De Novo Computational Design of a Lipase with Hydrolysis Activity towards Middle-Chained Fatty Acid Esters
Abstract
1. Introduction
2. Results
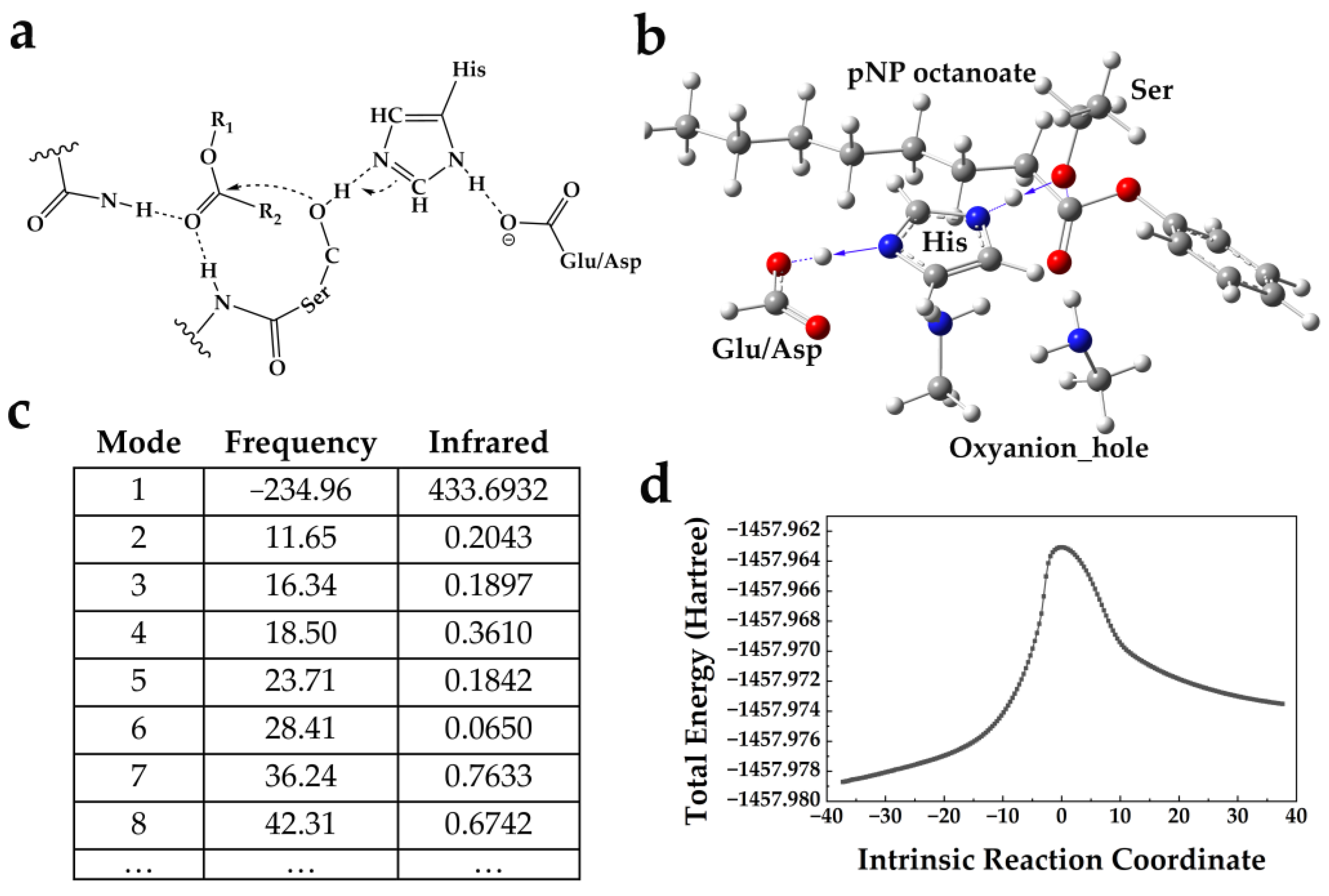
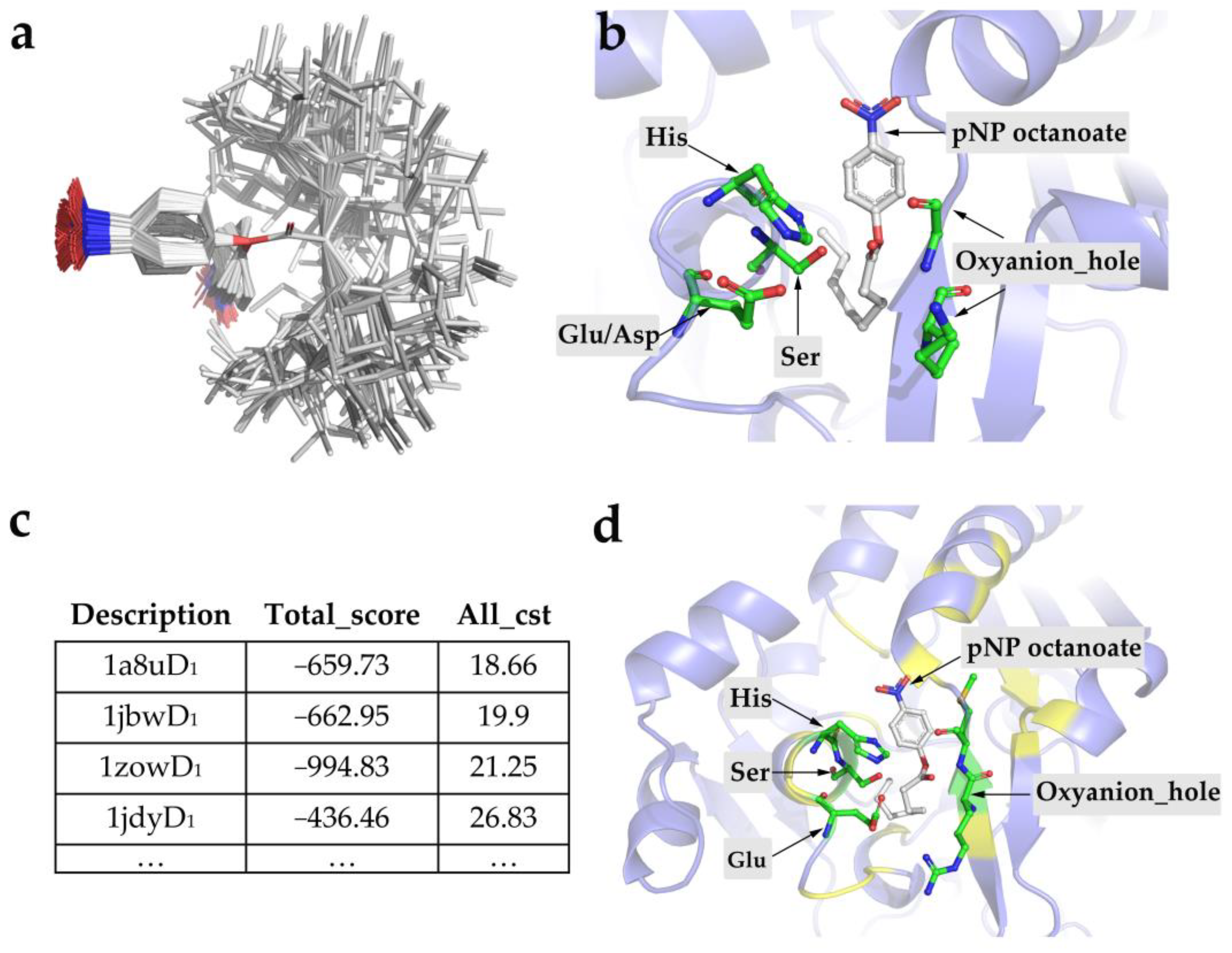
2.1. Computational Design of Enzymes from Scratch
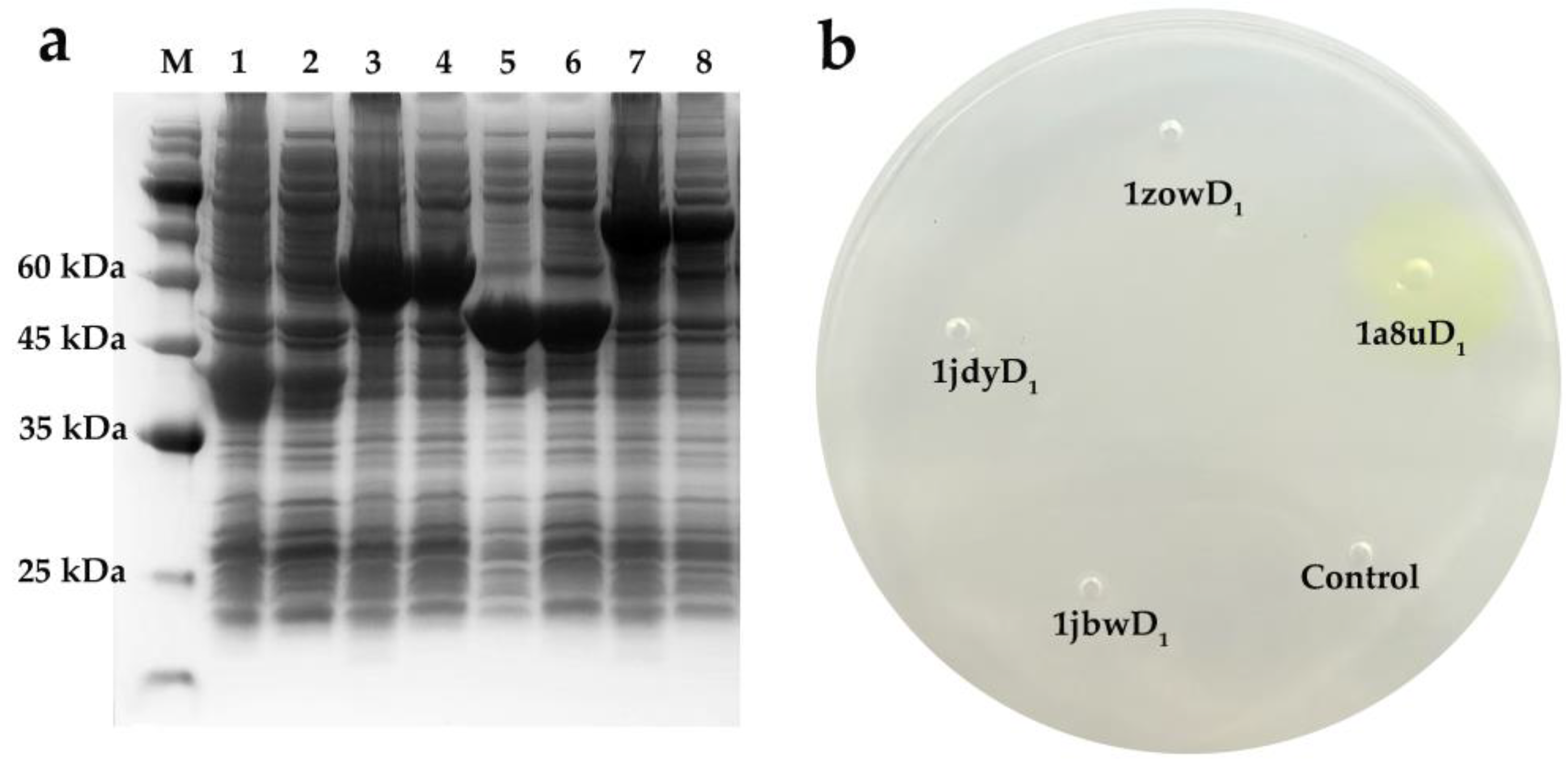
2.2. Preliminary Activity Screening
2.3. Computational Redesign of 1a8uD1 Based on Its Backbone
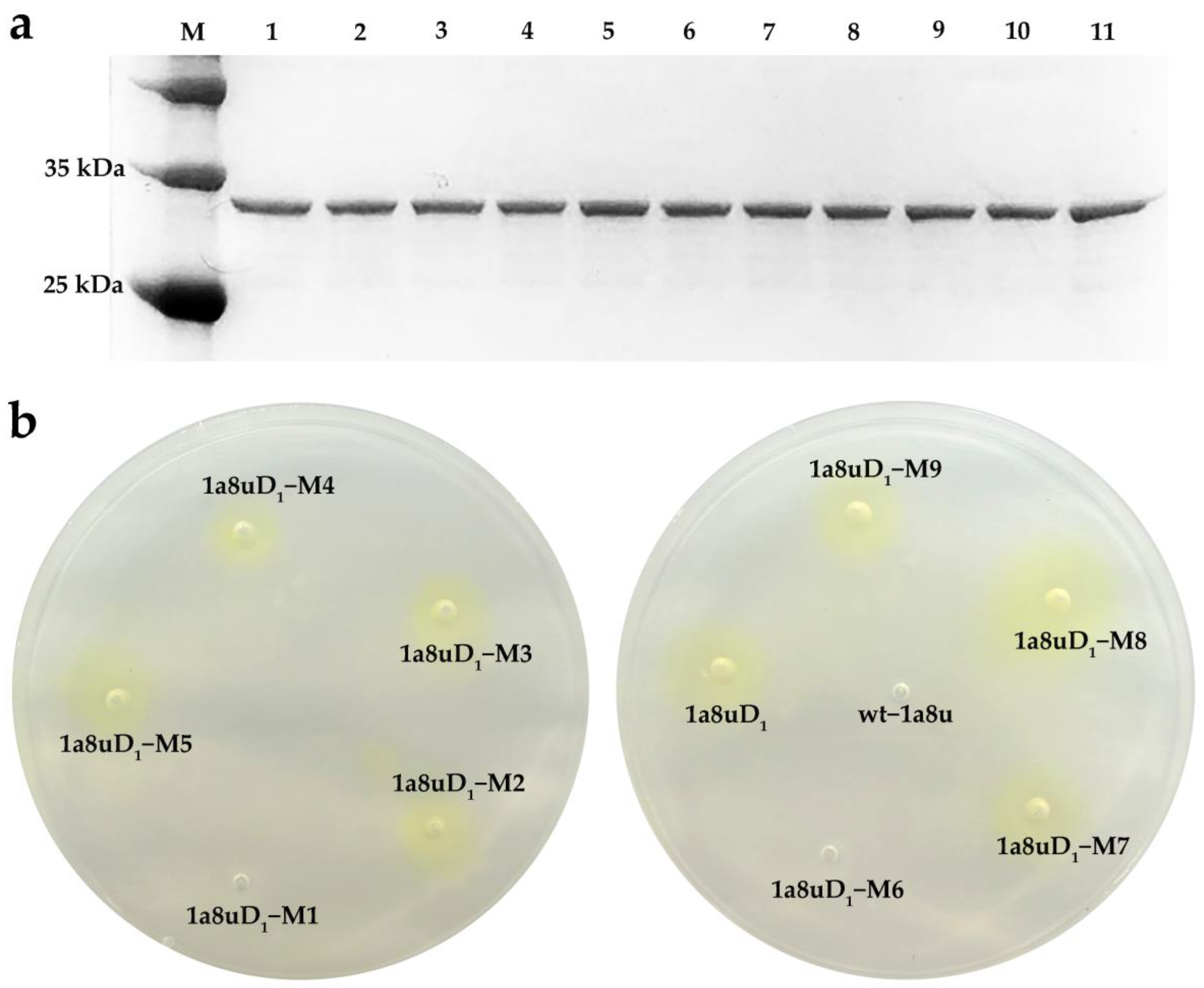
2.4. Activity Assay of the Redesigned Enzyme
2.5. Qualitative and Quantitative Evaluation of Positive Designed 1a8uD1 and 1a8uD1–M8 Using Glyceryl Trioctanoate as Natural Substrate
3. Discussion
4. Materials and Methods
4.1. General Information
4.2. Computational Design of Enzymes from Scratch
4.3. Experimental Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vivek, K.; Sandhia, G.S.; Subramaniyan, S. Extremophilic lipases for industrial applications: A general review. Biotechnol. Adv. 2022, 60, 108002. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, F.T.T.; Neto, F.S.; Rafael de Aguiar Falcão, I.; Erick da Silva Souza, J.; de Moura Junior, L.S.; da Silva Sousa, P.; Rocha, T.G.; de Sousa, I.G.; de Lima Gomes, P.H.; de Souza, M.C.M.; et al. Opportunities for improving biodiesel production via lipase catalysis. Fuel 2021, 288, 119577. [Google Scholar] [CrossRef]
- Kumar, A.; Khan, A.; Malhotra, S.; Mosurkal, R.; Dhawan, A.; Pandey, M.K.; Singh, B.K.; Kumar, R.; Prasad, A.K.; Sharma, S.K.; et al. Synthesis of macromolecular systems via lipase catalyzed biocatalytic reactions: Applications and future perspectives. Chem. Soc. Rev. 2016, 45, 6855–6887. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Liu, Q.; Qu, G.; Feng, Y.; Reetz, M.T. Utility of B-factors in protein science: Interpreting rigidity, flexibility, and internal motion and engineering thermostability. Chem. Rev. 2019, 119, 1626–1665. [Google Scholar] [CrossRef]
- Yu, X.; Xu, Y.; Xiao, R. Lipases from the genus Rhizopus: Characteristics, expression, protein engineering and application. Prog. Lipid Res. 2016, 64, 57–68. [Google Scholar] [CrossRef]
