Silva, T.; Amorim, T.C.; Conceição, L.F.M.R.d.; Gomes, M.M.D.; Teixeira, M.B.; et al. Efficacy of Brazilian Green Propolis (EPP-AF®) as an Adjunct Treatment for Hospitalized COVID-19 Patients: A Randomized, Controlled Clinical Trial. Biomed. Pharmacother. 2021, 138, 111526. [Google Scholar] [CrossRef]
- Wilkinson, J.T.; Fraunfelder, F.W. Use of Herbal Medicines and Nutritional Supplements in Ocular Disorders: An Evidence-Based Review. Drugs 2011, 71, 2421–2434. [Google Scholar] [CrossRef]
- Tomida, I.; Perlwee, R.G.; Azuara-Blanco, A. Cannabinoids and Glaucoma. Br. J. Ophthalmol. 2004, 88, 708–713. [Google Scholar] [CrossRef]
- Dheyab, N.; Ali, F.; Ismail, A.; Othman, F.B. Anti-Diabetic Retinopathy Potential of Noni: The Beneficial Effect and Possible Mechanism | Auctores. Available online: https://www.auctoresonline.org/article/anti-diabetic-retinopathy-potential-of-noni-the-beneficial-effect-and-possible-mechanism (accessed on 27 January 2023).
- Torres, M.A.O.; de Fátima Braga Magalhães, I.; Mondêgo-Oliveira, R.; de Sá, J.C.; Rocha, A.L.; Abreu-Silva, A.L. One Plant, Many Uses: A Review of the Pharmacological Applications of Morinda Citrifolia. Phytother. Res. 2017, 31, 971–979. [Google Scholar] [CrossRef]
- Rajabian, A.; Sadeghnia, H.R.; Hosseini, A.; Mousavi, S.H.; Boroushaki, M.T. 3-Acetyl-11-Keto-β-Boswellic Acid Attenuated Oxidative Glutamate Toxicity in Neuron-like Cell Lines by Apoptosis Inhibition. J. Cell. Biochem. 2020, 121, 1778–1789. [Google Scholar] [CrossRef]
- Siddiqui, A.; Shah, Z.; Jahan, R.N.; Othman, I.; Kumari, Y. Mechanistic Role of Boswellic Acids in Alzheimer’s Disease: Emphasis on Anti-Inflammatory Properties. Biomed. Pharmacother. 2021, 144, 112250. [Google Scholar] [CrossRef]
- Upadhayay, S.; Mehan, S.; Prajapati, A.; Sethi, P.; Suri, M.; Zawawi, A.; Almashjary, M.N.; Tabrez, S. Nrf2/HO-1 Signaling Stimulation through Acetyl-11-Keto-Beta-Boswellic Acid (AKBA) Provides Neuroprotection in Ethidium Bromide-Induced Experimental Model of Multiple Sclerosis. Genes 2022, 13, 1324. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.W.; Hsu, S.K.; Chen, J.Y.F.; Lin, I.L.; Chen, K.J.; Lee, P.Y.; Ng, H.S.; Chiu, C.C.; Cheng, K.C. Curcumin Metabolite Tetrahydrocurcumin in the Treatment of Eye Diseases. Int. J. Mol. Sci. 2020, 22, 212. [Google Scholar] [CrossRef]
- Lulli, M.; Cammalleri, M.; Fornaciari, I.; Casini, G.; Dal Monte, M. Acetyl-11-Keto-β-Boswellic Acid Reduces Retinal Angiogenesis in a Mouse Model of Oxygen-Induced Retinopathy. Exp. Eye Res. 2015, 135, 67–80. [Google Scholar] [CrossRef]
- Hong, S.C.; Ha, J.H.; Lee, J.K.; Jung, S.H.; Kim, J.C. In Vivo Anti-Inflammation Potential of Aster Koraiensis Extract for Dry Eye Syndrome by the Protection of Ocular Surface. Nutrients 2020, 12, 3245. [Google Scholar] [CrossRef]
- Zhang, L.; Park, J.Y.; Zhao, D.; Kwon, H.C.; Yang, H.O. Neuroprotective Effect of Astersaponin I against Parkinson’s Disease through Autophagy Induction. Biomol. Ther. 2021, 29, 615–629. [Google Scholar] [CrossRef]
- Kim, J.; Jo, K.; Kim, C.S.; Kim, J.S. Aster Koraiensis Extract Prevents Diabetes-Induced Retinal Vascular Dysfunction in Spontaneously Diabetic Torii Rats. BMC Complement. Altern. Med. 2017, 17, 497. [Google Scholar] [CrossRef]
- Lim, E.Y.; Kim, J.G.; Lee, J.; Lee, C.; Shim, J.; Kim, Y.T. Analgesic Effects of Cnidium Officinale Extracts on Postoperative, Neuropathic, and Menopausal Pain in Rat Models. Evid. Based. Complement. Alternat. Med. 2019, 2019, 9698727. [Google Scholar] [CrossRef] [PubMed]
- Sureshkumar, D.; Begum, S.; Johannah, N.M.; Maliakel, B.; Krishnakumar, I.M. Toxicological Evaluation of a Saponin-Rich Standardized Extract of Fenugreek Seeds (FenuSMART®): Acute, Sub-Chronic and Genotoxicity Studies. Toxicol. Rep. 2018, 5, 1060–1068. [Google Scholar] [CrossRef]
- Lin, Y.; Shi, R.; Wang, X.; Shen, H.-M. Luteolin, a Flavonoid with Potential for Cancer Prevention and Therapy. Curr. Cancer Drug Targets 2008, 8, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Stojanović-Radić, Z.; Matejić, J.; Sharifi-Rad, M.; Anil Kumar, N.V.; Martins, N.; Sharifi-Rad, J. The Therapeutic Potential of Curcumin: A Review of Clinical Trials. Eur. J. Med. Chem. 2019, 163, 527–545. [Google Scholar] [CrossRef]
- Kang, J.M.; Lin, S. Ginkgo Biloba and Its Potential Role in Glaucoma. Curr. Opin. Ophthalmol. 2018, 29, 116–120. [Google Scholar] [CrossRef]
- Kim, K.A.; Kim, S.M.; Kang, S.W.; Jeon, S.I.; Um, B.H.; Jung, S.H. Edible Seaweed, Eisenia Bicyclis, Protects Retinal Ganglion Cells Death Caused by Oxidative Stress. Mar. Biotechnol. 2012, 14, 383–395. [Google Scholar] [CrossRef]
- Yang, C.; Zhao, Q.; Li, S.; Pu, L.; Yu, L.; Liu, Y.; Lai, X. Effects of Lycium Barbarum L. Polysaccharides on Vascular Retinopathy: An Insight Review. Molecules 2022, 27, 5628. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Choi, Y.R.; Shim, J.; Choi, Y.S.; Kim, Y.T.; Kim, M.K.; Kim, M.J. Suppressive Effect of Arctium Lappa L. Leaves on Retinal Damage Against A2E-Induced ARPE-19 Cells and Mice. Molecules 2020, 25, 1737. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.S.; Cheng, L.N.; Wu, J.H.; Chan, E.; Kwan, Y.W.; Lee, S.M.Y.; Leung, G.P.H.; Yu, P.H.F.; Chan, S.W. A Review of the Pharmacological Effects of Arctium Lappa (Burdock). Inflammopharmacology 2011, 19, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Varughese, R.S.; Lam, W.S.T.; Marican, A.A.; Bin, H.; Viganeshwari, S.H.; Bhave, A.S.; Syn, N.L.; Wang, J.; Wong, A.L.A.; Kumar, A.P.; et al. Biopharmacological Considerations for Accelerating Drug Development of Deguelin, a Rotenoid with Potent Chemotherapeutic and Chemopreventive Potential. Cancer 2019, 125, 1789–1798. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef]
- Ren, D.; Shen, Z.Y.; Qin, L.P.; Zhu, B. Pharmacology, Phytochemistry, and Traditional Uses of Scrophularia Ningpoensis Hemsl. J. Ethnopharmacol. 2021, 269, 113688. [Google Scholar] [CrossRef]
- Ong, C.P.; Lee, W.L.; Tang, Y.Q.; Yap, W.H. Honokiol: A Review of Its Anticancer Potential and Mechanisms. Cancers 2019, 12, 48. [Google Scholar] [CrossRef]
- Giannaccare, G.; Pellegrini, M.; Senni, C.; Bernabei, F.; Scorcia, V.; Cicero, A.F.G. Clinical Applications of Astaxanthin in the Treatment of Ocular Diseases: Emerging Insights. Mar. Drugs 2020, 18, 239. [Google Scholar] [CrossRef]
- Guo, X.; He, Q.; Qi, B.; Sun, C.; Lyu, D.; Zhang, H. A Poisoning Outbreak Caused by Anisodus Tanguticus—Maqin County, Qinghai Province, China, July 2021. China CDC Wkly. 2022, 4, 920–923. [Google Scholar] [PubMed]
- Rathnavelu, V.; Alitheen, N.B.; Sohila, S.; Kanagesan, S.; Ramesh, R. Potential Role of Bromelain in Clinical and Therapeutic Applications. Biomed. Rep. 2016, 5, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Ige, M.; Liu, J. Herbal Medicines in Glaucoma Treatment. Yale J. Biol. Med. 2020, 93, 347–353. [Google Scholar] [PubMed]
- Takeuchi, M.; Shieh, P.C.; Horng, C.T. Treatment of Symptomatic Vitreous Opacities with Pharmacologic Vitreolysis Using a Mixure of Bromelain, Papain and Ficin Supplement. Appl. Sci. 2020, 10, 5901. [Google Scholar] [CrossRef]
- Manabe, K.; Kaidzu, S.; Tsutsui, A.; Mochiji, M.; Matsuoka, Y.; Takagi, Y.; Miyamoto, E.; Tanito, M. Effects of French Maritime Pine Bark/Bilberry Fruit Extracts on Intraocular Pressure for Primary Open-Angle Glaucoma. J. Clin. Biochem. Nutr. 2021, 68, 67–72. [Google Scholar] [CrossRef]
- Kumar, S.; Modgil, S.; Bammidi, S.; Minhas, G.; Shri, R.; Kaushik, S.; Singh, V.; Anand, A. Allium Cepa Exerts Neuroprotective Effect on Retinal Ganglion Cells of Pterygopalatine Artery (PPA) Ligated Mice. J. Ayurveda Integr. Med. 2020, 11, 489–494. [Google Scholar] [CrossRef]
- Lee, Y.M.; Lee, Y.R.; Kim, C.S.; Jo, K.; Sohn, E.; Kim, J.S.; Kim, J. Cnidium Officinale Extract and Butylidenephthalide Inhibits Retinal Neovascularization in Vitro and in Vivo. BMC Complement. Altern. Med. 2016, 16, 231. [Google Scholar] [CrossRef]
- Ikonne, E.U.; Ikpeazu, V.O.; Ugbogu, E.A. Corrigendum to “The Potential Health Benefits of Dietary Natural Plant Products in Age Related Eye Diseases” [Heliyon 6 (7) (2020) E04408]. Heliyon 2021, 7, e07069. [Google Scholar] [CrossRef]
- Zheng, L.; Howell, S.J.; Hatala, D.A.; Huang, K.; Kern, T.S. Salicylate-Based Anti-Inflammatory Drugs Inhibit the Early Lesion of Diabetic Retinopathy. Diabetes 2007, 56, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Al-Zamil, W.M.; Yassin, S.A. Recent Developments in Age-Related Macular Degeneration: A Review. Clin. Interv. Aging 2017, 12, 1313. [Google Scholar] [CrossRef]
