5-ALA/SFC Ameliorates Endotoxin-Induced Ocular Inflammation in Rats by Inhibiting the NF-κB Signaling Pathway and Activating the HO-1/Nrf2 Signaling Pathway
Abstract
1. Introduction
2. Results
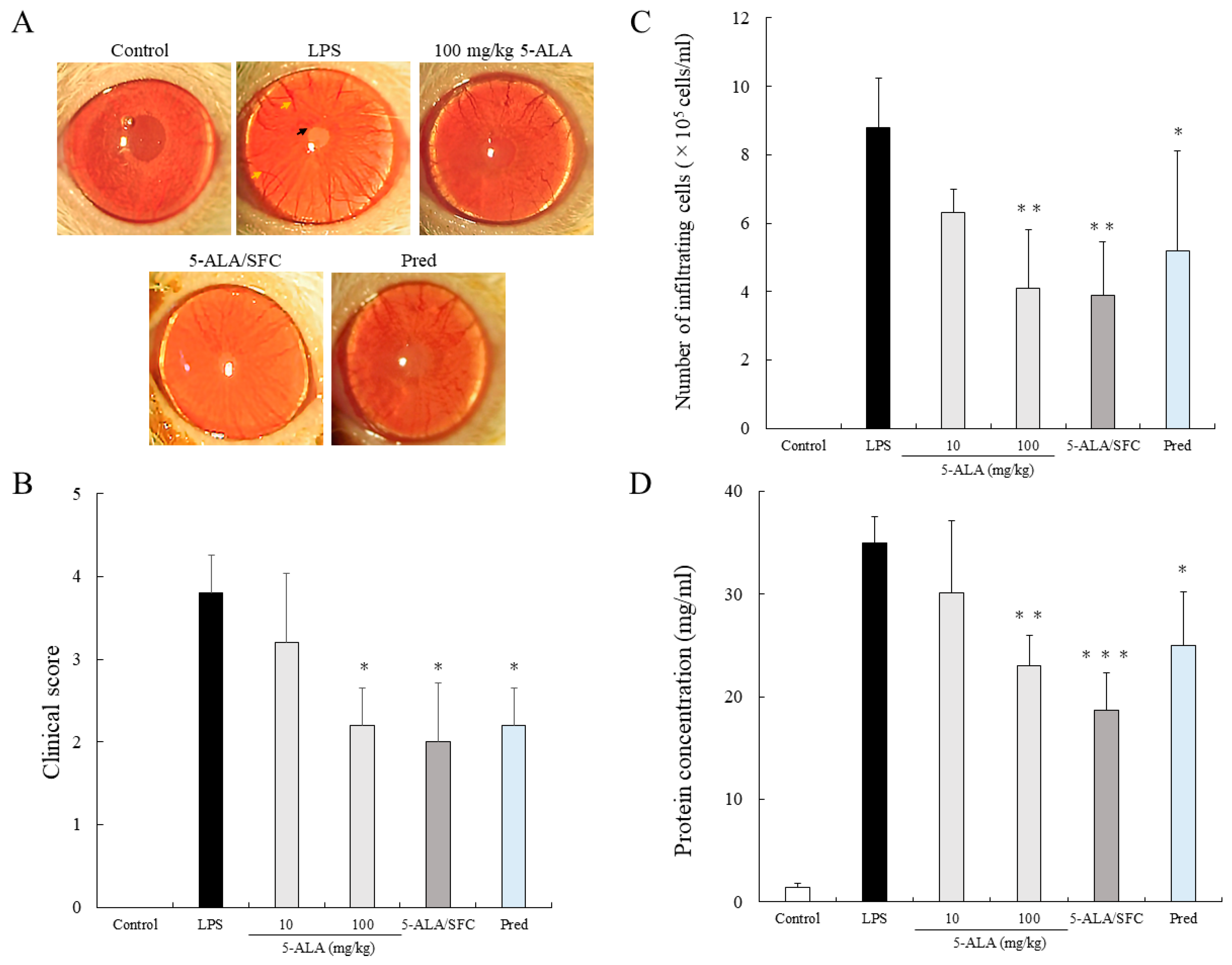
2.1. 5-ALA/SFC Suppresses Clinical Scoring and the Number of Infiltrating Cells and Protein Concentration in AqH
2.2. 5-ALA/SFC Improves Histopathologic Evaluation
2.3. 5-ALA/SFC Suppresses TNF-α, IL-6, NO, and PGE2 Levels in AqH
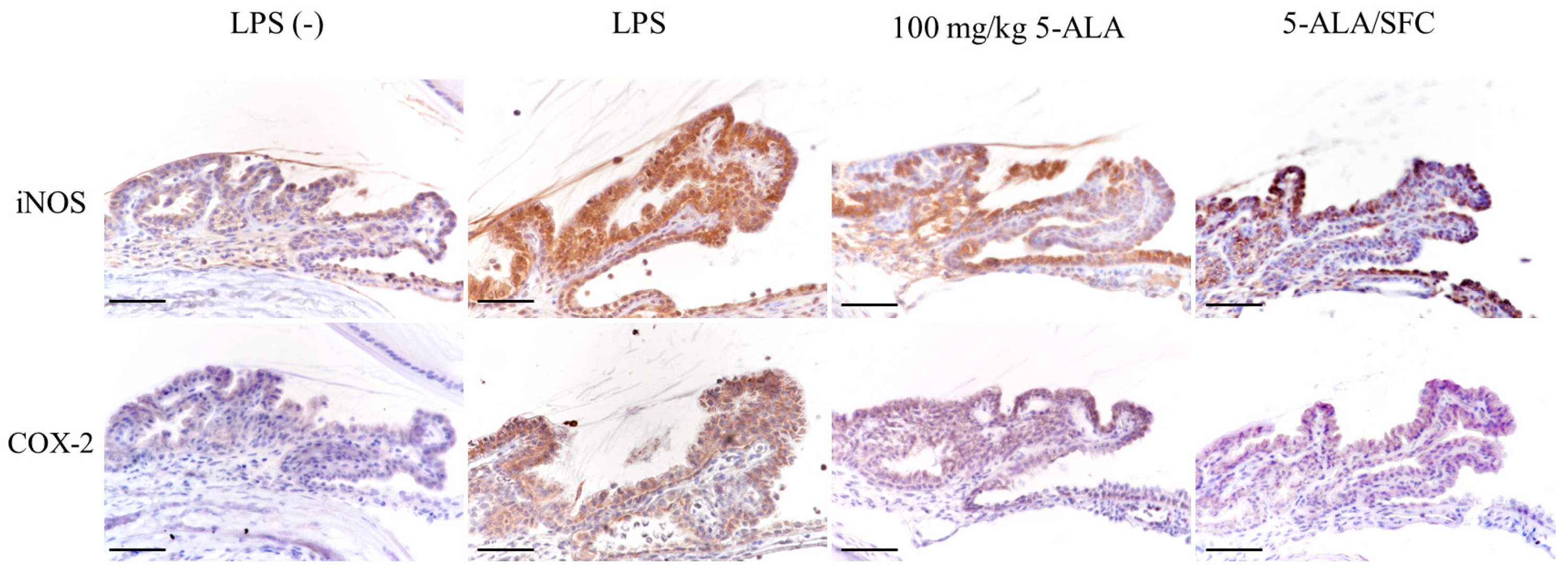
2.4. 5-ALA/SFC Downregulates the Expression of iNOS and COX-2 in ICB
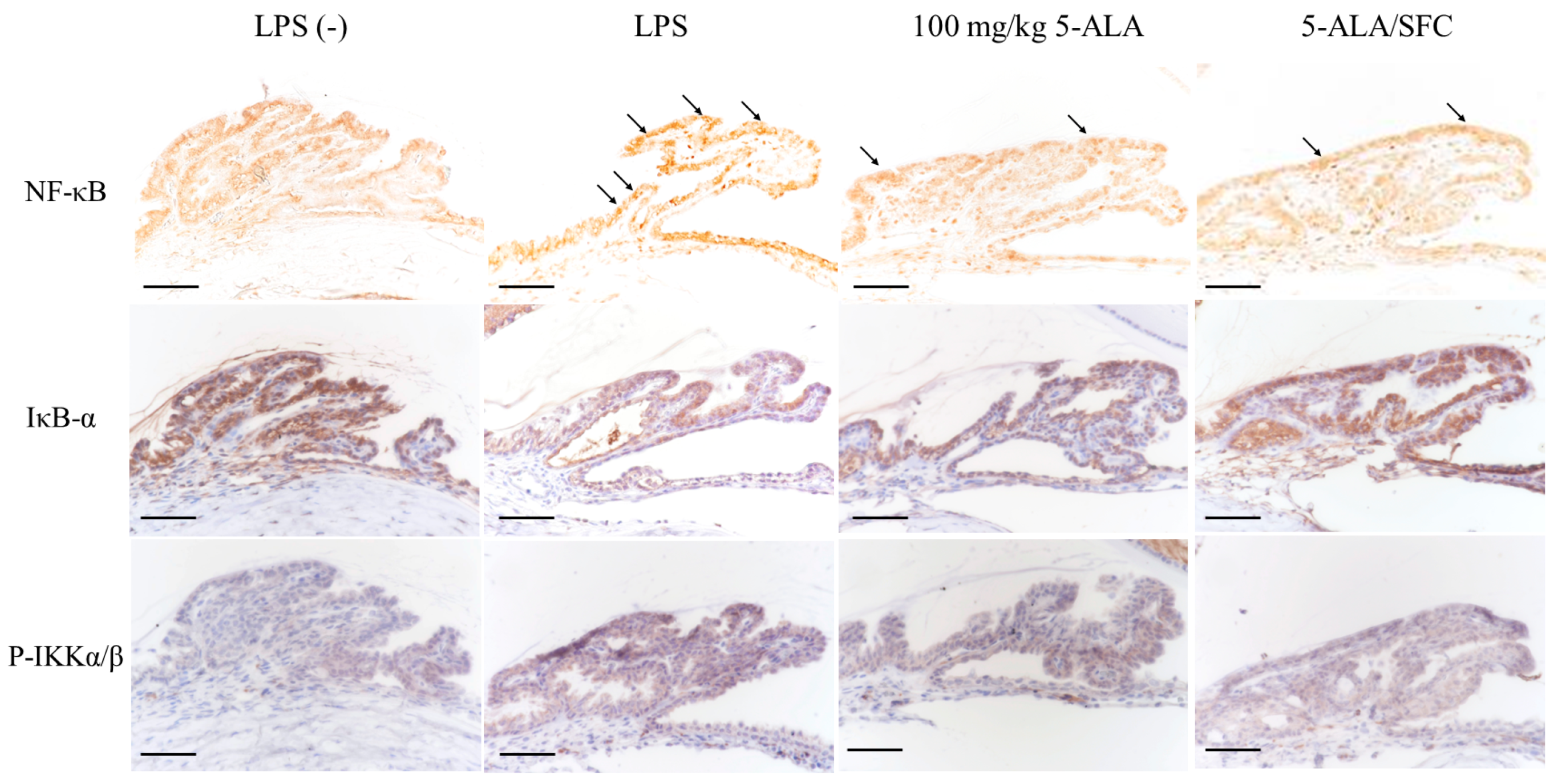
2.5. 5-ALA/SFC Downregulates the NF-κB Pathway
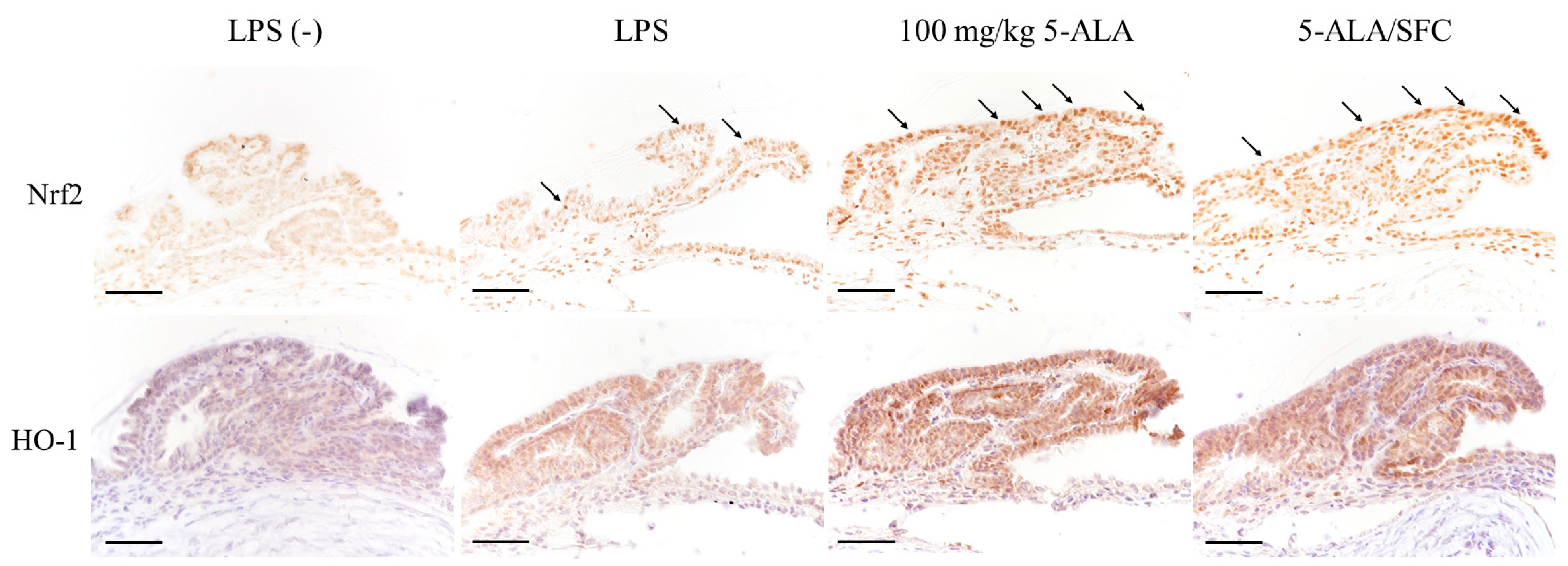
2.6. 5-ALA/SFC Upregulates the Nrf2/HO-1 Pathway
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Antibodies and Reagents
4.3. Induction of EIU Rats
4.4. Clinical Scoring
4.5. Number of Infiltrating Cells and Proteins in AqH
4.6. Histopathologic Evaluation
4.7. The Levels of TNF-α, IL-6, PGE2, and NO in AqH
4.8. Immunohistochemical Studies
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rosenbaum, J.T.; McDevitt, H.O.; Guss, R.B.; Egbert, P.R. Endotoxin-induced uveitis in rats as a model for human disease. Nature 1980, 286, 611–613. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.H.; Horng, C.T.; Chen, Y.H.; Chen, P.L.; Chen, C.L.; Liang, C.M.; Chien, M.W.; Chen, J.T. Inhibitory effects of glucosamine on endotoxin-induced uveitis in Lewis rats. Investig. Ophthalmol. Vis. Sci. 2008, 49, 5441–5449. [Google Scholar] [CrossRef] [PubMed]
- Bhattacherjee, P.; Williams, R.N.; Eakins, K.E. An evaluation of ocular inflammation following the injection of bacterial endotoxin into the rat foot pad. Investig. Ophthalmol. Vis. Sci. 1983, 24, 196–202. [Google Scholar]
- Uchida, T.; Honjo, M.; Yamagishi, R.; Aihara, M. The anti-inflammatory effect of ripasudil (K-115), a rho kinase (ROCK) inhibitor, on endotoxin-induced uveitis in rats. Investig. Ophthalmol. Vis. Sci. 2017, 58, 5584–5593. [Google Scholar] [CrossRef] [PubMed]