- Lovelock, S.L.; Crawshaw, R.; Basler, S.; Levy, C.; Baker, D.; Hilvert, D.; Green, A.P. The road to fully programmable protein catalysis. Nature 2022, 606, 49–58. [Google Scholar] [CrossRef]
- Qu, G.; Li, A.; Acevedo-Rocha, C.G.; Sun, Z.; Reetz, M.T. The crucial role of methodology development in directed evolution of selective enzymes. Angew. Chem. Int. Ed. 2020, 59, 13204–13231. [Google Scholar] [CrossRef] [PubMed]
- Vaissier Welborn, V.; Head-Gordon, T. Computational design of synthetic enzymes. Chem. Rev. 2019, 119, 6613–6630. [Google Scholar] [CrossRef]
- Huang, P.-S.; Boyken, S.E.; Baker, D. The coming of age of de novo protein design. Nature 2016, 537, 320–327. [Google Scholar] [CrossRef]
- Jemli, S.; Ayadi-Zouari, D.; Hlima, H.B.; Bejar, S. Biocatalysts: Application and engineering for industrial purposes. Crit. Rev. Biotechnol. 2016, 36, 246–258. [Google Scholar] [CrossRef]
- Li, C.; Zhang, R.; Wang, J.; Wilson, L.M.; Yan, Y. Protein engineering for improving and diversifying natural product biosynthesis. Trends Biotechnol. 2020, 38, 729–744. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Sun, J.; Wu, B. Computational enzyme redesign: Large jumps in function. Trends Chem. 2022, 4, 409–419. [Google Scholar] [CrossRef]
- Breslow, R. Biomimetic chemistry and artificial enzymes: Catalysis by design. Acc. Chem. Res. 1995, 28, 146–153. [Google Scholar] [CrossRef]
- Schwizer, F.; Okamoto, Y.; Heinisch, T.; Gu, Y.; Pellizzoni, M.M.; Lebrun, V.; Reuter, R.; Köhler, V.; Lewis, J.C.; Ward, T.R. Artificial metalloenzymes: Reaction scope and optimization strategies. Chem. Rev. 2018, 118, 142–231. [Google Scholar] [CrossRef]
- Arnold, F.H. Innovation by evolution: Bringing new chemistry to life (Nobel Lecture). Angew. Chem. Int. Ed. 2019, 58, 14420–14426. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Xu, Y.; Hu, X.; Liu, Y.; Liao, S.; Zhang, J.; Huang, C.; Hong, J.; Chen, Q.; Liu, H. A backbone-centred energy function of neural networks for protein design. Nature 2022, 602, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Xiong, P.; Hu, X.; Huang, B.; Zhang, J.; Chen, Q.; Liu, H. Increasing the efficiency and accuracy of the ABACUS protein sequence design method. Bioinformatics 2019, 36, 136–144. [Google Scholar] [CrossRef]
