The Role of Krüppel-like Factors in Pancreatic Physiology and Pathophysiology
Abstract
1. Introduction
2. Krüppel-like Factors in Regulation of Islet Function
2.1. Homeostatic Pancreatic Endocrine Function
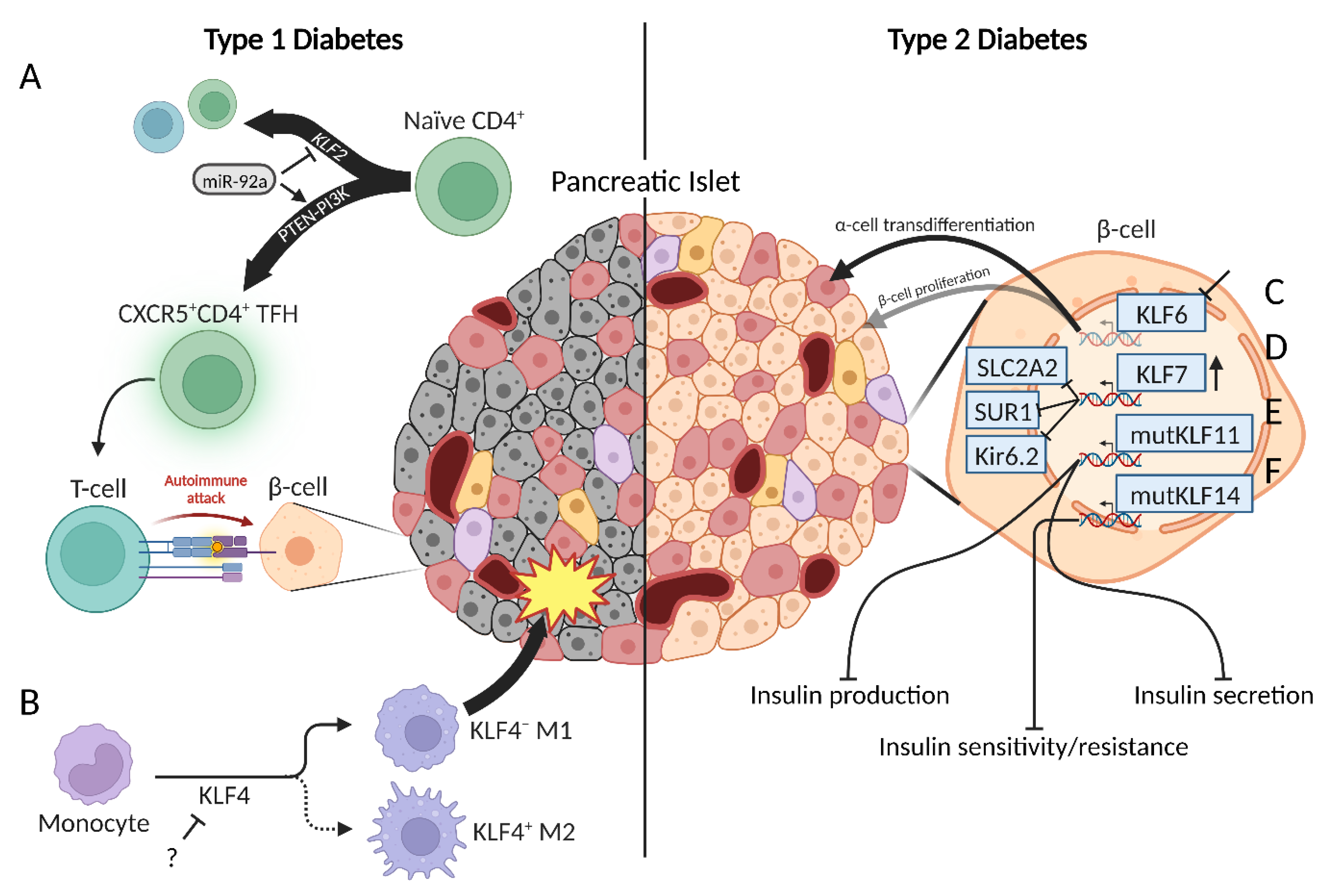
2.2. Type 1 Diabetes
2.3. Type 2 Diabetes
3. Regenerative Capabilities of Krüppel-like Factors
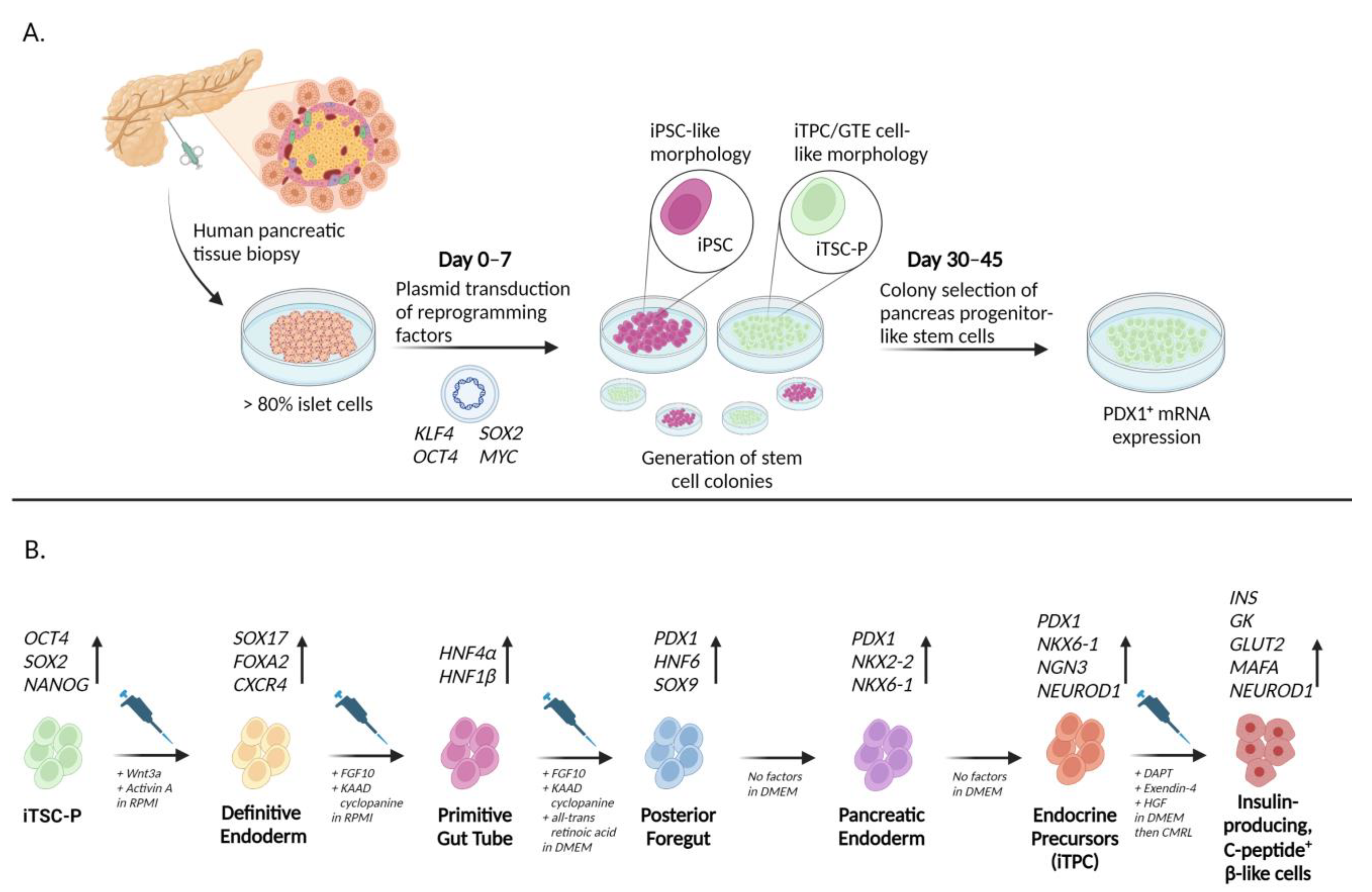
3.1. iPSCs from Non-Pancreatic-Specific Cell Types
3.2. iPSCs from Pancreatic-Lineage Specific Cell Types
4. Pancreatitis and Pancreatic Cancer
4.1. KLFs as Tumor Suppressors in PDAC
4.1.1. KLF2
4.1.2. KLF3
4.1.3. KLF6
4.1.4. KLF9
4.1.5. KLF10
4.1.6. KLF11
4.1.7. KLF13
4.2. KLFs as Oncogenes
4.2.1. KLF7
4.2.2. KLF8
4.2.3. KLF12
4.2.4. KLF16
4.3. KLFs with a Biphasic Role in PDAC
4.3.1. KLF4
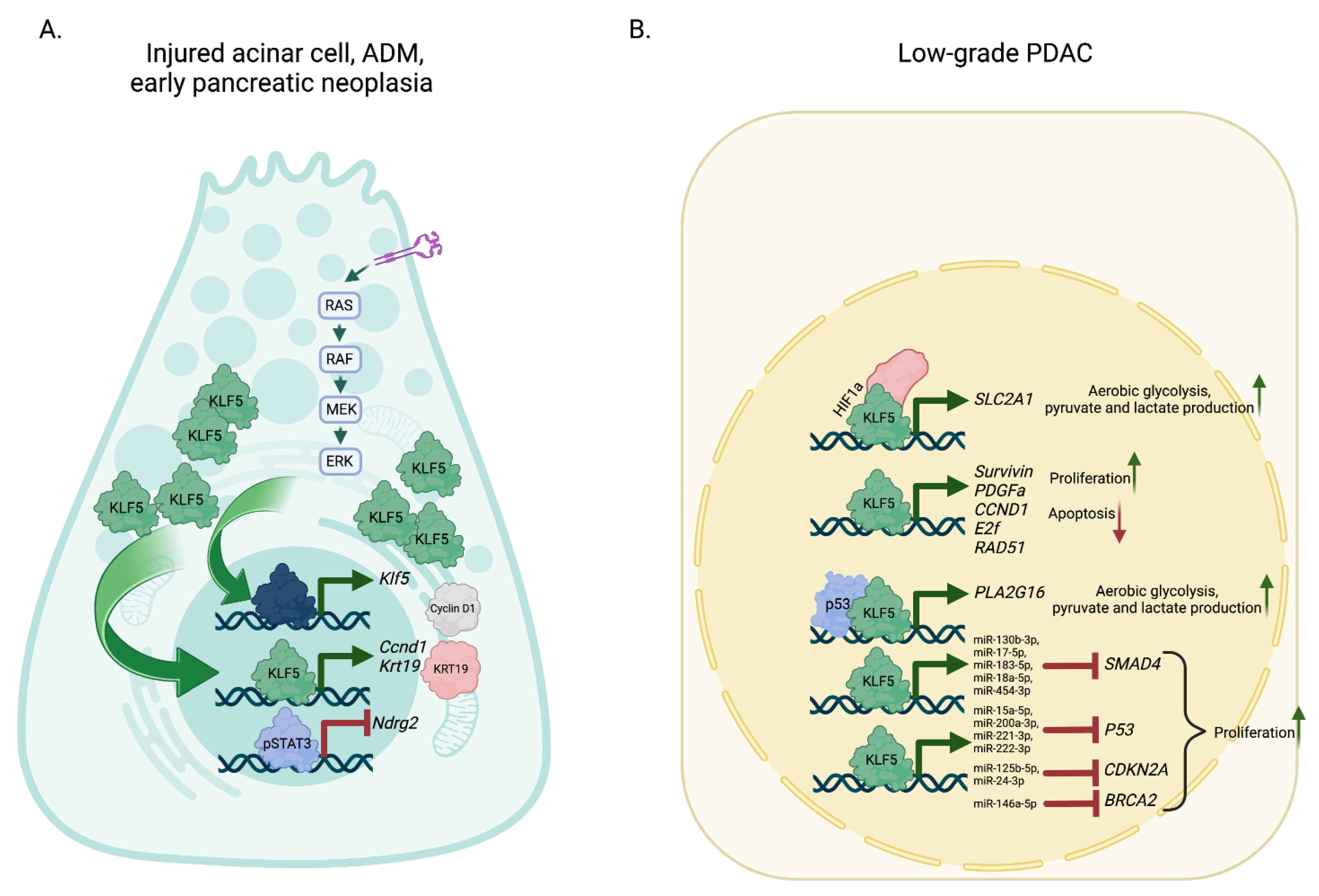
4.3.2. KLF5
4.4. KLFs as Potential Biomarkers
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leung, P.S. Overview of the pancreas. Adv. Exp. Med. Biol. 2010, 690, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Dolensek, J.; Rupnik, M.S.; Stozer, A. Structural similarities and differences between the human and the mouse pancreas. Islets 2015, 7, e1024405. [Google Scholar] [CrossRef] [PubMed]
- Leung, P.S. Physiology of the pancreas. Adv. Exp. Med. Biol. 2010, 690, 13–27. [Google Scholar] [CrossRef]
- Karpinska, M.; Czauderna, M. Pancreas-Its Functions, Disorders, and Physiological Impact on the Mammals’ Organism. Front. Physiol. 2022, 13, 807632. [Google Scholar] [CrossRef]
- Swamynathan, S.K. Kruppel-like factors: Three fingers in control. Hum. Genom. 2010, 4, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Kaczynski, J.; Cook, T.; Urrutia, R. Sp1- and Kruppel-like transcription factors. Genome Biol. 2003, 4, 206. [Google Scholar] [CrossRef]
- Presnell, J.S.; Schnitzler, C.E.; Browne, W.E. KLF/SP Transcription Factor Family Evolution: Expansion, Diversification, and Innovation in Eukaryotes. Genome Biol. Evol. 2015, 7, 2289–2309. [Google Scholar] [CrossRef]
- Kim, C.K.; He, P.; Bialkowska, A.B.; Yang, V.W. SP and KLF Transcription Factors in Digestive Physiology and Diseases. Gastroenterology 2017, 152, 1845–1875. [Google Scholar] [CrossRef]
- McConnell, B.B.; Yang, V.W. Mammalian Kruppel-like factors in health and diseases. Physiol. Rev. 2010, 90, 1337–1381. [Google Scholar] [CrossRef]
- Abe, M.; Saeki, N.; Ikeda, Y.; Ohba, S. Kruppel-like Factors in Skeletal Physiology and Pathologies. Int. J. Mol. Sci. 2022, 23, 15174. [Google Scholar] [CrossRef]
- Oishi, Y.; Manabe, I. Kruppel-Like Factors in Metabolic Homeostasis and Cardiometabolic Disease. Front. Cardiovasc. Med. 2018, 5, 69. [Google Scholar] [CrossRef] [PubMed]
- Atkins, G.B.; Jain, M.K. Role of Kruppel-like transcription factors in endothelial biology. Circ. Res. 2007, 100, 1686–1695. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, P.N.; Fan, L.; Sweet, D.R.; Jain, M.K. The Kruppel-Like Factors and Control of Energy Homeostasis. Endocr. Rev. 2019, 40, 137–152. [Google Scholar] [CrossRef]
- Shahid, Z.; Singh, G. Physiology, Islets of Langerhans. In StatPearls [Internet]; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Wu, M.J.; Wu, W.C.; Chang, H.W.; Lai, Y.T.; Lin, C.H.; Yu, W.C.; Chang, V.H. KLF10 affects pancreatic function via the SEI-1/p21Cip1 pathway. Int. J. Biochem. Cell Biol. 2015, 60, 53–59. [Google Scholar] [CrossRef]
- Poi, M.J.; Knobloch, T.J.; Yuan, C.; Tsai, M.D.; Weghorst, C.M.; Li, J. Evidence that P12, a specific variant of P16(INK4A), plays a suppressive role in human pancreatic carcinogenesis. Biochem. Biophys. Res. Commun. 2013, 436, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Neve, B.; Fernandez-Zapico, M.E.; Ashkenazi-Katalan, V.; Dina, C.; Hamid, Y.H.; Joly, E.; Vaillant, E.; Benmezroua, Y.; Durand, E.; Bakaher, N.; et al. Role of transcription factor KLF11 and its diabetes-associated gene variants in pancreatic beta cell function. Proc. Natl. Acad. Sci. USA 2005, 102, 4807–4812. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Perakakis, N.; Laubner, K.; Limbert, C.; Stahl, T.; Brendel, M.D.; Bretzel, R.G.; Seufert, J.; Path, G. Human Kruppel-like factor 11 inhibits human proinsulin promoter activity in pancreatic beta cells. Diabetologia 2007, 50, 1433–1441. [Google Scholar] [CrossRef]
- Fernandez-Zapico, M.E.; van Velkinburgh, J.C.; Gutierrez-Aguilar, R.; Neve, B.; Froguel, P.; Urrutia, R.; Stein, R. MODY7 gene, KLF11, is a novel p300-dependent regulator of Pdx-1 (MODY4) transcription in pancreatic islet beta cells. J. Biol. Chem. 2009, 284, 36482–36490. [Google Scholar] [CrossRef]
- Bonnefond, A.; Lomberk, G.; Buttar, N.; Busiah, K.; Vaillant, E.; Lobbens, S.; Yengo, L.; Dechaume, A.; Mignot, B.; Simon, A.; et al. Disruption of a novel Kruppel-like transcription factor p300-regulated pathway for insulin biosynthesis revealed by studies of the c.-331 INS mutation found in neonatal diabetes mellitus. J. Biol. Chem. 2011, 286, 28414–28424. [Google Scholar] [CrossRef]
- Perakakis, N.; Danassi, D.; Alt, M.; Tsaroucha, E.; Mehana, A.E.; Rimmer, N.; Laubner, K.; Wang, H.; Wollheim, C.B.; Seufert, J.; et al. Human Kruppel-like factor 11 differentially regulates human insulin promoter activity in beta-cells and non-beta-cells via p300 and PDX1 through the regulatory sites A3 and CACCC box. Mol. Cell. Endocrinol. 2012, 363, 20–26. [Google Scholar] [CrossRef]
- Nagare, T.; Sakaue, H.; Matsumoto, M.; Cao, Y.; Inagaki, K.; Sakai, M.; Takashima, Y.; Nakamura, K.; Mori, T.; Okada, Y.; et al. Overexpression of KLF15 transcription factor in adipocytes of mice results in down-regulation of SCD1 protein expression in adipocytes and consequent enhancement of glucose-induced insulin secretion. J. Biol. Chem. 2011, 286, 37458–37469. [Google Scholar] [CrossRef]
- Serr, I.; Furst, R.W.; Ott, V.B.; Scherm, M.G.; Nikolaev, A.; Gokmen, F.; Kalin, S.; Zillmer, S.; Bunk, M.; Weigmann, B.; et al. miRNA92a targets KLF2 and the phosphatase PTEN signaling to promote human T follicular helper precursors in T1D islet autoimmunity. Proc. Natl. Acad. Sci. USA 2016, 113, E6659–E6668. [Google Scholar] [CrossRef]
- Carlson, C.M.; Endrizzi, B.T.; Wu, J.; Ding, X.; Weinreich, M.A.; Walsh, E.R.; Wani, M.A.; Lingrel, J.B.; Hogquist, K.A.; Jameson, S.C. Kruppel-like factor 2 regulates thymocyte and T-cell migration. Nature 2006, 442, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Sebzda, E.; Zou, Z.; Lee, J.S.; Wang, T.; Kahn, M.L. Transcription factor KLF2 regulates the migration of naive T cells by restricting chemokine receptor expression patterns. Nat. Immunol. 2008, 9, 292–300. [Google Scholar] [CrossRef]
