(±)-3-Deoxyradicinin Induces Stomata Opening and Chloroplast Oxidative Stress in Tomato (Solanum lycopersicum L.)
Abstract
1. Introduction
2. Results
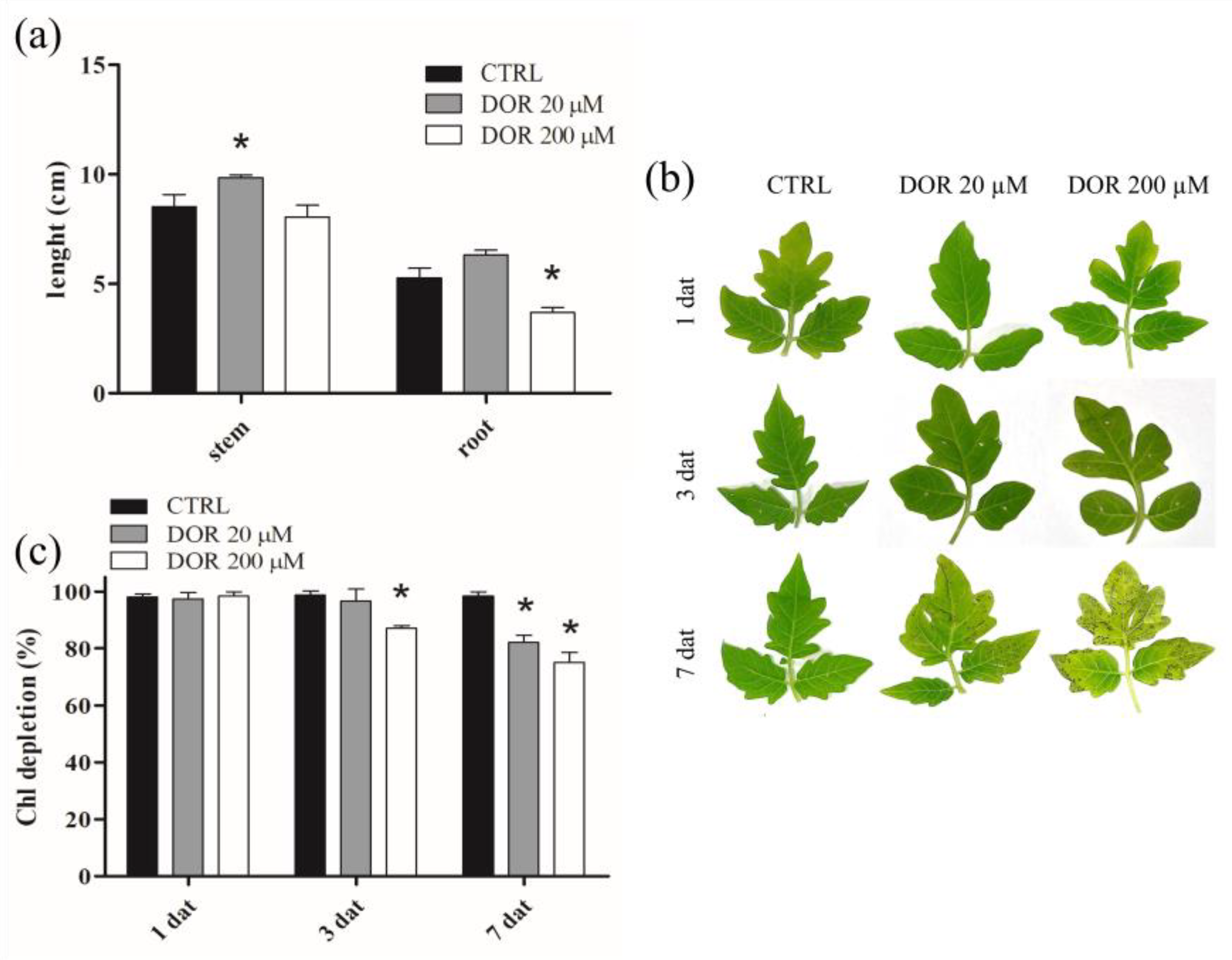
2.1. DOR Inhibited Root Growth and Induced Spot Lesions, Leaf Chlorosis, and Chlorophyll Loss in Tomato Leaves
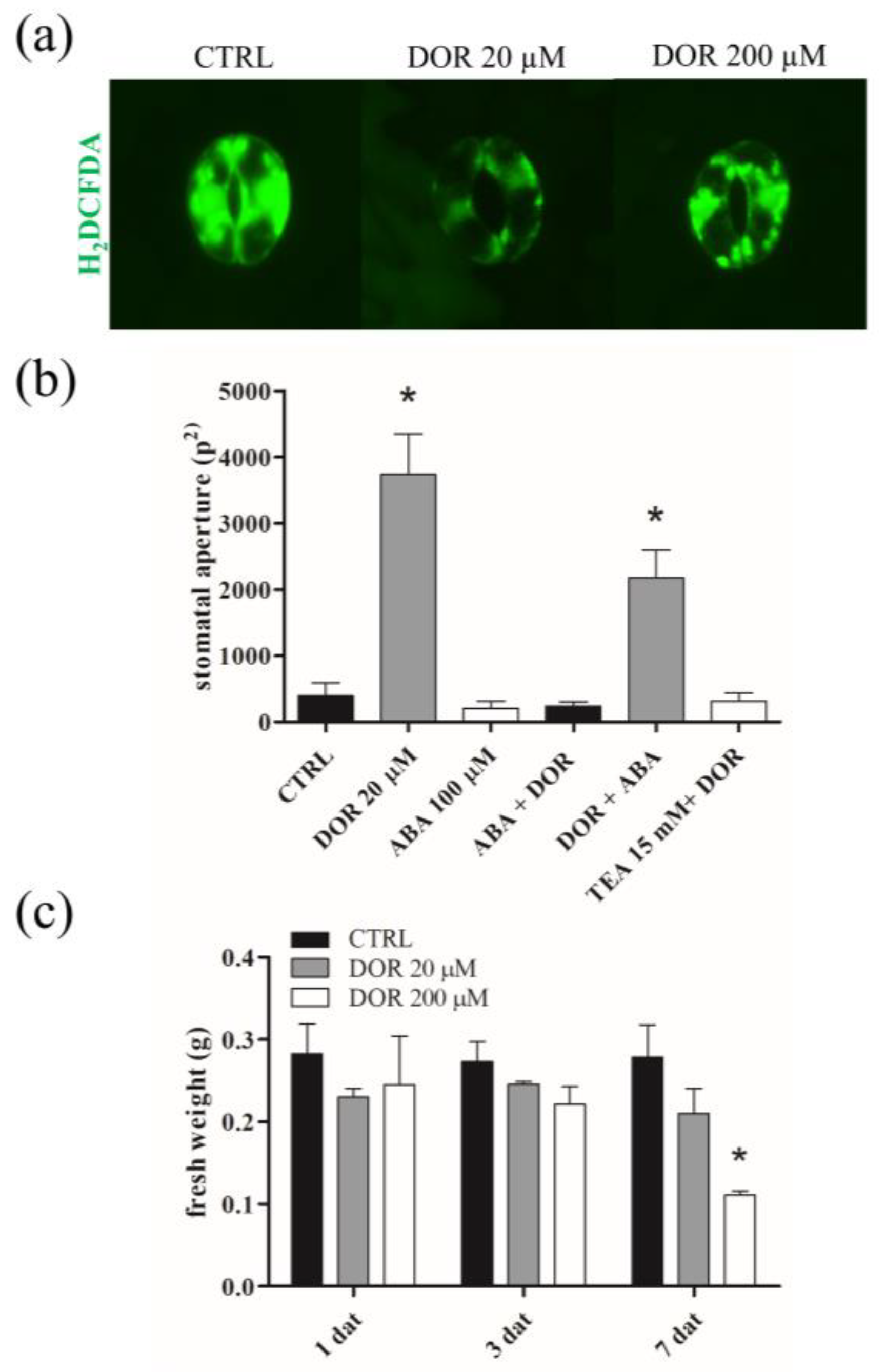
2.2. DOR Induced Stomata Opening and Wilting of Tomato Plants
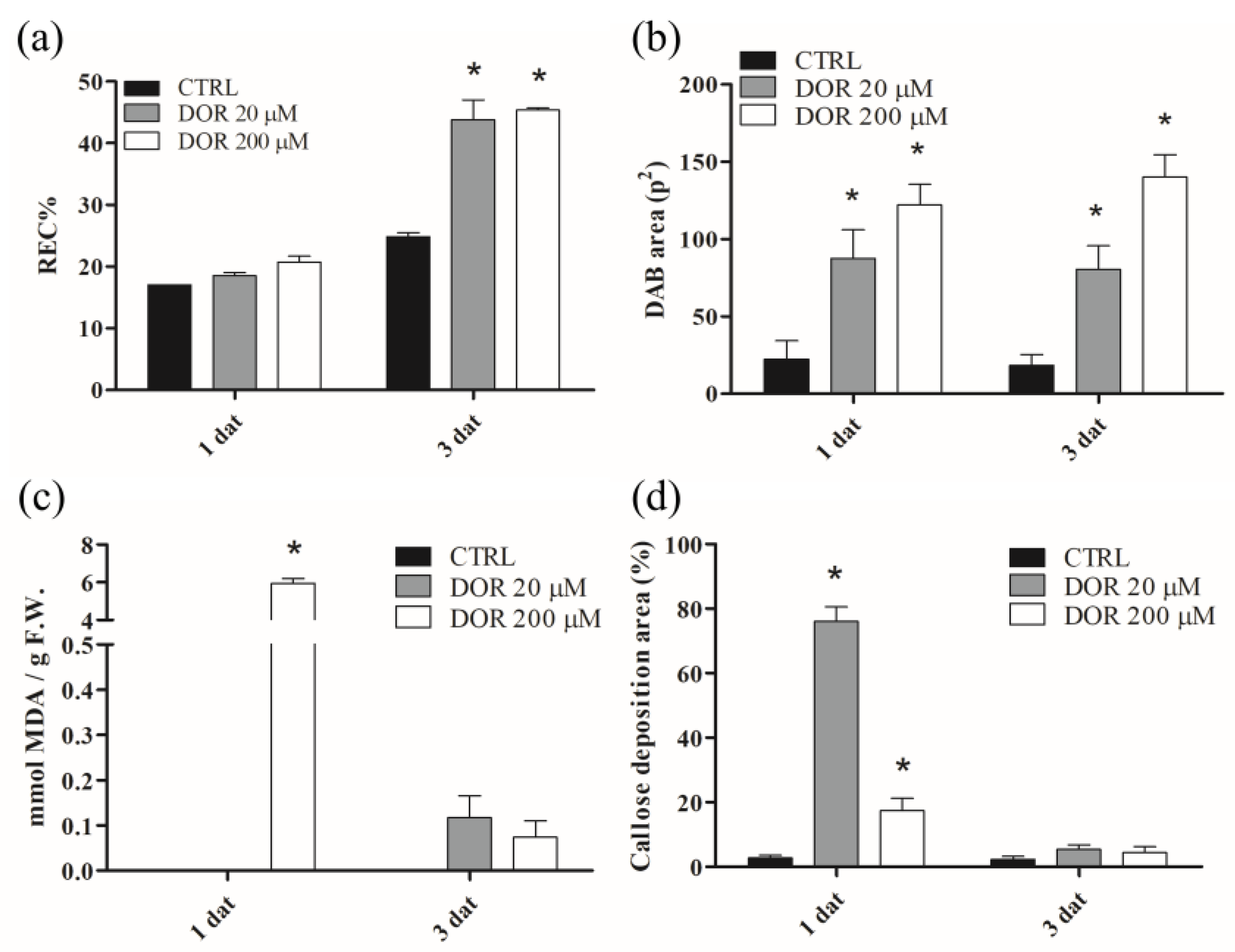
2.3. DOR Induced Ion Leakage, Hydrogen Peroxide Production, Membrane Lipid Peroxidation, and Callose Deposition in Tomato Leaves
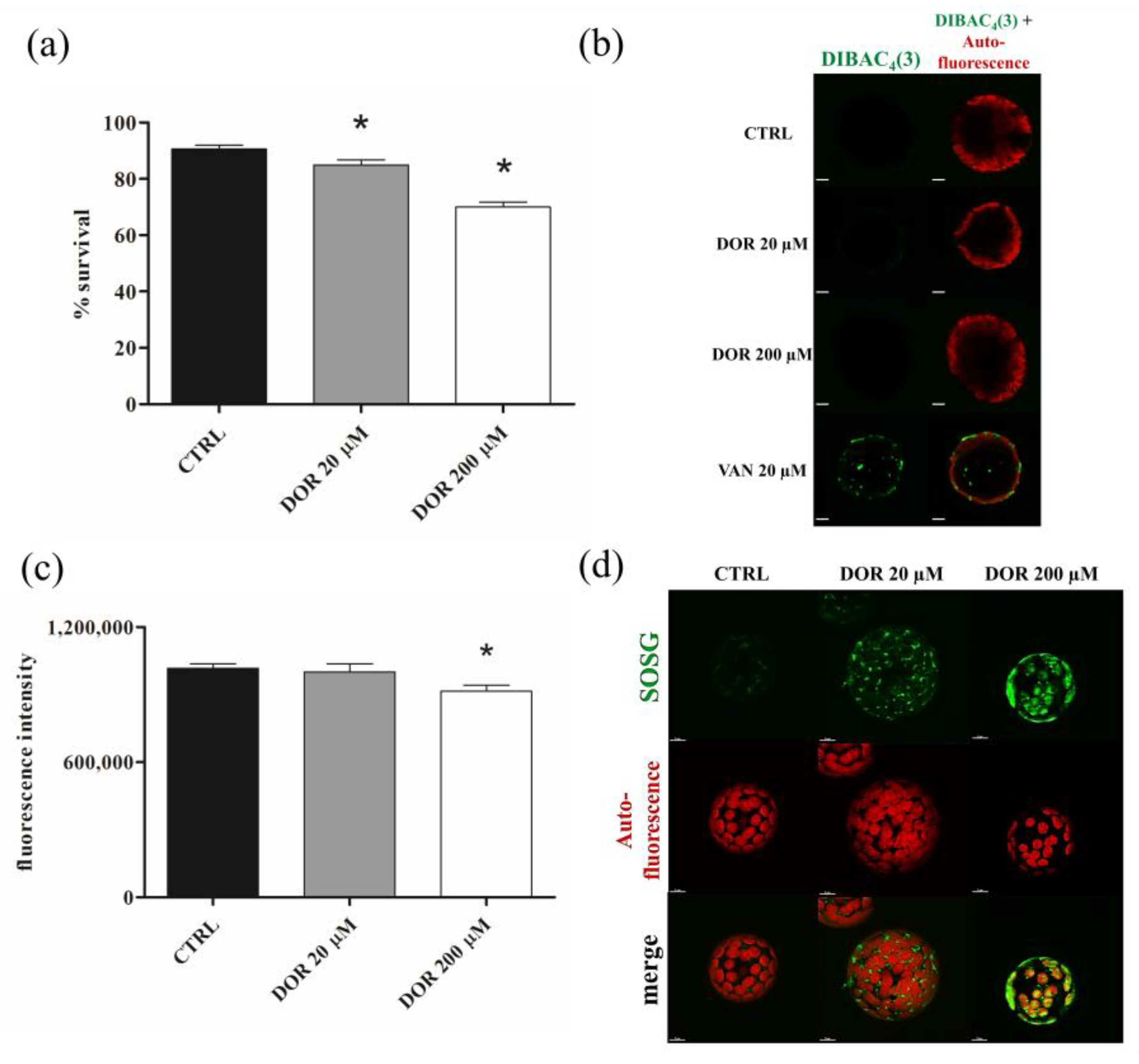
2.4. DOR Reduced Cell Viability and Induced Oxidative Stress in Chloroplasts from Tomato Leaf Protoplasts
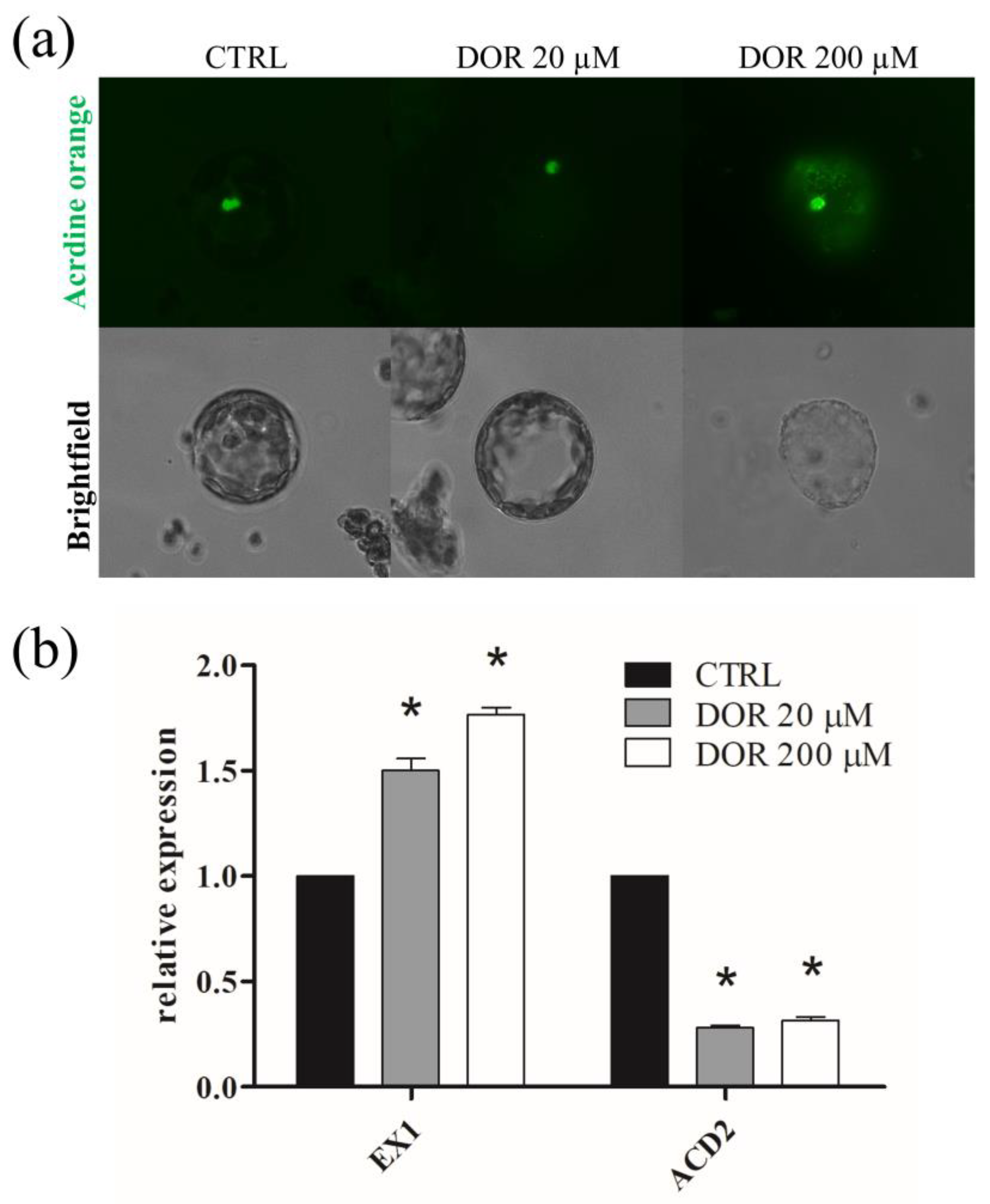
2.5. DOR Determined DNA Fragmentation in Protoplasts and Elicited the Transcription of Chloroplast-Induced Programmed Cell Death (PCD) Genes in Tomato Leaves
3. Discussion
4. Materials and Methods
4.1. (±)-3-Deoxyradicinin Preparation
4.2. Plant Growth and Treatments
4.3. Preparation of Protoplasts from Tomato Leaves
4.4. Total Chlorophyll Assay
4.5. Ion Leakage Assay
4.6. H2O2 Production Assay
4.7. Membrane Lipid Peroxidation Assay
4.8. Callose Deposition Assay
4.9. qRT-PCR Analysis of Genes Expression
4.10. Fluorescence and Confocal Microscopy
4.11. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
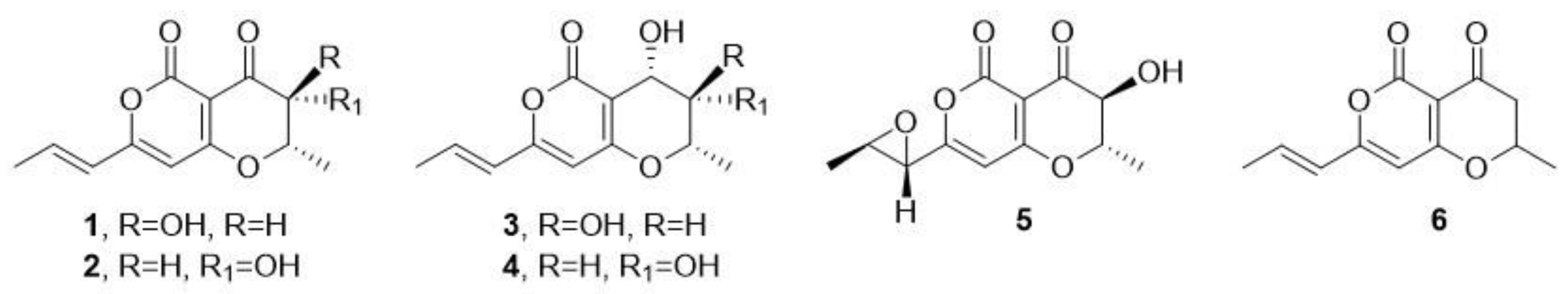
