The Effect of CaV1.2 Inhibitor Nifedipine on Chondrogenic Differentiation of Human Bone Marrow or Menstrual Blood-Derived Mesenchymal Stem Cells and Chondrocytes
Abstract
1. Introduction
2. Results
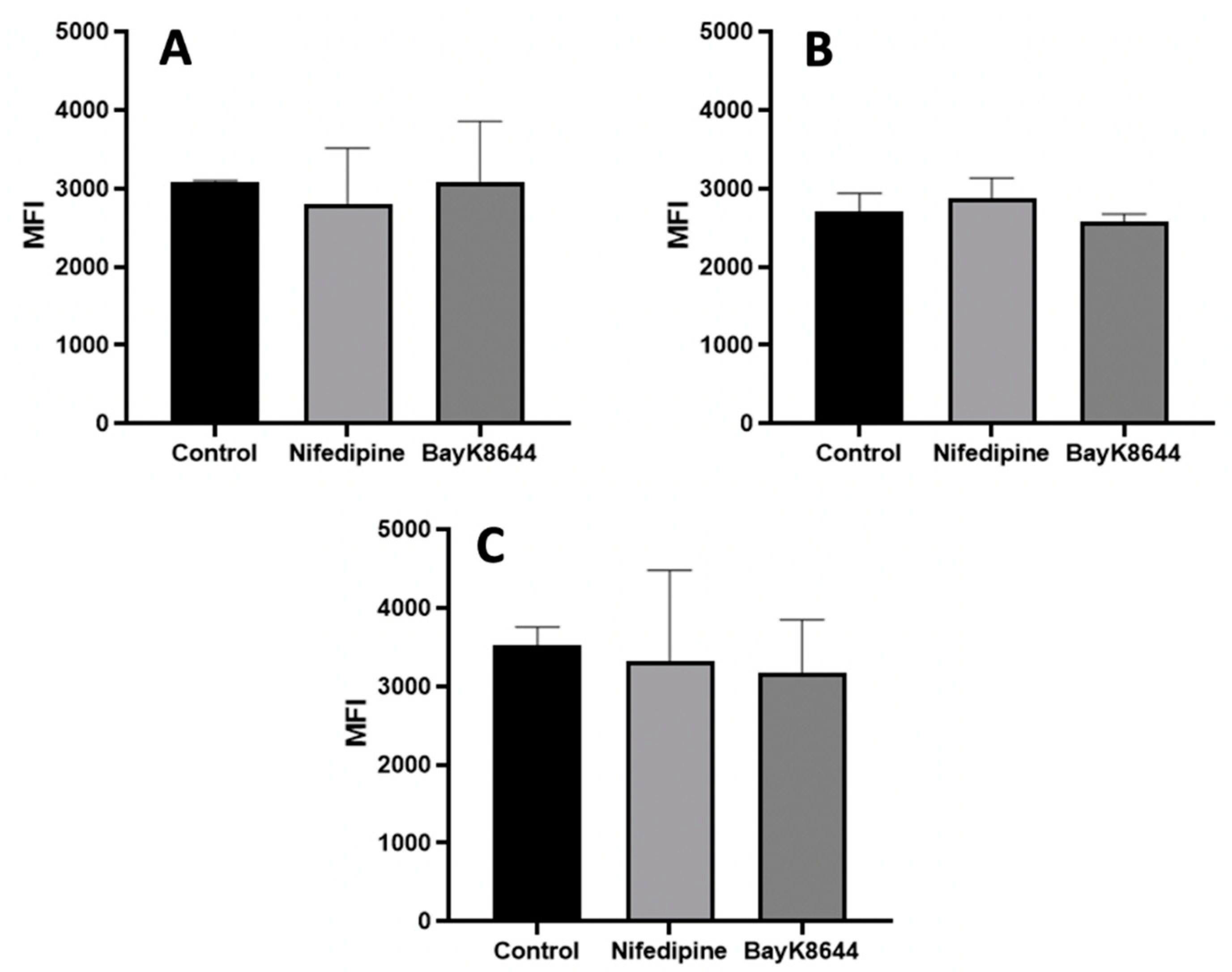
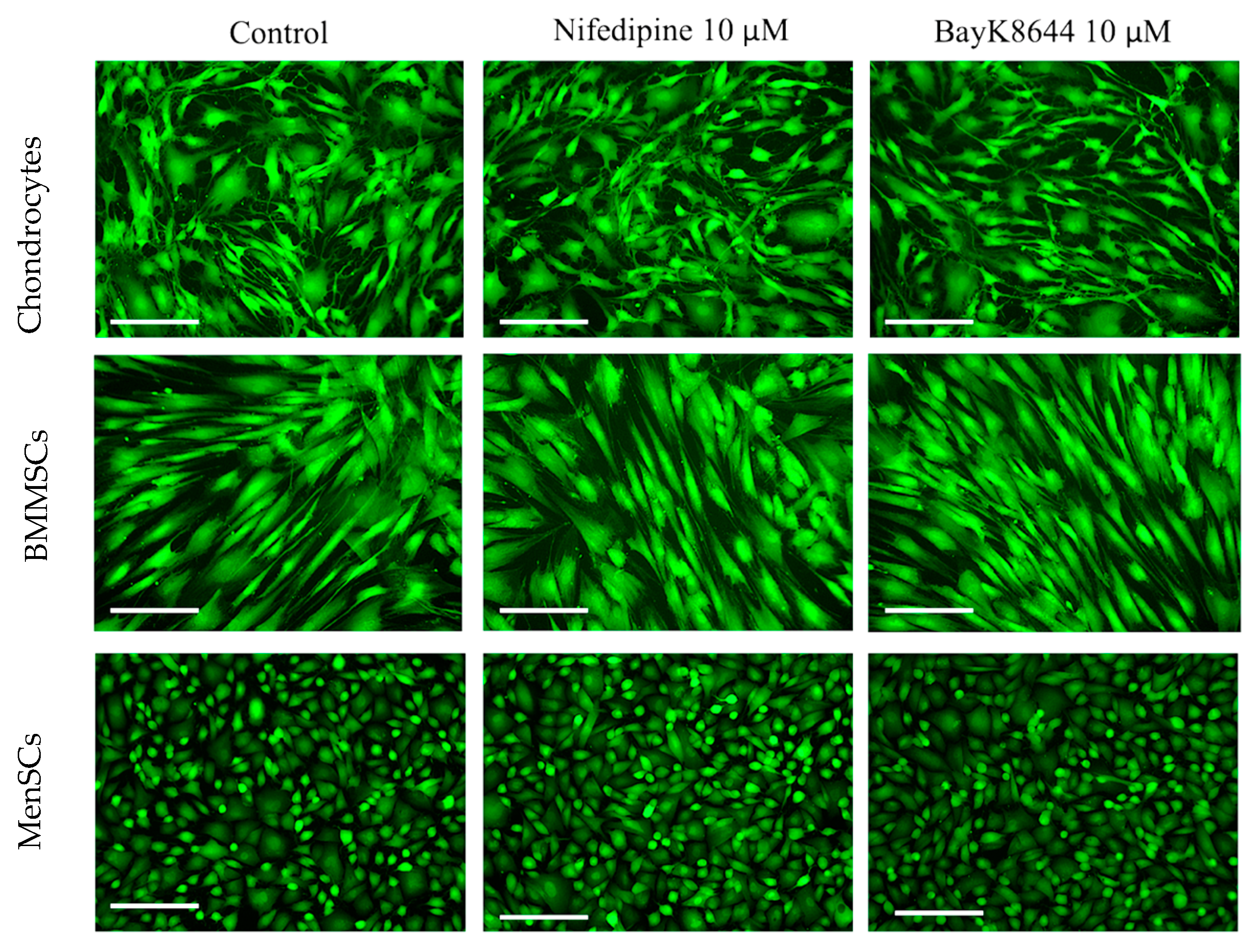
2.1. The Effects of Nifedipine and BayK8644 on iCa2+ Levels in MenSCs, BMMSCs and Chondrocytes
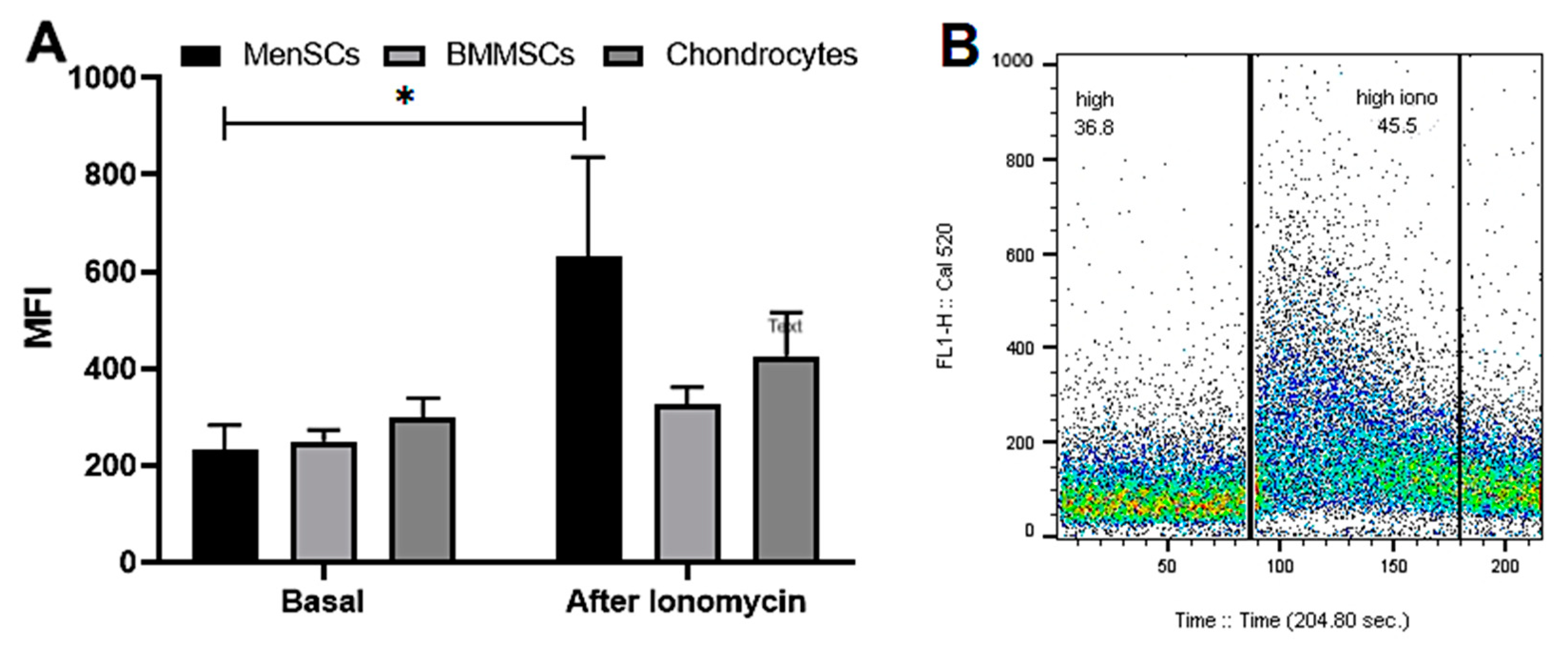
2.2. The Release of iCa2+ from Intracellular Stores in MenSCs, BMMSCs and Chondrocytes
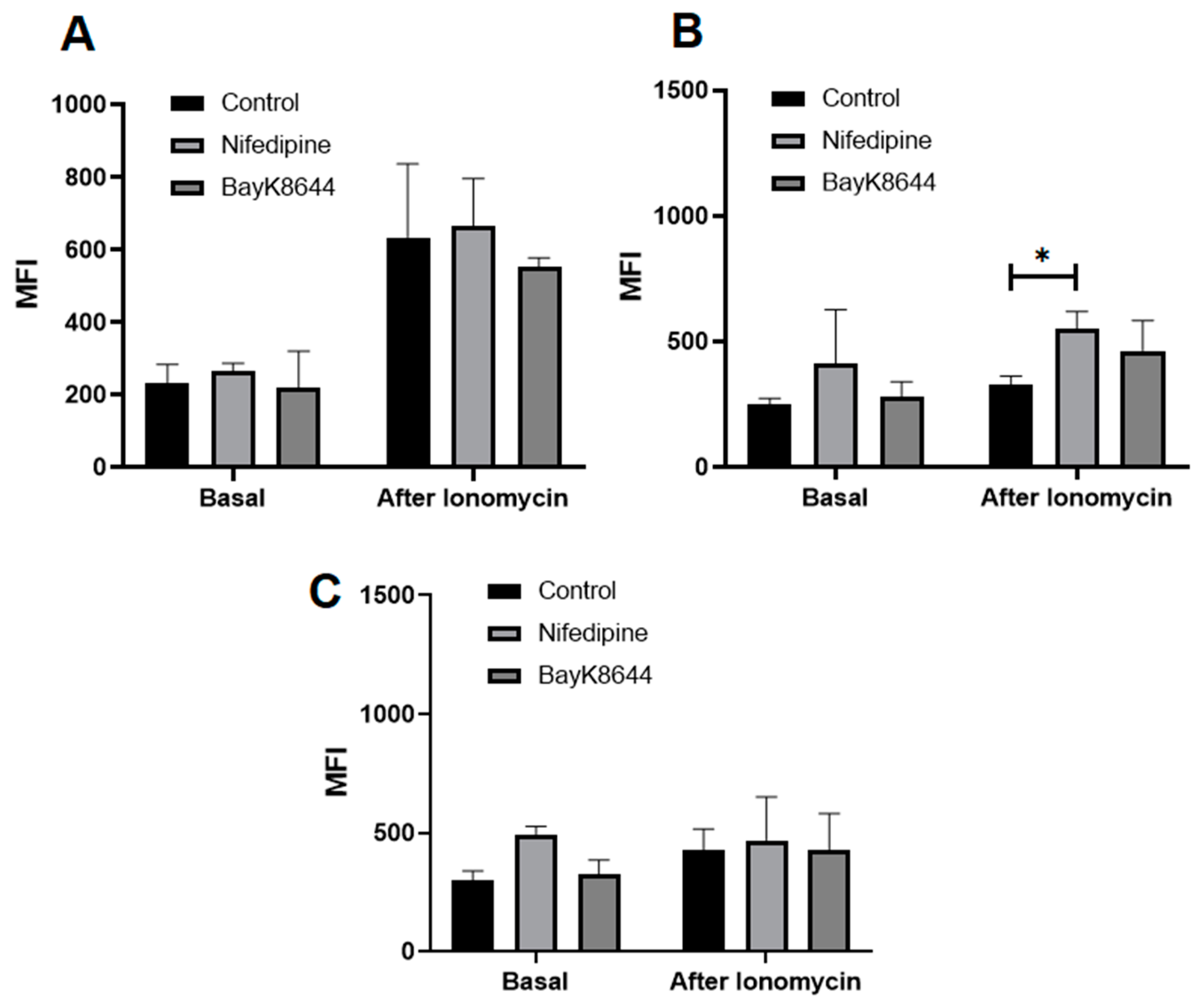
2.3. The Effect of Nifedipine on Basal Levels and Release of iCa2+ from Intracellular Stores of MenSCs, BMMSCs and Chondrocytes
2.4. The Effect of Nifedipine on Proliferation of MenSCs, BMMSCs and Chondrocytes
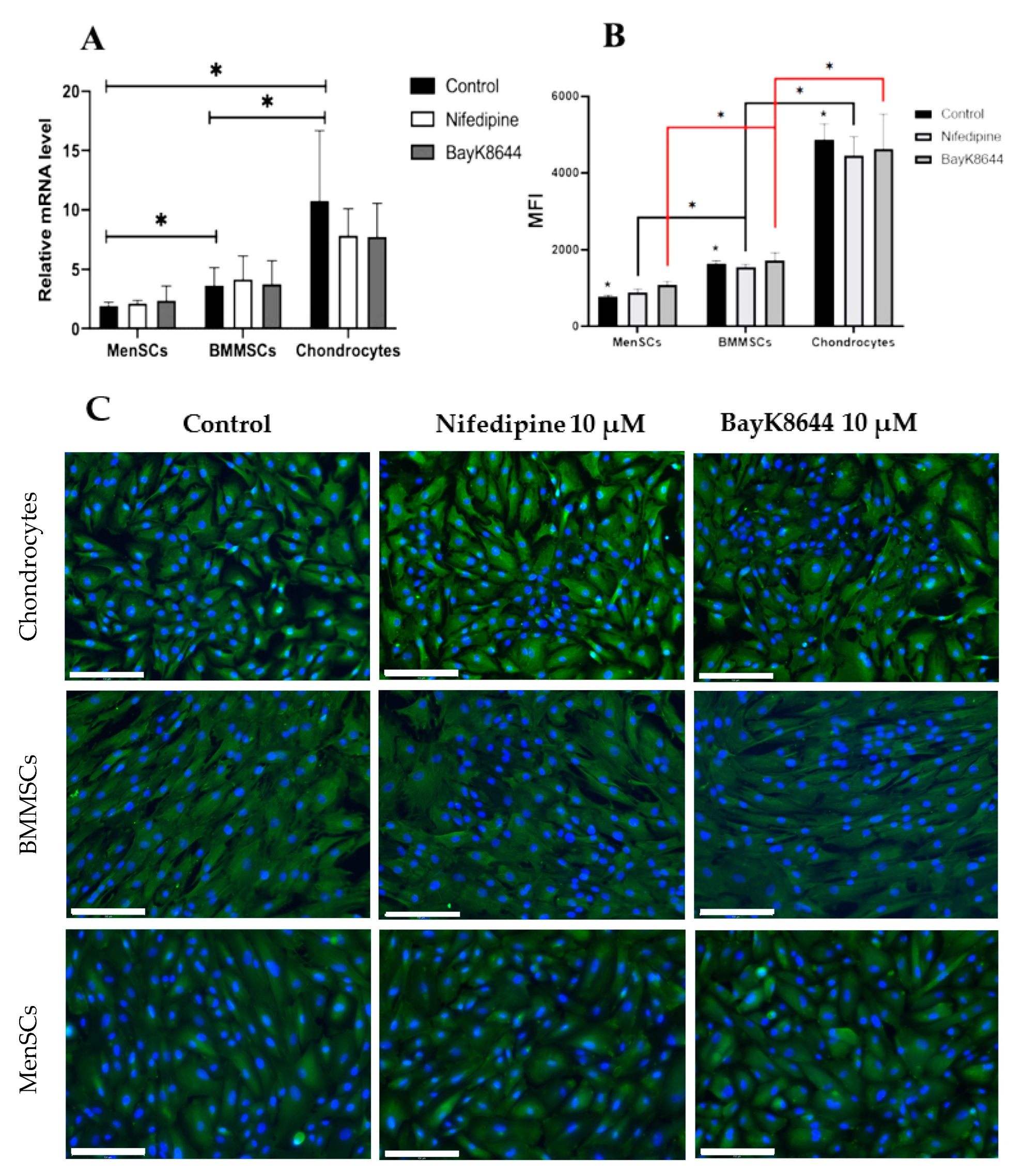
2.5. The Effect of Nifedipine on CaV1.2 Level in MenSCs, BMMSCs and Chondrocytes
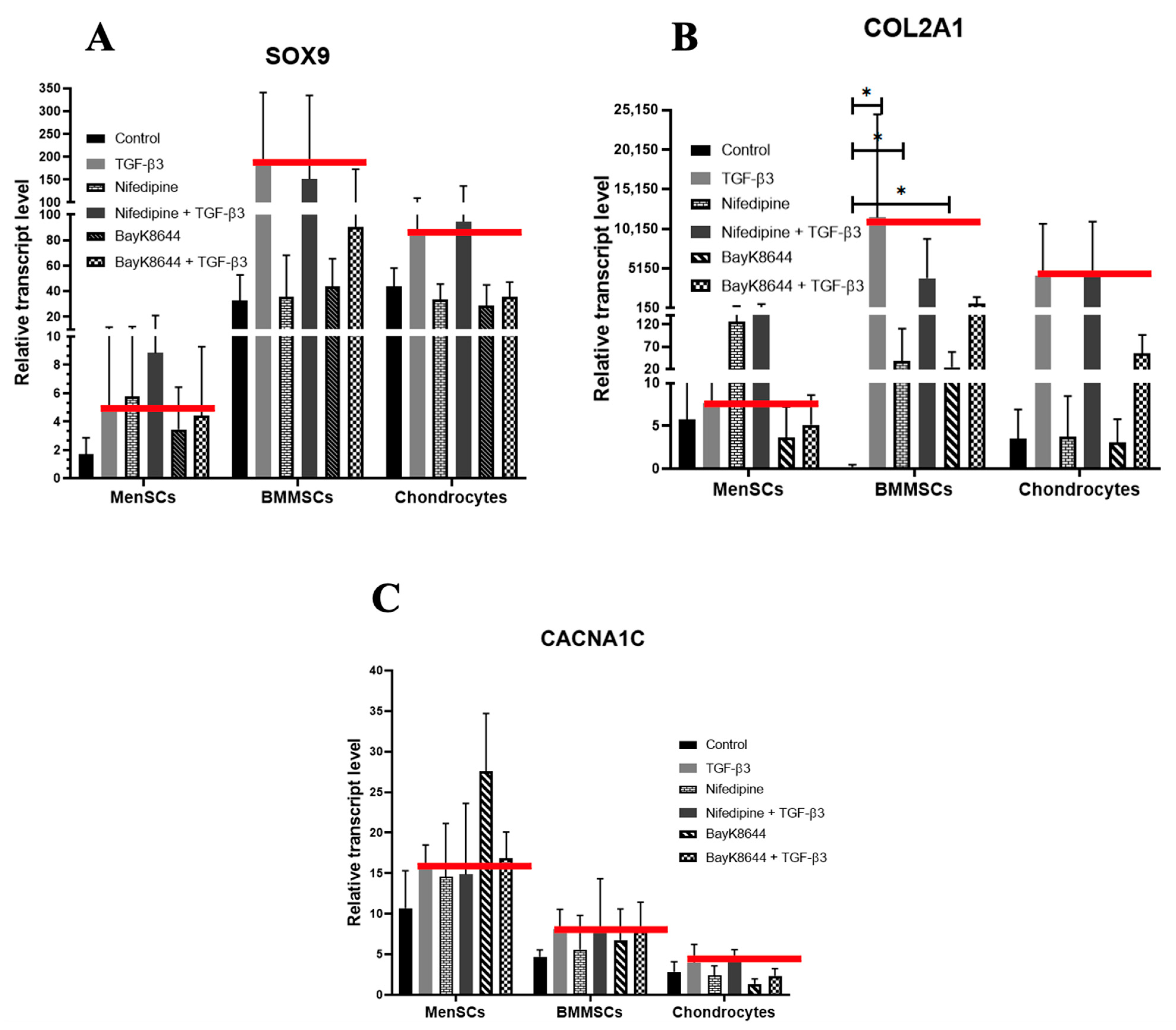
2.6. Different Effects of Nifedipine and BayK8644 on Expression of Chondrogenic Differentiation Markers in MenSCs, BMMSCs and Chondrocytes
3. Discussion
4. Materials and Methods
4.1. The Cell Isolation and Culture
4.2. Measurement of Intracellular Calcium (iCa2+) by Fluorescent Microscope and Flow Cytometry
4.3. Detection of CaV1.2 by Immunocytochemistry
4.4. Analysis of CaV1.2 by Flow Cytometer
4.5. Proliferation Assay
4.6. Chondrogenic Differentiation
4.7. Gene Expression Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMMSCs | bone marrow mesenchymal stem cells |
| BSA | bovine serum albumin |
| Ca2+ | calcium ions |
| COL2A1 | collagen type II gene |
| DAPI | 4′,6-diamidino-2-phenylindole |
| ER | endoplasmic reticulum |
| FBS | fetal bovine serum |
| FGF2 | fibroblast growth factor-2 |
| iCa2+ | intracellular Ca2+; |
| MenSCs | menstrual blood-derived mesenchymal stem cells |
| MFI | median fluorescence intensity |
| MSCs | mesenchymal stem cells |
| NSAIDs | non-steroidal anti-inflammatory drugs |
| OA | osteoarthritis |
| PFA | paraformaldehyde |
| TGF-β3 | transforming growth factor β3 |
| VOCC | voltage-operated calcium channels |
References
- Mobasheri, A.; Batt, M. An update on the pathophysiology of osteoarthritis. Ann. Phys. Rehabil. Med. 2016, 59, 333–339. [Google Scholar] [CrossRef]
- Mobasheri, A.; van Spil, W.E.; Budd, E.; Uzieliene, I.; Bernotiene, E.; Bay-Jensen, A.C.; Larkin, J.; Levesque, M.C.; Gualillo, O.; Henrotin, Y. Molecular taxonomy of osteoarthritis for patient stratification, disease management and drug development: Biochemical markers associated with emerging clinical phenotypes and molecular endotypes. Curr. Opin. Rheumatol. 2019, 31, 80–89. [Google Scholar] [CrossRef]
