Sex and HDAC4 Differently Affect the Pathophysiology of Amyotrophic Lateral Sclerosis in SOD1-G93A Mice
Abstract
1. Introduction
2. Results
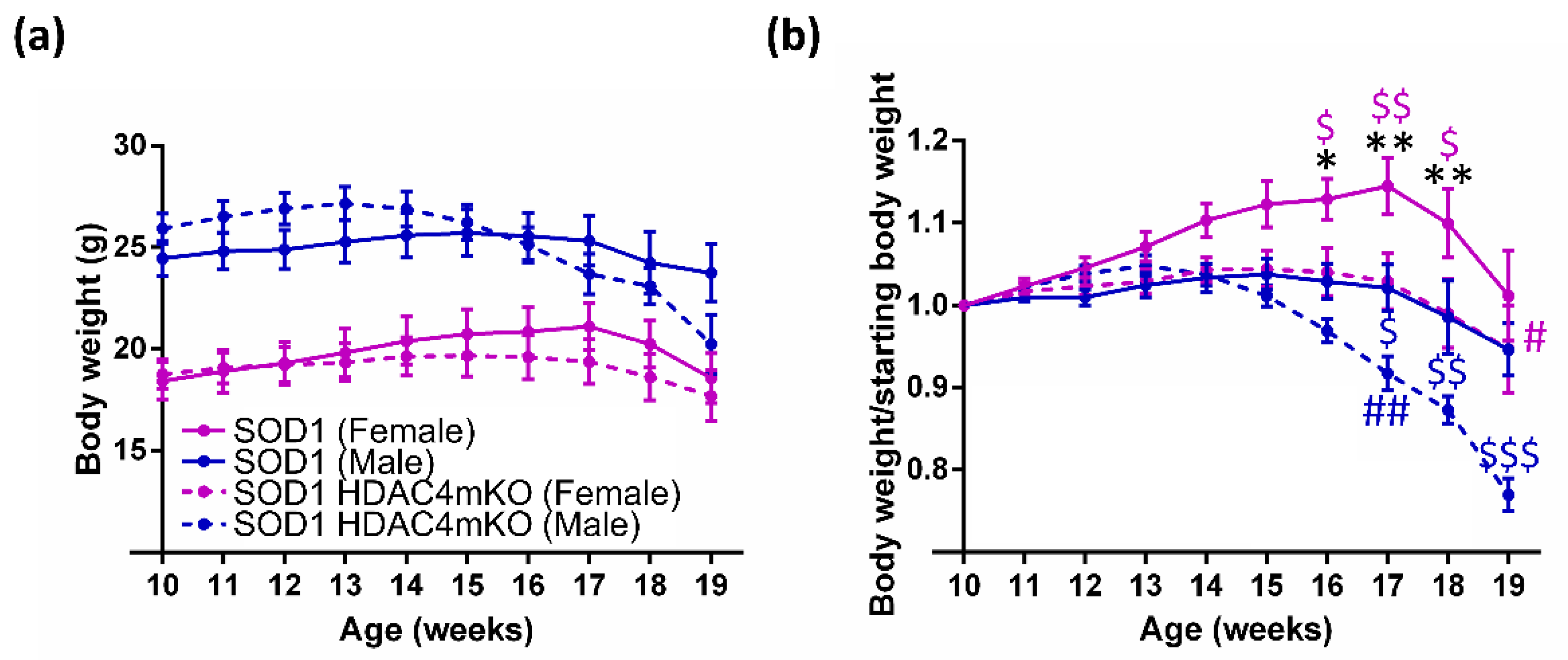
2.1. Sex and HDAC4 Influence Body Weight in SOD1 Mice
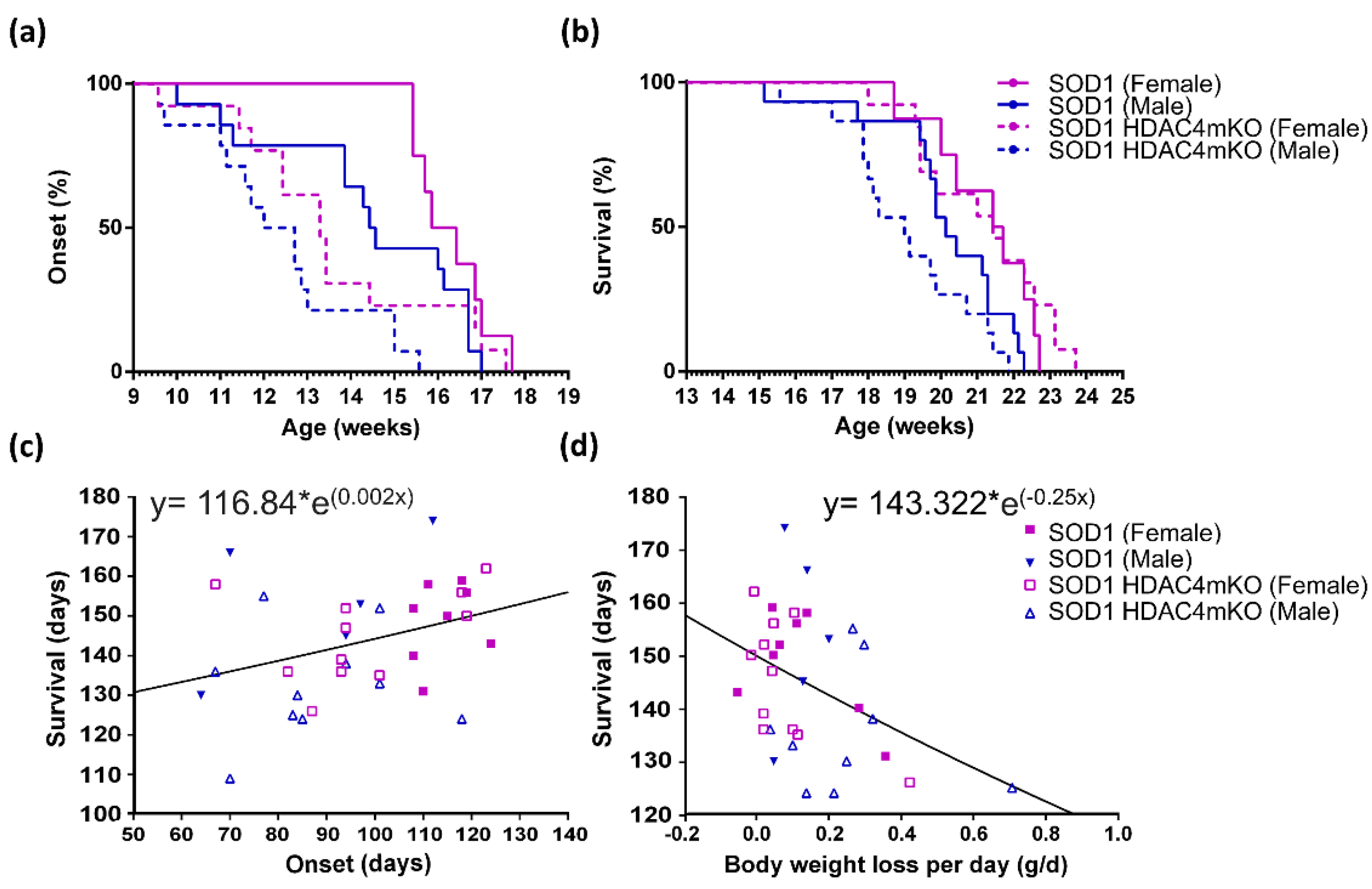
2.2. Sex and HDAC4 Differentially Affect ALS Onset and Survival in SOD1 Mice
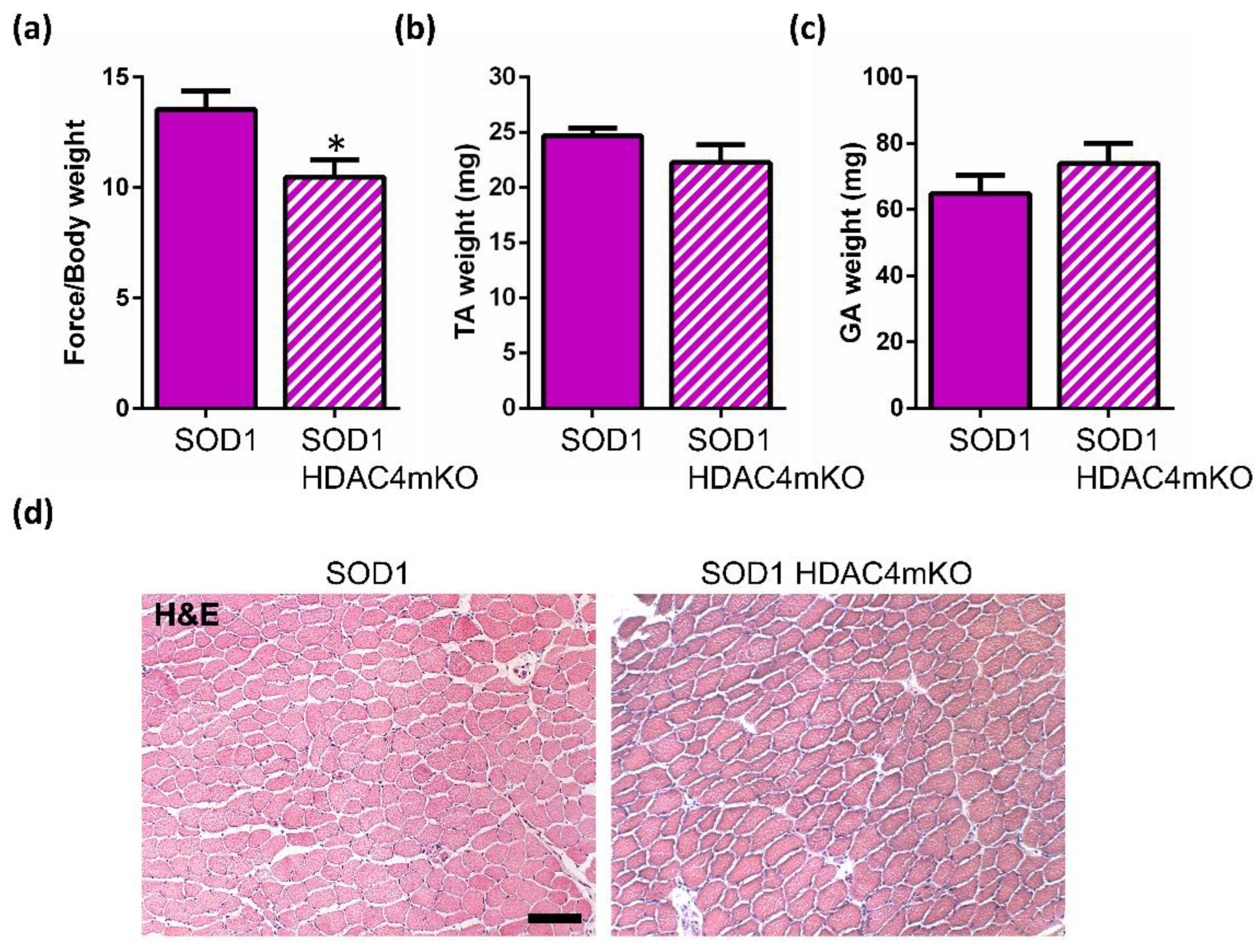
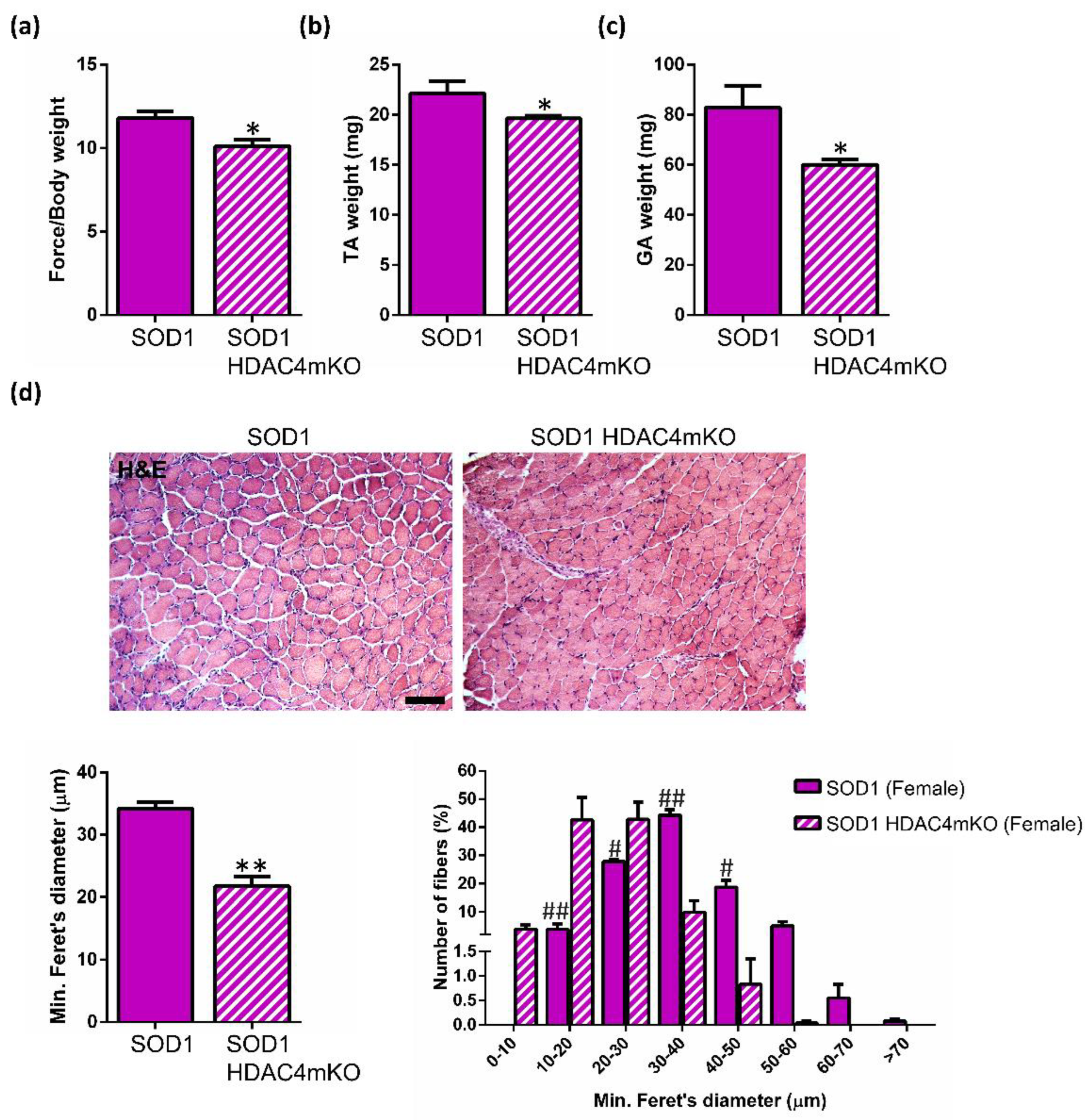
2.3. Deletion of HDAC4 Worsens the Pathological Features of Female SOD1 Muscles
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Functional Analyses
4.3. Histological Analyses
4.4. Morphometric Analyses
4.5. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosen, D. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 1993, 364, 362. [Google Scholar] [CrossRef] [PubMed]
- McCombe, P.A.; Henderson, R.D. Effects of gender in amyotrophic lateral sclerosis. Gend. Med. 2010, 7, 557–570. [Google Scholar] [CrossRef] [PubMed]
- Blasco, H.; Guennoc, A.M.; Veyrat-Durebex, C.; Gordon, P.H.; Andres, C.R.; Camu, W.; Corcia, P. Amyotrophic lateral sclerosis: A hormonal condition? Amyotroph. Lateral. Scler. 2012, 13, 585–588. [Google Scholar] [CrossRef] [PubMed]
- Portet, F.; Cadilhac, C.; Touchon, J.; Camu, W. Cognitive impairment in motor neuron disease with bulbar onset. Amyotroph. Lateral. Scler. Other Motor. Neuron. Disord. 2001, 2, 23–29. [Google Scholar] [CrossRef]
