Concentration-Dependent Efficacy of Recombinant Human Bone Morphogenetic Protein-2 Using a HA/β-TCP Hydrogel Carrier in a Mini-Pig Vertebral Oblique Lateral Interbody Fusion Model
Abstract
1. Introduction
2. Results
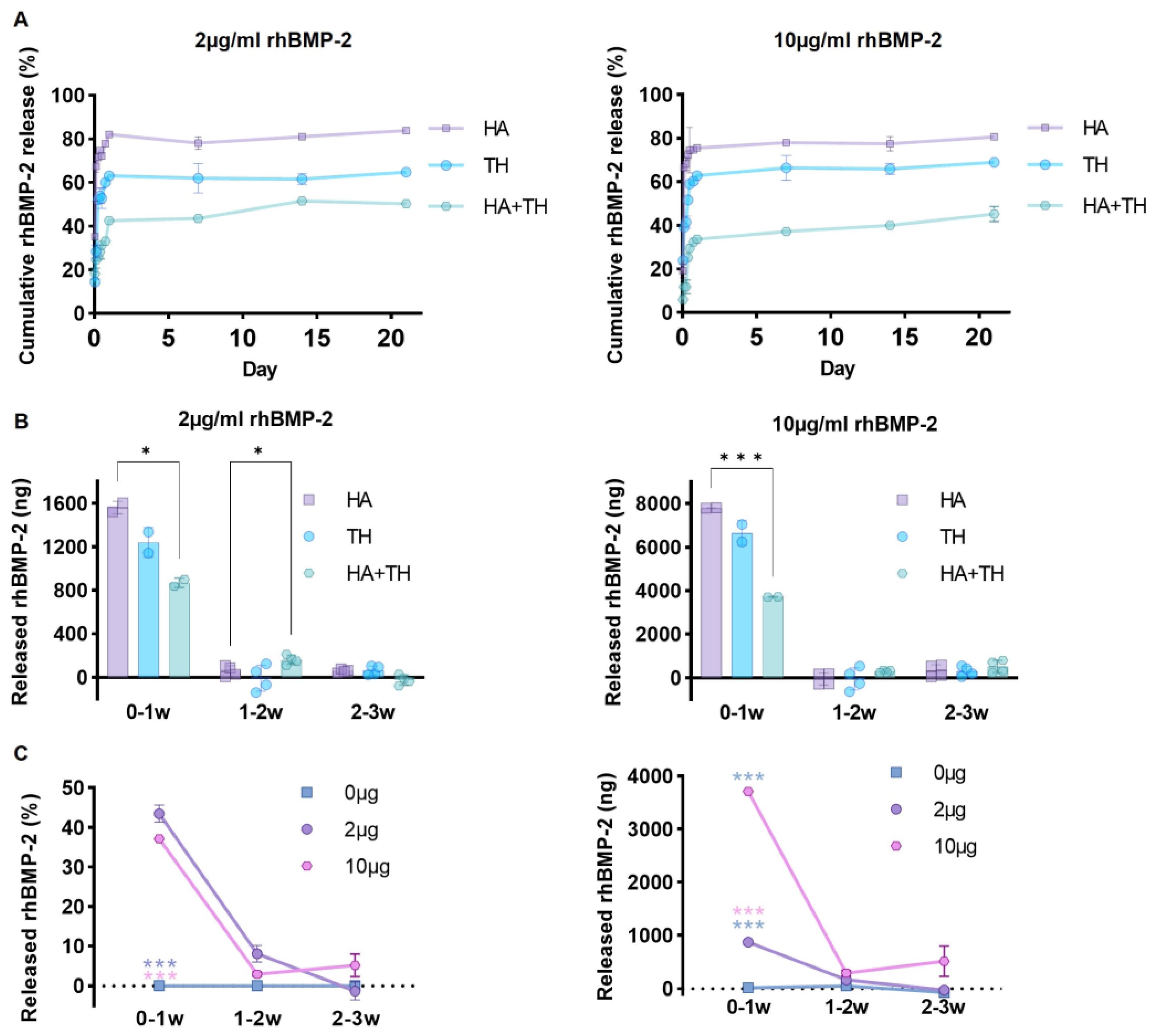
2.1. Advantages of Ceramic Carriers and Hydrogel Polymer Mixture for Sustained Release of Growth Factors
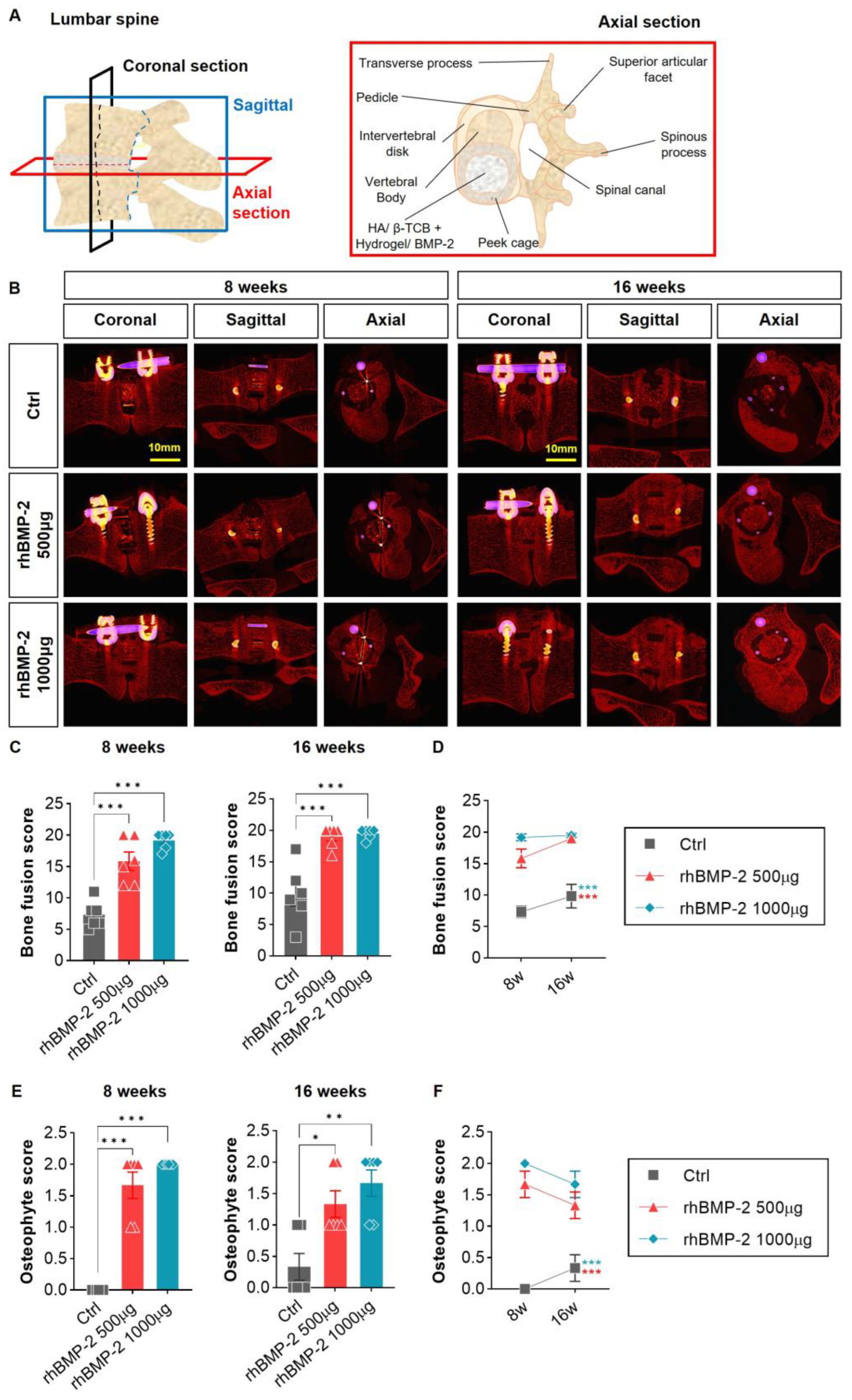
2.2. Appropriate Use of BMP-2 for Efficient Osteogenesis
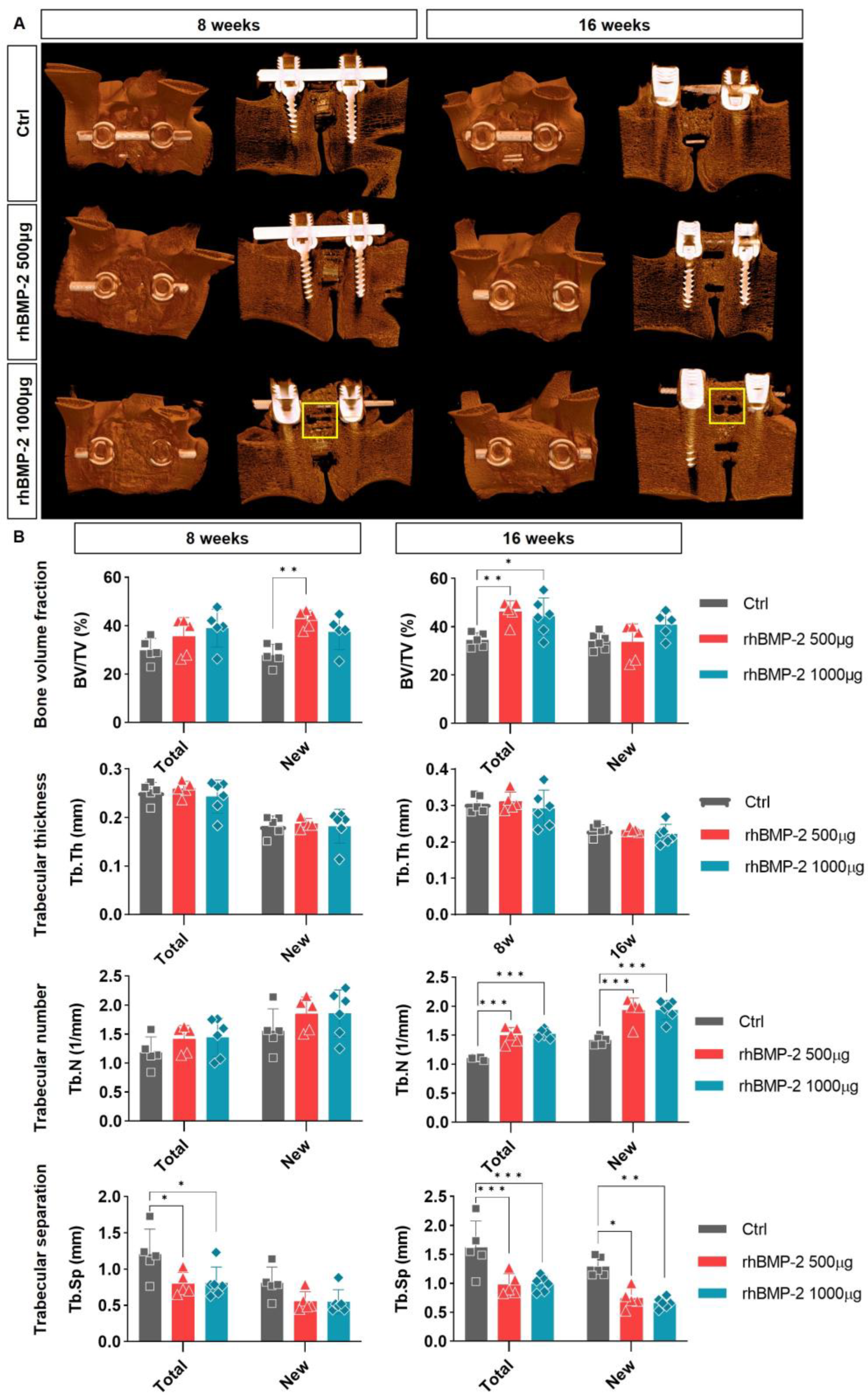
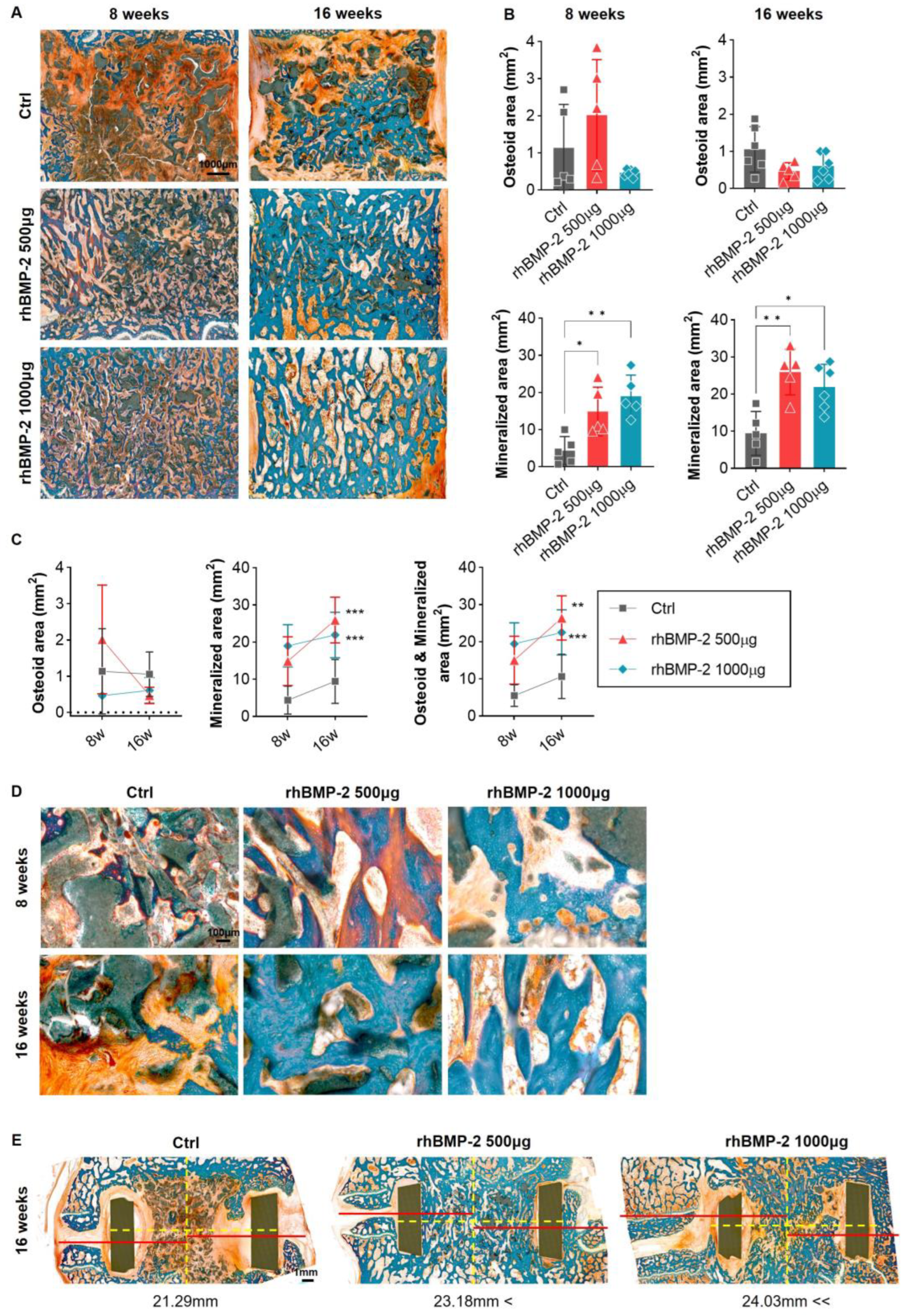
2.3. Appropriate Using of BMP-2 for Activation of Trabecular Bone Formation
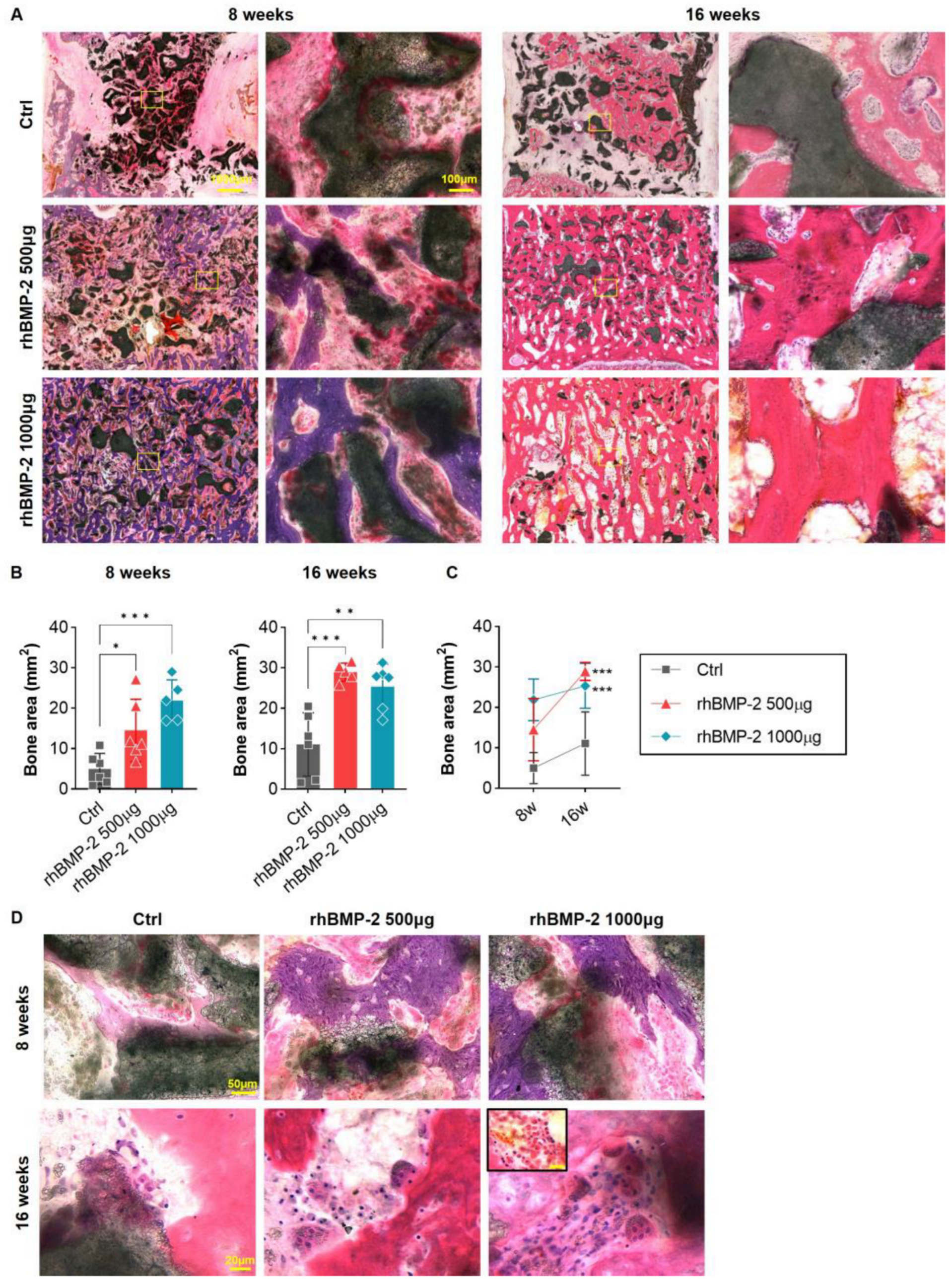
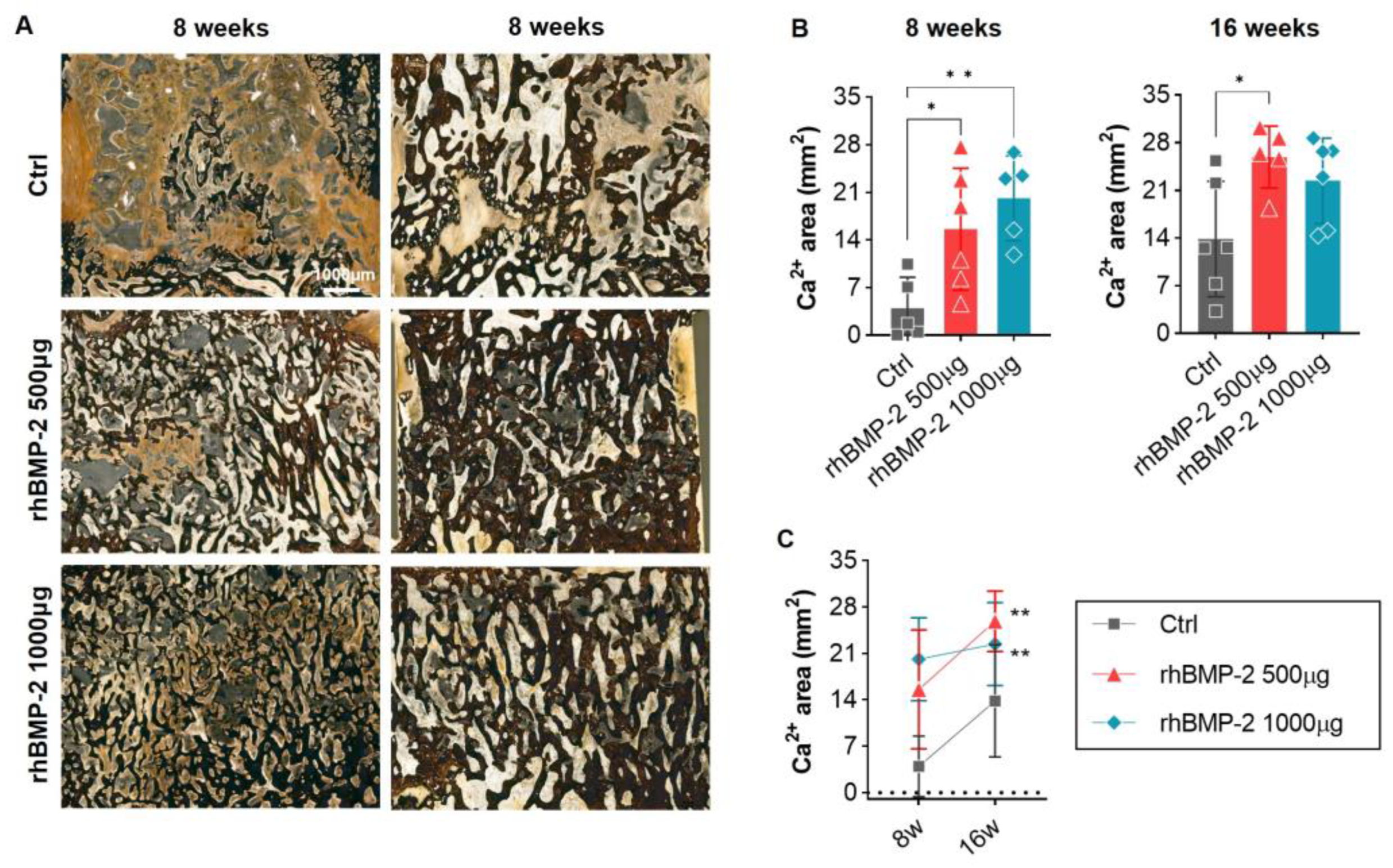
2.4. Appropriate Use of BMP-2 for Increased Bone Formation and Suppression of Inflammatory Response
2.5. Appropriate Use of BMP-2 for Organic and Inorganic Osteosynthesis
3. Discussion
4. Materials and Methods
4.1. Preparation of the Composite Carrier
4.2. ELISA Assay
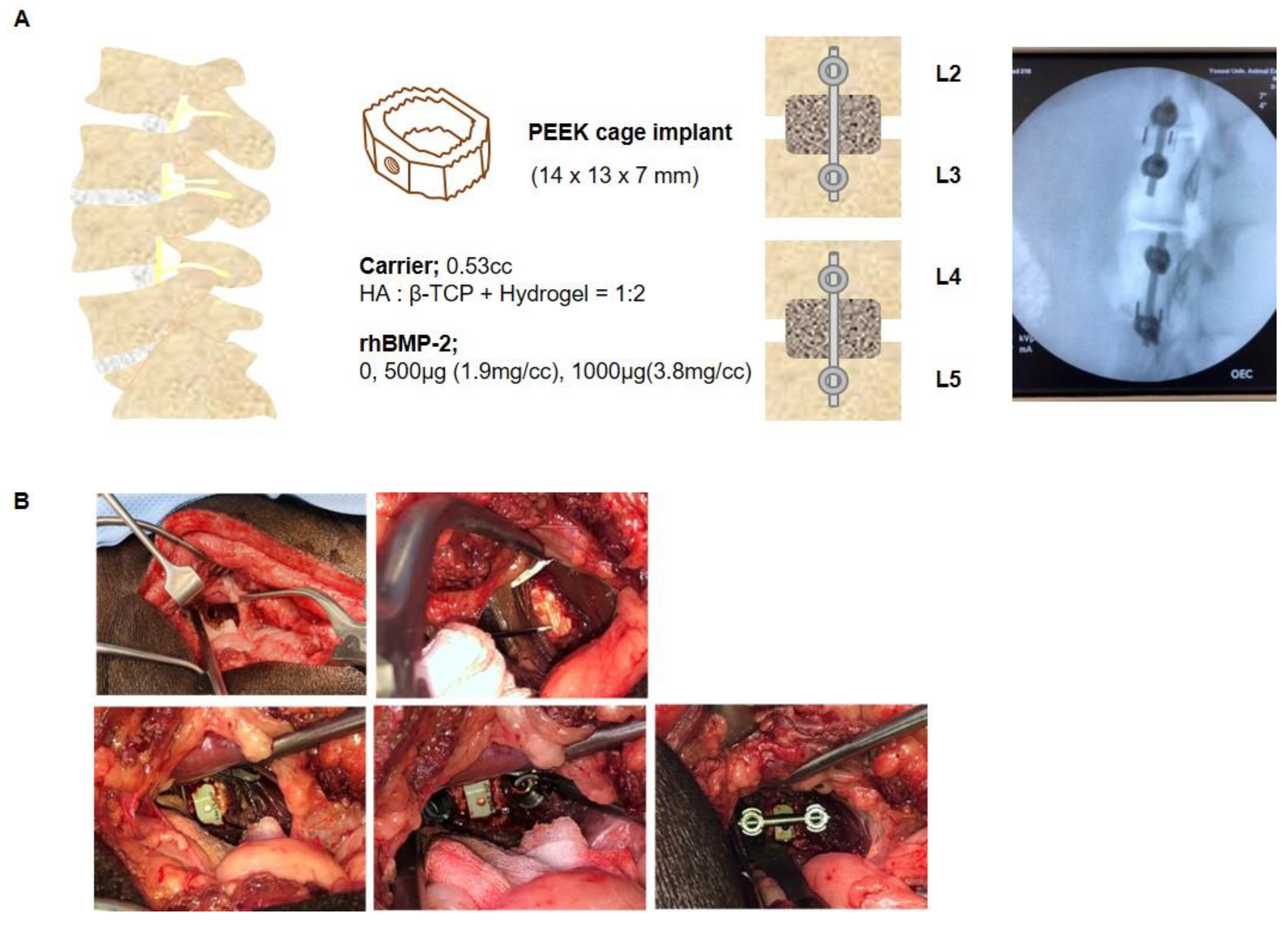
4.3. Mini-Pig Oblique Lateral Interbody Fusion Model
4.4. Fusion and Osteophyte Analysis
4.4.1. Fusion Rate
4.4.2. Osteophyte
4.5. Micro-CT Analysis
4.6. Sample Preparation
4.7. H&E Staining
4.8. Goldner’s Trichrome Staining
4.9. Von Kossa Staining
4.10. Slide Scanning and Image Analysis
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asahina, I.; Sampath, T.K.; Hauschka, P.V. Human osteogenic protein-1 induces chondroblastic, osteoblastic, and/or adipocytic differentiation of clonal murine target cells. Exp. Cell Res. 1996, 222, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Kempen, D.H.; Lu, L.; Heijink, A.; Hefferan, T.E.; Creemers, L.B.; Maran, A.; Yaszemski, M.J.; Dhert, W.J. Effect of local sequential vegf and bmp-2 delivery on ectopic and orthotopic bone regeneration. Biomaterials 2009, 30, 2816–2825. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.Y.; Hyun, S.J.; Lee, C.H.; Youn, J.H.; Ryu, M.Y.; Kim, K.J. Safety and efficacy of recombinant human bone morphogenetic protein-2 in multilevel posterolateral lumbar fusion in a prospective, randomized, controlled trial. Neurospine 2022, 19, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Kwon, O.H.; Park, J.Y. Comparison between 3-dimensional-printed titanium and polyetheretherketone cages: 1-year outcome after minimally invasive transforaminal interbody fusion. Neurospine 2022, 19, 524–532. [Google Scholar] [CrossRef]
- Won, Y.I.; Lee, C.H.; Yuh, W.T.; Kwon, S.W.; Kim, C.H.; Chung, C.K. Genetic odyssey to ossification of the posterior longitudinal ligament in the cervical spine: A systematic review. Neurospine 2022, 19, 299–306. [Google Scholar] [CrossRef]
- Benglis, D.; Wang, M.Y.; Levi, A.D. A comprehensive review of the safety profile of bone morphogenetic protein in spine surgery. Neurosurgery 2008, 62, 423–431. [Google Scholar]
- Lee, K.B.; Taghavi, C.E.; Murray, S.S.; Song, K.J.; Keorochana, G.; Wang, J.C. Bmp induced inflammation: A comparison of rhbmp-7 and rhbmp-2. J. Orthop. Res. 2012, 30, 1985–1994. [Google Scholar] [CrossRef]
- Lee, J.H.; Ryu, M.Y.; Baek, H.R.; Lee, K.M.; Seo, J.H.; Lee, H.K.; Ryu, H.S. Effects of porous beta-tricalcium phosphate-based ceramics used as an e. Coli-derived rhbmp-2 carrier for bone regeneration. J. Mater. Sci. Mater. Med. 2013, 24, 2117–2127. [Google Scholar] [CrossRef]
- Wozney, J.M.; Rosen, V.; Celeste, A.J.; Mitsock, L.M.; Whitters, M.J.; Kriz, R.W.; Hewick, R.M.; Wang, E.A. Novel regulators of bone formation: Molecular clones and activities. Science 1988, 242, 1528–1534. [Google Scholar] [CrossRef]
- Bessho, K.; Konishi, Y.; Kaihara, S.; Fujimura, K.; Okubo, Y.; Iizuka, T. Bone induction by Escherichia coli-derived recombinant human bone morphogenetic protein-2 compared with chinese hamster ovary cell-derived recombinant human bone morphogenetic protein-2. Br. J. Oral Maxillofac. Surg. 2000, 38, 645–649. [Google Scholar] [CrossRef]