- Lee, J.Y.; Skon, C.N.; Lee, Y.J.; Oh, S.; Taylor, J.J.; Malhotra, D.; Jenkins, M.K.; Rosenfeld, M.G.; Hogquist, K.A.; Jameson, S.C. The transcription factor KLF2 restrains CD4(+) T follicular helper cell differentiation. Immunity 2015, 42, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, J.; Yan, M.; Jiang, J.; Bian, A. MiRNA-92a protects pancreatic B-cell function by targeting KLF2 in diabetes mellitus. Biochem. Biophys. Res. Commun. 2018, 500, 577–582. [Google Scholar] [CrossRef]
- Park, S.W.; Goodpaster, B.H.; Strotmeyer, E.S.; de Rekeneire, N.; Harris, T.B.; Schwartz, A.V.; Tylavsky, F.A.; Newman, A.B. Decreased muscle strength and quality in older adults with type 2 diabetes: The health, aging, and body composition study. Diabetes 2006, 55, 1813–1818. [Google Scholar] [CrossRef]
- Leenders, M.; Verdijk, L.B.; van der Hoeven, L.; Adam, J.J.; van Kranenburg, J.; Nilwik, R.; van Loon, L.J. Patients with type 2 diabetes show a greater decline in muscle mass, muscle strength, and functional capacity with aging. J. Am. Med. Dir. Assoc. 2013, 14, 585–592. [Google Scholar] [CrossRef]
- Workeneh, B.; Bajaj, M. The regulation of muscle protein turnover in diabetes. Int. J. Biochem. Cell Biol. 2013, 45, 2239–2244. [Google Scholar] [CrossRef] [PubMed]
- Lecker, S.H.; Solomon, V.; Mitch, W.E.; Goldberg, A.L. Muscle protein breakdown and the critical role of the ubiquitin-proteasome pathway in normal and disease states. J. Nutr. 1999, 129, 227S–237S. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Wang, X.; Miereles, C.; Bailey, J.L.; Debigare, R.; Zheng, B.; Price, S.R.; Mitch, W.E. Activation of caspase-3 is an initial step triggering accelerated muscle proteolysis in catabolic conditions. J. Clin. Investig. 2004, 113, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Forsberg, N.E. Role of calpain in skeletal-muscle protein degradation. Proc. Natl. Acad. Sci. USA 1998, 95, 12100–12105. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Yeon, M.H.; Jun, H.S. Schisandrae chinensis Fructus Extract Ameliorates Muscle Atrophy in Streptozotocin-Induced Diabetic Mice by Downregulation of the CREB-KLF15 and Autophagy-Lysosomal Pathways. Cells 2021, 10, 2283. [Google Scholar] [CrossRef]
- Cosentino, C.; Regazzi, R. Crosstalk between Macrophages and Pancreatic beta-Cells in Islet Development, Homeostasis and Disease. Int. J. Mol. Sci. 2021, 22, 1765. [Google Scholar] [CrossRef]
- Liao, X.; Sharma, N.; Kapadia, F.; Zhou, G.; Lu, Y.; Hong, H.; Paruchuri, K.; Mahabeleshwar, G.H.; Dalmas, E.; Venteclef, N.; et al. Kruppel-like factor 4 regulates macrophage polarization. J. Clin. Investig. 2011, 121, 2736–2749. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Ou, L.; Han, S.; Li, M.; Pena, M.M.; Pena, E.A.; Liu, C.; Nagarkatti, M.; Fan, D.; Ai, W. Deficiency of Kruppel-like factor KLF4 in myeloid-derived suppressor cells inhibits tumor pulmonary metastasis in mice accompanied by decreased fibrocytes. Oncogenesis 2014, 3, e129. [Google Scholar] [CrossRef] [PubMed]
- Ou, L.; Shi, Y.; Dong, W.; Liu, C.; Schmidt, T.J.; Nagarkatti, P.; Nagarkatti, M.; Fan, D.; Ai, W. Kruppel-like factor KLF4 facilitates cutaneous wound healing by promoting fibrocyte generation from myeloid-derived suppressor cells. J. Investig. Dermatol. 2015, 135, 1425–1434. [Google Scholar] [CrossRef]
- Husseini, M.; Wang, G.S.; Patrick, C.; Crookshank, J.A.; MacFarlane, A.J.; Noel, J.A.; Strom, A.; Scott, F.W. Heme Oxygenase-1 Induction Prevents Autoimmune Diabetes in Association With Pancreatic Recruitment of M2-Like Macrophages, Mesenchymal Cells, and Fibrocytes. Endocrinology 2015, 156, 3937–3949. [Google Scholar] [CrossRef]
- Ohtake, K.; Saito, T.; Satoh, Y.; Kenjo, A.; Kimura, T.; Asawa, S.; Anazawa, T.; Gotoh, M. Bone marrow traffic to regenerating islets induced by streptozotocin injection and partial pancreatectomy in mice. Transplant. Proc. 2008, 40, 449–451. [Google Scholar] [CrossRef]
- Liu, J.; Joglekar, M.V.; Sumer, H.; Hardikar, A.A.; Teede, H.; Verma, P.J. Integration-Free Human Induced Pluripotent Stem Cells From Type 1 Diabetes Patient Skin Fibroblasts Show Increased Abundance of Pancreas-Specific microRNAs. Cell. Med. 2014, 7, 15–24. [Google Scholar] [CrossRef]
- Shrestha, S.; Erikson, G.; Lyon, J.; Spigelman, A.F.; Bautista, A.; Manning Fox, J.E.; Dos Santos, C.; Shokhirev, M.; Cartailler, J.P.; Hetzer, M.W.; et al. Aging compromises human islet beta cell function and identity by decreasing transcription factor activity and inducing ER stress. Sci. Adv. 2022, 8, eabo3932. [Google Scholar] [CrossRef] [PubMed]
- Dumayne, C.; Tarussio, D.; Sanchez-Archidona, A.R.; Picard, A.; Basco, D.; Berney, X.P.; Ibberson, M.; Thorens, B. Klf6 protects beta-cells against insulin resistance-induced dedifferentiation. Mol. Metab. 2020, 35, 100958. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, N.; Laub, F.; Aldabe, R.; Zhang, W.; Ramirez, F.; Yoshida, T.; Terada, M. Cloning the cDNA for a new human zinc finger protein defines a group of closely related Kruppel-like transcription factors. J. Biol. Chem. 1998, 273, 28229–28237. [Google Scholar] [CrossRef]
- Kawamura, Y.; Tanaka, Y.; Kawamori, R.; Maeda, S. Overexpression of Kruppel-like factor 7 regulates adipocytokine gene expressions in human adipocytes and inhibits glucose-induced insulin secretion in pancreatic beta-cell line. Mol. Endocrinol. 2006, 20, 844–856. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, T.; Sparso, T.; Grarup, N.; Jorgensen, T.; Pisinger, C.; Witte, D.R.; Diabetes Genetics, R.; Meta-analysis, C.; Hansen, T.; Pedersen, O. Type 2 diabetes risk allele near CENTD2 is associated with decreased glucose-stimulated insulin release. Diabetologia 2011, 54, 1052–1056. [Google Scholar] [CrossRef]
- Dimas, A.S.; Lagou, V.; Barker, A.; Knowles, J.W.; Magi, R.; Hivert, M.F.; Benazzo, A.; Rybin, D.; Jackson, A.U.; Stringham, H.M.; et al. Impact of type 2 diabetes susceptibility variants on quantitative glycemic traits reveals mechanistic heterogeneity. Diabetes 2014, 63, 2158–2171. [Google Scholar] [CrossRef]
- Bacos, K.; Gillberg, L.; Volkov, P.; Olsson, A.H.; Hansen, T.; Pedersen, O.; Gjesing, A.P.; Eiberg, H.; Tuomi, T.; Almgren, P.; et al. Blood-based biomarkers of age-associated epigenetic changes in human islets associate with insulin secretion and diabetes. Nat. Commun. 2016, 7, 11089. [Google Scholar] [CrossRef]
- Tateishi, K.; He, J.; Taranova, O.; Liang, G.; D’Alessio, A.C.; Zhang, Y. Generation of insulin-secreting islet-like clusters from human skin fibroblasts. J. Biol. Chem. 2008, 283, 31601–31607. [Google Scholar] [CrossRef]
- Miyagi-Shiohira, C.; Nakashima, Y.; Kobayashi, N.; Saitoh, I.; Watanabe, M.; Noguchi, H. Characterization of induced tissue-specific stem cells from pancreas by a synthetic self-replicative RNA. Sci. Rep. 2018, 8, 12341. [Google Scholar] [CrossRef]
