Regulation of PIN-FORMED Protein Degradation
Abstract
1. Introduction
2. PIN Degradation
3. Ubiquitin Modification
4. Ubiquitin E3 Ligases
5. PI3K Complexes
6. SNAREs
7. The Retrograde Systems
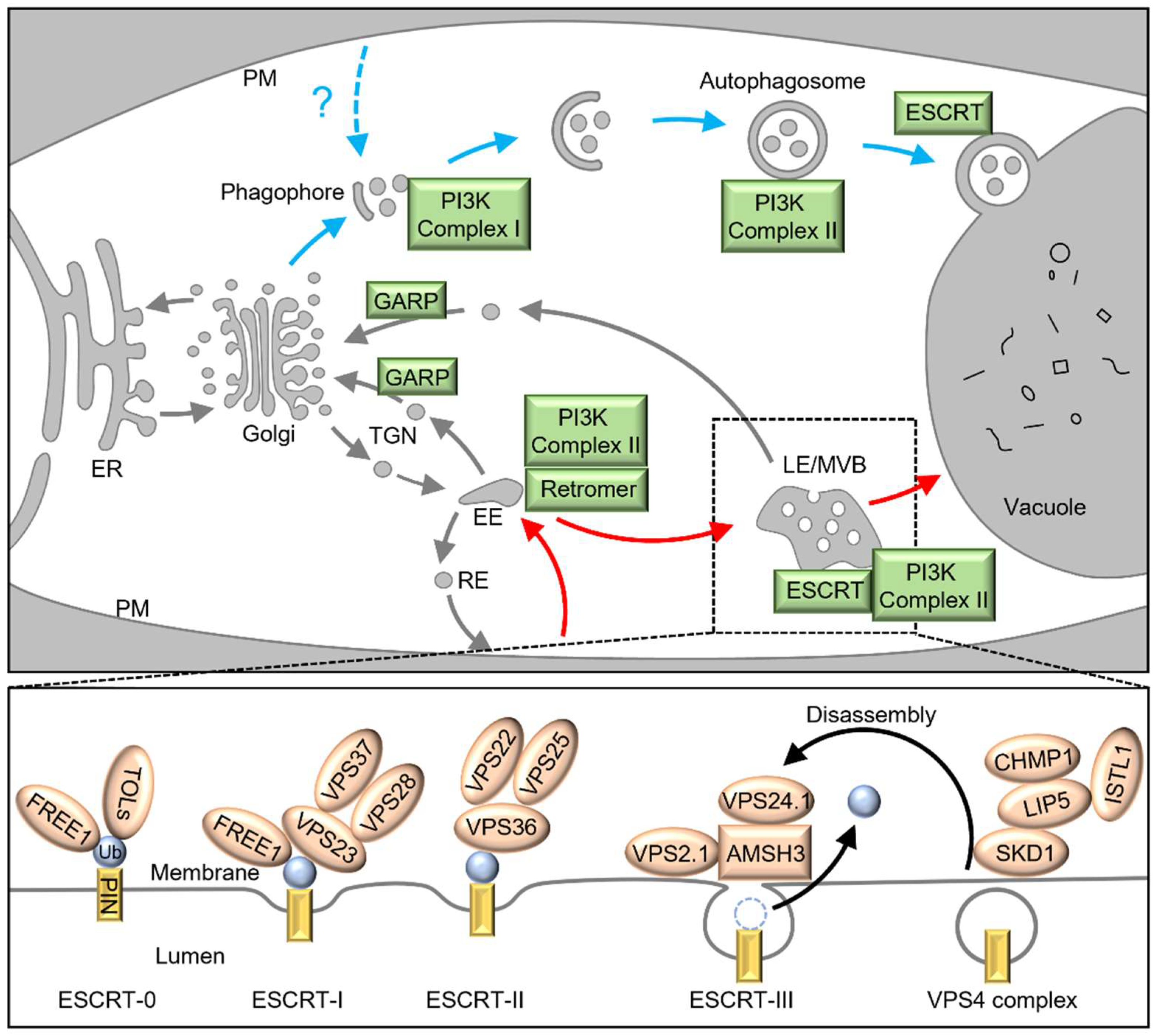
8. The ESCRT Pathway
9. Cytoskeletons
10. Plant Hormones
11. Environmental Stimuli
12. Other Regulators
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gälweiler, L.; Guan, C.; Müller, A.; Wisman, E.; Mendgen, K.; Yephremov, A.; Palme, K. Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science 1998, 282, 2226–2230. [Google Scholar] [CrossRef] [PubMed]
- Muller, A.; Guan, C.; Galweiler, L.; Tanzler, P.; Huijser, P.; Marchant, A.; Parry, G.; Bennett, M.; Wisman, E.; Palme, K. AtPIN2 defines a locus of Arabidopsis for root gravitropism control. EMBO J. 1998, 17, 6903–6911. [Google Scholar] [CrossRef] [PubMed]
- Petrasek, J.; Friml, J. Auxin transport routes in plant development. Development 2009, 136, 2675–2688. [Google Scholar] [CrossRef] [PubMed]
- Mravec, J.; Skupa, P.; Bailly, A.; Hoyerova, K.; Krecek, P.; Bielach, A.; Petrasek, J.; Zhang, J.; Gaykova, V.; Stierhof, Y.-D.; et al. Subcellular homeostasis of phytohormone auxin is mediated by the ER-localized PIN5 transporter. Nature 2009, 459, 1136–1140. [Google Scholar] [CrossRef] [PubMed]
- Dal Bosco, C.; Dovzhenko, A.; Liu, X.; Woerner, N.; Rensch, T.; Eismann, M.; Eimer, S.; Hegermann, J.; Paponov, I.A.; Ruperti, B.; et al. The endoplasmic reticulum localized PIN8 is a pollen-specific auxin carrier involved in intracellular auxin homeostasis. Plant J. 2012, 71, 860–870. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Wang, B.; Moreno, I.; Dupláková, N.; Simon, S.; Carraro, N.; Reemmer, J.; Pěnčík, A.; Chen, X.; Tejos, R.; et al. ER-localized auxin transporter PIN8 regulates auxin homeostasis and male gametophyte development in Arabidopsis. Nat. Commun. 2012, 3, 941. [Google Scholar] [CrossRef] [PubMed]
- Ditengou, F.A.; Gomes, D.; Nziengui, H.; Kochersperger, P.; Lasok, H.; Medeiros, V.; Paponov, I.A.; Nagy, S.K.; Nádai, T.V.; Mészáros, T.; et al. Characterization of auxin transporter PIN6 plasma membrane targeting reveals a function for PIN6 in plant bolting. New Phytol. 2018, 217, 1610–1624. [Google Scholar] [CrossRef] [PubMed]
- Simon, S.; Skupa, P.; Viaene, T.; Zwiewka, M.; Tejos, R.; Klima, P.; Carna, M.; Rolcik, J.; De Rycke, R.; Moreno, I.; et al. PIN6 auxin transporter at endoplasmic reticulum and plasma membrane mediates auxin homeostasis and organogenesis in Arabidopsis. New Phytol. 2016, 211, 65–74. [Google Scholar] [CrossRef]
