Electrochemical Chemically Based Sensors and Emerging Enzymatic Biosensors for Antidepressant Drug Detection: A Review
Abstract
1. Introduction
1.1. Overview of Major Antidepressants
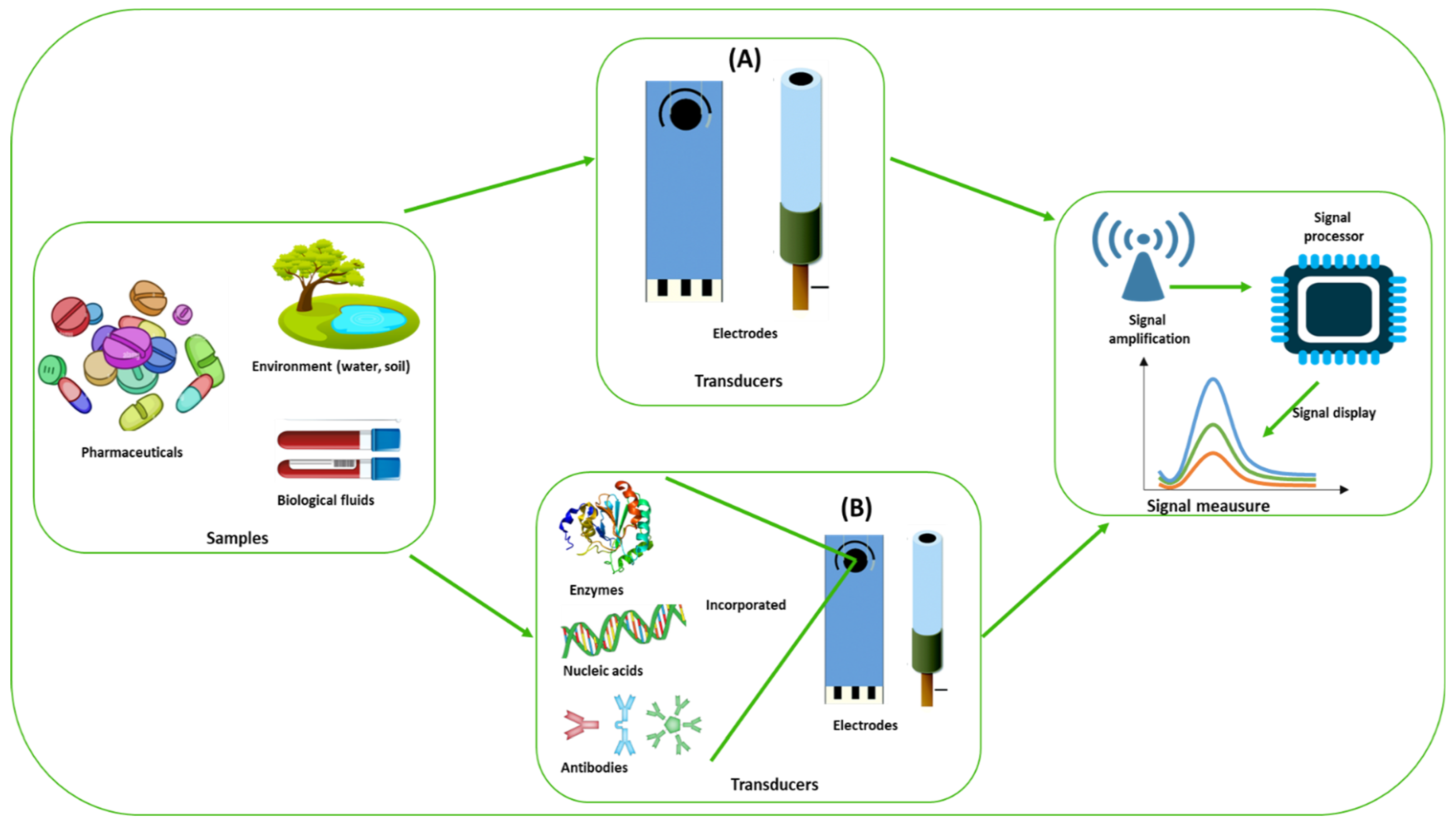
1.2. Traditional Analytical Techniques and Electrochemical Based (Bio)Sensing Techniques
1.3. Aim
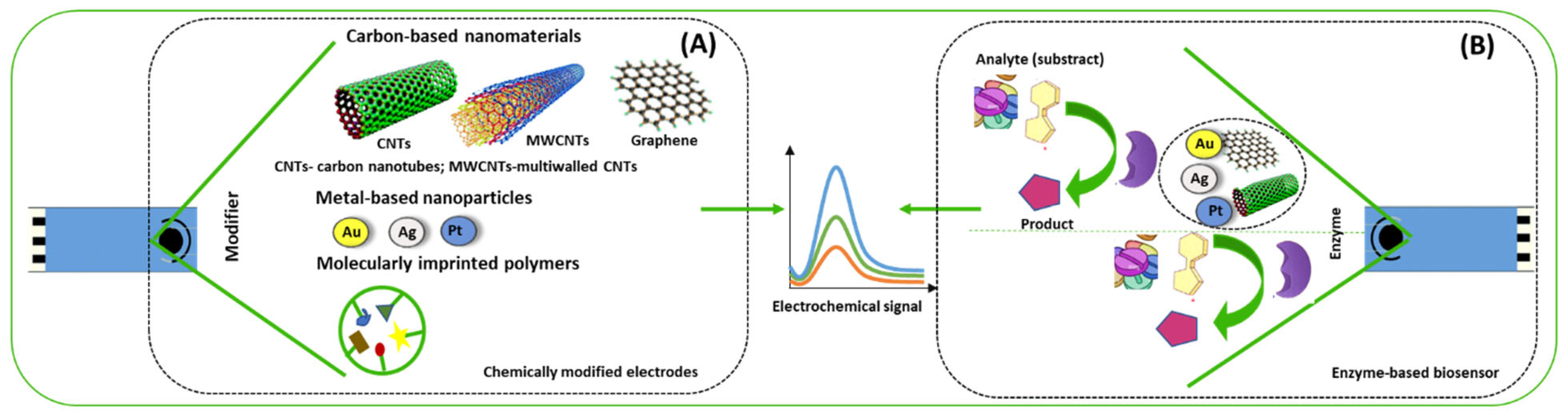
2. Chemically Modified Electrodes (CME) for the Detection of Antidepressants
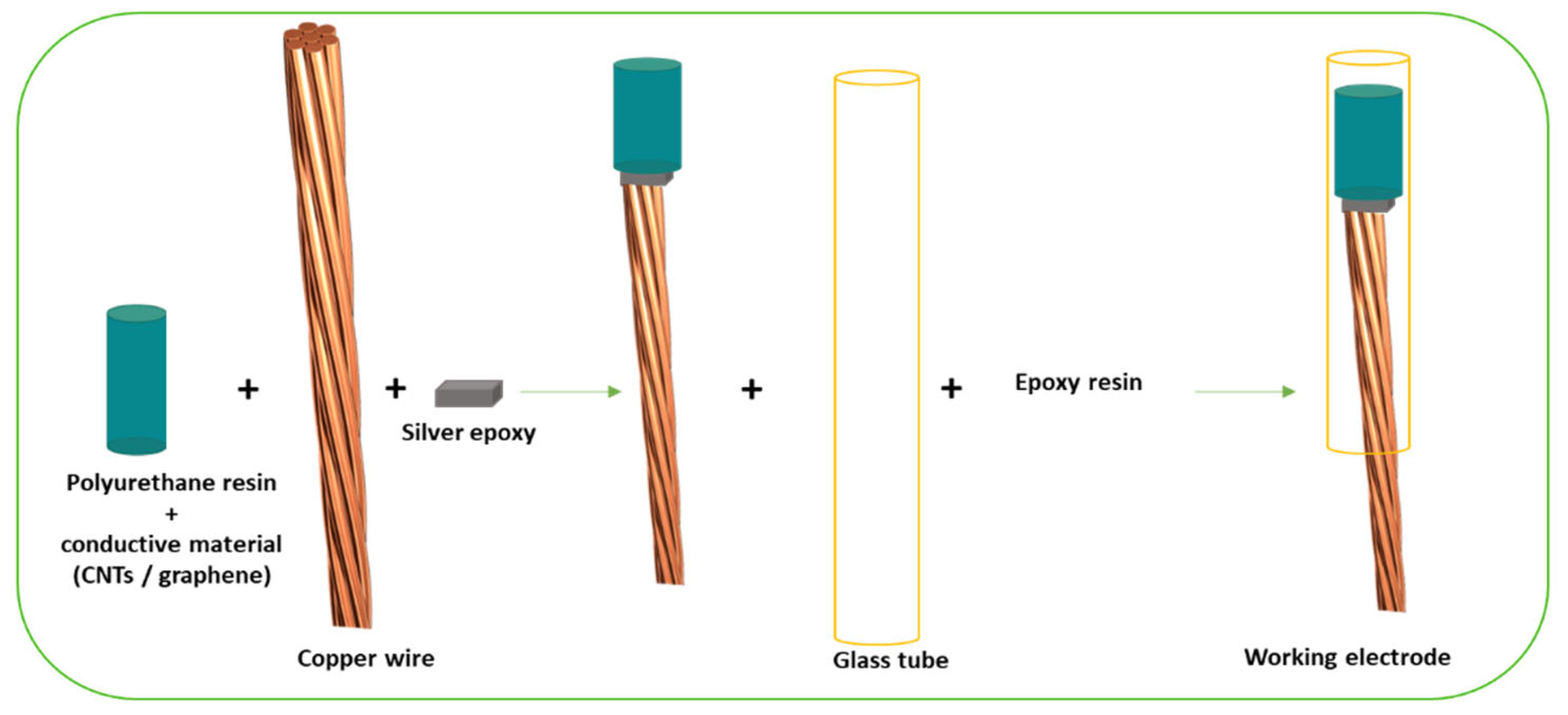
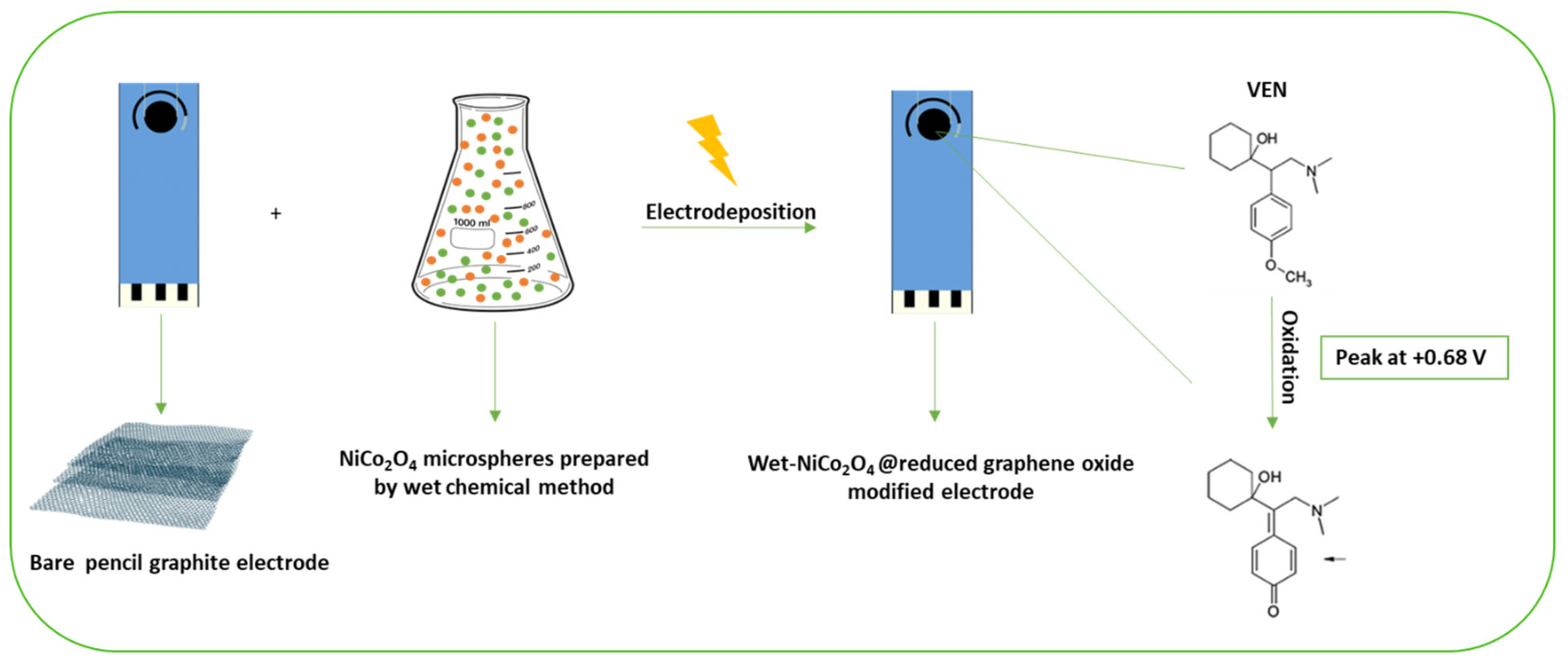
2.1. Carbon-Based Nanomaterials
2.2. Metal-Based Nanoparticles
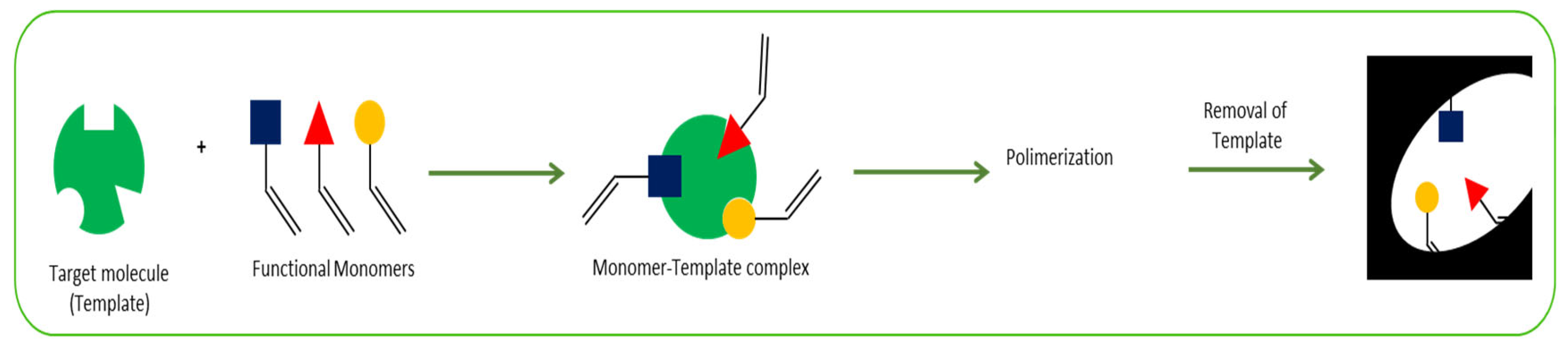
2.3. Molecularly Imprinted Polymers
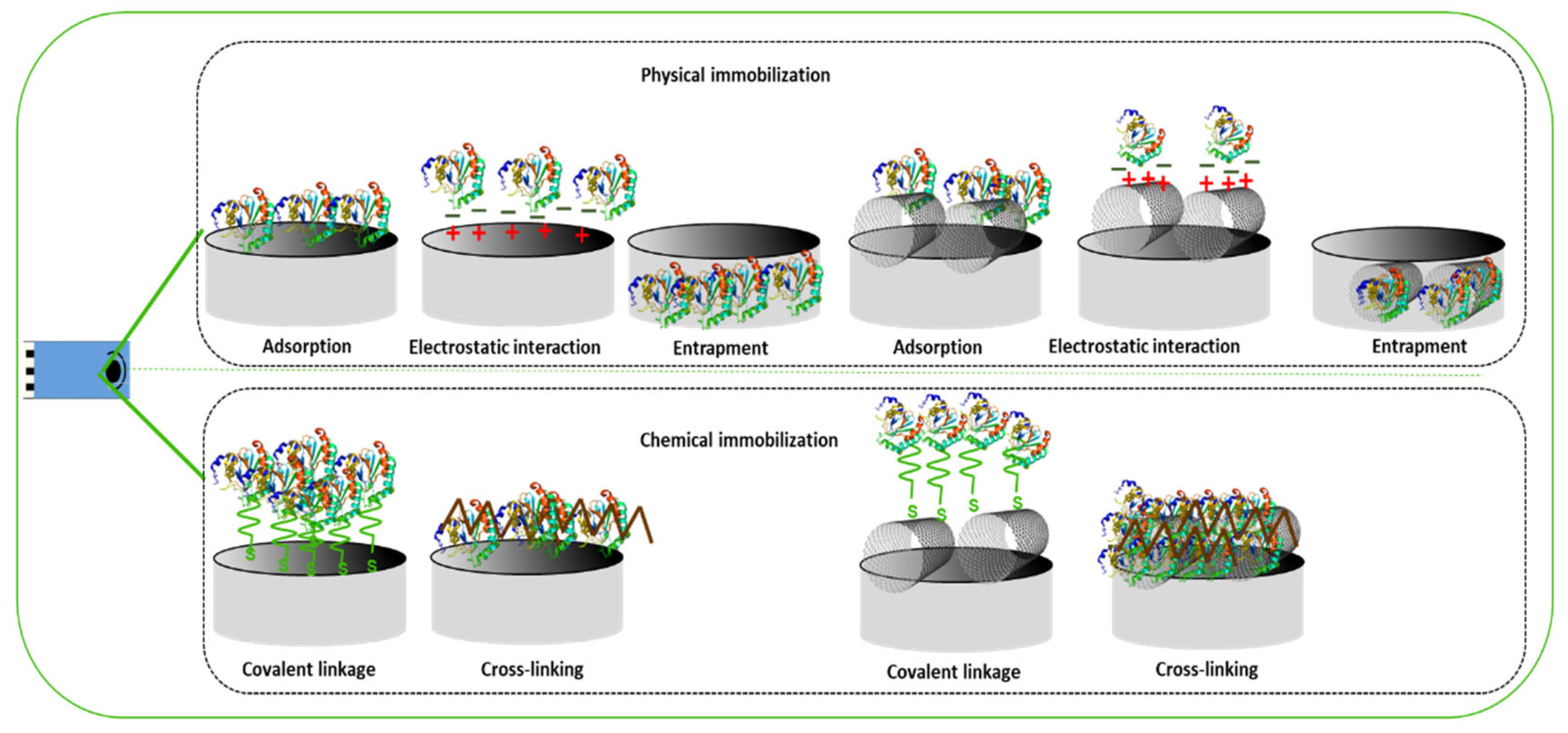
3. Enzyme-Based Electrochemical Biosensors for the Detection of Antidepressants
| Platform | Enzyme | Inhibitor (Molecule) | Sample | Detection Method | Linear Range | LOD | References |
|---|---|---|---|---|---|---|---|
| Au/PANSA/CYPD6 | CYP2D6 | Paroxetine | Pharmaceutical | CV, DPV, SWV | 0.002 µmolL−1 | [78] | |