- Duh, E.J.; Sun, J.K.; Stitt, A.W. Diabetic Retinopathy: Current Understanding, Mechanisms, and Treatment Strategies. JCI Insight 2017, 2, e93751. [Google Scholar] [CrossRef] [PubMed]
- Iu, L.P.L.; Kwok, A.K.H. An Update of Treatment Options for Neovascular Age-Related Macular Degeneration. Hong Kong Med. J. 2007, 13, 460–470. [Google Scholar]
- Adkins, J.C.; Balfour, J.A. Brimonidine A Review of Its Pharmacological Properties and Clinical Potential in the Management of Open-Angle Glaucoma and Ocular Hypertension. Drugs Aging 1998, 12, 225–241. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.M.; Knuckles, M.; Minni, J.P.; Johnson, S.M.; Belasco, K.T. The Role of Brimonidine Tartrate Gel in the Treatment of Rosacea. Clin. Cosmet. Investig. Dermatol. 2015, 8, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, L.A.; Lai, R.; Woldemussie, E. From the Lab to the Clinic: Activation of an Alpha-2 Agonist Pathway Is Neuroprotective in Models of Retinal and Optic Nerve Injury. Eur. J. Ophthalmol. 1999, 9 (Suppl. 1), S17–S21. [Google Scholar] [CrossRef] [PubMed]
- Dugel, P.U.; Jaffe, G.J.; Sallstig, P.; Warburton, J.; Weichselberger, A.; Wieland, M.; Singerman, L. Brolucizumab Versus Aflibercept in Participants with Neovascular Age-Related Macular Degeneration: A Randomized Trial. Ophthalmology 2017, 124, 1296–1304. [Google Scholar] [CrossRef]
- Motevasseli, T.; Mohammadi, S.; Abdi, F.; Freeman, W.R. Side Effects of Brolucizumab. J. Ophthalmic Vis. Res. 2021, 16, 670–675. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, Y.; Xie, J.; Li, D.; Hu, Q.; Li, X.; Zheng, W.; He, R. Conbercept for Patients with Age-Related Macular Degeneration: A Systematic Review. BMC Ophthalmol. 2018, 18, 142. [Google Scholar] [CrossRef]
- Theodossiadis, P.G.; Liarakos, V.S.; Sfikakis, P.P.; Vergados, I.A.; Theodossiadis, G.P. Intravitreal Administration of the Anti-Tumor Necrosis Factor Agent Infliximab for Neovascular Age-Related Macular Degeneration. Am. J. Ophthalmol. 2009, 147, 825–830.e1. [Google Scholar] [CrossRef]
- Vavvas, D.; D’Amico, D.J. Pegaptanib (Macugen): Treating Neovascular Age-Related Macular Degeneration and Current Role in Clinical Practice. Ophthalmol. Clin. N. Am. 2006, 19, 353–360. [Google Scholar]
- Gaudreault, J.; Fei, D.; Beyer, J.C.; Ryan, A.; Rangell, L.; Shiu, V.; Damico, L.A. Pharmacokinetics and Retinal Distribution of Ranibizumab, a Humanized Antibody Fragment Directed against VEGF-A, Following Intravitreal Administration in Rabbits. Retina 2007, 27, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- Kourlas, H.; Abrams, P. Ranibizumab for the Treatment of Neovascular Age-Related Macular Degeneration: A Review. Clin. Ther. 2007, 29, 1850–1861. [Google Scholar] [CrossRef]
- Hasan, N.; Chawla, R.; Shaikh, N.; Kandasamy, S.; Azad, S.V.; Sundar, M.D. A Comprehensive Review of Intravitreal Immunosuppressants andbiologicals Used in Ophthalmology. Ther. Adv. Ophthalmol. 2022, 14, 251584142210974. [Google Scholar] [CrossRef]
- Kaszuba-Bartkowiak, K.; Nowak, S.; Jurowski, P. The Role of Trimetazidine in the Protection of the Retina. Arch. Med. Sci. 2007, 3, S65–S66. [Google Scholar]
- Schmidt, K.-G.; Bergert, H.; Funk, R. Neurodegenerative Diseases of the Retina and Potential for Protection and Recovery. Curr. Neuropharmacol. 2008, 6, 164–178. [Google Scholar] [CrossRef] [PubMed]
- Hernández, C.; García-Ramírez, M.; Corraliza, L.; Fernández-Carneado, J.; Farrera-Sinfreu, J.; Ponsati, B.; González-Rodríguez, Á.; Valverde, Á.M.; Simó, R. Topical Administration of Somatostatin Prevents Retinal Neurodegeneration in Experimental Diabetes. Diabetes 2013, 62, 2569–2578. [Google Scholar] [CrossRef]
- Pöstyéni, E.; Kovács-Valasek, A.; Dénes, V.; Mester, A.; Sétáló, G.; Gábriel, R. PACAP for Retinal Health: Model for Cellular Aging and Rescue. Int. J. Mol. Sci. 2021, 22, 444. [Google Scholar] [CrossRef]
- Vaudry, D.; Falluel-Morel, A.; Bourgault, S.; Basille, M.; Burel, D.; Wurtz, O.; Fournier, A.; Chow, B.K.C.C.; Hashimoto, H.; Galas, L.; et al. Pituitary Adenylate Cyclase-Activating Polypeptide and Its Receptors: 20 Years after the Discovery. Pharmacol. Rev. 2009, 61, 283–357. [Google Scholar] [CrossRef]
- Szabadfi, K.; Atlasz, T.; Kiss, P.; Reglodi, D.; Szabo, A.; Kovacs, K.; Szalontai, B.; Setalo, G.; Banki, E.; Csanaky, K.; et al. Protective Effects of the Neuropeptide PACAP in Diabetic Retinopathy. Cell Tissue Res. 2012, 348, 37–46. [Google Scholar] [CrossRef]
- Kovacs, A.K.; Atlasz, T.; Werling, D.; Szabo, E.; Reglodi, D.; Toth, G.K. Stability Test of PACAP in Eye Drops. J. Mol. Neurosci. 2021, 71, 1567–1574. [Google Scholar] [CrossRef] [PubMed]
- Szabo, E.; Patko, E.; Vaczy, A.; Molitor, D.; Csutak, A.; Toth, G.; Reglodi, D.; Atlasz, T. Retinoprotective Effects of PACAP Eye Drops in Microbead-Induced Glaucoma Model in Rats. Int. J. Mol. Sci. 2021, 22, 8825. [Google Scholar] [CrossRef] [PubMed]
- IDF Diabetes Atlas | Tenth Edition. Available online: https://diabetesatlas.org/ (accessed on 27 January 2023).
- Yau, J.W.Y.; Rogers, S.L.; Kawasaki, R.; Lamoureux, E.L.; Kowalski, J.W.; Bek, T.; Chen, S.J.; Dekker, J.M.; Fletcher, A.; Grauslund, J.; et al. Global Prevalence and Major Risk Factors of Diabetic Retinopathy. Diabetes Care 2012, 35, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Holekamp, N.M. Review of Neovascular Age-Related Macular Degeneration Treatment Options. Am. J. Manag. Care 2019, 25, S172–S181. [Google Scholar]
- Nian, S.; Lo, A.C.Y.; Mi, Y.; Ren, K.; Yang, D. Neurovascular Unit in Diabetic Retinopathy: Pathophysiological Roles and Potential Therapeutical Targets. Eye Vis. 2021, 8, 15. [Google Scholar] [CrossRef]
- Wilkinson-Berka, J.L. Angiotensin and Diabetic Retinopathy. Int. J. Biochem. Cell Biol. 2006, 38, 752–765. [Google Scholar] [CrossRef]
- Musat, O.; Cernat, C.; Labib, M.; Gheorghe, A.; Toma, O.; Zamfir, M.; Boureanu, A.M. DIABETIC MACULAR EDEMA. Rom. J. Ophthalmol. 2015, 59, 133. [Google Scholar] [CrossRef]
- Browning, D.J.; Stewart, M.W.; Lee, C. Diabetic Macular Edema: Evidence-Based Management. Indian J. Ophthalmol. 2018, 66, 1736. [Google Scholar] [CrossRef]
- Meyer, C.H. Current Treatment Approaches in Diabetic Macular Edema. Ophthalmologica. 2007, 221, 118–131. [Google Scholar] [CrossRef]
- Chauhan, M.Z.; Rather, P.A.; Samarah, S.M.; Elhusseiny, A.M.; Sallam, A.B. Current and Novel Therapeutic Approaches for Treatment of Diabetic Macular Edema. Cells 2022, 11, 1950. [Google Scholar] [CrossRef]
- Chung, Y.R.; Kim, Y.H.; Ha, S.J.; Byeon, H.E.; Cho, C.H.; Kim, J.H.; Lee, K. Role of Inflammation in Classification of Diabetic Macular Edema by Optical Coherence Tomography. J. Diabetes Res. 2019, 2019, 8164250. [Google Scholar] [CrossRef] [PubMed]
- Otani, T.; Kishi, S.; Maruyama, Y. Patterns of Diabetic Macular Edema with Optical Coherence Tomography. Am. J. Ophthalmol. 1999, 127, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, M.; Wickremasinghe, S.; Osborne, A.; Van Wijngaarden, P.; Martin, K.R. Diabetic Retinopathy: A Complex Pathophysiology Requiring Novel Therapeutic Strategies. Expert Opin. Biol. Ther. 2018, 18, 1257–1270. [Google Scholar] [CrossRef] [PubMed]
- Tarr, J.M.; Kaul, K.; Chopra, M.; Kohner, E.M.; Chibber, R. Pathophysiology of Diabetic Retinopathy. ISRN Ophthalmol. 2013, 2013, 343560. [Google Scholar] [CrossRef]
- Adornetto, A.; Gesualdo, C.; Laganà, M.L.; Trotta, M.C.; Rossi, S.; Russo, R. Autophagy: A Novel Pharmacological Target in Diabetic Retinopathy. Front. Pharmacol. 2021, 12. [Google Scholar] [CrossRef]
- Chang, Y.C.; Chuang, L.M. The Role of Oxidative Stress in the Pathogenesis of Type 2 Diabetes: From Molecular Mechanism to Clinical Implication. Am. J. Transl. Res. 2010, 2, 316–331. [Google Scholar]
- Cai, X.; Li, J.; Wang, M.; She, M.; Tang, Y.; Li, J.; Li, H.; Hui, H. GLP-1 Treatment Improves Diabetic Retinopathy by Alleviating Autophagy through GLP-1R-ERK1/2-HDAC6 Signaling Pathway. Int. J. Med. Sci. 2017, 14, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
- Kang, Q.; Yang, C. Oxidative Stress and Diabetic Retinopathy: Molecular Mechanisms, Pathogenetic Role and Therapeutic Implications. Redox Biol. 2020, 37, 101799. [Google Scholar] [CrossRef]
- Gong, Q.; Wang, H.; Yu, P.; Qian, T.; Xu, X. Protective or Harmful: The Dual Roles of Autophagy in Diabetic Retinopathy. Front. Med. 2021, 8, 644121. [Google Scholar] [CrossRef]