- Ubhayasekera, W. Homology modeling for enzyme design. In Cellulases: Methods and Protocols; Lübeck, M., Ed.; Springer: New York, NY, USA, 2018; pp. 301–320. [Google Scholar]
- Gainza-Cirauqui, P.; Correia, B.E. Computational protein design—The next generation tool to expand synthetic biology applications. Curr. Opin. Biotechnol. 2018, 52, 145–152. [Google Scholar] [CrossRef]
- Bershtein, S.; Serohijos, A.W.R.; Shakhnovich, E.I. Bridging the physical scales in evolutionary biology: From protein sequence space to fitness of organisms and populations. Curr. Opin. Struct. Biol. 2017, 42, 31–40. [Google Scholar] [CrossRef]
- Richter, F.; Leaver-Fay, A.; Khare, S.D.; Bjelic, S.; David, B. De Novo Enzyme Design Using Rosetta3. PLoS ONE 2011, 6, e19230. [Google Scholar] [CrossRef]
- Richter, F.; Blomberg, R.; Khare, S.D.; Kiss, G.; Kuzin, A.P.; Smith, A.J.T.; Gallaher, J.; Pianowski, Z.; Helgeson, R.C.; Grjasnow, A.; et al. Computational design of catalytic dyads and oxyanion holes for ester hydrolysis. J. Am. Chem. Soc. 2012, 134, 16197–16206. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, S.; Wang, C.; Yu, K.; Kuzin, A.P.; Richter, F.; Lew, S.; Miklos, A.E.; Matthews, M.L.; Seetharaman, J.; Su, M.; et al. Design of activated serine–containing catalytic triads with atomic-level accuracy. Nat. Chem. Biol. 2014, 10, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Althoff, E.A.; Clemente, F.R.; Doyle, L.; Röthlisberger, D.; Zanghellini, A.; Gallaher, J.L.; Betker, J.L.; Tanaka, F.; Barbas, C.F.; et al. De novo computational design of Retro-Aldol enzymes. Science 2008, 319, 1387–1391. [Google Scholar] [CrossRef] [PubMed]
- Röthlisberger, D.; Khersonsky, O.; Wollacott, A.M.; Jiang, L.; DeChancie, J.; Betker, J.; Gallaher, J.L.; Althoff, E.A.; Zanghellini, A.; Dym, O.; et al. Kemp elimination catalysts by computational enzyme design. Nature 2008, 453, 190–195. [Google Scholar] [CrossRef]