- Clarke, D.D.; Nord, F.F. Radicinin: A new pigment from Stemphyllium radicinum. Arch. Biochem. Biophys. 1953, 45, 643–657. [Google Scholar] [CrossRef] [PubMed]
- Nukina, M.; Marumo, S. Radicinol, a new metabolite of Cochliobolus lunatus, and absolute stereochemistry of radicinin. Tetrahedron Lett. 1977, 37, 3271–3272. [Google Scholar] [CrossRef]
- Noordelos, M.E.; De Gruyter, J.; Van Eijl, G.V.; Roeijmans, H.J. Production of dendritic crystals in pure cultures of Phoma and Ascochyta and its value as taxonomic character relative to morphology, pathology and cultural characteristics. Mycol. Res. 1993, 97, 1343–1350. [Google Scholar] [CrossRef]
- Kadam, S.; Poddig, J.; Humprey, P.; Karwowski, J.; Jackson, M.; Tennent, S.; Fung, L.; Hochlowski, J.; Rasmussen, R.; McAlpine, J. Citrinin hydrate and radicinin: Human rhinovirus 3 C-protease inhibitors discovered in a target-directed microbial screen. J. Antibiot. 1994, 47, 836–839. [Google Scholar] [CrossRef] [PubMed]
- Robeson, D.J.; Gary, G.R.; Strobel, G.A. Production of the phytotoxins radicinin and radicinol by Alternaria chrysanthemi. Phytochemistry 1982, 21, 1821–1823. [Google Scholar] [CrossRef]
- Robeson, D.J.; Burke, B.A.; Aasen, A.J. Phytotoxins from Alternaria helianthi: Radicinin and the structures of deoxyradicinol and radianthin. Phytochemistry 1985, 24, 729–731. [Google Scholar]
