Dopamine-Dependent Ketamine Modulation of Glutamatergic Synaptic Plasticity in the Prelimbic Cortex of Adult Rats Exposed to Acute Stress
Abstract
1. Introduction
2. Results
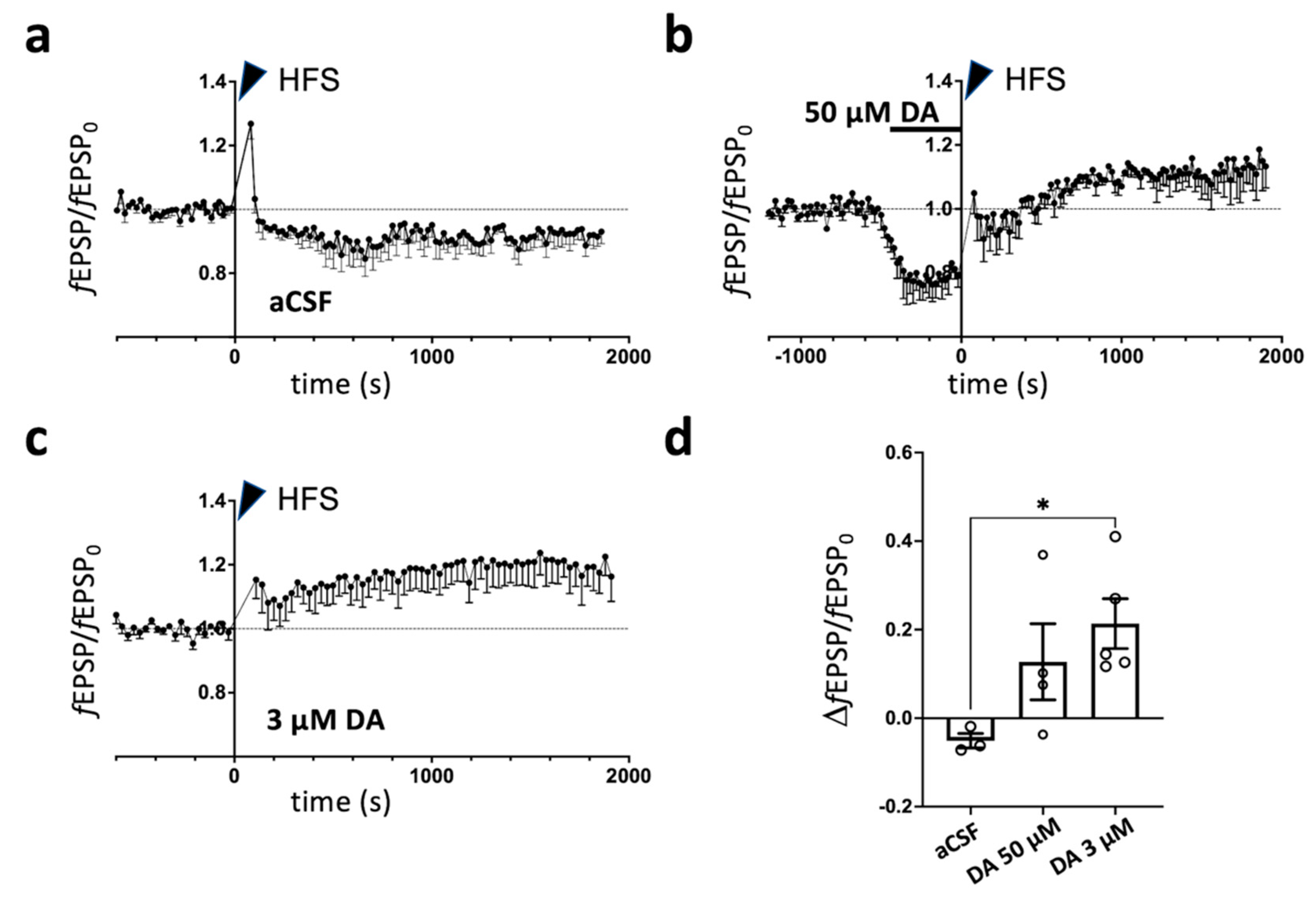
2.1. Dopamine-Dependent Modulation of Long-Term Potentiation in Prelimbic Cortex of Rats Subjected to Acute Footshock Stress and Ketamine Treatment
2.2. Changes in Dopamine Release Induced by Acute Footshock Stress in the Prefrontal Cortex
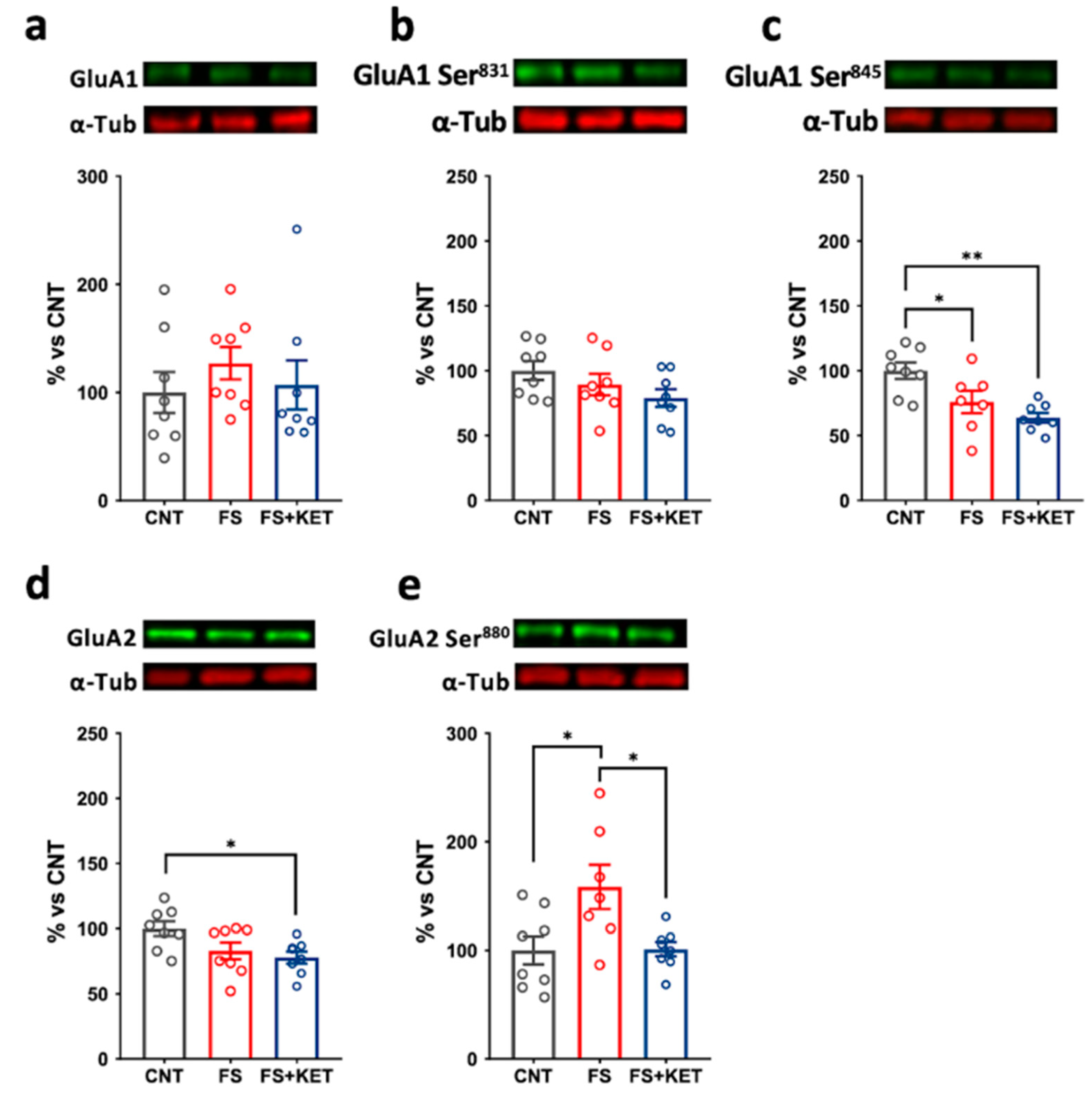
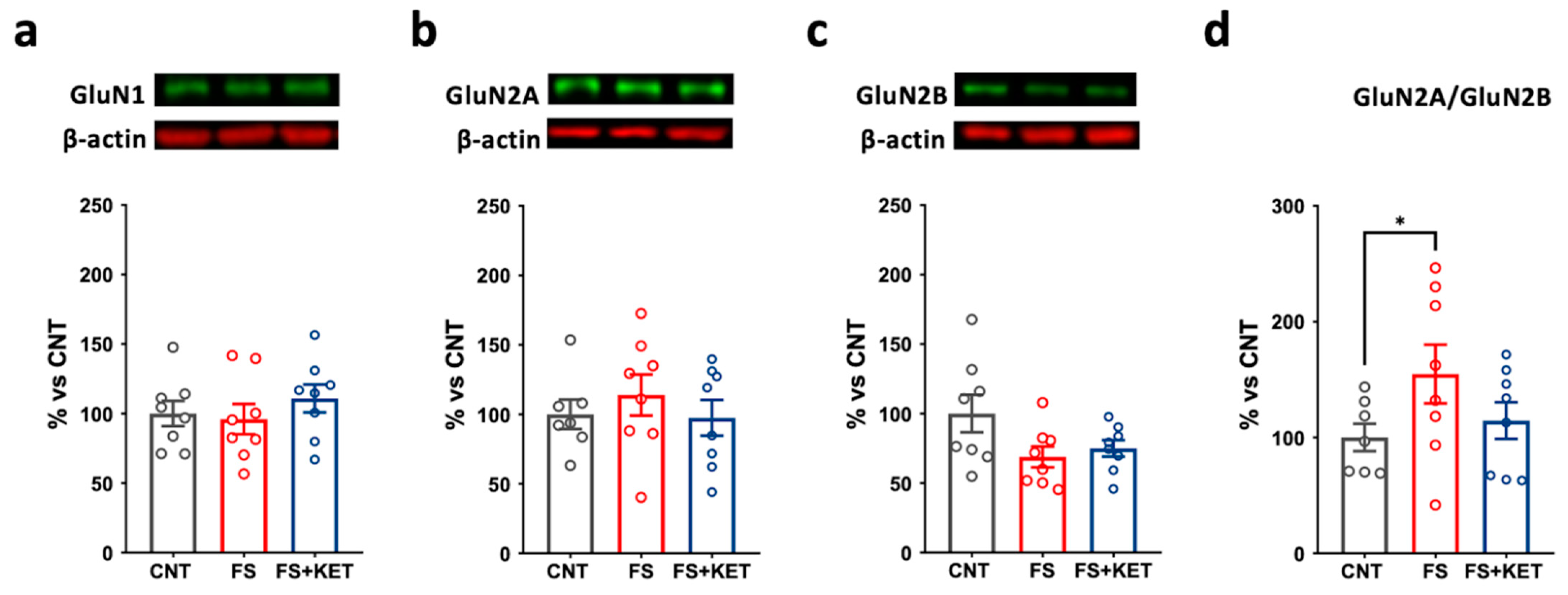
2.3. Modulation of Ionotropic Glutamate Receptor Subunit Expression and Phosphorylation Levels Induced by Acute Footshock and Ketamine in The Prefrontal Cortex
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Drug Treatment
4.3. Footshock Stress
4.4. Brain Slices Preparation
4.5. Field Recordings of Excitatory Postsynaptic Potentials (fEPSPs)
4.6. Synaptosomes Preparation and Release Experiments
4.7. Western Blotting
4.8. Analysis and Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Musazzi, L.; Tornese, P.; Sala, N.; Popoli, M. What Acute Stress Protocols Can Tell Us About PTSD and Stress-Related Neuropsychiatric Disorders. Front. Pharmacol. 2018, 9, 758. [Google Scholar] [CrossRef] [PubMed]
- Sanacora, G.; Yan, Z.; Popoli, M. The Stressed Synapse 2.0: Pathophysiological Mechanisms in Stress-Related Neuropsychiatric Disorders. Nat. Rev. Neurosci. 2022, 23, 86–103. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, V.; Nestler, E.J. The Molecular Neurobiology of Depression. Nature 2008, 455, 894–902. [Google Scholar] [CrossRef]
- Klengel, T.; Binder, E.B. Epigenetics of Stress-Related Psychiatric Disorders and Gene × Environment Interactions. Neuron 2015, 86, 1343–1357. [Google Scholar] [CrossRef] [PubMed]
- Cathomas, F.; Murrough, J.W.; Nestler, E.J.; Han, M.-H.; Russo, S.J. Neurobiology of Resilience: Interface Between Mind and Body. Biol. Psychiatry 2019, 86, 410–420. [Google Scholar] [CrossRef]
