Blockers of Skeletal Muscle Nav1.4 Channels: From Therapy of Myotonic Syndrome to Molecular Determinants of Pharmacological Action and Back
Abstract
1. Introduction
2. Sodium Channel Blockers as Therapeutic Options in Non-Dystrophic Myotonias
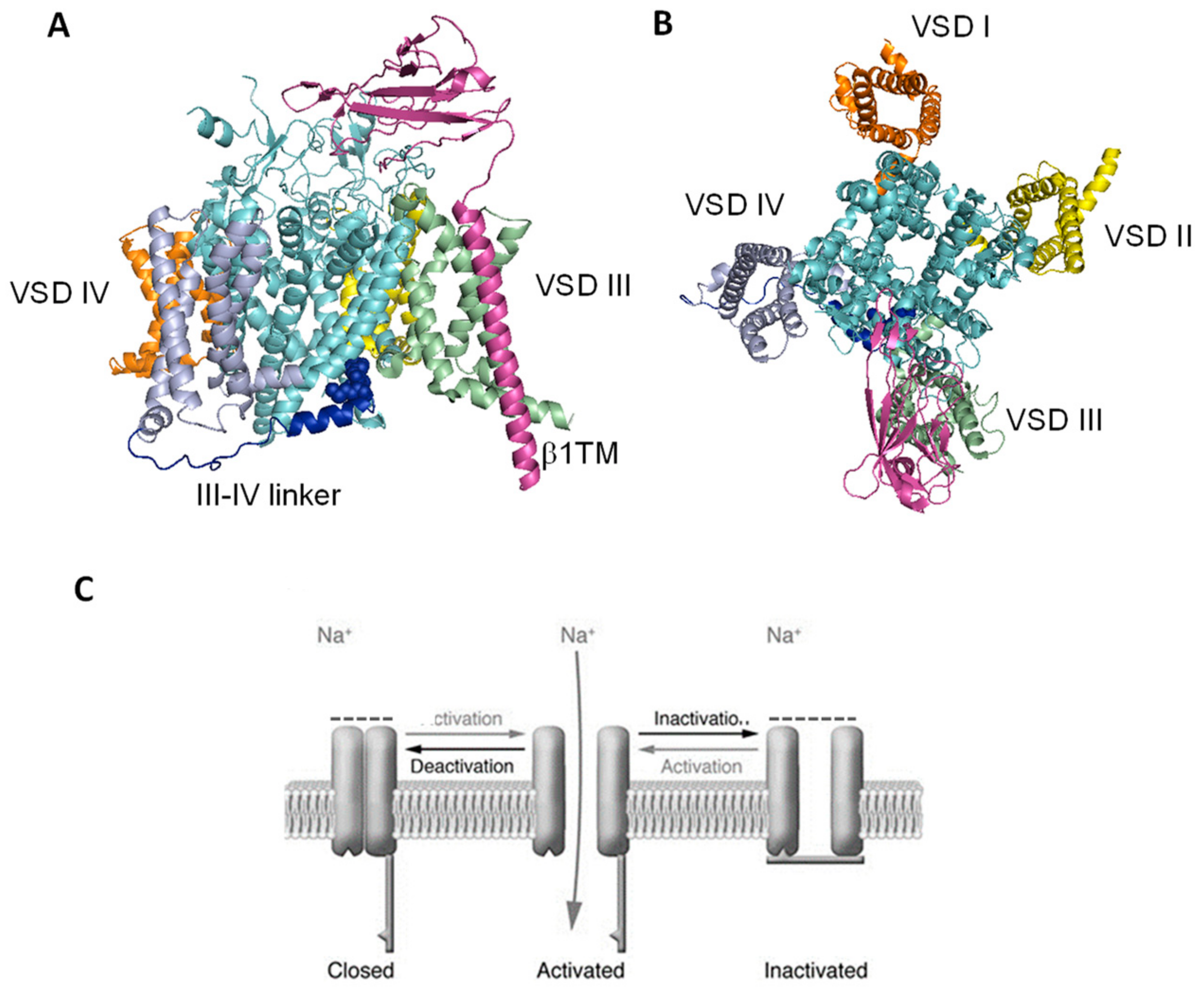
3. State-Dependent Block of Sodium Channels: Molecular Basis and Pre-Clinical Drug Studies
4. Structure-Based Drug Design for Nav1.4 Blockers with Antimyotonic Activity
- Increased steric hindrance on the stereogenic center;
- Increased distance between the chiral carbon atom and the amino-terminal group, different position of the chiral center in the elongated alkyl chain and modifications of the amino group;
- Introduction of tetramethyl—pirroline moiety of the amino group of Mex.
