Mitochondrial Dysfunction: At the Nexus between Alcohol-Associated Immunometabolic Dysregulation and Tissue Injury
Abstract
1. Introduction
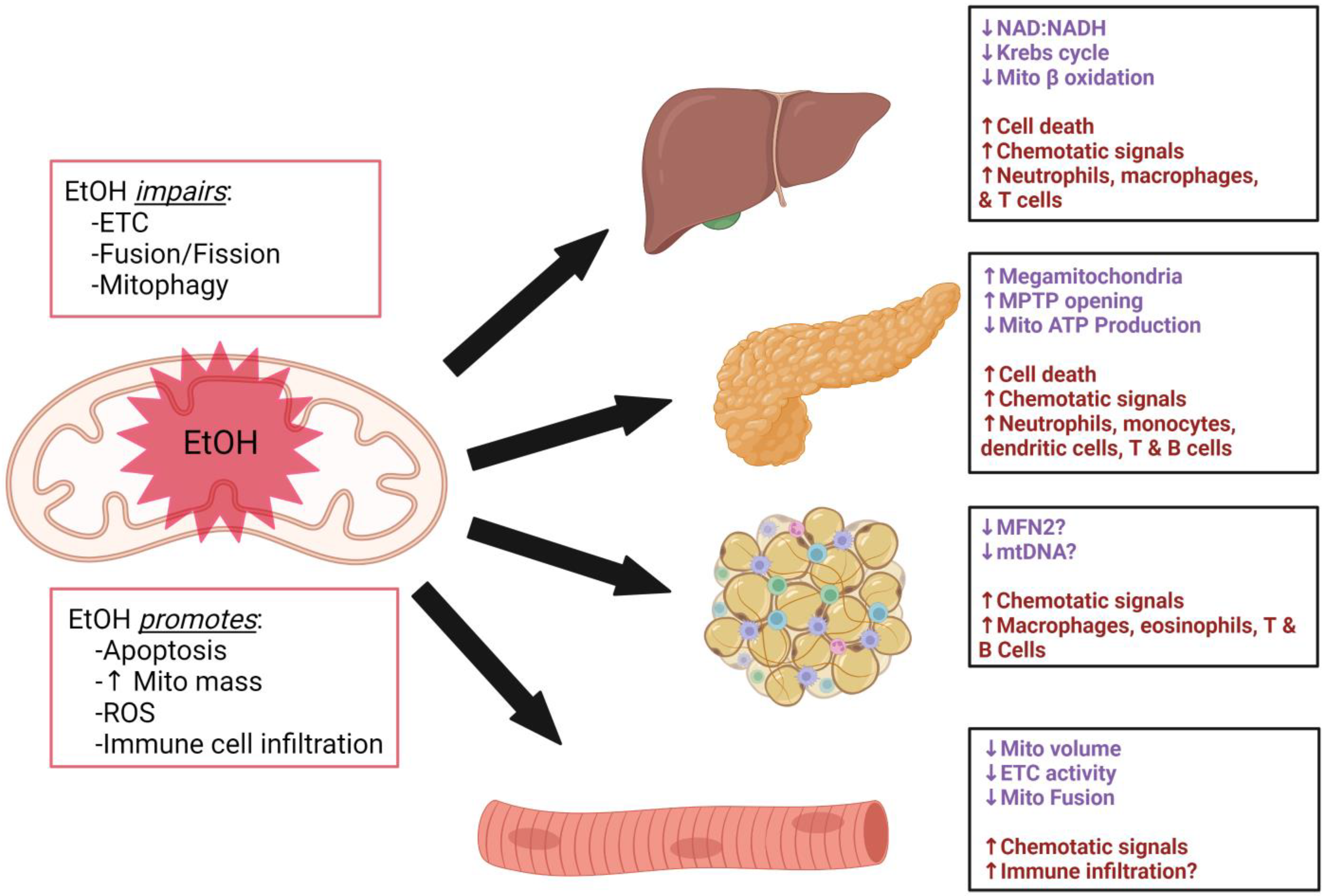
2. Impact of Alcohol on Tissues Regulating Metabolic Homeostasis
2.1. Liver
2.2. Pancreas
2.3. Adipose
2.4. Skeletal Muscle
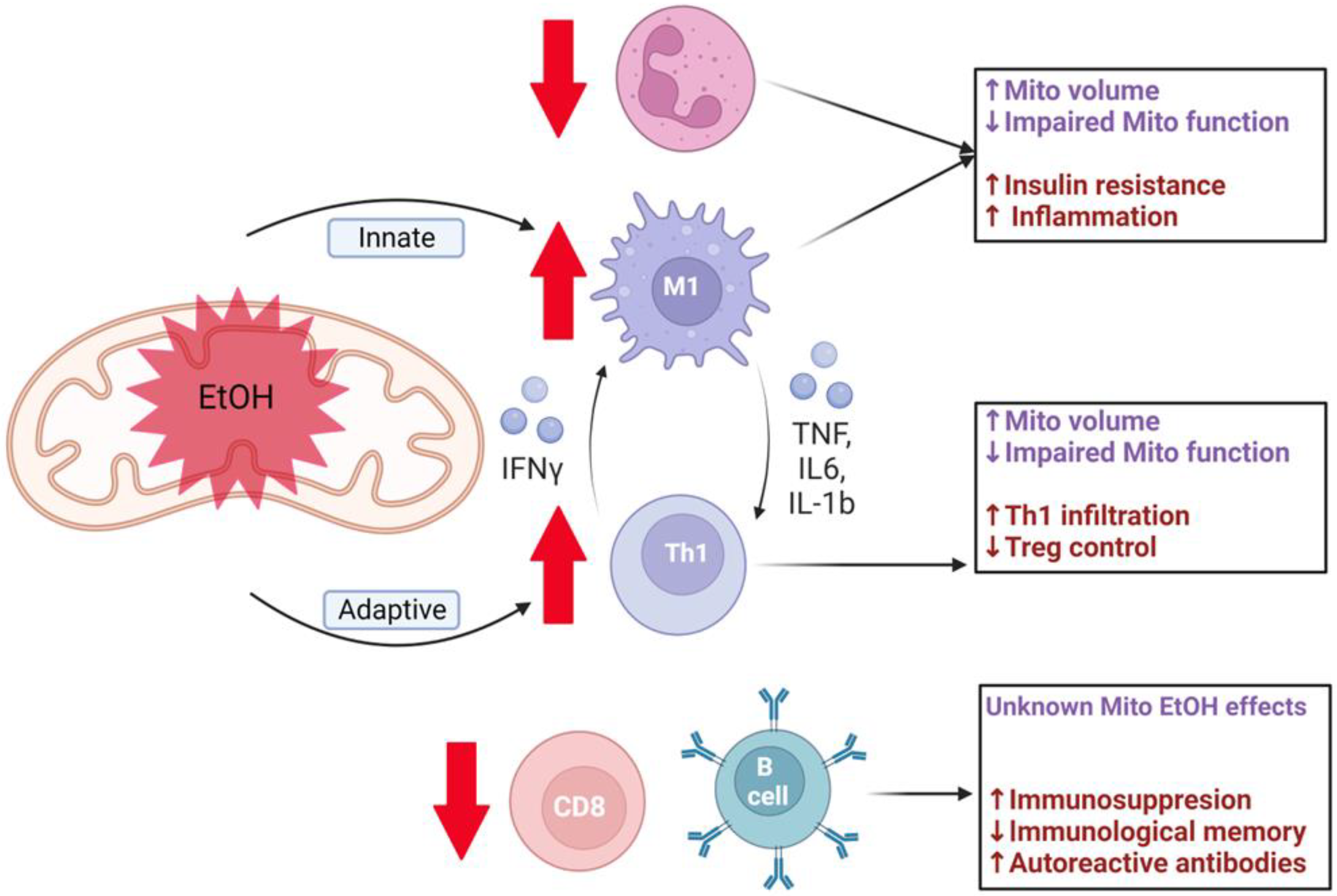
3. Impact of Alcohol on Immune Cell Mitochondrial Function
3.1. Innate Immune Cells
3.1.1. Neutrophils
3.1.2. Macrophages
3.2. Adaptive Immune Cells
3.2.1. CD4+ T Cells
3.2.2. CD8+ T Cells
3.2.3. B Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Simon, L.; Souza-Smith, F.M.; Molina, P.E. Alcohol-Associated Tissue Injury: Current Views on Pathophysiological Mechanisms. Annu. Rev. Physiol. 2022, 10, 87–112. [Google Scholar] [CrossRef] [PubMed]
- Seitz, H.K.; Bataller, R.; Cortez-Pinto, H.; Gao, B.; Gual, A.; Lackner, C.; Mathurin, P.; Mueller, S.; Szabo, G.; Tsukamoto, H. Alcoholic liver disease. Nat. Rev. Dis. Primers 2018, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- Apte, M.V.; Pirola, R.C.; Wilson, J.S. Mechanisms of alcoholic pancreatitis. J. Gastroenterol. Hepatol. 2010, 25, 1816–1826. [Google Scholar] [CrossRef] [PubMed]
- Steiner, J.L.; Lang, C.H. Alcohol, Adipose Tissue and Lipid Dysregulation. Biomolecules 2017, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Preedy, V.R.; Peters, T.J. Alcohol and skeletal muscle disease. Alcohol Alcohol. 1990, 25, 177–187. [Google Scholar] [CrossRef]
- Ferrao, K.; Ali, N.; Mehta, K.J. Iron and iron-related proteins in alcohol consumers: Cellular and clinical aspects. J. Mol. Med. 2022, 100, 1673–1689. [Google Scholar] [CrossRef]
- Harrison-Findik, D.D. Role of alcohol in the regulation of iron metabolism. World J. Gastroenterol. 2007, 13, 4925–4930. [Google Scholar] [CrossRef]
- Molina, P.E.; Gardner, J.D.; Souza-Smith, F.M.; Whitaker, A.M. Alcohol Abuse: Critical Pathophysiological Processes and Contribution to Disease Burden. Physiology 2014, 29, 203–215. [Google Scholar] [CrossRef]
- Hoek, J.B.; Cahill, A.; Pastorino, J.G. Alcohol and mitochondria: A dysfunctional relationship. Gastroenterology 2002, 122, 2049–2063. [Google Scholar] [CrossRef]
- Wang, H.J.; Zakhari, S.; Jung, M.K. Alcohol, inflammation, and gut-liver-brain interactions in tissue damage and disease development. World J. Gastroenterol. 2010, 16, 1304–1313. [Google Scholar] [CrossRef]
- Sastre, J.; Serviddio, G.; Pereda, J.; Minana, J.B.; Arduini, A.; Vendemiale, G.; Poli, G.; Pallardo, F.V.; Vina, J. Mitochondrial function in liver disease. Front. Biosci. 2007, 12, 1200–1209. [Google Scholar] [CrossRef] [PubMed]
- Hekimi, S.; Wang, Y.; Noë, A. Mitochondrial ROS and the Effectors of the Intrinsic Apoptotic Pathway in Aging Cells: The Discerning Killers! Front. Genet. 2016, 7, 161. [Google Scholar] [CrossRef] [PubMed]
- Bassoy, E.Y.; Walch, M.; Martinvalet, D. Reactive Oxygen Species: Do They Play a Role in Adaptive Immunity? Front. Immunol. 2021, 12, 755856. [Google Scholar] [CrossRef] [PubMed]
- Ceni, E.; Mello, T.; Galli, A. Pathogenesis of alcoholic liver disease: Role of oxidative metabolism. World J. Gastroenterol. 2014, 20, 17756–17772. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.K.; Jampana, S.C.; Weinman, S.A. Antioxidants as therapeutic agents for liver disease. Liver Int. 2011, 31, 1432–1448. [Google Scholar] [CrossRef]
- Chavakis, T. Immunometabolism: Where Immunology and Metabolism Meet. J. Innate Immun. 2022, 14, 16. [Google Scholar] [CrossRef]
- Caslin, H.L.; Hasty, A.H. Extrinsic and Intrinsic Immunometabolism Converge: Perspectives on Future Research and Therapeutic Development for Obesity. Curr. Obes. Rep. 2019, 8, 210–219. [Google Scholar] [CrossRef]
- Simon, L.; Molina, P.E. Cellular bioenergetics: Experimental evidence for alcohol-induced adaptations. Function 2022, 3, zqac039. [Google Scholar] [CrossRef]
- Man, K.; Kutyavin, V.I.; Chawla, A. Tissue Immunometabolism: Development, Physiology, and Pathobiology. Cell Metab. 2017, 25, 11–26. [Google Scholar] [CrossRef]
- Cederbaum, A.I. Alcohol Metabolism. Clin. Liver Dis. 2012, 16, 667–685. [Google Scholar] [CrossRef]
- Lieber, C.S. Alcoholic fatty liver: Its pathogenesis and mechanism of progression to inflammation and fibrosis. Alcohol 2004, 34, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Abdelmegeed, M.A.; Ha, S.-K.; Choi, Y.; Akbar, M.; Song, B.-J. Role of CYP2E1 in Mitochondrial Dysfunction and Hepatic Injury by Alcohol and Non-Alcoholic Substances. Curr. Mol. Pharmacol. 2017, 10, 207–225. [Google Scholar] [CrossRef] [PubMed]
- Hyun, J.; Han, J.; Lee, C.; Yoon, M.; Jung, Y. Pathophysiological Aspects of Alcohol Metabolism in the Liver. Int. J. Mol. Sci. 2021, 22, 5717. [Google Scholar] [CrossRef]
- Cahill, A.; Cunningham, C.C.; Adachi, M.; Ishii, H.; Bailey, S.M.; Fromenty, B.; Davies, A. Effects of alcohol and oxidative stress on liver pathology: The role of the mitochondrion. Alcohol. Clin. Exp. Res. 2002, 26, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Ybanez, M.D.; Johnson, H.S.; McDonald, J.N.; Mesropyan, L.; Sancheti, H.; Martin, G.; Martin, A.; Lim, A.M.; Dara, L.; et al. Dynamic Adaptation of Liver Mitochondria to Chronic Alcohol Feeding in Mice: Biogenesis, remodeling, and functional alterations. J. Biol. Chem. 2012, 287, 42165–42179. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Johnson, H.S.; Rao, M.P.; Martin, G.; Sancheti, H.; Silkwood, K.H.; Decker, C.W.; Nguyen, K.T.; Casian, J.G.; Cadenas, E.; et al. Mitochondrial remodeling in the liver following chronic alcohol feeding to rats. Free. Radic. Biol. Med. 2017, 102, 100–110. [Google Scholar] [CrossRef]
- Prasun, P.; Ginevic, I.; Oishi, K. Mitochondrial dysfunction in nonalcoholic fatty liver disease and alcohol related liver disease. Transl. Gastroenterol. Hepatol. 2021, 6, 4. [Google Scholar] [CrossRef]