| Au/PANSA/CYPD6 | CYP2D6 | Sertraline | Pharmaceutical | CV, DPV, SWV | 0.2–1.4 µmolL−1 | 0.13 µmolL−1 | [77] |
| SPE-CNTs-GO/MAO | MAO | Moclobemide | Pharmaceutical | CV, AMP | 10 nmolL−1–0.1 mmolL−1 | 5.0 nmolL−1 | [84] |
| SPE-CNTs-GO/MAO | MAO | Amitriptyline | Pharmaceutical | CV, AMP | 10 nmolL−1–0.1 mmolL−1 | 8.0 nmolL−1 | [69] |
| MWCNTs-AgNP-MAO | MAO | Amitriptyline | Pharmaceutical, urine | CV, AMP | 10 nmolL−1–0.1 mmolL−1 | 8.0 nmolL−1 | [88] |
| MWCNTs-AgNP-MAO | MAO | Imipramine | Pharmaceutical, urine | CV, AMP | 10 nmolL−1–0.1 mmolL−1 | 7.0 nmolL−1 | [88] |
| Pt-SPE/MAO r | MAO | Fluoxetine | Pharmaceutical | CV, AMP | 1.0 nmolL−1–0.1 mmolL−1 | 0.8 nmolL−1 | [86] |
| Pt-SPE/MAO r | MAO | Petylyl | Pharmaceutical | CV, AMP | 10 nmolL−1–0.1 mmolL−1 | 8 nmolL−1 | [86] |
| Pt-SPE/MAO r | MAO | Pyrazidol | Pharmaceutical | CV, AMP | 0.1 µmolL−1–0.1 mmol L−1 | 0.8 µmolL−1 | [86] |
| PT-SPE/PPy/MAO | MAO | Fluoxetine | Pharmaceutical | FIA | 0.674.33 mmolL−1 | 0.1 mmolL−1 | [85] |
