The Role of Vitamins in the Pathogenesis of Asthma
Abstract
1. Introduction
2. Healthy Lifestyle, Nutrition, and Asthma
3. General Characteristics of the Vitamins
3.1. Vitamin A and Carotenoids
3.2. Vitamin B Group
3.3. Vitamin C
3.4. Vitamin D
3.5. Vitamin E
3.6. Vitamin K
4. Vitamins and the Risk of Asthma
4.1. Vitamin A
4.2. Vitamin B Family
4.3. Vitamin C
4.4. Vitamin D
4.5. Vitamin E
5. Maternal Intake of Vitamins and Asthma in the Offspring
5.1. Vitamin A
5.2. Vitamin B Group
5.3. Vitamin C
5.4. Vitamin D
5.5. Vitamin E
5.6. Vitamin K
5.7. Vitamins and the Gut Microbiota
6. Vitamins, Vitamin Levels and Asthma Features
6.1. Vitamin Levels in Asthma
6.2. Main Features of Asthma
6.2.1. Airway Hyperreactivity and Lung Function in Asthma
6.2.2. Oxidative Stress
6.2.3. Airway Inflammation and Cellular Influx into the Airways
6.2.4. Mucus Production and Structural Changes within the Airways (Airway Remodeling)
6.3. Vitamin A
6.3.1. Vitamin A Levels in Asthma
6.3.2. Vitamin A, Lung Function, and Airway Hyperresponsiveness
6.3.3. Vitamin A and Oxidative Stress
6.3.4. Vitamin A, Inflammation and Cellular Influx into the Airways
6.3.5. Vitamin A, Mucus Production, and Airway Remodeling
6.4. Vitamin B Group
6.4.1. Vitamin B Group Levels in Asthma
6.4.2. Vitamins of the B Group, Lung Function and Airway Hyperresponsiveness
6.4.3. Vitamins of the B Group and Oxidative Stress
6.4.4. Vitamins of the B Group, Inflammation and Cellular Influx into the Airways
6.4.5. Vitamins of the B Group, Mucus Production, and Airway Remodeling
6.5. Vitamin C
6.5.1. Vitamin C Levels in Asthma
6.5.2. Vitamin C, Lung Function and Airway Hyperresponsiveness
6.5.3. Vitamin C and Oxidative Stress
6.5.4. Vitamin C, Inflammation, and Cellular Influx into the Airways
6.5.5. Vitamin C, Mucus Production, and Airway Remodeling
6.6. Vitamin D
6.6.1. Vitamin D Levels in Asthma
6.6.2. Vitamin D, Lung Function and Airway Hyperresponsiveness
6.6.3. Vitamin D and Oxidative Stress
6.6.4. Vitamin D, Inflammation, and Cellular Influx into the Airways
6.6.5. Vitamin D, Mucus Production, and Airway Remodeling
6.7. Vitamin E
6.7.1. Vitamin E Levels in Asthma
6.7.2. Vitamin E, Lung Function and Airway Hyperresponsiveness
6.7.3. Vitamin E and Oxidative Stress
6.7.4. Vitamin E, Inflammation, and Cellular Influx into the Airways
6.7.5. Vitamin E, Mucus Production, and Airway Remodeling
7. Vitamins and Asthma Exacerbations
8. Summary
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. 2022. Available online: www.ginasthma.org (accessed on 10 January 2023).
- Gans, M.D.; Gavrilova, T. Understanding the Immunology of Asthma: Pathophysiology, Biomarkers, and Treatments for Asthma Endotypes. Paediatr. Respir. Rev. 2020, 36, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Papi, A.; Brightling, C.; Pedersen, S.E.; Reddel, H.K. Asthma. Lancet 2018, 391, 783–800. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.R.; Peters, S.P.; Busse, W.W. Asthma Exacerbations: Pathogenesis, Prevention, and Treatment. J. Allergy Clin. Immunol. Pract. 2017, 5, 918–927. [Google Scholar] [CrossRef] [PubMed]
- Zajac, D. Mineral Micronutrients in Asthma. Nutrients 2021, 13, 4001. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Gao, X.; Li, W.; Zhu, Y.; Thompson, P.J. Observational Studies on the Effect of Dietary Antioxidants on Asthma: A Meta-Analysis. Respirology 2008, 13, 528–536. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Kim, B.-S.; Kwon, S.-O.; Oh, S.-Y.; Shin, H.L.; Jung, Y.-H.; Lee, E.; Yang, S.-I.; Kim, H.Y.; Seo, J.-H.; et al. Modification of Additive Effect between Vitamins and ETS on Childhood Asthma Risk According to GSTP1 Polymorphism: A Cross -Sectional Study. BMC Pulm. Med. 2015, 15, 125. [Google Scholar] [CrossRef]
- Mai, X.-M.; Langhammer, A.; Chen, Y.; Camargo, C.A. Cod Liver Oil Intake and Incidence of Asthma in Norwegian Adults—The HUNT Study. Thorax 2013, 68, 25–30. [Google Scholar] [CrossRef]
- Palacios, C.; Gonzalez, L. Is Vitamin D Deficiency a Major Global Public Health Problem? J. Steroid Biochem. Mol. Biol. 2014, 144, 138–145. [Google Scholar] [CrossRef]
- Hejazi, M.; Modarresi-Ghazani, F.; Entezari-Maleki, T. A Review of Vitamin D Effects on Common Respiratory Diseases: Asthma, Chronic Obstructive Pulmonary Disease, and Tuberculosis. J. Res. Pharm. Pract. 2016, 5, 7. [Google Scholar] [CrossRef]
- Holick, M.F. The Vitamin D Deficiency Pandemic: Approaches for Diagnosis, Treatment and Prevention. Rev. Endocr. Metab. Disord. 2017, 18, 153–165. [Google Scholar] [CrossRef]
- Paul, G.; Brehm, J.M.; Alcorn, J.F.; Holguín, F.; Aujla, S.J.; Celedón, J.C. Vitamin D and Asthma. Am. J. Respir. Crit. Care Med. 2012, 185, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Black, P.N.; Scragg, R. Relationship between Serum 25-Hydroxyvitamin d and Pulmonary Function in the Third National Health and Nutrition Examination Survey. Chest 2005, 128, 3792–3798. [Google Scholar] [CrossRef] [PubMed]
- Brehm, J.M.; Celedón, J.C.; Soto-Quiros, M.E.; Avila, L.; Hunninghake, G.M.; Forno, E.; Laskey, D.; Sylvia, J.S.; Hollis, B.W.; Weiss, S.T.; et al. Serum Vitamin D Levels and Markers of Severity of Childhood Asthma in Costa Rica. Am. J. Respir. Crit. Care Med. 2009, 179, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Zosky, G.R.; Berry, L.J.; Elliot, J.G.; James, A.L.; Gorman, S.; Hart, P.H. Vitamin D Deficiency Causes Deficits in Lung Function and Alters Lung Structure. Am. J. Respir. Crit. Care Med. 2011, 183, 1336–1343. [Google Scholar] [CrossRef] [PubMed]
- Larange, A.; Cheroutre, H. Retinoic Acid and Retinoic Acid Receptors as Pleiotropic Modulators of the Immune System. Annu. Rev. Immunol. 2016, 34, 369–394. [Google Scholar] [CrossRef]
- Mora, J.R.; Iwata, M.; von Andrian, U.H. Vitamin Effects on the Immune System: Vitamins A and D Take Centre Stage. Nat. Rev. Immunol. 2008, 8, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Shoseyov, D.; Bibi, H.; Biesalski, H.; Reifen, R. Repeated Allergen Challenge in Rats Increases Vitamin A Consumption. Chest 2002, 122, 1407–1411. [Google Scholar] [CrossRef]
- Benson, M.J.; Pino-Lagos, K.; Rosemblatt, M.; Noelle, R.J. All-Trans Retinoic Acid Mediates Enhanced T Reg Cell Growth, Differentiation, and Gut Homing in the Face of High Levels of Co-Stimulation. J. Exp. Med. 2007, 204, 1765–1774. [Google Scholar] [CrossRef]
- Engedal, N. Survival of Activated Human T Lymphocytes Is Promoted by Retinoic Acid via Induction of IL-2. Int. Immunol. 2004, 16, 443–453. [Google Scholar] [CrossRef]
- Sun, C.-M.; Hall, J.A.; Blank, R.B.; Bouladoux, N.; Oukka, M.; Mora, J.R.; Belkaid, Y. Small Intestine Lamina Propria Dendritic Cells Promote de Novo Generation of Foxp3 T Reg Cells via Retinoic Acid. J. Exp. Med. 2007, 204, 1775–1785. [Google Scholar] [CrossRef]
- Milani, A.; Basirnejad, M.; Shahbazi, S.; Bolhassani, A. Carotenoids: Biochemistry, Pharmacology and Treatment: Carotenoids: Pharmacology and Treatment. Br. J. Pharmacol. 2017, 174, 1290–1324. [Google Scholar] [CrossRef] [PubMed]
- Maret, M.; Ruffie, C.; Periquet, B.; Campo, A.-M.; Menevret, M.; Phelep, A.; Dziewiszek, K.; Druilhe, A.; Pretolani, M. Liposomal Retinoic Acids Modulate Asthma Manifestations in Mice. J. Nutr. 2007, 137, 2730–2736. [Google Scholar] [CrossRef] [PubMed]
- Reifen, R.; Berkovich, Z.; Mandelberg, A. Vitamin A Supplementation via Aerosol Spray in Asthmatic Children. Pediatr. Allergy Immunol. 2015, 26, 578–579. [Google Scholar] [CrossRef] [PubMed]
- Kull, I.; Bergstrom, A.; Melen, E.; Lilja, G.; Vanhage, M.; Pershagen, G.; Wickman, M. Early-Life Supplementation of Vitamins A and D, in Water-Soluble Form or in Peanut Oil, and Allergic Diseases during Childhood. J. Allergy Clin. Immunol. 2006, 118, 1299–1304. [Google Scholar] [CrossRef]
- Romieu, I.; Varraso, R.; Avenel, V.; Leynaert, B.; Kauffmann, F.; Clavel-Chapelon, F. Fruit and Vegetable Intakes and Asthma in the E3N Study. Thorax 2006, 61, 209–215. [Google Scholar] [CrossRef]
- Wood, L.G.; Garg, M.L.; Smart, J.M.; Scott, H.A.; Barker, D.; Gibson, P.G. Manipulating Antioxidant Intake in Asthma: A Randomized Controlled Trial. Am. J. Clin. Nutr. 2012, 96, 534–543. [Google Scholar] [CrossRef]
- Wood, L.G.; Garg, M.L.; Powell, H.; Gibson, P.G. Lycopene-Rich Treatments Modify Noneosinophilic Airway Inflammation in Asthma: Proof of Concept. Free Radic. Res. 2008, 42, 94–102. [Google Scholar] [CrossRef]
- Harijith, A.; Natarajan, V.; Fu, P. The Role of Nicotinamide Adenine Dinucleotide Phosphate Oxidases in Lung Architecture Remodeling. Antioxidants 2017, 6, 104. [Google Scholar] [CrossRef]
- Chang, S.; Linderholm, A.; Franzi, L.; Kenyon, N.; Grasberger, H.; Harper, R. Dual Oxidase Regulates Neutrophil Recruitment in Allergic Airways. Free Radic. Biol. Med. 2013, 65, 38–46. [Google Scholar] [CrossRef]
- Ueland, P.M.; McCann, A.; Midttun, Ø.; Ulvik, A. Inflammation, Vitamin B6 and Related Pathways. Mol. Asp. Med. 2017, 53, 10–27. [Google Scholar] [CrossRef]
- Sharma, S.; Litonjua, A. Asthma, Allergy, and Responses to Methyl Donor Supplements and Nutrients. J. Allergy Clin. Immunol. 2014, 133, 1246–1254. [Google Scholar] [CrossRef]
- Rautiainen, S.; Manson, J.E.; Lichtenstein, A.H.; Sesso, H.D. Dietary Supplements and Disease Prevention—A Global Overview. Nat. Rev. Endocrinol. 2016, 12, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Tamura, J.; Kubota, K.; Murakami, H.; Sawamura, M.; Matsushima, T.; Tamura, T.; Saitoh, T.; Kurabayshi, H.; Naruse, T. Immunomodulation by Vitamin B12: Augmentation of CD8+ T Lymphocytes and Natural Killer (NK) Cell Activity in Vitamin B12-Deficient Patients by Methyl-B12 Treatment. Clin. Exp. Immunol. 1999, 116, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-H.; Chen, C.-S.; Lin, J.-Y. High Dose Vitamin C Supplementation Increases the Th1/Th2 Cytokine Secretion Ratio, but Decreases Eosinophilic Infiltration in Bronchoalveolar Lavage Fluid of Ovalbumin-Sensitized and Challenged Mice. J. Agric. Food Chem. 2009, 57, 10471–10476. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.-Y.; Blatter, J.; Brehm, J.M.; Forno, E.; Litonjua, A.A.; Celedón, J.C. Diet and Asthma: Vitamins and Methyl Donors. Lancet Respir. Med. 2013, 1, 813–822. [Google Scholar] [CrossRef]
- Hemilä, H. Vitamin C and Common Cold-Induced Asthma: A Systematic Review and Statistical Analysis. Allergy Asthma Clin. Immunol. 2013, 9, 46. [Google Scholar] [CrossRef] [PubMed]
- Omenaas, E.; Fluge, Ø.; Buist, A.S.; Vollmer, W.M.; Gulsvik, A. Dietary Vitamin C Intake Is Inversely Related to Cough and Wheeze in Young Smokers. Respir. Med. 2003, 97, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Hemilä, H. The Effect of Vitamin C on Bronchoconstriction and Respiratory Symptoms Caused by Exercise: A Review and Statistical Analysis. Allergy Asthma Clin. Immunol. 2014, 10, 58. [Google Scholar] [CrossRef]
- Hernandez, M.; Zhou, H.; Zhou, B.; Robinette, C.; Crissman, K.; Hatch, G.; Alexis, N.E.; Peden, D. Combination Treatment with High-Dose Vitamin C and Alpha-Tocopherol Does Not Enhance Respiratory-Tract Lining Fluid Vitamin C Levels in Asthmatics. Inhal. Toxicol. 2009, 21, 173–181. [Google Scholar] [CrossRef]
- Wiester, M.J.; Winsett, D.W.; Richards, J.H.; Jackson, M.C.; Crissman, K.M.; Costa, D.L. Ozone Adaptation in Mice and Its Association with Ascorbic Acid in the Lung. Inhal. Toxicol. 2000, 12, 577–590. [Google Scholar] [CrossRef]
- Hornung, T.C.; Biesalski, H.-K. Glut-1 Explains the Evolutionary Advantage of the Loss of Endogenous Vitamin C-Synthesis. Evol. Med. Public Health 2019, 2019, 221–231. [Google Scholar] [CrossRef]
- Kerley, C.P.; Elnazir, B.; Faul, J.; Cormican, L. Vitamin D as an Adjunctive Therapy in Asthma. Part 1: A Review of Potential Mechanisms. Pulm. Pharmacol. Ther. 2015, 32, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Kerley, C.P.; Elnazir, B.; Faul, J.; Cormican, L. Vitamin D as an Adjunctive Therapy in Asthma. Part 2: A Review of Human Studies. Pulm. Pharmacol. Ther. 2015, 32, 75–92. [Google Scholar] [CrossRef] [PubMed]
- Chirumbolo, S.; Bjørklund, G.; Sboarina, A.; Vella, A. The Role of Vitamin D in the Immune System as a Pro-Survival Molecule. Clin. Ther. 2017, 39, 894–916. [Google Scholar] [CrossRef] [PubMed]
- Hewison, M. Vitamin D and Immune Function: An Overview. Proc. Nutr. Soc. 2012, 71, 50–61. [Google Scholar] [CrossRef]
- Hewison, M. Vitamin D and Innate and Adaptive Immunity. In Vitamins & Hormones; Elsevier: Amsterdam, The Netherlands, 2011; Volume 86, pp. 23–62. [Google Scholar]
- Bikle, D.D. Vitamin D Regulation of Immune Function. In Vitamins & Hormones; Elsevier: Amsterdam, The Netherlands, 2011; Volume 86, pp. 1–21. [Google Scholar]
- Colotta, F.; Jansson, B.; Bonelli, F. Modulation of Inflammatory and Immune Responses by Vitamin D. J. Autoimmun. 2017, 85, 78–97. [Google Scholar] [CrossRef]
- Mathyssen, C.; Gayan-Ramirez, G.; Bouillon, R.; Janssens, W. Vitamin D Supplementation in Respiratory Diseases: Evidence from Randomized Controlled Trials. Pol. Arch. Intern. Med. 2017, 127, 775–784. [Google Scholar] [CrossRef]
- Bossé, Y.; Maghni, K.; Hudson, T.J. 1α,25-Dihydroxy-Vitamin D 3 Stimulation of Bronchial Smooth Muscle Cells Induces Autocrine, Contractility, and Remodeling Processes. Physiol. Genom. 2007, 29, 161–168. [Google Scholar] [CrossRef]
- Hansdottir, S.; Monick, M.M.; Hinde, S.L.; Lovan, N.; Look, D.C.; Hunninghake, G.W. Respiratory Epithelial Cells Convert Inactive Vitamin D to Its Active Form: Potential Effects on Host Defense. J. Immunol. 2008, 181, 7090–7099. [Google Scholar] [CrossRef]
- Herr, C.; Greulich, T.; Koczulla, R.A.; Meyer, S.; Zakharkina, T.; Branscheidt, M.; Eschmann, R.; Bals, R. The Role of Vitamin D in Pulmonary Disease: COPD, Asthma, Infection, and Cancer. Respir. Res. 2011, 12, 31. [Google Scholar] [CrossRef]
- Adorini, L. Tolerogenic Dendritic Cells Induced by Vitamin D Receptor Ligands Enhance Regulatory T Cells Inhibiting Autoimmune Diabetes. Ann. N. Y. Acad. Sci. 2003, 987, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Hansdottir, S.; Monick, M.M. Vitamin D Effects on Lung Immunity and Respiratory Diseases. In Vitamins & Hormones; Elsevier: Amsterdam, The Netherlands, 2011; Volume 86, pp. 217–237. ISBN 978-0-12-386960-9. [Google Scholar]