- Tortella-Feliu, M.; Fullana, M.A.; Pérez-Vigil, A.; Torres, X.; Chamorro, J.; Littarelli, S.A.; Solanes, A.; Ramella-Cravaro, V.; Vilar, A.; González-Parra, J.A.; et al. Risk Factors for Posttraumatic Stress Disorder: An Umbrella Review of Systematic Reviews and Meta-Analyses. Neurosci. Biobehav. Rev. 2019, 107, 154–165. [Google Scholar] [CrossRef]
- Chattarji, S.; Tomar, A.; Suvrathan, A.; Ghosh, S.; Rahman, M.M. Neighborhood Matters: Divergent Patterns of Stress-Induced Plasticity across the Brain. Nat. Neurosci. 2015, 18, 1364–1375. [Google Scholar] [CrossRef]
- Alexandra Kredlow, M.; Fenster, R.J.; Laurent, E.S.; Ressler, K.J.; Phelps, E.A. Prefrontal Cortex, Amygdala, and Threat Processing: Implications for PTSD. Neuropsychopharmacology 2022, 47, 247–259. [Google Scholar] [CrossRef]
- Maren, S.; Phan, K.L.; Liberzon, I. The Contextual Brain: Implications for Fear Conditioning, Extinction and Psychopathology. Nat. Rev. Neurosci. 2013, 14, 417–428. [Google Scholar] [CrossRef]
- Ressler, K.J.; Berretta, S.; Bolshakov, V.Y.; Rosso, I.M.; Meloni, E.G.; Rauch, S.L.; Carlezon, W.A. Post-Traumatic Stress Disorder: Clinical and Translational Neuroscience from Cells to Circuits. Nat. Rev. Neurol. 2022, 18, 273–288. [Google Scholar] [CrossRef]
- Sosa, J.L.R.; Buonomano, D.; Izquierdo, A. The Orbitofrontal Cortex in Temporal Cognition. Behav. Neurosci. 2021, 135, 154–164. [Google Scholar] [CrossRef]
- Musazzi, L.; Tornese, P.; Sala, N.; Popoli, M. Acute or Chronic? A Stressful Question. Trends Neurosci. 2017, 40, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Musazzi, L.; Treccani, G.; Popoli, M. Functional and Structural Remodeling of Glutamate Synapses in Prefrontal and Frontal Cortex Induced by Behavioral Stress. Front. Psychiatry 2015, 6, 60. [Google Scholar] [CrossRef] [PubMed]