- Yano, K.; Hoshino, M.; Ohta, Y.; Manaka, T.; Naka, Y.; Imai, Y.; Sebald, W.; Takaoka, K. Osteoinductive capacity and heat stability of recombinant human bone morphogenetic protein-2 produced by Escherichia coli and dimerized by biochemical processing. J. Bone Miner. Metab. 2009, 27, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.Y.; Oh, J.H.; Lee, B.S.; Seo, Y.K.; Hwang, S.J.; Kim, I.S. The effect of dose on rhbmp-2 signaling, delivered via collagen sponge, on osteoclast activation and in vivo bone resorption. Biomaterials 2014, 35, 1869–1881. [Google Scholar] [CrossRef] [PubMed]
- Burkus, J.K.; Sandhu, H.S.; Gornet, M.F.; Longley, M.C. Use of rhbmp-2 in combination with structural cortical allografts: Clinical and radiographic outcomes in anterior lumbar spinal surgery. J. Bone Jt. Surg. Am. 2005, 87, 1205–1212. [Google Scholar] [CrossRef]
- Burkus, J.K.; Heim, S.E.; Gornet, M.F.; Zdeblick, T.A. Is infuse bone graft superior to autograft bone? An integrated analysis of clinical trials using the lt-cage lumbar tapered fusion device. J. Spinal Disord. Tech. 2003, 16, 113–122. [Google Scholar] [CrossRef] [PubMed]
- El Bialy, I.; Jiskoot, W.; Reza Nejadnik, M. Formulation, delivery and stability of bone morphogenetic proteins for effective bone regeneration. Pharm. Res. 2017, 34, 1152–1170. [Google Scholar] [CrossRef] [PubMed]
- James, A.W.; LaChaud, G.; Shen, J.; Asatrian, G.; Nguyen, V.; Zhang, X.; Ting, K.; Soo, C. A review of the clinical side effects of bone morphogenetic protein-2. Tissue Eng. Part B Rev. 2016, 22, 284–297. [Google Scholar] [CrossRef]
- Woo, B.H.; Fink, B.F.; Page, R.; Schrier, J.A.; Jo, Y.W.; Jiang, G.; DeLuca, M.; Vasconez, H.C.; DeLuca, P.P. Enhancement of bone growth by sustained delivery of recombinant human bone morphogenetic protein-2 in a polymeric matrix. Pharm. Res. 2001, 18, 1747–1753. [Google Scholar] [CrossRef]
