The Preventive Role of the Vitamin D Endocrine System in Cervical Cancer
Abstract
1. Introduction
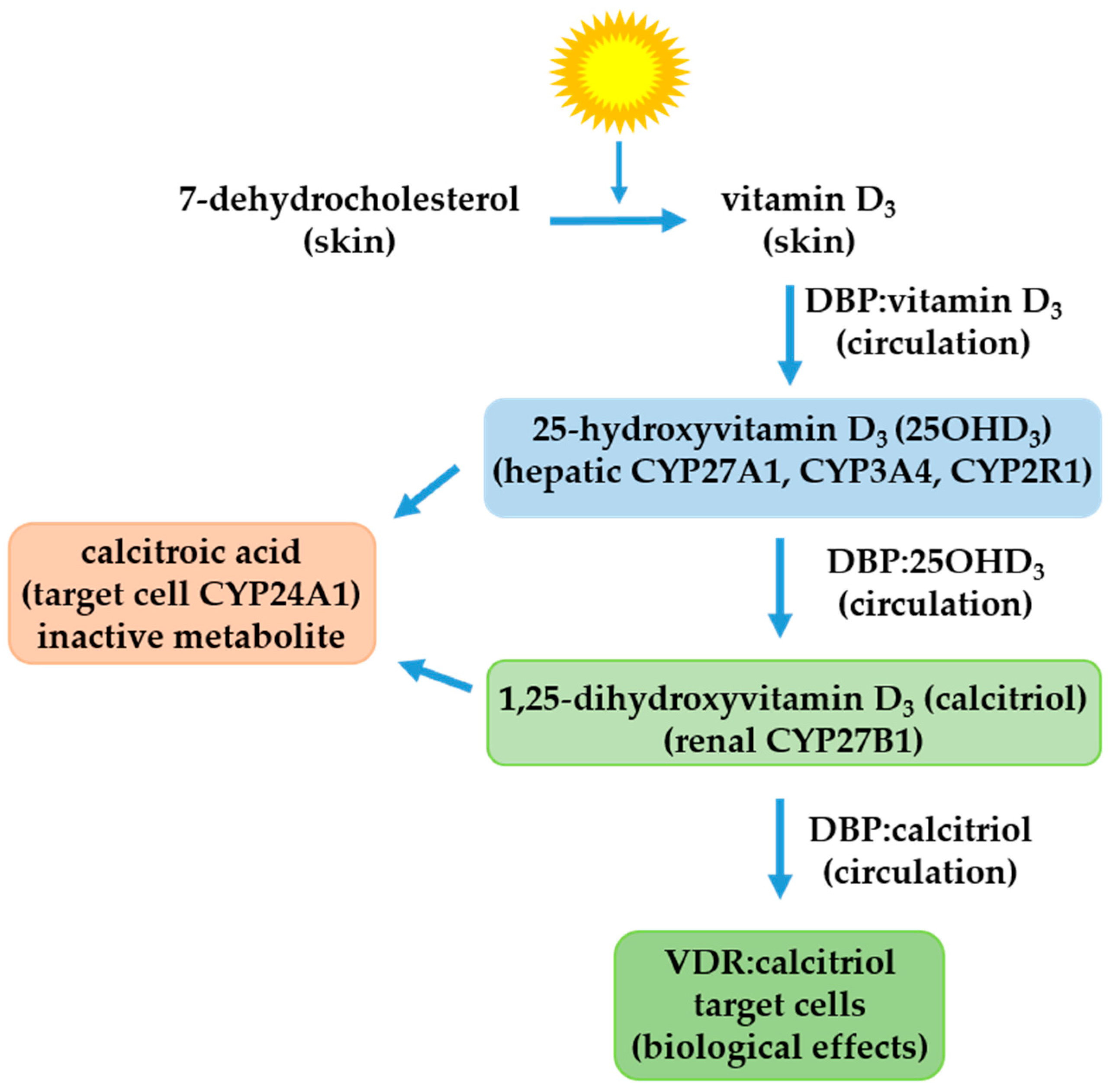
2. The Vitamin D Endocrine System
3. The Vitamin D Endocrine System in Cervical Cancer: Preclinical Studies
4. The Vitamin D Endocrine System in Cervical Cancer: Clinical Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.K.; Azuine, R.E.; Siahpush, M. Global Inequalities in Cervical Cancer Incidence and Mortality are Linked to Deprivation, Low Socioeconomic Status, and Human Development. Int. J. MCH AIDS 2012, 1, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Arbyn, M.; Xu, L.; Simoens, C.; Martin-Hirsch, P.P. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst. Rev. 2018, 5, CD009069. [Google Scholar] [CrossRef] [PubMed]
- Burd, E.M. Human papillomavirus and cervical cancer. Clin. Microbiol. Rev. 2003, 16, 1. [Google Scholar] [CrossRef] [PubMed]
- Mishra, G.A.; Pimple, S.A.; Shastri, S.S. An overview of prevention and early detection of cervical cancers. Indian J. Med. Paediatr. Oncol. 2011, 32, 125–132. [Google Scholar] [CrossRef]
- Arbyn, M.; Weiderpass, E.; Bruni, L.; de Sanjose, S.; Saraiya, M.; Ferlay, J.; Bray, F. Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. Lancet Glob. Health 2020, 8, e191–e203. [Google Scholar] [CrossRef]
- Paulino, E.; de Melo, A.C.; de Andrade, D.A.P.; de Almeida, M.S. Systemic therapy for advanced cervical cancer: Leveraging the historical threshold of overall survival. Crit. Rev. Oncol. Hematol. 2023, 183, 103925. [Google Scholar] [CrossRef]
- Alrajjal, A.; Pansare, V.; Choudhury, M.S.R.; Khan, M.Y.A.; Shidham, V.B. Squamous intraepithelial lesions (SIL: LSIL, HSIL, ASCUS, ASC-H, LSIL-H) of Uterine Cervix and Bethesda System. Cytojournal 2021, 18, 16. [Google Scholar] [CrossRef]
- Brinton, L.A.; Reeves, W.C.; Brenes, M.M.; Herrero, R.; de Britton, R.C.; Gaitan, E.; Tenorio, F.; Garcia, M.; Rawls, W.E. Parity as a risk factor for cervical cancer. Am. J. Epidemiol. 1989, 130, 486–496. [Google Scholar] [CrossRef]
- Vaccarella, S.; Franceschi, S.; Herrero, R.; Munoz, N.; Snijders, P.J.; Clifford, G.M.; Smith, J.S.; Lazcano-Ponce, E.; Sukvirach, S.; Shin, H.R.; et al. Sexual behavior, condom use, and human papillomavirus: Pooled analysis of the IARC human papillomavirus prevalence surveys. Cancer Epidemiol. Biomark. Prev. 2006, 15, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Louie, K.S.; de Sanjose, S.; Diaz, M.; Castellsague, X.; Herrero, R.; Meijer, C.J.; Shah, K.; Franceschi, S.; Munoz, N.; Bosch, F.X.; et al. Early age at first sexual intercourse and early pregnancy are risk factors for cervical cancer in developing countries. Br. J. Cancer 2009, 100, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wu, X.; Lin, Y.; Li, W.; Liu, J.; Song, B. Multiple sexual partners and vaginal microecological disorder are associated with HPV infection and cervical carcinoma development. Oncol. Lett. 2020, 20, 1915–1921. [Google Scholar] [CrossRef]
- de Abreu, A.L.; Malaguti, N.; Souza, R.P.; Uchimura, N.S.; Ferreira, E.C.; Pereira, M.W.; Carvalho, M.D.; Pelloso, S.M.; Bonini, M.G.; Gimenes, F.; et al. Association of human papillomavirus, Neisseria gonorrhoeae and Chlamydia trachomatis co-infections on the risk of high-grade squamous intraepithelial cervical lesion. Am. J. Cancer Res. 2016, 6, 1371–1383. [Google Scholar] [PubMed]
- Stone, K.M.; Zaidi, A.; Rosero-Bixby, L.; Oberle, M.W.; Reynolds, G.; Larsen, S.; Nahmias, A.J.; Lee, F.K.; Schachter, J.; Guinan, M.E. Sexual behavior, sexually transmitted diseases, and risk of cervical cancer. Epidemiology 1995, 6, 409–414. [Google Scholar] [CrossRef]
- Garcia-Quiroz, J.; Vazquez-Almazan, B.; Garcia-Becerra, R.; Diaz, L.; Avila, E. The Interaction of Human Papillomavirus Infection and Prostaglandin E2 Signaling in Carcinogenesis: A Focus on Cervical Cancer Therapeutics. Cells 2022, 11, 2528. [Google Scholar] [CrossRef]
- Abraham, A.G.; D’Souza, G.; Jing, Y.; Gange, S.J.; Sterling, T.R.; Silverberg, M.J.; Saag, M.S.; Rourke, S.B.; Rachlis, A.; Napravnik, S.; et al. Invasive cervical cancer risk among HIV-infected women: A North American multicohort collaboration prospective study. J. Acquir. Immune Defic. Syndr. 2013, 62, 405–413. [Google Scholar] [CrossRef]