- Shoeb, M.; Zhang, M.; Xiao, T.; Syed, M.F.; Ansari, N.H. Amelioration of endotoxin-induced inflammatory toxic response by a metal chelator in rat eyes. Investig. Ophthalmol. Vis. Sci. 2018, 59, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Yadav, U.C.; Ramana, K.V. Endotoxin-induced uveitis in rodents. Methods Mol. Biol. 2013, 1031, 155–162. [Google Scholar] [PubMed]
- de Vos, A.F.; van Haren, M.A.; Verhagen, C.; Hoekzema, R.; Kijlstra, A. Kinetics of intraocular tumor necrosis factor and interleukin-6 in endotoxin-induced uveitis in the rat. Investig. Ophthalmol. Vis. Sci. 1994, 35, 1100–1106. [Google Scholar]
- Planck, S.R.; Huang, X.N.; Robertson, J.E.; Rosenbaum, J.T. Cytokine mRNA levels in rat ocular tissues after systemic endotoxin treatment. Investig. Ophthalmol. Vis. Sci. 1994, 35, 924–930. [Google Scholar]
- Iwama, D.; Miyahara, S.; Tamura, H.; Miyamoto, K.; Hirose, F.; Yoshimura, N. Lack of inducible nitric oxide synthases attenuates leukocyte-endothelial cell interactions in retinal microcirculation. Br. J. Ophthalmol. 2008, 92, 694–698. [Google Scholar] [CrossRef]
- Smith, J.R.; Hart, P.H.; Williams, K.A. Basic pathogenic mechanisms operating in experimental models of acute anterior uveitis. Immunol. Cell Biol. 1998, 76, 497–512. [Google Scholar] [CrossRef]
- Yoshida, M.; Yoshimura, N.; Hangai, M.; Tanihara, H.; Honda, Y. Interleukin-1 alpha, interleukin-1 beta, and tumor necrosis factor gene expression in endotoxin-induced uveitis. Investig. Ophthalmol. Vis. Sci. 1994, 35, 1107–1113. [Google Scholar]
- Kanai, K.; Itoh, N.; Yoshioka, K.; Yonezawa, T.; Ikadai, H.; Hori, Y.; Ito, Y.; Nagai, N.; Chikazawa, S.; Hoshi, F.; et al. Inhibitory effects of oral disulfiram on endotoxin-induced uveitis in rats. Curr. Eye Res. 2010, 35, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Kubota, S.; Kurihara, T.; Mochimaru, H.; Satofuka, S.; Noda, K.; Ozawa, Y.; Oike, Y.; Ishida, S.; Tsubota, K. Prevention of ocular inflammation in endotoxin-induced uveitis with resveratrol by inhibiting oxidative damage and nuclear factor-kappaB activation. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3512–3519. [Google Scholar] [CrossRef] [PubMed]