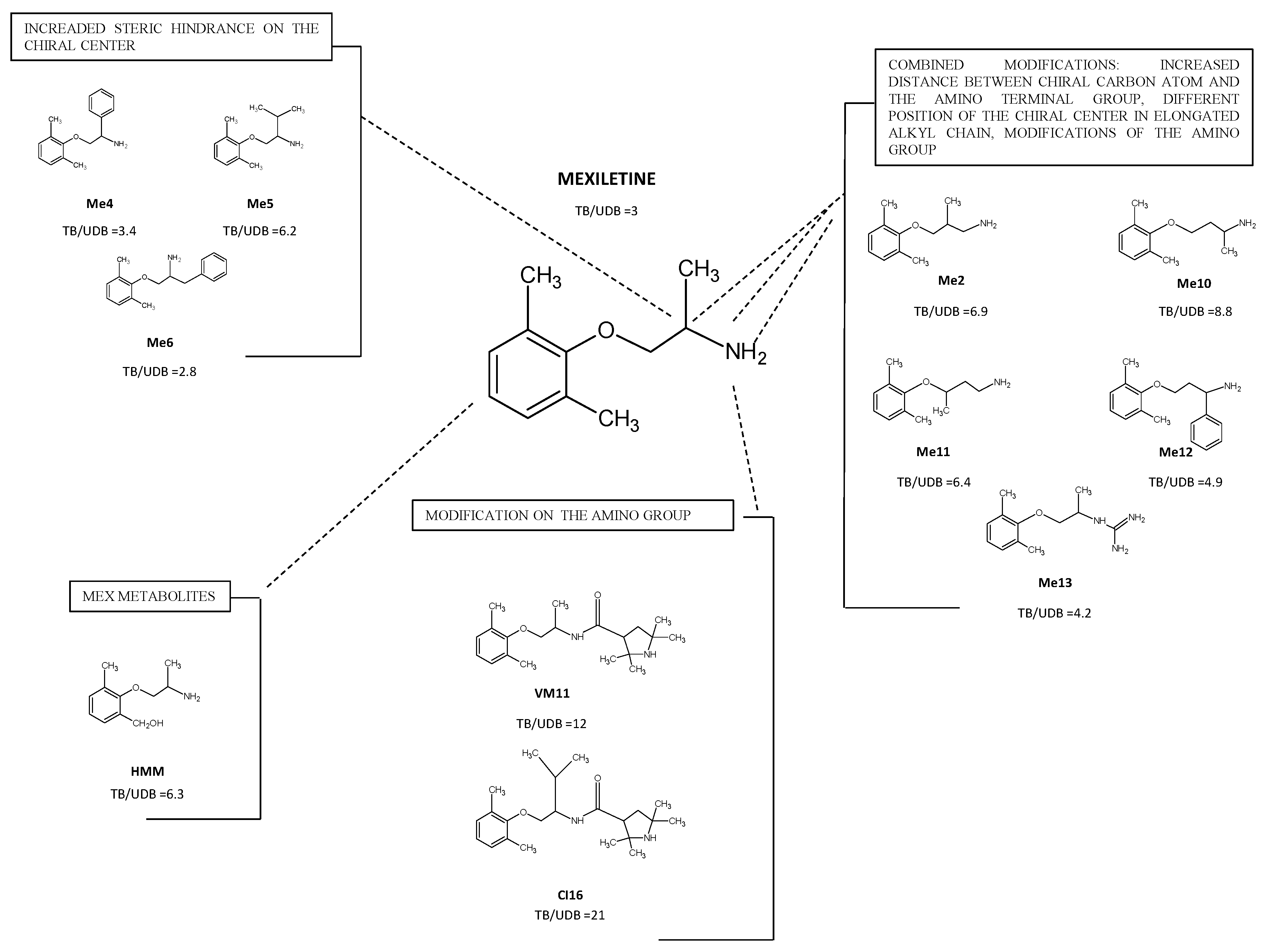
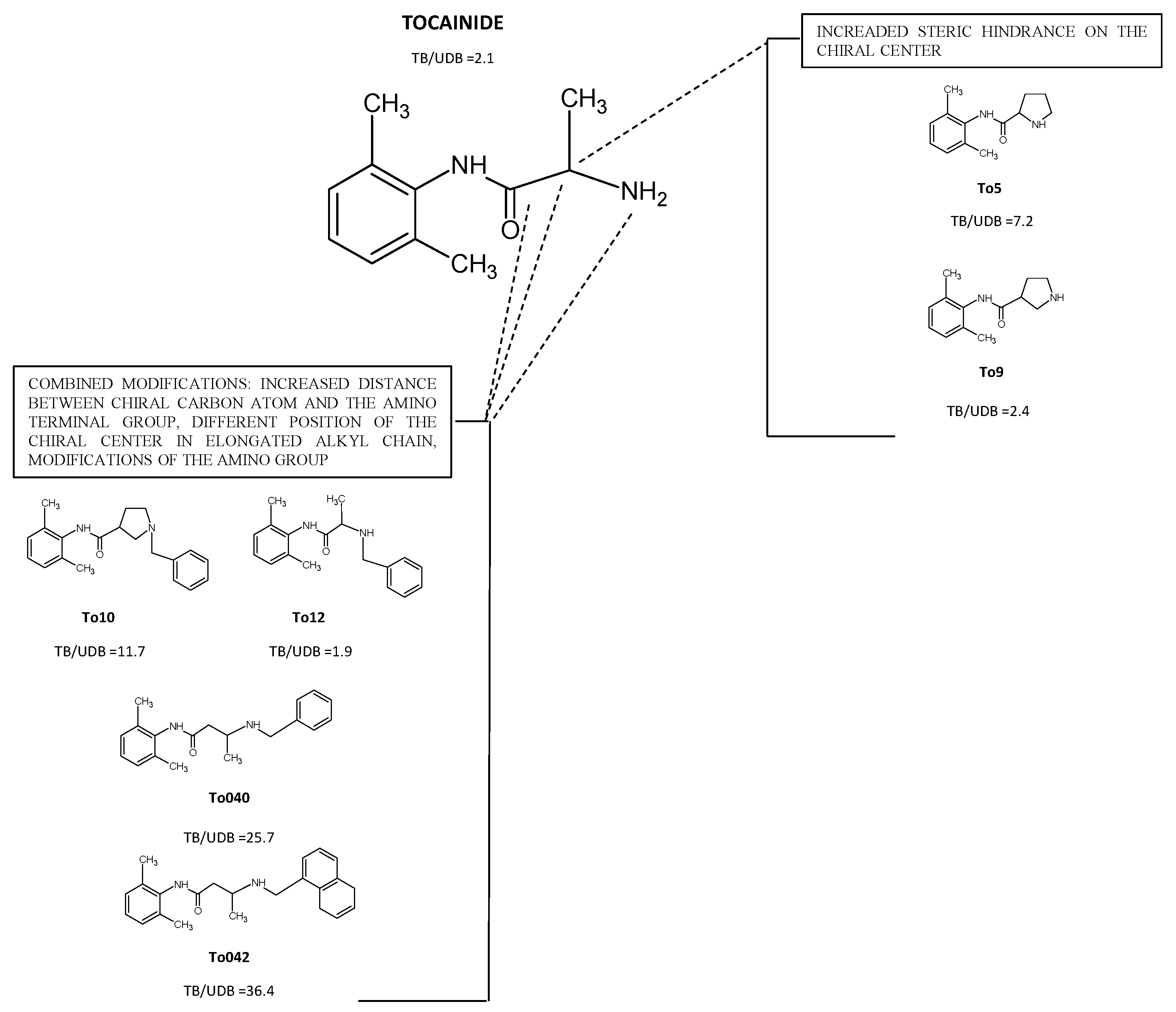
4.1. Derivatives with Increased Steric Hindrance on the Stereogenic Center
4.2. Derivatives with Increased Distance between the Chiral Carbon Atom and the Amino Terminal Group and/or, Modifications of the Amino Group
4.3. Derivatives with Tetramethyl—Pyrroline Moiety of the Amino Group of Mex
4.4. Pharmacological Role of Active Metabolites of Mexiletine
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matthews, E.; Balestrini, S.; Sisodiya, S.M.; Hanna, M.G. Muscle and brain sodium channelopathies: Genetic causes, clinical phenotypes, and management approaches. Lancet Child Adolesc. Health 2020, 4, 536–547. [Google Scholar] [CrossRef]
- Catterall, W.A.; Lenaeus, M.J.; Gamal El-Din, T.M. Structure and Pharmacology of Voltage-Gated Sodium and Calcium Channels. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 133–154. [Google Scholar] [CrossRef]
- Black, J.A.; Waxman, S.G. Noncanonical roles of voltage-gated sodium channels. Neuron 2013, 80, 280–291. [Google Scholar] [CrossRef]
- de Lera Ruiz, M.; Kraus, R.L. Voltage-Gated Sodium Channels: Structure, Function, Pharmacology, and Clinical Indications. J. Med. Chem. 2015, 58, 7093–7118. [Google Scholar] [CrossRef]
- Mantegazza, M.; Cestèle, S.; Catteral, W.A. Sodium channelopathies of skeletal muscle and brain. Physiol. Rev. 2021, 101, 1633–1689. [Google Scholar] [CrossRef]
- Meisler, M.; Hill, S.F.; Yu, W. Sodium channelopathies in neurodevelopmental disorders. Nat. Rev. Neurosci. 2021, 22, 152–166. [Google Scholar] [CrossRef]
- Li, Z.M.; Chen, L.X.; Li, H. Voltage-gated Sodium Channels and Blockers: An Overview and Where Will They Go? Curr. Med. Sci. 2019, 39, 863–873. [Google Scholar] [CrossRef]
- Imbrici, P.; Liantonio, A.; Camerino, G.M.; De Bellis, M.; Camerino, C.; Mele, A.; Giustino, A.; Pierno, S.; De Luca, A.; Tricarico, D.; et al. Therapeutic Approaches to Genetic Ion Channelopathies and Perspectives in Drug Discovery. Front. Pharmacol. 2016, 7, 121. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.; Zhang, J.; Körner, H.; Jiang, Y.; Ying, S. The Emerging Role of Voltage-Gated Sodium Channels in Tumor Biology. Front. Oncol. 2019, 9, 124. [Google Scholar] [CrossRef] [PubMed]
- Angus, M.; Ruben, P. Voltage gated sodium channels in cancer and their potential mechanisms of action. Channels 2019, 13, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jing, Y.; Pan, R.; Ding, K.; Chen, R.; Meng, Q. Mechanisms of Cancer Inhibition by Local Anesthetics. Front. Pharmacol. 2021, 12, 770694. [Google Scholar] [CrossRef] [PubMed]
- Altamura, C.; Gavazzo, P.; Pusch, M.; Desaphy, J.-F. Ion Channel Involvement in Tumor Drug Resistance. J. Pers. Med. 2022, 12, 210. [Google Scholar] [CrossRef] [PubMed]
- Miren, D.; Martínez-Mármol, R.; Felipe, T.G.A.; Valenzuela, C. Differential Regulation of Na(v)Beta Subunits during Myogenesis. Biochem. Biophys. Res. Commun. 2008, 368, 761–766. [Google Scholar]
- Kotsias, B.A.; Venosa, R.A. Sodium Influx during Action Potential in Innervated and Denervated Rat Skeletal Muscles. Muscle Nerve 2001, 24, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, K.; Kanda, F.; Mitsui, S.; Kohara, N.; Chihara, K. Fibrillation Potentials of Denervated Rat Skeletal Muscle Are Associated with Expression of Cardiac-Type Voltage-Gated Sodium Channel Isoform Nav1.5. Clin. Neurophysiol. 2012, 123, 1650–1655. [Google Scholar] [CrossRef]
- Cappellari, O.; Mantuano, P.; De Luca, A. The Social Network and Muscular Dystrophies: The Lesson Learnt about the Niche Environment as a Target for Therapeutic Strategies. Cells 2020, 9, 1659. [Google Scholar] [CrossRef]
- Cannon, S.C. Sodium channelopathies of skeletal muscle. Handb. Exp. Pharmacol. 2018, 246, 309–330. [Google Scholar]
- Vivekanandam, V.; Munot, P.; Hanna, M.G.; Matthews, E. Skeletal Muscle channelopathies. Neurol. Clin. 2020, 38, 481–491. [Google Scholar] [CrossRef]
- Simkin, D.; Bendahhou, S. Skeletal Muscle Na+ Channel Disorders. Front. Pharmacol. 2011, 2, 63. [Google Scholar] [CrossRef]