- Moreno, V.; Bosch, F.X.; Munoz, N.; Meijer, C.J.; Shah, K.V.; Walboomers, J.M.; Herrero, R.; Franceschi, S.; International Agency for Research on Cancer (IARC) Multicentric Cervical Cancer Study Group. Effect of oral contraceptives on risk of cervical cancer in women with human papillomavirus infection: The IARC multicentric case-control study. Lancet 2002, 359, 1085–1092. [Google Scholar] [CrossRef]
- Hellberg, D.; Nilsson, S.; Haley, N.J.; Hoffman, D.; Wynder, E. Smoking and cervical intraepithelial neoplasia: Nicotine and cotinine in serum and cervical mucus in smokers and nonsmokers. Am. J. Obs. Gynecol. 1988, 158, 910–913. [Google Scholar] [CrossRef]
- Bosch, F.X.; Munoz, N.; de Sanjose, S.; Izarzugaza, I.; Gili, M.; Viladiu, P.; Tormo, M.J.; Moreo, P.; Ascunce, N.; Gonzalez, L.C.; et al. Risk factors for cervical cancer in Colombia and Spain. Int. J. Cancer 1992, 52, 750–758. [Google Scholar] [CrossRef]
- Siegel, E.M.; Salemi, J.L.; Villa, L.L.; Ferenczy, A.; Franco, E.L.; Giuliano, A.R. Dietary consumption of antioxidant nutrients and risk of incident cervical intraepithelial neoplasia. Gynecol. Oncol. 2010, 118, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, C.A.; Travier, N.; Lujan-Barroso, L.; Castellsague, X.; Bosch, F.X.; Roura, E.; Bueno-de-Mesquita, H.B.; Palli, D.; Boeing, H.; Pala, V.; et al. Dietary factors and in situ and invasive cervical cancer risk in the European prospective investigation into cancer and nutrition study. Int. J. Cancer 2011, 129, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Negri, E.; La Vecchia, C.; Bosetti, C.; Franceschi, S.; Parazzini, F. Risk of cervical cancer in women with a family history of breast and female genital tract neoplasms. Int. J. Cancer 2005, 117, 880–881. [Google Scholar] [CrossRef]
- Poorolajal, J.; Jenabi, E. The association between BMI and cervical cancer risk: A meta-analysis. Eur. J. Cancer Prev. 2016, 25, 232–238. [Google Scholar] [CrossRef]
- Barrea, L.; Frias-Toral, E.; Pugliese, G.; Garcia-Velasquez, E.; Savastano, S.; Colao, A.; Muscogiuri, G. Vitamin D in obesity and obesity-related diseases: An overview. Minerva Endocrinol. 2021, 46, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Hosono, S.; Matsuo, K.; Kajiyama, H.; Hirose, K.; Suzuki, T.; Kawase, T.; Kidokoro, K.; Nakanishi, T.; Hamajima, N.; Kikkawa, F.; et al. Association between dietary calcium and vitamin D intake and cervical carcinogenesis among Japanese women. Eur. J. Clin. Nutr. 2010, 64, 400–409. [Google Scholar] [CrossRef]
- Grant, W.B. Does solar ultraviolet irradiation affect cancer mortality rates in China? Asian Pac. J. Cancer Prev. 2007, 8, 236–242. [Google Scholar]
- Grant, W.B. An ecological study of cancer incidence and mortality rates in France with respect to latitude, an index for vitamin D production. Dermatoendocrinology 2010, 2, 62–67. [Google Scholar] [CrossRef]
- Ozgu, E.; Yilmaz, N.; Baser, E.; Gungor, T.; Erkaya, S.; Yakut, H.I. Could 25-OH vitamin D deficiency be a reason for HPV infection persistence in cervical premalignant lesions? J. Exp. Ther. Oncol. 2016, 11, 177–180. [Google Scholar]
- Shim, J.; Perez, A.; Symanski, E.; Nyitray, A.G. Association Between Serum 25-Hydroxyvitamin D Level and Human Papillomavirus Cervicovaginal Infection in Women in the United States. J. Infect. Dis. 2016, 213, 1886–1892. [Google Scholar] [CrossRef]
- Vahedpoor, Z.; Jamilian, M.; Bahmani, F.; Aghadavod, E.; Karamali, M.; Kashanian, M.; Asemi, Z. Effects of Long-Term Vitamin D Supplementation on Regression and Metabolic Status of Cervical Intraepithelial Neoplasia: A Randomized, Double-Blind, Placebo-Controlled Trial. Horm. Cancer. 2017, 8, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Vahedpoor, Z.; Mahmoodi, S.; Samimi, M.; Gilasi, H.R.; Bahmani, F.; Soltani, A.; Sharifi Esfahani, M.; Asemi, Z. Long-Term Vitamin D Supplementation and the Effects on Recurrence and Metabolic Status of Cervical Intraepithelial Neoplasia Grade 2 or 3: A Randomized, Double-Blind, Placebo-Controlled Trial. Ann. Nutr. Metab. 2018, 72, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Photosynthesis of vitamin D in the skin: Effect of environmental and life-style variables. Fed. Proc. 1987, 46, 1876–1882. [Google Scholar] [PubMed]
- Zhu, J.; DeLuca, H.F. Vitamin D 25-hydroxylase—Four decades of searching, are we there yet? Arch. Biochem. Biophys. 2012, 523, 30–36. [Google Scholar] [CrossRef]