- Yadav, U.C.; Subramanyam, S.; Ramana, K.V. Prevention of endotoxin-induced uveitis in rats by benfotiamine, a lipophilic analogue of vitamin B1. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2276–2282. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Mihara, Y.; Kanai, K.; Yamashita, Y.; Kimura, Y.; Itoh, N. Tyrosol ameliorates lipopolysaccharide-induced ocular inflammation in rats via inhibition of nuclear factor (NF)-κB activation. J. Vet. Med. Sci. 2016, 78, 1429–1438. [Google Scholar] [CrossRef]
- Li, Q.; Verma, I.M. NF-kappaB regulation in the immune system. Nat. Rev. Immunol. 2002, 2, 725–734. [Google Scholar] [CrossRef]
- Silverman, N.; Maniatis, T. NF-kappaB signaling pathways in mammalian and insect innate immunity. Genes. Dev. 2001, 15, 2321–2342. [Google Scholar] [CrossRef]
- Surh, Y.J.; Chun, K.S.; Cha, H.H.; Han, S.S.; Keum, Y.S.; Park, K.K.; Lee, S.S. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: Down-regulation of COX-2 and iNOS through suppression of NF-kappa B activation. Mutat. Res. 2001, 480–481, 243–268. [Google Scholar] [CrossRef]
- Lennikov, A.; Kitaichi, N.; Noda, K.; Mizuuchi, K.; Ando, R.; Dong, Z.; Fukuhara, J.; Kinoshita, S.; Namba, K.; Ohno, S.; et al. Amelioration of endotoxin-induced uveitis treated with the sea urchin pigment echinochrome in rats. Mol. Vis. 2014, 20, 171–177. [Google Scholar]
- Srivastava, S.K.; Ramana, K.V. Focus on molecules: Nuclear factor-kappaB. Exp. Eye Res. 2009, 88, 2–3. [Google Scholar] [CrossRef]