- Jeon, S.; Carr, R. Alcohol effects on hepatic lipid metabolism. J. Lipid Res. 2020, 61, 470–479. [Google Scholar] [CrossRef]
- Nakajima, T.; Kamijo, Y.; Tanaka, N.; Sugiyama, E.; Tanaka, E.; Kiyosawa, K.; Fukushima, Y.; Peters, J.M.; Gonzalez, F.J.; Aoyama, T. Peroxisome proliferator-activated receptor alpha protects against alcohol-induced liver damage. Hepatology 2004, 40, 972–980. [Google Scholar] [CrossRef]
- Kong, L.; Ren, W.; Li, W.; Zhao, S.; Mi, H.; Wang, R.; Zhang, Y.; Wu, W.; Nan, Y.; Yu, J. Activation of peroxisome proliferator activated receptor alpha ameliorates ethanol induced steatohepatitis in mice. Lipids Health Dis. 2011, 10, 246. [Google Scholar] [CrossRef]
- You, M.; Arteel, G.E. Effect of ethanol on lipid metabolism. J. Hepatol. 2019, 70, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Liang, X.; Jogasuria, A.; Davidson, N.O.; You, M. miR-217 Regulates Ethanol-Induced Hepatic Inflammation by Disrupting Sirtuin 1–Lipin-1 Signaling. Am. J. Pathol. 2015, 185, 1286–1296. [Google Scholar] [CrossRef] [PubMed]
- You, M.; Jogasuria, A.; Taylor, C.; Wu, J. Sirtuin 1 signaling and alcoholic fatty liver disease. Hepatobiliary Surg. Nutr. 2015, 4, 88–100. [Google Scholar] [CrossRef]
- French, S.W. Chronic alcohol binging injures the liver and other organs by reducing NAD+ levels required for sirtuin’s deacetylase activity. Exp. Mol. Pathol. 2016, 100, 303–306. [Google Scholar] [CrossRef]
- You, M.; Jogasuria, A.; Lee, K.; Wu, J.; Zhang, Y.; Lee, Y.K.; Sadana, P. Signal Transduction Mechanisms of Alcoholic Fatty Liver Disease: Emer ging Role of Lipin-1. Curr. Mol. Pharmacol. 2017, 10, 226–236. [Google Scholar] [CrossRef]
- Duan, Y.; Llorente, C.; Lang, S.; Brandl, K.; Chu, H.; Jiang, L.; White, R.C.; Clarke, T.H.; Nguyen, K.; Torralba, M.; et al. Bacteriophage targeting of gut bacterium attenuates alcoholic liver disease. Nature 2019, 575, 505–511. [Google Scholar] [CrossRef]
- Li, S.; Tan, H.-Y.; Wang, N.; Feng, Y.; Wang, X.; Feng, Y. Recent Insights Into the Role of Immune Cells in Alcoholic Liver Disease. Front. Immunol. 2019, 10, 1328. [Google Scholar] [CrossRef] [PubMed]
- Bruellman, R.; Llorente, C. A perspective of intestinal immune-microbiome interactions in alcohol-associated Liver Disease. Int. J. Biol. Sci. 2021, 17, 307–327. [Google Scholar] [CrossRef]
- Nath, B.; Szabo, G. Alcohol-induced Modulation of Signaling Pathways in Liver Parenchymal and Nonparenchymal Cells: Implications for Immunity. Semin. Liver Dis. 2009, 29, 166–177. [Google Scholar] [CrossRef]
- Riva, A.; Patel, V.; Kurioka, A.; Jeffery, H.C.; Wright, G.; Tarff, S.; Shawcross, D.; Ryan, J.M.; Evans, A.; Azarian, S.; et al. Mucosa-associated invariant T cells link intestinal immunity with antibacterial immune defects in alcoholic liver disease. Gut 2018, 67, 918–930. [Google Scholar] [CrossRef]
- Lee, J.; Shim, Y.; Seo, W.; Kim, M.; Choi, W.; Kim, H.; Kim, Y.E.; Yang, K.; Ryu, T.; Jeong, J.; et al. Mitochondrial Double-Stranded RNA in Exosome Promotes Interleukin-17 Production Through Toll-Like Receptor 3 in Alcohol-associated Liver Injury. Hepatology 2020, 72, 609–625. [Google Scholar] [CrossRef] [PubMed]
- Parfieniuk-Kowerda, A.; Świderska, M.; Szulzyk, T.; Jaroszewicz, J.; Lapinski, T.; Flisiak, R. Serum Concentrations of Th17-Associated Interleukins and Autoimmune Phenomena are Associated with the Degree of Liver Damage in Alcoholic Liver Disease. J. Gastrointest. Liver Dis. 2017, 26, 269–274. [Google Scholar] [CrossRef]
- Almeida, J.; Polvorosa, M.A.; Gonzalez-Quintela, A.; Marcos, M.; Pastor, I.; Hernandez Cerceño, M.L.; Orfao, A.; Laso, F.-J. Decreased Peripheral Blood CD4+/CD25+ Regulatory T Cells in Patients with Alcoholic Hepatitis. Alcohol. Clin. Exp. Res. 2013, 37, 1361–1369. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Wu, X.; Allende, D.S.; Nagy, L.E. Gene Deconvolution Reveals Aberrant Liver Regeneration and Immune Cell Infiltration in Alcohol-Associated Hepatitis. Hepatology 2021, 74, 987–1002. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, M.; Vidigal, P.T.; Guerra, M.T.; Hundt, M.A.; Robert, M.E.; Olave-Martinez, M.; Aoki, S.; Khamphaya, T.; Kersten, R.; Kruglov, E.; et al. Neutrophils interact with cholangiocytes to cause cholestatic changes in alcoholic hepatitis. Gut 2021, 70, 342–356. [Google Scholar] [CrossRef] [PubMed]
- Guerra, M.T.; Nathanson, M.H. Calcium signaling and secretion in cholangiocytes. Pancreatology 2015, 15, S44–S48. [Google Scholar] [CrossRef] [PubMed]
- Avila, M.A.; Dufour, J.-F.; Gerbes, A.L.; Zoulim, F.; Bataller, R.; Burra, P.; Cortez-Pinto, H.; Gao, B.; Gilmore, I.; Mathurin, P.; et al. Recent advances in alcohol-related liver disease (ALD): Summary of a Gut round table meeting. Gut 2020, 69, 764–780. [Google Scholar] [CrossRef]
- Asrani, S.K.; Mellinger, J.; Arab, J.P.; Shah, V.H. Reducing the Global Burden of Alcohol-Associated Liver Disease: A Blueprint for Action. Hepatology 2021, 73, 2039–2050. [Google Scholar] [CrossRef]
- Wheeler, M.D.; Nakagami, M.; Bradford, B.U.; Uesugi, T.; Mason, R.P.; Connor, H.D.; Dikalova, A.; Kadiiska, M.; Thurman, R.G. Overexpression of Manganese Superoxide Dismutase Prevents Alcohol-induced Liver Injury in the Rat. J. Biol. Chem. 2001, 276, 36664–36672. [Google Scholar] [CrossRef]
- Gopal, T.; Kumar, N.; Perriotte-Olson, C.; Casey, C.A.; Donohue, T.M., Jr.; Harris, E.N.; Talmon, G.; Kabanov, A.V.; Saraswathi, V. Nanoformulated SOD1 ameliorates the combined NASH and alcohol-associated liver disease partly via regulating CYP2E1 expression in adipose tissue and liver. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 318, G428–G438. [Google Scholar] [CrossRef]
- Pi, A.; Jiang, K.; Ding, Q.; Lai, S.; Yang, W.; Zhu, J.; Guo, R.; Fan, Y.; Chi, L.; Li, S. Alcohol Abstinence Rescues Hepatic Steatosis and Liver Injury via Improving Metabolic Reprogramming in Chronic Alcohol-Fed Mice. Front. Pharmacol. 2021, 12, 752148. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Kim, M.-B.; Park, Y.-K.; Lee, J.-Y. A mouse model of the regression of alcoholic hepatitis: Monitoring the regression of hepatic steatosis, inflammation, oxidative stress, and NAD+ metabolism upon alcohol withdrawal. J. Nutr. Biochem. 2022, 99, 108852. [Google Scholar] [CrossRef] [PubMed]