- Neogi, T. The epidemiology and impact of pain in osteoarthritis. Osteoarthr. Cartil. 2013, 21, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, A.; Rayman, M.P.; Gualillo, O.; Sellam, J.; van der Kraan, P.; Fearon, U. The role of metabolism in the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2017, 13, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Davies, P.S.; Graham, S.M.; MacFarlane, R.J.; Leonidou, A.; Mantalaris, A.; Tsiridis, E. Disease-modifying osteoarthritis drugs: In vitro and in vivo data on the development of DMOADs under investigation. Expert Opin. Investig. Drugs 2013, 22, 423–441. [Google Scholar] [CrossRef]
- Karsdal, M.A.; Michaelis, M.; Ladel, C.; Siebuhr, A.S.; Bihlet, A.R.; Andersen, J.R.; Guehring, H.; Christiansen, C.; Bay-Jensen, A.C.; Kraus, V.B. Disease-modifying treatments for osteoarthritis (DMOADs) of the knee and hip: Lessons learned from failures and opportunities for the future. Osteoarthr. Cartil. 2016, 24, 2013–2021. [Google Scholar] [CrossRef]
- Ghouri, A.; Conaghan, P.G. Update on novel pharmacological therapies for osteoarthritis. Ther. Adv. Musculoskelet Dis. 2019, 11, 1759720x19864492. [Google Scholar] [CrossRef]
- Mancuso, P.; Raman, S.; Glynn, A.; Barry, F.; Murphy, J.M. Mesenchymal Stem Cell Therapy for Osteoarthritis: The Critical Role of the Cell Secretome. Front. Bioeng. Biotechnol. 2019, 7, 9. [Google Scholar] [CrossRef]
- Iijima, H.; Isho, T.; Kuroki, H.; Takahashi, M.; Aoyama, T. Effectiveness of mesenchymal stem cells for treating patients with knee osteoarthritis: A meta-analysis toward the establishment of effective regenerative rehabilitation. NPJ Regen. Med. 2018, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Diekman, B.O.; Guilak, F. Stem cell-based therapies for osteoarthritis: Challenges and opportunities. Curr. Opin. Rheumatol. 2013, 25, 119–126. [Google Scholar] [CrossRef]
- Harrell, C.R.; Markovic, B.S.; Fellabaum, C.; Arsenijevic, A.; Volarevic, V. Mesenchymal stem cell-based therapy of osteoarthritis: Current knowledge and future perspectives. Biomed. Pharmacother. 2019, 109, 2318–2326. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.; Mobasheri, A.; Táncos, Z.; Kobolák, J.; Dinnyés, A. The Potency of Induced Pluripotent Stem Cells in Cartilage Regeneration and Osteoarthritis Treatment. Adv. Exp. Med. Biol. 2018, 1079, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Cheli, V.T.; Santiago González, D.A.; Namgyal Lama, T.; Spreuer, V.; Handley, V.; Murphy, G.G.; Paez, P.M. Conditional Deletion of the L-Type Calcium Channel Cav1.2 in Oligodendrocyte Progenitor Cells Affects Postnatal Myelination in Mice. J. Neurosci. 2016, 36, 10853–10869. [Google Scholar] [CrossRef]