- Jin, X.H.; Ohgami, K.; Shiratori, K.; Suzuki, Y.; Hirano, T.; Koyama, Y.; Yoshida, K.; Ilieva, I.; Iseki, K.; Ohno, S. Inhibitory effects of lutein on endotoxin-induced uveitis in Lewis rats. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2562–2568. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, J.T.; Lee, S.J.; Kim, J.C. The anti-inflammatory effects of angiogenin in an endotoxin induced uveitis in rats. Int. J. Mol. Sci. 2020, 21, 413. [Google Scholar] [CrossRef]
- Maines, M.D. The heme oxygenase system: A regulator of second messenger gases. Annu. Rev. Pharmacol. Toxicol. 1997, 37, 517–554. [Google Scholar] [CrossRef] [PubMed]
- Suematsu, M.; Wakabayashi, Y.; Ishimura, Y. Gaseous monoxides: A new class of microvascular regulator in the liver. Cardiovasc. Res. 1996, 32, 679–686. [Google Scholar] [CrossRef]
- Ohta, K.; Kikuchi, T.; Arai, S.; Yoshida, N.; Sato, A.; Yoshimura, N. Protective role of heme oxygenase-1 against endotoxin-induced uveitis in rats. Exp. Eye Res. 2003, 77, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Kapitulnik, J.; Maines, M.D. Pleiotropic functions of biliverdin reductase: Cellular signaling and generation of cytoprotective and cytotoxic bilirubin. Trends Pharmacol. Sci. 2009, 30, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Otterbein, L.E.; Bach, F.H.; Alam, J.; Soares, M.; Tao Lu, H.; Wysk, M.; Davis, R.J.; Flavell, R.A.; Choi, A.M. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat. Med. 2000, 6, 422–428. [Google Scholar] [CrossRef]
- Chen, L.G.; Zhang, Y.Q.; Wu, Z.Z.; Hsieh, C.W.; Chu, C.S.; Wung, B.S. Peanut arachidin-1 enhances Nrf2-mediated protective mechanisms against TNF-α-induced ICAM-1 expression and NF-κB activation in endothelial cells. Int. J. Mol. Med. 2018, 41, 541–547. [Google Scholar] [CrossRef]
- Alam, J.; Stewart, D.; Touchard, C.; Boinapally, S.; Choi, A.M.; Cook, J.L. Nrf2, a Cap’n’Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J. Biol. Chem. 1999, 274, 26071–26078. [Google Scholar] [CrossRef]
- Kobayashi, A.; Kang, M.I.; Watai, Y.; Tong, K.I.; Shibata, T.; Uchida, K.; Yamamoto, M. Oxidative and electrophilic stresses activate Nrf2 through inhibition of ubiquitination activity of Keap1. Mol. Cell. Biol. 2006, 26, 221–229. [Google Scholar] [CrossRef]