- Desaphy, J.F.; Carbonara, R.; D’Amico, A.; Modoni, A.; Roussel, J.; Imbrici, P.; Pagliarani, S.; Lucchiari, S.; Lo Monaco, M.; Conte Camerino, D. Translational approach to address therapy in myotonia permanens due to a new SCN4A mutation. Neurology 2016, 22, 2010–2018. [Google Scholar]
- Camerino, G.M.; Pierno, S.; Liantonio, A.; De Bellis, M.; Cannone, M.; Sblendorio, V.; Conte, E.; Mele, A.; Tricarico, D.; Tavella, S.; et al. Effects of pleiotrophin overexpression on mouse skeletal muscles in normal loading and in actual and simulated microgravity. PLoS ONE 2013, 8, e72028. [Google Scholar] [CrossRef] [PubMed]
- Altamura, C.; Desaphy, J.F.; Conte, D.; De Luca, A.; Imbrici, P. Skeletal muscle ClC1 chloride channels in health and diseases. Pflugers Arch. 2020, 472, 961–975. [Google Scholar] [CrossRef] [PubMed]
- Hirn, C.; Shapovalov, G.; Petermann, O.; Roulet, E.; Ruegg, U.T. Nav1.4 deregulation in dystrophic skeletal muscle leads to Na+ overload and enhanced cell death. J. Gen. Physiol. 2008, 132, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Trip, J.; Drost, G.; van Engelen, B.G.M.; Faber, G.C. Drug treatment for myotonia. Cochrane Database Syst. Rev. 2006, 1, CD004762. [Google Scholar] [CrossRef]
- Alfonsi, E.; Merlo, I.M.; Tonini, M.; Ravaglia, S.; Brugnoni, R.; Gozzini, A.; Moglia, A. Efficacy of propafenone in paramyotonia congenita. Neurology 2007, 68, 1080–1081. [Google Scholar] [CrossRef]
- De Luca, A.; Pierno, S.; Natuzzi, F.; Franchini, C.; Duranti, A.; Lentini, G.; Tortorella, V.; Jockusch, H.; Camerino, D.C. Evaluation of the antimyotonic activity of mexiletine and some new analogs on sodium currents of single muscle fibers and on the abnormal excitability of the myotonic ADR mouse. J. Pharmacol. Exp. Ther. 1997, 282, 93–100. [Google Scholar]
- De Luca, A.; Pierno, S.; Liantonio, A.; Desaphy, J.-F.; Natuzzi, F.; Didonna, M.P.; Ferrannini, E.; Jockusch, H.; Franchini, C.; Lentini, G.; et al. New potent mexiletine and tocainide analogues evaluated in vivo and in vitro as antimyotonic agents on the myotonic ADR mouse. Neuromuscul. Disord. 2004, 14, 405–416. [Google Scholar] [CrossRef]
- Stunnenberg, B.C.; LoRusso, S.; Arnold, W.D.; Barohn, R.J.; Cannon, S.C.; Fontaine, B.; Griggs, R.C.; Hanna, M.G.; Matthews, E.; Meola, G.; et al. Guidelines on clinical presentation and management of nondystrophic myotonias. Muscle Nerve 2020, 62, 430–444. [Google Scholar] [CrossRef]
- Hoffman, E.P.; Kaminski, H.J. Mexiletine for treatment of myotonia. JAMA 2012, 308, 1377–1378. [Google Scholar] [CrossRef][Green Version]
- Statland, J.M.; Bundy, B.N.; Wang, Y.; Rayan, D.R.; Trivedi, J.R.; Sansone, V.A.; Salajegheh, M.K.; Venance, S.L.; Ciafaloni, E.; Matthews, E.; et al. Mexiletine for symptoms andsigns of myotonia in nondystrophic myotonia: A randomized con-trolled trial. JAMA 2012, 308, 1357–1365. [Google Scholar]
- Suetterlin, K.J.; Bugiardini, E.; Kaski, J.P.; Morrow, J.M.; Matthews, E.; Hanna, M.G.; Fialho, D. Long-term safety and efficacy of mexiletine for patients with skeletal muscle channelopathies. JAMA Neurol. 2015, 72, 1531–1533. [Google Scholar] [CrossRef] [PubMed]
- Stunnenberg, B.C.; Raaphorst, J.; Groenewoud, H.M.; Statland, J.M.; Robert, C.; Griggs, R.C.; Woertman, W.; Stegeman, D.F.; Timmermans, J.; Trivedi, J.; et al. Effect of mexiletine on muscle stiffness in patients with nondystrophic myotonia evaluated using aggregated N-of-1 trials. JAMA 2018, 320, 2344–2353. [Google Scholar] [CrossRef]
- Modoni, A.; D’Amico, A.; Primiano, G.; Capozzoli, F.; Desaphy, J.-F.; Monaco, M.L. Long-Term Safety and Usefulness of Mexiletine in a Large Cohort of Patients Affected by Non-dystrophic Myotonias. Front. Neurol. 2020, 11, 300. [Google Scholar] [CrossRef]
- Lo Monaco, M.; D’Amico, A.; Luigetti, M.; Desaphy, J.F.; Modoni, A. Effect of mexiletine on transitory depression of compound motor action potential in recessive myotonia congenita. Clin. Neurophysiol. 2015, 126, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Matthews, E.; Hanna, M.G. Repurposing of sodium channel antagonists as potential new anti-myotonic drugs. Exp. Neurol. 2014, 261, 812–815. [Google Scholar] [CrossRef] [PubMed]