- Fowler, J.L.; Ang, L.T.; Loh, K.M. A critical look: Challenges in differentiating human pluripotent stem cells into desired cell types and organoids. Wiley Interdiscip. Rev. Dev. Biol. 2020, 9, e368. [Google Scholar] [CrossRef]
- Abad, M.; Mosteiro, L.; Pantoja, C.; Canamero, M.; Rayon, T.; Ors, I.; Grana, O.; Megias, D.; Dominguez, O.; Martinez, D.; et al. Reprogramming in vivo produces teratomas and iPS cells with totipotency features. Nature 2013, 502, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Stadtfeld, M.; Brennand, K.; Hochedlinger, K. Reprogramming of pancreatic beta cells into induced pluripotent stem cells. Curr. Biol. 2008, 18, 890–894. [Google Scholar] [CrossRef] [PubMed]
- Papp, B.; Plath, K. Reprogramming to pluripotency: Stepwise resetting of the epigenetic landscape. Cell Res. 2011, 21, 486–501. [Google Scholar] [CrossRef] [PubMed]
- Park, W.; Chawla, A.; O’Reilly, E.M. Pancreatic Cancer: A Review. JAMA 2021, 326, 851–862. [Google Scholar] [CrossRef]
- Saitoh, I.; Sato, M.; Soda, M.; Inada, E.; Iwase, Y.; Murakami, T.; Ohshima, H.; Hayasaki, H.; Noguchi, H. Tissue-Specific Stem Cells Obtained by Reprogramming of Non-Obese Diabetic (NOD) Mouse-Derived Pancreatic Cells Confer Insulin Production in Response to Glucose. PLoS ONE 2016, 11, e0163580. [Google Scholar] [CrossRef] [PubMed]
- Krog, R.T.; de Miranda, N.; Vahrmeijer, A.L.; Kooreman, N.G. The Potential of Induced Pluripotent Stem Cells to Advance the Treatment of Pancreatic Ductal Adenocarcinoma. Cancers 2021, 13, 5789. [Google Scholar] [CrossRef]
- Maehr, R.; Chen, S.; Snitow, M.; Ludwig, T.; Yagasaki, L.; Goland, R.; Leibel, R.L.; Melton, D.A. Generation of pluripotent stem cells from patients with type 1 diabetes. Proc. Natl. Acad. Sci. USA 2009, 106, 15768–15773. [Google Scholar] [CrossRef]
- Santamaria, P.; Rodriguez-Piza, I.; Clemente-Casares, X.; Yamanouchi, J.; Mulero-Perez, L.; Aasen, T.; Raya, A.; Izpisua Belmonte, J.C. Turning human epidermis into pancreatic endoderm. Rev. Diabet. Stud. 2010, 7, 158–167. [Google Scholar] [CrossRef]
- Noguchi, H.; Miyagi-Shiohira, C.; Nakashima, Y.; Kinjo, T.; Kobayashi, N.; Saitoh, I.; Watanabe, M.; Shapiro, A.M.J.; Kin, T. Induction of Expandable Tissue-Specific Progenitor Cells from Human Pancreatic Tissue through Transient Expression of Defined Factors. Mol. Ther. Methods Clin. Dev. 2019, 13, 243–252. [Google Scholar] [CrossRef]
- Gupta, M.K.; De Jesus, D.F.; Kahraman, S.; Valdez, I.A.; Shamsi, F.; Yi, L.; Swensen, A.C.; Tseng, Y.H.; Qian, W.J.; Kulkarni, R.N. Insulin receptor-mediated signaling regulates pluripotency markers and lineage differentiation. Mol. Metab. 2018, 18, 153–163. [Google Scholar] [CrossRef]
- Ma, F.; Chen, F.; Chi, Y.; Yang, S.; Lu, S.; Han, Z. Isolation of pancreatic progenitor cells with the surface marker of hematopoietic stem cells. Int. J. Endocrinol. 2012, 2012, 948683. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, C.M.; Kim, S.C. Adult human pancreas-derived cells expressing stage-specific embryonic antigen 4 differentiate into Sox9-expressing and Ngn3-expressing pancreatic ducts in vivo. Stem Cell Res. Ther. 2016, 7, 162. [Google Scholar] [CrossRef] [PubMed]
- Montanucci, P.; Pennoni, I.; Pescara, T.; Blasi, P.; Bistoni, G.; Basta, G.; Calafiore, R. The functional performance of microencapsulated human pancreatic islet-derived precursor cells. Biomaterials 2011, 32, 9254–9262. [Google Scholar] [CrossRef]
- Peery, A.F.; Crockett, S.D.; Barritt, A.S.; Dellon, E.S.; Eluri, S.; Gangarosa, L.M.; Jensen, E.T.; Lund, J.L.; Pasricha, S.; Runge, T.; et al. Burden of Gastrointestinal, Liver, and Pancreatic Diseases in the United States. Gastroenterology 2015, 149, 1731–1741.e1733. [Google Scholar] [CrossRef] [PubMed]
- Habtezion, A.; Gukovskaya, A.S.; Pandol, S.J. Acute Pancreatitis: A Multifaceted Set of Organelle and Cellular Interactions. Gastroenterology 2019, 156, 1941–1950. [Google Scholar] [CrossRef]
- Forsmark, C.E.; Vege, S.S.; Wilcox, C.M. Acute Pancreatitis. N. Engl. J. Med. 2016, 375, 1972–1981. [Google Scholar] [CrossRef]
- Stram, M.; Liu, S.; Singhi, A.D. Chronic Pancreatitis. Surg. Pathol. Clin. 2016, 9, 643–659. [Google Scholar] [CrossRef]
- Lee, B.; Zhao, Q.; Habtezion, A. Immunology of pancreatitis and environmental factors. Curr. Opin. Gastroenterol. 2017, 33, 383–389. [Google Scholar] [CrossRef]
- Xue, J.; Sharma, V.; Habtezion, A. Immune cells and immune-based therapy in pancreatitis. Immunol. Res. 2014, 58, 378–386. [Google Scholar] [CrossRef]
- Storz, P. Acinar cell plasticity and development of pancreatic ductal adenocarcinoma. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 296–304. [Google Scholar] [CrossRef]
- Jensen, J.N.; Cameron, E.; Garay, M.V.; Starkey, T.W.; Gianani, R.; Jensen, J. Recapitulation of elements of embryonic development in adult mouse pancreatic regeneration. Gastroenterology 2005, 128, 728–741. [Google Scholar] [CrossRef] [PubMed]
- Siveke, J.T.; Lubeseder-Martellato, C.; Lee, M.; Mazur, P.K.; Nakhai, H.; Radtke, F.; Schmid, R.M. Notch signaling is required for exocrine regeneration after acute pancreatitis. Gastroenterology 2008, 134, 544–555. [Google Scholar] [CrossRef] [PubMed]
- Daniluk, J.; Liu, Y.; Deng, D.; Chu, J.; Huang, H.; Gaiser, S.; Cruz-Monserrate, Z.; Wang, H.; Ji, B.; Logsdon, C.D. An NF-kappaB pathway-mediated positive feedback loop amplifies Ras activity to pathological levels in mice. J. Clin. Investig. 2012, 122, 1519–1528. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.A.; Yan, W.; Sebolt-Leopold, J.S.; Pasca di Magliano, M. MAPK signaling is required for dedifferentiation of acinar cells and development of pancreatic intraepithelial neoplasia in mice. Gastroenterology 2014, 146, 822–834.e827. [Google Scholar] [CrossRef] [PubMed]
- Halbrook, C.J.; Wen, H.J.; Ruggeri, J.M.; Takeuchi, K.K.; Zhang, Y.; di Magliano, M.P.; Crawford, H.C. Mitogen-activated Protein Kinase Kinase Activity Maintains Acinar-to-Ductal Metaplasia and Is Required for Organ Regeneration in Pancreatitis. Cell. Mol. Gastroenterol. Hepatol. 2017, 3, 99–118. [Google Scholar] [CrossRef]
- Murtaugh, L.C.; Stanger, B.Z.; Kwan, K.M.; Melton, D.A. Notch signaling controls multiple steps of pancreatic differentiation. Proc. Natl. Acad. Sci. USA 2003, 100, 14920–14925. [Google Scholar] [CrossRef]
- Kopp, J.L.; von Figura, G.; Mayes, E.; Liu, F.F.; Dubois, C.L.; Morris, J.P.t.; Pan, F.C.; Akiyama, H.; Wright, C.V.; Jensen, K.; et al. Identification of Sox9-dependent acinar-to-ductal reprogramming as the principal mechanism for initiation of pancreatic ductal adenocarcinoma. Cancer Cell 2012, 22, 737–750. [Google Scholar] [CrossRef]
- Stanger, B.Z.; Hebrok, M. Control of cell identity in pancreas development and regeneration. Gastroenterology 2013, 144, 1170–1179. [Google Scholar] [CrossRef]