- Siegel, J.B.; Zanghellini, A.; Lovick, H.M.; Kiss, G.; Lambert, A.R.; St.Clair, J.L.; Gallaher, J.L.; Hilvert, D.; Gelb, M.H.; Stoddard, B.L.; et al. Computational design of an enzyme catalyst for a stereoselective bimolecular Diels-Alder reaction. Science 2010, 329, 309–313. [Google Scholar] [CrossRef]
- Lapidoth, G.; Khersonsky, O.; Lipsh, R.; Dym, O.; Albeck, S.; Rogotner, S.; Fleishman, S.J. Highly active enzymes by automated combinatorial backbone assembly and sequence design. Nat. Commun. 2018, 9, 2780. [Google Scholar] [CrossRef]
- Ożga, K.; Berlicki, Ł. Miniprotein-based artificial retroaldolase. ACS Catal. 2022, 12, 15424–15430. [Google Scholar] [CrossRef]
- Li, G.; Xu, L.; Zhang, H.; Liu, J.; Yan, J.; Yan, Y. A de novo designed esterase with p-nitrophenyl acetate hydrolysis activity. Molecules 2020, 25, 4658. [Google Scholar] [CrossRef]
- Arnold, F.H. Directed evolution: Bringing new chemistry to life. Angew. Chem. Int. Ed. 2018, 57, 4143–4148. [Google Scholar] [CrossRef]
- Goldsmith, M.; Tawfik, D.S. Enzyme engineering: Reaching the maximal catalytic efficiency peak. Curr. Opin. Struct. Biol. 2017, 47, 140–150. [Google Scholar] [CrossRef]
- Packer, M.S.; Liu, D.R. Methods for the directed evolution of proteins. Nat. Rev. Genet. 2015, 16, 379–394. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Dai, S.; Chen, X.; Xu, L.; Yan, J.; Yang, M.; Yan, Y. Alteration of chain-length selectivity and thermostability of Rhizopus oryzae lipase via virtual saturation mutagenesis coupled with disulfide bond design. Appl. Environ. Microbiol. 2023, 89, e01878-22. [Google Scholar] [CrossRef] [PubMed]
- Barber-Zucker, S.; Mateljak, I.; Goldsmith, M.; Kupervaser, M.; Alcalde, M.; Fleishman, S.J. Designed high-redox potential laccases exhibit high functional diversity. ACS Catal. 2022, 12, 13164–13173. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; DeChancie, J.; Gunaydin, H.; Chowdry, A.B.; Clemente, F.R.; Smith, A.J.; Handel, T.M.; Houk, K.N. Quantum mechanical design of enzyme active sites. J. Org. Chem. 2008, 73, 889–899. [Google Scholar] [CrossRef]