- Curtis, A.F.; Masellis, M.; Hsiung, G.Y.R.; Moineddin, R.; Zhang, K.; Au, B.; Millett, G.; MacKenzie, I.; Rogaeva, E.; Tierney, M.C. Sex differences in the prevalence of genetic mutations in FTD and ALS: A meta-analysis. Neurology 2017, 89, 1633–1642. [Google Scholar] [CrossRef] [PubMed]
- Murdock, B.J.; Famie, J.P.; Piecuch, C.E.; Raue, K.D.; Mendelson, F.E.; Pieroni, C.H.; Iniguez, S.D.; Zhao, L.; Goutman, S.A.; Feldman, E.L. NK cells associate with ALS in a sex- and age-dependent manner. JCI Insight 2021, 6, e147129. [Google Scholar]
- Cui, C.; Ingre, C.; Yin, L.; Li, X.; Andersson, J.; Seitz, C.; Ruffin, N.; Pawitan, Y.; Piehl, F.; Fang, F. Correlation between leukocyte phenotypes and prognosis of amyotrophic lateral sclerosis. Elife 2022, 11, e74065. [Google Scholar] [CrossRef]
- Pape, J.A.; Grose, J.H. The effects of diet and sex in amyotrophic lateral sclerosis. Rev. Neurol. 2020, 176, 301–315. [Google Scholar] [CrossRef]
- Nakamura, R.; Kurihara, M.; Ogawa, N.; Kitamura, A.; Yamakawa, I.; Bamba, S.; Sanada, M.; Sasaki, M.; Urushitani, M. Investigation of the prognostic predictive value of serum lipid profiles in amyotrophic lateral sclerosis: Roles of sex and hypermetabolism. Sci. Rep. 2022, 12, 1826. [Google Scholar] [CrossRef]