- Desaphy, J.-F.; Modoni, A.; LoMonaco, M.; Camerino, D.C. Dramatic improvement of myotonia permanens with flecainide: A two-case report of a possible bench-to-bedside pharmacogenetics strategy. Eur. J. Clin. Pharmacol. 2013, 69, 1037–1039. [Google Scholar] [CrossRef]
- Maggi, L.; Bonanno, S.; Altamura, C.; Desaphy, J.-F. Ion Channel Gene Mutations Causing Skeletal Muscle Disorders: Pathomechanisms and Opportunities for Therapy. Cells 2021, 10, 1521. [Google Scholar] [CrossRef] [PubMed]
- Desaphy, J.-F.; Luca, A.D.E.; Didonna, M.P.; George, A.L.; Camerino, D.C. Different flecainide sensitivity of hNav1.4 channels and myotonic mutants explained by state-dependent block. J. Physiol. 2004, 554, 321–334. [Google Scholar] [CrossRef]
- Farinato, A.; Altamura, C.; Desaphy, J.-F. Effects of benzothiazolamines on voltage-gated sodium channels. Handb. Exp. Pharmacol. 2018, 246, 233–250. [Google Scholar]
- Cavalli, M.; Fossati, B.; Vitale, R.; Brigonzi, E.; Ricigliano, V.A.G.; Saraceno, L.; Cardani, R.; Pappone, C.; Meola, G. Flecainide-Induced Brugada Syndrome in a Patient with Skeletal Muscle Sodium Channelopathy: A Case Report with Critical Therapeutical Implications and Review of the Literature. Front. Neurol. 2018, 9, 385. [Google Scholar] [CrossRef]
- Streib, E.W. Paramyotonia congenita: Successful treatment with tocainide. Clinical and electrophysiologic findings in seven patients. Muscle Nerve 1987, 10, 155–162. [Google Scholar] [CrossRef]
- Soff, G.A.; Kadin, M.E. Tocainide-induced reversible agranulocytosis and anemia. Srch. Intern. Med. 1987, 147, 598–599. [Google Scholar] [CrossRef]
- Andersen, G.; Hedermann, G.; Witting, N.; Duno, M.; Andersen, H.; Vissing, J. The antimyotonic effect of lamotrigine in non-dystrophic myotonias: A double-blind randomized study. Brain 2017, 140, 2295–2305. [Google Scholar] [CrossRef] [PubMed]
- Vereb, N.; Montagnese, F.; Gläser, D.; Schoser, B. Non-dystrophic myotonias: Clinical and mutation spectrum of 70 German patients. J. Neurol. 2021, 268, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Novak, K.R.; Norman, J.; Mitchell, J.R.; Pinter, M.J.; Rich, M.M. Sodium channel slow inactivation as a therapeutic target for myotonia congenita. Ann. Neurol. 2015, 77, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Arnold, W.D.; Kline, D.; Sanderson, A.; Hawash, A.A.; Bartlett, A.; Novak, K.R.; Rich, M.M.; Kissel, J.T. Open-label trial of ranolazine for the treatment of myotonia congenita. Neurology 2017, 89, 710–713. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, S.; Kline, D.; Bartlett, A.; Freimer, M.; Agriesti, J.; Hawash, A.A.; Rich, M.M.; Kissel, J.T.; Arnold, W.D. Open-label trial of ranolazine for the treatment of paramyotonia congenital. Muscle Nerve 2019, 59, 240–243. [Google Scholar] [CrossRef] [PubMed]
- Ravaglia, S.; Maggi, L.; Zito, A.; Arceri, S.; Gallotti, P.; Altamura, C.; Desaphy, J.F.; Bernasconi, P.; Alfonsi, E. Buprenorphine may be effective for treatment of paramyotonia congenita. Muscle Nerve 2021, 64, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Montagnese, F.; Stahl, K.; Wenninger, S.; Schoser, B. A role for cannabinoids in the treatment of myotonia? Report of compassionate use in a small cohort of patients. J. Neurol. 2020, 267, 415–421. [Google Scholar] [CrossRef]
- Ghovanloo, M.-R.; Choudhury, K.; Bandaru, T.S.; Fouda, M.A.; Rayani, K.; Rusinova, R.; Phaterpekar, T.; Nelkenbrecher, K.; Watkins, A.R.; Poburko, D.; et al. Cannabidiol inhibits the skeletal muscle Nav1.4 by blocking its pore and by altering membrane elasticity. J. Gen. Physiol. 2021, 153, e202012701. [Google Scholar] [CrossRef]
- Philips, L.; Trivedi, J. Skeletal Muscle Channelopathies. Neurotherapeutics 2018, 4, 954–965. [Google Scholar] [CrossRef] [PubMed]
- Heatwole, C.R.; Statland, J.M.; Logigian, E.L. The diagnosis and treatment of myotonic disorders. Muscle Nerve 2013, 47, 632–648. [Google Scholar] [CrossRef] [PubMed]