- Kwasnieski, O.; Verdier, L.; Malacria, M.; Derat, E. Fixation of the two Tabun isomers in acetylcholinesterase: A QM/MM study. J. Phys. Chem. B 2009, 113, 10001–10007. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, Y.; Tian, W.; Bu, Y.; Li, T.; Cui, X.; Zhu, T.; Li, R.; Wu, B. Development of a versatile and efficient C–N lyase platform for asymmetric hydroamination via computational enzyme redesign. Nat. Catal. 2021, 4, 364–373. [Google Scholar] [CrossRef]
- Kuhlman, B.; Baker, D. Native protein sequences are close to optimal for their structures. Proc. Natl. Acad. Sci. USA 2000, 97, 10383–10388. [Google Scholar] [CrossRef]
- Kiss, G.; Röthlisberger, D.; Baker, D.; Houk, K.N. Evaluation and ranking of enzyme designs. Protein Sci. 2010, 19, 1760–1773. [Google Scholar] [CrossRef]
- Li, R.; Wijma, H.J.; Song, L.; Cui, Y.; Otzen, M.; Tian, Y.e.; Du, J.; Li, T.; Niu, D.; Chen, Y.; et al. Computational redesign of enzymes for regio- and enantioselective hydroamination. Nat. Chem. Biol. 2018, 14, 664–670. [Google Scholar] [CrossRef]
- Corey, D.R.; Craik, C.S. An investigation into the minimum requirements for peptide hydrolysis by mutation of the catalytic triad of trypsin. J. Am. Chem. Soc. 1992, 114, 1784–1790. [Google Scholar] [CrossRef]
- Kast, P.; Asif-Ullah, M.; Jiang, N.; Hilvert, D. Exploring the active site of chorismate mutase by combinatorial mutagenesis and selection: The importance of electrostatic catalysis. Proc. Natl. Acad. Sci. USA 1996, 93, 5043–5048. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Xun, G.; Feng, Y. The state-of-the-art strategies of protein engineering for enzyme stabilization. Biotechnol. Adv. 2019, 37, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Gomez de Santos, P.; Mateljak, I.; Hoang, M.D.; Fleishman, S.J.; Hollmann, F.; Alcalde, M. Repertoire of computationally designed peroxygenases for enantiodivergent C–H oxyfunctionalization reactions. J. Am. Chem. Soc. 2023, 145, 3443–3453. [Google Scholar] [CrossRef]