- Caplan, A.I. Mesenchymal Stem Cells: Time to Change the Name! Stem Cells Transl. Med. 2017, 6, 1445–1451. [Google Scholar] [CrossRef]
- Craft, A.M.; Ahmed, N.; Rockel, J.S.; Baht, G.S.; Alman, B.A.; Kandel, R.A.; Grigoriadis, A.E.; Keller, G.M. Specification of chondrocytes and cartilage tissues from embryonic stem cells. Development 2013, 140, 2597–2610. [Google Scholar] [CrossRef] [PubMed]
- Ou, L.; Wang, X.; Zou, F. Is iPS cell the panacea? IUBMB Life 2010, 62, 170–175. [Google Scholar] [CrossRef]
- Wang, M.; Yuan, Z.; Ma, N.; Hao, C.; Guo, W.; Zou, G.; Zhang, Y.; Chen, M.; Gao, S.; Peng, J.; et al. Advances and Prospects in Stem Cells for Cartilage Regeneration. Stem Cells Int. 2017, 2017, 4130607. [Google Scholar] [CrossRef]
- Ansboro, S.; Greiser, U.; Barry, F.; Murphy, M. Strategies for improved targeting of therapeutic cells: Implications for tissue repair. Eur. Cell Mater. 2012, 23, 310–318. [Google Scholar] [CrossRef]
- Somoza, R.A.; Welter, J.F.; Correa, D.; Caplan, A.I. Chondrogenic differentiation of mesenchymal stem cells: Challenges and unfulfilled expectations. Tissue Eng. Part B Rev. 2014, 20, 596–608. [Google Scholar] [CrossRef]
- Murata, D.; Fujimoto, R.; Nakayama, K. Osteochondral Regeneration Using Adipose Tissue-Derived Mesenchymal Stem Cells. Int. J. Mol. Sci. 2020, 21, 3589. [Google Scholar] [CrossRef]
- Fernandez-Pernas, P.; Rodríguez-Lesende, I.; de la Fuente, A.; Mateos, J.; Fuentes, I.; De Toro, J.; Blanco, F.J.; Arufe, M.C. CD105+-mesenchymal stem cells migrate into osteoarthritis joint: An animal model. PLoS ONE 2017, 12, e0188072. [Google Scholar] [CrossRef] [PubMed]
- Nejadnik, H.; Hui, J.H.; Feng Choong, E.P.; Tai, B.C.; Lee, E.H. Autologous bone marrow-derived mesenchymal stem cells versus autologous chondrocyte implantation: An observational cohort study. Am. J. Sports Med. 2010, 38, 1110–1116. [Google Scholar] [CrossRef]
- Spakova, T.; Plsikova, J.; Harvanova, D.; Lacko, M.; Stolfa, S.; Rosocha, J. Influence of Kartogenin on Chondrogenic Differentiation of Human Bone Marrow-Derived MSCs in 2D Culture and in Co-Cultivation with OA Osteochondral Explant. Molecules 2018, 23, 181. [Google Scholar] [CrossRef] [PubMed]
- Brittberg, M.; Lindahl, A.; Nilsson, A.; Ohlsson, C.; Isaksson, O.; Peterson, L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N. Engl. J. Med. 1994, 331, 889–895. [Google Scholar] [CrossRef]