- King, A.L.; Mantena, S.K.; Andringa, K.K.; Millender-Swain, T.; Dunham-Snary, K.J.; Oliva, C.R.; Griguer, C.E.; Bailey, S.M. The methyl donor S -adenosylmethionine prevents liver hypoxia and dysregulation of mitochondrial bioenergetic function in a rat model of alcohol-induced fatty liver disease. Redox Biol. 2016, 9, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Barbier-Torres, L.; Murray, B.; Yang, J.W.; Wang, J.; Matsuda, M.; Robinson, A.; Binek, A.; Fan, W.; Fernández-Ramos, D.; Lopitz-Otsoa, F.; et al. Depletion of mitochondrial methionine adenosyltransferase α1 triggers mitochondrial dysfunction in alcohol-associated liver disease. Nat. Commun. 2022, 13, 557. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Zhang, W.; Zhong, W.; Sun, X.; Zhou, Z. Pharmacological inhibition of NOX4 ameliorates alcohol-induced liver injury in mice through improving oxidative stress and mitochondrial function. Biochim. Biophys. Acta-Gen. Subj. 2017, 1861, 2912–2921. [Google Scholar] [CrossRef]
- Silva, J.; Spatz, M.H.; Folk, C.; Chang, A.; Cadenas, E.; Liang, J.; Davies, D.L. Dihydromyricetin improves mitochondrial outcomes in the liver of alcohol-fed mice via the AMPK/Sirt-1/PGC-1α signaling axis. Alcohol 2021, 91, 1–9. [Google Scholar] [CrossRef]
- Criddle, D.N.; Murphy, J.; Fistetto, G.; Barrow, S.; Tepikin, A.V.; Neoptolemos, J.P.; Sutton, R.; Petersen, O.H. Fatty Acid Ethyl Esters Cause Pancreatic Calcium Toxicity via Inositol Trisphosphate Receptors and Loss of ATP Synthesis. Gastroenterology 2006, 130, 781–793. [Google Scholar] [CrossRef]
- Petersen, O.; Tepikin, A.; Gerasimenko, J.V.; Gerasimenko, O.; Sutton, R.; Criddle, D. Fatty acids, alcohol and fatty acid ethyl esters: Toxic Ca2+ signal generation and pancreatitis. Cell Calcium 2009, 45, 634–642. [Google Scholar] [CrossRef]
- Criddle, D.N.; Sutton, R.; Petersen, O.H. Role of Ca2+ in pancreatic cell death induced by alcohol metabolites. J Gastroenterol Hepatol. 2006, 21, S14–S17. [Google Scholar] [CrossRef]
- Huang, W.; Booth, D.M.; Cane, M.C.; Chvanov, M.; Javed, M.A.; Elliott, V.L.; Armstrong, J.A.; Dingsdale, H.; Cash, N.; Li, Y.; et al. Fatty acid ethyl ester synthase inhibition ameliorates ethanol-induced Ca2+-dependent mitochondrial dysfunction and acute pancreatitis. Gut 2014, 63, 1313–1324. [Google Scholar] [CrossRef]
- Mukherjee, R.; Mareninova, O.A.; Odinokova, I.V.; Huang, W.; Murphy, J.; Chvanov, M.; Javed, M.A.; Wen, L.; Booth, D.M.; Cane, M.C.; et al. Mechanism of mitochondrial permeability transition pore induction and damage in the pancreas: Inhibition prevents acute pancreatitis by protecting production of ATP. Gut 2016, 65, 1333–1346. [Google Scholar] [CrossRef] [PubMed]
- Fortunato, F.; Berger, I.; Gross, M.-L.; Rieger, P.; Buechler, M.; Werner, J. Immune-compromised state in the rat pancreas after chronic alcohol exposure: The role of peroxisome proliferator-activated receptor γ. J. Pathol. 2007, 213, 441–452. [Google Scholar] [CrossRef]
- Shalbueva, N.; Mareninova, O.A.; Gerloff, A.; Yuan, J.; Waldron, R.T.; Pandol, S.J.; Gukovskaya, A. Effects of Oxidative Alcohol Metabolism on the Mitochondrial Permeability Transition Pore and Necrosis in a Mouse Model of Alcoholic Pancreatitis. Gastroenterology 2013, 144, 437–446.e6. [Google Scholar] [CrossRef] [PubMed]
- Javed, M.A.; Wen, L.; Awais, M.; Latawiec, D.; Huang, W.; Chvanov, M.; Schaller, S.; Bordet, T.; Michaud, M.; Pruss, R.; et al. TRO40303 Ameliorates Alcohol-Induced Pancreatitis Through Reduction of Fatty Acid Ethyl Ester–Induced Mitochondrial Injury and Necrotic Cell Death. Pancreas 2018, 47, 18–24. [Google Scholar] [CrossRef]
- Srinivasan, P.; Nabokina, S.; Said, H.M. Chronic alcohol exposure affects pancreatic acinar mitochondrial thiamin pyrophosphate uptake: Studies with mouse 266-6 cell line and primary cells. Am. J. Physiol. Liver Physiol. 2015, 309, G750–G758. [Google Scholar] [CrossRef]
- de Andrade, J.A.A.; Gayer, C.R.M.; Nogueira, N.P.D.A.; Paes, M.C.; Bastos, V.L.F.C.; Neto, J.D.C.B.; Alves, S.C.; Coelho, R.M.; da Cunha, M.G.A.T.; Gomes, R.N.; et al. The effect of thiamine deficiency on inflammation, oxidative stress and cellular migration in an experimental model of sepsis. J. Inflamm. 2014, 11, 11. [Google Scholar] [CrossRef]
- Molina, P.E.; Yousef, K.A.; Smith, R.M.; Tepper, P.G.; Lang, C.H.; Abumrad, N.N. Thiamin deficiency impairs endotoxin-induced increases in hepatic glucose output. Am. J. Clin. Nutr. 1994, 59, 1045–1049. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, R.; Thompson, C.; Ganguly, K.; Singh, S.; Batra, S.K.; Kumar, S. Alcohol and Smoking Mediated Modulations in Adaptive Immunity in Pancreatitis. Cells 2020, 9, 1880. [Google Scholar] [CrossRef]
- Moo-Young, T.A.; Larson, J.W.; Belt, B.A.; Tan, M.C.; Hawkins, W.G.; Eberlein, T.J.; Goedegebuure, P.S.; Linehan, D.C. Tumor-derived TGF-β Mediates Conversion of CD4+Foxp3+ Regulatory T Cells in a Murine Model of Pancreas Cancer. J. Immunother. 2009, 32, 12–21. [Google Scholar] [CrossRef]
- Manzo, T.; Prentice, B.M.; Anderson, K.G.; Raman, A.; Schalck, A.; Codreanu, G.S.; Lauson, C.B.N.; Tiberti, S.; Raimondi, A.; Jones, M.A.; et al. Accumulation of long-chain fatty acids in the tumor microenvironment drives dysfunction in intrapancreatic CD8+ T cells. J. Exp. Med. 2020, 217, e20191920. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Sun, Z.; Zhan, H. Macrophages in pancreatic cancer: An immunometabolic perspective. Cancer Lett. 2020, 498, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Park, A.; Kim, W.K.; Bae, K.-H. Distinction of white, beige and brown adipocytes derived from mesenchymal stem cells. World J. Stem Cells 2014, 6, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Barbatelli, G.; Murano, I.; Madsen, L.; Hao, Q.; Jimenez, M.; Kristiansen, K.; Giacobino, J.P.; De Matteis, R.; Cinti, S. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E1244–E1253. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Park, A.; Oh, K.-J.; Kim, W.K.; Bae, K.-H. The Role of Adipose Tissue Mitochondria: Regulation of Mitochondrial Function for the Treatment of Metabolic Diseases. Int. J. Mol. Sci. 2019, 20, 4924. [Google Scholar] [CrossRef] [PubMed]