- Yawn, J.; Lawrence, L.A.; Carroll, W.W.; Mulligan, J.K. Vitamin D for the Treatment of Respiratory Diseases: Is It the End or Just the Beginning? J. Steroid Biochem. Mol. Biol. 2015, 148, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-Q.; Li, X.-X.; Qiu, S.-Q.; Yu, Y.; Li, M.-G.; Yang, L.-T.; Li, L.-J.; Wang, S.; Zheng, P.-Y.; Liu, Z.-G.; et al. Vitamin D Contributes to Mast Cell Stabilization. Allergy 2017, 72, 1184–1192. [Google Scholar] [CrossRef] [PubMed]
- Goleva, E.; Searing, D.A.; Jackson, L.P.; Richers, B.N.; Leung, D.Y.M. Steroid Requirements and Immune Associations with Vitamin D Are Stronger in Children than Adults with Asthma. J. Allergy Clin. Immunol. 2012, 129, 1243–1251. [Google Scholar] [CrossRef]
- Zhang, Y.; Leung, D.Y.M.; Goleva, E. Anti-Inflammatory and Corticosteroid-Enhancing Actions of Vitamin D in Monocytes of Patients with Steroid-Resistant and Those with Steroid-Sensitive Asthma. J. Allergy Clin. Immunol. 2014, 133, 1744–1752.e1. [Google Scholar] [CrossRef]
- Pfeffer, P.E.; Hawrylowicz, C.M. Vitamin D in Asthma. Chest 2018, 153, 1229–1239. [Google Scholar] [CrossRef]
- Xystrakis, E.; Kusumakar, S.; Boswell, S.; Peek, E.; Urry, Z.; Richards, D.F.; Adikibi, T.; Pridgeon, C.; Dallman, M.; Loke, T.-K.; et al. Reversing the Defective Induction of IL-10–Secreting Regulatory T Cells in Glucocorticoid-Resistant Asthma Patients. J. Clin. Investig. 2006, 116, 146–155. [Google Scholar] [CrossRef]
- Cook-Mills, J.M.; Averill, S.H.; Lajiness, J.D. Asthma, Allergy and Vitamin E: Current and Future Perspectives. Free Radic. Biol. Med. 2022, 179, 388–402. [Google Scholar] [CrossRef]
- McCary, C.A.; Abdala-Valencia, H.; Berdnikovs, S.; Cook-Mills, J.M. Supplemental and Highly Elevated Tocopherol Doses Differentially Regulate Allergic Inflammation: Reversibility of α-Tocopherol and γ-Tocopherol’s Effects. J. Immunol. 2011, 186, 3674–3685. [Google Scholar] [CrossRef]
- Wagner, J.G.; Harkema, J.R.; Jiang, Q.; Hernandez, M.; Peden, D.B. Two Faces of Vitamin E in the Lung. Am. J. Respir. Crit. Care Med. 2014, 190, 841–842. [Google Scholar] [CrossRef]
- Abdala-Valencia, H.; Berdnikovs, S.; Cook-Mills, J.M. Vitamin E Isoforms Differentially Regulate Intercellular Adhesion Molecule-1 Activation of PKCα in Human Microvascular Endothelial Cells. PLoS ONE 2012, 7, e41054. [Google Scholar] [CrossRef] [PubMed]
- Berdnikovs, S.; Abdala-Valencia, H.; McCary, C.; Somand, M.; Cole, R.; Garcia, A.; Bryce, P.; Cook-Mills, J.M. Isoforms of Vitamin E Have Opposing Immunoregulatory Functions during Inflammation by Regulating Leukocyte Recruitment. J. Immunol. 2009, 182, 4395–4405. [Google Scholar] [CrossRef] [PubMed]
- Abdala-Valencia, H.; Berdnikovs, S.; Cook-Mills, J.M. Vitamin E Isoforms as Modulators of Lung Inflammation. Nutrients 2013, 5, 4347–4363. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Im, S.; Wagner, J.G.; Hernandez, M.L.; Peden, D.B. Gamma-Tocopherol, a Major Form of Vitamin E in Diets: Insights into Antioxidant and Anti-Inflammatory Effects, Mechanisms, and Roles in Disease Management. Free Radic. Biol. Med. 2022, 178, 347–359. [Google Scholar] [CrossRef]
- Azzi, A. Many Tocopherols, One Vitamin E. Mol. Asp. Med. 2018, 61, 92–103. [Google Scholar] [CrossRef]
- Lim, Y.; Vasu, V.T.; Valacchi, G.; Leonard, S.; Hnin Aung, H.; Schock, B.C.; Kenyon, N.J.; Li, C.-S.; Traber, M.G.; Cross, C.E. Severe Vitamin E Deficiency Modulates Airway Allergic Inflammatory Responses in the Murine Asthma Model. Free Radic. Res. 2008, 42, 387–396. [Google Scholar] [CrossRef]
- Cook-Mills, J.M.; McCary, C.A. Isoforms of Vitamin E Differentially Regulate Inflammation. Endocr. Metab. Immune Disord. Drug Targets 2010, 10, 348–366. [Google Scholar] [CrossRef]
- Sen, C.K.; Khanna, S.; Roy, S. Tocotrienols in Health and Disease: The Other Half of the Natural Vitamin E Family. Mol. Asp. Med. 2007, 28, 692–728. [Google Scholar] [CrossRef]
- Peh, H.Y.; Ho, W.E.; Cheng, C.; Chan, T.K.; Seow, A.C.G.; Lim, A.Y.H.; Fong, C.W.; Seng, K.Y.; Ong, C.N.; Wong, W.S.F. Vitamin E Isoform γ-Tocotrienol Downregulates House Dust Mite–Induced Asthma. J. Immunol. 2015, 195, 437–444. [Google Scholar] [CrossRef]
- Ahn, K.S.; Sethi, G.; Krishnan, K.; Aggarwal, B.B. γ-Tocotrienol Inhibits Nuclear Factor-ΚB Signaling Pathway through Inhibition of Receptor-Interacting Protein and TAK1 Leading to Suppression of Antiapoptotic Gene Products and Potentiation of Apoptosis. J. Biol. Chem. 2007, 282, 809–820. [Google Scholar] [CrossRef]
- Xiao, H.; Chen, J.; Duan, L.; Li, S. Role of Emerging Vitamin K-dependent Proteins: Growth Arrest-specific Protein 6, Gla-rich Protein and Periostin (Review). Int. J. Mol. Med. 2021, 47, 2. [Google Scholar] [CrossRef] [PubMed]
- Kimur, I.; Tanizaki, Y.; Sato, S.; Saito, K.; Takahashi, K. Menaquinone (Vitamin K2) Therapy for Bronchial Asthma. II. Clinical Effect of Menaquinone on Bronchial Asthma. Acta Med. Okayama 1975, 29, 127–135. [Google Scholar] [PubMed]
- Zheng, X.; Hou, Y.; He, H.; Chen, Y.; Zhou, R.; Wang, X.; Gong, T.; Jiang, W. Synthetic Vitamin K Analogs Inhibit Inflammation by Targeting the NLRP3 Inflammasome. Cell. Mol. Immunol. 2021, 18, 2422–2430. [Google Scholar] [CrossRef] [PubMed]
- Kohli, V.; Sharma, D.; Sandur, S.K.; Suryavanshi, S.; Sainis, K.B. Immune Responses to Novel Allergens and Modulation of Inflammation by Vitamin K3 Analogue: A ROS Dependent Mechanism. Int. Immunopharmacol. 2011, 11, 233–243. [Google Scholar] [CrossRef]
- Ohsaki, Y.; Shirakawa, H.; Hiwatashi, K.; Furukawa, Y.; Mizutani, T.; Komai, M. Vitamin K Suppresses Lipopolysaccharide-Induced Inflammation in the Rat. Biosci. Biotechnol. Biochem. 2006, 70, 926–932. [Google Scholar] [CrossRef]
- Checker, R.; Sharma, D.; Sandur, S.K.; Khan, N.M.; Patwardhan, R.S.; Kohli, V.; Sainis, K.B. Vitamin K3 Suppressed Inflammatory and Immune Responses in a Redox-Dependent Manner. Free Radic. Res. 2011, 45, 975–985. [Google Scholar] [CrossRef]
- Gozzi-Silva, S.C.; Teixeira, F.M.E.; Duarte, A.J.d.S.; Sato, M.N.; Oliveira, L.d.M. Immunomodulatory Role of Nutrients: How Can Pulmonary Dysfunctions Improve? Front. Nutr. 2021, 8, 674258. [Google Scholar] [CrossRef]
- Koumpagioti, D.; Boutopoulou, B.; Moriki, D.; Priftis, K.N.; Douros, K. Does Adherence to the Mediterranean Diet Have a Protective Effect against Asthma and Allergies in Children? A Systematic Review. Nutrients 2022, 14, 1618. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, J.; Fu, W.; Liu, S.; Gong, C.; Dai, J. Mediterranean Diet during Pregnancy and Childhood for Asthma in Children: A Systematic Review and Meta-Analysis of Observational Studies. Pediatr. Pulmonol. 2019, 54, 949–961. [Google Scholar] [CrossRef]
- Lee, Y.-L.; Lee, Y.-C.; Guo, Y.L. Associations of Glutathione S-Transferase P1, M1, and Environmental Tobacco Smoke with Wheezing Illness in School Children. Allergy 2007, 62, 641–647. [Google Scholar] [CrossRef]
- Huang, S.-L.; Pan, W.-H. Dietary Fats and Asthma in Teenagers: Analyses of the First Nutrition and Health Survey in Taiwan (NAHSIT): Dietary Fats and Asthma in Teenagers. Clin. Exp. Allergy 2001, 31, 1875–1880. [Google Scholar] [CrossRef] [PubMed]
- Checkley, W.; West, K.P.; Wise, R.A.; Wu, L.; LeClerq, S.C.; Khatry, S.; Katz, J.; Christian, P.; Tielsch, J.M.; Sommer, A. Supplementation with Vitamin A Early in Life and Subsequent Risk of Asthma. Eur. Respir. J. 2011, 38, 1310–1319. [Google Scholar] [CrossRef] [PubMed]
- Green, A.S.; Fascetti, A.J. Meeting the Vitamin A Requirement: The Efficacy and Importance of β-Carotene in Animal Species. Sci. World J. 2016, 2016, 7393620. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Sang, J.; Hao, F.; Liu, L. Association between Vitamin A and Asthma: A Meta-Analysis with Trial Sequential Analysis. Front. Pharmacol. 2023, 14, 1100002. [Google Scholar] [CrossRef]
- Quyen, D.T.; Irei, A.V.; Sato, Y.; Ota, F.; Fujimaki, Y.; Sakai, T.; Kunii, D.; Khan, N.C.; Yamamoto, S. Nutritional Factors, Parasite Infection and Allergy in Rural and Suburban Vietnamese School Children. J. Med. Investig. JMI 2004, 51, 171–177. [Google Scholar] [CrossRef]
- Sur, S.; Camara, M.; Buchmeier, A.; Morgan, S.; Nelson, H.S. Double-Blind Trial of Pyridoxine (Vitamin B6) in the Treatment of Steroid-Dependent Asthma. Ann. Allergy 1993, 70, 147–152. [Google Scholar]
- Collipp, P.J.; Goldzier, S.; Weiss, N.; Soleymani, Y.; Snyder, R. Pyridoxine Treatment of Childhood Bronchial Asthma. Ann. Allergy 1975, 35, 93–97. [Google Scholar]
- Seyedrezazadeh, E.; Pour Moghaddam, M.; Ansarin, K.; Reza Vafa, M.; Sharma, S.; Kolahdooz, F. Fruit and Vegetable Intake and Risk of Wheezing and Asthma: A Systematic Review and Meta-Analysis. Nutr. Rev. 2014, 72, 411–428. [Google Scholar] [CrossRef]
- Matsui, E.C.; Matsui, W. Higher Serum Folate Levels Are Associated with a Lower Risk of Atopy and Wheeze. J. Allergy Clin. Immunol. 2009, 123, 1253–1259.e2. [Google Scholar] [CrossRef]
- Chatzi, L.; Apostolaki, G.; Bibakis, I.; Skypala, I.; Bibaki-Liakou, V.; Tzanakis, N.; Kogevinas, M.; Cullinan, P. Protective Effect of Fruits, Vegetables and the Mediterranean Diet on Asthma and Allergies among Children in Crete. Thorax 2007, 62, 677–683. [Google Scholar] [CrossRef]
- Hosseini, B.; Berthon, B.S.; Wark, P.; Wood, L.G. Effects of Fruit and Vegetable Consumption on Risk of Asthma, Wheezing and Immune Responses: A Systematic Review and Meta-Analysis. Nutrients 2017, 9, 341. [Google Scholar] [CrossRef] [PubMed]
- Shidfar, F.; Baghai, N.; Keshavarz, A.; Ameri, A.; Shidfar, S. Comparison of Plasma and Leukocyte Vitamin C Status between Asthmatic and Healthy Subjects. East. Mediterr. Health J. 2005, 11, 87–95. [Google Scholar] [PubMed]
- de Luis, D.A.; Armentia, A.; Aller, R.; Asensio, A.; Sedano, E.; Izaola, O.; Cuellar, L. Dietary Intake in Patients with Asthma: A Case Control Study. Nutrition 2005, 21, 320–324. [Google Scholar] [CrossRef]
- Misso, N.L.A.; Brooks-Wildhaber, J.; Ray, S.; Vally, H.; Thompson, P.J. Plasma Concentrations of Dietary and Nondietary Antioxidants Are Low in Severe Asthma. Eur. Respir. J. 2005, 26, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Soutar, A.; Seaton, A.; Brown, K. Bronchial Reactivity and Dietary Antioxidants. Thorax 1997, 52, 166–170. [Google Scholar] [CrossRef]
- Devereux, G.; Seaton, A. Diet as a Risk Factor for Atopy and Asthma. J. Allergy Clin. Immunol. 2005, 115, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Gilliland, F.D.; Berhane, K.T.; Li, Y.-F.; Gauderman, W.J.; McConnell, R.; Peters, J. Children’s Lung Function and Antioxidant Vitamin, Fruit, Juice, and Vegetable Intake. Am. J. Epidemiol. 2003, 158, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Milan, S.J.; Hart, A.; Wilkinson, M. Vitamin C for Asthma and Exercise-Induced Bronchoconstriction. Cochrane Database Syst. Rev. 2013, 2018, CD010391. [Google Scholar] [CrossRef]
- Troisi, R.J.; Willett, W.C.; Weiss, S.T.; Trichopoulos, D.; Rosner, B.; Speizer, F.E. A Prospective Study of Diet and Adult-Onset Asthma. Am. J. Respir. Crit. Care Med. 1995, 151, 1401–1408. [Google Scholar] [CrossRef]
- Hollams, E.M.; Hart, P.H.; Holt, B.J.; Serralha, M.; Parsons, F.; de Klerk, N.H.; Zhang, G.; Sly, P.D.; Holt, P.G. Vitamin D and Atopy and Asthma Phenotypes in Children: A Longitudinal Cohort Study. Eur. Respir. J. 2011, 38, 1320–1327. [Google Scholar] [CrossRef]
- Sikorska-Szaflik, H.; Sozańska, B. The Role of Vitamin D in Respiratory Allergies Prevention. Why the Effect Is so Difficult to Disentangle? Nutrients 2020, 12, 1801. [Google Scholar] [CrossRef] [PubMed]
- Hollams, E.M.; Teo, S.M.; Kusel, M.; Holt, B.J.; Holt, K.E.; Inouye, M.; De Klerk, N.H.; Zhang, G.; Sly, P.D.; Hart, P.H.; et al. Vitamin D over the First Decade and Susceptibility to Childhood Allergy and Asthma. J. Allergy Clin. Immunol. 2017, 139, 472–481.e9. [Google Scholar] [CrossRef] [PubMed]
- Wittke, A.; Chang, A.; Froicu, M.; Harandi, O.F.; Weaver, V.; August, A.; Paulson, R.F.; Cantorna, M.T. Vitamin D Receptor Expression by the Lung Micro-Environment Is Required for Maximal Induction of Lung Inflammation. Arch. Biochem. Biophys. 2007, 460, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Wittke, A.; Weaver, V.; Mahon, B.D.; August, A.; Cantorna, M.T. Vitamin D Receptor-Deficient Mice Fail to Develop Experimental Allergic Asthma. J. Immunol. 2004, 173, 3432–3436. [Google Scholar] [CrossRef]
- Kompauer, I.; Heinrich, J.; Wolfram, G.; Linseisen, J. Association of Carotenoids, Tocopherols and Vitamin C in Plasma with Allergic Rhinitis and Allergic Sensitisation in Adults. Public Health Nutr. 2006, 9, 472–479. [Google Scholar] [CrossRef]
- Hoskins, A.; Roberts, J.L.; Milne, G.; Choi, L.; Dworski, R. Natural-Source d-α-Tocopheryl Acetate Inhibits Oxidant Stress and Modulates Atopic Asthma in Humans in Vivo. Allergy 2012, 67, 676–682. [Google Scholar] [CrossRef]
- Ghaffari, J.; Hossaini, R.F.; Khalilian, A.; Nahanmoghadam, N.; Salehifar, E.; Rafatpanah, H. Vitamin E Supplementation, Lung Functions and Clinical Manifestations in Children with Moderate Asthma: A Randomized Double Blind Placebo- Controlled Trial. Iran. J. Allergy Asthma Immunol. 2014, 13, 98–103. [Google Scholar]
- Pearson, P.J.K.; Lewis, S.A.; Britton, J.; Fogarty, A. Vitamin E Supplements in Asthma: A Parallel Group Randomised Placebo Controlled Trial. Thorax 2004, 59, 652–656. [Google Scholar] [CrossRef]
- Rubin, R.N.; Navon, L.; Cassano, P.A. Relationship of Serum Antioxidants to Asthma Prevalence in Youth. Am. J. Respir. Crit. Care Med. 2004, 169, 393–398. [Google Scholar] [CrossRef]
- Allen, S.; Britton, J.R.; Leonardi-Bee, J.A. Association between Antioxidant Vitamins and Asthma Outcome Measures: Systematic Review and Meta-Analysis. Thorax 2009, 64, 610–619. [Google Scholar] [CrossRef]