- Volpe, C.M.O.; Villar-Delfino, P.H.; Dos Anjos, P.M.F.; Nogueira-Machado, J.A. Cellular Death, Reactive Oxygen Species (ROS) and Diabetic Complications. Cell Death Dis. 2018, 9, 119. [Google Scholar] [CrossRef]
- Fu, D.; Yu, J.Y.; Yang, S.; Wu, M.; Hammad, S.M.; Connell, A.R.; Du, M.; Chen, J.; Lyons, T.J. Survival or Death: A Dual Role for Autophagy in Stress-Induced Pericyte Loss in Diabetic Retinopathy. Diabetologia 2016, 59, 2251–2261. [Google Scholar] [CrossRef]
- Dehdashtian, E.; Mehrzadi, S.; Yousefi, B.; Hosseinzadeh, A.; Reiter, R.J.; Safa, M.; Ghaznavi, H.; Naseripour, M. Diabetic Retinopathy Pathogenesis and the Ameliorating Effects of Melatonin; Involvement of Autophagy, Inflammation and Oxidative Stress. Life Sci. 2018, 193, 20–33. [Google Scholar] [CrossRef]
- De Faria, J.M.L.; Duarte, D.A.; Montemurro, C.; Papadimitriou, A.; Consonni, S.R.; De Faria, J.B.L. Defective Autophagy in Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2016, 57, 4356–4366. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zhang, Z.; Wang, X.; Li, R.; Hou, W.; Bi, W.; Zhang, X. Inhibition of Autophagy Induces IL-1β Release from ARPE-19 Cells via ROS Mediated NLRP3 Inflammasome Activation under High Glucose Stress. Biochem. Biophys. Res. Commun. 2015, 463, 1071–1076. [Google Scholar] [CrossRef]
- Piano, I.; Novelli, E.; Della Santina, L.; Strettoi, E.; Cervetto, L.; Gargini, C. Involvement of Autophagic Pathway in the Progression of Retinal Degeneration in a Mouse Model of Diabetes. Front. Cell. Neurosci. 2016, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Madrakhimov, S.B.; Yang, J.Y.; Kim, J.H.; Han, J.W.; Park, T.K. MTOR-Dependent Dysregulation of Autophagy Contributes to the Retinal Ganglion Cell Loss in Streptozotocin-Induced Diabetic Retinopathy. Cell Commun. Signal. 2021, 19, 1–16. [Google Scholar] [CrossRef]
- Park, H.Y.L.; Kim, J.H.; Park, C.K. Different Contributions of Autophagy to Retinal Ganglion Cell Death in the Diabetic and Glaucomatous Retinas. Sci. Rep. 2018, 8, 13321. [Google Scholar] [CrossRef]
- Kreutzberg, G.W. Microglia: A Sensor for Pathological Events in the CNS. Trends Neurosci. 1996, 19, 312–318. [Google Scholar] [CrossRef]
- Tambuyzer, B.R.; Ponsaerts, P.; Nouwen, E.J. Microglia: Gatekeepers of Central Nervous System Immunology. J. Leukoc. Biol. 2009, 85, 352–370. [Google Scholar] [CrossRef]
- Dick, A.D.; Carter, D.; Robertson, M.; Broderick, C.; Hughes, E.; Forrester, J.V.; Liversidge, J. Control of Myeloid Activity during Retinal Inflammation. J. Leukoc. Biol. 2003, 74, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Karlstetter, M.; Ebert, S.; Langmann, T. Microglia in the Healthy and Degenerating Retina: Insights from Novel Mouse Models. Immunobiology 2010, 215, 685–691. [Google Scholar] [CrossRef]
- Esposito, K.; Nappo, F.; Marfella, R.; Giugliano, G.; Giugliano, F.; Ciotola, M.; Quagliaro, L.; Ceriello, A.; Giugliano, D. Inflammatory Cytokine Concentrations Are Acutely Increased by Hyperglycemia in Humans: Role of Oxidative Stress. Circulation 2002, 106, 2067–2072. [Google Scholar] [CrossRef]
- Semeraro, F.; Cancarini, A.; Dell’Omo, R.; Rezzola, S.; Romano, M.R.; Costagliola, C. Diabetic Retinopathy: Vascular and Inflammatory Disease. J. Diabetes Res. 2015, 2015, 582060. [Google Scholar] [CrossRef]
- Yang, L.P.; Sun, H.L.; Wu, L.M.; Guo, X.J.; Dou, H.L.; Tso, M.O.M.; Zhao, L.; Li, S.M. Baicalein Reduces Inflammatory Process in a Rodent Model of Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2319–2327. [Google Scholar] [CrossRef]
- Tang, J.; Kern, T.S. Inflammation in Diabetic Retinopathy. Prog. Retin. Eye Res. 2011, 30, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Rangasamy, S.; McGuire, P.G.; Das, A. Diabetic Retinopathy and Inflammation: Novel Therapeutic Targets. Middle East Afr. J. Ophthalmol. 2012, 19, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Krady, J.K.; Basu, A.; Allen, C.M.; Xu, Y.; LaNoue, K.F.; Gardner, T.W.; Levison, S.W. Minocycline Reduces Proinflammatory Cytokine Expression, Microglial Activation, and Caspase-3 Activation in a Rodent Model of Diabetic Retinopathy. Diabetes 2005, 54, 1559–1565. [Google Scholar] [CrossRef] [PubMed]
- Khalfaoui, T.; Lizard, G.; Ouertani-Meddeb, A. Adhesion Molecules (ICAM-1 and VCAM-1) and Diabetic Retinopathy in Type 2 Diabetes. J. Mol. Histol. 2008, 39, 243–249. [Google Scholar] [CrossRef]
- Joussen, A.M.; Poulaki, V.; Le, M.L.; Koizumi, K.; Esser, C.; Janicki, H.; Schraermeyer, U.; Kociok, N.; Fauser, S.; Kirchhof, B.; et al. A Central Role for Inflammation in the Pathogenesis of Diabetic Retinopathy. FASEB J. 2004, 18, 1450–1452. [Google Scholar] [CrossRef]
- Grant, M.B.; Afzal, A.; Spoerri, P.; Pan, H.; Shaw, L.C.; Mames, R.N. The Role of Growth Factors in the Pathogenesis of Diabetic Retinopathy. Expert Opin. Investig. Drugs 2004, 13, 1275–1293. [Google Scholar] [CrossRef]
- Rangasamy, S.; McGuire, P.G.; Nitta, C.F.; Monickaraj, F.; Oruganti, S.R.; Das, A. Chemokine Mediated Monocyte Trafficking into the Retina: Role of Inflammation in Alteration of the Blood-Retinal Barrier in Diabetic Retinopathy. PLoS ONE 2014, 9, e108508. [Google Scholar] [CrossRef]
- Maier, R.; Weger, M.; Haller-Schober, E.M.; El-Shabrawi, Y.; Wedrich, A.; Theisl, A.; Aigner, R.; Barth, A.; Haas, A. Multiplex Bead Analysis of Vitreous and Serum Concentrations of Inflammatory and Proangiogenic Factors in Diabetic Patients. Mol. Vis. 2008, 14, 637–643. [Google Scholar]
- QY, G.; GY, H.; SQ, Y.; TW, Q.; X, X. Comprehensive Assessment of Growth Factors, Inflammatory Mediators, and Cytokines in Vitreous from Patients with Proliferative Diabetic Retinopathy. Int. J. Ophthalmol. 2022, 15, 1736–1742. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, H.; Gong, Y.; Wei, S.; Zhang, M. Early Spatiotemporal Characterization of Microglial Activation in the Retinas of Rats with Streptozotocin-Induced Diabetes. Graefes Arch. Clin. Exp. Ophthalmol. 2015, 253, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.X.; Ng, Y.K.; Ling, E.A. Neuronal and Microglial Response in the Retina of Streptozotocin-Induced Diabetic Rats. Vis. Neurosci. 2000, 17, 463–471. [Google Scholar] [CrossRef]
- Ke, M.; Hu, X.Q.; Ouyang, J.; Dai, B.; Xu, Y. The Effect of Astragalin on the VEGF Production of Cultured Müller Cells under High Glucose Conditions. Biomed. Mater. Eng. 2012, 22, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Dean, C.; Dunning, F.M.; Liu, H.; Bomba-Warczak, E.; Martens, H.; Bharat, V.; Ahmed, S.; Chapman, E.R. Axonal and Dendritic Synaptotagmin Isoforms Revealed by a PHluorin-Syt Functional Screen. Mol. Biol. Cell 2012, 23, 1715–1727. [Google Scholar] [CrossRef]
- Liu, Y.; Leo, L.F.; McGregor, C.; Grivitishvili, A.; Barnstable, C.J.; Tombran-Tink, J. Pigment Epithelium-Derived Factor (PEDF) Peptide Eye Drops Reduce Inflammation, Cell Death and Vascular Leakage in Diabetic Retinopathy in Ins2(Akita) Mice. Mol. Med. 2012, 18, 1387–1401. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, E.; Elliott, M.H.; Zhu, M.; Le, Y.Z. Müller Cell-Derived VEGF Is Essential for Diabetes-Induced Retinal Inflammation and Vascular Leakage. Diabetes 2010, 59, 2297–2305. [Google Scholar] [CrossRef]
- Zong, H.; Ward, M.; Madden, A.; Yong, P.H.; Limb, G.A.; Curtis, T.M.; Stitt, A.W. Hyperglycaemia-Induced pro-Inflammatory Responses by Retinal Müller Glia Are Regulated by the Receptor for Advanced Glycation End-Products (RAGE). Diabetologia 2010, 53, 2656–2666. [Google Scholar] [CrossRef]
- Coughlin, B.A.; Trombley, B.T.; Mohr, S. Interleukin-6 (IL-6) Mediates Protection against Glucose Toxicity in Human Müller Cells via Activation of VEGF-A Signaling. Biochem. Biophys. Res. Commun. 2019, 517, 227–232. [Google Scholar] [CrossRef]
- Huang, H.C.; Wang, H.R.; Hsieh, L.M. Antiproliferative Effect of Baicalein, a Flavonoid from a Chinese Herb, on Vascular Smooth Muscle Cell. Eur. J. Pharmacol. 1994, 251, 91–93. [Google Scholar] [CrossRef]
- Bucolo, C.; Leggio, G.M.; Drago, F.; Salomone, S. Eriodictyol Prevents Early Retinal and Plasma Abnormalities in Streptozotocin-Induced Diabetic Rats. Biochem. Pharmacol. 2012, 84, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Silva, K.C.; Rosales, M.A.B.; Hamassaki, D.E.; Saito, K.C.; Faria, A.M.; Ribeiro, P.A.O.; Lopes de Faria, J.B.; Lopes de Faria, J.M. Green Tea Is Neuroprotective in Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1325–1336. [Google Scholar] [CrossRef]