- Nassir, F.; Ibdah, J.A. Role of mitochondria in alcoholic liver disease. World J. Gastroenterol. 2014, 20, 2136–2142. [Google Scholar] [CrossRef]
- Li, Z.; Gurung, M.; Rodrigues, R.R.; Padiadpu, J.; Newman, N.K.; Manes, N.P.; Pederson, J.W.; Greer, R.L.; Vasquez-Perez, S.; You, H.; et al. Microbiota and adipocyte mitochondrial damage in type 2 diabetes are linked by Mmp12+ macrophages. J. Exp. Med. 2022, 219, e20220017. [Google Scholar] [CrossRef]
- Vernochet, C.; Damilano, F.; Mourier, A.; Bezy, O.; Mori, M.A.; Smyth, G.; Rosenzweig, A.; Larsson, N.; Kahn, C.R. Adipose tissue mitochondrial dysfunction triggers a lipodystrophic syndrome with insulin resistance, hepatosteatosis, and cardiovascular complications. FASEB J. 2014, 28, 4408–4419. [Google Scholar] [CrossRef]
- Hertzel, A.V.; Yong, J.; Chen, X.; Bernlohr, D.A. Immune Modulation of Adipocyte Mitochondrial Metabolism. Endocrinology 2022, 163, bqac094. [Google Scholar] [CrossRef]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef]
- Manzo-Avalos, S.; Saavedra-Molina, A. Cellular and Mitochondrial Effects of Alcohol Consumption. Int. J. Environ. Res. Public Health 2010, 7, 4281–4304. [Google Scholar] [CrossRef]
- Tang, H.; Sebastian, B.M.; Axhemi, A.; Chen, X.; Hillian, A.D.; Jacobsen, D.W.; Nagy, L.E. Ethanol-Induced Oxidative Stress via the CYP2E1 Pathway Disrupts Adiponectin Secretion from Adipocytes. Alcohol. Clin. Exp. Res. 2012, 36, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, B.M.; Roychowdhury, S.; Tang, H.; Hillian, A.D.; Feldstein, A.E.; Stahl, G.; Takahashi, K.; Nagy, L.E. Identification of a Cytochrome P4502E1/Bid/C1q-dependent Axis Mediating Inflammation in Adipose Tissue after Chronic Ethanol Feeding to Mice. J. Biol. Chem. 2011, 286, 35989–35997. [Google Scholar] [CrossRef] [PubMed]
- Fulham, M.A.; Ratna, A.; Gerstein, R.M.; Kurt-Jones, E.A.; Mandrekar, P. Alcohol-induced adipose tissue macrophage phenotypic switching is independent of myeloid Toll-like receptor 4 expression. Am. J. Physiol. Cell Physiol. 2019, 317, C687–C700. [Google Scholar] [CrossRef] [PubMed]
- Souza-Smith, F.M.; Simon, L.; Siggins, R.; Molina, P.E. Alcohol-Induced Mesenteric Lymphatic Permeability: Link to Immunometabolic Modulation of Perilymphatic Adipose Tissue. Int. J. Mol. Sci. 2019, 20, 4097. [Google Scholar] [CrossRef] [PubMed]
- Souza-Smith, F.M.; Ford, S.M.; Simon, L.; Molina, P.E. Repeated Binge-Like Alcohol Intoxication: Depot-Specific Adi-pose Tissue Immuno-Metabolic Dysregulation. Shock 2017, 48, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Pafili, K.; Kahl, S.; Mastrototaro, L.; Strassburger, K.; Pesta, D.; Herder, C.; Pützer, J.; Dewidar, B.; Hendlinger, M.; Granata, C.; et al. Mitochondrial respiration is decreased in visceral but not subcutaneous adipose tissue in obese individuals with fatty liver disease. J. Hepatol. 2022, 77, 1504–1514. [Google Scholar] [CrossRef]
- Trounce, I.; Byrne, E.; Dennett, X.; Santamaria, J.; Doery, J.C.G.; Peppard, R. Chronic alcoholic proximal wasting: Physiological, morphological and biochemical studies in skeletal muscle. Aust. N. Z. J. Med. 1987, 17, 413–419. [Google Scholar] [CrossRef]
- Cardellach, F.; Galofré, J.; Grau, J.M.; Casademont, J.; Hoek, J.B.; Rubin, E.; Urbano-Márquez, A. Oxidative metabolism in muscle mitochondria from patients with chronic alcoholism. Ann. Neurol. 1992, 31, 515–518. [Google Scholar] [CrossRef]
- Thapaliya, S.; Runkana, A.; McMullen, M.R.; Nagy, L.E.; McDonald, C.; Prasad, S.V.N.; Dasarathy, S. Alcohol-induced autophagy contributes to loss in skeletal muscle mass. Autophagy 2014, 10, 677–690. [Google Scholar] [CrossRef]
- Ismaeel, A.; Laudato, J.A.; Fletcher, E.; Papoutsi, E.; Tice, A.; Hwa, L.S.; Miserlis, D.; Jamurtas, A.Z.; Steiner, J.; Koutakis, P. High-Fat Diet Augments the Effect of Alcohol on Skeletal Muscle Mitochondrial Dysfunction in Mice. Nutrients 2022, 14, 1016. [Google Scholar] [CrossRef]
- Eisner, V.; Lenaers, G.; Hajnóczky, G. Mitochondrial fusion is frequent in skeletal muscle and supports excitation–contraction coupling. J. Cell Biol. 2014, 205, 179–195. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.H.; Sheoran, S.; Richmond, J.E.; Kim, H. Alcohol induces mitochondrial fragmentation and stress responses to maintain normal muscle function in Caenorhabditis elegans. FASEB J. 2020, 34, 8204–8216. [Google Scholar] [CrossRef] [PubMed]
- Molina, P.E.; Lang, C.H.; McNurlan, M.; Bagby, G.J.; Nelson, S. Chronic Alcohol Accentuates Simian Acquired Immunodeficiency Syndrome-Associated Wasting. Alcohol. Clin. Exp. Res. 2008, 32, 138–147. [Google Scholar] [CrossRef] [PubMed]
- LeCapitaine, N.J.; Wang, Z.Q.; Dufour, J.P.; Potter, B.J.; Bagby, G.J.; Nelson, S.; Cefalu, W.T.; Molina, P.E. Disrupted Anabolic and Catabolic Processes May Contribute to Alcohol-Accentuated SAIDS-Associated Wasting. J. Infect. Dis. 2011, 204, 1246–1255. [Google Scholar] [CrossRef]
- Duplanty, A.A.; Simon, L.; Molina, P.E. Chronic Binge Alcohol-Induced Dysregulation of Mitochondrial-Related Genes in Skeletal Muscle of Simian Immunodeficiency Virus-Infected Rhesus Macaques at End-Stage Disease. Alcohol Alcohol. 2017, 52, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Duplanty, A.A.; Siggins, R.W.; Allerton, T.; Simon, L.; Molina, P.E. Myoblast mitochondrial respiration is decreased in chronic binge alcohol administered simian immunodeficiency virus-infected antiretroviral-treated rhesus macaques. Physiol. Rep. 2018, 6, e13625. [Google Scholar] [CrossRef]