- Yamamoto, M.; Takahashi, Y.; Tabata, Y. Enhanced bone regeneration at a segmental bone defect by controlled release of bone morphogenetic protein-2 from a biodegradable hydrogel. Tissue Eng. 2006, 12, 1305–1311. [Google Scholar] [CrossRef]
- Visser, R.; Arrabal, P.M.; Becerra, J.; Rinas, U.; Cifuentes, M. The effect of an rhbmp-2 absorbable collagen sponge-targeted system on bone formation in vivo. Biomaterials 2009, 30, 2032–2037. [Google Scholar] [CrossRef]
- Takahashi, Y.; Yamamoto, M.; Tabata, Y. Enhanced osteoinduction by controlled release of bone morphogenetic protein-2 from biodegradable sponge composed of gelatin and beta-tricalcium phosphate. Biomaterials 2005, 26, 4856–4865. [Google Scholar] [CrossRef]
- Patel, V.V.; Zhao, L.; Wong, P.; Kanim, L.; Bae, H.W.; Pradhan, B.B.; Delamarter, R.B. Controlling bone morphogenetic protein diffusion and bone morphogenetic protein-stimulated bone growth using fibrin glue. Spine 2006, 31, 1201–1206. [Google Scholar] [CrossRef] [PubMed]
- Daculsi, G.; Uzel, A.P.; Weiss, P.; Goyenvalle, E.; Aguado, E. Developments in injectable multiphasic biomaterials. The performance of microporous biphasic calcium phosphate granules and hydrogels. J. Mater. Sci. Mater. Med. 2010, 21, 855–861. [Google Scholar] [CrossRef]
- Ding, X.; Zhao, H.; Li, Y.; Lee, A.L.; Li, Z.; Fu, M.; Li, C.; Yang, Y.Y.; Yuan, P. Synthetic peptide hydrogels as 3D scaffolds for tissue engineering. Adv. Drug Deliv. Rev. 2020, 160, 78–104. [Google Scholar] [CrossRef]
- Bao, X.; Zhu, L.; Huang, X.; Tang, D.; He, D.; Shi, J.; Xu, G. 3d biomimetic artificial bone scaffolds with dual-cytokines spatiotemporal delivery for large weight-bearing bone defect repair. Sci. Rep. 2017, 7, 7814. [Google Scholar] [CrossRef]
- Lee, S.H.; Shin, H. Matrices and scaffolds for delivery of bioactive molecules in bone and cartilage tissue engineering. Adv. Drug Deliv. Rev. 2007, 59, 339–359. [Google Scholar] [CrossRef] [PubMed]