- Bame, M.; Pentiak, P.A.; Needleman, R.; Brusilow, W.S.A. Effect of sex on lifespan, disease progression, and the response to methionine sulfoximine in the SOD1 G93A mouse model for ALS. Gend. Med. 2012, 9, 524–535. [Google Scholar] [CrossRef]
- Choi-Il, C.; Lee, Y.D.; Gwag, B.J.; Cho, S.I.; Kim, S.S.; Suh-Kim, H. Effects of estrogen on lifespan and motor functions in female hSOD1 G93A transgenic mice. J. Neurol. Sci. 2008, 268, 40–47. [Google Scholar] [CrossRef]
- Shaw, C. What have cellular models taught us about ALS? Amyotroph. Lateral. Scler. Other Motor. Neuron. Disord. 2002, 3, 55–56. [Google Scholar] [CrossRef]
- Gurney, M.E.; Pu, H.; Chiu, A.Y.; Dal Canto, M.C.; Polchow, C.Y.; Alexander, D.D.; Caliendo, J.; Hentati, A.; Kwon, Y.W.; Deng, H.X.; et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science 1994, 264, 1772–1775. [Google Scholar] [CrossRef]
- Knippenberg, S.; Thau, N.; Dengler, R.; Petri, S. Significance of behavioural tests in a transgenic mouse model of amyotrophic lateral sclerosis (ALS). Behav. Brain Res. 2010, 213, 82–87. [Google Scholar] [CrossRef]
- Miana-Mena, F.J.; Muñoz, M.J.; Yagüe, G.; Mendez, M.; Moreno, M.; Ciriza, J.; Zaragoza, P.; Osta, R. Optimal methods to characterize the G93A mouse model of ALS. Amyotroph. Lateral. Scler. Other Motor. Neuron. Disord. 2005, 6, 55–62. [Google Scholar] [CrossRef]