- Rodriguez-Furlan, C.; Minina, E.A.; Hicks, G.R. Remove, recycle, degrade: Regulating plasma membrane protein accumulation. Plant Cell 2019, 31, 2833–2854. [Google Scholar] [CrossRef]
- Tanaka, H.; Kitakura, S.; Rakusová, H.; Uemura, T.; Feraru, M.I.; De Rycke, R.; Robert, S.; Kakimoto, T.; Friml, J. Cell polarity and patterning by PIN trafficking through early endosomal compartments in Arabidopsis thaliana. PLoS Genet. 2013, 9, e1003540. [Google Scholar] [CrossRef]
- Geldner, N.; Anders, N.; Wolters, H.; Keicher, J.; Kornberger, W.; Muller, P.; Delbarre, A.; Ueda, T.; Nakano, A.; Jürgens, G. The Arabidopsis GNOM ARF-GEF mediates endosomal recycling, auxin transport, and auxin-dependent plant growth. Cell 2003, 112, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Teh, O.-K.; Moore, I. An ARF-GEF acting at the Golgi and in selective endocytosis in polarized plant cells. Nature 2007, 448, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Kitakura, S.; Adamowski, M.; Matsuura, Y.; Santuari, L.; Kouno, H.; Arima, K.; Hardtke, C.S.; Friml, J.; Kakimoto, T.; Tanaka, H. BEN3/BIG2 ARF GEF is involved in brefeldin A-sensitive trafficking at the trans-Golgi network/early endosome in Arabidopsis thaliana. Plant Cell Physiol. 2017, 58, 1801–1811. [Google Scholar] [CrossRef] [PubMed]
- Armengot, L.; Marquès-Bueno, M.M.; Jaillais, Y. Regulation of polar auxin transport by protein and lipid kinases. J. Exp. Bot. 2016, 67, 4015–4037. [Google Scholar] [CrossRef]
- Adamowski, M.; Friml, J. PIN-dependent auxin transport: Action, regulation, and evolution. Plant Cell 2015, 27, 20–32. [Google Scholar] [CrossRef]
- Abas, L.; Benjamins, R.; Malenica, N.; Paciorek, T.; Wisniewska, J.; Moulinier-Anzola, J.C.; Sieberer, T.; Friml, J.; Luschnig, C. Intracellular trafficking and proteolysis of the Arabidopsis auxin-efflux facilitator PIN2 are involved in root gravitropism. Nat. Cell Biol. 2006, 8, 249–256. [Google Scholar] [CrossRef]
- Baster, P.; Robert, S.; Kleine-Vehn, J.; Vanneste, S.; Kania, U.; Grunewald, W.; De Rybel, B.; Beeckman, T.; Friml, J. SCFTIR1/AFB-auxin signalling regulates PIN vacuolar trafficking and auxin fluxes during root gravitropism. EMBO J. 2013, 32, 260–274. [Google Scholar] [CrossRef]
- Kleine-Vehn, J.; Leitner, J.; Zwiewka, M.; Sauer, M.; Abas, L.; Luschnig, C.; Friml, J. Differential degradation of PIN2 auxin efflux carrier by retromer-dependent vacuolar targeting. Proc. Natl. Acad. Sci. USA 2008, 105, 17812–17817. [Google Scholar] [CrossRef]