- Bonifacino, T.; Mingardi, J.; Facchinetti, R.; Sala, N.; Frumento, G.; Ndoj, E.; Valenza, M.; Paoli, C.; Ieraci, A.; Torazza, C.; et al. Changes at Glutamate Tripartite Synapses in the Prefrontal Cortex of a New Animal Model of Resilience/Vulnerability to Acute Stress. Transl. Psychiatry 2023, 13, 62. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Rein, B. Mechanisms of Synaptic Transmission Dysregulation in the Prefrontal Cortex: Pathophysiological Implications. Mol. Psychiatry 2022, 27, 445–465. [Google Scholar] [CrossRef]
- Jumaili, W.A.; Trivedi, C.; Chao, T.; Kubosumi, A.; Jain, S. The Safety and Efficacy of Ketamine NMDA Receptor Blocker as a Therapeutic Intervention for PTSD Review of a Randomized Clinical Trial. Behav. Brain Res. 2022, 424, 113804. [Google Scholar] [CrossRef]
- Ragnhildstveit, A.; Roscoe, J.; Bass, L.C.; Averill, C.L.; Abdallah, C.G.; Averill, L.A. The Potential of Ketamine for Posttraumatic Stress Disorder: A Review of Clinical Evidence. Ther. Adv. Psychopharmacol. 2023, 13, 204512532311541. [Google Scholar] [CrossRef]
- Sala, N.; Paoli, C.; Bonifacino, T.; Mingardi, J.; Schiavon, E.; La Via, L.; Milanese, M.; Tornese, P.; Datusalia, A.K.; Rosa, J.; et al. Acute Ketamine Facilitates Fear Memory Extinction in a Rat Model of PTSD Along with Restoring Glutamatergic Alterations and Dendritic Atrophy in the Prefrontal Cortex. Front. Pharmacol. 2022, 13, 9626. [Google Scholar] [CrossRef]
- Peters, A.; Reisch, C.; Langemann, D. LTP or LTD? Modeling the Influence of Stress on Synaptic Plasticity. eNeuro 2018, 5, ENEURO.0242-17.2018. [Google Scholar] [CrossRef]
- Maroun, M.; Richter-Levin, G. Exposure to Acute Stress Blocks the Induction of Long-Term Potentiation of the Amygdala–Prefrontal Cortex Pathway In Vivo. J. Neurosci. 2003, 23, 4406–4409. [Google Scholar] [CrossRef]
- Rocher, C. Acute Stress-Induced Changes in Hippocampal/Prefrontal Circuits in Rats: Effects of Antidepressants. Cereb. Cortex 2004, 14, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Ryan, B.K.; Vollmayr, B.; Klyubin, I.; Gass, P.; Rowan, M.J. Persistent Inhibition of Hippocampal Long-Term Potentiation in Vivo by Learned Helplessness Stress. Hippocampus 2010, 20, 758–767. [Google Scholar] [CrossRef]
