Rhein Induces Oral Cancer Cell Apoptosis and ROS via Suppresse AKT/mTOR Signaling Pathway In Vitro and In Vivo
Abstract
1. Introduction
2. Results
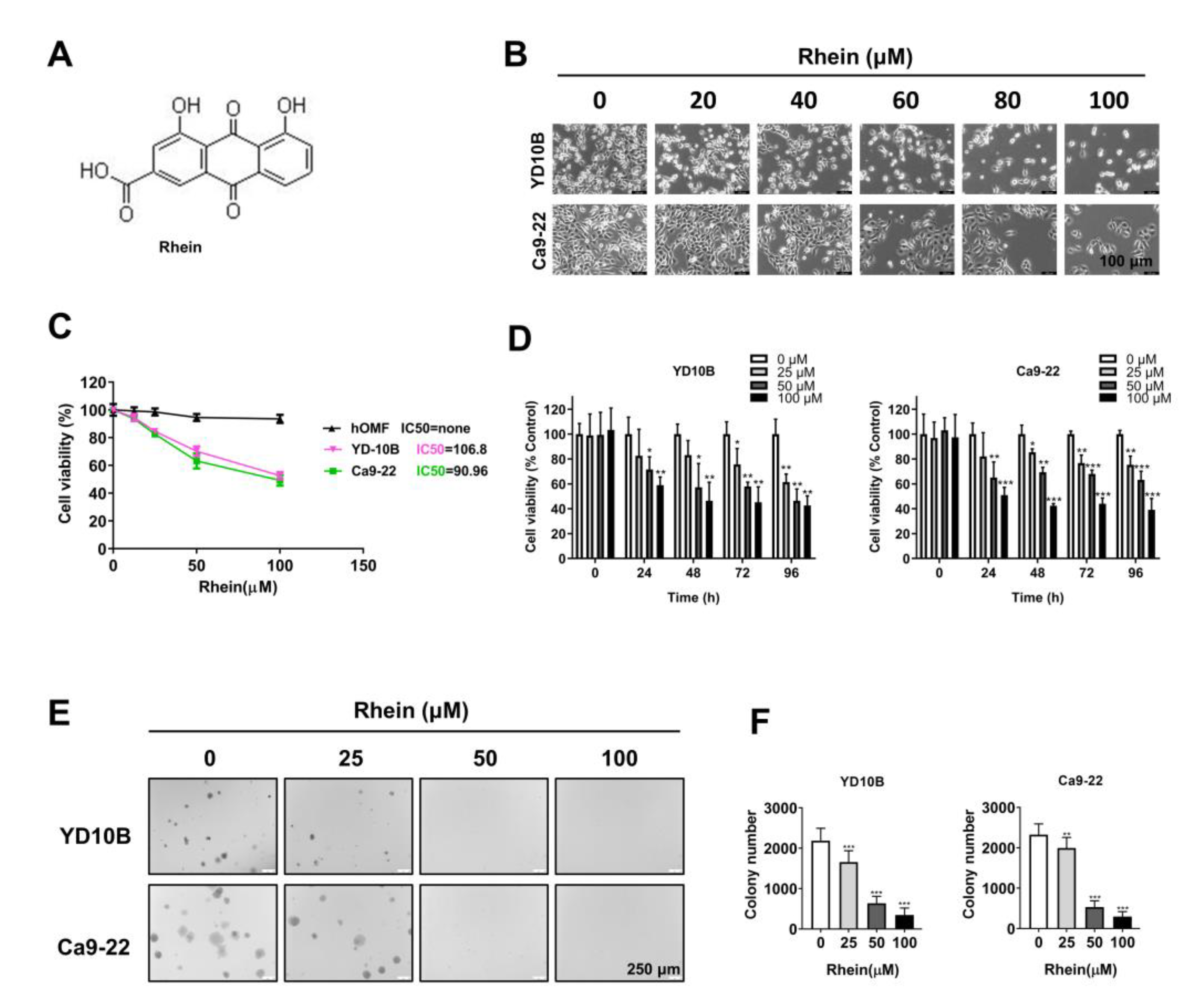
2.1. Rhein Exhibited Antiproliferative Effects in OC Cells
2.2. Rhein Inhibits the Migration and Invasion of OC Cells
2.3. Rhein Induces the S-Phase Cell Cycle Arrest of OC Cells
2.4. Rhein Induces the Apoptosis of OC Cells
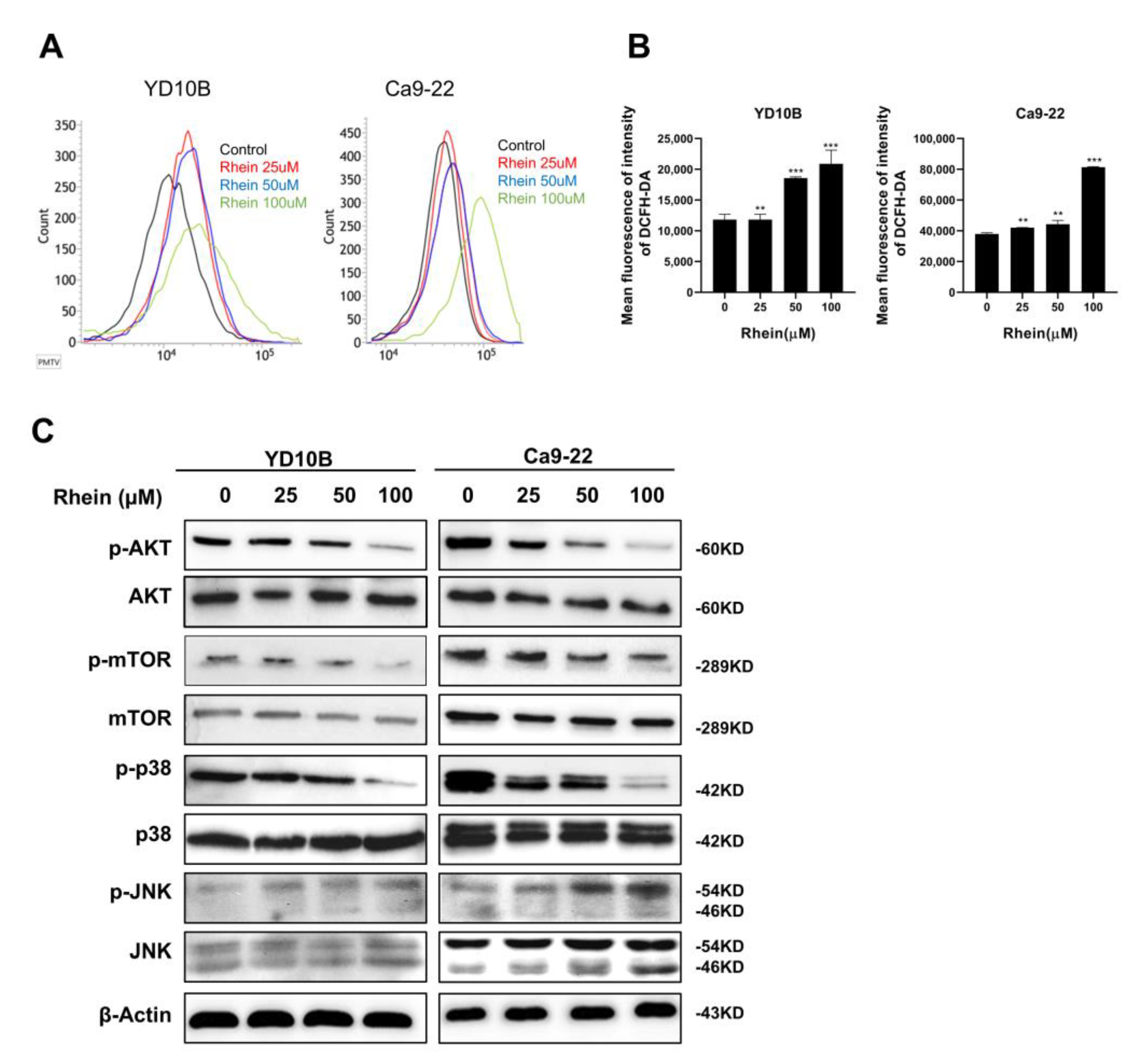
2.5. Rhein Induces ROS Generation and Suppresses the Akt/mTOR Signaling Pathway in OC Cells
2.6. Rhein Inhibits OC Cell Growth in a Xenograft Mouse Model
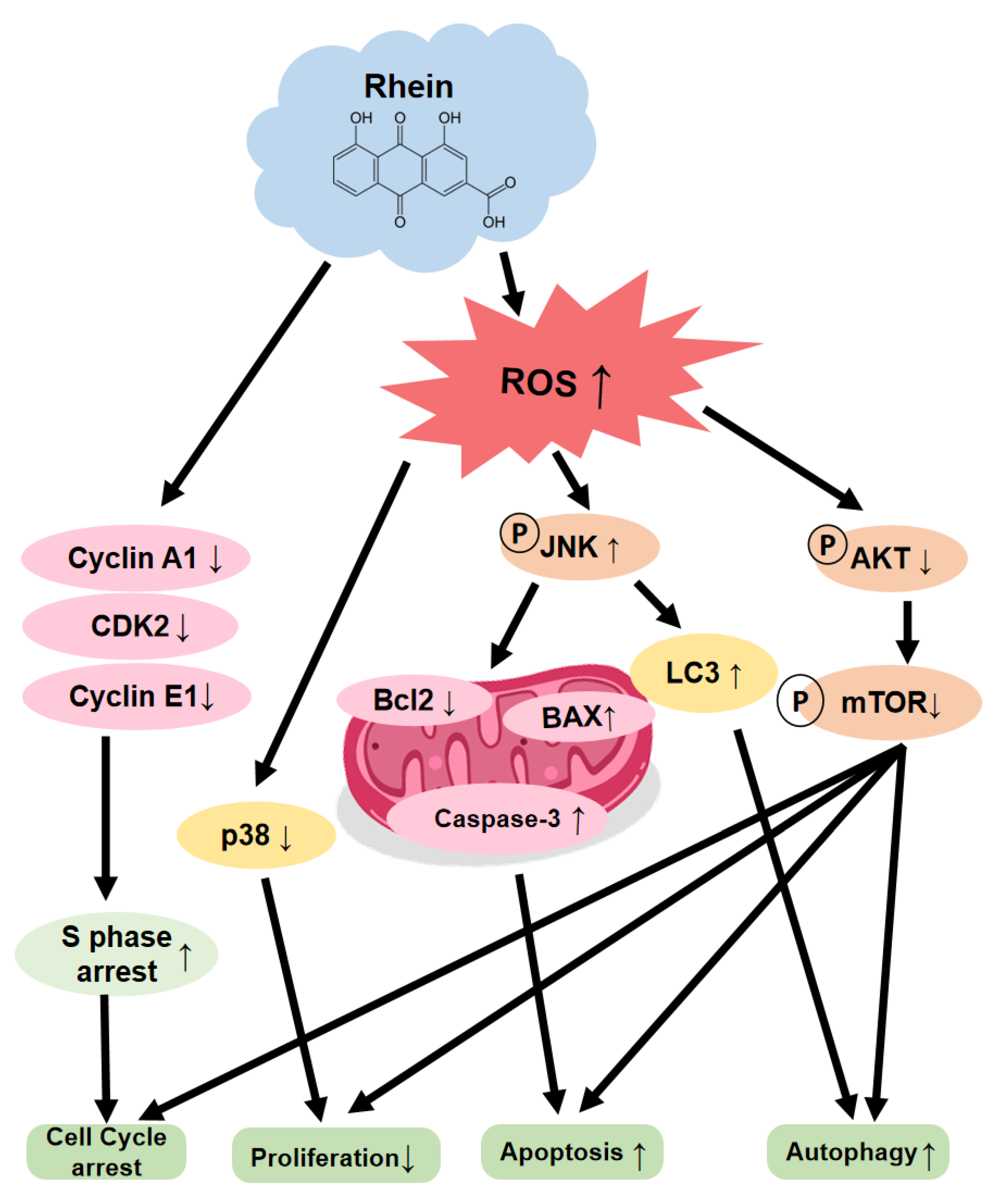
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Cell Culture
4.3. Cell Viability Assay
4.4. Soft Agar Colony Formation Assay
4.5. Cell Cycle and Apoptosis Analysis
4.6. Invasion and Migration Assays
4.7. Measurement of Intracellular ROS
4.8. Western Blotting Assay
4.9. Xenograft Tumor Model in Mice
4.10. IHC Staining
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rivera, C. Essentials of oral cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 11884–11894. [Google Scholar] [PubMed]
- Chi, A.C.; Day, T.A.; Neville, B.W. Oral cavity and oropharyngeal squamous cell carcinoma-an update. CA Cancer J. Clin. 2015, 65, 401–421. [Google Scholar] [CrossRef] [PubMed]
- Day, T.A.; Davis, B.K.; Gillespie, M.B.; Joe, J.K.; Kibbey, M.; Martin-Harris, B.; Neville, B.; Reed, S.G.; Richardson, M.S.; Rosenzweig, S.; et al. Oral cancer treatment. Curr. Treat. Options Oncol. 2003, 4, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Degenhardt, K.; Mathew, R.; Beaudoin, B.; Bray, K.; Anderson, D.; Chen, G.; Mukherjee, C.; Shi, Y.; Gélinas, C.; Fan, Y.; et al. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell 2006, 10, 51–64. [Google Scholar] [CrossRef]
- Turcotte, S.; Chan, D.A.; Sutphin, P.D.; Hay, M.; Denny, W.A.; Giaccia, A.J. A Molecule Targeting VHL-Deficient Renal Cell Carcinoma that Induces Autophagy. Cancer Cell 2008, 14, 90–102. [Google Scholar] [CrossRef]