- Hewison, M.; Zehnder, D.; Bland, R.; Stewart, P.M. 1alpha-Hydroxylase and the action of vitamin D. J. Mol. Endocrinol. 2000, 25, 141–148. [Google Scholar] [CrossRef]
- Olmos-Ortiz, A.; Avila, E.; Durand-Carbajal, M.; Diaz, L. Regulation of calcitriol biosynthesis and activity: Focus on gestational vitamin D deficiency and adverse pregnancy outcomes. Nutrients 2015, 7, 443–480. [Google Scholar] [CrossRef]
- Norman, A.W. Minireview: Vitamin D receptor: New assignments for an already busy receptor. Endocrinology 2006, 147, 5542–5548. [Google Scholar] [CrossRef]
- Jones, G.; Prosser, D.E.; Kaufmann, M. 25-Hydroxyvitamin D-24-hydroxylase (CYP24A1): Its important role in the degradation of vitamin D. Arch. Biochem. Biophys. 2012, 523, 9–18. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, J.; DeLuca, H.F. Where is the vitamin D receptor? Arch. Biochem. Biophys. 2012, 523, 123–133. [Google Scholar] [CrossRef]
- Deuster, E.; Jeschke, U.; Ye, Y.; Mahner, S.; Czogalla, B. Vitamin D and VDR in Gynecological Cancers–A Systematic Review. Int. J. Mol. Sci. 2017, 18, 2328. [Google Scholar] [CrossRef]
- Yoshizawa, T.; Handa, Y.; Uematsu, Y.; Takeda, S.; Sekine, K.; Yoshihara, Y.; Kawakami, T.; Arioka, K.; Sato, H.; Uchiyama, Y.; et al. Mice lacking the vitamin D receptor exhibit impaired bone formation, uterine hypoplasia and growth retardation after weaning. Nat. Genet. 1997, 16, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.P. Vitamin D in health and disease. Clin. J. Am. Soc. Nephrol. 2008, 3, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C.; Velleuer, E. Vitamin D and the risk for cancer: A molecular analysis. Biochem. Pharm. 2021, 196, 114735. [Google Scholar] [CrossRef] [PubMed]
- Diaz, L.; Diaz-Munoz, M.; Garcia-Gaytan, A.C.; Mendez, I. Mechanistic Effects of Calcitriol in Cancer Biology. Nutrients 2015, 7, 5020–5050. [Google Scholar] [CrossRef]
- Shao, T.; Klein, P.; Grossbard, M.L. Vitamin D and breast cancer. Oncologist 2012, 17, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Salih, M.M.; Almehmadi, M.; Shafie, A.; Alsharif, A.; Alsiwiehri, N.; El-Askary, A.; Alzahrani, K.; Aljuaid, A.; Abdulaziz, O.; Alrehaili, A.A.; et al. Evaluation of CD4+:CD8+ Ratio in Patients With Cervical Cancer and the Levels of Inflammatory Markers. Vivo 2022, 36, 2414–2421. [Google Scholar] [CrossRef]
- Keeratichamroen, S.; Subhasitanont, P.; Chokchaichamnankit, D.; Weeraphan, C.; Saharat, K.; Sritana, N.; Kantathavorn, N.; Wiriyaukaradecha, K.; Sricharunrat, T.; Paricharttanakul, N.M.; et al. Identification of potential cervical cancer serum biomarkers in Thai patients. Oncol. Lett. 2020, 19, 3815–3826. [Google Scholar] [CrossRef]
- Friedrich, M.; Rafi, L.; Mitschele, T.; Tilgen, W.; Schmidt, W.; Reichrath, J. Analysis of the vitamin D system in cervical carcinomas, breast cancer and ovarian cancer. Recent. Results Cancer Res. 2003, 164, 239–246. [Google Scholar] [CrossRef]
- Reichrath, J.; Rafi, L.; Muller, S.M.; Mink, D.; Reitnauer, K.; Tilgen, W.; Schmidt, W.; Friedrich, M. Immunohistochemical analysis of 1,25-dihydroxyvitamin D3 receptor in cervical carcinoma. Histochem. J. 1998, 30, 561–567. [Google Scholar] [CrossRef]
- Friedrich, M.; Villena-Heinsen, C.; Axt-Fliedner, R.; Meyberg, R.; Tilgen, W.; Schmidt, W.; Reichrath, J. Analysis of 25-hydroxyvitamin D3-1alpha-hydroxylase in cervical tissue. Anticancer Res. 2002, 22, 183–186. [Google Scholar]
- Kloss, M.; Fischer, D.; Thill, M.; Friedrich, M.; Cordes, T.; Salehin, D.; Diedrich, K.; Koster, F. Vitamin D, calcidiol and calcitriol regulate vitamin D metabolizing enzymes in cervical and ovarian cancer cells. Anticancer Res. 2010, 30, 4429–4434. [Google Scholar] [PubMed]
- Avila, E.; Garcia-Becerra, R.; Rodriguez-Rasgado, J.A.; Diaz, L.; Ordaz-Rosado, D.; Zugel, U.; Steinmeyer, A.; Barrera, D.; Halhali, A.; Larrea, F.; et al. Calcitriol down-regulates human ether a go-go 1 potassium channel expression in cervical cancer cells. Anticancer Res. 2010, 30, 2667–2672. [Google Scholar] [PubMed]