| SPE/MWCNT/MAO | MAO | Imipramine | Pharmaceutical, urine | CV | 1 × 10−9–1 × 10−4 molL−1 | 0.8 nmolL−1 | [87] |
| MnO2/SPE | MAO | Selegeline | Pharmaceuticals | FIA | 0.513.25 µgmL−1 | 0.15 µgmL−1 | [83] |
| SPGE/RGO/CoNPs/MAO | MAO | Fluoxetine | Pharmaceutical, urine | CV | 5.0 nmolL−1–0.1 mmolL−1 | 0.8 nmolL−1 | [90] |
| SPGE/RGO/CoNPs/MAO | MAO | Thioridazine | Pharmaceutical, urine | CV | 5.0 nmolL−1–0.1 m mmolL−1 | 8.0 nmolL−1 | [90] |
| SPGE/RGO/CoNPs/MAO | MAO | Tianeptine | Pharmaceutical, urine | CV | 5.0 nmolL−1–0.1 m mmolL−1 | 7.0 nmolL−1 | [90] |
| SPE/MWCNTs/AuNPAgNP/MAO | MAO | Amitryptiline | Pharmaceutical, urine | CV | -- | -- | [89] |
| SPE/MWCNTs/AuNPAgNP/MAO | MAO | Moclobemide | Pharmaceutical, urine | CV | 5.0 nmolL−1–0.1 mmolL−1 | 0.8 nmolL−1 | [89] |
| SPE/MWCNTs/AuNPAgNP/MAO | MAO | Tianeptine | Pharmaceutical, urine | CV | 10 nmolL−1–0.1 mmolL−1 | 7.0 nmolL−1 | [89] |
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Orgnaztion. Depression. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 12 October 2022).