- Veldink, J.H.; Bär, P.R.; Joosten, E.A.J.; Otten, M.; Wokke, J.H.J.; Van Den Berg, L.H. Sexual differences in onset of disease and response to exercise in a transgenic model of ALS. Neuromuscul. Disord. 2003, 13, 737–743. [Google Scholar] [CrossRef]
- Bruneteau, G.; Simonet, T.; Bauché, S.; Mandjee, N.; Malfatti, E.; Girard, E.; Tanguy, M.-L.; Behin, A.; Khiami, F.; Sariali, E.; et al. Muscle histone deacetylase 4 upregulation in amyotrophic lateral sclerosis: Potential role in reinnervation ability and disease progression. Brain 2013, 136, 2359–2368. [Google Scholar] [CrossRef]
- Di Pietro, L.; Baranzini, M.; Berardinelli, M.G.; Lattanzi, W.; Monforte, M.; Tasca, G.; Conte, A.; Logroscino, G.; Michetti, F.; Ricci, E.; et al. Potential therapeutic targets for ALS: MIR206, MIR208b and MIR499 are modulated during disease progression in the skeletal muscle of patients. Sci. Rep. 2017, 7, 9538. [Google Scholar] [CrossRef]
- Pigna, E.; Simonazzi, E.; Sanna, K.; Bernadzki, K.M.; Proszynski, T.; Heil, C.; Palacios, D.; Adamo, S.; Moresi, V. Histone deacetylase 4 protects from denervation and skeletal muscle atrophy in a murine model of amyotrophic lateral sclerosis. EBioMedicine 2019, 40, 717–732. [Google Scholar] [CrossRef]