- Goto, Y.; Yang, C.R.; Otani, S. Functional and Dysfunctional Synaptic Plasticity in Prefrontal Cortex: Roles in Psychiatric Disorders. Biol. Psychiatry 2010, 67, 199–207. [Google Scholar] [CrossRef]
- Negrón-Oyarzo, I.; Dagnino-Subiabre, A.; Muñoz Carvajal, P. Synaptic Impairment in Layer 1 of the Prefrontal Cortex Induced by Repeated Stress During Adolescence Is Reversed in Adulthood. Front. Cell. Neurosci. 2015, 9, 442. [Google Scholar] [CrossRef] [PubMed]
- Otani, S.; Bai, J.; Blot, K. Dopaminergic Modulation of Synaptic Plasticity in Rat Prefrontal Neurons. Neurosci. Bull. 2015, 31, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Lamanna, J.; Isotti, F.; Ferro, M.; Racchetti, G.; Anchora, L.; Rucco, D.; Malgaroli, A. Facilitation of Dopamine-dependent Long-term Potentiation in the Medial Prefrontal Cortex of Male Rats Follows the Behavioral Effects of Stress. J. Neurosci. Res. 2021, 99, 662–678. [Google Scholar] [CrossRef]
- Baik, J.-H. Stress and the Dopaminergic Reward System. Exp. Mol. Med. 2020, 52, 1879–1890. [Google Scholar] [CrossRef]
- Musazzi, L.; Milanese, M.; Farisello, P.; Zappettini, S.; Tardito, D.; Barbiero, V.S.; Bonifacino, T.; Mallei, A.; Baldelli, P.; Racagni, G.; et al. Acute Stress Increases Depolarization-Evoked Glutamate Release in the Rat Prefrontal/Frontal Cortex: The Dampening Action of Antidepressants. PLoS ONE 2010, 5, e8566. [Google Scholar] [CrossRef]
- Sheynikhovich, D.; Otani, S.; Bai, J.; Arleo, A. Long-Term Memory, Synaptic Plasticity and Dopamine in Rodent Medial Prefrontal Cortex: Role in Executive Functions. Front. Behav. Neurosci. 2023, 16, 1068271. [Google Scholar] [CrossRef]
- Douma, E.H.; de Kloet, E.R. Stress-Induced Plasticity and Functioning of Ventral Tegmental Dopamine Neurons. Neurosci. Biobehav. Rev. 2020, 108, 48–77. [Google Scholar] [CrossRef]
- Feenstra, M.G.P. Dopamine and Noradrenaline Release in the Prefrontal Cortex in Relation to Unconditioned and Conditioned Stress and Reward. Prog. Brain Res. 2000, 126, 133–163. [Google Scholar]