- Mills, J.C.; Sansom, O.J. Reserve stem cells: Differentiated cells reprogram to fuel repair, metaplasia, and neoplasia in the adult gastrointestinal tract. Sci. Signal. 2015, 8, re8. [Google Scholar] [CrossRef]
- Apte, M.V.; Haber, P.S.; Darby, S.J.; Rodgers, S.C.; McCaughan, G.W.; Korsten, M.A.; Pirola, R.C.; Wilson, J.S. Pancreatic stellate cells are activated by proinflammatory cytokines: Implications for pancreatic fibrogenesis. Gut 1999, 44, 534–541. [Google Scholar] [CrossRef]
- Jaster, R.; Emmrich, J. Crucial role of fibrogenesis in pancreatic diseases. Best Pract. Res. Clin. Gastroenterol. 2008, 22, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Kusiak, A.A.; Szopa, M.D.; Jakubowska, M.A.; Ferdek, P.E. Signaling in the Physiology and Pathophysiology of Pancreatic Stellate Cells—A Brief Review of Recent Advances. Front. Physiol. 2020, 11, 78. [Google Scholar] [CrossRef] [PubMed]
- Komar, H.M.; Serpa, G.; Kerscher, C.; Schwoegl, E.; Mace, T.A.; Jin, M.; Yang, M.C.; Chen, C.S.; Bloomston, M.; Ostrowski, M.C.; et al. Inhibition of Jak/STAT signaling reduces the activation of pancreatic stellate cells in vitro and limits caerulein-induced chronic pancreatitis in vivo. Sci. Rep. 2017, 7, 1787. [Google Scholar] [CrossRef] [PubMed]
- Bailey, P.; Chang, D.K.; Nones, K.; Johns, A.L.; Patch, A.M.; Gingras, M.C.; Miller, D.K.; Christ, A.N.; Bruxner, T.J.; Quinn, M.C.; et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 2016, 531, 47–52. [Google Scholar] [CrossRef]
- De La, O.J.; Emerson, L.L.; Goodman, J.L.; Froebe, S.C.; Illum, B.E.; Curtis, A.B.; Murtaugh, L.C. Notch and Kras reprogram pancreatic acinar cells to ductal intraepithelial neoplasia. Proc. Natl. Acad. Sci. USA 2008, 105, 18907–18912. [Google Scholar] [CrossRef]
- Habbe, N.; Shi, G.; Meguid, R.A.; Fendrich, V.; Esni, F.; Chen, H.; Feldmann, G.; Stoffers, D.A.; Konieczny, S.F.; Leach, S.D.; et al. Spontaneous induction of murine pancreatic intraepithelial neoplasia (mPanIN) by acinar cell targeting of oncogenic Kras in adult mice. Proc. Natl. Acad. Sci. USA 2008, 105, 18913–18918. [Google Scholar] [CrossRef]
- Logsdon, C.D.; Ji, B. Ras activity in acinar cells links chronic pancreatitis and pancreatic cancer. Clin. Gastroenterol. Hepatol. 2009, 7, S40–S43. [Google Scholar] [CrossRef]
- Huang, H.; Daniluk, J.; Liu, Y.; Chu, J.; Li, Z.; Ji, B.; Logsdon, C.D. Oncogenic K-Ras requires activation for enhanced activity. Oncogene 2014, 33, 532–535. [Google Scholar] [CrossRef]
- Liou, G.Y.; Doppler, H.; DelGiorno, K.E.; Zhang, L.; Leitges, M.; Crawford, H.C.; Murphy, M.P.; Storz, P. Mutant KRas-Induced Mitochondrial Oxidative Stress in Acinar Cells Upregulates EGFR Signaling to Drive Formation of Pancreatic Precancerous Lesions. Cell Rep. 2016, 14, 2325–2336. [Google Scholar] [CrossRef]
- Swidnicka-Siergiejko, A.K.; Gomez-Chou, S.B.; Cruz-Monserrate, Z.; Deng, D.; Liu, Y.; Huang, H.; Ji, B.; Azizian, N.; Daniluk, J.; Lu, W.; et al. Chronic inflammation initiates multiple forms of K-Ras-independent mouse pancreatic cancer in the absence of TP53. Oncogene 2017, 36, 3149–3158. [Google Scholar] [CrossRef]
- Vincent, A.; Herman, J.; Schulick, R.; Hruban, R.H.; Goggins, M. Pancreatic cancer. Lancet 2011, 378, 607–620. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Dai, Y.; Cai, Y.; Suo, T.; Liu, H.; Wang, Y.; Cheng, Z.; Liu, H. KLF2 is downregulated in pancreatic ductal adenocarcinoma and inhibits the growth and migration of cancer cells. Tumour Biol. 2016, 37, 3425–3431. [Google Scholar] [CrossRef] [PubMed]
- Lian, Y.; Wang, J.; Feng, J.; Ding, J.; Ma, Z.; Li, J.; Peng, P.; De, W.; Wang, K. Long non-coding RNA IRAIN suppresses apoptosis and promotes proliferation by binding to LSD1 and EZH2 in pancreatic cancer. Tumour Biol. 2016, 37, 14929–14937. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Huang, H.; Wang, J.; Zhou, Y.; Pu, F.; Zhao, Q.; Peng, P.; Hui, B.; Ji, H.; Wang, K. Long non-coding RNA SNHG15 inhibits P15 and KLF2 expression to promote pancreatic cancer proliferation through EZH2-mediated H3K27me3. Oncotarget 2017, 8, 84153–84167. [Google Scholar] [CrossRef]
- Lian, Y.; Yang, J.; Lian, Y.; Xiao, C.; Hu, X.; Xu, H. DUXAP8, a pseudogene derived lncRNA, promotes growth of pancreatic carcinoma cells by epigenetically silencing CDKN1A and KLF2. Cancer Commun. 2018, 38, 64. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Kong, L.; Xiao, Y.; Yuan, H.; Song, Y.; Wang, J.; Yu, H.; Mao, S.; Xu, W. CDK8 regulates the angiogenesis of pancreatic cancer cells in part via the CDK8-beta-catenin-KLF2 signal axis. Exp. Cell Res. 2018, 369, 304–315. [Google Scholar] [CrossRef]
- Yuedi, D.; Houbao, L.; Pinxiang, L.; Hui, W.; Min, T.; Dexiang, Z. KLF2 induces the senescence of pancreatic cancer cells by cooperating with FOXO4 to upregulate p21. Exp. Cell Res. 2020, 388, 111784. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Luo, H.; Yang, M.; Tian, X.; Peng, B.; Zhan, T.; Chen, X.; Ding, Y.; He, J.; Cheng, X.; et al. miR-324-5p Contributes to Cell Proliferation and Apoptosis in Pancreatic Cancer by Targeting KLF3. Mol. Ther. Oncolytics 2020, 18, 432–442. [Google Scholar] [CrossRef]
- Chang, J.; Li, H.; Zhu, Z.; Mei, P.; Hu, W.; Xiong, X.; Tao, J. microRNA-21-5p from M2 macrophage-derived extracellular vesicles promotes the differentiation and activity of pancreatic cancer stem cells by mediating KLF3. Cell Biol. Toxicol. 2022, 38, 577–590. [Google Scholar] [CrossRef]
- Brembeck, F.H.; Moffett, J.; Wang, T.C.; Rustgi, A.K. The keratin 19 promoter is potent for cell-specific targeting of genes in transgenic mice. Gastroenterology 2001, 120, 1720–1728. [Google Scholar] [CrossRef]
- Brembeck, F.H.; Rustgi, A.K. The tissue-dependent keratin 19 gene transcription is regulated by GKLF/KLF4 and Sp1. J. Biol. Chem. 2000, 275, 28230–28239. [Google Scholar] [CrossRef] [PubMed]
- Prasad, N.B.; Biankin, A.V.; Fukushima, N.; Maitra, A.; Dhara, S.; Elkahloun, A.G.; Hruban, R.H.; Goggins, M.; Leach, S.D. Gene expression profiles in pancreatic intraepithelial neoplasia reflect the effects of Hedgehog signaling on pancreatic ductal epithelial cells. Cancer Res. 2005, 65, 1619–1626. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Jin, Y.; Wu, J.; Zhu, H.; Ye, D. MiR-135b-5p is an oncogene in pancreatic cancer to regulate GPRC5A expression by targeting transcription factor KLF4. Cell Death Discov. 2022, 8, 23. [Google Scholar] [CrossRef]