- Cazares-Ordonez, V.; Gonzalez-Duarte, R.J.; Diaz, L.; Ishizawa, M.; Uno, S.; Ortiz, V.; Ordonez-Sanchez, M.L.; Makishima, M.; Larrea, F.; Avila, E. A cis-acting element in the promoter of human ether a go-go 1 potassium channel gene mediates repression by calcitriol in human cervical cancer cells. Biochem. Cell. Biol. 2015, 93, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Duarte, R.J.; Cazares-Ordonez, V.; Romero-Cordoba, S.; Diaz, L.; Ortiz, V.; Freyre-Gonzalez, J.A.; Hidalgo-Miranda, A.; Larrea, F.; Avila, E. Calcitriol increases Dicer expression and modifies the microRNAs signature in SiHa cervical cancer cells. Biochem. Cell. Biol. 2015, 93, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M.; Meyberg, R.; Axt-Fliedner, R.; Villena-Heinsen, C.; Tilgen, W.; Schmidt, W.; Reichrath, J. Vitamin D receptor (VDR) expression is not a prognostic factor in cervical cancer. Anticancer Res. 2002, 22, 299–304. [Google Scholar]
- Li, D.; Liu, Y.; Kong, D.; Papukashvili, D.; Rcheulishvili, N.; Zhao, H.; Li, Y.; Hou, C.; Ma, J.; Lu, X.; et al. Vitamin D Receptor Gene Polymorphisms and the Risk of CIN2+ in Shanxi Population. Biomed. Res. Int. 2022, 2022, 6875996. [Google Scholar] [CrossRef]
- Phuthong, S.; Settheetham-Ishida, W.; Natphopsuk, S.; Ishida, T. Genetic Polymorphisms of Vitamin D Receptor Gene are Associated with Cervical Cancer Risk in Northeastern Thailand. Asian Pac. J. Cancer. Prev. 2020, 21, 2935–2939. [Google Scholar] [CrossRef]
- Gnagnarella, P.; Pasquali, E.; Serrano, D.; Raimondi, S.; Disalvatore, D.; Gandini, S. Vitamin D receptor polymorphism FokI and cancer risk: A comprehensive meta-analysis. Carcinogenesis 2014, 35, 1913–1919. [Google Scholar] [CrossRef]
- Feldman, D.; Krishnan, A.V.; Swami, S.; Giovannucci, E.; Feldman, B.J. The role of vitamin D in reducing cancer risk and progression. Nat. Rev. Cancer 2014, 14, 342–357. [Google Scholar] [CrossRef]
- Wang, G.; Lei, L.; Zhao, X.; Zhang, J.; Zhou, M.; Nan, K. Calcitriol Inhibits Cervical Cancer Cell Proliferation Through Downregulation of HCCR1 Expression. Oncol. Res. 2014, 22, 301–309. [Google Scholar] [CrossRef]
- Bhoora, S.; Pillay, T.S.; Punchoo, R. Cholecalciferol induces apoptosis via autocrine metabolism in epidermoid cervical cancer cells. Biochem. Cell Biol. 2022, 100, 387–402. [Google Scholar] [CrossRef] [PubMed]
- Pardo, L.A.; del Camino, D.; Sanchez, A.; Alves, F.; Bruggemann, A.; Beckh, S.; Stuhmer, W. Oncogenic potential of EAG K(+) channels. EMBO J. 1999, 18, 5540–5547. [Google Scholar] [CrossRef] [PubMed]
- Farias, L.M.; Ocana, D.B.; Diaz, L.; Larrea, F.; Avila-Chavez, E.; Cadena, A.; Hinojosa, L.M.; Lara, G.; Villanueva, L.A.; Vargas, C.; et al. Ether a go-go potassium channels as human cervical cancer markers. Cancer Res. 2004, 64, 6996–7001. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, C.S.; Montante-Montes, D.; Saqui-Salces, M.; Hinojosa, L.M.; Gamboa-Dominguez, A.; Hernandez-Gallegos, E.; Martinez-Benitez, B.; Del Rosario Solis-Pancoatl, M.; Garcia-Villa, E.; Ramirez, A.; et al. Eag1 potassium channels as markers of cervical dysplasia. Oncol. Rep. 2011, 26, 1377–1383. [Google Scholar] [CrossRef] [PubMed]
- Diaz, L.; Ceja-Ochoa, I.; Restrepo-Angulo, I.; Larrea, F.; Avila-Chavez, E.; Garcia-Becerra, R.; Borja-Cacho, E.; Barrera, D.; Ahumada, E.; Gariglio, P.; et al. Estrogens and human papilloma virus oncogenes regulate human ether-a-go-go-1 potassium channel expression. Cancer Res. 2009, 69, 3300–3307. [Google Scholar] [CrossRef] [PubMed]
- Cho, G.W.; Shin, S.M.; Kim, H.K.; Ha, S.A.; Kim, S.; Yoon, J.H.; Hur, S.Y.; Kim, T.E.; Kim, J.W. HCCR-1, a novel oncogene, encodes a mitochondrial outer membrane protein and suppresses the UVC-induced apoptosis. BMC Cell Biol. 2007, 8, 50. [Google Scholar] [CrossRef]
- Setiawan, I.; Lesmana, R.; Goenawan, H.; Suardi, D.; Gatera, V.A.; Abdulah, R.; Judistiani, R.T.D.; Supratman, U.; Setiabudiawan, B. Calcitriol potentially alters HeLa cell viability via inhibition of autophagy. J. Cancer Res. 2022, 18, 1144–1151. [Google Scholar] [CrossRef]
- Bhoora, S.; Pather, Y.; Marais, S.; Punchoo, R. Cholecalciferol Inhibits Cell Growth and Induces Apoptosis in the CaSki Cell Line. Med. Sci. 2020, 8, 12. [Google Scholar] [CrossRef]
- Punchoo, R.; Dreyer, G.; Pillay, T.S. 25-Hydroxycholecalciferol inhibits cell growth and induces apoptosis in SiHa cervical cells via autocrine vitamin D metabolism. Biomedicines 2023, 11, 871. [Google Scholar] [CrossRef]