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Arlington County, VA, USA, 2013. [Google Scholar] [CrossRef]
- Chiriţă, A.L.; Gheorman, V.; Bondari, D.; Rogoveanu, I. Current understanding of the neurobiology of major depressive disorder. Rom. J. Morphol. Embryol. 2015, 56, 651–658. [Google Scholar] [PubMed]
- Lopez-Munoz, F.; Alamo, C. Monoaminergic Neurotransmission: The History of the Discovery of Antidepressants from 1950s Until Today. Curr. Pharm. Des. 2009, 15, 1563–1586. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, L.; Lapidus, K.A.B.; Iosifescu, D.V. Diagnosis and Treatment of Major Depressive Disorder. Neurol. Clin. 2011, 29, 177–193. [Google Scholar] [CrossRef] [PubMed]
- Zajecka, J.M. Clinical issues in long-term treatment with antidepressants. J. Clin. Psychiatry 2000, 61 (Suppl. 2), 20–25. [Google Scholar]
- Dunbar, G.C.; Claghorn, J.L.; Kiev, A.; Rickels, K.; Smith, W.T. A comparison of paroxetine and placebo in depressed outpatients. Acta Psychiatr. Scand. 1993, 87, 302–305. [Google Scholar] [CrossRef]
- Khawam, E.A.; Laurencic, G.; Malone, D.A. Side effects of antidepressants: An overview. Cleve. Clin. J. Med. 2006, 73, 351–361. [Google Scholar] [CrossRef]
- Shelton, R.C.; Tollefson, G.D.; Tohen, M.; Stahl, S.; Gannon, K.S.; Jacobs, T.G.; Buras, W.R.; Bymaster, F.P.; Zhang, W.; Spencer, K.A.; et al. A novel augmentation strategy for treating resistant major depression. Am. J. Psychiatry 2001, 158, 131–134. [Google Scholar] [CrossRef]
- Roszkowska, A.; Plenis, A.; Kowalski, P.; Bączek, T.; Olędzka, I. Recent advancements in techniques for analyzing modern, atypical antidepressants in complex biological matrices and their application in biomedical studies. TrAC-Trends Anal. Chem. 2022, 152, 116609. [Google Scholar] [CrossRef]
- Montenarh, D.; Wernet, M.P.; Hopf, M.; Maurer, H.H.; Schmidt, P.H.; Ewald, A.H. Quantification of 33 antidepressants by LC-MS/MS-Comparative validation in whole blood, plasma, and serum. Anal. Bioanal. Chem. 2014, 406, 5939–5953. [Google Scholar] [CrossRef]
- Manousi, N.; Samanidou, V.F. Recent Advances in the HPLC Analysis of Tricyclic Antidepressants in Bio-Samples. Mini-Rev. Med. Chem. 2019, 20, 24–38. [Google Scholar] [CrossRef]
- Truta, L.; Castro, A.L.; Tarelho, S.; Costa, P.; Sales, M.G.F.; Teixeira, H.M. Antidepressants detection and quantification in whole blood samples by GC–MS/MS, for forensic purposes. J. Pharm. Biomed. Anal. 2016, 128, 496–503. [Google Scholar] [CrossRef]
- Perumal, V.; Hashim, U. Advances in biosensors: Principle, architecture and applications. J. Appl. Biomed. 2014, 12, 1–15. [Google Scholar] [CrossRef]
- Orazio, P.D. Biosensors in clinical chemistry. Clin. Chim. Acta 2003, 334, 41–69. [Google Scholar] [CrossRef]
- Ronkainen, N.J.; Halsall, H.B.; Heineman, W.R. Electrochemical biosensors. Chem. Soc. Rev. 2010, 39, 1747–1763. [Google Scholar] [CrossRef]
- Pänke, O.; Balkenhohl, T.; Kafka, J.; Schäfer, D.; Lisdat, F. Impedance spectroscopy and biosensing. Adv. Biochem. Eng. Biotechnol. 2007, 109, 195–237. [Google Scholar]
- Lima, H.R.S.; da Silva, J.S.; de Oliveira Farias, E.A.; Teixeira, P.R.S.; Eiras, C.; Nunes, L.C.C. Electrochemical sensors and biosensors for the analysis of antineoplastic drugs. Biosens. Bioelectron. 2018, 108, 27–37. [Google Scholar] [CrossRef]
- Qian, L.; Thiruppathi, A.R.; Elmahdy, R.; van der Zalm, J.; Chen, A. Graphene-oxide-based electrochemical sensors for the sensitive detection of pharmaceutical drug naproxen. Sensors 2020, 20, 1252. [Google Scholar] [CrossRef]
- Yang, Z.; Ding, X.; Guo, Q.; Wang, Y.; Lu, Z.; Ou, H.; Luo, Z.; Lou, X. Second generation of signaling-probe displacement electrochemical aptasensor for detection of picomolar ampicillin and sulfadimethoxine. Sens. Actuators B Chem. 2017, 253, 1129–1136. [Google Scholar] [CrossRef]
- Kalanur, S.S.; Seetharamappa, J.; Prashanth, S.N. Voltammetric sensor for buzepide methiodide determination based on TiO2 nanoparticle-modified carbon paste electrode. Colloids Surf. B Biointerfaces 2010, 78, 217–221. [Google Scholar] [CrossRef]
- Stelmachowski, P.; Maj, D.; Grzybek, G.; Kruczała, K.; Kotarba, A. Functionalization of Graphite with Oxidative Plasma. Int. J. Mol. Sci. 2022, 23, 9650. [Google Scholar] [CrossRef]
- Myasoedova, T.N.; Kalusulingam, R.; Mikhailova, T.S. Sol-Gel Materials for Electrochemical Applications: Recent Advances. Coatings 2022, 12, 1625. [Google Scholar] [CrossRef]
- Ammarullah, M.I.; Afif, I.Y.; Maula, M.I.; Winarni, T.I.; Tauviqirrahman, M.; Akbar, I.; Basri, H.; van der Heide, E.; Jamari, J. Tresca stress simulation of metal-on-metal total hip arthroplasty during normal walking activity. Materials 2021, 14, 7554. [Google Scholar] [CrossRef] [PubMed]
- Soto, R.J.; Hall, J.R.; Brown, M.D.; Taylor, J.B.; Schoenfisch, M.H. In Vivo Chemical Sensors: Role of Biocompatibility on Performance and Utility. Anal. Chem. 2017, 89, 276–299. [Google Scholar] [CrossRef] [PubMed]
- Gilmartin, M.A.T.; Hart, J.P. Sensing with chemically and biologically modified carbon electrodes: A review. Analyst 1995, 120, 1029–1045. [Google Scholar] [CrossRef]