- Bussi, C.; Iribarren, P.; Rodriguez, C.M. Microtubule-associated protein 1A/1B-light chain 3 (LC3) ‘decorates’ intracytoplasmic inclusions in a patient with chronic lymphocytic leukaemia. Br. J. Haematol. 2017, 179, 529. [Google Scholar] [CrossRef]
- Cheng, X.; Liu, H.; Jiang, C.-C.; Fang, L.; Chen, C.; Zhang, X.-D.; Jiang, Z.-W. Connecting endoplasmic reticulum stress to autophagy through IRE1/JNK/beclin-1 in breast cancer cells. Int. J. Mol. Med. 2014, 34, 772–781. [Google Scholar] [CrossRef]
- Cayo, A.; Segovia, R.; Venturini, W.; Moore-Carrasco, R.; Valenzuela, C.; Brown, N. mTOR Activity and Autophagy in Senescent Cells, a Complex Partnership. Int. J. Mol. Sci. 2021, 22, 8149. [Google Scholar] [CrossRef]
- Deleyto-Seldas, N.; Efeyan, A. The mTOR-Autophagy Axis and the Control of Metabolism. Front. Cell Dev. Biol. 2021, 9, 655731. [Google Scholar] [CrossRef]
- Laplante, M.; Sabatini, D.M. mTOR Signaling. Cold Spring Harb Perspect. Biol. 2012, 4, a011593. [Google Scholar] [CrossRef]
- Laplante, M.; Sabatini, D.M. mTOR Signaling in Growth Control and Disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef] [PubMed]
- Conciatori, F.; Bazzichetto, C.; Falcone, I.; Pilotto, S.; Bria, E.; Cognetti, F.; Milella, M.; Ciuffreda, L. Role of mTOR Signaling in Tumor Microenvironment: An Overview. Int. J. Mol. Sci. 2018, 19, 2453. [Google Scholar] [CrossRef] [PubMed]
- Kaminskyy, V.; Piskunova, T.; Zborovskaya, I.B.; Tchevkina, E.M.; Zhivotovsky, B. Suppression of basal autophagy reduces lung cancer cell proliferation and enhances caspase-dependent and -independent apoptosis by stimulating ROS formation. Autophagy 2012, 8, 1032–1044. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Ding, Z.; Du, K.; Ye, X.; Cheng, S. Reactive Oxygen Species as a Link between Antioxidant Pathways and Autophagy. Oxidative Med. Cell. Longev. 2021, 2021, 5583215. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, X.; Tang, M.; Li, L.; Lei, Y.; Cheng, P.; Guo, W.; Zheng, Y.; Wang, W.; Luo, N.; et al. The role of ROS and subsequent DNA-damage response in PUMA-induced apoptosis of ovarian cancer cells. Oncotarget 2017, 8, 23492–23506. [Google Scholar] [CrossRef]