- Raiteri, L.; Raiteri, M. Synaptosomes Still Viable after 25 Years of Superfusion. Neurochem. Res. 2000, 25, 1265–1274. [Google Scholar] [CrossRef] [PubMed]
- Kokkinou, M.; Ashok, A.H.; Howes, O.D. The Effects of Ketamine on Dopaminergic Function: Meta-Analysis and Review of the Implications for Neuropsychiatric Disorders. Mol. Psychiatry 2018, 23, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Belujon, P.; Grace, A.A. Restoring Mood Balance in Depression: Ketamine Reverses Deficit in Dopamine-Dependent Synaptic Plasticity. Biol. Psychiatry 2014, 76, 927–936. [Google Scholar] [CrossRef]
- Tornese, P.; Sala, N.; Bonini, D.; Bonifacino, T.; La Via, L.; Milanese, M.; Treccani, G.; Seguini, M.; Ieraci, A.; Mingardi, J.; et al. Chronic Mild Stress Induces Anhedonic Behavior and Changes in Glutamate Release, BDNF Trafficking and Dendrite Morphology Only in Stress Vulnerable Rats. The Rapid Restorative Action of Ketamine. Neurobiol. Stress 2019, 10, 100160. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, B. Stress Activation of Glutamate Neurotransmission in the Prefrontal Cortex: Implications for Dopamine-Associated Psychiatric Disorders. Biol. Psychiatry 2002, 51, 775–787. [Google Scholar] [CrossRef]
- Izquierdo, I.; Furini, C.R.G.; Myskiw, J.C. Fear Memory. Physiol. Rev. 2016, 96, 695–750. [Google Scholar] [CrossRef]
- Yang, Y.; Ju, W.; Zhang, H.; Sun, L. Effect of Ketamine on LTP and NMDAR EPSC in Hippocampus of the Chronic Social Defeat Stress Mice Model of Depression. Front. Behav. Neurosci. 2018, 12, 229. [Google Scholar] [CrossRef]
- Aleksandrova, L.R.; Wang, Y.T.; Phillips, A.G. Ketamine and Its Metabolite, (2R,6R)-HNK, Restore Hippocampal LTP and Long-Term Spatial Memory in the Wistar-Kyoto Rat Model of Depression. Mol. Brain 2020, 13, 92. [Google Scholar] [CrossRef]
- Hansen, K.B.; Wollmuth, L.P.; Bowie, D.; Furukawa, H.; Menniti, F.S.; Sobolevsky, A.I.; Swanson, G.T.; Swanger, S.A.; Greger, I.H.; Nakagawa, T.; et al. Structure, Function, and Pharmacology of Glutamate Receptor Ion Channels. Pharmacol. Rev. 2021, 73, 1469–1658. [Google Scholar] [CrossRef]