- Durst, R.A.; Bäumner, A.J.; Murray, R.W.; Buck, R.P.; Andrieux, C.P. Chemically modified electrodes: Recommended terminology and definitions. Pure Appl. Chem. 1997, 69, 1317–1323. [Google Scholar] [CrossRef]
- McClure, E.W.; Daniels, R.N. Classics in Chemical Neuroscience: Amitriptyline. ACS Chem. Neurosci. 2021, 12, 354–362. [Google Scholar] [CrossRef]
- Henrique Duarte, E.; Dos Santos, W.P.; Fantinato Hudari, F.; Bott Neto, J.L.; Romão Sartori, E.; Dallantonia, L.H.; César Pereira, A.; Teixeira Tarley, C.R. A highly improved method for sensitive determination of amitriptyline in pharmaceutical formulations using an unmodified carbon nanotube electrode in the presence of sulfuric acid. Talanta 2014, 127, 26–32. [Google Scholar] [CrossRef]
- Jat, M.S.; Meena, K.; Jhankal, K.K.; Sharma, D.K. Sensitive electro-chemical determination of antidepressant drug clomipramine at f-mwcnts nano clusters modified glassy carbon electrode. J. Pharm. Sci. Res. 2019, 11, 700–707. [Google Scholar]
- Jiwanti, P.K.; Wardhana, B.Y.; Sutanto, L.G.; Dewi, D.M.M.; Putri, I.Z.D.; Savitri, I.N.I. Recent Development of Nano-Carbon Material in Pharmaceutical Application: A Review. Molecules 2022, 27, 7578. [Google Scholar] [CrossRef]
- Pastoor, D.; Gobburu, J. Clinical pharmacology review of escitalopram for the treatment of depression. Expert Opin. Drug Metab. Toxicol. 2014, 10, 121–128. [Google Scholar] [CrossRef]
- Adjei, K.; Adunlin, G.; Ali, A.A. Impact of Sertraline, Fluoxetine, and Escitalopram on Psychological Distress among United States Adult Outpatients with a Major Depressive Disorder. Healthcare 2023, 11, 740. [Google Scholar] [CrossRef]
- Baccarin, M.; Cervini, P.; Cavalheiro, E.T.G. Comparative performances of a bare graphite-polyurethane composite electrode unmodified and modified with graphene and carbon nanotubes in the electrochemical determination of escitalopram. Talanta 2018, 178, 1024–1032. [Google Scholar] [CrossRef]
- dos Santos Neto, A.G.; de Sousa, C.S.; da Silva Freires, A.; Silva, S.M.; Zanin, H.; Damos, F.S.; de Cássia Silva Luz, R. Electrochemical sensor for detection of imipramine antidepressant at low potential based on oxidized carbon nanotubes, ferrocenecarboxylic acid, and cyclodextrin: Application in psychotropic drugs and urine samples. J. Solid State Electrochem. 2018, 22, 1385–1394. [Google Scholar] [CrossRef]
- De Vane, C.L.; Liston, H.L.; Markowitz, J.S. Clinical pharmacokinetics of sertraline. Clin. Pharmacokinet. 2002, 41, 1247–1266. [Google Scholar] [CrossRef]
- Atty, S.A.; Ibrahim, A.H.; Ibrahim, H.; Abdelzaher, A.M.; Abdel-Raoof, A.M.; Fouad, F.A. Simultaneous voltammetric detection of anti-depressant drug, sertraline HCl and paracetamol in biological fluid at CNT-cesium modified electrode in micellar media. Microchem. J. 2020, 159, 105524. [Google Scholar] [CrossRef]
- Berger, M.; Gastpar, M. Trimipramine: A challenge to current concepts on antidepressives. Eur. Arch. Psychiatry Clin. Neurosci. 1996, 246, 235–239. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Rabiei, S.; Rezaei, B.; Jafari-Asl, M. Combined microporous membrane-based liquid-liquid-liquid microextraction and in situ differential pulse voltammetry for highly sensitive detection of trimipramine. Anal. Methods 2013, 5, 4027–4033. [Google Scholar] [CrossRef]
- Scott, M.A.; Shelton, P.S.; Gattis, W. Therapeutic options for treating major depression, and the role of venlafaxine. Pharmacotherapy 1996, 16, 352–365. [Google Scholar]
- Ding, L.; Li, L.; You, W.; Gao, Z.N.; Yang, T.L. Electrocatalytic oxidation of venlafaxine at a multiwall carbon nanotubes-ionic liquid gel modified glassy carbon electrode and its electrochemical determination. Croat. Chem. Acta 2015, 88, 81–87. [Google Scholar] [CrossRef]
- Peña-Bahamonde, J.; Nguyen, H.N.; Fanourakis, S.K.; Rodrigues, D.F. Recent advances in graphene-based biosensor technology with applications in life sciences. J. Nanobiotechnol. 2018, 16, 75. [Google Scholar] [CrossRef]
- Pumera, M. Graphene in biosensing. Mater. Today 2011, 14, 308–315. [Google Scholar] [CrossRef]
- De Toledo, R.A.; Santos, M.C.; Honório, K.M.; Da Silva, A.B.F.; Cavalheiro, E.T.G.; Mazo, L.H. Use of graphite polyurethane composite electrode for imipramine oxidation-Mechanism proposal and electroanalytical determination. Anal. Lett. 2006, 39, 507–520. [Google Scholar] [CrossRef]
- Oghli, A.H.; Soleymanpour, A. Polyoxometalate/reduced graphene oxide modified pencil graphite sensor for the electrochemical trace determination of paroxetine in biological and pharmaceutical media. Mater. Sci. Eng. C 2020, 108, 110407. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.F.B.; El-Zahry, M.R. A comparative study of different electrodeposited NiCo2O4 microspheres anchored on a reduced graphene oxide platform: Electrochemical sensor for anti-depressant drug venlafaxine. RSC Adv. 2019, 9, 31609–31620. [Google Scholar] [CrossRef]
- Deahl, M. Pharmacological treatment of depression: The role of paroxetine. Hosp. Med. 2001, 62, 38–42. [Google Scholar] [CrossRef]