- Jitpimolmard, N.; Matthews, E.; Fialho, D. Treatment Updates for Neuromuscular Channelopathies. Curr. Treat. Options Neurol. 2020, 22, 34. [Google Scholar] [CrossRef]
- Dharmadasa, T.; Kiernan, M.C. Riluzole, disease stage and survival in ALS. Lancet Neurol. 2018, 17, 385–386. [Google Scholar] [CrossRef] [PubMed]
- Desaphy, J.-F.; Carbonara, R.; Costanza, T.; Camerino, D.C. Preclinical evaluation of marketed sodium channel blockers in a rat model of myotonia discloses promising antimyotonic drugs. Exp. Neurol. 2014, 255, 96–102. [Google Scholar] [CrossRef]
- Caccia, C.; Maj, R.; Calabresi, M.; Maestroni, S.; Faravelli, L.; Curatolo, L.; Salvati, P.; Fariello, R.G. Safinamide: From molecular targets to a new anti-Parkinson drug. Neurology 2006, 67 (Suppl. 2), S18–S23. [Google Scholar] [CrossRef] [PubMed]
- Fariello, R.G. Safinamide. Nheurotherapeutics 2007, 4, 110–119. [Google Scholar] [CrossRef]
- Desaphy, J.F.; Farinato, A.; Altamura, C.; De Bellis, M.; Imbrici, P.; Tarantino, N.; Caccia, C.; Melloni, E.; Padoani, G.; Vailati, S.; et al. Safinamide’s potential in treating nondystrophic myotonias: Inhibition of skeletal muscle voltage-gated sodium channels and skeletal muscle hyperexcitability in vitro and in vivo. Exp. Neurol. 2020, 328, 113287. [Google Scholar] [CrossRef]
- Skov, M.; de Paoli, F.V.; Nielsen, O.B.; Pedersen, T.H. The anticonvulsants lacosamide, lamotrigine, and rufinamide reduce myotonia in isolated human and rat skeletal muscle. Muscle Nerve 2017, 56, 136–142. [Google Scholar] [CrossRef]
- Fozzard, H.A.; Sheets, M.F.; Hanck, D.A. The Sodium Channel as a Target for Local Anesthetic Drugs. Front. Pharmacol. 2011, 2, 68. [Google Scholar] [CrossRef]
- Payandeh, J.; Scheuer, T.; Zheng, N.; Catterall, W.A. The crystal structure of a voltage-gated sodium channel. Nature 2011, 475, 353–358. [Google Scholar] [CrossRef]
- Shen, H.; Zhou, Q.; Pan, X.; Li, Z.; Wu, J.; Yan, N. Structure of a eukaryotic voltage-gated sodium channel at nearatomic resolution. Science 2017, 355, eaal4326. [Google Scholar] [CrossRef]
- Li, T.; Chen, J. Voltage-Gated Sodium Channels in Drug Discovery. In Ion Channels Health Sickness; IntechOpen: London, UK, 2018; Chapter 2; pp. 13–44. [Google Scholar]
- Pan, X.; Li, Z.; Zhou, Q.; Shen, H.; Wu, K.; Huang, X.; Chen, J.; Zhang, J.; Zhu, X.; Lei, J.; et al. Structure of the human voltage-gated sodium channel Nav 1.4 in complex with β1. Science 2018, 362, eaau2486. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Shi, H.; Tonggu, L.; El-Din, T.M.G.; Lenaeus, M.J.; Zhao, Y.; Yoshioka, C.; Zheng, N.; Catterall, W.A. Structure of the Cardiac Sodium Channel. Cell 2020, 180, 122–134.e10. [Google Scholar] [CrossRef] [PubMed]
- George, A.L., Jr. Inherited disorders of voltage-gated sodium channels. J. Clin. Invest. 2005, 115, 1990–1999. [Google Scholar] [CrossRef] [PubMed]
- Catteral, W.A. Sodium channels, inherited epilepsy, and antiepileptic drugs. Annu. Rev. Pharmacol. Toxicol. 2014, 54, 317–338. [Google Scholar] [CrossRef]
- Ragsdale, D.S.; McPhee, J.C.; Scheuer, T.; Catterall, W.A. Molecular determinants of state-dependent block of Na+ channels by local anesthetics. Science 1994, 265, 1724–1728. [Google Scholar] [CrossRef] [PubMed]
- Ragsdale, D.S.; McPhee, J.C.; Scheuer, T.; Catterall, W.A. Common molecular determinants of local anesthetic, antiarrhythmic, and anticonvulsant block of voltage-gated Na+ channels. Proc. Natl. Acad. Sci. USA 1996, 93, 9270–9275. [Google Scholar] [CrossRef]
- Yarov-Yarovoy, V.; Brown, J.; Sharp, E.M.; Clare, J.J.; Scheuer, T.; Catterall, W.A. Molecular determinants of voltage-dependent gating and binding of pore-blocking drugs in transmembrane segment IIIS6 of the Na+ channel alpha subunit. J. Biol. Chem. 2001, 276, 20–27. [Google Scholar] [CrossRef]
- Yarov-Yarovoy, V.; McPhee, J.C.; Idsvoog, D.; Pate, C.; Scheuer, T.; Catterall, W.A. Role of amino acid residues in transmembrane segments IS6 and IIS6 of the Na+ channel alpha subunit in voltage-dependent gating and drug block. J. Biol. Chem. 2002, 277, 35393–35401. [Google Scholar] [CrossRef]