- Pali, T.; Dixon, N.; Kee, T.P.; Marsh, D. Incorporation of the V-ATPase inhibitors concanamycin and indole pentadiene in lipid membranes. Spin-label EPR studies. Biochim. Biophys. Acta 2004, 1663, 14–18. [Google Scholar] [CrossRef][Green Version]
- Matsuoka, K.; Bassham, D.C.; Raikhel, N.V.; Nakamura, K. Different sensitivity to wortmannin of two vacuolar sorting signals indicates the presence of distinct sorting machineries in tobacco cells. J. Cell Biol. 1995, 130, 1307–1318. [Google Scholar] [CrossRef]
- Paudyal, R.; Jamaluddin, A.; Warren, J.P.; Doyle, S.M.; Robert, S.; Warriner, S.L.; Baker, A. Trafficking modulator TENin1 inhibits endocytosis, causes endomembrane protein accumulation at the pre-vacuolar compartment and impairs gravitropic response in Arabidopsis thaliana. Biochem. J. 2014, 460 Pt 2, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Robert, H.S.; Friml, J. Auxin and other signals on the move in plants. Nat. Chem. Biol. 2009, 5, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Laxmi, A.; Pan, J.; Morsy, M.; Chen, R. Light plays an essential role in intracellular distribution of auxin efflux carrier PIN2 in Arabidopsis thaliana. PLoS ONE 2008, 3, e1510. [Google Scholar] [CrossRef]
- Nguyen, H.N.; Kim, J.H.; Jeong, C.Y.; Hong, S.W.; Lee, H. Inhibition of histone deacetylation alters Arabidopsis root growth in response to auxin via PIN1 degradation. Plant Cell Rep. 2013, 32, 1625–1636. [Google Scholar] [CrossRef]
- Yuan, X.; Xu, P.; Yu, Y.; Xiong, Y. Glucose-TOR signaling regulates PIN2 stability to orchestrate auxin gradient and cell expansion in Arabidopsis root. Proc. Natl. Acad. Sci. USA 2020, 117, 32223–32225. [Google Scholar] [CrossRef] [PubMed]
- Retzer, K.; Weckwerth, W. The TOR-Auxin connection upstream of root hair growth. Plants 2021, 10, 150. [Google Scholar] [CrossRef]
- Marshall, R.S.; Vierstra, R.D. Autophagy: The master of bulk and selective recycling. Annu. Rev. Plant Biol. 2018, 69, 173–208. [Google Scholar] [CrossRef]
- Huang, L.; Yu, L.J.; Zhang, X.; Fan, B.; Wang, F.Z.; Dai, Y.S.; Qi, H.; Zhou, Y.; Xie, L.J.; Xiao, S. Autophagy regulates glucose-mediated root meristem activity by modulating ROS production in Arabidopsis. Autophagy 2019, 15, 407–422. [Google Scholar] [CrossRef]
- Fu, L.; Wang, P.; Xiong, Y. Target of Rapamycin signaling in plant stress responses. Plant Physiol. 2020, 182, 1613–1623. [Google Scholar] [CrossRef]
- MacGurn, J.A.; Hsu, P.C.; Emr, S.D. Ubiquitin and membrane protein turnover: From cradle to grave. Annu. Rev. Biochem. 2012, 81, 231–259. [Google Scholar] [CrossRef]