- Krzystanek, M.; Bogus, K.; Pałasz, A.; Krzystanek, E.; Worthington, J.J.; Wiaderkiewicz, R. Effects of Long-Term Treatment with the Neuroleptics Haloperidol, Clozapine and Olanzapine on Immunoexpression of NMDA Receptor Subunits NR1, NR2A and NR2B in the Rat Hippocampus. Pharmacol. Reports 2015, 67, 965–969. [Google Scholar] [CrossRef]
- Barbon, A.; Caracciolo, L.; Orlandi, C.; Musazzi, L.; Mallei, A.; Via, L.L.; Bonini, D.; Mora, C.; Tardito, D.; Gennarelli, M.; et al. Chronic Antidepressant Treatments Induce a Time-Dependent up-Regulation of AMPA Receptor Subunit Protein Levels. Neurochem. Int. 2011, 59, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Elhussiny, M.E.A.; Carini, G.; Mingardi, J.; Tornese, P.; Sala, N.; Bono, F.; Fiorentini, C.; La Via, L.; Popoli, M.; Musazzi, L.; et al. Modulation by Chronic Stress and Ketamine of Ionotropic AMPA/NMDA and Metabotropic Glutamate Receptors in the Rat Hippocampus. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 104, 110033. [Google Scholar] [CrossRef]
- Lester, R.A.J.; Clements, J.D.; Westbrook, G.L.; Jahr, C.E. Channel Kinetics Determine the Time Course of NMDA Receptor-Mediated Synaptic Currents. Nature 1990, 346, 565–567. [Google Scholar] [CrossRef]
- Erreger, K.; Dravid, S.M.; Banke, T.G.; Wyllie, D.J.A.; Traynelis, S.F. Subunit-Specific Gating Controls Rat NR1/NR2A and NR1/NR2B NMDA Channel Kinetics and Synaptic Signalling Profiles. J. Physiol. 2005, 563, 345–358. [Google Scholar] [CrossRef]
- Chen, N.; Luo, T.; Raymond, L.A. Subtype-Dependence of NMDA Receptor Channel Open Probability. J. Neurosci. 1999, 19, 6844–6854. [Google Scholar] [CrossRef] [PubMed]
- Diering, G.H.; Huganir, R.L. The AMPA Receptor Code of Synaptic Plasticity. Neuron 2018, 100, 314–329. [Google Scholar] [CrossRef]