- Stokovic, N.; Ivanjko, N.; Pecin, M.; Erjavec, I.; Karlovic, S.; Smajlovic, A.; Capak, H.; Milosevic, M.; Bubic Spoljar, J.; Vnuk, D.; et al. Evaluation of synthetic ceramics as compression resistant matrix to promote osteogenesis of autologous blood coagulum containing recombinant human bone morphogenetic protein 6 in rabbit posterolateral lumbar fusion model. Bone 2020, 140, 115544. [Google Scholar] [CrossRef]
- Tateiwa, D.; Nakagawa, S.; Tsukazaki, H.; Okada, R.; Kodama, J.; Kushioka, J.; Bal, Z.; Ukon, Y.; Hirai, H.; Kaito, T. A novel bmp-2-loaded hydroxyapatite/beta-tricalcium phosphate microsphere/hydrogel composite for bone regeneration. Sci. Rep. 2021, 11, 16924. [Google Scholar] [CrossRef] [PubMed]
- Begam, H.; Nandi, S.K.; Kundu, B.; Chanda, A. Strategies for delivering bone morphogenetic protein for bone healing. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 70, 856–869. [Google Scholar] [CrossRef]
- Sanda, M.; Shiota, M.; Fujii, M.; Kon, K.; Fujimori, T.; Kasugai, S. Capability of new bone formation with a mixture of hydroxyapatite and beta-tricalcium phosphate granules. Clin. Oral Implants Res. 2015, 26, 1369–1374. [Google Scholar] [CrossRef]
- Gupta, V.; Lyne, D.V.; Barragan, M.; Berkland, C.J.; Detamore, M.S. Microsphere-based scaffolds encapsulating tricalcium phosphate and hydroxyapatite for bone regeneration. J. Mater. Sci. Mater. Med. 2016, 27, 121. [Google Scholar] [CrossRef]
- Lee, J.H.; Ryu, M.Y.; Baek, H.R.; Lee, H.K.; Seo, J.H.; Lee, K.M.; Lee, A.Y.; Zheng, G.B.; Chang, B.S.; Lee, C.K. The effects of recombinant human bone morphogenetic protein-2-loaded tricalcium phosphate microsphere-hydrogel composite on the osseointegration of dental implants in minipigs. Artif. Organs 2014, 38, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.K.; Kim, J.Y.; Han, M.S.; Lee, J.K.; Moon, B.J. Neurologic deficit due to vertebral body osteophytes after oblique lumbar interbody fusion: A case report. Medicine 2021, 100, e28095. [Google Scholar] [CrossRef] [PubMed]