- Leitner, J.; Petrášek, J.; Tomanov, K.; Retzer, K.; Pařezová, M.; Korbei, B.; Bachmair, A.; Zažímalová, E.; Luschnig, C. Lysine63-linked ubiquitylation of PIN2 auxin carrier protein governs hormonally controlled adaptation of Arabidopsis root growth. Proc. Natl. Acad. Sci. USA 2012, 109, 8322–8327. [Google Scholar] [CrossRef] [PubMed]
- Leitner, J.; Retzer, K.; Korbei, B.; Luschnig, C. Dynamics in PIN2 auxin carrier ubiquitylation in gravity-responding Arabidopsis roots. Plant Signal. Behav. 2012, 7, 1271–1273. [Google Scholar] [CrossRef] [PubMed]
- Löfke, C.; Scheuring, D.; Dünser, K.; Schöller, M.; Luschnig, C.; Kleine-Vehn, J. Tricho- and atrichoblast cell files show distinct PIN2 auxin efflux carrier exploitations and are jointly required for defined auxin-dependent root organ growth. J. Exp. Bot. 2015, 66, 5103–5112. [Google Scholar] [CrossRef] [PubMed]
- Isono, E.; Katsiarimpa, A.; Müller, I.K.; Anzenberger, F.; Stierhof, Y.-D.; Geldner, N.; Chory, J.; Schwechheimer, C. The deubiquitinating enzyme AMSH3 is required for intracellular trafficking and vacuole biogenesis in Arabidopsis thaliana. Plant Cell 2010, 22, 1826–1837. [Google Scholar] [CrossRef]
- Katsiarimpa, A.; Munoz, A.; Kalinowska, K.; Uemura, T.; Rojo, E.; Isono, E. The ESCRT-III-interacting deubiquitinating enzyme AMSH3 is essential for degradation of ubiquitinated membrane proteins in Arabidopsis thaliana. Plant Cell Physiol. 2014, 55, 727–736. [Google Scholar] [CrossRef]
- Katsiarimpa, A.; Kalinowska, K.; Anzenberger, F.; Weis, C.; Ostertag, M.; Tsutsumi, C.; Schwechheimer, C.; Brunner, F.; Huckelhoven, R.; Isono, E. The deubiquitinating enzyme AMSH1 and the ESCRT-III subunit VPS2.1 are required for autophagic degradation in Arabidopsis. Plant Cell 2013, 25, 2236–2252. [Google Scholar] [CrossRef]
- Kerscher, O.; Felberbaum, R.; Hochstrasser, M. Modification of proteins by ubiquitin and ubiquitin-like proteins. Annu. Rev. Cell. Dev. Biol. 2006, 22, 159–180. [Google Scholar] [CrossRef]
- Yin, X.J.; Volk, S.; Ljung, K.; Mehlmer, N.; Dolezal, K.; Ditengou, F.; Hanano, S.; Davis, S.J.; Schmelzer, E.; Sandberg, G.; et al. Ubiquitin lysine 63 chain forming ligases regulate apical dominance in Arabidopsis. Plant Cell 2007, 19, 1898–1911. [Google Scholar] [CrossRef]
- Sassi, M.; Lu, Y.; Zhang, Y.; Wang, J.; Dhonukshe, P.; Blilou, I.; Dai, M.; Li, J.; Gong, X.; Jaillais, Y.; et al. COP1 mediates the coordination of root and shoot growth by light through modulation of PIN1- and PIN2-dependent auxin transport in Arabidopsis. Development 2012, 139, 3402–3412. [Google Scholar] [CrossRef]