- Wang, J.Q.; Guo, M.-L.; Jin, D.-Z.; Xue, B.; Fibuch, E.E.; Mao, L.-M. Roles of Subunit Phosphorylation in Regulating Glutamate Receptor Function. Eur. J. Pharmacol. 2014, 728, 183–187. [Google Scholar] [CrossRef]
- Lu, W.; Roche, K.W. Posttranslational Regulation of AMPA Receptor Trafficking and Function. Curr. Opin. Neurobiol. 2012, 22, 470–479. [Google Scholar] [CrossRef]
- Musazzi, L.; Sala, N.; Tornese, P.; Gallivanone, F.; Belloli, S.; Conte, A.; Di Grigoli, G.; Chen, F.; Ikinci, A.; Treccani, G.; et al. Acute Inescapable Stress Rapidly Increases Synaptic Energy Metabolism in Prefrontal Cortex and Alters Working Memory Performance. Cereb. Cortex 2019, 29, 4948–4957. [Google Scholar] [CrossRef] [PubMed]
- Martini, P.; Mingardi, J.; Carini, G.; Mattevi, S.; Ndoj, E.; La Via, L.; Magri, C.; Gennarelli, M.; Russo, I.; Popoli, M.; et al. Transcriptional Profiling of Rat Prefrontal Cortex after Acute Inescapable Footshock Stress. Genes 2023, 14, 740. [Google Scholar] [CrossRef] [PubMed]
- Mingardi, J.; Paoli, C.; La Via, L.; Carini, G.; Misztak, P.; Cifani, C.; Popoli, M.; Barbon, A.; Musazzi, L. Involvement of MiR-135a-5p Downregulation in Acute and Chronic Stress Response in the Prefrontal Cortex of Rats. Int. J. Mol. Sci. 2023, 24, 1552. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 7th ed.; Academic Press: San Diego, CA, USA, 2013; ISBN 9780124157521. [Google Scholar]
- Raiteri, L.; Zappettini, S.; Milanese, M.; Fedele, E.; Raiteri, M.; Bonanno, G. Mechanisms of Glutamate Release Elicited in Rat Cerebrocortical Nerve Endings by ‘Pathologically’ Elevated Extraterminal K + Concentrations. J. Neurochem. 2007, 103, 952–961. [Google Scholar] [CrossRef] [PubMed]
- Grilli, M.; Pittaluga, A.; Merlo-Pich, E.; Marchi, M. NMDA-Mediated Modulation of Dopamine Release Is Modified in Rat Prefrontal Cortex and Nucleus Accumbens after Chronic Nicotine Treatment. J. Neurochem. 2009, 108, 408–416. [Google Scholar] [CrossRef]