- Akhtar, M.F.; Saleem, A.; Rasul, A.; Baig, M.M.; Bin-Jumah, M.; Daim, M.M. Anticancer natural medicines: An overview of cell signaling and other targets of anticancer phytochemicals. Eur. J. Pharmacol. 2020, 888, 173488. [Google Scholar] [CrossRef]
- Lee, T.-Y.; Tseng, Y.-H. The Potential of Phytochemicals in Oral Cancer Prevention and Therapy: A Review of the Evidence. Biomolecules 2020, 10, 1150. [Google Scholar] [CrossRef]
- Zhou, Y.-X.; Xia, W.; Yue, W.; Peng, C.; Rahman, K.; Zhang, H. Rhein: A Review of Pharmacological Activities. Evid. Based Complement. Altern. Med. 2015, 2015, 578107. [Google Scholar] [CrossRef]
- Wang, A.; Jiang, H.; Liu, Y.; Chen, J.; Zhou, X.; Zhao, C.; Chen, X.; Lin, M. Rhein induces liver cancer cells apoptosis via activating ROS-dependent JNK/Jun/caspase-3 signaling pathway. J. Cancer 2020, 11, 500–507. [Google Scholar] [CrossRef]
- Zhuang, Y.; Bai, Y.; Hu, Y.; Guo, Y.; Xu, L.; Hu, W.; Yang, L.; Zhao, C.; Li, X.; Zhao, H. Rhein sensitizes human colorectal cancer cells to EGFR inhibitors by inhibiting STAT3 pathway. OncoTargets Ther. 2019, 12, 5281–5291. [Google Scholar] [CrossRef]
- Yao, C.; Wang, F. Inhibition of hypoxia-induced HIF-1α-mediated autophagy enhances the in vitro antitumor activity of rhein in pancreatic cancer cells. J. Appl. Toxicol. 2022, 42, 1937–1947. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, J.; Xu, L.; Lin, S.; Xiang, Y.; Dai, X.; Liang, G.; Huang, X.; Zhu, J.; Zhao, C. Rhein shows potent efficacy against non-small-cell lung cancer through inhibiting the STAT3 pathway. Cancer Manag. Res. 2019, 11, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- You, L.; Dong, X.; Yin, X.; Yang, C.; Leng, X.; Wang, W.; Ni, J. Rhein Induces Cell Death in HepaRG Cells through Cell Cycle Arrest and Apoptotic Pathway. Int. J. Mol. Sci. 2018, 19, 1060. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-J.; Zhen, Y.-S. Rhein lysinate suppresses the growth of breast cancer cells and potentiates the inhibitory effect of Taxol in athymic mice. Anti Cancer Drugs 2009, 20, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Guo, W.; Tang, Y.; Zhang, J.; Xiao, N.; Zhang, L.; Li, W. Rhein Inhibits the Migration of Ovarian Cancer Cells through Down-Regulation of Matrix Metalloproteinases. Biol. Pharm. Bull. 2019, 42, 568–572. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Tian, W.; Hua, Y.; Hu, L.; Yang, J.; Xie, J.; Hu, J.; Wang, F. Rhein enhances the cytotoxicity of effector lymphocytes in colon cancer under hypoxic conditions. Exp. Ther. Med. 2018, 16, 5350–5358. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.; Qin, J.; Wu, J.; Dai, X.; Xu, J. OSR1 phosphorylates the Smad2/3 linker region and induces TGF-beta1 autocrine to promote EMT and metastasis in breast cancer. Oncogene 2020, 40, 68–84. [Google Scholar] [CrossRef]
- Du, P.; Zeng, H.; Xiao, Y.; Zhao, Y.; Zheng, B.; Deng, Y.; Liu, J.; Huang, B.; Zhang, X.; Yang, K.; et al. Chronic stress promotes EMT-mediated metastasis through activation of STAT3 signaling pathway by miR-337-3p in breast cancer. Cell Death Dis. 2020, 11, 761. [Google Scholar] [CrossRef]
- Johnson, D.E. Src family kinases and the MEK/ERK pathway in the regulation of myeloid differentiation and myeloid leukemogenesis. Adv. Enzyme Regul. 2008, 48, 98–112. [Google Scholar] [CrossRef]
- Kabil, A.; Silva, E.; Kortenkamp, A. Estrogens and genomic instability in human breast cancer cells--involvement of Src/Raf/Erk signaling in micronucleus formation by estrogenic chemicals. Carcinogenesis 2008, 29, 1862–1868. [Google Scholar] [CrossRef]
- Zhao, Z.; Wu, Z. Epithelial-mesenchymal transition and tumor metastasis. Zhongguo Fei Ai Za Zhi 2011, 14, 620–624. [Google Scholar] [PubMed]