- Zara, J.N.; Siu, R.K.; Zhang, X.; Shen, J.; Ngo, R.; Lee, M.; Li, W.; Chiang, M.; Chung, J.; Kwak, J.; et al. High doses of bone morphogenetic protein 2 induce structurally abnormal bone and inflammation in vivo. Tissue Eng. Part A 2011, 17, 1389–1399. [Google Scholar] [CrossRef] [PubMed]
- Birmingham, E.; Niebur, G.L.; McHugh, P.E.; Shaw, G.; Barry, F.P.; McNamara, L.M. Osteogenic differentiation of mesenchymal stem cells is regulated by osteocyte and osteoblast cells in a simplified bone niche. Eur. Cell Mater. 2012, 23, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Martin-Iglesias, S.; Milian, L.; Sancho-Tello, M.; Salvador-Clavell, R.; Martin de Llano, J.J.; Carda, C.; Mata, M. Bmp-2 enhances osteogenic differentiation of human adipose-derived and dental pulp stem cells in 2D and 3D in vitro models. Stem Cells Int. 2022, 2022, 4910399. [Google Scholar] [CrossRef]
- Tadross, M.R.; Tsien, R.W.; Yue, D.T. Ca2+ channel nanodomains boost local Ca2+ amplitude. Proc. Natl. Acad. Sci. USA 2013, 110, 15794–15799. [Google Scholar] [CrossRef] [PubMed]
- Melcrova, A.; Pokorna, S.; Pullanchery, S.; Kohagen, M.; Jurkiewicz, P.; Hof, M.; Jungwirth, P.; Cremer, P.S.; Cwiklik, L. The complex nature of calcium cation interactions with phospholipid bilayers. Sci. Rep. 2016, 6, 38035. [Google Scholar] [CrossRef]
- Hasegawa, T.; Hongo, H.; Yamamoto, T.; Abe, M.; Yoshino, H.; Haraguchi-Kitakamae, M.; Ishizu, H.; Shimizu, T.; Iwasaki, N.; Amizuka, N. Matrix vesicle-mediated mineralization and osteocytic regulation of bone mineralization. Int. J. Mol. Sci. 2022, 23, 9941. [Google Scholar] [CrossRef]
- Hasegawa, T.; Li, M.; Hara, K.; Sasaki, M.; Tabata, C.; de Freitas, P.H.; Hongo, H.; Suzuki, R.; Kobayashi, M.; Inoue, K.; et al. Morphological assessment of bone mineralization in tibial metaphyses of ascorbic acid-deficient ods rats. Biomed. Res. 2011, 32, 259–269. [Google Scholar] [CrossRef]