- Trenca, C.A.; Koenig, J.Q.; Williams, P.V. Dietary Antioxidants and Ozone-Induced Bronchial Hyperresponsiveness in Adults with Asthma. Arch. Environ. Health Int. J. 2001, 56, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Grievink, L.; Zijlstra, A.G.; Ke, X.; Brunekreef, B. Double-Blind Intervention Trial on Modulation of Ozone Effects on Pulmonary Function by Antioxidant Supplements. Am. J. Epidemiol. 1999, 149, 306–314. [Google Scholar] [CrossRef]
- Romieu, I.; Meneses, F.; Ramirez, M.; Ruiz, S.; Padilla, R.P.; Sienra, J.J.; Gerber, M.; Grievink, L.; Dekker, R.; Walda, I.; et al. Antioxidant Supplementation and Respiratory Functions among Workers Exposed to High Levels of Ozone. Am. J. Respir. Crit. Care Med. 1998, 158, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Kurti, S.P.; Murphy, J.D.; Ferguson, C.S.; Brown, K.R.; Smith, J.R.; Harms, C.A. Improved Lung Function Following Dietary Antioxidant Supplementation in Exercise-Induced Asthmatics. Respir. Physiol. Neurobiol. 2016, 220, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Cook-Mills, J.M.; Abdala-Valencia, H.; Hartert, T. Two Faces of Vitamin E in the Lung. Am. J. Respir. Crit. Care Med. 2013, 188, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Fogarty, A.; Lewis, S.; Weiss, S.; Britton, J. Dietary Vitamin E, IgE Concentrations, and Atopy. Lancet 2000, 356, 1573–1574. [Google Scholar] [CrossRef]
- Beasley, R.; Semprini, A.; Mitchell, E.A. Risk Factors for Asthma: Is Prevention Possible? Lancet 2015, 386, 1075–1085. [Google Scholar] [CrossRef]
- Maslova, E.; Hansen, S.; Strøm, M.; Halldorsson, T.I.; Olsen, S.F. Maternal Intake of Vitamins A, E and K in Pregnancy and Child Allergic Disease: A Longitudinal Study from the Danish National Birth Cohort. Br. J. Nutr. 2014, 111, 1096–1108. [Google Scholar] [CrossRef]
- Checkley, W.; West, K.P.; Wise, R.A.; Baldwin, M.R.; Wu, L.; LeClerq, S.C.; Christian, P.; Katz, J.; Tielsch, J.M.; Khatry, S.; et al. Maternal Vitamin A Supplementation and Lung Function in Offspring. N. Engl. J. Med. 2010, 362, 1784–1794. [Google Scholar] [CrossRef]
- Stelmach, I.; Grzelewski, T.; Bobrowska-Korzeniowska, M.; Kopka, M.; Majak, P.; Jerzynska, J.; Stelmach, W.; Polańska, K.; Sobala, W.; Gromadzińska, J.; et al. The Role of Zinc, Copper, Plasma Glutathione Peroxidase Enzyme, and Vitamins in the Development of Allergic Diseases in Early Childhood: The Polish Mother and Child Cohort Study. Allergy Asthma Proc. 2014, 35, 227–232. [Google Scholar] [CrossRef]
- Parr, C.L.; Magnus, M.C.; Karlstad, Ø.; Holvik, K.; Lund-Blix, N.A.; Haugen, M.; Page, C.M.; Nafstad, P.; Ueland, P.M.; London, S.J.; et al. Vitamin A and D Intake in Pregnancy, Infant Supplementation, and Asthma Development: The Norwegian Mother and Child Cohort. Am. J. Clin. Nutr. 2018, 107, 789–798. [Google Scholar] [CrossRef] [PubMed]
- McGuire, S. WHO Guideline: Vitamin A Supplementation in Pregnant Women. Geneva: WHO, 2011; WHO Guideline: Vitamin A Supplementation in Postpartum Women. Geneva: WHO, 2011. Adv. Nutr. 2012, 3, 215–216. [Google Scholar] [CrossRef] [PubMed]
- Miyake, Y.; Sasaki, S.; Tanaka, K.; Hirota, Y. Maternal B Vitamin Intake during Pregnancy and Wheeze and Eczema in Japanese Infants Aged 16–24 Months: The Osaka Maternal and Child Health Study: B Vitamin Intake and Wheeze and Eczema. Pediatr. Allergy Immunol. 2011, 22, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.B.; Reeves, K.W.; Bertone-Johnson, E.R. Maternal Folate Exposure in Pregnancy and Childhood Asthma and Allergy: A Systematic Review. Nutr. Rev. 2014, 72, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Haberg, S.E.; London, S.J.; Stigum, H.; Nafstad, P.; Nystad, W. Folic Acid Supplements in Pregnancy and Early Childhood Respiratory Health. Arch. Dis. Child. 2009, 94, 180–184. [Google Scholar] [CrossRef]
- Håberg, S.E.; London, S.J.; Nafstad, P.; Nilsen, R.M.; Ueland, P.M.; Vollset, S.E.; Nystad, W. Maternal Folate Levels in Pregnancy and Asthma in Children at Age 3 Years. J. Allergy Clin. Immunol. 2011, 127, 262–264.e1. [Google Scholar] [CrossRef]
- Kiefte-de Jong, J.C.; Timmermans, S.; Jaddoe, V.W.V.; Hofman, A.; Tiemeier, H.; Steegers, E.A.; de Jongste, J.C.; Moll, H.A. High Circulating Folate and Vitamin B-12 Concentrations in Women During Pregnancy Are Associated with Increased Prevalence of Atopic Dermatitis in Their Offspring. J. Nutr. 2012, 142, 731–738. [Google Scholar] [CrossRef]
- Okuda, M.; Bando, N.; Terao, J.; Sasaki, S.; Sugiyama, S.; Kunitsugu, I.; Hobara, T. Association of Serum Carotenoids and Tocopherols with Atopic Diseases in Japanese Children and Adolescents: Serum Antioxidant and Atopy in Young Japanese. Pediatr. Allergy Immunol. 2010, 21, e705–e710. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, H.-P.; Zhang, X.; Liang, Z.-A.; Ji, Y.-L.; Wang, G. Is Folate Status a Risk Factor for Asthma or Other Allergic Diseases? Allergy Asthma Immunol. Res. 2015, 7, 538. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, L.; Bi, M.; Jia, X.; Wang, Y.; He, C.; Yao, Y.; Wang, J.; Wang, Z. High Dose of Maternal Folic Acid Supplementation Is Associated to Infant Asthma. Food Chem. Toxicol. 2015, 75, 88–93. [Google Scholar] [CrossRef]
- Whitrow, M.J.; Moore, V.M.; Rumbold, A.R.; Davies, M.J. Effect of Supplemental Folic Acid in Pregnancy on Childhood Asthma: A Prospective Birth Cohort Study. Am. J. Epidemiol. 2009, 170, 1486–1493. [Google Scholar] [CrossRef] [PubMed]
- Alfonso, V.H.; Bandoli, G.; von Ehrenstein, O.; Ritz, B. Early Folic Acid Supplement Initiation and Risk of Adverse Early Childhood Respiratory Health: A Population-Based Study. Matern. Child Health J. 2018, 22, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Veeranki, S.P.; Gebretsadik, T.; Mitchel, E.F.; Tylavsky, F.A.; Hartert, T.V.; Cooper, W.O.; Dupont, W.D.; Dorris, S.L.; Hartman, T.J.; Carroll, K.N. Maternal Folic Acid Supplementation During Pregnancy and Early Childhood Asthma. Epidemiology 2015, 26, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Bekkers, M.B.M.; Elstgeest, L.E.M.; Scholtens, S.; Haveman-Nies, A.; de Jongste, J.C.; Kerkhof, M.; Koppelman, G.H.; Gehring, U.; Smit, H.A.; Wijga, A.H. Maternal Use of Folic Acid Supplements during Pregnancy, and Childhood Respiratory Health and Atopy. Eur. Respir. J. 2012, 39, 1468–1474. [Google Scholar] [CrossRef] [PubMed]
- Blatter, J.; Han, Y.-Y.; Forno, E.; Brehm, J.; Bodnar, L.; Celedón, J.C. Folate and Asthma. Am. J. Respir. Crit. Care Med. 2013, 188, 12–17. [Google Scholar] [CrossRef]
- Granell, R.; Heron, J.; Lewis, S.; Davey Smith, G.; Sterne, J.A.C.; Henderson, J. The Association between Mother and Child MTHFR C677T Polymorphisms, Dietary Folate Intake and Childhood Atopy in a Population-Based, Longitudinal Birth Cohort. Clin. Exp. Allergy 2008, 38, 320–328. [Google Scholar] [CrossRef]
- Liu, J.; Li, Z.; Ye, R.; Liu, J.; Ren, A. Periconceptional Folic Acid Supplementation and Risk of Parent-Reported Asthma in Children at 4–6 Years of Age. ERJ Open Res. 2020, 6, 00250–02019. [Google Scholar] [CrossRef]
- Magdelijns, F.J.H.; Mommers, M.; Penders, J.; Smits, L.; Thijs, C. Folic Acid Use in Pregnancy and the Development of Atopy, Asthma, and Lung Function in Childhood. Pediatrics 2011, 128, e135–e144. [Google Scholar] [CrossRef]
- Martinussen, M.P.; Risnes, K.R.; Jacobsen, G.W.; Bracken, M.B. Folic Acid Supplementation in Early Pregnancy and Asthma in Children Aged 6 Years. Am. J. Obstet. Gynecol. 2012, 206, 72.e1–72.e7. [Google Scholar] [CrossRef]
- van der Valk, R.J.P.; Kiefte-de Jong, J.C.; Sonnenschein-van der Voort, A.M.M.; Duijts, L.; Hafkamp-de Groen, E.; Moll, H.A.; Tiemeier, H.; Steegers, E.A.P.; Hofman, A.; Jaddoe, V.W.V.; et al. Neonatal Folate, Homocysteine, Vitamin B12 Levels and Methylenetetrahydrofolate Reductase Variants in Childhood Asthma and Eczema. Allergy 2013, 68, 788–795. [Google Scholar] [CrossRef]
- Shorey-Kendrick, L.E.; McEvoy, C.T.; Ferguson, B.; Burchard, J.; Park, B.S.; Gao, L.; Vuylsteke, B.H.; Milner, K.F.; Morris, C.D.; Spindel, E.R. Vitamin C Prevents Offspring DNA Methylation Changes Associated with Maternal Smoking in Pregnancy. Am. J. Respir. Crit. Care Med. 2017, 196, 745–755. [Google Scholar] [CrossRef]
- Yieh, L.; McEvoy, C.T.; Hoffman, S.W.; Caughey, A.B.; MacDonald, K.D.; Dukhovny, D. Cost Effectiveness of Vitamin c Supplementation for Pregnant Smokers to Improve Offspring Lung Function at Birth and Reduce Childhood Wheeze/Asthma. J. Perinatol. 2018, 38, 820–827. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, C.T.; Shorey-Kendrick, L.E.; Milner, K.; Schilling, D.; Tiller, C.; Vuylsteke, B.; Scherman, A.; Jackson, K.; Haas, D.M.; Harris, J.; et al. Oral Vitamin C (500 Mg/d) to Pregnant Smokers Improves Infant Airway Function at 3 Months (VCSIP). A Randomized Trial. Am. J. Respir. Crit. Care Med. 2019, 199, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, C.T.; Shorey-Kendrick, L.E.; Milner, K.; Schilling, D.; Tiller, C.; Vuylsteke, B.; Scherman, A.; Jackson, K.; Haas, D.M.; Harris, J.; et al. Vitamin C to Pregnant Smokers Persistently Improves Infant Airway Function to 12 Months of Age: A Randomised Trial. Eur. Respir. J. 2020, 56, 1902208. [Google Scholar] [CrossRef]
- McEvoy, C.T.; Shorey-Kendrick, L.E.; Milner, K.; Harris, J.; Vuylsteke, B.; Cunningham, M.; Tiller, C.; Stewart, J.; Schilling, D.; Brownsberger, J.; et al. Effect of Vitamin C Supplementation for Pregnant Smokers on Offspring Airway Function and Wheeze at Age 5 Years: Follow-up of a Randomized Clinical Trial. JAMA Pediatr. 2023, 177, 16–24. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, C.T.; Milner, K.F.; Scherman, A.J.; Schilling, D.G.; Tiller, C.J.; Vuylsteke, B.; Shorey-Kendrick, L.E.; Spindel, E.R.; Schuff, R.; Mitchell, J.; et al. Vitamin C to Decrease the Effects of Smoking in Pregnancy on Infant Lung Function (VCSIP): Rationale, Design, and Methods of a Randomized, Controlled Trial of Vitamin C Supplementation in Pregnancy for the Primary Prevention of Effects of in Utero Tobacco Smoke Exposure on Infant Lung Function and Respiratory Health. Contemp. Clin. Trials 2017, 58, 66–77. [Google Scholar] [CrossRef]
- Allan, K.M.; Prabhu, N.; Craig, L.C.A.; McNeill, G.; Kirby, B.; McLay, J.; Helms, P.J.; Ayres, J.G.; Seaton, A.; Turner, S.W.; et al. Maternal Vitamin D and E Intakes during Pregnancy Are Associated with Asthma in Children. Eur. Respir. J. 2015, 45, 1027–1036. [Google Scholar] [CrossRef]
- Devereux, G. Maternal Diet during Pregnancy: An Emerging Risk Factor for Childhood Asthma. Expert Rev. Clin. Immunol. 2008, 4, 663–668. [Google Scholar] [CrossRef]
- Devereux, G.; Litonjua, A.A.; Turner, S.W.; Craig, L.C.A.; McNeill, G.; Martindale, S.; Helms, P.J.; Seaton, A.; Weiss, S.T. Maternal Vitamin D Intake during Pregnancy and Early Childhood Wheezing. Am. J. Clin. Nutr. 2007, 85, 853–859. [Google Scholar] [CrossRef]
- Drozdenko, G.; Heine, G.; Worm, M. Oral Vitamin D Increases the Frequencies of CD38 + Human B Cells and Ameliorates IL-17-Producing T Cells. Exp. Dermatol. 2014, 23, 107–112. [Google Scholar] [CrossRef]
- Erkkola, M.; Kaila, M.; Nwaru, B.I.; Kronberg-Kippilä, C.; Ahonen, S.; Nevalainen, J.; Veijola, R.; Pekkanen, J.; Ilonen, J.; Simell, O.; et al. Maternal Vitamin D Intake during Pregnancy Is Inversely Associated with Asthma and Allergic Rhinitis in 5-Year-Old Children. Clin. Exp. Allergy 2009, 39, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Hollis, B.W.; Wagner, C.L. Vitamin D Supplementation during Pregnancy: Improvements in Birth Outcomes and Complications through Direct Genomic Alteration. Mol. Cell. Endocrinol. 2017, 453, 113–130. [Google Scholar] [CrossRef]
- Zosky, G.R.; Hart, P.H.; Whitehouse, A.J.O.; Kusel, M.M.; Ang, W.; Foong, R.E.; Chen, L.; Holt, P.G.; Sly, P.D.; Hall, G.L. Vitamin D Deficiency at 16 to 20 Weeks’ Gestation Is Associated with Impaired Lung Function and Asthma at 6 Years of Age. Ann. Am. Thorac. Soc. 2014, 11, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Beckhaus, A.A.; Garcia-Marcos, L.; Forno, E.; Pacheco-Gonzalez, R.M.; Celedón, J.C.; Castro-Rodriguez, J.A. Maternal Nutrition during Pregnancy and Risk of Asthma, Wheeze, and Atopic Diseases during Childhood: A Systematic Review and Meta-Analysis. Allergy 2015, 70, 1588–1604. [Google Scholar] [CrossRef] [PubMed]
- Camargo, C.A.; Rifas-Shiman, S.L.; Litonjua, A.A.; Rich-Edwards, J.W.; Weiss, S.T.; Gold, D.R.; Kleinman, K.; Gillman, M.W. Maternal Intake of Vitamin D during Pregnancy and Risk of Recurrent Wheeze in Children at 3 y of Age. Am. J. Clin. Nutr. 2007, 85, 788–795. [Google Scholar] [CrossRef]
- Litonjua, A.A. Fat-Soluble Vitamins and Atopic Disease: What Is the Evidence? Proc. Nutr. Soc. 2012, 71, 67–74. [Google Scholar] [CrossRef]
- Litonjua, A.A.; Weiss, S.T. Is Vitamin D Deficiency to Blame for the Asthma Epidemic? J. Allergy Clin. Immunol. 2007, 120, 1031–1035. [Google Scholar] [CrossRef]
- Wolsk, H.M.; Harshfield, B.J.; Laranjo, N.; Carey, V.J.; O’Connor, G.; Sandel, M.; Strunk, R.C.; Bacharier, L.B.; Zeiger, R.S.; Schatz, M.; et al. Vitamin D Supplementation in Pregnancy, Prenatal 25(OH)D Levels, Race, and Subsequent Asthma or Recurrent Wheeze in Offspring: Secondary Analyses from the Vitamin D Antenatal Asthma Reduction Trial. J. Allergy Clin. Immunol. 2017, 140, 1423–1429.e5. [Google Scholar] [CrossRef]
- Akhtar, E.; Mily, A.; Haq, A.; Al-Mahmud, A.; El-Arifeen, S.; Hel Baqui, A.; Roth, D.E.; Raqib, R. Prenatal High-Dose Vitamin D3 Supplementation Has Balanced Effects on Cord Blood Th1 and Th2 Responses. Nutr. J. 2016, 15, 75. [Google Scholar] [CrossRef]
- Miller, D.R.; Turner, S.W.; Spiteri-Cornish, D.; Scaife, A.R.; Danielian, P.J.; Devereux, G.S.; Walsh, G.M. Maternal Vitamin D and E Intakes during Early Pregnancy Are Associated with Airway Epithelial Cell Responses in Neonates. Clin. Exp. Allergy 2015, 45, 920–927. [Google Scholar] [CrossRef]
- Foong, R.E.; Bosco, A.; Jones, A.C.; Gout, A.; Gorman, S.; Hart, P.H.; Zosky, G.R. The Effects of In Utero Vitamin D Deficiency on Airway Smooth Muscle Mass and Lung Function. Am. J. Respir. Cell Mol. Biol. 2015, 53, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Lykkedegn, S.; Sorensen, G.L.; Beck-Nielsen, S.S.; Christesen, H.T. The Impact of Vitamin D on Fetal and Neonatal Lung Maturation. A Systematic Review. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2015, 308, L587–L602. [Google Scholar] [CrossRef] [PubMed]