- Backer, J.M. The intricate regulation and complex functions of the Class III phosphoinositide 3-kinase Vps34. Biochem. J. 2016, 473, 2251–2271. [Google Scholar] [CrossRef]
- Nakatogawa, H. Mechanisms governing autophagosome biogenesis. Nat. Rev. Mol. Cell Biol. 2020, 21, 439–458. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Xia, F.-N.; Xiao, S. Autophagy in plants: Physiological roles and post-translational regulation. J. Integr. Plant Biol. 2020, 63, 161–179. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Vierstra, R.D. Autophagy: A multifaceted intracellular system for bulk and selective recycling. Trends Plant Sci. 2012, 17, 526–537. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, X.; Luo, S.; Fan, B.; Zhu, C.; Chen, Z. Coordination and crosstalk between autophagosome and multivesicular body pathways in plant stress responses. Cells 2020, 9, 119. [Google Scholar] [CrossRef]
- Liu, F.; Hu, W.; Vierstra, R.D. The Vacuolar Protein Sorting-38 subunit of the Arabidopsis phosphatidylinositol-3-kinase complex plays critical roles in autophagy, endosome sorting, and gravitropism. Front. Plant Sci. 2018, 9, 781. [Google Scholar] [CrossRef]
- Lee, H.N.; Zarza, X.; Kim, J.H.; Yoon, M.J.; Kim, S.H.; Lee, J.H.; Paris, N.; Munnik, T.; Otegui, M.S.; Chung, T. Vacuolar trafficking protein VPS38 is dispensable for autophagy. Plant Physiol. 2018, 176, 1559–1572. [Google Scholar] [CrossRef]
- Yuan, R.; Lan, J.; Fang, Y.; Yu, H.; Zhang, J.; Huang, J.; Qin, G. The Arabidopsis USL1 controls multiple aspects of development by affecting late endosome morphology. New Phytol. 2018, 219, 1388–1405. [Google Scholar] [CrossRef]
- Gomez, R.E.; Chambaud, C.; Lupette, J.; Castets, J.; Pascal, S.; Brocard, L.; Noack, L.; Jaillais, Y.; Joubès, J.; Bernard, A. Phosphatidylinositol-4-phosphate controls autophagosome formation in Arabidopsis thaliana. Nat. Commun. 2022, 13, 4385. [Google Scholar] [CrossRef]
- Starodubtseva, A.; Kalachova, T.; Retzer, K.; Jelinkova, A.; Dobrev, P.; Lacek, J.; Pospichalova, R.; Angelini, J.; Guivarc’h, A.; Pateyron, S.; et al. An Arabidopsis mutant deficient in phosphatidylinositol-4-phosphate kinases ß1 and ß2 displays altered auxin-related responses in roots. Sci. Rep. 2022, 12, 6947. [Google Scholar] [CrossRef]
- Bock, J.B.; Matern, H.T.; Peden, A.A.; Scheller, R.H. A genomic perspective on membrane compartment organization. Nature 2001, 409, 839–841. [Google Scholar] [CrossRef]