- Xiao, J.; Bondarenko, V.; Wang, Y.; Suma, A.; Wells, M.; Chen, Q.; Tillman, T.; Luo, Y.; Yu, B.; Dailey, W.P.; et al. Regulation and drug modulation of a voltage-gated sodium channel: Pivotal role of the S4-S5 linker in activation and slow inactivation. Proc. Natl. Acad. Sci. USA 2021, 118, e2102285118. [Google Scholar] [CrossRef] [PubMed]
- Thikhonov, D.B.; Zhorov, B.S. Mechanism of sodium channel block by local anesthetics, antiarrhythmics, and anticonvulsants. J. Gen. Physiol. 2017, 149, 465–481. [Google Scholar] [CrossRef] [PubMed]
- Hille, B.; Campbell, D.T. An Improved Vaseline Gap Voltage Clamp for Skeletal Muscle Fibers. J. Gen. Physiol. 1976, 67, 265–293. [Google Scholar] [CrossRef] [PubMed]
- De Luca, A.; Natuzzi, F.; Falcone, G.; Duranti, A.; Lentini, G.; Franchini, C.; Tortorella, V.; Camerino, D.C. Inhibition of frog skeletal muscle sodium channels by newly synthesized chiral derivatives of mexiletine and tocainide. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1997, 356, 777–787. [Google Scholar] [CrossRef]
- De Bellis, M.; Carbonara, R.; Roussel, J.; Farinato, A.; Massari, A.; Pierno, S.; Muraglia, M.; Corbo, F.; Franchini, C.; Carratù, M.R.; et al. Increased sodium channel use-dependent inhibition by a new potent analogue of tocainide greatly enhances in vivo antimyotonic activity. Neuropharmacology 2017, 113 Pt A, 206–216. [Google Scholar] [CrossRef]
- Milani, G.; Cavalluzzi, M.M.; Altamura, C.; Santoro, A.; Perrone, M.; Muraglia, M.; Colabufo, N.A.; Corbo, F.; Casalino, E.; Franchini, C.; et al. Bioisosteric Modification of To042: Synthesis and Evaluation of Promising Use-Dependent Inhibitors of Voltage-Gated Sodium Channels. ChemMedChem 2022, 17, e202200131. [Google Scholar] [CrossRef] [PubMed]
- Farinato, A.; Altamura, C.; Imbrici, P.; Maggi, L.; Bernasconi, P.; Mantegazza, R.; Pasquali, L.; Siciliano, G.; Lo Monaco, M.; Vial, C.; et al. Pharmacogenetics of myotonic hNav1.4 sodium channel variants situated near the fast inactivation gatez. Pharmacol. Res. 2019, 141, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Talon, S.; De Luca, A.; De Bellis, M.; Desaphy, J.F.; Lentini, G.; Scilimati, A.; Corbo, F.; Franchini, C.; Tortorella, P.; Jockusch, H.; et al. Increased rigidity of the chiral centre of tocainide favours stereoselectivity and use-dependent block of skeletal muscle Na+ channels enhancing the antimyotonic activity in vivo. Br. J. Pharmacol. 2001, 134, 1523–1531. [Google Scholar] [CrossRef]
- Adrian, R.H.; Bryant, S.H. On the repetitive discharge in myotonic muscle fibres. J. Physiol. 1974, 240, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Cannon, S.C. Ion-channel defects and aberrant excitability in myotonia and periodic paralysis. Trends Neurosci. 1996, 19, 3–10. [Google Scholar] [CrossRef]
- Bryant, S.H.; Morales-Aguilera, A. Chloride conductance in normal and myotonic muscle fibres and the action of monocarboxylic aromatic acids. J. Physiol. 1971, 219, 367–383. [Google Scholar] [CrossRef] [PubMed]
- Estévez, R.; Schroeder, B.C.; Accardi, A.; Jentsch, T.J.; Pusch, M. Conservation of chloride channel structure revealed by an inhibitor binding site in ClC-1. Neuron 2003, 38, 47–59. [Google Scholar] [CrossRef] [PubMed]
- De Luca, A.; Franchini, C.; Tortorella, V.; Camerino, D.C. Enantiomers of tocainide and mexiletine: Antimyotonic activity and use-dependent block of skeletal muscle Na+ channels. Pharmacol. Res. 1992, 26 (Suppl. 1), 27. [Google Scholar] [CrossRef]
- Hille, B. Local anesthetics: Hydrophilic and hydrophobic pathways for the drug-receptor reaction. J. Gen. Physiol. 1977, 69, 497–515. [Google Scholar] [CrossRef]
- Sheldon, R.S.; Cannon, N.J.; Nies, A.S.; Duff, H. Stereospecific interaction of tocainide with the cardiac sodium channel. Mol. Pharmacol. 1987, 33, 327–331. [Google Scholar]