- Moglia, C.; Calvo, A.; Grassano, M.; Canosa, A.; Manera, U.; D’ovidio, F.; Bombaci, A.; Bersano, E.; Mazzini, L.; Mora, G.; et al. Early weight loss in amyotrophic lateral sclerosis: Outcome relevance and clinical correlates in a population-based cohort. J. Neurol. Neurosurg. Psychiatry 2019, 90, 666–673. [Google Scholar] [CrossRef]
- Shimizu, T.; Nagaoka, U.; Nakayama, Y.; Kawata, A.; Kugimoto, C.; Kuroiwa, Y.; Kawai, M.; Shimohata, T.; Nishizawa, M.; Mihara, B.; et al. Reduction rate of body mass index predicts prognosis for survival in amyotrophic lateral sclerosis: A multicenter study in Japan. Amyotroph. Lateral. Scler. 2012, 13, 363–366. [Google Scholar] [CrossRef]
- Shimizu, T.; Nakayama, Y.; Matsuda, C.; Haraguchi, M.; Bokuda, K.; Ishikawa-Takata, K.; Kawata, A.; Isozaki, E. Prognostic significance of body weight variation after diagnosis in ALS: A single-centre prospective cohort study. J. Neurol. 2019, 266, 1412–1420. [Google Scholar] [CrossRef]
- Egger, M.; Hoenigl, M.; Thompson, G.R.; Carvalho, A.; Jenks, J.D. Let’s talk about sex characteristics-As a risk factor for invasive fungal diseases. Mycoses 2022, 65, 599–612. [Google Scholar] [CrossRef]
- Argirò, A.; Ho, C.; Day, S.M.; van der Velden, J.; Cerbai, E.; Saberi, S.; Tardiff, J.C.; Lakdawala, N.K.; Olivotto, I. Sex-Related Differences in Genetic Cardiomyopathies. J. Am. Heart Assoc. 2022, 11, e024947. [Google Scholar] [CrossRef]