- Pryor, B.M.; Gilbertson, R.L. Relationships and taxonomic status of Alternaria radicina, A. carotiincultae and A. petroselini, based upon morphological, biochemical and molecular characteristics. Mycologia 2002, 94, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Grove, J.F. Metabolic products of Stemphylium radicinum. Part I. Radicinin. J. Chem. Soc. 1964, 1964, 3234–3239. [Google Scholar] [CrossRef]
- Santoro, E.; Mazzeo, G.; Marsico, G.; Masi, M.; Longhi, G.; Superchi, S.; Evidente, A.; Abbate, S. Assignment through chiroptical methods of the absolute configuration of fungal dihydropyranpyran-4-5-diones phytotoxins, potential herbicides for buffelgrass (Cenchrus Ciliaris) biocontrol. Molecules 2019, 24, 3022. [Google Scholar] [CrossRef]
- Masi, M.; Meyer, S.; Clement, S.; Cimmino, A.; Cristofaro, M.; Evidente, A. Cochliotoxin, a dihydropyranpyran-4-5-dione, and its analogues produced by Cochliobolus australiensis display phytotoxic activity against buffelgrass (Cenchrus ciliaris). J. Nat. Prod. 2017, 80, 1241–1247. [Google Scholar] [CrossRef]
- Masi, M.; Freda, F.; Sangermano, F.; Calabrò, V.; Cimmino, A.; Cristofaro, M.; Meyer, S.; Evidente, A. Radicinin, a fungal phytotoxin as a target-specific bioherbicide for invasive buffelgrass (Cenchrus ciliaris) control. Molecules 2019, 24, 1086. [Google Scholar] [CrossRef]
- Masi, M.; Freda, F.; Sangermano, F.; Calabrò, V.; Cimmino, A.; Cristofaro, M.; Meyer, S.; Evidente, A. Phytotoxic activity and structure-activity relationships of radicinin derivatives against the invasive weed buffelgrass (Cenchrus ciliaris). Molecules 2019, 24, 2793. [Google Scholar] [CrossRef] [PubMed]