- Diaz, L.; Bernadez-Vallejo, S.V.; Vargas-Castro, R.; Avila, E.; Gomez-Ceja, K.A.; Garcia-Becerra, R.; Segovia-Mendoza, M.; Prado-Garcia, H.; Lara-Sotelo, G.; Camacho, J.; et al. The Phytochemical alpha-Mangostin Inhibits Cervical Cancer Cell Proliferation and Tumor Growth by Downregulating E6/E7-HPV Oncogenes and KCNH1 Gene Expression. Int. J. Mol. Sci. 2023, 24, 3055. [Google Scholar] [CrossRef]
- Gonzalez-Duarte, R.J.; Cazares-Ordonez, V.; Diaz, L.; Ortiz, V.; Larrea, F.; Avila, E. The expression of RNA helicase DDX5 is transcriptionally upregulated by calcitriol through a vitamin D response element in the proximal promoter in SiHa cervical cells. Mol. Cell Biochem. 2015, 410, 65–73. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Wongjampa, W.; Ekalaksananan, T.; Chopjitt, P.; Chuerduangphui, J.; Kleebkaow, P.; Patarapadungkit, N.; Pientong, C. Suppression of miR-22, a tumor suppressor in cervical cancer, by human papillomavirus 16 E6 via a p53/miR-22/HDAC6 pathway. PLoS ONE 2018, 13, e0206644. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.; Gao, Y.; Qie, M. MiR-29c-3p inhibits epithelial-mesenchymal transition to inhibit the proliferation, invasion and metastasis of cervical cancer cells by targeting SPARC. Ann. Transl. Med. 2021, 9, 125. [Google Scholar] [CrossRef]
- Fu, J.; Zhang, Y.; Wang, M.; Hu, J.; Fang, Y. Inhibition of the long non-coding RNA UNC5B-AS1/miR-4455/RSPO4 axis reduces cervical cancer growth in vitro and in vivo. J. Gene Med. 2021, 23, e3382. [Google Scholar] [CrossRef]
- Lappe, J.M.; Travers-Gustafson, D.; Davies, K.M.; Recker, R.R.; Heaney, R.P. Vitamin D and calcium supplementation reduces cancer risk: Results of a randomized trial. Am. J. Clin. Nutr. 2007, 85, 1586–1591. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Y.; Liu, Z.; Pei, Y.; Xu, P.; Chong, W.; Hai, Y.; He, L.; He, Y.; Yu, J.; et al. Association between Vitamin D Supplementation and Cancer Mortality: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 3717. [Google Scholar] [CrossRef]
- Adams, S.; Lin, J.; Brown, D.; Shriver, C.D.; Zhu, K. Ultraviolet Radiation Exposure and the Incidence of Oral, Pharyngeal and Cervical Cancer and Melanoma: An Analysis of the SEER Data. Anticancer Res. 2016, 36, 233–237. [Google Scholar]
- Fleischer, A.B., Jr.; Fleischer, S.E. Solar radiation and the incidence and mortality of leading invasive cancers in the United States. Dermatoendocrinology 2016, 8, e1162366. [Google Scholar] [CrossRef]
- Grant, W.B. A meta-analysis of second cancers after a diagnosis of nonmelanoma skin cancer: Additional evidence that solar ultraviolet-B irradiance reduces the risk of internal cancers. J. Steroid. Biochem. Mol. Biol. 2007, 103, 668–674. [Google Scholar] [CrossRef]
- De Jaeghere, E.A.; Tuyaerts, S.; Van Nuffel, A.M.T.; Belmans, A.; Bogaerts, K.; Baiden-Amissah, R.; Lippens, L.; Vuylsteke, P.; Henry, S.; Trinh, X.B.; et al. Pembrolizumab, radiotherapy, and an immunomodulatory five-drug cocktail in pretreated patients with persistent, recurrent, or metastatic cervical or endometrial carcinoma: Results of the phase II PRIMMO study. Cancer Immunol. Immunother. 2023, 72, 475–491. [Google Scholar] [CrossRef] [PubMed]
- Schulte-Uebbing, C.; Schlett, S.; Craiut, I.; Antal, L.; Olah, H. Chronical cervical infections and dysplasia (CIN I, CIN II): Vaginal vitamin D (high dose) treatment: A new effective method? Dermatoendocrinology 2014, 6, e27791. [Google Scholar] [CrossRef] [PubMed]
- Troja, C.; Hoofnagle, A.N.; Szpiro, A.; Stern, J.E.; Lin, J.; Winer, R.L. Understanding the Role of Emerging Vitamin D Biomarkers on Short-term Persistence of High-Risk Human Papillomavirus Infection Among Mid-Adult Women. J. Infect. Dis. 2021, 224, 123–132. [Google Scholar] [CrossRef]
- Singh, A.K.; Devi, K.N.; Bist, J.S. A comparative evaluation of therapeutic response in warts to intralesional vitamin D3 versus intralesional measles, mumps, and rubella vaccine. Dermatol. Ther. 2022, 35, e15813. [Google Scholar] [CrossRef] [PubMed]
- Mohta, A.; Kushwaha, R.K.; Agrawal, A.; Sharma, M.K.; Gautam, U.; Jain, S.K. Evaluation of the Efficacy of Intralesional Measles, Mumps, and Rubella Vaccine with Intralesional Vitamin D3 as Immunotherapies in the Treatment of Recalcitrant Cutaneous Warts in Adult- A Randomized Placebo-Controlled Study. Indian Derm. Online J. 2021, 12, 879–887. [Google Scholar] [CrossRef]
- Kavya, M.; Shashikumar, B.M.; Harish, M.R.; Shweta, B.P. Safety and Efficacy of Intralesional Vitamin D3 in Cutaneous Warts: An Open Uncontrolled Trial. J. Cutan. Aesthet. Surg. 2017, 10, 90–94. [Google Scholar] [CrossRef]