- Jani, H.; Ritala, M.; Leskela, M. Atomic Layer Deposition of Noble Metals and Their Oxides Jani. Chem. Mater. 2014, 26, 786–801. [Google Scholar]
- Phetsang, S.; Kidkhunthod, P.; Chanlek, N.; Jakmunee, J. Copper/reduced graphene oxide film modified electrode for non-enzymatic glucose sensing application. Sci. Rep. 2021, 11, 9302. [Google Scholar] [CrossRef]
- Mazaheri, M.; Aashuri, H.; Simchi, A. Three-dimensional hybrid graphene/nickel electrodes on zinc oxide nanorod arrays as non-enzymatic glucose biosensors. Sens. Actuators B. Chem. 2017, 251, 462–471. [Google Scholar] [CrossRef]
- Hwang, J.; Wang, X.; Zhao, D.; Rex, M.M.; Cho, H.J.; Hyoung, W. Electrochimica Acta A novel nanoporous bismuth electrode sensor for in situ heavy metal detection. Electrochim. Acta 2019, 298, 440–448. [Google Scholar] [CrossRef]
- Keabadile, O.P.; Aremu, A.O.; Elugoke, S.E.; Fayemi, O.E. Green and traditional synthesis of copper oxide nanoparticles—Comparative study. Nanomaterials 2020, 10, 2502. [Google Scholar] [CrossRef]
- Nooranian, S.; Mohammadinejad, A.; Mohajeri, T.; Aleyaghoob, G.; Kazemi Oskuee, R. Biosensors based on aptamer-conjugated gold nanoparticles: A review. Biotechnol. Appl. Biochem. 2022, 69, 1517–1534. [Google Scholar] [CrossRef]
- Vandebriel, R.J.; De Jong, W.H. A review of mammalian toxicity of ZnO nanoparticles. Nanotechnol. Sci. Appl. 2012, 5, 61–71. [Google Scholar] [CrossRef]
- Tajik, S.; Safaei, M.; Beitollahi, H. A sensitive voltammetric sertraline nanosensor based on ZnFe2O4 nanoparticles modified screen printed electrode. Meas. J. Int. Meas. Confed. 2019, 143, 51–57. [Google Scholar] [CrossRef]
- Sultan, S.; Shah, A.; Firdous, N.; Nisar, J.; Ashiq, M.N.; Shah, I. A Novel Electrochemical Sensing Platform for the Detection of the Antidepressant Drug, Venlafaxine, in Water and Biological Specimens. Chemosensors 2022, 10, 400. [Google Scholar] [CrossRef]
- Madrakian, T.; Soleimani, M.; Afkhami, A. Electrochemical determination of fluvoxamine on mercury nanoparticle multi-walled carbon nanotube modified glassy carbon electrode. Sens. Actuators B Chem. 2015, 210, 259–266. [Google Scholar] [CrossRef]
- Shoja, Y.; Rafati, A.A.; Ghodsi, J. Electropolymerization of Ni-LD metallopolymers on gold nanoparticles enriched multi-walled carbon nanotubes as nano-structure electrocatalyst for efficient voltammetric sertraline detection in human serum. Electrochim. Acta 2016, 203, 281–291. [Google Scholar] [CrossRef]
- Rebelo, P.; Costa-Rama, E.; Seguro, I.; Pacheco, J.G.; Nouws, H.P.A.; Cordeiro, M.N.D.S.; Delerue-Matos, C. Molecularly imprinted polymer-based electrochemical sensors for environmental analysis. Biosens. Bioelectron. 2021, 172, 112719. [Google Scholar] [CrossRef]
- Seguro, I.; Rebelo, P.; Pacheco, J.G.; Delerue-Matos, C. Screen-Printed Electrode—A Simple, Fast, and Disposable Voltammetric Sensor for Trazodone. Sensors 2022, 22, 2819. [Google Scholar] [CrossRef]
- Rebelo, P.; Pacheco, J.G.; Voroshylova, I.V.; Seguro, I.; Cordeiro, M.N.D.S.; Delerue-Matos, C. Computational Modelling and Sustainable Synthesis of a Highly Selective Electrochemical MIP-Based Sensor for Citalopram Detection. Molecules 2022, 27, 3315. [Google Scholar] [CrossRef]
- Rebelo, P.; Pacheco, J.G.; Voroshylova, I.V.; Cordeiro, M.N.D.S.; Delerue-Matos, C. Development of a molecular imprinted electrochemiluminescence sensor for amitriptyline detection: From MD simulations to experimental implementation. Electrochim. Acta 2021, 397, 139273. [Google Scholar] [CrossRef]
- Branger, C.; Brisset, H. Advanced Electrochemical Molecularly Imprinted Polymer as Sensor Interfaces. Multidiscip. Digit. Publ. Inst. Proc. 2019, 15, 22. [Google Scholar]
- Hossain, M.F.; Heo, J.S.; Nelson, J.; Kim, I. Paper-based flexible electrode using chemically-modified graphene and functionalized multiwalled carbon nanotube composites for electrophysiological signal sensing. Information 2019, 10, 325. [Google Scholar] [CrossRef]
- Perez-Caballero, L.; Torres-Sanchez, S.; Bravo, L.; Mico, J.A.; Berrocoso, E. Fluoxetine: A case history of its discovery and preclinical development. Expert Opin. Drug Discov. 2014, 9, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, T.; Azizi, S. Graphene/graphite paste electrode incorporated with molecularly imprinted polymer nanoparticles as a novel sensor for differential pulse voltammetry determination of fluoxetine. Biosens. Bioelectron. 2016, 81, 198–206. [Google Scholar] [CrossRef]
- Khosrokhavar, R.; Motaharian, A.; Milani Hosseini, M.R.; Mohammadsadegh, S. Screen-printed carbon electrode (SPCE) modified by molecularly imprinted polymer (MIP) nanoparticles and graphene nanosheets for determination of sertraline antidepressant drug. Microchem. J. 2020, 159, 105348. [Google Scholar] [CrossRef]
- Akhoundian, M.; Alizadeh, T.; Ganjali, M.R.; Rafiei, F. A new carbon paste electrode modified with MWCNTs and nano-structured molecularly imprinted polymer for ultratrace determination of trimipramine: The crucial effect of electrode components mixing on its performance. Biosens. Bioelectron. 2018, 111, 27–33. [Google Scholar] [CrossRef]