- Casas-Godoy, L.; Gasteazoro, F.; Duquesne, S.; Bordes, F.; Marty, A.; Sandoval, G. Lipases: An overview. In Lipases and Phospholipases: Methods and Protocols; Sandoval, G., Ed.; Springer: New York, NY, USA, 2018; pp. 3–38. [Google Scholar]
- Adlercreutz, P. Immobilisation and application of lipases in organic media. Chem. Soc. Rev. 2013, 42, 6406–6436. [Google Scholar] [CrossRef] [PubMed]
- Biermann, U.; Bornscheuer, U.T.; Feussner, I.; Meier, M.A.R.; Metzger, J.O. Fatty acids and their derivatives as renewable platform molecules for the chemical industry. Angew. Chem. Int. Ed. 2021, 60, 20144–20165. [Google Scholar] [CrossRef] [PubMed]
- Mak, W.S.; Siegel, J.B. Computational enzyme design: Transitioning from catalytic proteins to enzymes. Curr. Opin. Struct. Biol. 2014, 27, 87–94. [Google Scholar] [CrossRef]
- Smith, A.J.T.; Müller, R.; Toscano, M.D.; Kast, P.; Hellinga, H.W.; Hilvert, D.; Houk, K.N. Structural reorganization and preorganization in enzyme active sites: Comparisons of experimental and theoretically ideal active site geometries in the multistep serine esterase reaction cycle. J. Am. Chem. Soc. 2008, 130, 15361–15373. [Google Scholar] [CrossRef]
- Kiss, G.; Çelebi-Ölçüm, N.; Moretti, R.; Baker, D.; Houk, K.N. Computational enzyme design. Angew. Chem. Int. Ed. 2013, 52, 5700–5725. [Google Scholar] [CrossRef]
- Goldenzweig, A.; Goldsmith, M.; Hill, S.E.; Gertman, O.; Laurino, P.; Ashani, Y.; Dym, O.; Unger, T.; Albeck, S.; Prilusky, J.; et al. Automated structure- and sequence-based design of proteins for high bacterial expression and stability. Mol. Cell 2016, 63, 337–346. [Google Scholar] [CrossRef]
- Korkegian, A.; Black, M.E.; Baker, D.; Stoddard, B.L. Computational thermostabilization of an enzyme. Science 2005, 308, 857–860. [Google Scholar] [CrossRef]
- Leaver-Fay, A.; Tyka, M.; Lewis, S.M.; Lange, O.F.; Thompson, J.; Jacak, R.; Kaufman, K.W.; Renfrew, P.D.; Smith, C.A.; Sheffler, W.; et al. Chapter nineteen—ROSETTA3: An object-oriented software suite for the simulation and design of macromolecules. In Methods in Enzymology; Johnson, M.L., Brand, L., Eds.; Academic Press: Cambridge, MA, USA, 2011; Volume 487, pp. 545–574. [Google Scholar]