- Marotti, G. The osteocyte as a wiring transmission system. J. Musculoskelet. Neuron. Interact. 2000, 1, 133–136. [Google Scholar]
- Repp, F.; Kollmannsberger, P.; Roschger, A.; Berzlanovich, A.; Gruber, G.M.; Roschger, P.; Wagermaier, W.; Weinkamer, R. Coalignment of osteocyte canaliculi and collagen fibers in human osteonal bone. J. Struct. Biol. 2017, 199, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Guo, J.; Zhu, W.; Su, Y.; Tong, W.; Zheng, L.; Chang, L.; Wang, X.; Lai, Y.; Qin, L.; et al. Controlled release of bone morphogenetic protein-2 augments the coupling of angiogenesis and osteogenesis for accelerating mandibular defect repair. Pharmaceutics 2022, 14, 2397. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Yao, H.; Li, X.; Chang, L.; Wang, Z.; Zhu, W.; Su, Y.; Qin, L.; Xu, J. Advanced hydrogel systems for mandibular reconstruction. Bioact. Mater. 2023, 21, 175–193. [Google Scholar] [CrossRef]
- Zhang, B.; Huang, J.; Liu, J.; Lin, F.; Ding, Z.; Xu, J. Injectable composite hydrogel promotes osteogenesis and angiogenesis in spinal fusion by optimizing the bone marrow mesenchymal stem cell microenvironment and exosomes secretion. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 123, 111782. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, S.; Okada, R.; Kushioka, J.; Kodama, J.; Tsukazaki, H.; Bal, Z.; Tateiwa, D.; Ukon, Y.; Hirai, H.; Makino, T.; et al. Effects of rhbmp-2-loaded hydroxyapatite granules/beta-tricalcium phosphate hydrogel (ha/beta-tcp/hydrogel) composite on a rat model of caudal intervertebral fusion. Sci. Rep. 2022, 12, 7906. [Google Scholar] [CrossRef]
- Brambilla, E.; Locarno, S.; Gallo, S.; Orsini, F.; Pini, C.; Farronato, M.; Thomaz, D.V.; Lenardi, C.; Piazzoni, M.; Tartaglia, G. Poloxamer-based hydrogel as drug delivery system: How polymeric excipients influence the chemical-physical properties. Polymers 2022, 14, 3624. [Google Scholar] [CrossRef]