- Kumar, B.; Gupta, S.K.; Nag, T.C.; Srivastava, S.; Saxena, R. Green Tea Prevents Hyperglycemia-Induced Retinal Oxidative Stress and Inflammation in Streptozotocin-Induced Diabetic Rats. Ophthalmic Res. 2012, 47, 103–108. [Google Scholar] [CrossRef]
- Dos Santos, M.D.; Almeida, M.C.; Lopes, N.P.; De Souza, G.E.P. Evaluation of the Anti-Inflammatory, Analgesic and Antipyretic Activities of the Natural Polyphenol Chlorogenic Acid. Biol. Pharm. Bull. 2006, 29, 2236–2240. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Yu, H.G.; Sohn, J. The Anti-Angiogenic Effect of Chlorogenic Acid on Choroidal Neovascularization. Korean J. Ophthalmol. 2010, 24, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.Y.; Sohn, J.; Park, K.H. Chlorogenic Acid Decreases Retinal Vascular Hyperpermeability in Diabetic Rat Model. J. Korean Med. Sci. 2013, 28, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.S.; Naqvi, S.; Gupta, S.K.; Srivastava, S. Prevention and Management of Diabetic Retinopathy in STZ Diabetic Rats by Tinospora Cordifolia and Its Molecular Mechanisms. Food Chem. Toxicol. 2012, 50, 3126–3132. [Google Scholar] [CrossRef]
- Rathi, S.S.; Grover, J.K.; Vikrant, V.; Biswas, N.R. Prevention of Experimental Diabetic Cataract by Indian Ayurvedic Plant Extracts. Phytother. Res. 2002, 16, 774–777. [Google Scholar] [CrossRef]
- Gupta, S.K.; Kumar, B.; Nag, T.C.; Agrawal, S.S.; Agrawal, R.; Agrawal, P.; Saxena, R.; Srivastava, S. Curcumin Prevents Experimental Diabetic Retinopathy in Rats through Its Hypoglycemic, Antioxidant, and Anti-Inflammatory Mechanisms. J. Ocul. Pharmacol. Ther. 2011, 27, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.F.; Zhang, Q.; Liu, X.Z. Protective Effects of Curcumin on Retinal Müller Cell in Early Diabetic Rats. Int. J. Ophthalmol. 2013, 6, 422–424. [Google Scholar] [PubMed]
- Ilieva, I.; Ohgami, K.; Shiratori, K.; Koyama, Y.; Yoshida, K.; Kase, S.; Kitamei, H.; Takemoto, Y.; Yazawa, K.; Ohno, S. The Effects of Ginkgo Biloba Extract on Lipopolysaccharide-Induced Inflammation in Vitro and in Vivo. Exp. Eye Res. 2004, 79, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A.; Menon, B.; Gierhart, D.L. Beneficial Effect of Zeaxanthin on Retinal Metabolic Abnormalities in Diabetic Rats. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1645–1651. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Ozawa, Y.; Kurihara, T.; Kubota, S.; Yuki, K.; Noda, K.; Kobayashi, S.; Ishida, S.; Tsubota, K. Neurodegenerative Influence of Oxidative Stress in the Retina of a Murine Model of Diabetes. Diabetologia 2010, 53, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.Y.; Zhou, S.W. Effect of Berberine on PPARalpha/Delta/Gamma Expression in Type 2 Diabetic Rat Retinae. Yao Xue Xue Bao 2007, 42, 1243–1249. [Google Scholar]
- Wang, C.Z.; Aung, H.H.; Zhang, B.; Sun, S.; Li, X.L.; He, H.; Xie, J.T.; He, T.C.; Du, W.; Yuan, C.S. Chemopreventive Effects of Heat-Processed Panax Quinquefolius Root on Human Breast Cancer Cells. Anticancer Res. 2008, 28, 2545–2551. [Google Scholar]
- Kim, S.H.; Park, K.S. Effects of Panax Ginseng Extract on Lipid Metabolism in Humans. Pharmacol. Res. 2003, 48, 511–513. [Google Scholar] [CrossRef]
- Pietrucha-Dutczak, M.; Amadio, M.; Govoni, S.; Lewin-Kowalik, J.; Smedowski, A. The Role of Endogenous Neuroprotective Mechanisms in the Prevention of Retinal Ganglion Cells Degeneration. Front. Neurosci. 2018, 12, 834. [Google Scholar] [CrossRef]
- Lei, X.; Zhang, J.; Shen, J.; Hu, L.M.; Wu, Y.; Mou, L.; Xu, G.; Li, W.; Xu, G.T. EPO Attenuates Inflammatory Cytokines by Muller Cells in Diabetic Retinopathy. Front. Biosci. 2011, 3, 201–211. [Google Scholar] [CrossRef]
- Wang, P.; Xia, F. EPO Protects Müller Cell under High Glucose State through BDNF/TrkB Pathway. Int. J. Clin. Exp. Pathol. 2015, 8, 8083–8090. [Google Scholar]
- Tsai, J.C.; Wu, L.; Worgul, B.; Forbes, M.; Cao, J. Intravitreal Administration of Erythropoietin and Preservation of Retinal Ganglion Cells in an Experimental Rat Model of Glaucoma. Curr. Eye Res. 2005, 30, 1025–1031. [Google Scholar] [CrossRef]
- Garcia-Ramírez, M.; Hernández, C.; Ruiz-Meana, M.; Villarroel, M.; Corraliza, L.; García-Dorado, D.; Simó, R. Erythropoietin Protects Retinal Pigment Epithelial Cells against the Increase of Permeability Induced by Diabetic Conditions: Essential Role of JAK2/ PI3K Signaling. Cell. Signal. 2011, 23, 1596–1602. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Duncan, D.S.; Echevarria, F.; McLaughlin, W.M.; Hatcher, J.B.; Sappington, R.M. Pressure-Induced Alterations in PEDF and PEDF-R Expression: Implications for Neuroprotective Signaling in Glaucoma. J. Clin. Exp. Ophthalmol. 2015, 6, 491. [Google Scholar] [CrossRef]
- Sterling, J.K.; Adetunji, M.O.; Guttha, S.; Bargoud, A.R.; Uyhazi, K.E.; Ross, A.G.; Dunaief, J.L.; Cui, Q.N. GLP-1 Receptor Agonist NLY01 Reduces Retinal Inflammation and Neuron Death Secondary to Ocular Hypertension. Cell Rep. 2020, 33, 108271. [Google Scholar] [CrossRef]
- Hernández, C.; Dal Monte, M.; Simó, R.; Casini, G. Neuroprotection as a Therapeutic Target for Diabetic Retinopathy. J. Diabetes Res. 2016, 2016, 9508541. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, M.; Song, B.W.; Lu, B.; Hu, P. Expression of Ciliary Neurotrophic Factor after Induction of Ocular Hypertension in the Retina of Rats. Chin. Med. J. 2007, 120, 1825–1829. [Google Scholar] [CrossRef] [PubMed]
- Pease, M.E.; Zack, D.J.; Berlinicke, C.; Bloom, K.; Cone, F.; Wang, Y.; Klein, R.L.; Hauswirth, W.W.; Quigley, H.A. Effect of CNTF on Retinal Ganglion Cell Survival in Experimental Glaucoma. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2194–2200. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.Z.; Elyaman, W.; Yip, H.K.; Lee, V.W.H.; Yick, L.W.; Hugon, J.; So, K.F. CNTF Promotes Survival of Retinal Ganglion Cells after Induction of Ocular Hypertension in Rats: The Possible Involvement of STAT3 Pathway. Eur. J. Neurosci. 2004, 19, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Seki, M.; Nawa, H.; Fukuchi, T.; Abe, H.; Takei, N. BDNF Is Upregulated by Postnatal Development and Visual Experience: Quantitative and Immunohistochemical Analyses of BDNF in the Rat Retina. Investig. Ophthalmol. Vis. Sci. 2003, 44, 3211–3218. [Google Scholar] [CrossRef]
- Feng, L.; Chen, H.; Yi, J.; Troy, J.B.; Zhang, H.F.; Liu, X. Long-Term Protection of Retinal Ganglion Cells and Visual Function by Brain-Derived Neurotrophic Factor in Mice With Ocular Hypertension. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3793–3802. [Google Scholar] [CrossRef] [PubMed]
- Trotta, M.C.; Gesualdo, C.; Herman, H.; Gharbia, S.; Balta, C.; Lepre, C.C.; Russo, M.; Itro, A.; D’Amico, G.; Peluso, L.; et al. Systemic Beta-Hydroxybutyrate Affects BDNF and Autophagy into the Retina of Diabetic Mice. Int. J. Mol. Sci. 2022, 23, 10184. [Google Scholar] [CrossRef]
- Telegina, D.V.; Kolosova, N.G.; Kozhevnikova, O.S. Immunohistochemical Localization of NGF, BDNF, and Their Receptors in a Normal and AMD-like Rat Retina. BMC Med. Genom. 2019, 12, 48. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.X.; Zhao, G.L.; Hu, X.; Zhou, H.; Li, S.Y.; Li, F.; Miao, Y.; Lei, B.; Wang, Z. P2X7/P2X4 Receptors Mediate Proliferation and Migration of Retinal Microglia in Experimental Glaucoma in Mice. Neurosci. Bull. 2022, 38, 901–915. [Google Scholar] [CrossRef]
- Amato, R.; Catalani, E.; Dal Monte, M.; Cammalleri, M.; Di Renzo, I.; Perrotta, C.; Cervia, D.; Casini, G. Autophagy-Mediated Neuroprotection Induced by Octreotide in an Ex Vivo Model of Early Diabetic Retinopathy. Pharmacol. Res. 2018, 128, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Medina, J.J.; Rubio-Velazquez, E.; Foulquie-Moreno, E.; Casaroli-Marano, R.P.; Pinazo-Duran, M.D.; Zanon-Moreno, V.; Del-Rio-vellosillo, M. Update on the Effects of Antioxidants on Diabetic Retinopathy: In Vitro Experiments, Animal Studies and Clinical Trials. Antioxidants 2020, 9, 561. [Google Scholar] [CrossRef]
- Evans, Z.P.; Ellett, J.D.; Fariss, M.W.; Schnellmann, R.G.; Schmidt, M.G.; Chavin, K. Vitamin E Succinate Reduces Ischemia/Reperfusion Injury in Steatotic Livers. Transplant. Proc. 2008, 40, 3327–3329. [Google Scholar] [CrossRef]
- Neha, K.; Haider, M.R.; Pathak, A.; Yar, M.S. Medicinal Prospects of Antioxidants: A Review. Eur. J. Med. Chem. 2019, 178, 687–704. [Google Scholar] [CrossRef] [PubMed]