- Wei, D.; Wang, L.; Yan, Y.; Jia, Z.; Gagea, M.; Li, Z.; Zuo, X.; Kong, X.; Huang, S.; Xie, K. KLF4 Is Essential for Induction of Cellular Identity Change and Acinar-to-Ductal Reprogramming during Early Pancreatic Carcinogenesis. Cancer Cell 2016, 29, 324–338. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Cui, J.; Quan, M.; Xie, D.; Jia, Z.; Wei, D.; Wang, L.; Gao, Y.; Ma, Q.; Xie, K. The Novel KLF4/MSI2 Signaling Pathway Regulates Growth and Metastasis of Pancreatic Cancer. Clin. Cancer Res. 2017, 23, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, Y.; Lin, Y.; Wang, X.; Cui, X.; Zhang, Z.; Xian, G.; Qin, C. Novel crosstalk between KLF4 and ZEB1 regulates gemcitabine resistance in pancreatic ductal adenocarcinoma. Int. J. Oncol. 2017, 51, 1239–1248. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Li, Z.; Kong, X.; Jia, Z.; Zuo, X.; Gagea, M.; Huang, S.; Wei, D.; Xie, K. KLF4-Mediated Suppression of CD44 Signaling Negatively Impacts Pancreatic Cancer Stemness and Metastasis. Cancer Res. 2016, 76, 2419–2431. [Google Scholar] [CrossRef] [PubMed]
- Walter, K.; Rodriguez-Aznar, E.; Ferreira, M.S.V.; Frappart, P.O.; Dittrich, T.; Tiwary, K.; Meessen, S.; Lerma, L.; Daiss, N.; Schulte, L.A.; et al. Telomerase and Pluripotency Factors Jointly Regulate Stemness in Pancreatic Cancer Stem Cells. Cancers 2021, 13, 3145. [Google Scholar] [CrossRef]
- Zhu, Z.; Yu, Z.; Wang, J.; Zhou, L.; Zhang, J.; Yao, B.; Dou, J.; Qiu, Z.; Huang, C. Kruppel-Like Factor 4 Inhibits Pancreatic Cancer Epithelial-to-Mesenchymal Transition and Metastasis by Down-Regulating Caveolin-1 Expression. Cell. Physiol. Biochem. 2018, 46, 238–252. [Google Scholar] [CrossRef]
- He, P.; Yang, J.W.; Yang, V.W.; Bialkowska, A.B. Kruppel-like Factor 5, Increased in Pancreatic Ductal Adenocarcinoma, Promotes Proliferation, Acinar-to-Ductal Metaplasia, Pancreatic Intraepithelial Neoplasia, and Tumor Growth in Mice. Gastroenterology 2018, 154, 1494–1508.e1413. [Google Scholar] [CrossRef]
- Diaferia, G.R.; Balestrieri, C.; Prosperini, E.; Nicoli, P.; Spaggiari, P.; Zerbi, A.; Natoli, G. Dissection of transcriptional and cis-regulatory control of differentiation in human pancreatic cancer. EMBO J. 2016, 35, 595–617. [Google Scholar] [CrossRef]
- Mori, A.; Moser, C.; Lang, S.A.; Hackl, C.; Gottfried, E.; Kreutz, M.; Schlitt, H.J.; Geissler, E.K.; Stoeltzing, O. Up-regulation of Kruppel-like factor 5 in pancreatic cancer is promoted by interleukin-1beta signaling and hypoxia-inducible factor-1alpha. Mol. Cancer Res. 2009, 7, 1390–1398. [Google Scholar] [CrossRef]
- Abjalimov, I.R.; Zinovyeva, M.V.; Nikolaev, L.G.; Kopantzeva, M.R.; Kopantzev, E.P.; Sverdlov, E.D. Expression of transcription factor genes in cell lines corresponding to different stages of pancreatic cancer progression. Dokl. Biochem. Biophys. 2017, 475, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Hu, J.; Chen, F.; Lecomte, N.; Basnet, H.; David, C.J.; Witkin, M.D.; Allen, P.J.; Leach, S.D.; Hollmann, T.J.; et al. ID1 Mediates Escape from TGFbeta Tumor Suppression in Pancreatic Cancer. Cancer Discov. 2020, 10, 142–157. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Bai, H.; Deng, Y.; Yang, Y. PLA2G16 is a mutant p53/KLF5 transcriptional target and promotes glycolysis of pancreatic cancer. J. Cell. Mol. Med. 2020, 24, 12642–12655. [Google Scholar] [CrossRef] [PubMed]
- David, C.J.; Huang, Y.H.; Chen, M.; Su, J.; Zou, Y.; Bardeesy, N.; Iacobuzio-Donahue, C.A.; Massague, J. TGF-beta Tumor Suppression through a Lethal EMT. Cell 2016, 164, 1015–1030. [Google Scholar] [CrossRef]
- Zinovyeva, M.V.; Nikolaev, L.G.; Kondratyeva, L.G.; Vinogradova, T.V.; Sverdlov, E.D. Correlation between Expression of KLF5 and ZEB1 Transcription Factor Genes in Pancreatic Cancer. Dokl. Biochem. Biophys. 2018, 481, 219–221. [Google Scholar] [CrossRef]
- Stati, G.; Passaretta, F.; Gindraux, F.; Centurione, L.; Di Pietro, R. The Role of the CREB Protein Family Members and the Related Transcription Factors in Radioresistance Mechanisms. Life 2021, 11, 1437. [Google Scholar] [CrossRef]
- Ma, L.; Fan, Z.; Du, G.; Wang, H. Leptin-elicited miRNA-342-3p potentiates gemcitabine resistance in pancreatic ductal adenocarcinoma. Biochem. Biophys. Res. Commun. 2019, 509, 845–853. [Google Scholar] [CrossRef]
- Gupta, R.; Malvi, P.; Parajuli, K.R.; Janostiak, R.; Bugide, S.; Cai, G.; Zhu, L.J.; Green, M.R.; Wajapeyee, N. KLF7 promotes pancreatic cancer growth and metastasis by up-regulating ISG expression and maintaining Golgi complex integrity. Proc. Natl. Acad. Sci. USA 2020, 117, 12341–12351. [Google Scholar] [CrossRef]
- Li, H.; Shen, H.; Xie, P.; Zhang, Z.; Wang, L.; Yang, Y.; Yu, Z.; Cheng, Z.; Zhou, J. Role of long intergenic non-protein coding RNA 00152 in pancreatic cancer glycolysis via the manipulation of the microRNA-185-5p/Kruppel-like factor 7 axis. J. Cancer 2021, 12, 6330–6343. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Li, Y.; Zai, H.; Long, X.; Li, W. KLF8 knockdown triggered growth inhibition and induced cell phase arrest in human pancreatic cancer cells. Gene 2016, 585, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Ji, P.; Fan, X.; Ma, X.; Wang, X.; Zhang, J.; Mao, Z. Kruppel-like factor 9 suppressed tumorigenicity of the pancreatic ductal adenocarcinoma by negatively regulating frizzled-5. Biochem. Biophys. Res. Commun. 2018, 499, 815–821. [Google Scholar] [CrossRef]
- Zhong, Z.; Zhou, F.; Wang, D.; Wu, M.; Zhou, W.; Zou, Y.; Li, J.; Wu, L.; Yin, X. Expression of KLF9 in pancreatic cancer and its effects on the invasion, migration, apoptosis, cell cycle distribution, and proliferation of pancreatic cancer cell lines. Oncol. Rep. 2018, 40, 3852–3860. [Google Scholar] [CrossRef]
- Lin, J.; Zhai, S.; Zou, S.; Xu, Z.; Zhang, J.; Jiang, L.; Deng, X.; Chen, H.; Peng, C.; Zhang, J.; et al. Positive feedback between lncRNA FLVCR1-AS1 and KLF10 may inhibit pancreatic cancer progression via the PTEN/AKT pathway. J. Exp. Clin. Cancer Res. 2021, 40, 316. [Google Scholar] [CrossRef]
- Weng, C.C.; Hawse, J.R.; Subramaniam, M.; Chang, V.H.S.; Yu, W.C.Y.; Hung, W.C.; Chen, L.T.; Cheng, K.H. KLF10 loss in the pancreas provokes activation of SDF-1 and induces distant metastases of pancreatic ductal adenocarcinoma in the Kras(G12D) p53(flox/flox) model. Oncogene 2017, 36, 5532–5543. [Google Scholar] [CrossRef]
- Tsai, Y.C.; Chen, S.L.; Peng, S.L.; Tsai, Y.L.; Chang, Z.M.; Chang, V.H.; Ch’ang, H.J. Upregulating sirtuin 6 ameliorates glycolysis, EMT and distant metastasis of pancreatic adenocarcinoma with kruppel-like factor 10 deficiency. Exp. Mol. Med. 2021, 53, 1623–1635. [Google Scholar] [CrossRef] [PubMed]
- Buck, A.; Buchholz, M.; Wagner, M.; Adler, G.; Gress, T.; Ellenrieder, V. The tumor suppressor KLF11 mediates a novel mechanism in transforming growth factor beta-induced growth inhibition that is inactivated in pancreatic cancer. Mol. Cancer Res. 2006, 4, 861–872. [Google Scholar] [CrossRef]