- Fujino, M.; Nishio, Y.; Ito, H.; Tanaka, T.; Li, X.K. 5-aminolevulinic acid regulates the inflammatory response and alloimmune reaction. Int. Immunopharmacol. 2016, 37, 71–78. [Google Scholar] [CrossRef]
- Ishizuka, M.; Abe, F.; Sano, Y.; Takahashi, K.; Inoue, K.; Nakajima, M.; Kohda, T.; Komatsu, N.; Ogura, S.I.; Tanaka, T. Novel development of 5-aminolevurinic acid (ALA) in cancer diagnoses and therapy. Int. Immunopharmacol. 2011, 11, 358–365. [Google Scholar] [CrossRef]
- Rodriguez, B.L.; Curb, J.D.; Davis, J.; Shintani, T.; Perez, M.H.; Apau-Ludlum, N.; Johnson, C.; Harrigan, R.C. Use of the dietary supplement 5-aminiolevulinic acid (5-ALA) and its relationship with glucose levels and hemoglobin A1C among individuals with prediabetes. Clin. Transl. Sci. 2012, 5, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Miura, M.; Ito, K.; Hayashi, M.; Nakajima, M.; Tanaka, T.; Ogura, S. The effect of 5-aminolevulinic acid on cytochrome P450-mediated prodrug activation. PLoS ONE 2015, 10, e0131793. [Google Scholar] [CrossRef]
- Liu, C.; Zhu, P.; Fujino, M.; Zhu, S.; Ito, H.; Takahashi, K.; Nakajima, M.; Tanaka, T.; Zhuang, J.; Li, X.K. 5-ALA/SFC Attenuated binge alcohol-induced gut leakiness and inflammatory liver disease in HIV transgenic rats. Alcohol. Clin. Exp. Res. 2019, 43, 1651–1661. [Google Scholar] [CrossRef]
- Hara, T.; Koda, A.; Nozawa, N.; Ota, U.; Kondo, H.; Nakagawa, H.; Kamiya, A.; Miyashita, K.; Itoh, H.; Nakajima, M.; et al. Combination of 5-aminolevulinic acid and ferrous ion reduces plasma glucose and hemoglobin A1c levels in Zucker diabetic fatty rats. FEBS Open Bio 2016, 6, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Shimura, M.; Nozawa, N.; Ogawa-Tominaga, M.; Fushimi, T.; Tajika, M.; Ichimoto, K.; Matsunaga, A.; Tsuruoka, T.; Kishita, Y.; Ishii, T.; et al. Effects of 5-aminolevulinic acid and sodium ferrous citrate on fibroblasts from individuals with mitochondrial diseases. Sci. Rep. 2019, 9, 10549. [Google Scholar] [CrossRef]