- Young, I.S.; Woodside, J.V. Antioxidants in Health and Disease. J. Clin. Pathol. 2001, 54, 176–186. [Google Scholar] [CrossRef]
- Takiishi, T.; Gysemans, C.; Bouillon, R.; Mathieu, C. Vitamin D and Diabetes. Endocrinol. Metab. Clin. N. Am. 2010, 39, 419–446. [Google Scholar] [CrossRef]
- Albert, D.M.; Scheef, E.A.; Wang, S.; Mehraein, F.; Darjatmoko, S.R.; Sorenson, C.M.; Sheibani, N. Calcitriol Is a Potent Inhibitor of Retinal Neovascularization. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2327–2334. [Google Scholar] [CrossRef]
- Ren, Z.; Li, W.; Zhao, Q.; Ma, L.; Zhu, J. The Impact of 1,25-Dihydroxy Vitamin D3 on the Expressions of Vascular Endothelial Growth Factor and Transforming Growth Factor-Β₁ in the Retinas of Rats with Diabetes. Diabetes Res. Clin. Pract. 2012, 98, 474–480. [Google Scholar] [CrossRef]
- Del Valle, L.G.; Noblet, M.C.; Martinez-Sanchez, G. Crosstalk between Oxidative Stress and Ocular Diseases. J. Clin. Res. Ophthalmol. 2020, 7, 037–047. [Google Scholar] [CrossRef]
- Ozawa, Y. Oxidative Stress in the Light-Exposed Retina and Its Implication in Age-Related Macular Degeneration. Redox Biol. 2020, 37, 101779. [Google Scholar] [CrossRef]
- Goyal, A.; Srivastava, A.; Sihota, R.; Kaur, J. Evaluation of Oxidative Stress Markers in Aqueous Humor of Primary Open Angle Glaucoma and Primary Angle Closure Glaucoma Patients. Curr. Eye Res. 2014, 39, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Gurler, B.; Vural, H.; Yilmaz, N.; Oguz, H.; Satici, A.; Aksoy, N. The Role of Oxidative Stress in Diabetic Retinopathy. Eye 2000, 14 Pt 5, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Terao, R.; Ahmed, T.; Suzumura, A.; Terasaki, H. Oxidative Stress-Induced Cellular Senescence in Aging Retina and Age-Related Macular Degeneration. Antioxidants 2022, 11, 2189. [Google Scholar] [CrossRef]
- Gherghel, D.; Mroczkowska, S.; Qin, L. Reduction in Blood Glutathione Levels Occurs Similarly in Patients with Primary-Open Angle or Normal Tension Glaucoma. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3333–3339. [Google Scholar] [CrossRef]
- Al-Dosari, D.I.; Ahmed, M.M.; Al-Rejaie, S.S.; Alhomida, A.S.; Ola, M.S. Flavonoid Naringenin Attenuates Oxidative Stress, Apoptosis and Improves Neurotrophic Effects in the Diabetic Rat Retina. Nutrients 2017, 9, 1161. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, E.S.M.; Abo-Salem, O.M.; Aly, H.A.; Mansour, A.M. Potential Antidiabetic and Hypolipidemic Effects of Propolis Extract in Streptozotocin-Induced Diabetic Rats. Pak. J. Pharm. Sci. 2009, 22, 168–174. [Google Scholar]
- Liles, M.R.; Newsome, D.A.; Oliver, P.D. Antioxidant Enzymes in the Aging Human Retinal Pigment Epithelium. Arch. Ophthalmol. 1991, 109, 1285–1288. [Google Scholar] [CrossRef]
- Moreno, M.C.; Campanelli, J.; Sande, P.; Sáenz, D.A.; Keller Sarmiento, M.I.; Rosenstein, R.E. Retinal Oxidative Stress Induced by High Intraocular Pressure. Free Radic. Biol. Med. 2004, 37, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, G.; Tolun, F.I.; Gul, M.; Imrek, S. Retinal Oxidative Stress Induced by Intraocular Hypertension in Rats May Be Ameliorated by Brimonidine Treatment and N-Acetyl Cysteine Supplementation. J. Glaucoma 2009, 18, 662–665. [Google Scholar] [CrossRef]
- Ramos, H.; Bogdanov, P.; Huerta, J.; Deàs-Just, A.; Hernández, C.; Simó, R. Antioxidant Effects of DPP-4 Inhibitors in Early Stages of Experimental Diabetic Retinopathy. Antioxidants 2022, 11, 1418. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, M.; Oh, S.B.; Kim, H.Y.; Kim, C.; Kim, T.Y.; Park, Y.H. Superoxide Dismutase 3 Prevents Early Stage Diabetic Retinopathy in Streptozotocin-Induced Diabetic Rat Model. PLoS ONE 2022, 17, e0262396. [Google Scholar] [CrossRef] [PubMed]
- Ferreira de Melo, I.M.; Martins Ferreira, C.G.; Lima da Silva Souza, E.H.; Almeida, L.L.; Bezerra de Sá, F.; Cavalcanti Lapa Neto, C.J.; Paz de Castro, M.V.; Teixeira, V.W.; Coelho Teixeira, Á.A. Melatonin Regulates the Expression of Inflammatory Cytokines, VEGF and Apoptosis in Diabetic Retinopathy in Rats. Chem. Biol. Interact. 2020, 327, 109183. [Google Scholar] [CrossRef] [PubMed]
- Ohia, S.E.; Bagchi, M.; Stohs, S.J. Age-Related Oxidative Damage in Long-Evans Rat Retina. Res. Commun. Mol. Pathol. Pharmacol. 1994, 85, 21–31. [Google Scholar]
- Dutta, R.K.; Lee, J.N.; Maharjan, Y.; Park, C.; Choe, S.K.; Ho, Y.S.; Kwon, H.M.; Park, R. Catalase-Deficient Mice Induce Aging Faster through Lysosomal Dysfunction. Cell. Commun. Signal. 2022, 20, 192. [Google Scholar] [CrossRef] [PubMed]
- Lopez, P.; Wagner, K.-D.; Hofman, P.; Van Obberghen, E. RNA Activation of the Vascular Endothelial Growth Factor Gene (VEGF) Promoter by Double-Stranded RNA and Hypoxia: Role of Noncoding VEGF Promoter Transcripts. Mol. Cell. Biol. 2016, 36, 1480–1493. [Google Scholar] [CrossRef]
- Koc, F.; Kansu, T.; Kavuncu, S.; Firat, E. Topical Apraclonidine Testing Discloses Pupillary Sympathetic Denervation in Diabetic Patients. J. Neuroophthalmol. 2006, 26, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Hoyng, P.F.J.; Van Beek, L.M. Pharmacological Therapy for Glaucoma: A Review. Drugs 2000, 59, 411–434. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, K.; Schwartz, S.G.; Relhan, N.; Kishor, K.; Flynn, H.W. New Therapeutic Approaches in Diabetic Retinopathy. Rev. Diabet. Stud. 2015, 12, 196. [Google Scholar] [CrossRef]
- Boyer, D.S.; Yoon, Y.H.; Belfort, R.; Bandello, F.; Maturi, R.K.; Augustin, A.J.; Li, X.Y.; Cui, H.; Hashad, Y.; Whitcup, S.M. Three-Year, Randomized, Sham-Controlled Trial of Dexamethasone Intravitreal Implant in Patients with Diabetic Macular Edema. Ophthalmology 2014, 121, 1904–1914. [Google Scholar] [CrossRef]
- Kusari, J.; Zhou, S.; Padillo, E.; Clarke, K.G.; Gil, D.W. Effect of Memantine on Neuroretinal Function and Retinal Vascular Changes of Streptozotocin-Induced Diabetic Rats. Investig. Ophthalmol. Vis. Sci. 2007, 48, 5152–5159. [Google Scholar] [CrossRef] [PubMed]
- Kern, T.S.; Miller, C.M.; Tang, J.; Du, Y.; Ball, S.L.; Berti-Matera, L. Comparison of Three Strains of Diabetic Rats with Respect to the Rate at Which Retinopathy and Tactile Allodynia Develop. Mol. Vis. 2010, 16, 1629–1639. [Google Scholar]
- Akiyode, O.; Dunkelly-Allen, N. Ranibizumab: A Review of Its Use in the Treatment of Diabetic Retinopathy in Patients With Diabetic Macular Edema. J. Pharm. Technol. 2016, 32, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Ohta, M.; Inoue, T.; Mizuno, K.; Isobe, T.; Tanabe, S.; Tanihara, H. Effects of K-115 (Ripasudil), a Novel ROCK Inhibitor, on Trabecular Meshwork and Schlemm’s Canal Endothelial Cells. Sci. Rep. 2016, 6, 19640. [Google Scholar] [CrossRef]
- Song, J.H.; Lee, J.J.; Lee, S.J. Comparison of the Short-Term Effects of Intravitreal Triamcinolone Acetonide and Bevacizumab Injection for Diabetic Macular Edema. Korean J. Ophthalmol. 2011, 25, 156–160. [Google Scholar] [CrossRef]
- Zhang, X.; Lai, D.; Bao, S.; Hambly, B.D.; Gillies, M.C. Triamcinolone Acetonide Inhibits P38MAPK Activation and Neuronal Apoptosis in Early Diabetic Retinopathy. Curr. Mol. Med. 2013, 13, 946–958. [Google Scholar] [CrossRef]
- Furino, C.; Boscia, F.; Reibaldi, M.; Alessio, G. Intravitreal Therapy for Diabetic Macular Edema: An Update. J. Ophthalmol. 2021, 2021, 6654168. [Google Scholar] [CrossRef]
- Boyer, D.S.; Hopkins, J.J.; Sorof, J.; Ehrlich, J.S. Anti-Vascular Endothelial Growth Factor Therapy for Diabetic Macular Edema. Ther. Adv. Endocrinol. Metab. 2013, 4, 151–169. [Google Scholar] [CrossRef] [PubMed]
- Kovács-Valasek, A.; Szabadfi, K.; Dénes, V.; Szalontai, B.; Tamás, A.; Kiss, P.; Szabó, A.; Setalo, G.; Reglődi, D.; Gábriel, R. Accelerated Retinal Aging in PACAP Knock-out Mice. Neuroscience 2017, 348, 1–10. [Google Scholar] [CrossRef]
- Szabadfi, K.; Szabo, A.; Kiss, P.; Reglodi, D.; Setalo, G.; Kovacs, K.; Tamas, A.; Toth, G.; Gabriel, R. PACAP Promotes Neuron Survival in Early Experimental Diabetic Retinopathy. Neurochem. Int. 2014, 64, 84–91. [Google Scholar] [CrossRef]
- Huang, H.; Gandhi, J.K.; Zhong, X.; Wei, Y.; Gong, J.; Duh, E.J.; Vinores, S.A. TNFalpha Is Required for Late BRB Breakdown in Diabetic Retinopathy, and Its Inhibition Prevents Leukostasis and Protects Vessels and Neurons from Apoptosis. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1336–1344. [Google Scholar] [CrossRef]