- He, Z.; Guo, X.; Tian, S.; Zhu, C.; Chen, S.; Yu, C.; Jiang, J.; Sun, C. MicroRNA-137 reduces stemness features of pancreatic cancer cells by targeting KLF12. J. Exp. Clin. Cancer Res. 2019, 38, 126. [Google Scholar] [CrossRef]
- Li, B.; Pang, S.; Dou, J.; Zhou, C.; Shen, B.; Zhou, Y. The inhibitory effect of LINC00261 upregulation on the pancreatic cancer EMT process is mediated by KLF13 via the mTOR signaling pathway. Clin. Transl. Oncol. 2022, 24, 1059–1072. [Google Scholar] [CrossRef]
- Mi, W.; Zheng, Z.; Lu, J.D.; Duan, S.Q.; Zhang, J.; Zhang, H.Q.; Ding, Y.X.; Yin, J.; Cao, F.; Zhang, J.; et al. KLF16 promotes pancreatic adenocarcinoma cell proliferation and migration by positively regulating SMAD6. World J. Gastrointest. Oncol. 2022, 14, 2157–2169. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Zhang, Z.; Yang, Y.; Xu, Y.; Zhou, Y.; Zhang, S.; Liu, J.; Zheng, Y.; Zhu, Q. Kruppel-like Factor 6 Suppresses the Progression of Pancreatic Cancer by Upregulating Activating Transcription Factor 3. J. Clin. Med. 2022, 12, 200. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.C.; Yang, C.H.; Lin, C.H.; Ch’ang, H.J.; Chang, V.H.S.; Yu, W.C.Y. Destabilization of KLF10, a tumor suppressor, relies on thr93 phosphorylation and isomerase association. Biochim. Biophys. Acta 2013, 1833, 3035–3045. [Google Scholar] [CrossRef] [PubMed]
- Chang, V.H.; Chu, P.Y.; Peng, S.L.; Mao, T.L.; Shan, Y.S.; Hsu, C.F.; Lin, C.Y.; Tsai, K.K.; Yu, W.C.; Ch’ang, H.J. Kruppel-like factor 10 expression as a prognostic indicator for pancreatic adenocarcinoma. Am. J. Pathol. 2012, 181, 423–430. [Google Scholar] [CrossRef]
- Memon, A.; Lee, W.K. KLF10 as a Tumor Suppressor Gene and Its TGF-beta Signaling. Cancers 2018, 10, 161. [Google Scholar] [CrossRef]
- Tachibana, I.; Imoto, M.; Adjei, P.N.; Gores, G.J.; Subramaniam, M.; Spelsberg, T.C.; Urrutia, R. Overexpression of the TGFbeta-regulated zinc finger encoding gene, TIEG, induces apoptosis in pancreatic epithelial cells. J. Clin. Investig. 1997, 99, 2365–2374. [Google Scholar] [CrossRef]
- Jiang, L.; Chen, Y.; Chan, C.Y.; Wang, X.; Lin, L.; He, M.L.; Lin, M.C.; Yew, D.T.; Sung, J.J.; Li, J.C.; et al. Down-regulation of stathmin is required for TGF-beta inducible early gene 1 induced growth inhibition of pancreatic cancer cells. Cancer Lett. 2009, 274, 101–108. [Google Scholar] [CrossRef]
- Chang, X.; Liu, X.; Wang, H.; Yang, X.; Gu, Y. Glycolysis in the progression of pancreatic cancer. Am. J. Cancer Res. 2022, 12, 861–872. [Google Scholar]
- Chang, V.H.; Tsai, Y.C.; Tsai, Y.L.; Peng, S.L.; Chen, S.L.; Chang, T.M.; Yu, W.C.; Ch’ang, H.J. Krupple-like factor 10 regulates radio-sensitivity of pancreatic cancer via UV radiation resistance-associated gene. Radiother. Oncol. 2017, 122, 476–484. [Google Scholar] [CrossRef]
- Ellenrieder, V.; Buck, A.; Harth, A.; Jungert, K.; Buchholz, M.; Adler, G.; Urrutia, R.; Gress, T.M. KLF11 mediates a critical mechanism in TGF-beta signaling that is inactivated by Erk-MAPK in pancreatic cancer cells. Gastroenterology 2004, 127, 607–620. [Google Scholar] [CrossRef]
- Hou, Y.S.; Li, X. Circ_0005273 induces the aggravation of pancreatic cancer by targeting KLF12. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 11578–11586. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shi, M.; He, X.; Cao, Y.; Liu, P.; Li, F.; Zou, S.; Wen, C.; Zhan, Q.; Xu, Z.; et al. LncRNA-PACERR induces pro-tumour macrophages via interacting with miR-671-3p and m6A-reader IGF2BP2 in pancreatic ductal adenocarcinoma. J. Hematol. Oncol. 2022, 15, 52. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, X.; Li, Y.; Xiao, B.; Pei, S.; Jiang, H.; Zhang, X. Transcription factor KLF16 activates MAGT1 to regulate the tumorigenesis and progression of breast cancer. Int. J. Mol. Med. 2022, 50, 115. [Google Scholar] [CrossRef] [PubMed]
- Bang, S.; Li, J.; Zhang, M.; Cui, R.; Wu, X.; Xin, Z.; Ma, D.; Zhang, J.; Zhang, H. The Clinical Relevance and Function of Kruppel-Like Factor 16 in Breast Cancer. Cancer Manag. Res. 2020, 12, 6373–6383. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yu, W.; Wang, X.; Hu, B.; Wu, D.; Shi, G. KLF16 Affects the MYC Signature and Tumor Growth in Prostate Cancer. Onco Targets Ther. 2020, 13, 1303–1310. [Google Scholar] [CrossRef]
- Ma, P.; Sun, C.Q.; Wang, Y.F.; Pan, Y.T.; Chen, Q.N.; Liu, W.T.; Liu, J.; Zhao, C.H.; Shu, Y.Q.; Li, W. KLF16 promotes proliferation in gastric cancer cells via regulating p21 and CDK4. Am. J. Transl. Res. 2017, 9, 3027–3036. [Google Scholar]
- Rowland, B.D.; Bernards, R.; Peeper, D.S. The KLF4 tumour suppressor is a transcriptional repressor of p53 that acts as a context-dependent oncogene. Nat. Cell Biol. 2005, 7, 1074–1082. [Google Scholar] [CrossRef]
- Zammarchi, F.; Morelli, M.; Menicagli, M.; Di Cristofano, C.; Zavaglia, K.; Paolucci, A.; Campani, D.; Aretini, P.; Boggi, U.; Mosca, F.; et al. KLF4 is a novel candidate tumor suppressor gene in pancreatic ductal carcinoma. Am. J. Pathol. 2011, 178, 361–372. [Google Scholar] [CrossRef]
- Jagust, P.; Alcala, S.; Sainz Jr, B.; Heeschen, C.; Sancho, P. Glutathione metabolism is essential for self-renewal and chemoresistance of pancreatic cancer stem cells. World J. Stem Cells 2020, 12, 1410–1428. [Google Scholar] [CrossRef]
- Funel, N.; Morelli, M.; Giovannetti, E.; Del Chiaro, M.; Pollina, L.E.; Mosca, F.; Boggi, U.; Cavazzana, A.; Campani, D. Loss of heterozygosity status of D9S105 marker is associated with downregulation of Kruppel-like factor 4 expression in pancreatic ductal adenocarcinoma and pancreatic intraepithelial lesions. Pancreatology 2011, 11, 30–42. [Google Scholar] [CrossRef]
- Fujikura, K.; Hosoda, W.; Felsenstein, M.; Song, Q.; Reiter, J.G.; Zheng, L.; Beleva Guthrie, V.; Rincon, N.; Dal Molin, M.; Dudley, J.; et al. Multiregion whole-exome sequencing of intraductal papillary mucinous neoplasms reveals frequent somatic KLF4 mutations predominantly in low-grade regions. Gut 2021, 70, 928–939. [Google Scholar] [CrossRef] [PubMed]
- Tokheim, C.; Karchin, R. CHASMplus Reveals the Scope of Somatic Missense Mutations Driving Human Cancers. Cell Syst. 2019, 9, 9–23.e8. [Google Scholar] [CrossRef] [PubMed]
- Xie, V.K.; Li, Z.; Yan, Y.; Jia, Z.; Zuo, X.; Ju, Z.; Wang, J.; Du, J.; Xie, D.; Xie, K.; et al. DNA-Methyltransferase 1 Induces Dedifferentiation of Pancreatic Cancer Cells through Silencing of Kruppel-Like Factor 4 Expression. Clin. Cancer 2017, 23, 5585–5597. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; He, Y.; Miao, X.; Yu, B. ATF4-mediated histone deacetylase HDAC1 promotes the progression of acute pancreatitis. Cell Death Dis. 2021, 12, 5. [Google Scholar] [CrossRef]