- Chawes, B.L.; Bønnelykke, K.; Stokholm, J.; Vissing, N.H.; Bjarnadóttir, E.; Schoos, A.-M.M.; Wolsk, H.M.; Pedersen, T.M.; Vinding, R.K.; Thorsteinsdóttir, S.; et al. Effect of Vitamin D3 Supplementation During Pregnancy on Risk of Persistent Wheeze in the Offspring: A Randomized Clinical Trial. JAMA 2016, 315, 353–361. [Google Scholar] [CrossRef]
- Hansen, S.; Maslova, E.; Strøm, M.; Linneberg, A.; Halldorsson, T.I.; Granström, C.; Dahl, R.; Hoffmann, H.J.; Olsen, S.F. The Long-Term Programming Effect of Maternal 25-Hydroxyvitamin D in Pregnancy on Allergic Airway Disease and Lung Function in Offspring after 20 to 25 Years of Follow-Up. J. Allergy Clin. Immunol. 2015, 136, 169–176.e2. [Google Scholar] [CrossRef] [PubMed]
- Morales, E.; Romieu, I.; Guerra, S.; Ballester, F.; Rebagliato, M.; Vioque, J.; Tardón, A.; Rodriguez Delhi, C.; Arranz, L.; Torrent, M.; et al. Maternal Vitamin D Status in Pregnancy and Risk of Lower Respiratory Tract Infections, Wheezing, and Asthma in Offspring. Epidemiology 2012, 23, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Pike, K.C.; Inskip, H.M.; Robinson, S.; Lucas, J.S.; Cooper, C.; Harvey, N.C.; Godfrey, K.M.; Roberts, G.; the Southampton Women’s Survey Study Group. Maternal Late-Pregnancy Serum 25-Hydroxyvitamin D in Relation to Childhood Wheeze and Atopic Outcomes. Thorax 2012, 67, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Rothers, J.; Wright, A.L.; Stern, D.A.; Halonen, M.; Camargo, C.A. Cord Blood 25-Hydroxyvitamin D Levels Are Associated with Aeroallergen Sensitization in Children from Tucson, Arizona. J. Allergy Clin. Immunol. 2011, 128, 1093–1099.e5. [Google Scholar] [CrossRef]
- Stone, C.A.; Cook-Mills, J.; Gebretsadik, T.; Rosas-Salazar, C.; Turi, K.; Brunwasser, S.M.; Connolly, A.; Russell, P.; Liu, Z.; Costello, K.; et al. Delineation of the Individual Effects of Vitamin E Isoforms on Early Life Incident Wheezing. J. Pediatr. 2019, 206, 156–163.e3. [Google Scholar] [CrossRef]
- Devereux, G.; Turner, S.W.; Craig, L.C.A.; McNeill, G.; Martindale, S.; Harbour, P.J.; Helms, P.J.; Seaton, A. Low Maternal Vitamin E Intake during Pregnancy Is Associated with Asthma in 5-Year-Old Children. Am. J. Respir. Crit. Care Med. 2006, 174, 499–507. [Google Scholar] [CrossRef]
- Devereux, G. Early Life Events in Asthma—Diet. Pediatr. Pulmonol. 2007, 42, 663–673. [Google Scholar] [CrossRef]
- Litonjua, A.A.; Rifas-Shiman, S.L.; Ly, N.P.; Tantisira, K.G.; Rich-Edwards, J.W.; Camargo, C.A.; Weiss, S.T.; Gillman, M.W.; Gold, D.R. Maternal Antioxidant Intake in Pregnancy and Wheezing Illnesses in Children at 2 y of Age. Am. J. Clin. Nutr. 2006, 84, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Martindale, S.; McNeill, G.; Devereux, G.; Campbell, D.; Russell, G.; Seaton, A. Antioxidant Intake in Pregnancy in Relation to Wheeze and Eczema in the First Two Years of Life. Am. J. Respir. Crit. Care Med. 2005, 171, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Greenough, A.; Shaheen, S.O.; Shennan, A.; Seed, P.T.; Poston, L. Respiratory Outcomes in Early Childhood Following Antenatal Vitamin C and E Supplementation. Thorax 2010, 65, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- Abdala-Valencia, H.; Soveg, F.; Cook-Mills, J.M. γ-Tocopherol Supplementation of Allergic Female Mice Augments Development of CD11c + CD11b + Dendritic Cells in Utero and Allergic Inflammation in Neonates. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2016, 310, L759–L771. [Google Scholar] [CrossRef] [PubMed]
- Abdala-Valencia, H.; Berdnikovs, S.; Soveg, F.W.; Cook-Mills, J.M. α-Tocopherol Supplementation of Allergic Female Mice Inhibits Development of CD11c+CD11b+ Dendritic Cells in Utero and Allergic Inflammation in Neonates. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 307, L482–L496. [Google Scholar] [CrossRef]
- Gao, Y.; Nanan, R.; Macia, L.; Tan, J.; Sominsky, L.; Quinn, T.P.; O’Hely, M.; Ponsonby, A.-L.; Tang, M.L.K.; Collier, F.; et al. The Maternal Gut Microbiome during Pregnancy and Offspring Allergy and Asthma. J. Allergy Clin. Immunol. 2021, 148, 669–678. [Google Scholar] [CrossRef]
- Gray, L.E.K.; O’Hely, M.; Ranganathan, S.; Sly, P.D.; Vuillermin, P. The Maternal Diet, Gut Bacteria, and Bacterial Metabolites during Pregnancy Influence Offspring Asthma. Front. Immunol. 2017, 8, 365. [Google Scholar] [CrossRef]
- Alsharairi, N.A. The Infant Gut Microbiota and Risk of Asthma: The Effect of Maternal Nutrition during Pregnancy and Lactation. Microorganisms 2020, 8, 1119. [Google Scholar] [CrossRef]
- Arrieta, M.-C.; Stiemsma, L.T.; Dimitriu, P.A.; Thorson, L.; Russell, S.; Yurist-Doutsch, S.; Kuzeljevic, B.; Gold, M.J.; Britton, H.M.; Lefebvre, D.L.; et al. Early Infancy Microbial and Metabolic Alterations Affect Risk of Childhood Asthma. Sci. Transl. Med. 2015, 7, 307ra152. [Google Scholar] [CrossRef]
- Padilha, M.; Danneskiold-Samsøe, N.B.; Brejnrod, A.; Hoffmann, C.; Cabral, V.P.; Iaucci, J.D.M.; Sales, C.H.; Fisberg, R.M.; Cortez, R.V.; Brix, S.; et al. The Human Milk Microbiota Is Modulated by Maternal Diet. Microorganisms 2019, 7, 502. [Google Scholar] [CrossRef]
- Borak, J.; Lefkowitz, R.Y. Bronchial Hyperresponsiveness. Occup. Med. 2016, 66, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Sears, M.R. Lung Function Decline in Asthma. Eur. Respir. J. 2007, 30, 411–413. [Google Scholar] [CrossRef]
- Manni, M.L.; Trudeau, J.B.; Scheller, E.V.; Mandalapu, S.; Elloso, M.M.; Kolls, J.K.; Wenzel, S.E.; Alcorn, J.F. The Complex Relationship between Inflammation and Lung Function in Severe Asthma. Mucosal Immunol. 2014, 7, 1186–1198. [Google Scholar] [CrossRef] [PubMed]
- Berend, N.; Salome, C.M.; King, G.G. Mechanisms of Airway Hyperresponsiveness in Asthma. Respirology 2008, 13, 624–631. [Google Scholar] [CrossRef] [PubMed]
- James, A.L.; Palmer, L.J.; Kicic, E.; Maxwell, P.S.; Lagan, S.E.; Ryan, G.F.; Musk, A.W. Decline in Lung Function in the Busselton Health Study: The Effects of Asthma and Cigarette Smoking. Am. J. Respir. Crit. Care Med. 2005, 171, 109–114. [Google Scholar] [CrossRef]
- Dijkstra, A. Lung Function Decline in Asthma: Association with Inhaled Corticosteroids, Smoking and Sex. Thorax 2006, 61, 105–110. [Google Scholar] [CrossRef]
- Psarras, S.; Caramori, G.; Contoli, M.; Papadopoulos, N.; Papi, A. Oxidants in Asthma and in Chronic Obstructive Pulmonary Disease (COPD). Curr. Pharm. Des. 2005, 11, 2053–2062. [Google Scholar] [CrossRef]
- King, M.R.; Ismail, A.S.; Davis, L.S.; Karp, D.R. Oxidative Stress Promotes Polarization of Human T Cell Differentiation toward a T Helper 2 Phenotype. J. Immunol. 2006, 176, 2765–2772. [Google Scholar] [CrossRef]
- Park, C.-S.; Kim, T.-B.; Lee, K.-Y.; Moon, K.-A.; Bae, Y.-J.; Jang, M.K.; Cho, Y.S.; Moon, H.-B. Increased Oxidative Stress in the Airway and Development of Allergic Inflammation in a Mouse Model of Asthma. Ann. Allergy Asthma Immunol. Off. Publ. Am. Coll. Allergy Asthma Immunol. 2009, 103, 238–247. [Google Scholar] [CrossRef]
- Riedl, M.A.; Nel, A.E. Importance of Oxidative Stress in the Pathogenesis and Treatment of Asthma. Curr. Opin. Allergy Clin. Immunol. 2008, 8, 49–56. [Google Scholar] [CrossRef]
- Peters, M.C.; Nguyen, M.-L.T.; Dunican, E.M. Biomarkers of Airway Type-2 Inflammation and Integrating Complex Phenotypes to Endotypes in Asthma. Curr. Allergy Asthma Rep. 2016, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Lambrecht, B.N.; Hammad, H.; Fahy, J.V. The Cytokines of Asthma. Immunity 2019, 50, 975–991. [Google Scholar] [CrossRef] [PubMed]
- Frey, A.; Lunding, L.P.; Ehlers, J.C.; Weckmann, M.; Zissler, U.M.; Wegmann, M. More Than Just a Barrier: The Immune Functions of the Airway Epithelium in Asthma Pathogenesis. Front. Immunol. 2020, 11, 761. [Google Scholar] [CrossRef] [PubMed]
- Morcillo, E.J.; Cortijo, J. Mucus and MUC in Asthma. Curr. Opin. Pulm. Med. 2006, 12, 1–6. [Google Scholar] [CrossRef]
- Shen, Y.; Huang, S.; Kang, J.; Lin, J.; Lai, K.; Sun, Y.; Xiao, W.; Yang, L.; Yao, W.; Cai, S.; et al. Management of Airway Mucus Hypersecretion in Chronic Airway Inflammatory Disease: Chinese Expert Consensus (English Edition). Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 399–407. [Google Scholar] [CrossRef]
- Camoretti-Mercado, B.; Lockey, R.F. Airway Smooth Muscle Pathophysiology in Asthma. J. Allergy Clin. Immunol. 2021, 147, 1983–1995. [Google Scholar] [CrossRef]
- Fang, L.; Sun, Q.; Roth, M. Immunologic and Non-Immunologic Mechanisms Leading to Airway Remodeling in Asthma. Int. J. Mol. Sci. 2020, 21, 757. [Google Scholar] [CrossRef]
- Sferrazza Papa, G.F.; Pellegrino, G.M.; Pellegrino, R. Asthma and Respiratory Physiology: Putting Lung Function into Perspective. Respirol. Carlton Vic 2014, 19, 960–969. [Google Scholar] [CrossRef]
- Boulet, L.-P. Airway Remodeling in Asthma: Update on Mechanisms and Therapeutic Approaches. Curr. Opin. Pulm. Med. 2018, 24, 56–62. [Google Scholar] [CrossRef]
- Luo, Z.-X.; Liu, E.-M.; Luo, J.; Li, F.-R.; Li, S.-B.; Zeng, F.-Q.; Qu, P.; Fu, Z.; Li, T.-Y. Vitamin A Deficiency and Wheezing. World J. Pediatr. 2010, 6, 81–84. [Google Scholar] [CrossRef]
- Al Senaidy, A.M. Serum Vitamin A and β-Carotene Levels in Children with Asthma. J. Asthma 2009, 46, 699–702. [Google Scholar] [CrossRef] [PubMed]
- Andino, D.; Moy, J.; Gaynes, B.I. Serum Vitamin A, Zinc and Visual Function in Children with Moderate to Severe Persistent Asthma. J. Asthma 2019, 56, 1198–1203. [Google Scholar] [CrossRef] [PubMed]
- Arora, P.; Kumar, V.; Batra, S. Vitamin A Status in Children with Asthma: Vitamin A Status in Asthmatic Children. Pediatr. Allergy Immunol. 2002, 13, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.-J.; Dai, R.-J. Serum Levels of Vitamin A and 25-Hydroxyvitamin D3 (25OHD3) as Reflectors of Pulmonary Function and Quality of Life (QOL) in Children with Stable Asthma: A Case–Control Study. Medicine 2018, 97, e9830. [Google Scholar] [CrossRef]
- Fabian, E.; Pölöskey, P.; Kósa, L.; Elmadfa, I.; Réthy, L.A. Nutritional Supplements and Plasma Antioxidants in Childhood Asthma. Wien. Klin. Wochenschr. 2013, 125, 309–315. [Google Scholar] [CrossRef]
- Riccioni, G.; Bucciarelli, T.; Mancini, B.; Ilio, C.D.; Vecchia, R.D.; D’Orazio, N. Plasma Lycopene and Antioxidant Vitamins in Asthma: The PLAVA Study. J. Asthma 2007, 44, 429–432. [Google Scholar] [CrossRef]
- Sackesen, C.; Ercan, H.; Dizdar, E.; Soyer, O.; Gumus, P.; Tosun, B.N.; Büyüktuncer, Z.; Karabulut, E.; Besler, T.; Kalayci, O. A Comprehensive Evaluation of the Enzymatic and Nonenzymatic Antioxidant Systems in Childhood Asthma. J. Allergy Clin. Immunol. 2008, 122, 78–85. [Google Scholar] [CrossRef]
- Kassaye, T.; Becklake, M.R.; Receveur, O.; Hanley, J.A.; Johns, T. Association between Vitamin A Status and Lung Function Level in Children Aged 6–9 Years in Wukro Wereda, Northern Ethiopia. Int. J. Epidemiol. 2001, 30, 457–464. [Google Scholar] [CrossRef]
- Marquez, H.A.; Cardoso, W.V. Vitamin A-Retinoid Signaling in Pulmonary Development and Disease. Mol. Cell. Pediatr. 2016, 3, 28. [Google Scholar] [CrossRef]
- Ochs-Balcom, H.M.; Grant, B.J.B.; Muti, P.; Sempos, C.T.; Freudenheim, J.L.; Browne, R.W.; McCann, S.E.; Trevisan, M.; Cassano, P.A.; Iacoviello, L.; et al. Antioxidants, Oxidative Stress, and Pulmonary Function in Individuals Diagnosed with Asthma or COPD. Eur. J. Clin. Nutr. 2006, 60, 991–999. [Google Scholar] [CrossRef]
- Rodrigues, L.C.; Mota, J.F.; Corgosinho, F.C.; Masquio, D.C.L.; Dâmaso, A.R.; Tufik, S.; de Mello, M.T.; Cheik, N.C.; da Silva Agostinho, P.L. Nutrient Intake Is a Predictor of Lung Function in Obese Asthmatic Adolescents Undergoing Interdisciplinary Therapy. Br. J. Nutr. 2019, 122, 974–985. [Google Scholar] [CrossRef] [PubMed]
- Talaei, M.; Hughes, D.A.; Mahmoud, O.; Emmett, P.M.; Granell, R.; Guerra, S.; Shaheen, S.O. Dietary Intake of Vitamin A, Lung Function and Incident Asthma in Childhood. Eur. Respir. J. 2021, 58, 2004407. [Google Scholar] [CrossRef] [PubMed]
- Tobias, T.A.M.; Wood, L.G.; Rastogi, D. Carotenoids, Fatty Acids and Disease Burden in Obese Minority Adolescents with Asthma. Clin. Exp. Allergy 2019, 49, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.A.; de Souza, F.I.S.; Melges, A.P.B.; Fonseca, F.A.; Solé, D.; Sarni, R.O.S. Retinol, Beta-Carotene, Oxidative Stress, and Metabolic Syndrome Components in Obese Asthmatic Children. Pediatr. Allergy Immunol. 2014, 25, 292–294. [Google Scholar] [CrossRef]
- Bantulà, M.; Roca-Ferrer, J.; Arismendi, E.; Picado, C. Asthma and Obesity: Two Diseases on the Rise and Bridged by Inflammation. J. Clin. Med. 2021, 10, 169. [Google Scholar] [CrossRef] [PubMed]
- Schock, B.C.; Young, I.S.; Brown, V.; Fitch, P.S.; Shields, M.D.; Ennis, M. Antioxidants and Oxidative Stress in BAL Fluid of Atopic Asthmatic Children. Pediatr. Res. 2003, 53, 375–381. [Google Scholar] [CrossRef]
- Laerum, B.N.; Wentzel-Larsen, T.; Gulsvik, A.; Omenaas, E.; Gíslason, T.; Janson, C.; Svanes, C. Relationship of Fish and Cod Oil Intake with Adult Asthma. Clin. Exp. Allergy 2007, 37, 1616–1623. [Google Scholar] [CrossRef]
- Rerksuppaphol, S.; Rerksuppaphol, L. Carotenoids Intake and Asthma Prevalence in Thai Children. Pediatr. Rep. 2012, 4, e12. [Google Scholar] [CrossRef]
- Wood, L.G.; Garg, M.L.; Blake, R.J.; Garcia-Caraballo, S.; Gibson, P.G. Airway and Circulating Levels of Carotenoids in Asthma and Healthy Controls. J. Am. Coll. Nutr. 2005, 24, 448–455. [Google Scholar] [CrossRef]
- Babu, T.A.; Sharmila, V. Vitamin A Supplementation in Late Pregnancy Can Decrease the Incidence of Bronchopulmonary Dysplasia in Newborns. J. Matern. Fetal Neonatal Med. 2010, 23, 1468–1469. [Google Scholar] [CrossRef]
- Chen, F.; Marquez, H.; Kim, Y.-K.; Qian, J.; Shao, F.; Fine, A.; Cruikshank, W.W.; Quadro, L.; Cardoso, W.V. Prenatal Retinoid Deficiency Leads to Airway Hyperresponsiveness in Adult Mice. J. Clin. Investig. 2014, 124, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Spears, K.; Cheney, C.; Zerzan, J. Low Plasma Retinol Concentrations Increase the Risk of Developing Bronchopulmonary Dysplasia and Long-Term Respiratory Disability in Very-Low-Birth-Weight Infants. Am. J. Clin. Nutr. 2004, 80, 1589–1594. [Google Scholar] [CrossRef] [PubMed]