- Zheng, L.; Szabó, C.; Kern, T.S. Poly(ADP-Ribose) Polymerase Is Involved in the Development of Diabetic Retinopathy via Regulation of Nuclear Factor-KappaB. Diabetes 2004, 53, 2960–2967. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Chen, L.; Chen, R.; Lou, Q.; Wang, H. Poly(ADP-Ribose) Polymerase-1: An Update on Its Role in Diabetic Retinopathy. Discov. Med. 2021, 32, 13–22. [Google Scholar] [PubMed]
- Valverde, A.M.; Miranda, S.; García-Ramírez, M.; González-Rodriguez, Á.; Hernández, C.; Simó, R. Proapoptotic and Survival Signaling in the Neuroretina at Early Stages of Diabetic Retinopathy. Mol. Vis. 2013, 19, 47–53. [Google Scholar]
- Mi, X.S.; Zhong, J.X.; Chang, R.C.C.; So, K.F. Research Advances on the Usage of Traditional Chinese Medicine for Neuroprotection in Glaucoma. J. Integr. Med. 2013, 11, 233–240. [Google Scholar] [CrossRef]
- Andrea, H.; Roland, M.; Tibor, R.; Adrienne, C. Effect of Steroid Therapy on Intraocular Pressure. Orv. Hetil. 2022, 163, 1345–1352. [Google Scholar] [CrossRef]
- Pang, I.H.; Clark, A.F. Inducible Rodent Models of Glaucoma. Prog. Retin. Eye Res. 2020, 75, 100799. [Google Scholar] [CrossRef]
- Ficarrotta, K.R.; Mohamed, Y.H.; Passaglia, C.L. Experimental Glaucoma Model with Controllable Intraocular Pressure History. Sci. Rep. 2020, 10, 126. [Google Scholar] [CrossRef]
- Rodrigo, M.J.; Garcia-Herranz, D.; Subias, M.; Martinez-Rincón, T.; Mendez-Martínez, S.; Bravo-Osuna, I.; Carretero, A.; Ruberte, J.; Garcia-Feijoo, J.; Pablo, L.E.; et al. Chronic Glaucoma Using Biodegradable Microspheres to Induce Intraocular Pressure Elevation. Six-Month Follow-Up. Biomedicines 2021, 9, 682. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, H.; Sun, Y.; Li, Y.; Luo, B.; Cui, J.; Zhu, M.; Bi, F.; Chen, K.; Liu, Y. Monosodium Glutamate-Induced Mouse Model With Unique Diabetic Retinal Neuropathy Features and Artificial Intelligence Techniques for Quantitative Evaluation. Front. Immunol. 2022, 13, 862702. [Google Scholar] [CrossRef] [PubMed]
- Sankalp; Dada, T.; Yadav, R.K.; Sharma, H.B.; Netam, R.K.; Kochhar, K.P. Effect of Tratak (Yogic Ocular Exercises) on Intraocular Pressure in Glaucoma: An RCT. Int. J. Yoga 2022, 15, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Gábriel, R. Neuropeptides and Diabetic Retinopathy. Br. J. Clin. Pharmacol. 2013, 75, 1189–1201. [Google Scholar] [CrossRef]
- Pöstyéni, E.; Ganczer, A.; Kovács-Valasek, A.; Gabriel, R. Relevance of Peptide Homeostasis in Metabolic Retinal Degenerative Disorders: Curative Potential in Genetically Modified Mice. Front. Pharmacol. 2022, 12, 808315. [Google Scholar] [CrossRef]
- Singh, R.; Letai, A.; Sarosiek, K. Regulation of Apoptosis in Health and Disease: The Balancing Act of BCL-2 Family Proteins. Nat. Rev. Mol. Cell Biol. 2019, 20, 175–193. [Google Scholar] [CrossRef]
- Yuan, Z.; Dewson, G.; Czabotar, P.E.; Birkinshaw, R.W. VDAC2 and the BCL-2 Family of Proteins. Biochem. Soc. Trans. 2021, 49, 2787–2795. [Google Scholar] [CrossRef]
- Roberts, A.W. Therapeutic Development and Current Uses of BCL-2 Inhibition. Hematol. Am. Soc. Hematol. Educ. Progr. 2020, 2020, 1–9. [Google Scholar] [CrossRef]
- Hu, Y.; Xu, Q.; Li, H.; Meng, Z.; Hao, M.; Ma, X.; Lin, W.; Kuang, H. Dapagliflozin Reduces Apoptosis of Diabetic Retina and Human Retinal Microvascular Endothelial Cells Through ERK1/2/CPLA2/AA/ROS Pathway Independent of Hypoglycemic. Front. Pharmacol. 2022, 13, 827896. [Google Scholar] [CrossRef]
- Huang, B.; Liang, J.J.; Zhuang, X.; Chen, S.W.; Ng, T.K.; Chen, H. Intravitreal Injection of Hydrogen Peroxide Induces Acute Retinal Degeneration, Apoptosis, and Oxidative Stress in Mice. Oxid. Med. Cell. Longev. 2018, 2018, 5489476. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, W.; Liu, A.; Tao, Y.; Wang, Q.; Yang, Y.; Wang, L.; Huang, Y. Protective Effect of Salvianolic Acid A against N-Methyl-N-Nitrosourea-Induced Retinal Degeneration. Evid. Based Complement. Alternat. Med. 2022, 2022, 1219789. [Google Scholar] [CrossRef] [PubMed]
- Edington, M.; Siempis, T.; Montgomery, D. Discontinuation of the Herbal Preparation Hypericum Perforatum, Also Known as St John’s Wort, Associated with Improved Intraocular Pressure Control in a Patient on Topical Beta-Blockers for Primary Open-Angle Glaucoma. Oman J. Ophthalmol. 2018, 11, 188–189. [Google Scholar] [PubMed]
- Mincione, F.; Scozzafava, A.; Supuran, C.T. The Development of Topically Acting Carbonic Anhydrase Inhibitors as Anti-Glaucoma Agents. Curr. Top. Med. Chem. 2007, 7, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Farzam, K.; Abdullah, M. Acetazolamide. Xpharm Compr. Pharmacol. Ref. 2022, 1–5. [Google Scholar] [CrossRef]
- Lesar, T.S. Comparison of Ophthalmic Beta-Blocking Agents. Clin. Pharm. 1987, 6, 451–463. [Google Scholar]
- Lim, K.S.; Nau, C.B.; O’Byrne, M.M.; Hodge, D.O.; Toris, C.B.; McLaren, J.W.; Johnson, D.H. Mechanism of Action of Bimatoprost, Latanoprost, and Travoprost in Healthy Subjects. A Crossover Study. Ophthalmology 2008, 115, 790–795.e4. [Google Scholar] [CrossRef]
- Brubaker, R.F. Mechanism of Action of Bimatoprost (LumiganTM). Surv. Ophthalmol. 2001, 45, S347–S351. [Google Scholar] [CrossRef]
- Easthope, S.E.; Perry, C.M. Topical Bimatoprost: A Review of Its Use in Open-Angle Glaucoma and Ocular Hypertension. Drugs Aging 2002, 19, 231–248. [Google Scholar] [CrossRef]
- Woodward, D.F.; Liang, Y.; Krauss, A.H.P. Prostamides (Prostaglandin-Ethanolamides) and Their Pharmacology. Br. J. Pharmacol. 2008, 153, 410–419. [Google Scholar] [CrossRef]
- Alm, A. Latanoprost in the Treatment of Glaucoma. Clin. Ophthalmol. 2014, 8, 1967–1985. [Google Scholar] [CrossRef]
- Wheeler, L.A.; Gil, D.W.; WoldeMussie, E. Role of Alpha-2 Adrenergic Receptors in Neuroprotection and Glaucoma. Surv. Ophthalmol. 2001, 45, S290–S294. [Google Scholar] [CrossRef] [PubMed]
- El-Kamel, A.; Al-Dosari, H.; Al-Jenoobi, F. Environmentally Responsive Ophthalmic Gel Formulation of Carteolol Hydrochloride. Drug Deliv. 2006, 13, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Arthur, S.; Cantor, L.B. Update on the Role of Alpha-Agonists in Glaucoma Management. Exp. Eye Res. 2011, 93, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Martens-Lobenhoffer, J.; Banditt, P. Clinical Pharmacokinetics of Dorzolamide. Clin. Pharmacokinet. 2002, 41, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Balfour, J.A.; Wilde, M.I. Dorzolamide. A Review of Its Pharmacology and Therapeutic Potential in the Management of Glaucoma and Ocular Hypertension. Drugs Aging 1997, 10, 384–403. [Google Scholar] [CrossRef]
- Loftsson, T.; Jansook, P.; Stefánsson, E. Topical Drug Delivery to the Eye: Dorzolamide. Acta Ophthalmol. 2012, 90, 603–608. [Google Scholar] [CrossRef]
- Schmidt, K.G.; Horowitz, Y.; Buckman, G.; Segev, E.; Levinger, E.; Geyer, O. Lowering of IOP by Echothiophate Iodide in Pseudophakic Eyes with Glaucoma. Curr. Eye Res. 2010, 35, 698–702. [Google Scholar] [CrossRef]
- Sjöquist, B.; Stjernschantz, J. Ocular and Systemic Pharmacokinetics of Latanoprost in Humans. Surv. Ophthalmol. 2002, 47, S6. [Google Scholar] [CrossRef]
- Gonzalez, J.P.; Clissold, S.P. Ocular Levobunolol. A Review of Its Pharmacodynamic and Pharmacokinetic Properties, and Therapeutic Efficacy. Drugs 1987, 34, 648–661. [Google Scholar] [CrossRef]
- Novack, G.D. Levobunolol for the Long-Term Treatment of Glaucoma. Gen. Pharmacol. 1986, 17, 373–377. [Google Scholar] [CrossRef]
- Ishibashi, T.; Yokoi, N.; Kinoshita, S. Comparison of the Effects of Topical Levobunolol and Timolol Solution on the Human Ocular Surface. Cornea 2003, 22, 709–715. [Google Scholar] [CrossRef]
- Skorobohach, B.J.; Ward, D.A.; Hendrix, D.V.H. Effects of Oral Administration of Methazolamide on Intraocular Pressure and Aqueous Humor Flow Rate in Clinically Normal Dogs. Am. J. Vet. Res. 2003, 64, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Ren, R.; Li, G.; Le, T.D.; Kopczynski, C.; Stamer, W.D.; Gong, H. Netarsudil Increases Outflow Facility in Human Eyes Through Multiple Mechanisms. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6197–6209. [Google Scholar] [CrossRef] [PubMed]
- Papadia, M.; Bagnis, A.; Scotto, R.; Traverso, C.E. Tafluprost for Glaucoma. Expert Opin. Pharmacother. 2011, 12, 2393–2401. [Google Scholar] [CrossRef] [PubMed]
- Mina, B.P.; Leonard, K.S.; Nida, S.A.; Malik, Y.K. Tafluprost: A Novel Prostaglandin Analog for Treatment of Glaucoma. Adv. Ther. 2011, 28, 707–715. [Google Scholar] [CrossRef]