- Lu, X.; Liu, Y.; Yang, Y.; Wang, S.; Wang, Q.; Wang, X.; Yan, Z.; Cheng, J.; Liu, C.; Yang, X.; et al. Constructing a synthetic pathway for acetyl-coenzyme A from one-carbon through enzyme design. Nat. Commun. 2019, 10, 1378. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Huang, X.; Xue, J.; Zhu, Y. Computational redesign of penicillin acylase for cephradine synthesis with high kinetic selectivity. Green Chem. 2018, 20, 5484–5490. [Google Scholar] [CrossRef]
- Wang, X.; Du, J.; Zhao, B.; Wang, H.; Rao, S.; Du, G.; Zhou, J.; Chen, J.; Liu, S. Significantly improving the thermostability and catalytic efficiency of Streptomyces mobaraenesis transglutaminase through combined rational design. J. Agric. Food Chem. 2021, 69, 15268–15278. [Google Scholar] [CrossRef] [PubMed]
- Kamerlin, S.C.L.; Chu, Z.T.; Warshel, A. On catalytic preorganization in oxyanion holes: Highlighting the problems with the gas-phase modeling of oxyanion holes and illustrating the need for complete enzyme models. J. Org. Chem. 2010, 75, 6391–6401. [Google Scholar] [CrossRef] [PubMed]
- Simón, L.; Goodman, J.M. Enzyme catalysis by hydrogen bonds: The balance between transition state binding and substrate binding in oxyanion holes. J. Org. Chem. 2010, 75, 1831–1840. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision D.01; Gaussian, Inc.: Wallingford, UK, 2009. [Google Scholar]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open babel: An open chemical toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef] [PubMed]
- Nagano, N.; Nakayama, N.; Ikeda, K.; Fukuie, M.; Yokota, K.; Doi, T.; Kato, T.; Tomii, K. EzCatDB: The enzyme reaction database, 2015 update. Nucleic Acids Res. 2014, 43, D453–D458. [Google Scholar] [CrossRef]
- Li, G.; Fang, X.; Su, F.; Chen, Y.; Xu, L.; Yan, Y. Enhancing the thermostability of Rhizomucor miehei lipase with a limited screening library by rational-design point mutations and disulfide bonds. Appl. Environ. Microbiol. 2018, 84, e02129-17. [Google Scholar] [CrossRef]