- Shih, W.; Yamada, S. N-cadherin as a key regulator of collective cell migration in a 3D environment. Cell Adhes. Migr. 2012, 6, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Abd ElMoneim, H.M.; Zaghloul, N.M. Expression of E-cadherin, N-cadherin and snail and their correlation with clinicopathological variants: An immunohistochemical study of 132 invasive ductal breast carcinomas in Egypt. Clinics 2011, 66, 1765–1771. [Google Scholar]
- Araki, K.; Shimura, T.; Suzuki, H.; Tsutsumi, S.; Wada, W.; Yajima, T.; Kobayahi, T.; Kubo, N.; Kuwano, H. E/N-cadherin switch mediates cancer progression via TGF-β-induced epithelial-to-mesenchymal transition in extrahepatic cholangiocarcinoma. Br. J. Cancer 2011, 105, 1885–1893. [Google Scholar] [CrossRef]
- Kim, E.-K.; Jang, M.; Song, M.-J.; Kim, D.; Kim, Y.; Jang, H.H. Redox-Mediated Mechanism of Chemoresistance in Cancer Cells. Antioxidants 2019, 8, 471. [Google Scholar] [CrossRef]
- Liou, Y.; Chen, P.; Chu, S.; Kao, S.; Chang, Y.; Hsieh, Y.; Chang, H. Thymoquinone suppresses the proliferation of renal cell carcinoma cells via reactive oxygen species-induced apoptosis and reduces cell stemness. Environ. Toxicol. 2019, 34, 1208–1220. [Google Scholar] [CrossRef]
- Takac, P.; Kello, M.; Vilkova, M.; Vaskova, J.; Michalkova, R.; Mojzisova, G.; Mojzis, J. Antiproliferative Effect of Acridine Chalcone Is Mediated by Induction of Oxidative Stress. Biomolecules 2020, 10, 345. [Google Scholar] [CrossRef]
- Chae, I.G.; Song, N.Y.; Kim, D.H.; Lee, M.Y.; Park, J.M.; Chun, K.S. Thymoquinone induces apoptosis of human renal carcinoma Caki-1 cells by inhibiting JAK2/STAT3 through pro-oxidant effect. Food Chem. Toxicol. 2020, 139, 111253. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, T.; Sun, W.; Wang, H.; Yin, F.; Wang, Z.; Zuo, D.; Sun, M.; Zhou, Z.; Lin, B.; et al. Arsenic sulfide induces apoptosis and autophagy through the activation of ROS/JNK and suppression of Akt/mTOR signaling pathways in osteosarcoma. Free. Radic. Biol. Med. 2017, 106, 24–37. [Google Scholar] [CrossRef]