- Mrugala, D.; Bony, C.; Neves, N.; Caillot, L.; Fabre, S.; Moukoko, D.; Jorgensen, C.; Noël, D. Phenotypic and functional characterisation of ovine mesenchymal stem cells: Application to a cartilage defect model. Ann. Rheum. Dis. 2008, 67, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Matas, J.; Orrego, M.; Amenabar, D.; Infante, C.; Tapia-Limonchi, R.; Cadiz, M.I.; Alcayaga-Miranda, F.; González, P.L.; Muse, E.; Khoury, M.; et al. Umbilical Cord-Derived Mesenchymal Stromal Cells (MSCs) for Knee Osteoarthritis: Repeated MSC Dosing Is Superior to a Single MSC Dose and to Hyaluronic Acid in a Controlled Randomized Phase I/II Trial. Stem Cells Transl. Med. 2019, 8, 215–224. [Google Scholar] [CrossRef]
- Rossignoli, F.; Caselli, A.; Grisendi, G.; Piccinno, S.; Burns, J.S.; Murgia, A.; Veronesi, E.; Loschi, P.; Masini, C.; Conte, P.; et al. Isolation, Characterization, and Transduction of Endometrial Decidual Tissue Multipotent Mesenchymal Stromal/Stem Cells from Menstrual Blood. BioMed Res. Int. 2013, 2013, 901821. [Google Scholar] [CrossRef]
- Fodor, J.; Matta, C.; Juhász, T.; Oláh, T.; Gönczi, M.; Szíjgyártó, Z.; Gergely, P.; Csernoch, L.; Zákány, R. Ionotropic purinergic receptor P2X4 is involved in the regulation of chondrogenesis in chicken micromass cell cultures. Cell Calcium 2009, 45, 421–430. [Google Scholar] [CrossRef]
- Matta, C.; Zakany, R. Calcium signalling in chondrogenesis: Implications for cartilage repair. Front. Biosci.-Sch. 2013, 5, 305–324. [Google Scholar] [CrossRef] [PubMed]
- Matta, C.; Mobasheri, A. Regulation of chondrogenesis by protein kinase C: Emerging new roles in calcium signalling. Cell. Signal. 2014, 26, 979–1000. [Google Scholar] [CrossRef]
- Uzieliene, I.; Bernotas, P.; Mobasheri, A.; Bernotiene, E. The Role of Physical Stimuli on Calcium Channels in Chondrogenic Differentiation of Mesenchymal Stem Cells. Int. J. Mol. Sci. 2018, 19, 2998. [Google Scholar] [CrossRef]
- Mobasheri, A.; Matta, C.; Uzielienè, I.; Budd, E.; Martín-Vasallo, P.; Bernotiene, E. The chondrocyte channelome: A narrative review. Jt. Bone Spine 2019, 86, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.E. Cytotoxic Swelling of Sick Excitable Cells-Impaired Ion Homeostasis and Membrane Tension Homeostasis in Muscle and Neuron. Curr. Top. Membr. 2018, 81, 457–496. [Google Scholar] [CrossRef]
- Uzieliene, I.; Bernotiene, E.; Rakauskiene, G.; Denkovskij, J.; Bagdonas, E.; Mackiewicz, Z.; Porvaneckas, N.; Kvederas, G.; Mobasheri, A. The Antihypertensive Drug Nifedipine Modulates the Metabolism of Chondrocytes and Human Bone Marrow-Derived Mesenchymal Stem Cells. Front. Endocrinol. 2019, 10, 756. [Google Scholar] [CrossRef]