- Liu, P.T.; Stenger, S.; Li, H.; Wenzel, L.; Tan, B.H.; Krutzik, S.R.; Ochoa, M.T.; Schauber, J.; Wu, K.; Meinken, C.; et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 2006, 311, 1770–1773. [Google Scholar] [CrossRef]
- El-Esawy, F.M.; Sorour, N.E.; Akl, E.M.; Abdou, A.G.; El-Sayed, S.E. Immunohistochemical Expression of Cathelicidin in Verruca Vulgaris before and after intralesional injection of Vitamin D3. Benha. J. Appl. Sci. 2018, 3, 89–92. [Google Scholar] [CrossRef]
- Jha, N. Complete clearance of condyloma acuminata using injection Vitamin D3. Australas J. Derm. 2021, 62, e417–e418. [Google Scholar] [CrossRef]
- Rind, T.; Oiso, N.; Kawada, A. Successful Treatment of Anogenital Wart with a Topical Vitamin D(3) Derivative in an Infant. Case Rep. Derm. 2010, 2, 46–49. [Google Scholar] [CrossRef]
- Park, M.R.; Lee, J.H.; Park, M.S.; Hwang, J.E.; Shim, H.J.; Cho, S.H.; Chung, I.J.; Bae, W.K. Suppressive effect of 19-nor-1alpha-25-dihydroxyvitamin D2 on gastric cancer cells and peritoneal metastasis model. J. Korean Med. Sci. 2012, 27, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, J.; Ge, X.; Du, J.; Deb, D.K.; Li, Y.C. Vitamin D receptor inhibits nuclear factor kappaB activation by interacting with IkappaB kinase beta protein. J. Biol. Chem. 2013, 288, 19450–19458. [Google Scholar] [CrossRef] [PubMed]
- Kostoglou-Athanassiou, I.; Athanassiou, P.; Lyraki, A.; Raftakis, I.; Antoniadis, C. Vitamin D and rheumatoid arthritis. Adv. Endocrinol. Metab. 2012, 3, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.; Fischer, R.; Schneider, M. Bone density and 25-OH vitamin D serum level in patients with systemic lupus erythematosus. Z. Rheumatol. 2001, 60, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J.; Cooper, S.C.; Ghosh, S.; Hewison, M. The Role of Vitamin D in Inflammatory Bowel Disease: Mechanism to Management. Nutrients 2019, 11, 1019. [Google Scholar] [CrossRef]
- Kim, S.C.; Glynn, R.J.; Giovannucci, E.; Hernandez-Diaz, S.; Liu, J.; Feldman, S.; Karlson, E.W.; Schneeweiss, S.; Solomon, D.H. Risk of high-grade cervical dysplasia and cervical cancer in women with systemic inflammatory diseases: A population-based cohort study. Ann. Rheum. Dis. 2015, 74, 1360–1367. [Google Scholar] [CrossRef]
- Garcia-Carrasco, M.; Mendoza-Pinto, C.; Munguia-Realpozo, P.; Rodriguez-Gallegos, A.; Vallejo-Ruiz, V.; Munoz-Guarneros, M.; Mendez-Martinez, S.; Soto-Santillan, P.; Pezzat-Said, E.; Reyes-Leyva, J.; et al. Lack of association between serum 25-hydroxyvitamin D levels and cervical human papillomavirus infection in systemic lupus erythematosus. Lupus 2015, 24, 606–612. [Google Scholar] [CrossRef]
- Gunville, C.F.; Mourani, P.M.; Ginde, A.A. The role of vitamin D in prevention and treatment of infection. Inflamm. Allergy Drug. Targets 2013, 12, 239–245. [Google Scholar] [CrossRef]
- Gombart, A.F. The vitamin D-antimicrobial peptide pathway and its role in protection against infection. Future Microbiol. 2009, 4, 1151–1165. [Google Scholar] [CrossRef]
- Wiens, M.E.; Smith, J.G. Alpha-Defensin HD5 Inhibits Human Papillomavirus 16 Infection via Capsid Stabilization and Redirection to the Lysosome. mBio 2017, 8, 1. [Google Scholar] [CrossRef]
- Sinopoli, A.; Caminada, S.; Isonne, C.; Santoro, M.M.; Baccolini, V. What Are the Effects of Vitamin A Oral Supplementation in the Prevention and Management of Viral Infections? A Systematic Review of Randomized Clinical Trials. Nutrients 2022, 14, 4081. [Google Scholar] [CrossRef] [PubMed]
- Piyathilake, C.J.; Macaluso, M.; Chambers, M.M.; Badiga, S.; Siddiqui, N.R.; Bell, W.C.; Edberg, J.C.; Partridge, E.E.; Alvarez, R.D.; Johanning, G.L. Folate and vitamin B12 may play a critical role in lowering the HPV 16 methylation-associated risk of developing higher grades of CIN. Cancer Prev. Res. 2014, 7, 1128–1137. [Google Scholar] [CrossRef]
- Kim, J.; Kim, M.K.; Lee, J.K.; Kim, J.H.; Son, S.K.; Song, E.S.; Lee, K.B.; Lee, J.P.; Lee, J.M.; Yun, Y.M. Intakes of vitamin A, C, and E, and beta-carotene are associated with risk of cervical cancer: A case-control study in Korea. Nutr. Cancer 2010, 62, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Markowska, A.; Antoszczak, M.; Markowska, J.; Huczynski, A. Role of Vitamin K in Selected Malignant Neoplasms in Women. Nutrients 2022, 14, 3401. [Google Scholar] [CrossRef]