- De Luca, A.; Natuzzi, F.; Desaphy, J.F.; Loni, G.; Lentini, G.; Franchini, C.; Tortorella, V.; Camerino, D.C. Molecular determinants of mexiletine structure for potent and use-dependent block of skeletal muscle sodium channels. Mol. Pharmacol. 2000, 57, 268–277. [Google Scholar]
- Catalano, A.; Carocci, A.; Corbo, F.; Franchini, C.; Muraglia, M.; Scilimati, A.; De Bellis, M.; De Luca, A.; Camerino, D.C.; Sinicropi, M.S.; et al. Constrained analogues of tocainide as potent skeletal muscle sodium channel blockers towards the development of antimyotonic agents. Eur. J. Med. Chem. 2008, 43, 2535–2540. [Google Scholar] [CrossRef]
- De Luca, A.; De Bellis, M.; Corbo, F.; Franchini, C.; Muraglia, M.; Catalano, A.; Carocci, A.; Camerino, D.C. Searching for novel anti-myotonic agents: Pharmacophore requirement for use-dependent block of skeletal muscle sodium channels by N-benzylated cyclic derivatives of tocainide. Neuromuscul. Disord. 2012, 22, 56–65. [Google Scholar] [CrossRef]
- Mehrke, G.; Brinkmeier, H.; Jockusch, H. The myotonic mouse mutant ADR: Electrophysiology of the muscle fiber. Muscle Nerve 1988, 11, 440–446. [Google Scholar] [CrossRef]
- Roden, D.M.; Woosley, R.J. Drug therapy. Tocainide. N. Engl. J. Med. 1986, 315, 41–45. [Google Scholar] [CrossRef]
- De Bellis, M.; De Luca, A.; Rana, F.; Cavalluzzi, M.M.; Catalano, A.; Lentini, G.; Franchini, C.; Tortorella, V.; Camerino, D.C. Evaluation of the pharmacological activity of the major mexiletine metabolites on skeletal muscle sodium currents. Br. J. Pharmacol. 2006, 149, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Catalano, A.; Carocci, A.; Fracchiolla, G.; Franchini, C.; Lentini, G.; Tortorella, V.; De Luca, A.; De Bellis, M.; Desaphy, J.-F.; Camerino, D.C. Stereospecific Synthesis of “para-Hydroxymexiletine” and Sodium Channel Blocking Activity Evaluation. Chirality 2004, 16, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Carrieri, A.; Muraglia, M.; Corbo, F.; Pacifico, C. 2D- and 3D-QSAR of Tocainide and Mexiletine analogues acting as Nav1.4 channel blockers. Eur. J. Med. Chem. 2009, 44, 1477–1485. [Google Scholar] [CrossRef] [PubMed]
- De Bellis, M.; De Luca, A.; Desaphy, J.F.; Carbonara, R.; Heiny, J.A.; Kennedy, A.; Carocci, A.; Cavalluzzi, M.M.; Lentini, G.; Franchini, C.; et al. Combined modifications of mexiletine pharmacophores for new lead blockers of Na(v)1.4 channels. Biophys. J. 2013, 104, 344–354. [Google Scholar] [CrossRef] [PubMed]
- De Luca, A.; Talon, S.; De Bellis, M.; Desaphy, J.F.; Lentini, G.; Corbo, F.; Scilimati, A.; Franchini, C.; Tortorella, V.; Camerino, D.C. Optimal requirements for high affinity and use-dependent block of skeletal muscle sodium channel by N-benzyl analogs of tocainide-like compounds. Mol. Pharmacol. 2003, 64, 932–945. [Google Scholar] [CrossRef]
- De Bellis, M.; Camerino, D.C.; Desaphy, J.-F. Toward precision medicine in myotonic syndromes. Oncotarget 2017, 8, 14279–14280. [Google Scholar] [CrossRef]
- Muraglia, M.; Franchini, C.; Corbo, F.; Scilimati, A.; Tortorella, V.; Sinicropi, M.S.; De Luca, A.; De Bellis, M.; Camerino, D.C. Synthesis of beta-proline like derivatives and their evaluation as sodium channel blockers. J. Heterocycl. Chem. 2007, 44, 1099–1103. [Google Scholar] [CrossRef]
- Muraglia, M.; De Bellis, M.; Catalano, A.; Carocci, A.; Franchini, C.; Carrieri, A.; Fortugno, C.; Bertucci, C.; Desaphy, J.-F.; De Luca, A.; et al. N-aryl-2,6-dimethylbenzamides, a new generation of tocainide analogues as blockers of skeletal muscle voltage-gated sodium channels. J. Med. Chem. 2014, 57, 2589–2600. [Google Scholar] [CrossRef]
- Demirpençe, E.; Caner, H.; Bavbek, M.; Kilinç, K. Antioxidant action of the antiarrhythmic drug mexiletine in brain membranes. Jpn. J. Pharmacol. 1999, 81, 7–11. [Google Scholar] [CrossRef]
- Chang, C.-Z.; Winardi, D.; Loh, J.-K.; Kung, S.-S.; Howng, S.-L.; Jeng, A.Y.; Kwan, A.-L. Alteration of ischemic reperfusion injury in the rat neocortex by a potent antioxidant mexiletine. Acta Neurochir. 2002, 144, 189–193. [Google Scholar] [CrossRef]