- Levitt, D.E.; Chalapati, N.; Prendergast, M.J.; Simon, L.; Molina, P.E. Ethanol-Impaired Myogenic Differentiation is Associated With Decreased Myoblast Glycolytic Function. Alcohol. Clin. Exp. Res. 2020, 44, 2166–2176. [Google Scholar] [CrossRef] [PubMed]
- Levitt, D.E.; Ferguson, T.F.; Primeaux, S.D.; Zavala, J.A.; Ahmed, J.; Marshall, R.H.; Simon, L.; Molina, P.E. Skeletal muscle bioenergetic health and function in people living with HIV: Association with glucose tolerance and alcohol use. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2021, 321, R781–R790. [Google Scholar] [CrossRef]
- Steiner, J.L.; Lang, C.H. Dysregulation of skeletal muscle protein metabolism by alcohol. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E699–E712. [Google Scholar] [CrossRef]
- Fernandez-Solà, J.; Preedy, V.R.; Lang, C.H.; Gonzalez-Reimers, E.; Arno, M.; Lin, J.C.I.; Wiseman, H.; Zhou, S.; Emery, P.W.; Nakahara, T.; et al. Molecular and Cellular Events in Alcohol-Induced Muscle Disease. Alcohol. Clin. Exp. Res. 2007, 31, 1953–1962. [Google Scholar] [CrossRef]
- Preedy, V.R.; Paice, A.; Mantle, D.; Dhillon, A.S.; Palmer, T.; Peters, T.J. Alcoholic myopathy: Biochemical mechanisms. Drug Alcohol Depend. 2001, 63, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Kany, S.; Janicova, A.; Relja, B. Innate Immunity and Alcohol. J. Clin. Med. 2019, 8, 1981. [Google Scholar] [CrossRef]
- Daemen, S.; Schilling, J.D. The Interplay Between Tissue Niche and Macrophage Cellular Metabolism in Obesity. Front. Immunol. 2019, 10, 3133. [Google Scholar] [CrossRef] [PubMed]
- Ryan, D.G.; O’Neill, L.A. Krebs Cycle Reborn in Macrophage Immunometabolism. Annu. Rev. Immunol. 2020, 38, 289–313. [Google Scholar] [CrossRef]
- Rosales, C. Neutrophil: A Cell with Many Roles in Inflammation or Several Cell Types? Front. Physiol. 2018, 9, 113. [Google Scholar] [CrossRef] [PubMed]
- Bainton, D.F.; Ullyot, J.L.; Farquhar, M.G. The development of neutrophilic polymorphonuclear leukocytes in human bone marrow. J. Exp. Med. 1971, 134, 907–934. [Google Scholar] [CrossRef] [PubMed]
- Riffelmacher, T.; Clarke, A.; Richter, F.C.; Stranks, A.; Pandey, S.; Danielli, S.; Hublitz, P.; Yu, Z.; Johnson, E.; Schwerd, T.; et al. Autophagy-Dependent Generation of Free Fatty Acids Is Critical for Normal Neutrophil Differentiation. Immunity 2017, 47, 466–480.e5. [Google Scholar] [CrossRef]
- Borregaard, N.; Herlin, T. Energy Metabolism of Human Neutrophils during Phagocytosis. J. Clin. Investig. 1982, 70, 550–557. [Google Scholar] [CrossRef]
- van Raam, B.J.; Sluiter, W.; de Wit, E.; Roos, D.; Verhoeven, A.J.; Kuijpers, T.W. Mitochondrial Membrane Potential in Human Neutrophils Is Maintained by Complex III Activity in the Absence of Supercomplex Organisation. PLoS ONE 2008, 3, e2013. [Google Scholar] [CrossRef]
- Fossati, G.; Moulding, D.A.; Spiller, D.G.; Moots, R.J.; White, M.R.H.; Edwards, S.W. The Mitochondrial Network of Human Neutrophils: Role in Chemotaxis, Phagocytosis, Respiratory Burst Activation, and Commitment to Apoptosis. J. Immunol. 2003, 170, 1964–1972. [Google Scholar] [CrossRef]
- Bao, Y.; Ledderose, C.; Graf, A.F.; Brix, B.; Birsak, T.; Lee, A.; Zhang, J.; Junger, W.G. mTOR and differential activation of mitochondria orchestrate neutrophil chemotaxis. J. Cell Biol. 2015, 210, 1153–1164. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Corriden, R.; Inoue, Y.; Yip, L.; Hashiguchi, N.; Zinkernagel, A.; Nizet, V.; Insel, P.A.; Junger, W.G. ATP Release Guides Neutrophil Chemotaxis via P2Y2 and A3 Receptors. Science 2006, 314, 1792–1795. [Google Scholar] [CrossRef] [PubMed]
- Monceaux, V.; Chiche-Lapierre, C.; Chaput, C.; Witko-Sarsat, V.; Prevost, M.-C.; Taylor, C.T.; Ungeheuer, M.-N.; Sansonetti, P.J.; Marteyn, B.S. Anoxia and glucose supplementation preserve neutrophil viability and function. Blood 2016, 128, 993–1002. [Google Scholar] [CrossRef] [PubMed]
- Maianski, N.A.; Mul, F.P.J.; van Buul, J.D.; Roos, D.; Kuijpers, T.W. Granulocyte colony-stimulating factor inhibits the mitochondria-dependent activation of caspase-3 in neutrophils. Blood 2002, 99, 672–679. [Google Scholar] [CrossRef]
- Maianski, N.A.; Geissler, J.; Srinivasula, S.M.; Alnemri, E.S.; Roos, D.; Kuijpers, T.W. Functional characterization of mitochondria in neutrophils: A role restricted to apoptosis. Cell Death Differ. 2004, 11, 143–153. [Google Scholar] [CrossRef]
- Jeon, J.-H.; Hong, C.-W.; Kim, E.Y.; Lee, J.M. Current Understanding on the Metabolism of Neutrophils. Immune Netw. 2020, 20, e46. [Google Scholar] [CrossRef]
- Injarabian, L.; Devin, A.; Ransac, S.; Marteyn, B.S. Neutrophil Metabolic Shift during Their Lifecycle: Impact on Their Survival and Activation. Int. J. Mol. Sci. 2019, 21, 287. [Google Scholar] [CrossRef]
- Le, A.; Lane, A.N.; Hamaker, M.; Bose, S.; Gouw, A.; Barbi, J.; Tsukamoto, T.; Rojas, C.J.; Slusher, B.S.; Zhang, H.; et al. Glucose-Independent Glutamine Metabolism via TCA Cycling for Proliferation and Survival in B Cells. Cell Metab. 2012, 15, 110–121. [Google Scholar] [CrossRef]
- Singhal, P.C.; Patel, P.; Nàhàr, N.; Franki, N.; Kapasi, A.; Reddy, K.; Shah, N.; Nwakoby, I.E.; Mehrotra, B. Ethanol-induced neutrophil apoptosis is mediated through nitric oxide. J. Leukoc. Biol. 1999, 66, 930–936. [Google Scholar] [CrossRef]
- Todorović, V.; Koko, V.; Lacković, V.; Milin, J.; Varagić, J. Effect of chronic alcohol feeding on the ultrastructure of rat peripheral blood neutrophils: A morphometric study. J. Stud. Alcohol 1994, 55, 239–248. [Google Scholar] [CrossRef]
- Gacouin, A.; Roussel, M.; Gros, A.; Sauvadet, E.; Uhel, F.; Chimot, L.; Marqué, S.; Camus, C.; Fest, T.; Le Tulzo, Y. Chronic alcohol exposure, infection, extended circulating white blood cells differentiated by flow cytometry and neutrophil CD64 expression: A prospective, descriptive study of critically ill medical patients. Ann. Intensiv. Care 2012, 2, 50. [Google Scholar] [CrossRef] [PubMed]