- Courbebaisse, M.; Cavalier, E. Vitamin D in 2020: An Old Pro-Hormone with Potential Effects beyond Mineral Metabolism. Nutrients 2020, 12, 3378. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Li, Z.; Tang, L.; Shen, M.; Zhou, Z.; Wei, Y.; Zhao, Y.; Bai, S.; Song, L. Associations of Dietary Intakes with Gynecological Cancers: Findings from a Cross-Sectional Study. Nutrients 2022, 14, 5026. [Google Scholar] [CrossRef]
- Ono, A.; Koshiyama, M.; Nakagawa, M.; Watanabe, Y.; Ikuta, E.; Seki, K.; Oowaki, M. The Preventive Effect of Dietary Antioxidants on Cervical Cancer Development. Medicina 2020, 56, 604. [Google Scholar] [CrossRef]
- Vuolo, L.; Di Somma, C.; Faggiano, A.; Colao, A. Vitamin D and cancer. Front. Endocrinol. 2012, 3, 58. [Google Scholar] [CrossRef]
- Grant, W.B. Review of Recent Advances in Understanding the Role of Vitamin D in Reducing Cancer Risk: Breast, Colorectal, Prostate, and Overall Cancer. Anticancer Res. 2020, 40, 491–499. [Google Scholar] [CrossRef]
- Zhang, F.; Yu, Y.; Song, S.; Wang, M.; Ma, Y.; Xing, L. Calcitriol does not significantly enhance the efficacy of radiation of human cervical tumors in mice. Eur. J. Gynaecol. Oncol. 2015, 36, 452–456. [Google Scholar]
- Batman, A.; Ciftciler, R. The effect of hypervitaminosis D and intoxication on hematological parameters. Minerva Endocrinol. 2022, 47, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Pini, S.; Scaparrotta, G.; Fragasso, A.; Stefanelli, L.F.; Nalesso, F.; Calo, L.A. Vitamin D intoxication induced severe hypercalcemia from self-medication for COVID-19 infection: A public health problem? Minerva Endocrinol. 2022, 47, 371–374. [Google Scholar] [CrossRef] [PubMed]

| Patient Population (n) | Diagnostic | Principal Findings | Ref. |
|---|---|---|---|
| Japanese women Cases: 405 Controls: 11,814 | Invasive cervical cancer (333 cases) or CIN3 (72 cases) | Inverse association between dietary calcium and vitamin D intake and cervical neoplasia risk. | [26] |
| Chinese women | The annual cancer mortality rate data were obtained from 65 rural counties included in the nationwide survey of all deaths over three years | The indices of solar UV radiation, latitude, and heat index were correlated with reduced mortality rates for cervical cancer. It suggests that vitamin D production via solar UVB reduces the risk of cervical cancer. | [27] |
| French women Incidence data were presented as estimates for 2000 patients | Uterine cancer | Significant positive correlations with the latitude for uterine cervix cancer, indicating that solar UVB reduces the risk of cancer through the production of vitamin D. | [28] |
| Turkish women Cases: 23 Controls: 62 | HPV DNA-positive patients | Serum vitamin D levels were significantly lower in the study group (8085 IU/mL) than in the control group (11,472 IU/mL). | [29] |
| American woman Cases: 2353 | Cervicovaginal HPV infection | The vitamin D level is inversely associated with the prevalence of cervicovaginal HPV infection in sexually active women. | [30] |
| Iranian women Cases: 29 Controls: 29 | Patients with CIN1 | Vitamin D3 administration (50,000 IU vitamin D3 every two weeks for six months) resulted in CIN1 regression and had beneficial effects on glucose homeostasis parameters, plasma levels of NO and MDA. | [31] |
| Iranian women Cases: 29 Controls: 29 | Patients with CIN2/3 | Vitamin D3 administration (50,000 IU vitamin D3 every two weeks for six months) had beneficial effects on the metabolic status; however, it did not affect CIN2/3 recurrence. | [32] |
| Chinese women Cases: 188 Controls: 188 | Patients with CIN2 with HPV16-positive | Association between VDR polymorphisms (FokI and TaqI) in HPV16-positive cervical lesions and the risk of CIN2. | [56] |
| Thai women Cases: 204 Controls: 204 | Patients with squamous cell carcinoma of the cervix | Association between VDR polymorphisms (TaqI) and cervical cancer risk. | [57] |
| American woman Cases: 71,209 | Cervical cancer | Inverse association between UVR exposure and the incidence of cervical cancer. | [78] |
| American woman The cancer statistics incidence and mortality were obtained from the CDCP | Cervix uteri cancer | Association between increasing solar energy and increasing cervix uteri cancer incidence. No association between solar energy and cervix uteri cancer mortality. | [79] |