- Bonini, D.; Mora, C.; Tornese, P.; Sala, N.; Filippini, A.; La Via, L.; Milanese, M.; Calza, S.; Bonanno, G.; Racagni, G.; et al. Acute Footshock Stress Induces Time-Dependent Modifications of AMPA/NMDA Protein Expression and AMPA Phosphorylation. Neural Plast. 2016, 2016, 1–10. [Google Scholar] [CrossRef]
- Farrell, M.R.; Sengelaub, D.R.; Wellman, C.L. Sex Differences and Chronic Stress Effects on the Neural Circuitry Underlying Fear Conditioning and Extinction. Physiol. Behav. 2013, 122, 208–215. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forti, L.; Ndoj, E.; Mingardi, J.; Secchi, E.; Bonifacino, T.; Schiavon, E.; Carini, G.; La Via, L.; Russo, I.; Milanese, M.; et al. Dopamine-Dependent Ketamine Modulation of Glutamatergic Synaptic Plasticity in the Prelimbic Cortex of Adult Rats Exposed to Acute Stress. Int. J. Mol. Sci. 2023, 24, 8718. https://doi.org/10.3390/ijms24108718
Forti L, Ndoj E, Mingardi J, Secchi E, Bonifacino T, Schiavon E, Carini G, La Via L, Russo I, Milanese M, et al. Dopamine-Dependent Ketamine Modulation of Glutamatergic Synaptic Plasticity in the Prelimbic Cortex of Adult Rats Exposed to Acute Stress. International Journal of Molecular Sciences. 2023; 24(10):8718. https://doi.org/10.3390/ijms24108718
Chicago/Turabian StyleForti, Lia, Elona Ndoj, Jessica Mingardi, Emanuele Secchi, Tiziana Bonifacino, Emanuele Schiavon, Giulia Carini, Luca La Via, Isabella Russo, Marco Milanese, and et al. 2023. "Dopamine-Dependent Ketamine Modulation of Glutamatergic Synaptic Plasticity in the Prelimbic Cortex of Adult Rats Exposed to Acute Stress" International Journal of Molecular Sciences 24, no. 10: 8718. https://doi.org/10.3390/ijms24108718
APA StyleForti, L., Ndoj, E., Mingardi, J., Secchi, E., Bonifacino, T., Schiavon, E., Carini, G., La Via, L., Russo, I., Milanese, M., Gennarelli, M., Bonanno, G., Popoli, M., Barbon, A., & Musazzi, L. (2023). Dopamine-Dependent Ketamine Modulation of Glutamatergic Synaptic Plasticity in the Prelimbic Cortex of Adult Rats Exposed to Acute Stress. International Journal of Molecular Sciences, 24(10), 8718. https://doi.org/10.3390/ijms24108718