- Ito, H.; Nishio, Y.; Hara, T.; Sugihara, H.; Tanaka, T.; Li, X.K. Oral administration of 5-aminolevulinic acid induces heme oxygenase-1 expression in peripheral blood mononuclear cells of healthy human subjects in combination with ferrous iron. Eur. J. Pharmacol. 2018, 833, 25–33. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Z.; Hu, X.; Ito, H.; Takahashi, K.; Nakajima, M.; Tanaka, T.; Zhu, P.; Li, X.K. 5-aminolevulinic acid combined with sodium ferrous citrate ameliorated lupus nephritis in a mouse chronic graft-versus-host disease model. Int. Immunopharmacol. 2021, 96, 107626. [Google Scholar] [CrossRef] [PubMed]
- Otaka, Y.; Kanai, K.; Okada, D.; Nagai, N.; Yamashita, Y.; Ichikawa, Y.; Tajima, K. Effects of oral 5-aminolevulinic acid on lipopolysaccharide-induced ocular inflammation in rats. Vet. Sci. 2023, 10, 207. [Google Scholar] [CrossRef]
- Ohgami, K.; Ilieva, I.; Shiratori, K.; Koyama, Y.; Jin, X.H.; Yoshida, K.; Kase, S.; Kitaichi, N.; Suzuki, Y.; Tanaka, T.; et al. Anti-inflammatory effects of aronia extract on rat endotoxin-induced uveitis. Investig. Ophthalmol. Vis. Sci. 2005, 46, 275–281. [Google Scholar] [CrossRef]
- Hou, J.; Zhang, Q.; Fujino, M.; Cai, S.; Ito, H.; Takahashi, K.; Abe, F.; Nakajima, M.; Tanaka, T.; Xu, J.; et al. 5-aminolevulinic acid with ferrous iron induces permanent cardiac allograft acceptance in mice via induction of regulatory cells. J. Heart Lung Transplant. 2015, 34, 254–263. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, W.; Lu, H.; Hu, X.; Li, S.; Wang, J.; Zhao, L. The expression of cytokines in the aqueous humor and serum during endotoxin-induced uveitis in C3H/HeN mice. Mol. Vis. 2010, 16, 1689–1695. [Google Scholar] [PubMed]
- Tilton, R.G.; Chang, K.; Corbett, J.A.; Misko, T.P.; Currie, M.G.; Bora, N.S.; Kaplan, H.J.; Williamson, J.R. Endotoxin-induced uveitis in the rat is attenuated by inhibition of nitric oxide production. Investig. Ophthalmol. Vis. Sci. 1994, 35, 3278–3288. [Google Scholar]