- McGowan, S.E.; Smith, J.; Holmes, A.J.; Smith, L.A.; Businga, T.R.; Madsen, M.T.; Kopp, U.C.; Kline, J.N. Vitamin A Deficiency Promotes Bronchial Hyperreactivity in Rats by Altering Muscarinic M 2 Receptor Function. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2002, 282, L1031–L1039. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Shao, F.; Hinds, A.; Yao, S.; Ram-Mohan, S.; Norman, T.A.; Krishnan, R.; Fine, A. Retinoic Acid Signaling Is Essential for Airway Smooth Muscle Homeostasis. JCI Insight 2018, 3, e120398. [Google Scholar] [CrossRef]
- Cui, W.; Zhang, P.; Gu, J.; Tian, Y.; Gao, X.; Liu, Y.; Jin, Z.; Yan, D.; Zhu, X.; Li, D. Vitamin A Deficiency Promotes Inflammation by Induction of Type 2 Cytokines in Experimental Ovalbumin-Induced Asthma Murine Model. Inflammation 2016, 39, 1798–1804. [Google Scholar] [CrossRef]
- Fujii, U.; Miyahara, N.; Taniguchi, A.; Oda, N.; Morichika, D.; Murakami, E.; Nakayama, H.; Waseda, K.; Kataoka, M.; Kakuta, H.; et al. Effect of a Retinoid X Receptor Partial Agonist on Airway Inflammation and Hyperresponsiveness in a Murine Model of Asthma. Respir. Res. 2017, 18, 23. [Google Scholar] [CrossRef]
- Denburg, J.A.; Sehmi, R.; Upham, J. Regulation of IL-5 Receptor on Eosinophil Progenitors in Allergic Inflammation: Role of Retinoic Acid. Int. Arch. Allergy Immunol. 2001, 124, 246–248. [Google Scholar] [CrossRef]
- Spiegl, N.; Didichenko, S.; McCaffery, P.; Langen, H.; Dahinden, C.A. Human Basophils Activated by Mast Cell–Derived IL-3 Express Retinaldehyde Dehydrogenase-II and Produce the Immunoregulatory Mediator Retinoic Acid. Blood 2008, 112, 3762–3771. [Google Scholar] [CrossRef]
- Kanagaratham, C.; Kalivodová, A.; Najdekr, L.; Friedecký, D.; Adam, T.; Hajduch, M.; De Sanctis, J.B.; Radzioch, D. Fenretinide Prevents Inflammation and Airway Hyperresponsiveness in a Mouse Model of Allergic Asthma. Am. J. Respir. Cell Mol. Biol. 2014, 51, 783–792. [Google Scholar] [CrossRef]
- Sakamoto, H.; Koya, T.; Tsukioka, K.; Shima, K.; Watanabe, S.; Kagamu, H.; Kimura, Y.; Sakagami, T.; Hasegawa, T.; Suzuki, E.; et al. The Effects of All-Trans Retinoic Acid on the Induction of Oral Tolerance in a Murine Model of Bronchial Asthma. Int. Arch. Allergy Immunol. 2015, 167, 167–176. [Google Scholar] [CrossRef]
- Schuster, G.U.; Kenyon, N.J.; Stephensen, C.B. Vitamin A Deficiency Decreases and High Dietary Vitamin A Increases Disease Severity in the Mouse Model of Asthma. J. Immunol. 2008, 180, 1834–1842. [Google Scholar] [CrossRef] [PubMed]
- Schünemann, H.J.; Grant, B.J.B.; Freudenheim, J.L.; Muti, P.; Browne, R.W.; Drake, J.A.; Klocke, R.A.; Trevisan, M. The Relation of Serum Levels of Antioxidant Vitamins C and E, Retinol and Carotenoids with Pulmonary Function in the General Population. Am. J. Respir. Crit. Care Med. 2001, 163, 1246–1255. [Google Scholar] [CrossRef] [PubMed]
- Bishopp, A.; Sathyamurthy, R.; Manney, S.; Webbster, C.; Krishna, M.T.; Mansur, A.H. Biomarkers of Oxidative Stress and Antioxidants in Severe Asthma. Ann. Allergy. Asthma Immunol. 2017, 118, 445–451. [Google Scholar] [CrossRef] [PubMed]
- de Bittencourt Pasquali, M.A.; Schnorr, C.E.; Feistauer, L.B.H.; Gelain, D.P.; Moreira, J.C.F. Vitamin A Supplementation to Pregnant and Breastfeeding Female Rats Induces Oxidative Stress in the Neonatal Lung. Reprod. Toxicol. 2010, 30, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Sommer, A.; Vyas, K.S. A Global Clinical View on Vitamin A and Carotenoids. Am. J. Clin. Nutr. 2012, 96, 1204S–1206S. [Google Scholar] [CrossRef]
- Neuman, I.; Nahum, H.; Ben-Amotz, A. Reduction of Exercise-Induced Asthma Oxidative Stress by Lycopene, a Natural Antioxidant. Allergy 2000, 55, 1184–1189. [Google Scholar] [CrossRef]
- Wood, L.G.; Gibson, P.G. Reduced Circulating Antioxidant Defences Are Associated with Airway Hyper-Responsiveness, Poor Control and Severe Disease Pattern in Asthma. Br. J. Nutr. 2010, 103, 735–741. [Google Scholar] [CrossRef]
- Heine, G.; Hollstein, T.; Treptow, S.; Radbruch, A.; Worm, M. 9-Cis Retinoic Acid Modulates the Type I Allergic Immune Response. J. Allergy Clin. Immunol. 2018, 141, 650–658.e5. [Google Scholar] [CrossRef]
- Son, H.-L.; Park, H.-R.; Park, Y.-J.; Kim, S.-W. Effect of Retinoic Acid in a Mouse Model of Allergic Rhinitis. Allergy Asthma Immunol. Res. 2015, 7, 590. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, Y.; Liu, Q.; Zhong, W.; Xia, Z. All-Trans Retinoic Acid Attenuates Airway Inflammation by Inhibiting Th2 and Th17 Response in Experimental Allergic Asthma. BMC Immunol. 2013, 14, 28. [Google Scholar] [CrossRef]
- Maruya, M.; Suzuki, K.; Fujimoto, H.; Miyajima, M.; Kanagawa, O.; Wakayama, T.; Fagarasan, S. Vitamin A-Dependent Transcriptional Activation of the Nuclear Factor of Activated T Cells C1 (NFATc1) Is Critical for the Development and Survival of B1 Cells. Proc. Natl. Acad. Sci. USA 2011, 108, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Tian, Q.; Wu, Y.; Peng, X.; Chen, Y.; Li, Q.; Zhang, G.; Tian, X.; Ren, L.; Luo, Z. Vitamin A Supplement after Neonatal Streptococcus Pneumoniae Pneumonia Inhibits the Progression of Experimental Asthma by Altering CD4+T Cell Subsets. Sci. Rep. 2020, 10, 4214. [Google Scholar] [CrossRef] [PubMed]
- Takamura, K.; Nasuhara, Y.; Kobayashi, M.; Betsuyaku, T.; Tanino, Y.; Kinoshita, I.; Yamaguchi, E.; Matsukura, S.; Schleimer, R.P.; Nishimura, M. Retinoic Acid Inhibits Interleukin-4-Induced Eotaxin Production in a Human Bronchial Epithelial Cell Line. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2004, 286, L777–L785. [Google Scholar] [CrossRef]
- Fang, H.; Jin, H.; Wang, H. Effect of All-Trans Retinoic Acid on Airway Inflammation in Asthmatic Rats and Its Mechanism. J. Huazhong Univ. Sci. Technol. Med. Sci. 2004, 24, 229–232. [Google Scholar] [CrossRef]
- Goswami, S.; Angkasekwinai, P.; Shan, M.; Greenlee, K.J.; Barranco, W.T.; Polikepahad, S.; Seryshev, A.; Song, L.; Redding, D.; Singh, B.; et al. Divergent Functions for Airway Epithelial Matrix Metalloproteinase 7 and Retinoic Acid in Experimental Asthma. Nat. Immunol. 2009, 10, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Liu, N.; Liu, J.; Zhang, M.; Ying, L.; Wang, L.; Tian, D.; Dai, J.; Luo, Z.; Liu, E.; et al. Vitamin A Maintains the Airway Epithelium in a Murine Model of Asthma by Suppressing Glucocorticoid-Induced Leucine Zipper. Clin. Exp. Allergy 2016, 46, 848–860. [Google Scholar] [CrossRef]
- Hazlewood, L.C.; Wood, L.G.; Hansbro, P.M.; Foster, P.S. Dietary Lycopene Supplementation Suppresses Th2 Responses and Lung Eosinophilia in a Mouse Model of Allergic Asthma. J. Nutr. Biochem. 2011, 22, 95–100. [Google Scholar] [CrossRef]
- Lee, C.-M.; Chang, J.-H.; Moon, D.-O.; Choi, Y.H.; Choi, I.-W.; Park, Y.-M.; Kim, G.-Y. Lycopene Suppresses Ovalbumin-Induced Airway Inflammation in a Murine Model of Asthma. Biochem. Biophys. Res. Commun. 2008, 374, 248–252. [Google Scholar] [CrossRef]
- Aggarwal, S.; Kim, S.-W.; Cheon, K.; Tabassam, F.H.; Yoon, J.-H.; Koo, J.S. Nonclassical Action of Retinoic Acid on the Activation of the CAMP Response Element-Binding Protein in Normal Human Bronchial Epithelial Cells. Mol. Biol. Cell 2006, 17, 566–575. [Google Scholar] [CrossRef]
- Defnet, A.E.; Shah, S.D.; Huang, W.; Shapiro, P.; Deshpande, D.A.; Kane, M.A. Dysregulated Retinoic Acid Signaling in Airway Smooth Muscle Cells in Asthma. FASEB J. 2021, 35, e22016. [Google Scholar] [CrossRef]
- Day, R.M.; Lee, Y.H.; Park, A.-M.; Suzuki, Y.J. Retinoic Acid Inhibits Airway Smooth Muscle Cell Migration. Am. J. Respir. Cell Mol. Biol. 2006, 34, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Husemoen, L.L.N.; Toft, U.; Fenger, M.; Jørgensen, T.; Johansen, N.; Linneberg, A. The Association between Atopy and Factors Influencing Folate Metabolism: Is Low Folate Status Causally Related to the Development of Atopy? Int. J. Epidemiol. 2006, 35, 954–961. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, J.; Hölscher, B.; Bolte, G.; Winkler, G. Allergic Sensitization and Diet: Ecological Analysis in Selected European Cities. Eur. Respir. J. 2001, 17, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Comhair, S.A.A.; McDunn, J.; Bennett, C.; Fettig, J.; Erzurum, S.C.; Kalhan, S.C. Metabolomic Endotype of Asthma. J. Immunol. 2015, 195, 643–650. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Zhang, L.; Oliver, B.G.; Wang, H.G.; Liu, Z.P.; Chen, Z.H.; Wood, L.; Hsu, A.C.-Y.; Xie, M.; et al. Sputum Metabolomic Profiling Reveals Metabolic Pathways and Signatures Associated With Inflammatory Phenotypes in Patients With Asthma. Allergy Asthma Immunol. Res. 2022, 14, 393–411. [Google Scholar] [CrossRef]
- Delport, R.; Ubbink, J.B.; Serfontein, W.J.; Becker, P.J.; Walters, L. Vitamin B6 Nutritional Status in Asthma: The Effect of Theophylline Therapy on Plasma Pyridoxal-5′-Phosphate and Pyridoxal Levels. Int. J. Vitam. Nutr. Res. Int. Z. Vitam.-Ernahrungsforschung J. Int. Vitaminol. Nutr. 1988, 58, 67–72. [Google Scholar]
- Shimizu, T.; Maeda, S.; Mochizuki, H.; Tokuyama, K.; Morikawa, A. Theophylline Attenuates Circulating Vitamin B6 Levels in Children with Asthma. Pharmacology 1994, 49, 392–397. [Google Scholar] [CrossRef]
- Nicholson, A.; Pollard, S.L.; Lima, J.J.; Romero, K.M.; Tarazona-Meza, C.; Malpartida-Guzmán, G.; Mougey, E.; Hansel, N.N.; Checkley, W. Serum Folate Concentrations, Asthma, Atopy, and Asthma Control in Peruvian Children. Respir. Med. 2017, 133, 29–35. [Google Scholar] [CrossRef]
- Papamichael, M.M.; Katsardis, C.; Tsoukalas, D.; Lambert, K.; Erbas, B.; Itsiopoulos, C. Potential Role of Folate Status on Pulmonary Function in Pediatric Asthma. Nutrition 2021, 90, 111267. [Google Scholar] [CrossRef]
- Farres, M.N.; Shahin, R.Y.; Melek, N.A.; El-Kabarity, R.H.; Arafa, N.A. Study of Folate Status Among Egyptian Asthmatics. Intern. Med. 2011, 50, 205–211. [Google Scholar] [CrossRef]
- Thuesen, B.H.; Husemoen, L.L.N.; Ovesen, L.; Jørgensen, T.; Fenger, M.; Gilderson, G.; Linneberg, A. Atopy, Asthma, and Lung Function in Relation to Folate and Vitamin B12 in Adults: Atopy and Asthma in Relation to Folate and B12. Allergy 2010, 65, 1446–1454. [Google Scholar] [CrossRef] [PubMed]
- Blatter, J.; Brehm, J.M.; Sordillo, J.; Forno, E.; Boutaoui, N.; Acosta-Pérez, E.; Alvarez, M.; Colón-Semidey, A.; Weiss, S.T.; Litonjua, A.A.; et al. Folate Deficiency, Atopy, and Severe Asthma Exacerbations in Puerto Rican Children. Ann. Am. Thorac. Soc. 2016, 13, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Skaaby, T.; Taylor, A.E.; Jacobsen, R.K.; Møllehave, L.T.; Friedrich, N.; Thuesen, B.H.; Shabanzadeh, D.M.; Paternoster, L.; Völker, U.; Nauck, M.; et al. Associations of Genetic Determinants of Serum Vitamin B12 and Folate Concentrations with Hay Fever and Asthma: A Mendelian Randomization Meta-Analysis. Eur. J. Clin. Nutr. 2018, 72, 264–271. [Google Scholar] [CrossRef]
- Han, Y.-Y.; Forno, E.; Rosser, F.; Celedón, J.C. Serum Folate Metabolites, Asthma, and Lung Function in a Nationwide US Study. J. Allergy Clin. Immunol. 2020, 146, 220–222.e8. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.H.; Matsui, W.; Aloe, C.; Peng, R.D.; Diette, G.B.; Breysse, P.N.; Matsui, E.C. Relationships between Folate and Inflammatory Features of Asthma. J. Allergy Clin. Immunol. 2013, 131, 918–920.e6. [Google Scholar] [CrossRef] [PubMed]
- Bekier, E.; Szyc, H.; Czerwińska, U.; Maśliński, C. The Influence of Nicotinamide on the Course of Experimental Bronchial Asthma in Guinea Pig. Agents Actions 1973, 3, 176. [Google Scholar] [CrossRef]
- Wu, C.-H.; Huang, T.-C.; Lin, B.-F. Folate Deficiency Affects Dendritic Cell Function and Subsequent T Helper Cell Differentiation. J. Nutr. Biochem. 2017, 41, 65–72. [Google Scholar] [CrossRef]
- Okupa, A.Y.; Lemanske, R.F.; Jackson, D.J.; Evans, M.D.; Wood, R.A.; Matsui, E.C. Early-Life Folate Levels Are Associated with Incident Allergic Sensitization. J. Allergy Clin. Immunol. 2013, 131, 226–228.e2. [Google Scholar] [CrossRef]
- İscan, B.; Tuzun, F.; Eroglu Filibeli, B.; Cilekar Micili, S.; Ergur, B.U.; Duman, N.; Ozkan, H.; Kumral, A. Effects of Maternal Folic Acid Supplementation on Airway Remodeling and Allergic Airway Disease Development. J. Matern. Fetal Neonatal Med. 2019, 32, 2970–2978. [Google Scholar] [CrossRef]
- Hollingsworth, J.W.; Maruoka, S.; Boon, K.; Garantziotis, S.; Li, Z.; Tomfohr, J.; Bailey, N.; Potts, E.N.; Whitehead, G.; Brass, D.M.; et al. In Utero Supplementation with Methyl Donors Enhances Allergic Airway Disease in Mice. J. Clin. Investig. 2008, 118, 3462–3469. [Google Scholar] [CrossRef]
- Siripornpanich, S.; Chongviriyaphan, N.; Manuyakorn, W.; Matangkasombut, P. Zinc and Vitamin C Deficiencies Associate with Poor Pulmonary Function in Children with Persistent Asthma. Asian Pac. J. Allergy Immunol. 2022, 40, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Harik-Khan, R.I. Serum Vitamin Levels and the Risk of Asthma in Children. Am. J. Epidemiol. 2004, 159, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Mudway, I.S.; Stenfors, N.; Blomberg, A.; Helleday, R.; Dunster, C.; Marklund, S.L.; Frew, A.J.; Sandström, T.; Kelly, F.J. Differences in Basal Airway Antioxidant Concentrations Are Not Predictive of Individual Responsiveness to Ozone: A Comparison of Healthy and Mild Asthmatic Subjects. Free Radic. Biol. Med. 2001, 31, 962–974. [Google Scholar] [CrossRef] [PubMed]
- Allan, K.; Devereux, G. Diet and Asthma: Nutrition Implications from Prevention to Treatment. J. Am. Diet. Assoc. 2011, 111, 258–268. [Google Scholar] [CrossRef]
- Picado, C.; Deulofeu, R.; Lleonart, R.; Agusti, M.; Mullol, J.; Torra, M.; Quinto, L. Dietary Micronutrients/Antioxidants and Their Relationship with Bronchial Asthma Severity. Allergy 2001, 56, 43–49. [Google Scholar] [CrossRef]