- Aminikhah, M.; Babaei, A.; Taheri, A. A novel electrochemical sensor based on molecularly imprinted polymer nanocomposite platform for sensitive and ultra-selective determination of citalopram. J. Electroanal. Chem. 2022, 918, 116493. [Google Scholar] [CrossRef]
- Zhang, S.; Wright, G.; Yang, Y. Materials and techniques for electrochemical biosensor design and construction. Biosens. Bioelectron. 2000, 15, 273–282. [Google Scholar] [CrossRef]
- Rogers, K.R.; Mascini, M. Biosensors for Field Analytical Monitoring. Field Anal. Chem. Technol. 1998, 2, 317–331. [Google Scholar] [CrossRef]
- Rocchitta, G.; Spanu, A.; Babudieri, S.; Latte, G.; Madeddu, G.; Galleri, G.; Nuvoli, S.; Bagella, P.; Demartis, M.I.; Fiore, V.; et al. Enzyme biosensors for biomedical applications: Strategies for safeguarding analytical performances in biological fluids. Sensors 2016, 16, 780. [Google Scholar] [CrossRef]
- Zhao, W.W.; Xu, J.J.; Chen, H.Y. Photoelectrochemical enzymatic biosensors. Biosens. Bioelectron. 2017, 92, 294–304. [Google Scholar] [CrossRef]
- Poulos, T.L. Cytochrome P450. Curr. Opin. Struct. Biol. 1995, 5, 767–774. [Google Scholar] [CrossRef]
- Zanger, U.M.; Schwab, M. Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther. 2013, 138, 103–141. [Google Scholar] [CrossRef]
- Bistolas, N.; Wollenberger, U.; Jung, C.; Scheller, F.W. Cytochrome P450 biosensors—A review. Biosens. Bioelectron. 2005, 20, 2408–2423. [Google Scholar] [CrossRef]
- Iwuoha, E.; Ngece, R.; Klink, M.; Baker, P. Amperometric responses of CYP2D6 drug metabolism nanobiosensor for sertraline: A selective serotonin reuptake inhibitor. IET Nanobiotechnol. 2007, 1, 62–67. [Google Scholar] [CrossRef]
- Ajayi, R.F.; Nxusani, E.; Douman, S.F.; Jonnas, A.; Baker, P.G.L.; Iwuoha, E.I. An amperometric cytochrome P450-2D6 biosensor system for the detection of the selective serotonin reuptake inhibitors (SSRIs) paroxetine and fluvoxamine. J. Nano Res. 2016, 44, 208–228. [Google Scholar] [CrossRef]
- Jones, D.N.; Raghanti, M.A. The role of monoamine oxidase enzymes in the pathophysiology of neurological disorders. J. Chem. Neuroanat. 2021, 114, 101957. [Google Scholar] [CrossRef]
- Pitsillou, E.; Bresnehan, S.M.; Kagarakis, E.A.; Wijoyo, S.J.; Liang, J.; Hung, A.; Karagiannis, T.C. The cellular and molecular basis of major depressive disorder: Towards a unified model for understanding clinical depression. Mol. Biol. Rep. 2020, 47, 753–770. [Google Scholar] [CrossRef]
- Werner, F.M.; Coveñas, R. Classical neurotransmitters and neuropeptides involved in major depression: A review. Int. J. Neurosci. 2010, 120, 455–470. [Google Scholar] [CrossRef]
- Ishikawa, T.; Okano, M.; Minami, A.; Tsunekawa, H.; Satoyoshi, H.; Tsukamoto, Y.; Takahata, K.; Muraoka, S. Selegiline ameliorates depression-like behaviors in rodents and modulates hippocampal dopaminergic transmission and synaptic plasticity. Behav. Brain Res. 2019, 359, 353–361. [Google Scholar] [CrossRef]
- Aigner, M.; Preissegger, P.; Kalcher, K.; Mehmeti, E.; Macheroux, P.; Edmondson, D.; Ortner, A. Biosensor for the characterisation of hMAO B inhibitors and the quantification of selegiline. Talanta 2017, 174, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Brusnitsyn, D.V.; Medyantseva, E.P.; Varlamova, R.M.; Sitdikova, R.R.; Fattakhova, A.N.; Konovalova, O.A.; Budnikov, G.K. Carbon nanomaterials as electrode surface modifiers in development of amperometric monoamino oxidase biosensors. Inorg. Mater. 2016, 52, 1413–1419. [Google Scholar] [CrossRef]
- Vela, M.H.; De Jesus, D.S.; Couto, C.M.C.M.; Araújo, A.N.; Montenegro, M.C.B.S.M. Electroimmobilization of MAO into a polypyrrole film and its utilization for amperometric flow detection of antidepressant drugs. Electroanalysis 2003, 15, 133–138. [Google Scholar] [CrossRef]
- Medyantseva, E.P.; Varlamova, R.M.; Gimaletdinova, D.A.; Fattakhova, A.N.; Budnikov, G.K. An amperometric monoamine oxidase biosensor for determining some antidepressants. J. Anal. Chem. 2008, 63, 275–279. [Google Scholar] [CrossRef]
- Medyantseva, E.P.; Brusnitsyn, D.V.; Varlamova, R.M.; Beshevets, M.A.; Budnikov, H.C.; Fattakhova, A.N. Capabilities of amperometric monoamine oxidase biosensors based on screen-printed graphite electrodes modified with multiwall carbon nanotubes in the determination of some antidepressants. J. Anal. Chem. 2015, 70, 535–539. [Google Scholar] [CrossRef]
- Medyantseva, E.P.; Brusnitsyn, D.V.; Varlamova, R.M.; Maksimov, A.A.; Fattakhova, A.N.; Konovalova, O.A.; Budnikov, G.K. Effect of nanostructured materials as electrode surface modifiers on the analytical capacity of amperometric biosensors. Russ. J. Appl. Chem. 2015, 88, 40–49. [Google Scholar] [CrossRef]
- Medyantseva, E.P.; Brusnitsyn, D.V.; Varlamova, R.M.; Maksimov, A.A.; Konovalova, O.A.; Budnikov, H.C. Surface modification of electrodes by carbon nanotubes and gold and silver nanoparticles in monoaminoxidase biosensors for the determination of some antidepressants. J. Anal. Chem. 2017, 72, 362–370. [Google Scholar] [CrossRef]