- Mandai, M.; Yoshimura, N.; Yoshida, M.; Iwaki, M.; Honda, Y. The role of nitric oxide synthase in endotoxin-induced uveitis: Effects of NG-nitro L-arginine. Investig. Ophthalmol. Vis. Sci. 1994, 35, 3673–3680. [Google Scholar]
- Bellot, J.L.; Palmero, M.; García-Cabanes, C.; Espí, R.; Hariton, C.; Orts, A. Additive effect of nitric oxide and prostaglandin-E2 synthesis inhibitors in endotoxin-induced uveitis in the rabbit. Inflamm. Res. 1996, 45, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.H.; Ohgami, K.; Shiratori, K.; Suzuki, Y.; Koyama, Y.; Yoshida, K.; Ilieva, I.; Tanaka, T.; Onoe, K.; Ohno, S. Effects of blue honeysuckle (Lonicera caerulea L.) extract on lipopolysaccharide-induced inflammation in vitro and in vivo. Exp. Eye Res. 2006, 82, 860–867. [Google Scholar] [CrossRef]
- Kanai, K.; Ito, Y.; Nagai, N.; Itoh, N.; Hori, Y.; Chikazawa, S.; Hoshi, F.; Higuchi, S. Effects of instillation of eyedrops containing disulfiram and hydroxypropyl-β-cyclodextrin inclusion complex on endotoxin-induced uveitis in rats. Curr. Eye Res. 2012, 37, 124–131. [Google Scholar] [CrossRef]
- Kalariya, N.M.; Shoeb, M.; Ansari, N.H.; Srivastava, S.K.; Ramana, K.V. Antidiabetic drug metformin suppresses endotoxin-induced uveitis in rats. Investig. Ophthalmol. Vis. Sci. 2012, 53, 3431–3440. [Google Scholar] [CrossRef]
- Shi, X.; Zhu, S.; Jin, H.; Fang, J.; Xing, X.; Wang, Y.; Wang, H.; Wang, C.; Niu, T.; Liu, K. The anti-inflammatory effect of KS23, a novel peptide derived from globular adiponectin, on endotoxin-induced uveitis in rats. Front. Pharmacol. 2021, 11, 585446. [Google Scholar] [CrossRef]
- Yang, X.; Jin, H.; Liu, K.; Gu, Q.; Xu, X. A novel peptide derived from human pancreatitis-associated protein inhibits inflammation in vivo and in vitro and blocks NF-kappa B signaling pathway. PLoS ONE 2011, 6, e29155. [Google Scholar] [CrossRef] [PubMed]