- Ugidos, I.F.; Pistono, C.; Korhonen, P.; Gómez-Budia, M.; Sitnikova, V.; Klecki, P.; Stanová, I.; Jolkkonen, J.; Malm, T. Sex Differences in Poststroke Inflammation: A Focus on Microglia Across the Lifespan. Stroke 2022, 53, 1500–1509. [Google Scholar] [CrossRef]
- Bosello, O.; Vanzo, A. Obesity paradox and aging. Eat. Weight Disord. 2021, 26, 27–35. [Google Scholar] [CrossRef]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Horwich, T.B.; Oreopoulos, A.; Kovesdy, C.P.; Younessi, H.; Anker, S.D.; Morley, J.E. Risk factor paradox in wasting diseases. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 433–442. [Google Scholar] [CrossRef]
- Janse Van Mantgem, M.R.; Van Eijk, R.P.A.; Van Der Burgh, H.K.; Tan, H.H.G.; Westeneng, H.J.; Van Es, M.A.; Veldink, J.H.; Van Den Berg, L.H. Prognostic value of weight loss in patients with amyotrophic lateral sclerosis: A population-based study. J. Neurol. Neurosurg. Psychiatry 2020, 91, 867–875. [Google Scholar] [CrossRef]
- C57BL/6 Mice Nomenclature: C57BL/6NCrl Strain Origin Coat Color VAF/Elite ® (SOPF). Available online: https://www.criver.com/sites/default/files/resources/C57BL6MouseModelInformationSheet.pdf (accessed on 14 November 2022).