- Medyantseva, E.P.; Brusnitsyn, D.V.; Varlamova, R.M.; Konovalova, O.A.; Budnikov, H.K. Nanostructured Composites Based on Graphene and Cobalt Nanoparticles in Monoamine Oxidase Biosensors for Determining Antidepressants. Inorg. Mater. 2019, 55, 1390–1398. [Google Scholar] [CrossRef]

| Tricyclic Antidepressant | Selective Serotonin Reuptake Inhibitors | ||
|---|---|---|---|
| Antidepressant | Chemical Structure | Antidepressant | Chemical Structure |
| Amitriptyline | 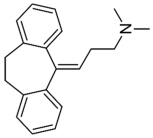 | Escitalopram | 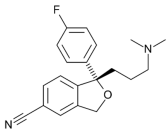 |
| Clomipramine | 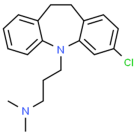 | Sertraline | 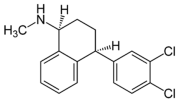 |
| Imipramine |  | Paroxetine | 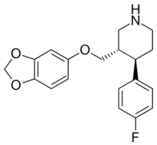 |
| Trimipramine |  | Fluvoxamine |  |
| Desipramine | 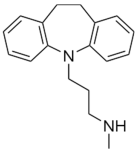 | Fluoxetine | 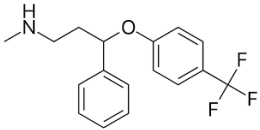 |
| Monoamine oxidase inhibitor | Serotonin antagonist and reuptake inhibitor | ||
| Selegiline | 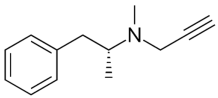 | Tradozone | 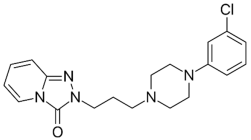 |
| Moclobemide |  | Serotonin and norepinephrine reuptake inhibitor | |
| Pirlindole |  | Venlafaxine |  |
| Antidepressant | Sample | Platform | Technique | Linear Range | LOD | References |
|---|---|---|---|---|---|---|
| Amitriptyline | Pharmaceutical | CNT/PE | CV, DPV, SWV | 1.61 µmolL−1 | [29] | |
| Buzepide Methiodide | Urine, human blood serum | TiO2/CPE | CV, DPV | µmolL−1 | 8.2 nmolL−1 | [21] |
| Clomipramine | Spiked human serum, urine | MWCNTs/GCE | DPV-AAdSV | mmol L−1 | 1.315 × 10−8 gmL−1 | [30] |
| Escitalopram | Urine, cerebrospinal fluid | EGPU-GR | DPV, SWV | 1.512 µmolL−1 | 0.25 µmolL−1 (SWV) 0.32 µmolL−1 (DPV) | [33] |
| Fluvoxamine | Urine, pharmaceuticals | HgNPs/MWCNT/GCE | CV, DPV | 0.0201.750 µmolL−1 | 0.01 µmolL−1 | [57] |
| Paroxetine | Urine, pharmaceuticals, blood serum | FCA-CD/CNT/GCE | CV, DPV | µmolL−1 | 0.03 µmolL−1 | [29] |
| Imipramine | Urine, pharmaceuticals | FCA-CD/CNT/GCE | CV, DPV | 10–350 µmolL−1 (CV) 0.1–10 µmolL−1 (DPV) | 0.03 µmolL−1 | [29] |
| Imipramine | Pharmaceuticals | EGRU | CV, SWV | --- | 4.60 nmolL−1 | [44] |
| Sertraline | Human serum | NiLD/AuNPs/MWCNTs/GCE | CV, DPV | 0.05–5.50 mmolL−1 | --- | [58] |
| Sertraline | Spiked plasma | CNT/CsMCPE/SDS | CV, SWV | 60.0 nM–15.0 µmolL−1 | 9.2 nmolL−1 | [37] |
| Sertraline | Pharmaceuticals, synthetic urine | ZnFe2O4NP/SPE | CV, DPV | 0.07–300 µmolL−1 | 0.02 µmolL−1 | [55] |
| Sertraline | Pharmaceuticals, human serum | MIP/Gr/SPE | CV, DPV | 5.0 nmolL−1mol L−1 | 1.99 nmolL−1 | [67] |
| Fluoxetine | Pharmaceuticals, plasms | Graphene/MIP/CPE | DPV | 6 nmolL−1–0.1 µmolL−1 | 2.8 nmol L−1 | [59] |
| Trimipramine | Human blood and serum | MWCNT/MIP/CPE | SWV | 0.10–25 nmolL−1 | 0.045 nmolL−1 | [61] |
| Trazodone | Tap water, human serum | MIP/SPCE | CV, DPV | 5–80 µmolL−1 | 1.6 µmolL−1 | [60] |
| Citalopram | Spiked river water | MIP/SPCE | CV, EIS | 0.10–1.25 µmolL−1 | 0.162 µmolL−1 | [61] |
| Citalopram | Pharmaceuticals, urine, serum | AMWCNTs@GONRs | CV, EIS, DPV | 0.5–10 µmolL−1 | 0.042 µmolL−1 | [69] |
| Trimipramine | Plasma, urine | MWCNT/CPE | DPV | ---- | 0.002 µmolL−1 | [31] |
| Venlafaxine | Pharmaceuticals | MWCNT/IL/GCE | CV, SWV | 2.0 µmolL−1–2.0mol L−1 | 1.69 µmolL−1 | [32] |
| Venlafaxine | Serum | CoPd@Al2O3/GCE | CV, SWASV | 1.95 mmolL−1–0.5 µmolL−1 | 1.96 pmolL−1 | [55] |
| Venlafaxine | Serum, pharmaceuticals | NiCO2O4/rGO/GCE | CV, SWV | 5.0500molL−1 | 3.4 nmolL−1 | [46] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caldevilla, R.; Morais, S.L.; Cruz, A.; Delerue-Matos, C.; Moreira, F.; Pacheco, J.G.; Santos, M.; Barroso, M.F. Electrochemical Chemically Based Sensors and Emerging Enzymatic Biosensors for Antidepressant Drug Detection: A Review. Int. J. Mol. Sci. 2023, 24, 8480. https://doi.org/10.3390/ijms24108480
Caldevilla R, Morais SL, Cruz A, Delerue-Matos C, Moreira F, Pacheco JG, Santos M, Barroso MF. Electrochemical Chemically Based Sensors and Emerging Enzymatic Biosensors for Antidepressant Drug Detection: A Review. International Journal of Molecular Sciences. 2023; 24(10):8480. https://doi.org/10.3390/ijms24108480
Chicago/Turabian StyleCaldevilla, Renato, Stephanie L. Morais, Agostinho Cruz, Cristina Delerue-Matos, Fernando Moreira, João G. Pacheco, Marlene Santos, and Maria Fátima Barroso. 2023. "Electrochemical Chemically Based Sensors and Emerging Enzymatic Biosensors for Antidepressant Drug Detection: A Review" International Journal of Molecular Sciences 24, no. 10: 8480. https://doi.org/10.3390/ijms24108480
APA StyleCaldevilla, R., Morais, S. L., Cruz, A., Delerue-Matos, C., Moreira, F., Pacheco, J. G., Santos, M., & Barroso, M. F. (2023). Electrochemical Chemically Based Sensors and Emerging Enzymatic Biosensors for Antidepressant Drug Detection: A Review. International Journal of Molecular Sciences, 24(10), 8480. https://doi.org/10.3390/ijms24108480