- Ihaka, R.; Gentleman, R. R: A Language for Data Analysis and Graphics. J. Comput. Graph. Stat. 1996, 5, 299–314. [Google Scholar]
- Behar-Cohen, F.F.; Savoldelli, M.; Parel, J.M.; Goureau, O.; Thillaye-Goldenberg, B.; Courtois, Y.; Pouliquen, Y.; de Kozak, Y. Reduction of corneal edema in endotoxin-induced uveitis after application of L-NAME as nitric oxide synthase inhibitor in rats by iontophoresis. Investig. Ophthalmol. Vis. Sci. 1998, 39, 897–904. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otaka, Y.; Kanai, K.; Mori, A.; Okada, D.; Nagai, N.; Yamashita, Y.; Ichikawa, Y.; Tajima, K. 5-ALA/SFC Ameliorates Endotoxin-Induced Ocular Inflammation in Rats by Inhibiting the NF-κB Signaling Pathway and Activating the HO-1/Nrf2 Signaling Pathway. Int. J. Mol. Sci. 2023, 24, 8653. https://doi.org/10.3390/ijms24108653
Otaka Y, Kanai K, Mori A, Okada D, Nagai N, Yamashita Y, Ichikawa Y, Tajima K. 5-ALA/SFC Ameliorates Endotoxin-Induced Ocular Inflammation in Rats by Inhibiting the NF-κB Signaling Pathway and Activating the HO-1/Nrf2 Signaling Pathway. International Journal of Molecular Sciences. 2023; 24(10):8653. https://doi.org/10.3390/ijms24108653
Chicago/Turabian StyleOtaka, Yuya, Kazutaka Kanai, Arisa Mori, Daiki Okada, Noriaki Nagai, Yohei Yamashita, Yoichiro Ichikawa, and Kazuki Tajima. 2023. "5-ALA/SFC Ameliorates Endotoxin-Induced Ocular Inflammation in Rats by Inhibiting the NF-κB Signaling Pathway and Activating the HO-1/Nrf2 Signaling Pathway" International Journal of Molecular Sciences 24, no. 10: 8653. https://doi.org/10.3390/ijms24108653
APA StyleOtaka, Y., Kanai, K., Mori, A., Okada, D., Nagai, N., Yamashita, Y., Ichikawa, Y., & Tajima, K. (2023). 5-ALA/SFC Ameliorates Endotoxin-Induced Ocular Inflammation in Rats by Inhibiting the NF-κB Signaling Pathway and Activating the HO-1/Nrf2 Signaling Pathway. International Journal of Molecular Sciences, 24(10), 8653. https://doi.org/10.3390/ijms24108653