- Takagi, Y.; Nakajima, T.; Shimazaki, A.; Kageyama, M.; Matsugi, T.; Matsumura, Y.; Gabelt, B.T.; Kaufman, P.L.; Hara, H. Pharmacological Characteristics of AFP-168 (Tafluprost), a New Prostanoid FP Receptor Agonist, as an Ocular Hypotensive Drug. Exp. Eye Res. 2004, 78, 767–776. [Google Scholar] [CrossRef]
- Watanabe, K.; Chiou, G.C.Y. Action Mechanism of Timolol to Lower the Intraocular Pressure in Rabbits. Ophthalmic Res. 1983, 15, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Volotinen, M.; Hakkola, J.; Pelkonen, O.; Vapaatalo, H.; Mäenpää, J. Metabolism of Ophthalmic Timolol: New Aspects of an Old Drug. Basic Clin. Pharmacol. Toxicol. 2011, 108, 297–303. [Google Scholar] [CrossRef]
- Arranz-Marquez, E.; Teus, M.A. Prostanoids for the Management of Glaucoma. Expert Opin. Drug Saf. 2008, 7, 801–808. [Google Scholar] [CrossRef]
- Costagliola, C.; Dell’Omo, R.; Romano, M.R.; Rinaldi, M.; Zeppa, L.; Parmeggiani, F. Pharmacotherapy of Intraocular Pressure—Part II. Carbonic Anhydrase Inhibitors, Prostaglandin Analogues and Prostamides. Expert Opin. Pharmacother. 2009, 10, 2859–2870. [Google Scholar] [CrossRef] [PubMed]
- Wan, P.; Su, W.; Zhang, Y.; Li, Z.; Deng, C.; Zhuo, Y. Trimetazidine Protects Retinal Ganglion Cells from Acute Glaucoma via the Nrf2/Ho-1 Pathway. Clin. Sci. 2017, 131, 2363–2375. [Google Scholar] [CrossRef] [PubMed]
- Fung, D.S.; Whitson, J.T. An Evidence-Based Review of Unoprostone Isopropyl Ophthalmic Solution 0.15% for Glaucoma: Place in Therapy. Clin. Ophthalmol. 2014, 8, 543. [Google Scholar] [CrossRef] [PubMed]
- Acheampong, A.A.; Shackleton, M.; John, B.; Burke, J.; Wheeler, L.; Tang-Liu, D. Distribution of Brimonidine into Anterior and Posterior Tissues of Monkey, Rabbit, and Rat Eyes. Drug Metab. Dispos. 2002, 30, 421–429. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, L.; Weinreb, R.N. Ophthalmic Drug Discovery: Novel Targets and Mechanisms for Retinal Diseases and Glaucoma. Nat. Rev. Drug Discov. 2012, 11, 541–559. [Google Scholar] [CrossRef]
- Costa, V.P.; Harris, A.; Stefánsson, E.; Flammer, J.; Krieglstein, G.K.; Orzalesi, N.; Heijl, A.; Renard, J.P.; Serra, L.M. The Effects of Antiglaucoma and Systemic Medications on Ocular Blood Flow. Prog. Retin. Eye Res. 2003, 22, 769–805. [Google Scholar] [CrossRef]
- Lewis, R.A.; Christie, W.C.; Day, D.G.; Craven, E.R.; Walters, T.; Bejanian, M.; Lee, S.S.; Goodkin, M.L.; Zhang, J.; Whitcup, S.M.; et al. Bimatoprost Sustained-Release Implants for Glaucoma Therapy: 6-Month Results From a Phase I/II Clinical Trial. Am. J. Ophthalmol. 2017, 175, 137–147. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, L.; Dai, Y.; Sheng, X.; Chen, S.; Xie, Q. Effects of Coconut Water on Retina in Diabetic Rats. Evid. Based Complement. Alternat. Med. 2020, 2020, 9450634. [Google Scholar] [CrossRef]
- Su, W.; Li, Z.; Jia, Y.; Zhuo, Y. Rapamycin Is Neuroprotective in a Rat Chronic Hypertensive Glaucoma Model. PLoS ONE 2014, 9, e99719. [Google Scholar] [CrossRef]
- Ishizaki, T.; Maekawa, M.; Fujisawa, K.; Okawa, K.; Iwamatsu, A.; Fujita, A.; Watanabe, N.; Saito, Y.; Kakizuka, A.; Morii, N.; et al. The Small GTP-Binding Protein Rho Binds to and Activates a 160 KDa Ser/Thr Protein Kinase Homologous to Myotonic Dystrophy Kinase. EMBO J. 1996, 15, 1885–1893. [Google Scholar] [CrossRef]
- Waki, M.; Yoshida, Y.; Oka, T.; Azuma, M. Reduction of Intraocular Pressure by Topical Administration of an Inhibitor of the Rho-Associated Protein Kinase. Curr. Eye Res. 2001, 22, 470–474. [Google Scholar] [CrossRef]
- Honjo, M.; Tanihara, H.; Inatani, M.; Kido, N.; Sawamura, T.; Yue, B.Y.J.T.; Narumiya, S.; Honda, Y. Effects of Rho-Associated Protein Kinase Inhibitor Y-27632 on Intraocular Pressure and Outflow Facility. Investig. Ophthalmol. Vis. Sci. 2001, 42, 137–144. [Google Scholar]
- Honjo, M.; Inatani, M.; Kido, N.; Sawamura, T.; Yue, B.Y.J.T.; Honda, Y.; Tanihara, H. Effects of Protein Kinase Inhibitor, HA1077, on Intraocular Pressure and Outflow Facility in Rabbit Eyes. Arch. Ophthalmol. 2001, 119, 1171–1178. [Google Scholar] [CrossRef]
- Lin, C.W.; Sherman, B.; Moore, L.A.; Laethem, C.L.; Lu, D.W.; Pattabiraman, P.P.; Rao, P.V.; Delong, M.A.; Kopczynski, C.C. Discovery and Preclinical Development of Netarsudil, a Novel Ocular Hypotensive Agent for the Treatment of Glaucoma. J. Ocul. Pharmacol. Ther. 2018, 34, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Arita, R.; Hata, Y.; Nakao, S.; Kita, T.; Miura, M.; Kawahara, S.; Zandi, S.; Almulki, L.; Tayyari, F.; Shimokawa, H.; et al. Rho Kinase Inhibition by Fasudil Ameliorates Diabetes-Induced Microvascular Damage. Diabetes 2009, 58, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Anwar, K.N.; Fazal, F.; Malik, A.B.; Rahman, A. RhoA/Rho-Associated Kinase Pathway Selectively Regulates Thrombin-Induced Intercellular Adhesion Molecule-1 Expression in Endothelial Cells via Activation of I Kappa B Kinase Beta and Phosphorylation of RelA/P65. J. Immunol. 2004, 173, 6965–6972. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Zhong, Y. Rho/Rho-Associated Kinase Pathway in Glaucoma (Review). Int. J. Oncol. 2013, 43, 1357–1367. [Google Scholar] [CrossRef]
- Tanihara, H.; Inoue, T.; Yamamoto, T.; Kuwayama, Y.; Abe, H.; Araie, M. Phase 1 Clinical Trials of a Selective Rho Kinase Inhibitor, K-115. JAMA Ophthalmol. 2013, 131, 1288–1295. [Google Scholar] [CrossRef] [PubMed]
- Tanihara, H.; Inoue, T.; Yamamoto, T.; Kuwayama, Y.; Abe, H.; Araie, M. Phase 2 Randomized Clinical Study of a Rho Kinase Inhibitor, K-115, in Primary Open-Angle Glaucoma and Ocular Hypertension. Am. J. Ophthalmol. 2013, 156, 731–736.e2. [Google Scholar] [CrossRef]
- Tanihara, H.; Inoue, T.; Yamamoto, T.; Kuwayama, Y.; Abe, H.; Suganami, H.; Araie, M. Intra-Ocular Pressure-Lowering Effects of a Rho Kinase Inhibitor, Ripasudil (K-115), over 24 Hours in Primary Open-Angle Glaucoma and Ocular Hypertension: A Randomized, Open-Label, Crossover Study. Acta Ophthalmol. 2015, 93, e254–e260. [Google Scholar] [CrossRef]
- Tanihara, H.; Inoue, T.; Yamamoto, T.; Kuwayama, Y.; Abe, H.; Suganami, H.; Araie, M. Additive Intraocular Pressure-Lowering Effects of the Rho Kinase Inhibitor Ripasudil (K-115) Combined With Timolol or Latanoprost: A Report of 2 Randomized Clinical Trials. JAMA Ophthalmol. 2015, 133, 755–761. [Google Scholar] [CrossRef]
- Apte, R.S. Age-Related Macular Degeneration. N. Engl. J. Med. 2021, 385, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S. Update in Retina: Photodynamic Therapy for the Treatment of Subfoveal Choroidal Neovascularization Secondary to Age-Related Macular Degeneration. Can. J. Ophthalmol. 2001, 36, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Ellis, D.; Burgess, P.I.; Kayange, P. Management of Diabetic Retinopathy. Malawi Med. J. 2013, 25, 116–120. [Google Scholar] [PubMed]
- Stitt, A.W.; Curtis, T.M.; Chen, M.; Medina, R.J.; McKay, G.J.; Jenkins, A.; Gardiner, T.A.; Lyons, T.J.; Hammes, H.P.; Simó, R.; et al. The Progress in Understanding and Treatment of Diabetic Retinopathy. Prog. Retin. Eye Res. 2016, 51, 156–186. [Google Scholar] [CrossRef]
- Mounirou, B.A.; Adam, N.D.; Yakoura, A.K.; Aminou, M.S.; Liu, Y.T.; Tan, L.Y. Diabetic Retinopathy: An Overview of Treatments. Indian J. Endocrinol. Metab. 2022, 26, 111. [Google Scholar] [CrossRef]
- Pande, G.S.; Tidake, P. Laser Treatment Modalities for Diabetic Retinopathy. Cureus 2022, 14, e30024. [Google Scholar] [CrossRef] [PubMed]
- Ulbig, M.W.; Kollias, A.N. Diabetic Retinopathy: Early Diagnosis and Effective Treatment. Dtsch. Arztebl. Int. 2010, 107, 75–84. [Google Scholar] [CrossRef]
- Brănişteanu, D.C.; Bilha, A.; Moraru, A. Vitrectomy Surgery of Diabetic Retinopathy Complications. Rom. J. Ophthalmol. 2016, 60, 31. [Google Scholar] [PubMed]
- Gedde, S.; Anderson, D.; Budenz, D.; Del Calvo, M.; Fantes, F.; Greenfield, D.; Hodapp, E.; Lee, R.; Marcellino, A.; Palmberg, P.; et al. Treatment Outcomes in the Tube versus Trabeculectomy Study after One Year of Follow-Up. Am. J. Ophthalmol. 2007, 143, 22.e2. [Google Scholar] [CrossRef]
- Rulli, E.; Biagioli, E.; Riva, I.; Gambirasio, G.; De Simone, I.; Floriani, I.; Quaranta, L. Efficacy and Safety of Trabeculectomy vs Nonpenetrating Surgical Procedures: A Systematic Review and Meta-Analysis. JAMA Ophthalmol. 2013, 131, 1573–1582. [Google Scholar] [CrossRef]
- Rao, P.K. Stem Cell Therapies for Intraocular Disease. Mo. Med. 2022, 119, 65–68. [Google Scholar]
- Home—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/home (accessed on 19 April 2023).