- Jadhav, A.; Liang, W.; Papageorgiou, P.C.; Shoker, A.; Kanthan, S.C.; Balsevich, J.; Levy, A.S.; Heximer, S.; Backx, P.H.; Gopalakrishnan, V. Catharanthine dilates small mesenteric arteries and decreases heart rate and cardiac contractility by inhibition of voltage-operated calcium channels on vascular smooth muscle cells and cardiomyocytes. J. Pharmacol. Exp. Ther. 2013, 345, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Kawano, S.; Shoji, S.; Ichinose, S.; Yamagata, K.; Tagami, M.; Hiraoka, M. Characterization of Ca(2+) signaling pathways in human mesenchymal stem cells. Cell Calcium 2002, 32, 165–174. [Google Scholar] [CrossRef]
- Shao, Y.; Alicknavitch, M.; Farach-Carson, M.C. Expression of voltage sensitive calcium channel (VSCC) L-type Cav1.2 (alpha1C) and T-type Cav3.2 (alpha1H) subunits during mouse bone development. Dev. Dyn. 2005, 234, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Vaiciuleviciute, R.; Bironaite, D.; Uzieliene, I.; Mobasheri, A.; Bernotiene, E. Cardiovascular Drugs and Osteoarthritis: Effects of Targeting Ion Channels. Cells 2021, 10, 2572. [Google Scholar] [CrossRef] [PubMed]
- Munaron, L.; Antoniotti, S.; Lovisolo, D. Intracellular calcium signals and control of cell proliferation: How many mechanisms? J. Cell Mol. Med. 2004, 8, 161–168. [Google Scholar] [CrossRef]
- Xiang, S.; Lin, Z.; Makarcyzk, M.J.; Riewruja, K.; Zhang, Y.; Zhang, X.; Li, Z.; Clark, K.L.; Li, E.; Liu, S.; et al. Differences in the intrinsic chondrogenic potential of human mesenchymal stromal cells and iPSC-derived multipotent cells. Clin. Transl. Med. 2022, 12, e1112. [Google Scholar] [CrossRef]
- Uzieliene, I.; Bironaite, D.; Pachaleva, J.; Bagdonas, E.; Sobolev, A.; Tsai, W.-B.; Kvedaras, G.; Bernotiene, E. Chondroitin Sulfate-Tyramine-Based Hydrogels for Cartilage Tissue Repair. Int. J. Mol. Sci. 2023, 24, 3451. [Google Scholar] [CrossRef]
- Vonk, L.A.; van Dooremalen, S.F.J.; Liv, N.; Klumperman, J.; Coffer, P.J.; Saris, D.B.F.; Lorenowicz, M.J. Mesenchymal Stromal/stem Cell-derived Extracellular Vesicles Promote Human Cartilage Regeneration In Vitro. Theranostics 2018, 8, 906–920. [Google Scholar] [CrossRef]
- Solchaga, L.A.; Penick, K.J.; Welter, J.F. Chondrogenic differentiation of bone marrow-derived mesenchymal stem cells: Tips and tricks. Methods Mol. Biol. 2011, 698, 253–278. [Google Scholar] [CrossRef]
- Narcisi, R.; Koevoet, W.; van Osch, G. Expansion and Chondrogenic Differentiation of Human Bone Marrow-Derived Mesenchymal Stromal Cells. Methods Mol. Biol. 2021, 2221, 15–28. [Google Scholar] [CrossRef]
- Uzieliene, I.; Bironaite, D.; Bagdonas, E.; Pachaleva, J.; Sobolev, A.; Tsai, W.-B.; Kvederas, G.; Bernotiene, E. The Effects of Mechanical Load on Chondrogenic Responses of Bone Marrow Mesenchymal Stem Cells and Chondrocytes Encapsulated in Chondroitin Sulfate-Based Hydrogel. Int. J. Mol. Sci. 2023, 24, 2915. [Google Scholar] [CrossRef] [PubMed]