- Hansen, R.O. Stemphylone, a root-killing substance from Stemphylium radicinum. Acta Chem. Scand. 1954, 8, 1332–1334. [Google Scholar] [CrossRef]
- Canning, A.M.; Hook, I.; Sheridan, H.; James, J.P.; Kelly, D.R. Bisradicinin: A novel dimer elicited in cultures of Alternaria chrysanthemi. J. Nat. Prod. 1992, 55, 487–490. [Google Scholar] [CrossRef]
- Nakajima, H.; Ishida, T.; Otsuka, Y.; Hamasaki, T.; Ichinoe, M. Phytotoxins and related metabolites produced by Bipolaris coicis, the pathogen of Job’s tears. Phytochemistry 1997, 45, 41–45. [Google Scholar] [CrossRef]
- Solfrizzo, M.; Vitti, C.; De Girolamo, A.; Visconti, A.; Logrieco, A.; Fanizzi, F.P. Radicinol and radicinin phytotoxins produced by Alternaria radicina on carrots. J. Agric. Food Chem. 2004, 52, 3655–3660. [Google Scholar] [CrossRef]
- Li, S.; Shao, M.-W.; Lu, Y.; Kong, L.-C.; Jiang, D.-H.; Zhang, I.-L. Phytotoxic and antibacterial metabolites from Fusarium proliferatum ZS07 isolated from the gut of long-horned grasshopppers. J. Agric. Food Chem. 2014, 62, 8997–9001. [Google Scholar] [CrossRef]
- Mathieu, V.; Superchi, S.; Masi, M.; Scafato, P.; Kornienko, A.; Evidente, A. In vitro effects of fungal phytotoxins on cancer cell viability: First insight into structure activity relationship of a potent metabolite of Cochliobolus australiensis radicinin. Toxins 2022, 14, 517. [Google Scholar] [CrossRef]