- Hirun, N.; Kraisit, P. Drug-polymers composite matrix tablets: Effect of hydroxypropyl methylcellulose (hpmc) k-series on porosity, compatibility, and release behavior of the tablet containing a bcs class i drug. Polymers 2022, 14, 3406. [Google Scholar] [CrossRef]
- Moussa, E.; Siepmann, F.; Flament, M.P.; Benzine, Y.; Penis, F.; Siepmann, J.; Karrout, Y. Controlled release tablets based on hpmc:Lactose blends. J. Drug Deliv. Sci. Technol. 2019, 52, 607–617. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.-Y.; Kang, J.-I.; Lee, H.-L.; Hwang, G.-Y.; Kim, K.-N.; Ha, Y. Concentration-Dependent Efficacy of Recombinant Human Bone Morphogenetic Protein-2 Using a HA/β-TCP Hydrogel Carrier in a Mini-Pig Vertebral Oblique Lateral Interbody Fusion Model. Int. J. Mol. Sci. 2023, 24, 892. https://doi.org/10.3390/ijms24010892
Lee H-Y, Kang J-I, Lee H-L, Hwang G-Y, Kim K-N, Ha Y. Concentration-Dependent Efficacy of Recombinant Human Bone Morphogenetic Protein-2 Using a HA/β-TCP Hydrogel Carrier in a Mini-Pig Vertebral Oblique Lateral Interbody Fusion Model. International Journal of Molecular Sciences. 2023; 24(1):892. https://doi.org/10.3390/ijms24010892
Chicago/Turabian StyleLee, Hye-Yeong, Ji-In Kang, Hye-Lan Lee, Gwang-Yong Hwang, Keung-Nyun Kim, and Yoon Ha. 2023. "Concentration-Dependent Efficacy of Recombinant Human Bone Morphogenetic Protein-2 Using a HA/β-TCP Hydrogel Carrier in a Mini-Pig Vertebral Oblique Lateral Interbody Fusion Model" International Journal of Molecular Sciences 24, no. 1: 892. https://doi.org/10.3390/ijms24010892
APA StyleLee, H.-Y., Kang, J.-I., Lee, H.-L., Hwang, G.-Y., Kim, K.-N., & Ha, Y. (2023). Concentration-Dependent Efficacy of Recombinant Human Bone Morphogenetic Protein-2 Using a HA/β-TCP Hydrogel Carrier in a Mini-Pig Vertebral Oblique Lateral Interbody Fusion Model. International Journal of Molecular Sciences, 24(1), 892. https://doi.org/10.3390/ijms24010892