- Park, M.S.; Kim, Y.H.; Jung, Y.; Kim, S.H.; Park, J.C.; Yoon, D.S.; Kim, S.-H.; Lee, J.W. In Situ Recruitment of Human Bone Marrow-Derived Mesenchymal Stem Cells Using Chemokines for Articular Cartilage Regeneration. Cell Transplant. 2015, 24, 1067–1083. [Google Scholar] [CrossRef]
- Doyle, E.C.; Wragg, N.M.; Wilson, S.L. Intraarticular injection of bone marrow-derived mesenchymal stem cells enhances regeneration in knee osteoarthritis. Knee Surg. Sport. Traumatol. Arthrosc. 2020, 28, 3827–3842. [Google Scholar] [CrossRef]
- Meng, X.; Ichim, T.E.; Zhong, J.; Rogers, A.; Yin, Z.; Jackson, J.; Wang, H.; Ge, W.; Bogin, V.; Chan, K.W.; et al. Endometrial regenerative cells: A novel stem cell population. J. Transl. Med. 2007, 5, 57. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, M.R.; Carvalhos, C.A.; Figueiredo-Dias, M. The Emerging Role of Menstrual-Blood-Derived Stem Cells in Endometriosis. Biomedicines 2023, 11, 39. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Stock, P.; Brückner, S.; Winkler, S.; Dollinger, M.M.; Christ, B. Human bone marrow mesenchymal stem cell-derived hepatocytes improve the mouse liver after acute acetaminophen intoxication by preventing progress of injury. Int. J. Mol. Sci. 2014, 15, 7004–7028. [Google Scholar] [CrossRef]
- Islam, M.S. Calcium Signaling: From Basic to Bedside. Adv. Exp. Med. Biol. 2020, 1131, 1–6. [Google Scholar] [CrossRef]
- Cui, C.; Merritt, R.; Fu, L.; Pan, Z. Targeting calcium signaling in cancer therapy. Acta Pharm. Sin. B 2017, 7, 3–17. [Google Scholar] [CrossRef]
- Klec, C.; Ziomek, G.; Pichler, M.; Malli, R.; Graier, W.F. Calcium Signaling in ß-cell Physiology and Pathology: A Revisit. Int. J. Mol. Sci. 2019, 20, 6110. [Google Scholar] [CrossRef]
- Glaser, T.; Arnaud Sampaio, V.F.; Lameu, C.; Ulrich, H. Calcium signalling: A common target in neurological disorders and neurogenesis. Semin. Cell Dev. Biol. 2019, 95, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Suryakumar, G.; Rathor, R. Role of defective Ca(2+) signaling in skeletal muscle weakness: Pharmacological implications. J. Cell Commun. Signal. 2018, 12, 645–659. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Sopher, B.L.; Furukawa, K.; Pham, D.G.; Robinson, N.; Martin, G.M.; Mattson, M.P. Alzheimer’s presenilin mutation sensitizes neural cells to apoptosis induced by trophic factor withdrawal and amyloid beta-peptide: Involvement of calcium and oxyradicals. J. Neurosci. 1997, 17, 4212–4222. [Google Scholar] [CrossRef] [PubMed]
- Carey, M.B.; Matsumoto, S.G. Spontaneous calcium transients are required for neuronal differentiation of murine neural crest. Dev. Biol. 1999, 215, 298–313. [Google Scholar] [CrossRef] [PubMed]