- Suzuki, M.; Sakuno, E.; Ishihara, A.; Tamura, J.I.; Nakajima, H. Conversions of deoxyradicinin to radicinin and of radicinin to 3-epi-radicinin in the phytopathogenic fungus Bipolaris coicis. Phytochemistry 2012, 75, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Marsico, G.; Ciccone, M.S.; Masi, M.; Freda, F.; Evidente, A.; Superchi, S.; Scafato, P. Synthesis and herbicidal activity against buffelgrass (Cenchrus ciliaris) of (±)-3-deoxyradicinin. Molecules 2019, 24, 3193. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, C.A.; Castro, C.A.; Blacutt, A.A.; Costa, E.A.; Brinton, K.C.; Corral, D.W.; Drodz, C.L.; Roper, M.C.; Rolshausen, P.E.; Maloney, K.N.; et al. Synthesis of deoxyradicinin an inhibitor of Xylella fastidiosa and Liberibacter crescens, a culturable surrogate for Candidatus Liberibacter asiaticus. J. Nat. Prod. 2020, 83, 1810–1816. [Google Scholar] [CrossRef] [PubMed]
- Aldrich, T.J.; Rolshausen, P.E.; Roper, M.C.; Reader, J.M.; Steinhaus, M.J.; Rapicavoli, J.; Vosburg, D.A.; Maloney, K.N. Radicinin from Cochliobolus sp. inhibits Xylella fastidiosa, the causal agent of Pierce’s disease of grapevine. Phytochemistry 2015, 116, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Marra, M.; Camoni, L.; Visconti, S.; Fiorillo, A.; Evidente, A. The surprising story of fusicoccin: A wilt-inducing toxin, a tool in plant physiology and a 14-3-3 targeted drug. Biomolecules 2021, 11, 1393. [Google Scholar] [CrossRef]