- Stadlbauer, V.; Horvath, A.; Komarova, I.; Schmerboeck, B.; Feldbacher, N.; Wurm, S.; Klymiuk, I.; Durdevic, M.; Rainer, F.; Blesl, A.; et al. A single alcohol binge impacts on neutrophil function without changes in gut barrier function and gut microbiome composition in healthy volunteers. PLoS ONE 2019, 14, e0211703. [Google Scholar] [CrossRef] [PubMed]
- Malacco, N.L.S.d.O.; Souza, J.A.M.; Martins, F.R.B.; Rachid, M.A.; Simplicio, J.A.; Tirapelli, C.R.; Sabino, A.d.P.; Queiroz-Junior, C.M.; Goes, G.R.; Vieira, L.Q.; et al. Chronic ethanol consumption compromises neutrophil function in acute pulmonary Aspergillus fumigatus infection. Elife 2020, 9, e58855. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Dikshit, M. Metabolic Insight of Neutrophils in Health and Disease. Front. Immunol. 2019, 10, 2099. [Google Scholar] [CrossRef]
- Trim, W.; Turner, J.E.; Thompson, D. Parallels in Immunometabolic Adipose Tissue Dysfunction with Ageing and Obesity. Front. Immunol. 2018, 9, 169. [Google Scholar] [CrossRef]
- Hwang, S.; Ren, T.; Gao, B. Obesity and binge alcohol intake are deadly combination to induce steatohepatitis: A model of high-fat diet and binge ethanol intake. Clin. Mol. Hepatol. 2020, 26, 586–594. [Google Scholar] [CrossRef]
- O’Brien, B.J.; Faraoni, E.Y.; Strickland, L.N.; Ma, Z.; Mota, V.; Mota, S.; Chen, X.; Mills, T.; Eltzschig, H.K.; DelGiorno, K.E.; et al. CD73-generated extracellular adenosine promotes resolution of neutrophil-mediated tissue injury and restrains metaplasia in pancreatitis. FASEB J. 2023, 37, e22684. [Google Scholar] [CrossRef]
- Manohar, M.; Verma, A.K.; Venkateshaiah, S.U.; Sanders, N.L.; Mishra, A. Pathogenic mechanisms of pancreatitis. World J. Gastrointest. Pharmacol. Ther. 2017, 8, 10–25. [Google Scholar] [CrossRef]
- Kasztelan-Szczerbinska, B.; Zygo, B.; Rycyk-Bojarzynska, A.; Surdacka, A.; Rolinski, J.; Cichoz-Lach, H. Blood concentrations of mediators released from activated neutrophils are related to the severity of alcohol-induced liver damage. PLoS ONE 2023, 18, e0280068. [Google Scholar] [CrossRef]
- Rice, C.M.; Davies, L.C.; Subleski, J.J.; Maio, N.; Gonzalez-Cotto, M.; Andrews, C.; Patel, N.L.; Palmieri, E.M.; Weiss, J.M.; Lee, J.-M.; et al. Tumour-elicited neutrophils engage mitochondrial metabolism to circumvent nutrient limitations and maintain immune suppression. Nat. Commun. 2018, 9, 5099. [Google Scholar] [CrossRef]
- Alba-Loureiro, T.C.; Hirabara, S.M.; Mendonça, J.R.; Curi, R.; Pithon-Curi, T.C. Diabetes causes marked changes in function and metabolism of rat neutrophils. J. Endocrinol. 2006, 188, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, S.; Oh, D.Y.; Bandyopadhyay, G.; Li, D.; Xu, J.; McNelis, J.; Lu, M.; Li, P.; Yan, Q.; Zhu, Y.; et al. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat. Med. 2012, 18, 1407–1412. [Google Scholar] [CrossRef]
- Houghton, A.M.; Rzymkiewicz, D.M.; Ji, H.; Gregory, A.D.; Egea, E.E.; Metz, H.E.; Stolz, D.B.; Land, S.R.; Marconcini, L.A.; Kliment, C.R.; et al. Neutrophil elastase-mediated degradation of IRS-1 accelerates lung tumor growth. Nat. Med. 2010, 16, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Ren, Y.; Yang, X.; Li, X.; Xia, L.; Lu, N. The Role of Neutrophils and Neutrophil Extracellular Traps in Acute Pancreatitis. Front. Cell Dev. Biol. 2021, 8, 565758. [Google Scholar] [CrossRef]
- Cho, Y.; Szabo, G. Two Faces of Neutrophils in Liver Disease Development and Progression. Hepatology 2021, 74, 503–512. [Google Scholar] [CrossRef]
- Ziol, M.; Tepper, M.; Lohez, M.; Arcangeli, G.; Ganne, N.; Christidis, C.; Trinchet, J.-C.; Beaugrand, M.; Guillet, J.-G.; Guettier, C. Clinical and biological relevance of hepatocyte apoptosis in alcoholic hepatitis. J. Hepatol. 2001, 34, 254–260. [Google Scholar] [CrossRef]
- Gauthier, T.; Chen, W. Modulation of Macrophage Immunometabolism: A New Approach to Fight Infections. Front. Immunol. 2022, 13, 780839. [Google Scholar] [CrossRef]
- Ramond, E.; Jamet, A.; Coureuil, M.; Charbit, A. Pivotal Role of Mitochondria in Macrophage Response to Bacterial Pathogens. Front. Immunol. 2019, 10, 2461. [Google Scholar] [CrossRef]
- Mills, C.D.; Kincaid, K.; Alt, J.M.; Heilman, M.J.; Hill, A.M. M-1/M-2 Macrophages and the Th1/Th2 Paradigm. J. Immunol. 2000, 164, 6166–6173. [Google Scholar] [CrossRef] [PubMed]
- Blouin, C.C.; Pagé, E.L.; Soucy, G.M.; Richard, D.E. Hypoxic gene activation by lipopolysaccharide in macrophages: Implication of hypoxia-inducible factor 1α. Blood 2004, 103, 1124–1130. [Google Scholar] [CrossRef]
- Viola, A.; Munari, F.; Sánchez-Rodríguez, R.; Scolaro, T.; Castegna, A. The Metabolic Signature of Macrophage Responses. Front. Immunol. 2019, 10, 1462. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Pore, N.; Behrooz, A.; Ismail-Beigi, F.; Maity, A. Regulation of glut1 mRNA by Hypoxia-inducible Factor-1. Interaction between H-ras and hypoxia. J. Biol. Chem. 2001, 276, 9519–9525. [Google Scholar] [CrossRef] [PubMed]
- Freemerman, A.J.; Johnson, A.R.; Sacks, G.N.; Milner, J.J.; Kirk, E.L.; Troester, M.A.; Macintyre, A.N.; Goraksha-Hicks, P.; Rathmell, J.C.; Makowski, L. Metabolic reprogramming of macrophages: Glucose transporter 1 (GLUT1)-mediated glucose metabolism drives a proinflammatory phenotype. J. Biol. Chem. 2014, 289, 7884–7896. [Google Scholar] [CrossRef] [PubMed]
- Riddle, S.R.; Ahmad, A.; Ahmad, S.; Deeb, S.S.; Malkki, M.; Schneider, B.K.; Allen, C.B.; White, C.W. Hypoxia induces hexokinase II gene expression in human lung cell line A549. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2000, 278, L407–L416. [Google Scholar] [CrossRef] [PubMed]