- Rizkiawan, D.E.; Evelyn, M.; Tjandra, K.C.; Setiawan, B. Utilization of Modified Induced Pluripotent Stem Cells as the Advance Therapy of Glaucoma: A Systematic Review. Clin. Ophthalmol. 2022, 16, 2851. [Google Scholar] [CrossRef]
- Cho, Y.K.; Park, D.H.; Jeon, I.C. Medication Trends for Age-Related Macular Degeneration. Int. J. Mol. Sci. 2021, 22, 11837. [Google Scholar] [CrossRef] [PubMed]
- Kern, T.S.; Miller, C.M.; Du, Y.; Zheng, L.; Mohr, S.; Ball, S.L.; Kim, M.; Jamison, J.A.; Bingaman, D.P. Topical Administration of Nepafenac Inhibits Diabetes-Induced Retinal Microvascular Disease and Underlying Abnormalities of Retinal Metabolism and Physiology. Diabetes 2007, 56, 373–379. [Google Scholar] [CrossRef]
- Vincent, J.A.; Mohr, S. Inhibition of Caspase-1/Interleukin-1beta Signaling Prevents Degeneration of Retinal Capillaries in Diabetes and Galactosemia. Diabetes 2007, 56, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Z.; Xi, X.; Gao, L.; Kern, T.S. Captopril Inhibits Capillary Degeneration in the Early Stages of Diabetic Retinopathy. Curr. Eye Res. 2007, 32, 883–889. [Google Scholar] [CrossRef] [PubMed]
- El-Remessy, A.B.; Bartoli, M.; Platt, D.H.; Fulton, D.; Caldwell, R.B. Oxidative Stress Inactivates VEGF Survival Signaling in Retinal Endothelial Cells via PI 3-Kinase Tyrosine Nitration. J. Cell Sci. 2016, 129, 3203. [Google Scholar] [CrossRef]
- Kompella, U.B.; Hartman, R.R.; Patil, M.A. Extraocular, Periocular, and Intraocular Routes for Sustained Drug Delivery for Glaucoma. Prog. Retin. Eye Res. 2021, 82, 100901. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Xu, X.; Zheng, Z.; Zhu, B.; Shi, Y.; Liu, K. Protective Effects of Rosiglitazone on Retinal Neuronal Damage in Diabetic Rats. Curr. Eye Res. 2011, 36, 673–679. [Google Scholar] [CrossRef]
- Pöstyéni, E.; Szabadfi, K.; Sétáló, G.; Gabriel, R. A Promising Combination: Pacap and Parp Inhibitor Have Therapeutic Potential in Models of Diabetic and Hypertensive Retinopathies. Cells 2021, 10, 3470. [Google Scholar] [CrossRef] [PubMed]
- Kiss, P.; Atlasz, T.; Szabadfi, K.; Horvath, G.; Griecs, M.; Farkas, J.; Matkovits, A.; Toth, G.; Lubics, A.; Tamas, A.; et al. Comparison between PACAP- and Enriched Environment-Induced Retinal Protection in MSG-Treated Newborn Rats. Neurosci. Lett. 2011, 487, 400–405. [Google Scholar] [CrossRef] [PubMed]
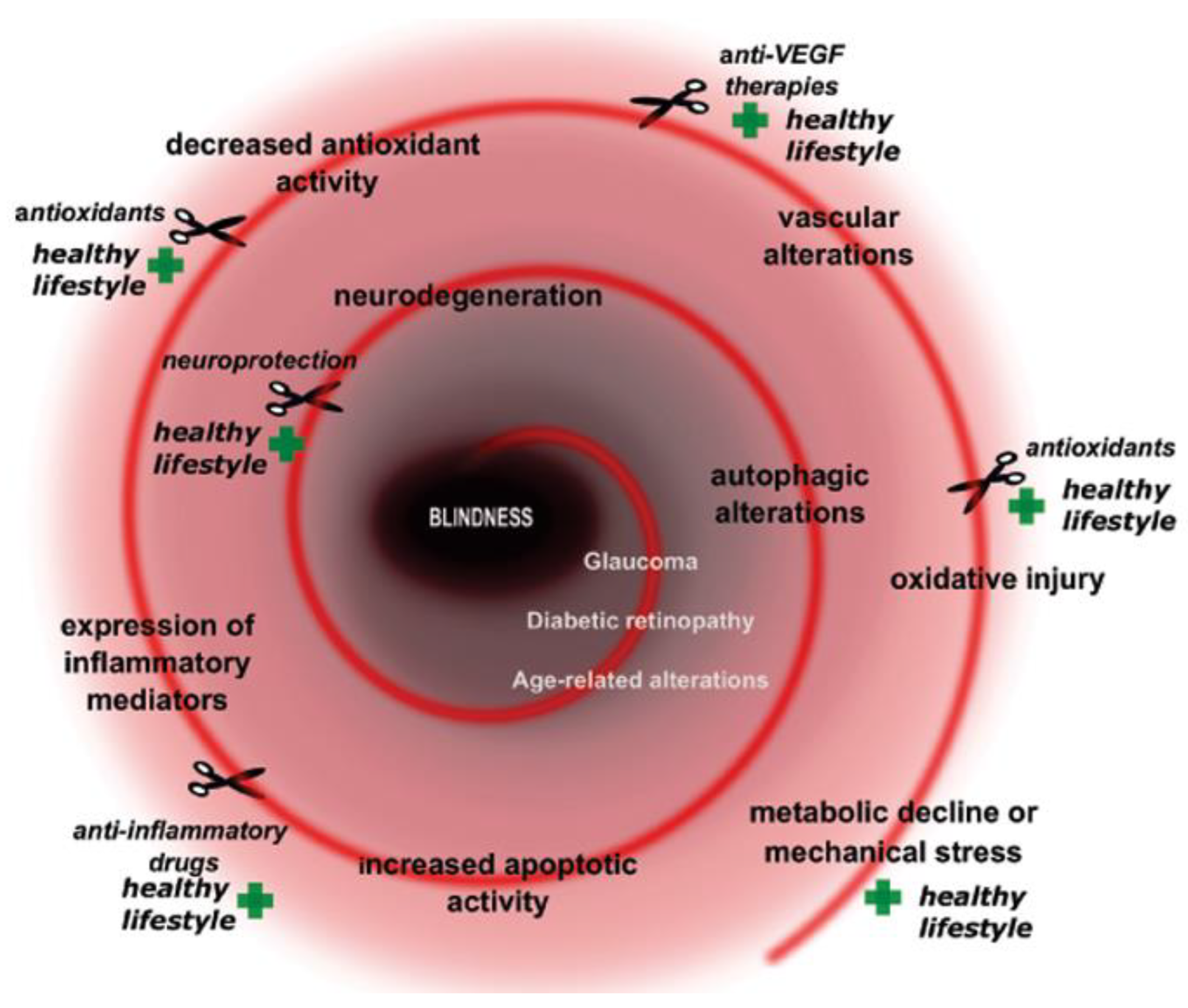
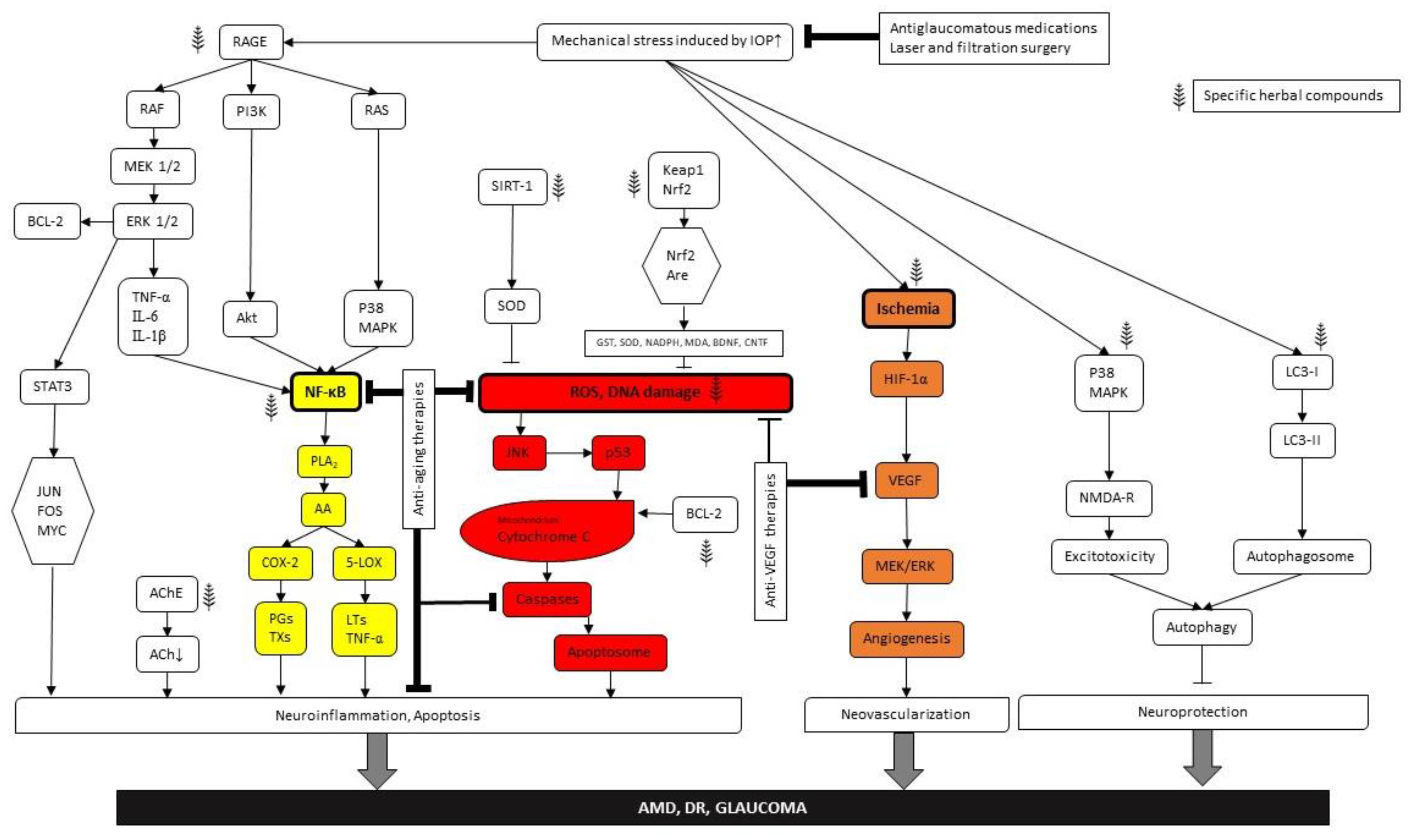
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovács-Valasek, A.; Rák, T.; Pöstyéni, E.; Csutak, A.; Gábriel, R. Three Major Causes of Metabolic Retinal Degenerations and Three Ways to Avoid Them. Int. J. Mol. Sci. 2023, 24, 8728. https://doi.org/10.3390/ijms24108728
Kovács-Valasek A, Rák T, Pöstyéni E, Csutak A, Gábriel R. Three Major Causes of Metabolic Retinal Degenerations and Three Ways to Avoid Them. International Journal of Molecular Sciences. 2023; 24(10):8728. https://doi.org/10.3390/ijms24108728
Chicago/Turabian StyleKovács-Valasek, Andrea, Tibor Rák, Etelka Pöstyéni, Adrienne Csutak, and Robert Gábriel. 2023. "Three Major Causes of Metabolic Retinal Degenerations and Three Ways to Avoid Them" International Journal of Molecular Sciences 24, no. 10: 8728. https://doi.org/10.3390/ijms24108728
APA StyleKovács-Valasek, A., Rák, T., Pöstyéni, E., Csutak, A., & Gábriel, R. (2023). Three Major Causes of Metabolic Retinal Degenerations and Three Ways to Avoid Them. International Journal of Molecular Sciences, 24(10), 8728. https://doi.org/10.3390/ijms24108728