- Ganguly, K.; Krishn, S.R.; Rachagani, S.; Jahan, R.; Shah, A.; Nallasamy, P.; Rauth, S.; Atri, P.; Cox, J.L.; Pothuraju, R.; et al. Secretory Mucin 5AC Promotes Neoplastic Progression by Augmenting KLF4-Mediated Pancreatic Cancer Cell Stemness. Cancer Res. 2021, 81, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Ma, Y.; Roy, S.K.; Srivastava, R.; Shankar, S.; Srivastava, R.K. Ethanol exposure of human pancreatic normal ductal epithelial cells induces EMT phenotype and enhances pancreatic cancer development in KC (Pdx1-Cre and LSL-Kras(G12D)) mice. J. Cell Mol. Med. 2022, 26, 399–409. [Google Scholar] [CrossRef]
- Wei, D.; Wang, L.; Kanai, M.; Jia, Z.; Le, X.; Li, Q.; Wang, H.; Xie, K. KLF4alpha up-regulation promotes cell cycle progression and reduces survival time of patients with pancreatic cancer. Gastroenterology 2010, 139, 2135–2145. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Kanai, M.; Jia, Z.; Le, X.; Xie, K. Kruppel-like factor 4 induces p27Kip1 expression in and suppresses the growth and metastasis of human pancreatic cancer cells. Cancer Res. 2008, 68, 4631–4639. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Li, L.; Li, Z.; Le, X.; Huang, C.; Jia, Z.; Cui, J.; Huang, S.; Wang, L.; Xie, K. Dysregulated expression of FOXM1 isoforms drives progression of pancreatic cancer. Cancer Res. 2013, 73, 3987–3996. [Google Scholar] [CrossRef]
- Ouyang, H.; Gore, J.; Deitz, S.; Korc, M. microRNA-10b enhances pancreatic cancer cell invasion by suppressing TIP30 expression and promoting EGF and TGF-beta actions. Oncogene 2017, 36, 4952. [Google Scholar] [CrossRef]
- Sureban, S.M.; May, R.; Qu, D.; Weygant, N.; Chandrakesan, P.; Ali, N.; Lightfoot, S.A.; Pantazis, P.; Rao, C.V.; Postier, R.G.; et al. DCLK1 regulates pluripotency and angiogenic factors via microRNA-dependent mechanisms in pancreatic cancer. PLoS ONE 2013, 8, e73940. [Google Scholar] [CrossRef] [PubMed]
- Sureban, S.M.; May, R.; Weygant, N.; Qu, D.; Chandrakesan, P.; Bannerman-Menson, E.; Ali, N.; Pantazis, P.; Westphalen, C.B.; Wang, T.C.; et al. XMD8-92 inhibits pancreatic tumor xenograft growth via a DCLK1-dependent mechanism. Cancer Lett. 2014, 351, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, K.; Eguchi, H.; Konno, M.; Kawamoto, K.; Nishida, N.; Koseki, J.; Wada, H.; Marubashi, S.; Nagano, H.; Doki, Y.; et al. Susceptibility of pancreatic cancer stem cells to reprogramming. Cancer Sci. 2015, 106, 1182–1187. [Google Scholar] [CrossRef]
- Tyagi, N.; Marimuthu, S.; Bhardwaj, A.; Deshmukh, S.K.; Srivastava, S.K.; Singh, A.P.; McClellan, S.; Carter, J.E.; Singh, S. p-21 activated kinase 4 (PAK4) maintains stem cell-like phenotypes in pancreatic cancer cells through activation of STAT3 signaling. Cancer Lett. 2016, 370, 260–267. [Google Scholar] [CrossRef]
- Zeng, S.; Pottler, M.; Lan, B.; Grutzmann, R.; Pilarsky, C.; Yang, H. Chemoresistance in Pancreatic Cancer. Int. J. Mol. Sci. 2019, 20, 4504. [Google Scholar] [CrossRef] [PubMed]
- Quinonero, F.; Mesas, C.; Doello, K.; Cabeza, L.; Perazzoli, G.; Jimenez-Luna, C.; Rama, A.R.; Melguizo, C.; Prados, J. The challenge of drug resistance in pancreatic ductal adenocarcinoma: A current overview. Cancer Biol. Med. 2019, 16, 688–699. [Google Scholar] [CrossRef]
- Sandforth, L.; Ammar, N.; Dinges, L.A.; Rocken, C.; Arlt, A.; Sebens, S.; Schafer, H. Impact of the Monocarboxylate Transporter-1 (MCT1)-Mediated Cellular Import of Lactate on Stemness Properties of Human Pancreatic Adenocarcinoma Cells dagger. Cancers 2020, 12, 581. [Google Scholar] [CrossRef]
- Kondratyeva, L.G.; Chernov, I.P.; Zinovyeva, M.V.; Kopantzev, E.P.; Sverdlov, E.D. Expression of master regulatory genes of embryonic development in pancreatic tumors. Dokl. Biochem. Biophys. 2017, 475, 250–252. [Google Scholar] [CrossRef]
- Gomez, D.L.; Farina, H.G.; Gomez, D.E. Telomerase regulation: A key to inhibition? (Review). Int. J. Oncol. 2013, 43, 1351–1356. [Google Scholar] [CrossRef]
- Petersen, G.M.; Amundadottir, L.; Fuchs, C.S.; Kraft, P.; Stolzenberg-Solomon, R.Z.; Jacobs, K.B.; Arslan, A.A.; Bueno-de-Mesquita, H.B.; Gallinger, S.; Gross, M.; et al. A genome-wide association study identifies pancreatic cancer susceptibility loci on chromosomes 13q22.1, 1q32.1 and 5p15.33. Nat. Genet. 2010, 42, 224–228. [Google Scholar] [CrossRef]
- Childs, E.J.; Mocci, E.; Campa, D.; Bracci, P.M.; Gallinger, S.; Goggins, M.; Li, D.; Neale, R.E.; Olson, S.H.; Scelo, G.; et al. Common variation at 2p13.3, 3q29, 7p13 and 17q25.1 associated with susceptibility to pancreatic cancer. Nat. Genet. 2015, 47, 911–916. [Google Scholar] [CrossRef] [PubMed]
- Ghaleb, A.M.; Yang, V.W. The Pathobiology of Kruppel-like Factors in Colorectal Cancer. Curr. Color. Cancer Rep. 2008, 4, 59–64. [Google Scholar] [CrossRef]
- Liu, X.; Zhong, L.; Jiang, W.; Wen, D. Repression of circRNA_000684 inhibits malignant phenotypes of pancreatic ductal adenocarcinoma cells via miR-145-mediated KLF5. Pancreatology 2021, 21, 406–417. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kong, R.; Chen, H.; Zhao, Z.; Li, L.; Li, J.; Hu, J.; Zhang, G.; Pan, S.; Wang, Y.; et al. Overexpression of KLF5 is associated with poor survival and G1/S progression in pancreatic cancer. Aging 2019, 11, 5035–5057. [Google Scholar] [CrossRef] [PubMed]
- Pardali, K.; Moustakas, A. Actions of TGF-beta as tumor suppressor and pro-metastatic factor in human cancer. Biochim. Biophys. Acta 2007, 1775, 21–62. [Google Scholar] [CrossRef]
- Seoane, J.; Gomis, R.R. TGF-beta Family Signaling in Tumor Suppression and Cancer Progression. Cold Spring Harb. Perspect. Biol. 2017, 9, a022277. [Google Scholar] [CrossRef]
- Frappart, P.O.; Hofmann, T.G. Pancreatic Ductal Adenocarcinoma (PDAC) Organoids: The Shining Light at the End of the Tunnel for Drug Response Prediction and Personalized Medicine. Cancers 2020, 12, 2750. [Google Scholar] [CrossRef]
- Hoskins, J.W.; Ibrahim, A.; Emmanuel, M.A.; Manmiller, S.M.; Wu, Y.; O’Neill, M.; Jia, J.; Collins, I.; Zhang, M.; Thomas, J.V.; et al. Functional characterization of a chr13q22.1 pancreatic cancer risk locus reveals long-range interaction and allele-specific effects on DIS3 expression. Hum. Mol. Genet. 2016, 25, 4726–4738. [Google Scholar] [CrossRef]
- Zhong, J.; Jermusyk, A.; Wu, L.; Hoskins, J.W.; Collins, I.; Mocci, E.; Zhang, M.; Song, L.; Chung, C.C.; Zhang, T.; et al. A Transcriptome-Wide Association Study Identifies Novel Candidate Susceptibility Genes for Pancreatic Cancer. J. Natl. Cancer Inst. 2020, 112, 1003–1012. [Google Scholar] [CrossRef]
- Jiang, F.; Huang, X.; Zhang, F.; Pan, J.; Wang, J.; Hu, L.; Chen, J.; Wang, Y. Integrated Analysis of Multi-Omics Data to Identify Prognostic Genes for Pancreatic Cancer. DNA Cell Biol. 2022, 41, 305–318. [Google Scholar] [CrossRef]
- Wei, Y.; Chen, G.; You, L.; Zhao, Y. Krupel-like factor 8 is a potential prognostic factor for pancreatic cancer. Chin. Med. J. 2014, 127, 856–859. [Google Scholar] [PubMed]