- Camoni, L.; Visconti, S.; Aducci, P.; Marra, M. From plant physiology to pharmacology: Fusicoccin leaves the leaves. Planta 2019, 249, 49–57. [Google Scholar] [CrossRef]
- Bharath, P.; Gahir, S.; Raghavendra, A.S. Abscisic acid-induced stomatal closure: An important component of plant defense against abiotic and biotic stress. Front. Plant Sci. 2021, 12, 615114. [Google Scholar] [CrossRef]
- Byzckowska, A.; Kunikowska, A.; Kazmierczak, A. Determination of ACC-induced cell programmed death in roots of Vicia faba ssp. minor seedlings by acridine orange and ethidium bromide staining. Protoplasma 2013, 250, 121–128. [Google Scholar] [CrossRef]
- Kim, W.C.; Meskauskiene, R.; Zhang, S.; Lee, K.P.; Ashok, L.M.; Blajecka, K.; Herrfurth, C.; Feussner, I.; Apela, K. Chloroplasts of Arabidopsis are the source and a primary target of a plant-specific programmed cell death signaling pathway. Plant Cell 2012, 24, 3026–3039. [Google Scholar] [CrossRef]
- Ambastha, V.; Chauhan, G.; Tiwari, B.S.; Tripathy, B.C. Execution of programmed cell death by singlet oxygen generated inside the chloroplast of Arabidospsis thaliana. Protoplasma 2020, 257, 841–851. [Google Scholar] [CrossRef]
- Pattanyak, G.K.; Venkataramani, S.; Hortensteiner, S.; Kunz, L.; Christ, B.; Moulin, M.; Smith, A.G.; Okamoto, Y.; Tamiaki, H.; Sugishima, M.; et al. Accelerated Cell Death 2 suppress mithocondrial oxidative burst and modulates cell death in Arabidopsis. Plant J. 2012, 69, 589–600. [Google Scholar]