- Freemerman, A.J.; Zhao, L.; Pingili, A.K.; Teng, B.; Cozzo, A.J.; Fuller, A.M.; Johnson, A.R.; Milner, J.J.; Lim, M.F.; Galanko, J.A.; et al. Myeloid Slc2a1-Deficient Murine Model Revealed Macrophage Activation and Metabolic Phenotype Are Fueled by GLUT1. J. Immunol. 2019, 202, 1265–1286. [Google Scholar] [CrossRef]
- Labonte, A.C.; Tosello-Trampont, A.-C.; Hahn, Y.S. The Role of Macrophage Polarization in Infectious and Inflammatory Diseases. Mol. Cells 2014, 37, 275–285. [Google Scholar] [CrossRef]
- Grumish, E.L.; Armstrong, A.R.; Voigt, R.M.; Forsyth, C.B.; Bishehsari, F. Alcohol-Induced Immune Dysregulation in the Colon Is Diurnally Variable. Visc. Med. 2020, 36, 212–219. [Google Scholar] [CrossRef]
- McTernan, P.M.; Levitt, D.E.; Welsh, D.A.; Simon, L.; Siggins, R.W.; Molina, P.E. Alcohol Impairs Immunometabolism and Promotes Naïve T Cell Differentiation to Pro-Inflammatory Th1 CD4+ T Cells. Front. Immunol. 2022, 13, 839390. [Google Scholar] [CrossRef]
- Mills, E.L.; Kelly, B.; Logan, A.; Costa, A.S.H.; Varma, M.; Bryant, C.E.; Tourlomousis, P.; Däbritz, J.H.M.; Gottlieb, E.; Latorre, I.; et al. Succinate Dehydrogenase Supports Metabolic Repurposing of Mitochondria to Drive Inflammatory Macrophages. Cell 2016, 167, 457–470. [Google Scholar] [CrossRef]
- Vats, D.; Mukundan, L.; Odegaard, J.I.; Zhang, L.; Smith, K.L.; Morel, C.R.; Wagner, R.A.; Greaves, D.R.; Murray, P.J.; Chawla, A. Oxidative metabolism and PGC-1β attenuate macrophage-mediated inflammation. Cell Metab. 2006, 4, 13–24. [Google Scholar] [CrossRef]
- Mandrekar, P.; Bala, S.; Catalano, D.; Kodys, K.; Szabo, G. The Opposite Effects of Acute and Chronic Alcohol on Lipopolysaccharide-Induced Inflammation Are Linked to IRAK-M in Human Monocytes. J. Immunol. 2009, 183, 1320–1327. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.; Bruneau, J.C.; Kodys, K.; Szabo, G. Alcohol-Induced miR-27a Regulates Differentiation and M2 Macrophage Polarization of Normal Human Monocytes. J. Immunol. 2015, 194, 3079–3087. [Google Scholar] [CrossRef] [PubMed]
- Louvet, A.; Teixeira-Clerc, F.; Chobert, M.-N.; Deveaux, V.; Pavoine, C.; Zimmer, A.; Pecker, F.; Mallat, A.; Lotersztajn, S. Cannabinoid CB2 receptors protect against alcoholic liver disease by regulating Kupffer cell polarization in mice. Hepatology 2011, 54, 1217–1226. [Google Scholar] [CrossRef]
- Mandal, P.; Pratt, B.T.; Barnes, M.; McMullen, M.R.; Nagy, L.E. Molecular Mechanism for Adiponectin-dependent M2 Macrophage Polarization: Link between the metabolic and innate immune activity of full-length adiponectin. J. Biol. Chem. 2011, 286, 13460–13469. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Zhong, Z.; Kim, S.Y.; Uchiyama, R.; Roh, Y.S.; Matsushita, H.; Gottlieb, R.A.; Seki, E. Murine macrophage autophagy protects against alcohol-induced liver injury by degrading interferon regulatory factor 1 (IRF1) and removing damaged mitochondria. J. Biol. Chem. 2019, 294, 12359–12369. [Google Scholar] [CrossRef] [PubMed]
- Agoro, R.; Taleb, M.; Quesniaux, V.F.J.; Mura, C. Cell iron status influences macrophage polarization. PLoS ONE 2018, 13, e0196921. [Google Scholar] [CrossRef]
- Xiong, S.; She, H.; Zhang, A.-S.; Wang, J.; Mkrtchyan, H.; Dynnyk, A.; Gordeuk, V.R.; French, S.W.; Enns, C.A.; Tsukamoto, H. Hepatic macrophage iron aggravates experimental alcoholic steatohepatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, G512–G521. [Google Scholar] [CrossRef]
- Morris, N.L.; Harris, F.L.; Brown, L.A.S.; Yeligar, S.M. Alcohol induces mitochondrial derangements in alveolar macrophages by upregulating NADPH oxidase 4. Alcohol 2021, 90, 27–38. [Google Scholar] [CrossRef]
- Panday, A.; Sahoo, M.K.; Osorio, D.; Batra, S. NADPH oxidases: An overview from structure to innate immunity-associated pathologies. Cell. Mol. Immunol. 2015, 12, 5–23. [Google Scholar] [CrossRef]
- Kumar, B.V.; Connors, T.; Farber, D.L. Human T Cell Development, Localization, and Function throughout Life. Immunity 2018, 48, 202–213. [Google Scholar] [CrossRef]
- Zloza, A.; Al-Harthi, L. Multiple populations of T lymphocytes are distinguished by the level of CD4 and CD8 coexpression and require individual consideration. J. Leukoc. Biol. 2006, 79, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Pearce, E.L.; Poffenberger, M.C.; Chang, C.-H.; Jones, R.G. Fueling Immunity: Insights into Metabolism and Lymphocyte Function. Science 2013, 342, 1242454. [Google Scholar] [CrossRef] [PubMed]
- Waters, L.R.; Ahsan, F.M.; Wolf, D.M.; Shirihai, O.; Teitell, M.A. Initial B Cell Activation Induces Metabolic Reprogramming and Mitochondrial Remodeling. iScience 2018, 5, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.; Cronin, J.G.; Dolton, G.; Panetti, S.; Schauenburg, A.J.; Galloway, S.A.E.; Sewell, A.K.; Cole, D.K.; Thornton, C.A.; Francis, N.J. Metabolic Adaptation of Human CD4+ and CD8+ T-Cells to T-Cell Receptor-Mediated Stimulation. Front. Immunol. 2017, 8, 1516. [Google Scholar] [CrossRef] [PubMed]
- Ripperger, T.J.; Bhattacharya, D. Transcriptional and Metabolic Control of Memory B Cells and Plasma Cells. Annu. Rev. Immunol. 2021, 39, 345–368. [Google Scholar] [CrossRef]
- Corrado, M.; Pearce, E.L. Targeting memory T cell metabolism to improve immunity. J. Clin. Investig. 2022, 132, e148546. [Google Scholar] [CrossRef]
- MacIver, N.J.; Michalek, R.D.; Rathmell, J.C. Metabolic Regulation of T Lymphocytes. Annu. Rev. Immunol. 2013, 31, 259–283. [Google Scholar] [CrossRef]
- Palmer, C.S.; Ostrowski, M.; Balderson, B.; Christian, N.; Crowe, S.M. Glucose Metabolism Regulates T Cell Activation, Differentiation, and Functions. Front. Immunol. 2015, 6, 1. [Google Scholar] [CrossRef]
- Reina-Campos, M.; Scharping, N.E.; Goldrath, A.W. CD8+ T cell metabolism in infection and cancer. Nat. Rev. Immunol. 2021, 21, 718–738. [Google Scholar] [CrossRef]
- Meyerholz, D.K.; Edsen-Moore, M.; McGill, J.; Coleman, R.A.; Cook, R.T.; Legge, K.L. Chronic Alcohol Consumption Increases the Severity of Murine Influenza Virus Infections. J. Immunol. 2008, 181, 641–648. [Google Scholar] [CrossRef]