- Shain, A.H.; Salari, K.; Giacomini, C.P.; Pollack, J.R. Integrative genomic and functional profiling of the pancreatic cancer genome. BMC Genom. 2013, 14, 624. [Google Scholar] [CrossRef]
- Zhu, P.; Liu, H.Y.; Liu, F.C.; Gu, F.M.; Yuan, S.X.; Huang, J.; Pan, Z.Y.; Wang, W.J. Circulating Tumor Cells Expressing Kruppel-Like Factor 8 and Vimentin as Predictors of Poor Prognosis in Pancreatic Cancer Patients. Cancer Control 2021, 28, 10732748211027163. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.C. Circulating tumor cells. Proc. Natl. Acad. Sci. USA 2013, 110, 4861. [Google Scholar] [CrossRef] [PubMed]
- Tazi, J.; Bakkour, N.; Stamm, S. Alternative splicing and disease. Biochim. Biophys. Acta 2009, 1792, 14–26. [Google Scholar] [CrossRef]
- Hartel, M.; Narla, G.; Wente, M.N.; Giese, N.A.; Martignoni, M.E.; Martignetti, J.A.; Friess, H.; Friedman, S.L. Increased alternative splicing of the KLF6 tumour suppressor gene correlates with prognosis and tumour grade in patients with pancreatic cancer. Eur. J. Cancer 2008, 44, 1895–1903. [Google Scholar] [CrossRef]
- Yu, M.; Hong, W.; Ruan, S.; Guan, R.; Tu, L.; Huang, B.; Hou, B.; Jian, Z.; Ma, L.; Jin, H. Genome-Wide Profiling of Prognostic Alternative Splicing Pattern in Pancreatic Cancer. Front. Oncol. 2019, 9, 773. [Google Scholar] [CrossRef]
- Yang, Z.; Li, D.; Liu, Z.; Miao, X.; Yang, L.; Zou, Q.; Yuan, Y. BIRC7 and KLF4 expression in benign and malignant lesions of pancreas and their clinicopathological significance. Cancer Biomark. 2016, 17, 437–444. [Google Scholar] [CrossRef]
- Knoedler, J.R.; Subramani, A.; Denver, R.J. The Kruppel-like factor 9 cistrome in mouse hippocampal neurons reveals predominant transcriptional repression via proximal promoter binding. BMC Genom. 2017, 18, 299. [Google Scholar] [CrossRef]
- Mao, Z.; Fan, X.; Zhang, J.; Wang, X.; Ma, X.; Michalski, C.W.; Zhang, Y. KLF9 Is a Prognostic Indicator in Human Pancreatic Ductal Adenocarcinoma. Anticancer Res. 2017, 37, 3795–3799. [Google Scholar] [CrossRef]
- Jin, Y.; Gong, S.; Shang, G.; Hu, L.; Li, G. Profiling of a novel circadian clock-related prognostic signature and its role in immune function and response to molecular targeted therapy in pancreatic cancer. Aging 2023, 15, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Pen, S.L.; Shan, Y.S.; Hsiao, C.F.; Liu, T.W.; Chen, J.S.; Ho, C.L.; Chou, W.C.; Hsieh, R.K.; Chen, L.T.; Ch’ang, H.J. High expression of kruppel-like factor 10 or Smad4 predicts clinical benefit of adjuvant chemoradiotherapy in curatively resected pancreatic adenocarcinoma: From a randomized phase III trial. Radiother. Oncol. 2021, 158, 146–154. [Google Scholar] [CrossRef] [PubMed]

| Initial Cell Type | Species | Induction Factors | Result | References |
|---|---|---|---|---|
| Dermal fibroblasts | Human | Yamanaka retroviral transduction, basic fibroblast growth factor supplementation | α- and β-like cells produced low levels of glucagon and c-peptide | [49] |
| Dermal fibroblasts from T1D patients | Human | KLF4, OCT4, SOX2 retroviral transduction, genetic/growth factor supplementation | Teratoma potential, embryoid bodies of cell types from all three germ layers produced, patient-specific disease modeling in vitro | [58] |
| Keratinocytes | Human | KLF4, OXT4, SOX2 retroviral transduction, growth factor supplementation | Higher reprogramming speed and efficiency compared to fibroblasts | [59] |
| In vivo | Mouse | rtTA, lentiviral doxycycline-inducible cassette encoding Yamanaka factors, DOX treatment | Well-differentiated teratomas emerged from multiple organs, highlighting diverse capabilities of in vivo iPSCs | [53] |
| Pancreatic cells | Mouse | Yamanaka retroviral transduction, genetic/growth factor supplementation | Expressed biomarkers of β-cell lineage, released insulin in response to glucose stimulation. Did not develop in-vivo teratomas | [56] |
| Pancreatic-specific progenitor cells | Human | Yamanaka and p53 shRNA transient expression, genetic/growth factor supplementation | Highest differentiation efficiency of insulin and c-peptide positive cells, highest levels of insulin released, lacked tumorigenic markers | [60] |
| Name | Status in Pancreatic Cancer | Downstream Targets | References |
|---|---|---|---|
| KLF2 | Downregulated | Cyclin D1, JUN, MMP9, MYC, p21, SEMA3F, SNAI1, VEGF, VEGFR2 | [93,94,95,96,97,98] |
| KLF3 | Downregulated | BAX, NANOG, OCT4, PCNA, | [99,100] |
| KLF4 | Stage-dependent | CD44, cyclin D1, E-cadherin, GPRC5A, KRT19, miR-183, miR-200b, MSI2, NANOG, OCT3/4, p21, p27, SKP2, SOX2, caveolin-1 | [101,102,103,104,105,106,107,108,109,110] |
| KLF5 | Stage-dependent | BRCA2, cyclin D1, E-cadherin, E2F1, KRT19, miR-130b-3p, miR-15a-5p, miR-17-5p, miR-183-5p, miR-18a-5p, miR-200a-3p, miR-221-3p, miR-222-3p, miR-454-3p, miR-125b-5p, miR-146a-5p, miR-24-3p, NDRG2, PDGFα, PLA2G16, PTF1α, p16, p53 RAD51, SLC2A1, SMAD4, survivin | [111,112,113,114,115,116,117,118] |
| KLF6 | Downregulated | ATF3, MMP2, N-cadherin, vimentin | [119,120] |
| KLF7 | Upregulated | DSG3, HK2, IFIT1, IFIT3, PDK1, PFKBF3 | [121,122] |
| KLF8 | Upregulated | CDK1/CDC2, Cyclin B1, cyclin D1, FHL2, p21, p27, SNAI2 | [123] |
| KLF9 | Downregulated | BAX, BCL2, CDK4, cyclin B, cyclin D1, E-cadherin, FZD5, MMP9, MMP2, N-cadherin, TP53 | [124,125] |
| KLF10 | Downregulated | ABCG2, CD133, CD44, CXCL-12, cyclin D1, FLVCR1-AS1, MYC, nestin, survivin, TWIST2 | [126,127,128] |
| KLF11 | Downregulated | c-MYC, SMAD7 | [129] |
| KLF12 | Upregulated | BMI1, CCND1, c-MYC, CD44, DVL2, LGR5, MMP7, NANOG, OCT4, SOX2, TCF4, TWIST1 | [130] |
| KLF13 | Upregulated | LINC00261, E-cadherin, MMP2, vimentin | [131] |
| KLF16 | Upregulated | SMAD6 | [132] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giarrizzo, M.; LaComb, J.F.; Bialkowska, A.B. The Role of Krüppel-like Factors in Pancreatic Physiology and Pathophysiology. Int. J. Mol. Sci. 2023, 24, 8589. https://doi.org/10.3390/ijms24108589
Giarrizzo M, LaComb JF, Bialkowska AB. The Role of Krüppel-like Factors in Pancreatic Physiology and Pathophysiology. International Journal of Molecular Sciences. 2023; 24(10):8589. https://doi.org/10.3390/ijms24108589
Chicago/Turabian StyleGiarrizzo, Michael, Joseph F. LaComb, and Agnieszka B. Bialkowska. 2023. "The Role of Krüppel-like Factors in Pancreatic Physiology and Pathophysiology" International Journal of Molecular Sciences 24, no. 10: 8589. https://doi.org/10.3390/ijms24108589
APA StyleGiarrizzo, M., LaComb, J. F., & Bialkowska, A. B. (2023). The Role of Krüppel-like Factors in Pancreatic Physiology and Pathophysiology. International Journal of Molecular Sciences, 24(10), 8589. https://doi.org/10.3390/ijms24108589