- Fogarty, A.; Lewis, S.A.; Scrivener, S.L.; Antoniak, M.; Pacey, S.; Pringle, M.; Britton, J. Corticosteroid Sparing Effects of Vitamin C and Magnesium in Asthma: A Randomised Trial. Respir. Med. 2006, 100, 174–179. [Google Scholar] [CrossRef]
- Fogarty, A.; Lewis, S.A.; Scrivener, S.L.; Antoniak, M.; Pacey, S.; Pringle, M.; Britton, J. Oral Magnesium and Vitamin C Supplements in Asthma: A Parallel Group Randomized Placebo-Controlled Trial: Oral Magnesium and Vitamin C Supplements in Asthma. Clin. Exp. Allergy 2003, 33, 1355–1359. [Google Scholar] [CrossRef]
- Tecklenburg, S.L.; Mickleborough, T.D.; Fly, A.D.; Bai, Y.; Stager, J.M. Ascorbic Acid Supplementation Attenuates Exercise-Induced Bronchoconstriction in Patients with Asthma. Respir. Med. 2007, 101, 1770–1778. [Google Scholar] [CrossRef]
- Jeong, Y.-J.; Kim, J.-H.; Kang, J.S.; Lee, W.J.; Hwang, Y. Mega-Dose Vitamin C Attenuated Lung Inflammation in Mouse Asthma Model. Anat. Cell Biol. 2010, 43, 294. [Google Scholar] [CrossRef]
- Nadi, E.; Tavakoli, F.; Zeraati, F.; Goodarzi, M.T.; Hashemi, S.H. Effect of Vitamin C Administration on Leukocyte Vitamin C Level and Severity of Bronchial Asthma. Acta Med. Iran. 2012, 50, 233–238. [Google Scholar]
- Hemilä, H. Vitamin C May Alleviate Exercise-Induced Bronchoconstriction: A Meta-Analysis. BMJ Open 2013, 3, e002416. [Google Scholar] [CrossRef] [PubMed]
- Hemilä, H. Vitamin C Should Be Tested against Exercise-Induced Bronchoconstriction. Am. J. Respir. Crit. Care Med. 2013, 188, 1370. [Google Scholar] [CrossRef] [PubMed]
- Davison, G.W.; Ashton, T.; Davies, B.; Bailey, D.M. In Vitro Electron Paramagnetic Resonance Characterization of Free Radicals: Relevance to Exercise-Induced Lipid Peroxidation and Implications of Ascorbate Prophylaxis. Free Radic. Res. 2008, 42, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Block, G.; Jensen, C.D.; Dalvi, T.B.; Norkus, E.P.; Hudes, M.; Crawford, P.B.; Holland, N.; Fung, E.B.; Schumacher, L.; Harmatz, P. Vitamin C Treatment Reduces Elevated C-Reactive Protein. Free Radic. Biol. Med. 2009, 46, 70–77. [Google Scholar] [CrossRef]
- Hemilä, H.; Louhiala, P. Vitamin C for Preventing and Treating Pneumonia. Cochrane Database Syst. Rev. 2013, 2013, CD005532. [Google Scholar] [CrossRef]
- Noh, K.; Lim, H.; Moon, S.; Kang, J.S.; Lee, W.J.; Lee, D.; Hwang, Y. Mega-Dose Vitamin C Modulates T Cell Functions in Balb/c Mice Only When Administered during T Cell Activation. Immunol. Lett. 2005, 98, 63–72. [Google Scholar] [CrossRef]
- Kianian, F.; Karimian, S.M.; Kadkhodaee, M.; Takzaree, N.; Seifi, B.; Sadeghipour, H.R. Protective Effects of Ascorbic Acid and Calcitriol Combination on Airway Remodelling in Ovalbumin-Induced Chronic Asthma. Pharm. Biol. 2020, 58, 107–115. [Google Scholar] [CrossRef]
- Ali, A.M.; Selim, S.; Abbassi, M.M.; Sabry, N.A. Effect of Alfacalcidol on the Pulmonary Function of Adult Asthmatic Patients: A Randomized Trial. Ann. Allergy. Asthma Immunol. 2017, 118, 557–563. [Google Scholar] [CrossRef]
- Boonpiyathad, T.; Chantveerawong, T.; Pradubpongsa, P.; Sangasapaviliya, A. Serum Vitamin D Levels and Vitamin D Supplement in Adult Patients with Asthma Exacerbation. J. Allergy 2016, 2016, 4070635. [Google Scholar] [CrossRef]
- Li, F.; Peng, M.; Jiang, L.; Sun, Q.; Zhang, K.; Lian, F.; Litonjua, A.A.; Gao, J.; Gao, X. Vitamin D Deficiency Is Associated with Decreased Lung Function in Chinese Adults with Asthma. Respiration 2011, 81, 469–475. [Google Scholar] [CrossRef]
- Tolppanen, A.-M.; Williams, D.; Henderson, J.; Lawlor, D.A. Serum 25-Hydroxy-Vitamin D and Ionised Calcium in Relation to Lung Function and Allergen Skin Tests. Eur. J. Clin. Nutr. 2011, 65, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Bener, A.; Ehlayel, M.; Bener, H.; Hamid, Q. The Impact of Vitamin D Deficiency on Asthma, Allergic Rhinitis and Wheezing in Children: An Emerging Public Health Problem. J. Fam. Community Med. 2014, 21, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Breysse, P.N.; McCormack, M.C.; Hansel, N.N.; Rusher, R.R.; Matsui, E.; Peng, R.; Curtin-Brosnan, J.; Diette, G.B.; for the Center for Childhood Asthma in the Urban Environment. Outdoor Exposure and Vitamin D Levels in Urban Children with Asthma. Nutr. J. 2013, 12, 81. [Google Scholar] [CrossRef]
- Brehm, J.M.; Acosta-Pérez, E.; Klei, L.; Roeder, K.; Barmada, M.; Boutaoui, N.; Forno, E.; Kelly, R.; Paul, K.; Sylvia, J.; et al. Vitamin D Insufficiency and Severe Asthma Exacerbations in Puerto Rican Children. Am. J. Respir. Crit. Care Med. 2012, 186, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.-Y.; Forno, E.; Celedón, J.C. Vitamin D Insufficiency and Asthma in a US Nationwide Study. J. Allergy Clin. Immunol. Pract. 2017, 5, 790–796.e1. [Google Scholar] [CrossRef] [PubMed]
- Hatami, G.; Ghasemi, K.; Motamed, N.; Firoozbakht, S.; Movahed, A.; Farrokhi, S. Relationship between Vitamin D and Childhood Asthma: A Case–Control Study. Iran J Pediatr 2014, 24, 710–714. [Google Scholar]
- Mirzakhani, H.; Al-Garawi, A.; Weiss, S.T.; Litonjua, A.A. Vitamin D and the Development of Allergic Disease: How Important Is It? Clin. Exp. Allergy 2015, 45, 114–125. [Google Scholar] [CrossRef]
- Samrah, S.; Khatib, I.; Omari, M.; Khassawneh, B.; Momany, S.; Daoud, A.; Malkawi, M.; Khader, Y. Vitamin D Deficiency and Level of Asthma Control in Women from North of Jordan: A Case–Control Study. J. Asthma 2014, 51, 832–838. [Google Scholar] [CrossRef]
- Wang, Q.; Ying, Q.; Zhu, W.; Chen, J. Vitamin D and Asthma Occurrence in Children: A Systematic Review and Meta-Analysis. J. Pediatr. Nurs. 2022, 62, e60–e68. [Google Scholar] [CrossRef]
- Gupta, A.; Sjoukes, A.; Richards, D.; Banya, W.; Hawrylowicz, C.; Bush, A.; Saglani, S. Relationship between Serum Vitamin D, Disease Severity, and Airway Remodeling in Children with Asthma. Am. J. Respir. Crit. Care Med. 2011, 184, 1342–1349. [Google Scholar] [CrossRef]
- Mann, E.H.; Chambers, E.S.; Pfeffer, P.E.; Hawrylowicz, C.M. Immunoregulatory Mechanisms of Vitamin D Relevant to Respiratory Health and Asthma: Immunoregulatory Mechanisms of Vitamin D in Asthma. Ann. N. Y. Acad. Sci. 2014, 1317, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Brehm, J.M.; Schuemann, B.; Fuhlbrigge, A.L.; Hollis, B.W.; Strunk, R.C.; Zeiger, R.S.; Weiss, S.T.; Litonjua, A.A. Serum Vitamin D Levels and Severe Asthma Exacerbations in the Childhood Asthma Management Program Study. J. Allergy Clin. Immunol. 2010, 126, 52–58.e5. [Google Scholar] [CrossRef] [PubMed]
- Forno, E.; Lescher, R.; Strunk, R.; Weiss, S.; Fuhlbrigge, A.; Celedón, J.C. Decreased Response to Inhaled Steroids in Overweight and Obese Asthmatic Children. J. Allergy Clin. Immunol. 2011, 127, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Searing, D.A.; Zhang, Y.; Murphy, J.R.; Hauk, P.J.; Goleva, E.; Leung, D.Y.M. Decreased Serum Vitamin D Levels in Children with Asthma Are Associated with Increased Corticosteroid Use. J. Allergy Clin. Immunol. 2010, 125, 995–1000. [Google Scholar] [CrossRef]
- Sutherland, E.R.; Goleva, E.; Jackson, L.P.; Stevens, A.D.; Leung, D.Y.M. Vitamin D Levels, Lung Function, and Steroid Response in Adult Asthma. Am. J. Respir. Crit. Care Med. 2010, 181, 699–704. [Google Scholar] [CrossRef]
- Wu, A.C.; Tantisira, K.; Li, L.; Fuhlbrigge, A.L.; Weiss, S.T.; Litonjua, A.; for the Childhood Asthma Management Program Research Group. Effect of Vitamin D and Inhaled Corticosteroid Treatment on Lung Function in Children. Am. J. Respir. Crit. Care Med. 2012, 186, 508–513. [Google Scholar] [CrossRef]
- Szentpetery, S.E.; Han, Y.-Y.; Brehm, J.M.; Acosta-Pérez, E.; Forno, E.; Boutaoui, N.; Canino, G.; Alcorn, J.F.; Celedón, J.C. Vitamin D Insufficiency, Plasma Cytokines, and Severe Asthma Exacerbations in School-Aged Children. J. Allergy Clin. Immunol. Pract. 2018, 6, 289–291.e2. [Google Scholar] [CrossRef]
- Turkeli, A.; Ayaz, O.; Uncu, A.; Ozhan, B.; Bas, V.N.; Tufan, A.K.; Yilmaz, O.; Yuksel, H. Effects of Vitamin D Levels on Asthma Control and Severity in Pre-School Children. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 26–36. [Google Scholar]
- Freishtat, R.J.; Iqbal, S.F.; Pillai, D.K.; Klein, C.J.; Ryan, L.M.; Benton, A.S.; Teach, S.J. High Prevalence of Vitamin D Deficiency among Inner-City African American Youth with Asthma in Washington, DC. J. Pediatr. 2010, 156, 948–952. [Google Scholar] [CrossRef]
- Gupta, A.; Bush, A.; Hawrylowicz, C.; Saglani, S. Vitamin D and Asthma in Children. Paediatr. Respir. Rev. 2012, 13, 236–243. [Google Scholar] [CrossRef]
- Majak, P.; Olszowiec-Chlebna, M.; Smejda, K.; Stelmach, I. Vitamin D Supplementation in Children May Prevent Asthma Exacerbation Triggered by Acute Respiratory Infection. J. Allergy Clin. Immunol. 2011, 127, 1294–1296. [Google Scholar] [CrossRef]
- Martineau, A.R.; Cates, C.J.; Urashima, M.; Jensen, M.; Griffiths, A.P.; Nurmatov, U.; Sheikh, A.; Griffiths, C.J. Vitamin D for the Management of Asthma. Cochrane Database Syst. Rev. 2016, 2019, CD011511. [Google Scholar] [CrossRef]
- Hibbs, A.M.; Ross, K.; Kerns, L.A.; Wagner, C.; Fuloria, M.; Groh-Wargo, S.; Zimmerman, T.; Minich, N.; Tatsuoka, C. Effect of Vitamin D Supplementation on Recurrent Wheezing in Black Infants Who Were Born Preterm: The D-Wheeze Randomized Clinical Trial. JAMA 2018, 319, 2086–2094. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.; Schaible, U.E. Macrophage Defense Mechanisms against Intracellular Bacteria. Immunol. Rev. 2015, 264, 182–203. [Google Scholar] [CrossRef]
- Devereux, G.; Wilson, A.; Avenell, A.; McNeill, G.; Fraser, W.D. A Case-Control Study of Vitamin D Status and Asthma in Adults. Allergy 2010, 65, 666–667. [Google Scholar] [CrossRef] [PubMed]
- Mai, X.-M.; Langhammer, A.; Camargo, C.A.; Chen, Y. Serum 25-Hydroxyvitamin D Levels and Incident Asthma in Adults: The HUNT Study. Am. J. Epidemiol. 2012, 176, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Camargo, C.A.; Ingham, T.; Wickens, K.; Thadhani, R.; Silvers, K.M.; Epton, M.J.; Town, G.I.; Pattemore, P.K.; Espinola, J.A.; Crane, J.; et al. Cord-Blood 25-Hydroxyvitamin D Levels and Risk of Respiratory Infection, Wheezing, and Asthma. Pediatrics 2011, 127, e180–e187. [Google Scholar] [CrossRef]
- Cheng, H.M.; Kim, S.; Park, G.-H.; Chang, S.E.; Bang, S.; Won, C.H.; Lee, M.W.; Choi, J.H.; Moon, K.C. Low Vitamin D Levels Are Associated with Atopic Dermatitis, but Not Allergic Rhinitis, Asthma, or IgE Sensitization, in the Adult Korean Population. J. Allergy Clin. Immunol. 2014, 133, 1048–1055. [Google Scholar] [CrossRef]
- Thuesen, B.H.; Skaaby, T.; Husemoen, L.L.N.; Fenger, M.; Jørgensen, T.; Linneberg, A. The Association of Serum 25-OH Vitamin D with Atopy, Asthma, and Lung Function in a Prospective Study of Danish Adults. Clin. Exp. Allergy 2015, 45, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.J.; Hesketh, K.; Power, C.; Hyppönen, E. Vitamin D Status Has a Linear Association with Seasonal Infections and Lung Function in British Adults. Br. J. Nutr. 2011, 106, 1433–1440. [Google Scholar] [CrossRef]
- Rehan, V.K.; Torday, J.S.; Peleg, S.; Gennaro, L.; Vouros, P.; Padbury, J.; Rao, D.S.; Reddy, G.S. 1a,25-Dihydroxy-3-Epi-Vitamin D3, a Natural Metabolite of 1a,25-Dihydroxy Vitamin D3: Production and Biological Activity Studies in Pulmonary Alveolar Type II Cellsq. Mol. Genet. Metab. 2002, 76, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Amrein, K.; Quraishi, S.A.; Litonjua, A.A.; Gibbons, F.K.; Pieber, T.R.; Camargo, C.A.; Giovannucci, E.; Christopher, K.B. Evidence for a U-Shaped Relationship Between Prehospital Vitamin D Status and Mortality: A Cohort Study. J. Clin. Endocrinol. Metab. 2014, 99, 1461–1469. [Google Scholar] [CrossRef] [PubMed]
- Quraishi, S.A.; Bittner, E.A.; Christopher, K.B.; Camargo, C.A. Vitamin D Status and Community-Acquired Pneumonia: Results from the Third National Health and Nutrition Examination Survey. PLoS ONE 2013, 8, e81120. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.S.; Nanji, K. A Review on the Role of Vitamin D in Asthma. Cureus 2017, 9, e1288. [Google Scholar] [CrossRef] [PubMed]
- Papamichael, M.M.; Itsiopoulos, C.; Lambert, K.; Katsardis, C.; Tsoukalas, D.; Erbas, B. Sufficient Vitamin D Status Positively Modified Ventilatory Function in Asthmatic Children Following a Mediterranean Diet Enriched with Fatty Fish Intervention Study. Nutr. Res. 2020, 82, 99–109. [Google Scholar] [CrossRef]
- Hufnagl, K.; Jensen-Jarolim, E. Vitamin A and D in Allergy: From Experimental Animal Models and Cellular Studies to Human Disease. Allergo J. Int. 2018, 27, 72–78. [Google Scholar] [CrossRef]
- Jolliffe, D.A.; Greenberg, L.; Hooper, R.L.; Griffiths, C.J.; Camargo, C.A.; Kerley, C.P.; Jensen, M.E.; Mauger, D.; Stelmach, I.; Urashima, M.; et al. Vitamin D Supplementation to Prevent Asthma Exacerbations: A Systematic Review and Meta-Analysis of Individual Participant Data. Lancet Respir. Med. 2017, 5, 881–890. [Google Scholar] [CrossRef]
- Tachimoto, H.; Mezawa, H.; Segawa, T.; Akiyama, N.; Ida, H.; Urashima, M. Improved Control of Childhood Asthma with Low-Dose, Short-Term Vitamin D Supplementation: A Randomized, Double-Blind, Placebo-Controlled Trial. Allergy 2016, 71, 1001–1009. [Google Scholar] [CrossRef]
- Yadav, M.; Mittal, K. Effect of Vitamin D Supplementation on Moderate to Severe Bronchial Asthma. Indian J. Pediatr. 2014, 81, 650–654. [Google Scholar] [CrossRef]
- Baris, S.; Kiykim, A.; Ozen, A.; Tulunay, A.; Karakoc-Aydiner, E.; Barlan, I.B. Vitamin D as an Adjunct to Subcutaneous Allergen Immunotherapy in Asthmatic Children Sensitized to House Dust Mite. Allergy 2014, 69, 246–253. [Google Scholar] [CrossRef]
- Kerley, C.P.; Hutchinson, K.; Cormican, L.; Faul, J.; Greally, P.; Coghlan, D.; Elnazir, B. Vitamin D3 for Uncontrolled Childhood Asthma: A Pilot Study. Pediatr. Allergy Immunol. 2016, 27, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Puranik, S.; Forno, E.; Bush, A.; Celedón, J.C. Predicting Severe Asthma Exacerbations in Children. Am. J. Respir. Crit. Care Med. 2017, 195, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, T.; Gupta, G.K.; Agrawal, D.K. Vitamin D Supplementation Reduces Airway Hyperresponsiveness and Allergic Airway Inflammation in a Murine Model. Clin. Exp. Allergy 2013, 43, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.D.; Hall, S.C.; Agrawal, D.K. Vitamin D Supplementation Reduces Induction of Epithelial-Mesenchymal Transition in Allergen Sensitized and Challenged Mice. PLoS ONE 2016, 11, e0149180. [Google Scholar] [CrossRef]