- Poonia, B.; Nelson, S.; Bagby, G.J.; Veazey, R.S. Intestinal Lymphocyte Subsets and Turnover Are Affected by Chronic Alcohol Consumption: Implications for SIV/HIV infection. J. Acquir. Immune Defic. Syndr. 2006, 41, 537–547. [Google Scholar] [CrossRef]
- Doughty, C.A.; Bleiman, B.F.; Wagner, D.J.; Dufort, F.J.; Mataraza, J.M.; Roberts, M.F.; Chiles, T.C. Antigen receptor–mediated changes in glucose metabolism in B lymphocytes: Role of phosphatidylinositol 3-kinase signaling in the glycolytic control of growth. Blood 2006, 107, 4458–4465. [Google Scholar] [CrossRef] [PubMed]
- Khalsa, J.K.; Chawla, A.S.; Prabhu, S.B.; Vats, M.; Dhar, A.; Dev, G.; Das, N.; Mukherjee, S.; Tanwar, S.; Banerjee, H.; et al. Functionally significant metabolic differences between B and T lymphocyte lineages. Immunology 2019, 158, 104–120. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Hong, M.J.; Sun, H.; Wang, L.; Shi, X.; Gilbert, B.E.; Corry, D.B.; Kheradmand, F.; Wang, J. Essential role for autophagy in the maintenance of immunological memory against influenza infection. Nat. Med. 2014, 20, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Kodali, S.; Jang, A.; Kuai, L.; Wang, J. Requirement for Autophagy in the Long-Term Persistence but not Initial Formation of Memory B cells. J. Immunol. 2015, 194, 2607–2615. [Google Scholar] [CrossRef] [PubMed]
- Raza, I.G.A.; Clarke, A.J. B Cell Metabolism and Autophagy in Autoimmunity. Front. Immunol. 2021, 12, 681105. [Google Scholar] [CrossRef]
- Mili, F.; Flanders, W.D.; Boring, J.R.; Annest, J.L.; DeStefano, F. The Associations of Alcohol Drinking and Drinking Cessation to Measures of the Immune System in Middle-Aged Men. Alcohol. Clin. Exp. Res. 1992, 16, 688–694. [Google Scholar] [CrossRef]
- Sacanella, E.; Estruch, R.; Gaya, A.; Fernandez-Sola, J.; Antunez, E.; Urbano-Marquez, A. Activated Lymphocytes (CD25+ CD69+ Cells) and Decreased CD19+ Cells in Well-Nourished Chronic Alcoholics without Ethanol-Related Diseases. Alcohol. Clin. Exp. Res. 1998, 22, 897–901. [Google Scholar] [CrossRef]
- Matos, L.C.; Batista, P.; Monteiro, N.; Ribeiro, J.; Cipriano, M.A.; Henriques, P.; Girão, F.; Carvalho, A. Lymphocyte subsets in alcoholic liver disease. World J. Hepatol. 2013, 5, 46–55. [Google Scholar] [CrossRef]
- Pasala, S.; Barr, T.; Messaoudi, I. Impact of Alcohol Abuse on the Adaptive Immune System. Alcohol Res. 2015, 37, 185–197. [Google Scholar]
- Scharf, R.E.; Aul, C. Alcohol-induced disorders of the hematopoietic system. Z. Gastroenterol. 1988, 26 (Suppl. 3), 75–83. [Google Scholar] [PubMed]
- Siggins, R.W.; Molina, P.; Zhang, P.; Bagby, G.J.; Nelson, S.; Dufour, J.; LeCapitaine, N.J.; Walsh, C.; Welsh, D.A. Dysregulation of Myelopoiesis by Chronic Alcohol Administration During Early SIV Infection of Rhesus Macaques. Alcohol. Clin. Exp. Res. 2014, 38, 1993–2000. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.; Gasparetto, M.; Jordan, C.; Pollyea, D.A.; Vasiliou, V. The Effects of Alcohol and Aldehyde Dehydrogenases on Disorders of Hematopoiesis. Adv. Exp. Med. Biol. 2015, 815, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Collier, J.J.; Oláhová, M.; McWilliams, T.G.; Taylor, R.W. Mitochondrial signalling and homeostasis: From cell biology to neurological disease. Trends Neurosci. 2023, 46, 137–152. [Google Scholar] [CrossRef]
- Diaz-Vegas, A.; Sanchez-Aguilera, P.; Krycer, J.R.; Morales, P.E.; Monsalves-Alvarez, M.; Cifuentes, M.; Rothermel, B.A.; Lavandero, S. Is Mitochondrial Dysfunction a Common Root of Noncommunicable Chronic Diseases? Endocr. Rev. 2020, 41, bnaa005. [Google Scholar] [CrossRef]
- Gabandé-Rodríguez, E.; Gomez de Las, M.M.; Mittelbrunn, M. Control of Inflammation by Calorie Restriction Mimetics: On the Crossroad of Autophagy and Mitochondria. Cells 2019, 9, 82. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Wei, Y.; Sowers, J.R. Role of Mitochondrial Dysfunction in Insulin Resistance. Circ. Res. 2008, 102, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Krako Jakovljevic, N.; Pavlovic, K.; Jotic, A.; Lalic, K.; Stoiljkovic, M.; Lukic, L.; Milicic, T.; Macesic, M.; Gajovic, J.S.; Lalic, N.M. Targeting Mitochondria in Diabetes. Int. J. Mol. Sci. 2021, 22, 6642. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, D.Y.; Lee, Y.J.; Park, K.J.; Kim, K.H.; Kim, J.W.; Kim, W.-H. Chronic alcohol consumption potentiates the development of diabetes through pancreatic β-cell dysfunction. World J. Biol. Chem. 2015, 6, 1–15. [Google Scholar] [CrossRef]
- Rock, K.L.; Kono, H. The Inflammatory Response to Cell Death. Annu. Rev. Pathol. Mech. Dis. 2008, 3, 99–126. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siggins, R.W.; McTernan, P.M.; Simon, L.; Souza-Smith, F.M.; Molina, P.E. Mitochondrial Dysfunction: At the Nexus between Alcohol-Associated Immunometabolic Dysregulation and Tissue Injury. Int. J. Mol. Sci. 2023, 24, 8650. https://doi.org/10.3390/ijms24108650
Siggins RW, McTernan PM, Simon L, Souza-Smith FM, Molina PE. Mitochondrial Dysfunction: At the Nexus between Alcohol-Associated Immunometabolic Dysregulation and Tissue Injury. International Journal of Molecular Sciences. 2023; 24(10):8650. https://doi.org/10.3390/ijms24108650
Chicago/Turabian StyleSiggins, Robert W., Patrick M. McTernan, Liz Simon, Flavia M. Souza-Smith, and Patricia E. Molina. 2023. "Mitochondrial Dysfunction: At the Nexus between Alcohol-Associated Immunometabolic Dysregulation and Tissue Injury" International Journal of Molecular Sciences 24, no. 10: 8650. https://doi.org/10.3390/ijms24108650
APA StyleSiggins, R. W., McTernan, P. M., Simon, L., Souza-Smith, F. M., & Molina, P. E. (2023). Mitochondrial Dysfunction: At the Nexus between Alcohol-Associated Immunometabolic Dysregulation and Tissue Injury. International Journal of Molecular Sciences, 24(10), 8650. https://doi.org/10.3390/ijms24108650