- Xue, S.; Zhou, Y.; Zhang, J.; Xiang, Z.; Liu, Y.; Miao, T.; Liu, G.; Liu, B.; Liu, X.; Shen, L.; et al. Anemoside B4 exerts anti-cancer effect by inducing apoptosis and autophagy through inhibiton of PI3K/Akt/mTOR pathway in hepatocellular carcinoma. Am. J. Transl. Res. 2019, 11, 2580–2589. [Google Scholar]
- Lee, J.H.; Parveen, A.; Do, M.H.; Kang, M.C.; Yumnam, S.; Kim, S.Y. Molecular mechanisms of methylglyoxal-induced aortic endothelial dysfunction in human vascular endothelial cells. Cell Death Dis. 2020, 11, 403. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Green, D.R. Autophagy-Independent Functions of the Autophagy Machinery. Cell 2019, 177, 1682–1699. [Google Scholar] [CrossRef] [PubMed]
- Dyshlovoy, S.A. Blue-Print Autophagy in 2020: A Critical Review. Mar. Drugs 2020, 18, 482. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Haybaeck, J.; Yang, Z. Therapeutic Potential of PI3K/AKT/mTOR Pathway in Gastrointestinal Stromal Tumors: Rationale and Progress. Cancers 2020, 12, 2972. [Google Scholar] [CrossRef] [PubMed]
- Demain, A.L.; Vaishnav, P. Natural products for cancer chemotherapy. Microb. Biotechnol. 2010, 4, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Yang, J.; Wei, G. Ginsenoside 20(S)-Rh2 exerts anti-cancer activity through the Akt/GSK3beta signaling pathway in human cervical cancer cells. Mol. Med. Rep. 2018, 17, 4811–4816. [Google Scholar] [PubMed]
- Gao, Q.; Zheng, J. Ginsenoside Rh2 inhibits prostate cancer cell growth through suppression of microRNA-4295 that activates CDKN1A. Cell Prolif. 2018, 51, e12438. [Google Scholar] [CrossRef]
- Ge, G.; Yan, Y.; Cai, H. Ginsenoside Rh2 Inhibited Proliferation by Inducing ROS Mediated ER Stress Dependent Apoptosis in Lung Cancer Cells. Biol. Pharm. Bull. 2017, 40, 2117–2124. [Google Scholar] [CrossRef]
- Zhang, B.P.; Li, B.; Cheng, J.Y.; Cao, R.; Gao, S.T.; Huang, C.J.; Li, R.P.; Ning, J.; Liu, B.; Li, Z.G. Anti-cancer Effect of 20(S)-Ginsenoside-Rh2 on Oral Squamous Cell Carcinoma Cells via the Decrease in ROS and Downregulation of MMP-2 and VEGF. Biomed. Environ. Sci. 2020, 33, 713–717. [Google Scholar]