- Heine, G.; Tabeling, C.; Hartmann, B.; González Calera, C.R.; Kühl, A.A.; Lindner, J.; Radbruch, A.; Witzenrath, M.; Worm, M. 25-Hydroxvitamin D 3 Promotes the Long-Term Effect of Specific Immunotherapy in a Murine Allergy Model. J. Immunol. 2014, 193, 1017–1023. [Google Scholar] [CrossRef]
- Schedel, M.; Jia, Y.; Michel, S.; Takeda, K.; Domenico, J.; Joetham, A.; Ning, F.; Strand, M.; Han, J.; Wang, M.; et al. 1,25D3 Prevents CD8+Tc2 Skewing and Asthma Development through VDR Binding Changes to the Cyp11a1 Promoter. Nat. Commun. 2016, 7, 10213. [Google Scholar] [CrossRef]
- Lan, N.; Luo, G.; Yang, X.; Cheng, Y.; Zhang, Y.; Wang, X.; Wang, X.; Xie, T.; Li, G.; Liu, Z.; et al. 25-Hydroxyvitamin D3-Deficiency Enhances Oxidative Stress and Corticosteroid Resistance in Severe Asthma Exacerbation. PLoS ONE 2014, 9, e111599. [Google Scholar] [CrossRef]
- Codoñer-Franch, P.; Tavárez-Alonso, S.; Simó-Jordá, R.; Laporta-Martín, P.; Carratalá-Calvo, A.; Alonso-Iglesias, E. Vitamin D Status Is Linked to Biomarkers of Oxidative Stress, Inflammation, and Endothelial Activation in Obese Children. J. Pediatr. 2012, 161, 848–854. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, H.; Sun, X.; Ren, L. The Protective Role of Vitamin D3 in a Murine Model of Asthma via the Suppression of TGF-β/Smad Signaling and Activation of the Nrf2/HO-1 Pathway. Mol. Med. Rep. 2016, 14, 2389–2396. [Google Scholar] [CrossRef]
- Adam-Bonci, T.-I.; Bonci, E.-A.; Pârvu, A.-E.; Herdean, A.-I.; Moț, A.; Taulescu, M.; Ungur, A.; Pop, R.-M.; Bocșan, C.; Irimie, A. Vitamin D Supplementation: Oxidative Stress Modulation in a Mouse Model of Ovalbumin-Induced Acute Asthmatic Airway Inflammation. Int. J. Mol. Sci. 2021, 22, 7089. [Google Scholar] [CrossRef]
- Adams, J.S.; Hewison, M. Unexpected Actions of Vitamin D: New Perspectives on the Regulation of Innate and Adaptive Immunity. Nat. Clin. Pract. Endocrinol. Metab. 2008, 4, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Chambers, E.S.; Hawrylowicz, C.M. The Impact of Vitamin D on Regulatory T Cells. Curr. Allergy Asthma Rep. 2011, 11, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Biggs, L.; Yu, C.; Fedoric, B.; Lopez, A.F.; Galli, S.J.; Grimbaldeston, M.A. Evidence That Vitamin D3 Promotes Mast Cell–Dependent Reduction of Chronic UVB-Induced Skin Pathology in Mice. J. Exp. Med. 2010, 207, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Bscheider, M.; Butcher, E.C. Vitamin D Immunoregulation through Dendritic Cells. Immunology 2016, 148, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, B.; Heine, G.; Babina, M.; Steinmeyer, A.; Zügel, U.; Radbruch, A.; Worm, M. Targeting the Vitamin D Receptor Inhibits the B Cell-Dependent Allergic Immune Response: VDR Targeting Inhibits the Allergic Immune Response. Allergy 2011, 66, 540–548. [Google Scholar] [CrossRef]
- Heine, G.; Niesner, U.; Chang, H.-D.; Steinmeyer, A.; Zügel, U.; Zuberbier, T.; Radbruch, A.; Worm, M. 1,25-Dihydroxyvitamin D3 Promotes IL-10 Production in Human B Cells. Eur. J. Immunol. 2008, 38, 2210–2218. [Google Scholar] [CrossRef]
- Litonjua, A.A. Dietary Factors and the Development of Asthma. Immunol. Allergy Clin. North Am. 2008, 28, 603–629. [Google Scholar] [CrossRef]
- Keating, P.; Munim, A.; Hartmann, J.X. Effect of Vitamin D on T-Helper Type 9 Polarized Human Memory Cells in Chronic Persistent Asthma. Ann. Allergy. Asthma. Immunol. 2014, 112, 154–162. [Google Scholar] [CrossRef]
- Yip, K.-H.; Kolesnikoff, N.; Yu, C.; Hauschild, N.; Taing, H.; Biggs, L.; Goltzman, D.; Gregory, P.A.; Anderson, P.H.; Samuel, M.S.; et al. Mechanisms of Vitamin D3 Metabolite Repression of IgE-Dependent Mast Cell Activation. J. Allergy Clin. Immunol. 2014, 133, 1356–1364.e14. [Google Scholar] [CrossRef]
- Taher, Y.A.; van Esch, B.C.A.M.; Hofman, G.A.; Henricks, P.A.J.; van Oosterhout, A.J.M. 1α,25-Dihydroxyvitamin D3 Potentiates the Beneficial Effects of Allergen Immunotherapy in a Mouse Model of Allergic Asthma: Role for IL-10 and TGF-β. J. Immunol. 2008, 180, 5211–5221. [Google Scholar] [CrossRef]
- Belderbos, M.E.; Houben, M.L.; Wilbrink, B.; Lentjes, E.; Bloemen, E.M.; Kimpen, J.L.L.; Rovers, M.; Bont, L. Cord Blood Vitamin D Deficiency Is Associated with Respiratory Syncytial Virus Bronchiolitis. Pediatrics 2011, 127, e1513–e1520. [Google Scholar] [CrossRef] [PubMed]
- Camargo, C.A.; Ganmaa, D.; Frazier, A.L.; Kirchberg, F.F.; Stuart, J.J.; Kleinman, K.; Sumberzul, N.; Rich-Edwards, J.W. Randomized Trial of Vitamin D Supplementation and Risk of Acute Respiratory Infection in Mongolia. Pediatrics 2012, 130, e561–e567. [Google Scholar] [CrossRef] [PubMed]
- Greiller, C.; Martineau, A. Modulation of the Immune Response to Respiratory Viruses by Vitamin D. Nutrients 2015, 7, 4240–4270. [Google Scholar] [CrossRef] [PubMed]
- Jartti, T.; Ruuskanen, O.; Mansbach, J.M.; Vuorinen, T.; Camargo, C.A. Low Serum 25-Hydroxyvitamin D Levels Are Associated with Increased Risk of Viral Coinfections in Wheezing Children. J. Allergy Clin. Immunol. 2010, 126, 1074–1076.e4. [Google Scholar] [CrossRef] [PubMed]
- Urashima, M.; Segawa, T.; Okazaki, M.; Kurihara, M.; Wada, Y.; Ida, H. Randomized Trial of Vitamin D Supplementation to Prevent Seasonal Influenza A in Schoolchildren. Am. J. Clin. Nutr. 2010, 91, 1255–1260. [Google Scholar] [CrossRef]
- Goodall, E.C.; Granados, A.C.; Luinstra, K.; Pullenayegum, E.; Coleman, B.L.; Loeb, M.; Smieja, M. Vitamin D3 and Gargling for the Prevention of Upper Respiratory Tract Infections: A Randomized Controlled Trial. BMC Infect. Dis. 2014, 14, 273. [Google Scholar] [CrossRef]
- Vasiliou, J.E.; Lui, S.; Walker, S.A.; Chohan, V.; Xystrakis, E.; Bush, A.; Hawrylowicz, C.M.; Saglani, S.; Lloyd, C.M. Vitamin D Deficiency Induces T H2 Skewing and Eosinophilia in Neonatal Allergic Airways Disease. Allergy 2014, 69, 1380–1389. [Google Scholar] [CrossRef]
- Lai, G.; Wu, C.; Hong, J.; Song, Y. 1,25-Dihydroxyvitamin D3 (1,25-(OH) 2 D3) Attenuates Airway Remodeling in a Murine Model of Chronic Asthma. J. Asthma 2013, 50, 133–140. [Google Scholar] [CrossRef]
- Matheu, V.; Bäck, O.; Mondoc, E.; Issazadeh-Navikas, S. Dual Effects of Vitamin D-Induced Alteration of TH1/TH2 Cytokine Expression: Enhancing IgE Production and Decreasing Airway Eosinophilia in Murine Allergic Airway Disease. J. Allergy Clin. Immunol. 2003, 112, 585–592. [Google Scholar] [CrossRef]
- Bose, S.; Diette, G.B.; Woo, H.; Koehler, K.; Romero, K.; Rule, A.M.; Detrick, B.; Brigham, E.; McCormack, M.C.; Hansel, N.N. Vitamin D Status Modifies the Response to Indoor Particulate Matter in Obese Urban Children with Asthma. J. Allergy Clin. Immunol. Pract. 2019, 7, 1815–1822.e2. [Google Scholar] [CrossRef]
- Szymczak-Pajor, I.; Kleniewska, P.; Wieczfinska, J.; Pawliczak, R. Wide-Range Effects of 1,25(OH)2D3 on Group 4A Phospholipases Is Related to Nuclear Factor κ-B and Phospholipase-A2 Activating Protein Activity in Mast Cells. Int. Arch. Allergy Immunol. 2020, 181, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Agrawal, D.K. Vitamin D and Inflammatory Diseases. J. Inflamm. Res. 2014, 7, 69–87. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Meng, T.; Qi, Y.; Athari, S.S.; Chen, X. Study Effect of Vitamin D on the Immunopathology Responses of the Bronchi in Murine Model of Asthma. Iran. J. Allergy Asthma Immunol. 2021, 20, 509–519. [Google Scholar] [CrossRef]
- Banerjee, A.; Panettieri, R. Vitamin D Modulates Airway Smooth Muscle Function in COPD. Curr. Opin. Pharmacol. 2012, 12, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Damera, G.; Fogle, H.; Lim, P.; Goncharova, E.; Zhao, H.; Banerjee, A.; Tliba, O.; Krymskaya, V.; Panettieri, R., Jr. Vitamin D Inhibits Growth of Human Airway Smooth Muscle Cells through Growth Factor-Induced Phosphorylation of Retinoblastoma Protein and Checkpoint Kinase 1: Airway Smooth Muscle Growth and Vitamin D. Br. J. Pharmacol. 2009, 158, 1429–1441. [Google Scholar] [CrossRef] [PubMed]
- Foley, S.C.; Mogas, A.K.; Olivenstein, R.; Fiset, P.O.; Chakir, J.; Bourbeau, J.; Ernst, P.; Lemière, C.; Martin, J.G.; Hamid, Q. Increased Expression of ADAM33 and ADAM8 with Disease Progression in Asthma. J. Allergy Clin. Immunol. 2007, 119, 863–871. [Google Scholar] [CrossRef]
- Johnson, L.A.; Sauder, K.L.; Rodansky, E.S.; Simpson, R.U.; Higgins, P.D.R. CARD-024, a Vitamin D Analog, Attenuates the pro-Fibrotic Response to Substrate Stiffness in Colonic Myofibroblasts. Exp. Mol. Pathol. 2012, 93, 91–98. [Google Scholar] [CrossRef]
- Slominski, A.T.; Li, W.; Bhattacharya, S.K.; Smith, R.A.; Johnson, P.L.; Chen, J.; Nelson, K.E.; Tuckey, R.C.; Miller, D.; Jiao, Y.; et al. Vitamin D Analogs 17,20S(OH)2pD and 17,20R(OH)2pD Are Noncalcemic and Exhibit Antifibrotic Activity. J. Investig. Dermatol. 2011, 131, 1167–1169. [Google Scholar] [CrossRef]
- Song, Y.; Qi, H.; Wu, C. Effect of 1,25-(OH) 2 D 3 (a Vitamin D Analogue) on Passively Sensitized Human Airway Smooth Muscle Cells. Respirology 2007, 12, 486–494. [Google Scholar] [CrossRef]
- Sundar, I.K.; Hwang, J.-W.; Wu, S.; Sun, J.; Rahman, I. Deletion of Vitamin D Receptor Leads to Premature Emphysema/COPD by Increased Matrix Metalloproteinases and Lymphoid Aggregates Formation. Biochem. Biophys. Res. Commun. 2011, 406, 127–133. [Google Scholar] [CrossRef]
- Al-Afaleg, N.O.; Al-Senaidy, A.; El-Ansary, A. Oxidative Stress and Antioxidant Status in Saudi Asthmatic Patients. Clin. Biochem. 2011, 44, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Hijazi, N.; Abalkhail, B.; Seaton, A. Diet and Childhood Asthma in a Society in Transition: A Study in Urban and Rural Saudi Arabia. Thorax 2000, 55, 775–779. [Google Scholar] [CrossRef] [PubMed]
- Wood, L.G.; Garg, M.L.; Blake, R.J.; Simpson, J.L.; Gibson, P.G. Oxidized Vitamin E and Glutathione as Markers of Clinical Status in Asthma. Clin. Nutr. 2008, 27, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, Y.; Furusho, T.; Yoshida, A.; Nakamura, H.; Matsuura, T.; Eto, Y. Serum Vitamin A Concentrations in Asthmatic Children in Japan. Pediatr. Int. 2006, 48, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Nwaru, B.I.; Virtanen, S.M.; Alfthan, G.; Karvonen, A.M.; Genuneit, J.; Lauener, R.P.; Dalphin, J.-C.; Hyvärinen, A.; Pfefferle, P.; Riedler, J.; et al. Serum Vitamin E Concentrations at 1 Year and Risk of Atopy, Atopic Dermatitis, Wheezing, and Asthma in Childhood: The PASTURE Study. Allergy 2014, 69, 87–94. [Google Scholar] [CrossRef]
- Butland, B.K. Diet, Lung Function, and Lung Function Decline in a Cohort of 2512 Middle Aged Men. Thorax 2000, 55, 102–108. [Google Scholar] [CrossRef]
- Hu, G.; Cassano, P.A. Antioxidant Nutrients and Pulmonary Function: The Third National Health and Nutrition Examination Survey (NHANES III). Am. J. Epidemiol. 2000, 151, 975–981. [Google Scholar] [CrossRef]
- Cui, H.; Huang, J.; Lu, M.; Zhang, Q.; Qin, W.; Zhao, Y.; Lu, X.; Zhang, J.; Xi, Z.; Li, R. Antagonistic Effect of Vitamin E on NAl2O3-Induced Exacerbation of Th2 and Th17-Mediated Allergic Asthma via Oxidative Stress. Environ. Pollut. 2019, 252, 1519–1531. [Google Scholar] [CrossRef]
- Li, J.; Li, L.; Chen, H.; Chang, Q.; Liu, X.; Wu, Y.; Wei, C.; Li, R.; Kwan, J.K.C.; Yeung, K.L.; et al. Application of Vitamin E to Antagonize SWCNTs-Induced Exacerbation of Allergic Asthma. Sci. Rep. 2014, 4, 4275. [Google Scholar] [CrossRef]
- Okamoto, N.; Murata, T.; Tamai, H.; Tanaka, H.; Nagai, H. Effects of Alpha Tocopherol and Probucol Supplements on Allergen-Induced Airway Inflammation and Hyperresponsiveness in a Mouse Model of Allergic Asthma. Int. Arch. Allergy Immunol. 2006, 141, 172–180. [Google Scholar] [CrossRef]
- Wagner, J.G.; Jiang, Q.; Harkema, J.R.; Illek, B.; Patel, D.D.; Ames, B.N.; Peden, D.B. Ozone Enhancement of Lower Airway Allergic Inflammation Is Prevented by γ-Tocopherol. Free Radic. Biol. Med. 2007, 43, 1176–1188. [Google Scholar] [CrossRef] [PubMed]
- Talati, M.; Meyrick, B.; Peebles, R.S.; Davies, S.S.; Dworski, R.; Mernaugh, R.; Mitchell, D.; Boothby, M.; Roberts, L.J.; Sheller, J.R. Oxidant Stress Modulates Murine Allergic Airway Responses. Free Radic. Biol. Med. 2006, 40, 1210–1219. [Google Scholar] [CrossRef] [PubMed]
- Nishio, K.; Horie, M.; Akazawa, Y.; Shichiri, M.; Iwahashi, H.; Hagihara, Y.; Yoshida, Y.; Niki, E. Attenuation of Lipopolysaccharide (LPS)-Induced Cytotoxicity by Tocopherols and Tocotrienols. Redox Biol. 2013, 1, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Saito, Y.; Jones, L.S.; Shigeri, Y. Chemical Reactivities and Physical Effects in Comparison between Tocopherols and Tocotrienols: Physiological Significance and Prospects as Antioxidants. J. Biosci. Bioeng. 2007, 104, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Liebner, F.; Netscher, T.; Mereiter, K.; Rosenau, T. Vitamin E Chemistry. Nitration of Non-Alpha-Tocopherols: Products and Mechanistic Considerations. J. Org. Chem. 2007, 72, 6504–6512. [Google Scholar] [CrossRef]
- Cook-Mills, J.; Gebretsadik, T.; Abdala-Valencia, H.; Green, J.; Larkin, E.K.; Dupont, W.D.; Shu, X.O.; Gross, M.; Bai, C.; Gao, Y.-T.; et al. Interaction of Vitamin E Isoforms on Asthma and Allergic Airway Disease. Thorax 2016, 71, 954–956. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, J.; Wu, Z.; Lu, Y.; You, H.; Li, R.; Li, B.; Yang, X.; Duan, L. Acute Exposure of Ozone Induced Pulmonary Injury and the Protective Role of Vitamin E through the Nrf2 Pathway in Balb/c Mice. Toxicol. Res. 2016, 5, 268–277. [Google Scholar] [CrossRef]
- Dworski, R.; Han, W.; Blackwell, T.S.; Hoskins, A.; Freeman, M.L. Vitamin E Prevents NRF2 Suppression by Allergens in Asthmatic Alveolar Macrophages in Vivo. Free Radic. Biol. Med. 2011, 51, 516–521. [Google Scholar] [CrossRef]