- De Bellis, M.; Sanarica, F.; Carocci, A.; Lentini, G.; Pierno, S.; Rolland, J.-F.; Camerino, D.C.; De Luca, A. Dual Action of Mexiletine and Its Pyrroline Derivatives as Skeletal Muscle Sodium Channel Blockers and Anti-oxidant Compounds: Toward Novel Therapeutic Potential. Front. Pharmacol. 2018, 8, 907. [Google Scholar] [CrossRef] [PubMed]
- Burdi, R.; Rolland, J.-F.; Fraysse, B.; Litvinova, K.; Cozzoli, A.; Giannuzzi, V.; Liantonio, A.; Camerino, G.M.; Sblendorio, V.; Capogrosso, R.F.; et al. Multiple pathological events in exercised dystrophic mdx mice are targeted by pentoxifylline: Outcome of a large array of in vivo and ex vivo tests. J. Appl. Physiol. 2009, 106, 1311–1324. [Google Scholar] [CrossRef] [PubMed]
- Roden, D.M.; Reele, S.B.; Higgins, S.B.; Wilkinson, G.R.; Smith, R.F.; Oates, J.A.; Woosley, R.L. Antiarrhythmic efficacy, pharmacokinetics and safety of N-acetylprocainamide in human subjects: Comparison with procainamide. Am. J. Cardiol. 1980, 46, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Burdi, R.; Didonna, M.P.; Pignol, B.; Nico, B.; Mangieri, D.; Rolland, J.-F.; Camerino, C.; Zallone, A.; Ferro, P.; Andreetta, F.; et al. First evaluation of the potential effectiveness in muscular dystrophy of a novel chimeric compound, BN 82270, acting as calpain-inhibitor and anti-oxidant. Neuromuscul. Disord. 2006, 16, 237–248. [Google Scholar] [CrossRef]
- Desaphy, J.F.; Dipalma, A.; Costanza, T.; Carbonara, R.; Dinardo, M.M.; Catalano, A.; Carocci, A.; Lentini, G.; Franchini, C.; Camerino, D.C. Molecular Insights into the Local Anesthetic Receptor within Voltage-Gated Sodium Channels Using Hydroxylated Analogs of Mexiletine. Front. Pharmacol. 2012, 3, 17. [Google Scholar] [CrossRef] [PubMed]
- March-Vila, E.; Pinzi, L.; Sturm, N.; Tinivella, A.; Engkvist, O.; Chen, H.; Rastelli, G. On the Integration of In Silico Drug Design Methods for Drug Repurposing. Front. Pharmacol. 2017, 8, 298. [Google Scholar] [CrossRef]
- Schattling, B.; Fazeli, W.; Engeland, B.; Liu, Y.; Lerche, H.; Isbrandt, D.; Friese, M.A. Activity of NaV1.2 promotes neurodegeneration in an animal model of multiple sclerosis. JCI Insight. 2016, 1, e89810. [Google Scholar] [CrossRef]
- Ciccone, R.; Franco, C.; Piccialli, I.; Boscia, F.; Casamassa, A.; de Rosa, V.; Cepparulo, P.; Cataldi, M.; Annunziato, L.; Pannaccione, A. Amyloid β-Induced Upregulation of Nav1.6 Underlies Neuronal Hyperactivity in Tg2576 Alzheimer’s Disease Mouse Model. Sci. Rep. 2019, 9, 13592. [Google Scholar] [CrossRef]
- Franklin, J.P.; Cooper-Knock, J.; Baheerathan, A.; Moll, T.; Männikkö, R.; Heverin, M.; Hardiman, O.; Shaw, P.J.; Hanna, M.G. Concurrent sodium channelopathies and amyotrophic lateral sclerosis supports shared pathogenesis. Amyotroph Lateral Scler. Front. Degener 2020, 21, 627–630. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Bellis, M.; Boccanegra, B.; Cerchiara, A.G.; Imbrici, P.; De Luca, A. Blockers of Skeletal Muscle Nav1.4 Channels: From Therapy of Myotonic Syndrome to Molecular Determinants of Pharmacological Action and Back. Int. J. Mol. Sci. 2023, 24, 857. https://doi.org/10.3390/ijms24010857
De Bellis M, Boccanegra B, Cerchiara AG, Imbrici P, De Luca A. Blockers of Skeletal Muscle Nav1.4 Channels: From Therapy of Myotonic Syndrome to Molecular Determinants of Pharmacological Action and Back. International Journal of Molecular Sciences. 2023; 24(1):857. https://doi.org/10.3390/ijms24010857
Chicago/Turabian StyleDe Bellis, Michela, Brigida Boccanegra, Alessandro Giovanni Cerchiara, Paola Imbrici, and Annamaria De Luca. 2023. "Blockers of Skeletal Muscle Nav1.4 Channels: From Therapy of Myotonic Syndrome to Molecular Determinants of Pharmacological Action and Back" International Journal of Molecular Sciences 24, no. 1: 857. https://doi.org/10.3390/ijms24010857
APA StyleDe Bellis, M., Boccanegra, B., Cerchiara, A. G., Imbrici, P., & De Luca, A. (2023). Blockers of Skeletal Muscle Nav1.4 Channels: From Therapy of Myotonic Syndrome to Molecular Determinants of Pharmacological Action and Back. International Journal of Molecular Sciences, 24(1), 857. https://doi.org/10.3390/ijms24010857