- Alves, C.J.; De Santana, L.P.; Dos Santos, A.J.D.; De Oliveira, G.P.; Duobles, T.; Scorisa, J.M.; Martins, R.S.; Maximino, J.R.; Chadi, G. Early motor and electrophysiological changes in transgenic mouse model of amyotrophic lateral sclerosis and gender differences on clinical outcome. Brain Res. 2011, 1394, 90–104. [Google Scholar] [CrossRef]
- Heiman-Patterson, T.D.; Deitch, J.S.; Blankenhorn, E.P.; Erwin, K.L.; Perreault, M.J.; Alexander, B.K.; Byers, N.; Toman, I.; Alexander, G.M. Background and gender effects on survival in the TgN(SOD1-G93A)1Gur mouse model of ALS. J. Neurol. Sci. 2005, 236, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Su, W.M.; Cheng, Y.F.; Jiang, Z.; Duan, Q.Q.; Yang, T.M.; Shang, H.F.; Chen, Y.P. Predictors of survival in patients with amyotrophic lateral sclerosis: A large meta-analysis. eBioMedicine 2021, 74, 103732. [Google Scholar] [CrossRef] [PubMed]
- Renzini, A.; Marroncelli, N.; Cavioli, G.; Di Francescantonio, S.; Forcina, L.; Lambridis, A.; Di Giorgio, E.; Valente, S.; Mai, A.; Brancolini, C.; et al. Cytoplasmic HDAC4 regulates the membrane repair mechanism in Duchenne muscular dystrophy. J. Cachexia. Sarcopenia Muscle 2022, 13, 1339–1359. [Google Scholar] [CrossRef]
- Pigna, E.; Renzini, A.; Greco, E.; Simonazzi, E.; Fulle, S.; Mancinelli, R.; Moresi, V.; Adamo, S. HDAC4 preserves skeletal muscle structure following long-term denervation by mediating distinct cellular responses. Skelet. Muscle 2018, 8, 6. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Renzini, A.; Pigna, E.; Rocchi, M.; Cedola, A.; Gigli, G.; Moresi, V.; Coletti, D. Sex and HDAC4 Differently Affect the Pathophysiology of Amyotrophic Lateral Sclerosis in SOD1-G93A Mice. Int. J. Mol. Sci. 2023, 24, 98. https://doi.org/10.3390/ijms24010098
Renzini A, Pigna E, Rocchi M, Cedola A, Gigli G, Moresi V, Coletti D. Sex and HDAC4 Differently Affect the Pathophysiology of Amyotrophic Lateral Sclerosis in SOD1-G93A Mice. International Journal of Molecular Sciences. 2023; 24(1):98. https://doi.org/10.3390/ijms24010098
Chicago/Turabian StyleRenzini, Alessandra, Eva Pigna, Marco Rocchi, Alessia Cedola, Giuseppe Gigli, Viviana Moresi, and Dario Coletti. 2023. "Sex and HDAC4 Differently Affect the Pathophysiology of Amyotrophic Lateral Sclerosis in SOD1-G93A Mice" International Journal of Molecular Sciences 24, no. 1: 98. https://doi.org/10.3390/ijms24010098
APA StyleRenzini, A., Pigna, E., Rocchi, M., Cedola, A., Gigli, G., Moresi, V., & Coletti, D. (2023). Sex and HDAC4 Differently Affect the Pathophysiology of Amyotrophic Lateral Sclerosis in SOD1-G93A Mice. International Journal of Molecular Sciences, 24(1), 98. https://doi.org/10.3390/ijms24010098