- Kuo, I.Y.; Ehrlich, B.E. Signaling in muscle contraction. Cold Spring Harb. Perspect. Biol. 2015, 7, a006023. [Google Scholar] [CrossRef]
- Winslow, R.L.; Walker, M.A.; Greenstein, J.L. Modeling calcium regulation of contraction, energetics, signaling, and transcription in the cardiac myocyte. Wiley Interdiscip. Rev. Syst. Biol. Med. 2016, 8, 37–67. [Google Scholar] [CrossRef]
- Buonanno, A.; Fields, R.D. Gene regulation by patterned electrical activity during neural and skeletal muscle development. Curr. Opin. Neurobiol. 1999, 9, 110–120. [Google Scholar] [CrossRef]
- Pchelintseva, E.; Djamgoz, M.B.A. Mesenchymal stem cell differentiation: Control by calcium-activated potassium channels. J. Cell. Physiol. 2018, 233, 3755–3768. [Google Scholar] [CrossRef] [PubMed]
- Patergnani, S.; Danese, A.; Bouhamida, E.; Aguiari, G.; Previati, M.; Pinton, P.; Giorgi, C. Various Aspects of Calcium Signaling in the Regulation of Apoptosis, Autophagy, Cell Proliferation, and Cancer. Int. J. Mol. Sci. 2020, 21, 8323. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Holt, M.; Philipova, R.; Moss, S.; Schulman, H.; Hidaka, H.; Whitaker, M. Calcium/calmodulin-dependent phosphorylation and activation of human Cdc25-C at the G2/M phase transition in HeLa cells. J. Biol. Chem. 1999, 274, 7958–7968. [Google Scholar] [CrossRef] [PubMed]
- Bonora, M.; Patergnani, S.; Ramaccini, D.; Morciano, G.; Pedriali, G.; Kahsay, A.E.; Bouhamida, E.; Giorgi, C.; Wieckowski, M.R.; Pinton, P. Physiopathology of the Permeability Transition Pore: Molecular Mechanisms in Human Pathology. Biomolecules 2020, 10, 998. [Google Scholar] [CrossRef] [PubMed]
- Morgan, A.J.; Jacob, R. Ionomycin enhances Ca2+ influx by stimulating store-regulated cation entry and not by a direct action at the plasma membrane. Biochem. J. 1994, 300 Pt 3, 665–672. [Google Scholar] [CrossRef]
- Yoshida, S.; Plant, S. Mechanism of release of Ca2+ from intracellular stores in response to ionomycin in oocytes of the frog Xenopus laevis. J. Physiol. 1992, 458, 307–318. [Google Scholar] [CrossRef]
- Uzieliene, I.; Bagdonas, E.; Hoshi, K.; Sakamoto, T.; Hikita, A.; Tachtamisevaite, Z.; Rakauskiene, G.; Kvederas, G.; Mobasheri, A.; Bernotiene, E. Different phenotypes and chondrogenic responses of human menstrual blood and bone marrow mesenchymal stem cells to activin A and TGF-β3. Stem Cell Res. Ther. 2021, 12, 251. [Google Scholar] [CrossRef]
- Piriz, J.; Rosato Siri, M.D.; Pagani, R.; Uchitel, O.D. Nifedipine-mediated mobilization of intracellular calcium stores increases spontaneous neurotransmitter release at neonatal rat motor nerve terminals. J. Pharmacol. Exp. Ther. 2003, 306, 658–663. [Google Scholar] [CrossRef]