| Different nationalities Data from the literature | Cervical cancer | UVB irradiance significantly reduces the risk of cervical cancer. | [80] |
| Belgian women Cases: cervical (18) and endometrial (25) cancer | Patients with pretreated persistent/recurrent/metastatic cervical or endometrial cancer | Patients who received a drug cocktail that included vitamin D had a modest but durable antitumor activity with acceptable but not negligible toxicity. | [81] |
| German women Cases: 200 | Women with chronic recurrent cervical infections and with cervix dysplasia (CIN 1, CIN 2) | Patients who received treatment with vaginal suppositories with 12,500 IU of vitamin D three nights per week for six weeks had good anti-inflammatory and antidysplastic effects in the CIN1 group. No beneficial effects were seen in the CIN2 group. | [82] |
| American women Cases: 72 | Patients with prevalent high-risk HPV | A positive association between vitamin D levels and 14 high-risk HPV types persistence per 10 ng/mL increase. | [83] |
| Indian women and men Group A: 50, MMR vaccine Group B: 50, vitamin D3 treatment | Patients with single or multiple warts | The intralesional vitamin D3 administration produced efficacious results in terms of warts clearance as well as side effects profile. | [84] |
| Indian women and men Group A: 33, MMR vaccine Group B: 31, vitamin D3 treatment Group C: 24, saline solution | Patients with two or more recalcitrant extragenital warts of any duration at various sites of the body | The intralesional vitamin D3 administration (600,000 IU every two weeks, four injections, or till the complete clearance of warts) was effective, safe, and tolerable, with low recurrence rates. | [85] |
| Indian women and men Cases: 42 | Patients with cutaneous warts | Intralesional vitamin D3 (600,000 IU every two weeks, four injections, or till the complete clearance of warts) was safe and effective for treating multiple cutaneous warts. | [86] |
| Egyptian women and men Cases: 20 | Patients with verruca vulgaris | Good clinical response and expression of cathelicidin in warts after an intralesional injection of vitamin D3. | [88] |
| Indian man | Patient with condyloma acuminata | Three intralesional injections of vitamin D3 (600,000 IU every two weeks) resulted in complete clearance of condyloma acuminata. No recurrence was seen in a period of 6 months of follow-up. | [89] |
| Japanese infant | Patient with anogenital wart | Applying topical calcipotriene (vitamin D3 derivative) in the affected lesion twice daily for four months showed complete regression. No recurrence was seen in 6 months of follow-up. | [90] |
| Mexican women Cases: 67 | HPV-infected women with systemic lupus erythematosus | No association between vitamin D deficiency and cervical HPV. | [97] |
| American women Cases: 162 Control: 12,275 | Cervical cancer | Intakes of vitamin D showed no relationship with gynecological cancers. | [106] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avila, E.; Noriega-Mejía, B.J.; González-Macías, J.; Cortes-Hernández, U.; García-Quiroz, J.; García-Becerra, R.; Díaz, L. The Preventive Role of the Vitamin D Endocrine System in Cervical Cancer. Int. J. Mol. Sci. 2023, 24, 8665. https://doi.org/10.3390/ijms24108665
Avila E, Noriega-Mejía BJ, González-Macías J, Cortes-Hernández U, García-Quiroz J, García-Becerra R, Díaz L. The Preventive Role of the Vitamin D Endocrine System in Cervical Cancer. International Journal of Molecular Sciences. 2023; 24(10):8665. https://doi.org/10.3390/ijms24108665
Chicago/Turabian StyleAvila, Euclides, Bryan Javier Noriega-Mejía, Jocelyn González-Macías, Ulises Cortes-Hernández, Janice García-Quiroz, Rocío García-Becerra, and Lorenza Díaz. 2023. "The Preventive Role of the Vitamin D Endocrine System in Cervical Cancer" International Journal of Molecular Sciences 24, no. 10: 8665. https://doi.org/10.3390/ijms24108665
APA StyleAvila, E., Noriega-Mejía, B. J., González-Macías, J., Cortes-Hernández, U., García-Quiroz, J., García-Becerra, R., & Díaz, L. (2023). The Preventive Role of the Vitamin D Endocrine System in Cervical Cancer. International Journal of Molecular Sciences, 24(10), 8665. https://doi.org/10.3390/ijms24108665