- Peyressatre, M.; Prével, C.; Pellerano, M.; Morris, M.C. Targeting Cyclin-Dependent Kinases in Human Cancers: From Small Molecules to Peptide Inhibitors. Cancers 2015, 7, 179–237. [Google Scholar] [CrossRef]
- Donjerkovic, D.; Scott, D.W. Regulation of the G1 phase of the mammalian cell cycle. Cell Res. 2000, 10, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Kim, J.; Lee, S.A.; Kim, E.-J.; Chun, Y.-C.; Ryu, M.H.; Yook, J.-I. Characterization of newly established oral cancer cell lines derived from six squamous cell carcinoma and two mucoepidermoid carcinoma cells. Exp. Mol. Med. 2005, 37, 379–390. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Ma, L.; Kim, E.; Yi, J.; Huang, H.; Kim, H.; Raza, M.A.; Park, S.; Jang, S.; Kim, K.; et al. Rhein Induces Oral Cancer Cell Apoptosis and ROS via Suppresse AKT/mTOR Signaling Pathway In Vitro and In Vivo. Int. J. Mol. Sci. 2023, 24, 8507. https://doi.org/10.3390/ijms24108507
Zhang H, Ma L, Kim E, Yi J, Huang H, Kim H, Raza MA, Park S, Jang S, Kim K, et al. Rhein Induces Oral Cancer Cell Apoptosis and ROS via Suppresse AKT/mTOR Signaling Pathway In Vitro and In Vivo. International Journal of Molecular Sciences. 2023; 24(10):8507. https://doi.org/10.3390/ijms24108507
Chicago/Turabian StyleZhang, Haibo, Lei Ma, Eungyung Kim, Junkoo Yi, Hai Huang, Hyeonjin Kim, Muhammad Atif Raza, Sijun Park, Soyoung Jang, Kirim Kim, and et al. 2023. "Rhein Induces Oral Cancer Cell Apoptosis and ROS via Suppresse AKT/mTOR Signaling Pathway In Vitro and In Vivo" International Journal of Molecular Sciences 24, no. 10: 8507. https://doi.org/10.3390/ijms24108507
APA StyleZhang, H., Ma, L., Kim, E., Yi, J., Huang, H., Kim, H., Raza, M. A., Park, S., Jang, S., Kim, K., Kim, S.-H., Lee, Y., Kim, E., Ryoo, Z. Y., & Kim, M. O. (2023). Rhein Induces Oral Cancer Cell Apoptosis and ROS via Suppresse AKT/mTOR Signaling Pathway In Vitro and In Vivo. International Journal of Molecular Sciences, 24(10), 8507. https://doi.org/10.3390/ijms24108507