- Berkels, R.; Mueller, A.; Roesen, R.; Klaus, W. Nifedipine and Bay K 8644 Induce an increase of. J. Cardiovasc. Pharmacol. Ther. 1999, 4, 175–181. [Google Scholar] [CrossRef]
- Carafoli, E.; Krebs, J. Why Calcium? How Calcium Became the Best Communicator. J. Biol. Chem. 2016, 291, 20849–20857. [Google Scholar] [CrossRef]
- Atsuta, Y.; Tomizawa, R.R.; Levin, M.; Tabin, C.J. L-type voltage-gated Ca(2+) channel Ca(V)1.2 regulates chondrogenesis during limb development. Proc. Natl. Acad. Sci. USA 2019, 116, 21592–21601. [Google Scholar] [CrossRef] [PubMed]
- Dieterle, M.P.; Husari, A.; Rolauffs, B.; Steinberg, T.; Tomakidi, P. Integrins, cadherins and channels in cartilage mechanotransduction: Perspectives for future regeneration strategies. Expert Rev. Mol. Med. 2021, 23, e14. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.; Ge, J.; Ren, X.; Zhu, X.; Xue, Z.; Feng, Y.; Zhao, S. Cav1.2 of L-type Calcium Channel Is a Key Factor for the Differentiation of Dental Pulp Stem Cells. J. Endod. 2015, 41, 1048–1055. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uzieliene, I.; Bironaite, D.; Miksiunas, R.; Bagdonas, E.; Vaiciuleviciute, R.; Mobasheri, A.; Bernotiene, E. The Effect of CaV1.2 Inhibitor Nifedipine on Chondrogenic Differentiation of Human Bone Marrow or Menstrual Blood-Derived Mesenchymal Stem Cells and Chondrocytes. Int. J. Mol. Sci. 2023, 24, 6730. https://doi.org/10.3390/ijms24076730
Uzieliene I, Bironaite D, Miksiunas R, Bagdonas E, Vaiciuleviciute R, Mobasheri A, Bernotiene E. The Effect of CaV1.2 Inhibitor Nifedipine on Chondrogenic Differentiation of Human Bone Marrow or Menstrual Blood-Derived Mesenchymal Stem Cells and Chondrocytes. International Journal of Molecular Sciences. 2023; 24(7):6730. https://doi.org/10.3390/ijms24076730
Chicago/Turabian StyleUzieliene, Ilona, Daiva Bironaite, Rokas Miksiunas, Edvardas Bagdonas, Raminta Vaiciuleviciute, Ali Mobasheri, and Eiva Bernotiene. 2023. "The Effect of CaV1.2 Inhibitor Nifedipine on Chondrogenic Differentiation of Human Bone Marrow or Menstrual Blood-Derived Mesenchymal Stem Cells and Chondrocytes" International Journal of Molecular Sciences 24, no. 7: 6730. https://doi.org/10.3390/ijms24076730
APA StyleUzieliene, I., Bironaite, D., Miksiunas, R., Bagdonas, E., Vaiciuleviciute, R., Mobasheri, A., & Bernotiene, E. (2023). The Effect of CaV1.2 Inhibitor Nifedipine on Chondrogenic Differentiation of Human Bone Marrow or Menstrual Blood-Derived Mesenchymal Stem Cells and Chondrocytes. International Journal of Molecular Sciences, 24(7), 6730. https://doi.org/10.3390/ijms24076730