- Doehlemann, G.; Ökmen, B.; Zhu, W.; Sharon, A. Plant pathogenic fungi. Microbiol. Spectr. 2016, 5, 1–20. [Google Scholar]
- Howlett, J.B. Secondary metabolite toxins and nutrition of plant pathogenic fungi. Curr. Opin. Plant Biol. 2006, 9, 371–375. [Google Scholar] [CrossRef]
- Samperna, S.; Boari, A.; Vurro, M.; Salzano, A.M.; Reveglia, P.; Evidente, A.; Gismondi, A.; Canini, A.; Scaloni, A.; Marra, M. Arabidopsis defense against the pathogenic fungus Drechslera gigantea is dependent on the integrity of the unfolded protein response. Biomolecules 2021, 11, 240. [Google Scholar] [CrossRef]
- Lee, K.P.; Kim, C.; Landgraf, F.; Apel, K. Executer1 and Executer2-dependent transfer of stress-related signals from the plastid to the nucleus of Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2007, 104, 1270–1275. [Google Scholar] [CrossRef] [PubMed]
- Resh, H.M.; Howard, M. Hydroponic Food Production: A Definitive Guidebook for the Advanced Home Gardener and the Commercial Hydroponic Grower; CRC Press: Santa Barbara, CA, USA, 2012; pp. 9–30. [Google Scholar]
- Fiorillo, A.; Mattei, M.; Aducci, P.; Visconti, A.; Camoni, L. The salt tolerance related protein (STRP) mediates cold stress responses and abscisic signaling in Arabidopsis thaliana. Front. Plant Sci. 2020, 11, 1251. [Google Scholar] [CrossRef]