- Quoc, Q.L.; Bich, T.C.T.; Kim, S.; Park, H.; Shin, Y.S. Administration of Vitamin E Attenuates Airway Inflammation through Restoration of Nrf2 in a Mouse Model of Asthma. J. Cell. Mol. Med. 2021, 25, 6721–6732. [Google Scholar] [CrossRef]
- Muti, A.D.; Pârvu, A.E.; Muti, L.A.; Moldovan, R.; Mureșan, A. Vitamin E effect in a rat model of toluene diisocyanate-induced asthma. Med. Pharm. Rep. 2016, 89, 499–505. [Google Scholar] [CrossRef]
- Shang, S.; Li, J.; Zhao, Y.; Xi, Z.; Lu, Z.; Li, B.; Yang, X.; Li, R. Oxidized Graphene-Aggravated Allergic Asthma Is Antagonized by Antioxidant Vitamin E in Balb/c Mice. Environ. Sci. Pollut. Res. 2017, 24, 1784–1793. [Google Scholar] [CrossRef] [PubMed]
- Pearson, P.; Lewis, S.A.; Britton, J.; Young, I.S.; Fogarty, A. The Pro-Oxidant Activity of High-Dose Vitamin E Supplements In Vivo. BioDrugs 2006, 20, 271–273. [Google Scholar] [CrossRef] [PubMed]
- Marchese, M.E.; Kumar, R.; Colangelo, L.A.; Avila, P.C.; Jacobs, D.R.; Gross, M.; Sood, A.; Liu, K.; Cook-Mills, J.M. The Vitamin E Isoforms α-Tocopherol and γ-Tocopherol Have Opposite Associations with Spirometric Parameters: The CARDIA Study. Respir. Res. 2014, 15, 31. [Google Scholar] [CrossRef]
- McCary, C.A.; Yoon, Y.; Panagabko, C.; Cho, W.; Atkinson, J.; Cook-Mills, J.M. Vitamin E Isoforms Directly Bind PKCα and Differentially Regulate Activation of PKCα. Biochem. J. 2012, 441, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Schock, B.C.; Van der Vliet, A.; Corbacho, A.M.; Leonard, S.W.; Finkelstein, E.; Valacchi, G.; Obermueller-Jevic, U.; Cross, C.E.; Traber, M.G. Enhanced Inflammatory Responses in α-Tocopherol Transfer Protein Null Mice. Arch. Biochem. Biophys. 2004, 423, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Singh, U.; Devaraj, S.; Jialal, I. Vitamin E, Oxidative Stress, and Inflammation. Annu. Rev. Nutr. 2005, 25, 151–174. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Li, J.; Ma, P.; Yang, X.; Xu, S. Vitamin E Antagonizes Ozone-Induced Asthma Exacerbation in Balb/c Mice through the Nrf2 Pathway. Food Chem. Toxicol. 2017, 107, 47–56. [Google Scholar] [CrossRef]
- Jiang, J.; Mehrabi Nasab, E.; Athari, S.M.; Athari, S.S. Effects of Vitamin E and Selenium on Allergic Rhinitis and Asthma Pathophysiology. Respir. Physiol. Neurobiol. 2021, 286, 103614. [Google Scholar] [CrossRef]
- Mabalirajan, U.; Aich, J.; Leishangthem, G.D.; Sharma, S.K.; Dinda, A.K.; Ghosh, B. Effects of Vitamin E on Mitochondrial Dysfunction and Asthma Features in an Experimental Allergic Murine Model. J. Appl. Physiol. 2009, 107, 1285–1292. [Google Scholar] [CrossRef]
- Nounou, H.A.; Deif, M.M.; Arafah, M. The Influence of Dexamethasone and the Role of Some Antioxidant Vitamins in the Pathogenesis of Experimental Bronchial Asthma. J. Exp. Pharmacol. 2010, 2, 93–103. [Google Scholar] [CrossRef]
- Salinthone, S.; Kerns, A.R.; Tsang, V.; Carr, D.W. α-Tocopherol (Vitamin E) Stimulates Cyclic AMP Production in Human Peripheral Mononuclear Cells and Alters Immune Function. Mol. Immunol. 2013, 53, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Pein, H.; Ville, A.; Pace, S.; Temml, V.; Garscha, U.; Raasch, M.; Alsabil, K.; Viault, G.; Dinh, C.-P.; Guilet, D.; et al. Endogenous Metabolites of Vitamin E Limit Inflammation by Targeting 5-Lipoxygenase. Nat. Commun. 2018, 9, 3834. [Google Scholar] [CrossRef] [PubMed]
- Centanni, S.; Santus, P.; Dimarco, F.; Fumagalli, F.; Zarini, S.; Sala, A. The Potential Role of Tocopherol in Asthma and Allergies: Modification of the Leukotriene Pathway. BioDrugs 2001, 15, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Burbank, A.J.; Duran, C.G.; Almond, M.; Wells, H.; Jenkins, S.; Jiang, Q.; Yang, C.; Wang, T.; Zhou, H.; Hernandez, M.L.; et al. A Short Course of Gamma-Tocopherol Mitigates LPS-Induced Inflammatory Responses in Humans Ex Vivo. J. Allergy Clin. Immunol. 2017, 140, 1179–1181.e4. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, M.L.; Wagner, J.G.; Kala, A.; Mills, K.; Wells, H.B.; Alexis, N.E.; Lay, J.C.; Jiang, Q.; Zhang, H.; Zhou, H.; et al. Vitamin E, γ-Tocopherol, Reduces Airway Neutrophil Recruitment after Inhaled Endotoxin Challenge in Rats and in Healthy Volunteers. Free Radic. Biol. Med. 2013, 60, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Wiser, J.; Alexis, N.E.; Jiang, Q.; Wu, W.; Robinette, C.; Roubey, R.; Peden, D.B. In Vivo γ-Tocopherol Supplementation Decreases Systemic Oxidative Stress and Cytokine Responses of Human Monocytes in Normal and Asthmatic Subjects. Free Radic. Biol. Med. 2008, 45, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Suchankova, J.; Voprsalova, M.; Kottova, M.; Semecky, V.; Visnovsky, P. Effects of Oral Alpha-Tocopherol on Lung Response in Rat Model of Allergic Asthma. Respirology 2006, 11, 414–421. [Google Scholar] [CrossRef]
- Kuhad, A.; Chopra, K. Tocotrienol Attenuates Oxidative–Nitrosative Stress and Inflammatory Cascade in Experimental Model of Diabetic Neuropathy. Neuropharmacology 2009, 57, 456–462. [Google Scholar] [CrossRef]
- Matsunaga, T.; Shoji, A.; Gu, N.; Joo, E.; Li, S.; Adachi, T.; Yamazaki, H.; Yasuda, K.; Kondoh, T.; Tsuda, K. γ-Tocotrienol Attenuates TNF-α-Induced Changes in Secretion and Gene Expression of MCP-1, IL-6 and Adiponectin in 3T3-L1 Adipocytes. Mol. Med. Rep. 2012, 5, 905–909. [Google Scholar] [CrossRef]
- Morante, M.; Sandoval, J.; Gómez-Cabrera, M.-C.; Rodríguez, J.L.; Pallardó, F.V.; Viña, J.R.; Torres, L.; Barber, T. Vitamin E Deficiency Induces Liver Nuclear Factor-KappaB DNA-Binding Activity and Changes in Related Genes. Free Radic. Res. 2005, 39, 1127–1138. [Google Scholar] [CrossRef]
- Wu, G.; Zhu, H.; Wu, X.; Liu, L.; Ma, X.; Yuan, Y.; Fu, X.; Zhang, L.; Lv, Y.; Li, D.; et al. Anti-Allergic Function of α-Tocopherol Is Mediated by Suppression of PI3K-PKB Activity in Mast Cells in Mouse Model of Allergic Rhinitis. Allergol. Immunopathol. 2020, 48, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Ramsahai, J.M.; Hansbro, P.M.; Wark, P.A.B. Mechanisms and Management of Asthma Exacerbations. Am. J. Respir. Crit. Care Med. 2019, 199, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Tierno, R.; Alkhraisat, M.H. Current Opinion on the Role of Vitamin D Supplementation in Respiratory Infections and Asthma/COPD Exacerbations: A Need to Establish Publication Guidelines for Overcoming the Unpublished Data. Clin. Nutr. 2022, 41, 755–777. [Google Scholar] [CrossRef] [PubMed]
- Jartti, T.; Bønnelykke, K.; Elenius, V.; Feleszko, W. Role of Viruses in Asthma. Semin. Immunopathol. 2020, 42, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Hansbro, N.G.; Horvat, J.C.; Wark, P.A.; Hansbro, P.M. Understanding the Mechanisms of Viral Induced Asthma: New Therapeutic Directions. Pharmacol. Ther. 2008, 117, 313–353. [Google Scholar] [CrossRef] [PubMed]
- Hershenson, M.B. Rhinovirus-Induced Exacerbations of Asthma and COPD. Scientifica 2013, 2013, 405876. [Google Scholar] [CrossRef] [PubMed]
- Coverstone, A.M.; Wang, L.; Sumino, K. Beyond Respiratory Syncytial Virus and Rhinovirus in the Pathogenesis and Exacerbation of Asthma. Immunol. Allergy Clin. North Am. 2019, 39, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Adir, Y.; Saliba, W.; Beurnier, A.; Humbert, M. Asthma and COVID-19: An Update. Eur. Respir. Rev. 2021, 30, 210152. [Google Scholar] [CrossRef]
- Skevaki, C.; Karsonova, A.; Karaulov, A.; Fomina, D.; Xie, M.; Chinthrajah, S.; Nadeau, K.C.; Renz, H. SARS-CoV-2 Infection and COVID-19 in Asthmatics: A Complex Relationship. Nat. Rev. Immunol. 2021, 21, 202–203. [Google Scholar] [CrossRef]
- Palmon, P.A.; Jackson, D.J.; Denlinger, L.C. COVID-19 Infections and Asthma. J. Allergy Clin. Immunol. Pract. 2022, 10, 658–663. [Google Scholar] [CrossRef]
- Salciccioli, J.D.; She, L.; Tulchinsky, A.; Rockhold, F.; Cardet, J.C.; Israel, E. Effect of COVID-19 on Asthma Exacerbation. J. Allergy Clin. Immunol. Pract. 2021, 9, 2896–2899.e1. [Google Scholar] [CrossRef] [PubMed]
- Stoodley, I.; Williams, L.; Thompson, C.; Scott, H.; Wood, L. Evidence for Lifestyle Interventions in Asthma. Breathe 2019, 15, e50–e61. [Google Scholar] [CrossRef] [PubMed]
- Sinopoli, A.; Caminada, S.; Isonne, C.; Santoro, M.M.; Baccolini, V. What Are the Effects of Vitamin A Oral Supplementation in the Prevention and Management of Viral Infections? A Systematic Review of Randomized Clinical Trials. Nutrients 2022, 14, 4081. [Google Scholar] [CrossRef] [PubMed]
- Vlieg-Boerstra, B.; de Jong, N.; Meyer, R.; Agostoni, C.; De Cosmi, V.; Grimshaw, K.; Milani, G.P.; Muraro, A.; Oude Elberink, H.; Pali- Schöll, I.; et al. Nutrient Supplementation for Prevention of Viral Respiratory Tract Infections in Healthy Subjects: A Systematic Review and Meta-analysis. Allergy 2022, 77, 1373–1388. [Google Scholar] [CrossRef] [PubMed]
- Cerullo, G.; Negro, M.; Parimbelli, M.; Pecoraro, M.; Perna, S.; Liguori, G.; Rondanelli, M.; Cena, H.; D’Antona, G. The Long History of Vitamin C: From Prevention of the Common Cold to Potential Aid in the Treatment of COVID-19. Front. Immunol. 2020, 11, 574029. [Google Scholar] [CrossRef]
- Hemilä, H.; Chalker, E. Vitamin C for Preventing and Treating the Common Cold. Cochrane Database Syst. Rev. 2013, 2013, CD000980. [Google Scholar] [CrossRef]
- Keya, T.A.; Leela, A.; Fernandez, K.; Habib, N.; Rashid, M. Effect of Vitamin C Supplements on Respiratory Tract Infections: A SystematicReview and Meta-Analysis. Curr. Rev. Clin. Exp. Pharmacol. 2022, 17, 205–215. [Google Scholar] [CrossRef]
- Abioye, A.I.; Bromage, S.; Fawzi, W. Effect of Micronutrient Supplements on Influenza and Other Respiratory Tract Infections among Adults: A Systematic Review and Meta-Analysis. BMJ Glob. Health 2021, 6, e003176. [Google Scholar] [CrossRef]
- BourBour, F.; Mirzaei Dahka, S.; Gholamalizadeh, M.; Akbari, M.E.; Shadnoush, M.; Haghighi, M.; Taghvaye-Masoumi, H.; Ashoori, N.; Doaei, S. Nutrients in Prevention, Treatment, and Management of Viral Infections; Special Focus on Coronavirus. Arch. Physiol. Biochem. 2023, 129, 16–25. [Google Scholar] [CrossRef]
- Calder, P.; Carr, A.; Gombart, A.; Eggersdorfer, M. Optimal Nutritional Status for a Well-Functioning Immune System Is an Important Factor to Protect against Viral Infections. Nutrients 2020, 12, 1181. [Google Scholar] [CrossRef]
- Jolliffe, D.A.; Camargo, C.A.; Sluyter, J.D.; Aglipay, M.; Aloia, J.F.; Ganmaa, D.; Bergman, P.; Bischoff-Ferrari, H.A.; Borzutzky, A.; Damsgaard, C.T.; et al. Vitamin D Supplementation to Prevent Acute Respiratory Infections: A Systematic Review and Meta-Analysis of Aggregate Data from Randomised Controlled Trials. Lancet Diabetes Endocrinol. 2021, 9, 276–292. [Google Scholar] [CrossRef] [PubMed]
- Martineau, A.R.; Jolliffe, D.A.; Hooper, R.L.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; et al. Vitamin D Supplementation to Prevent Acute Respiratory Tract Infections: Systematic Review and Meta-Analysis of Individual Participant Data. BMJ 2017, 356, i6583. [Google Scholar] [CrossRef] [PubMed]
- Trivillin, A.; Zanella, S.; Castaldo, R.J.; Prati, F.; Zanconato, S.; Carraro, S.; Ferraro, V.A. Early Oral Nutritional Supplements in the Prevention of Wheezing, Asthma, and Respiratory Infections. Front. Pediatr. 2022, 10, 866868. [Google Scholar] [CrossRef] [PubMed]
- Ferolla, F.M.; Yfran, E.W.; Ballerini, M.G.; Caratozzolo, A.; Toledano, A.; Giordano, A.C.; Acosta, P.L.; Cassinelli, H.; Bergada, I.; Ropelato, M.G.; et al. Serum Vitamin D Levels and Life-Threatening Respiratory Syncytial Virus Infection in Previously Healthy Infants. J. Infect. Dis. 2022, 226, 958–966. [Google Scholar] [CrossRef]
- Saraf, R.; Jensen, B.P.; Camargo, C.A.; Morton, S.M.B.; Jing, M.; Sies, C.W.; Grant, C.C. Vitamin D Status at Birth and Acute Respiratory Infection Hospitalisation during Infancy. Paediatr. Perinat. Epidemiol. 2021, 35, 540–548. [Google Scholar] [CrossRef]
- Charoenngam, N.; Holick, M.F. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef]
- Hong, M.; Xiong, T.; Huang, J.; Wu, Y.; Lin, L.; Zhang, Z.; Huang, L.; Gao, D.; Wang, H.; Kang, C.; et al. Association of Vitamin D Supplementation with Respiratory Tract Infection in Infants. Matern. Child. Nutr. 2020, 16, e12987. [Google Scholar] [CrossRef]
- Ramos-Martínez, E.; López-Vancell, M.R.; Fernández de Córdova-Aguirre, J.C.; Rojas-Serrano, J.; Chavarría, A.; Velasco-Medina, A.; Velázquez-Sámano, G. Reduction of Respiratory Infections in Asthma Patients Supplemented with Vitamin D Is Related to Increased Serum IL-10 and IFNγ Levels and Cathelicidin Expression. Cytokine 2018, 108, 239–246. [Google Scholar] [CrossRef]
- Sharma, S.; Yang, I.V.; Schwartz, D.A. Epigenetic Regulation of Immune Function in Asthma. J. Allergy Clin. Immunol. 2022, 150, 259–265. [Google Scholar] [CrossRef]
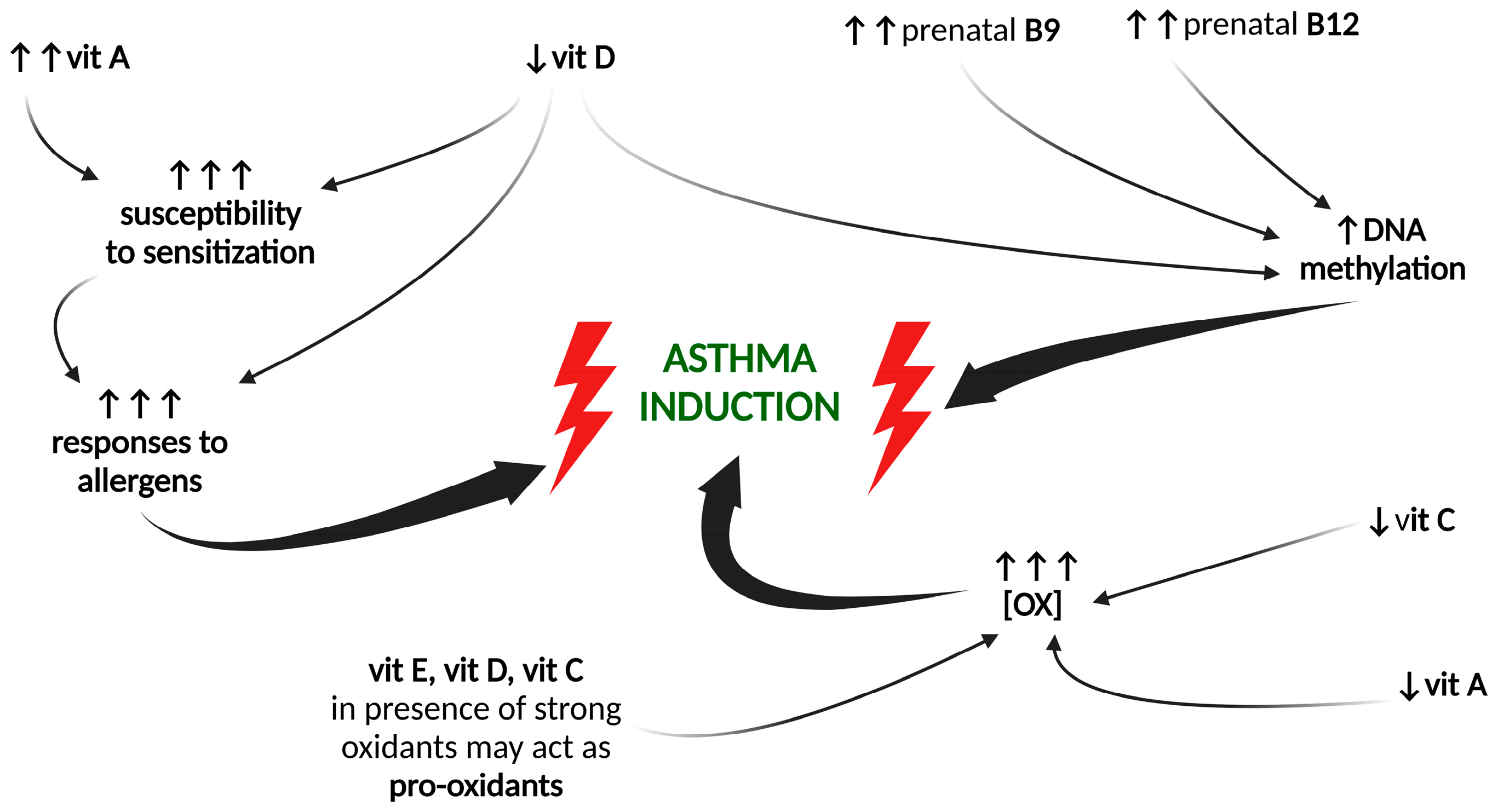
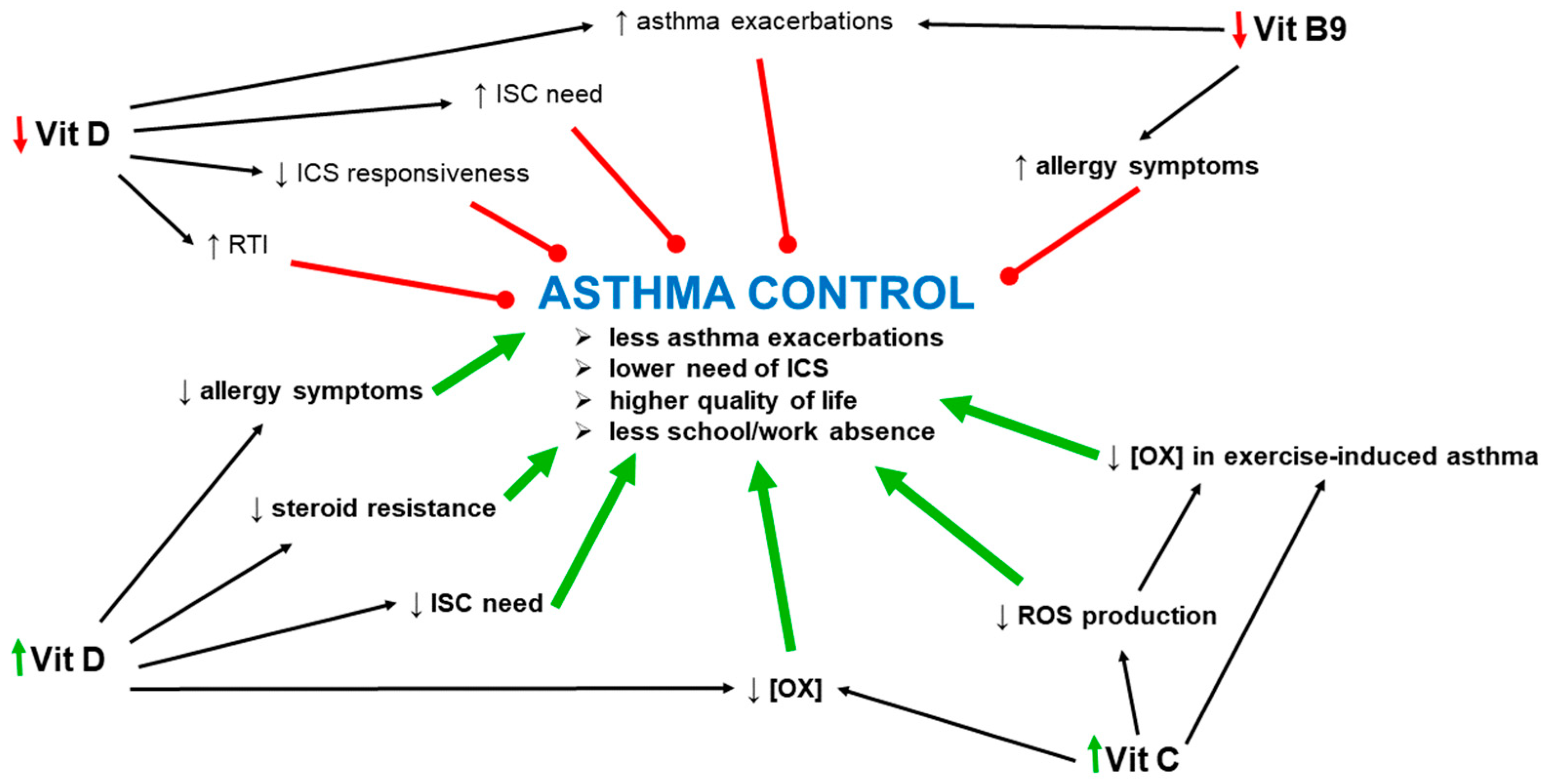

| Vitamin | Levels in Asthma | Increased Maternal Vitamin Intake and the Risk of Developing Asthma by the Child |
|---|---|---|
| A | ↓ | ↑ |
| B1, B2, B6, B7, B12 | ? | ? |
| B3 | ↑ | ? |
| B9 | ↓ | ↑ |
| C | ↓ | ↓ |
| D | ↓ | ↓ |
| E | ↓ | ↓ |
| Vitamin | Lung Function | AHR | Oxidative Stress | Inflammation | Airway Remodeling |
|---|---|---|---|---|---|
| A | ↑ | ↓ | ↓ | ↓ | ? |
| B | ? | ? | ? | ? | ? |
| C | ↑ | ↓ | ↓ | ↓ | ? |
| D | ↑ | ↓ | ↓ | ↓ | ↓ |
| E | ↓↑ * | ↓↑ * | ↓ | ↓↑ * | ↓ |
| K | ? | ? | ? | ↓ | ? |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zajac, D.; Wojciechowski, P. The Role of Vitamins in the Pathogenesis of Asthma. Int. J. Mol. Sci. 2023, 24, 8574. https://doi.org/10.3390/ijms24108574
Zajac D, Wojciechowski P. The Role of Vitamins in the Pathogenesis of Asthma. International Journal of Molecular Sciences. 2023; 24(10):8574. https://doi.org/10.3390/ijms24108574
Chicago/Turabian StyleZajac, Dominika, and Piotr Wojciechowski. 2023. "The Role of Vitamins in the Pathogenesis of Asthma" International Journal of Molecular Sciences 24, no. 10: 8574. https://doi.org/10.3390/ijms24108574
APA StyleZajac, D., & Wojciechowski, P. (2023). The Role of Vitamins in the Pathogenesis of Asthma. International Journal of Molecular Sciences, 24(10), 8574. https://doi.org/10.3390/ijms24108574