- Wang, S.; Xu, Y.; Yu, X.-W. Propeptide in Rhizopus chinensis lipase: New insights into its mechanism of activity and substrate selectivity by computational design. J. Agric. Food Chem. 2021, 69, 4263–4275. [Google Scholar] [CrossRef]

| Description | Concentration of Purified Protein (mg/mL) | Catalytic Activity (U/g) a |
|---|---|---|
| wt–1a8u | 0.83 ± 0.03 | N/A |
| 1a8uD1 | 0.62 ± 0.03 | 24.25 ± 0.57 |
| 1a8uD1–M1 | 0.54 ± 0.02 | N/A |
| 1a8uD1–M2 | 0.65 ± 0.03 | 43.03 ± 1.00 |
| 1a8uD1–M3 | 0.60 ± 0.03 | 36.63 ± 2.63 |
| 1a8uD1–M4 | 0.59 ± 0.04 | 20.47 ± 0.77 |
| 1a8uD1–M5 | 0.50 ± 0.02 | 32.13 ± 1.82 |
| 1a8uD1–M6 | 0.55 ± 0.02 | N/A |
| 1a8uD1–M7 | 0.63 ± 0.02 | 25.31 ± 1.07 |
| 1a8uD1–M8 | 0.63 ± 0.02 | 81.07 ± 4.59 |
| 1a8uD1–M9 | 0.62 ± 0.03 | 22.38 ± 0.92 |
| Mutations | wt–1a8u | 1a8uD1 | 1a8uD1–M8 |
|---|---|---|---|
| Catalytic activity (U/g) a | N/A | 7.28 ± 0.45 | 27.67 ± 0.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Xie, X.; Zheng, Z.; Ye, L.; Wang, P.; Xu, L.; Wu, Y.; Yan, J.; Yang, M.; Yan, Y. De Novo Computational Design of a Lipase with Hydrolysis Activity towards Middle-Chained Fatty Acid Esters. Int. J. Mol. Sci. 2023, 24, 8581. https://doi.org/10.3390/ijms24108581
Huang J, Xie X, Zheng Z, Ye L, Wang P, Xu L, Wu Y, Yan J, Yang M, Yan Y. De Novo Computational Design of a Lipase with Hydrolysis Activity towards Middle-Chained Fatty Acid Esters. International Journal of Molecular Sciences. 2023; 24(10):8581. https://doi.org/10.3390/ijms24108581
Chicago/Turabian StyleHuang, Jinsha, Xiaoman Xie, Zhen Zheng, Luona Ye, Pengbo Wang, Li Xu, Ying Wu, Jinyong Yan, Min Yang, and Yunjun Yan. 2023. "De Novo Computational Design of a Lipase with Hydrolysis Activity towards Middle-Chained Fatty Acid Esters" International Journal of Molecular Sciences 24, no. 10: 8581. https://doi.org/10.3390/ijms24108581
APA StyleHuang, J., Xie, X., Zheng, Z., Ye, L., Wang, P., Xu, L., Wu, Y., Yan, J., Yang, M., & Yan, Y. (2023). De Novo Computational Design of a Lipase with Hydrolysis Activity towards Middle-Chained Fatty Acid Esters. International Journal of Molecular Sciences, 24(10), 8581. https://doi.org/10.3390/ijms24108581