- Samperna, S.; Masi, M.; Vurro, M.; Evidente, A.; Marra, M. Cyclopaldic acid, the main phytotoxic metabolite of Diplodia cupressi, induces programmed cell death and autophagy in Arabidopsis thaliana. Toxins 2022, 14, 474. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samperna, S.; Zanotti, C.; Scafato, P.; Boari, A.; Visconti, S.; Vurro, M.; Superchi, S.; Evidente, A.; Marra, M. (±)-3-Deoxyradicinin Induces Stomata Opening and Chloroplast Oxidative Stress in Tomato (Solanum lycopersicum L.). Int. J. Mol. Sci. 2023, 24, 8467. https://doi.org/10.3390/ijms24108467
Samperna S, Zanotti C, Scafato P, Boari A, Visconti S, Vurro M, Superchi S, Evidente A, Marra M. (±)-3-Deoxyradicinin Induces Stomata Opening and Chloroplast Oxidative Stress in Tomato (Solanum lycopersicum L.). International Journal of Molecular Sciences. 2023; 24(10):8467. https://doi.org/10.3390/ijms24108467
Chicago/Turabian StyleSamperna, Simone, Clarissa Zanotti, Patrizia Scafato, Angela Boari, Sabina Visconti, Maurizio Vurro, Stefano Superchi, Antonio Evidente, and Mauro Marra. 2023. "(±)-3-Deoxyradicinin Induces Stomata Opening and Chloroplast Oxidative Stress in Tomato (Solanum lycopersicum L.)" International Journal of Molecular Sciences 24, no. 10: 8467. https://doi.org/10.3390/ijms24108467
APA StyleSamperna, S., Zanotti, C., Scafato, P., Boari, A., Visconti, S., Vurro, M., Superchi, S., Evidente, A., & Marra, M. (2023). (±)-3-Deoxyradicinin Induces Stomata Opening and Chloroplast Oxidative Stress in Tomato (Solanum lycopersicum L.). International Journal of Molecular Sciences, 24(10), 8467. https://doi.org/10.3390/ijms24108467