- Sanderfoot, A. Increases in the number of SNARE genes parallels the rise of multicellularity among the green plants. Plant Physiol. 2007, 144, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Shirakawa, M.; Ueda, H.; Shimada, T.; Nishiyama, C.; Hara-Nishimura, I. Vacuolar SNAREs function in the formation of the leaf vascular network by regulating auxin distribution. Plant Cell Physiol. 2009, 50, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Uemura, T.; Kim, H.; Saito, C.; Ebine, K.; Ueda, T.; Schulze-Lefert, P.; Nakano, A. Qa-SNAREs localized to the trans-Golgi network regulate multiple transport pathways and extracellular disease resistance in plants. Proc. Natl. Acad. Sci. USA 2012, 109, 1784–1789. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.J.; Kim, H.; Kang, H.; Jang, M.; Lee, D.W.; Lee, S.; Hwang, I. EpsinR2 interacts with clathrin, adaptor protein-3, AtVTI12, and phosphatidylinositol-3-phosphate. Implications for EpsinR2 function in protein trafficking in plant cells. Plant Physiol. 2007, 143, 1561–1575. [Google Scholar] [CrossRef]
- Feraru, E.; Paciorek, T.; Feraru, M.I.; Zwiewka, M.; De Groodt, R.; De Rycke, R.; Kleine-Vehn, J.; Friml, J. The AP-3 beta adaptin mediates the biogenesis and function of lytic vacuoles in Arabidopsis. Plant Cell 2010, 22, 2812–2824. [Google Scholar] [CrossRef]
- Zwiewka, M.; Feraru, E.; Moller, B.; Hwang, I.; Feraru, M.I.; Kleine-Vehn, J.; Weijers, D.; Friml, J. The AP-3 adaptor complex is required for vacuolar function in Arabidopsis. Cell Res. 2011, 21, 1711–1722. [Google Scholar] [CrossRef]
- Sancho-Andrés, G.; Soriano-Ortega, E.; Gao, C.; Bernabé-Orts, J.M.; Narasimhan, M.; Müller, A.O.; Tejos, R.; Jiang, L.; Friml, J.; Aniento, F.; et al. Sorting motifs involved in the trafficking and localization of the PIN1 auxin efflux carrier. Plant Physiol. 2016, 171, 1965–1982. [Google Scholar] [CrossRef]
- Liu, J.-J. Retromer-mediated protein sorting and vesicular trafficking. J. Genet. Genom. 2016, 43, 165–177. [Google Scholar] [CrossRef]
- Seaman, M.N. The retromer complex-endosomal protein recycling and beyond. J. Cell Sci. 2012, 125 Pt 20, 4693–4702. [Google Scholar] [CrossRef]
- Oliviusson, P.; Heinzerling, O.; Hillmer, S.; Hinz, G.; Tse, Y.C.; Jiang, L.; Robinson, D.G. Plant retromer, localized to the prevacuolar compartment and microvesicles in Arabidopsis, may interact with vacuolar sorting receptors. Plant Cell 2006, 18, 1239–1252. [Google Scholar] [CrossRef]
- Jaillais, Y.; Santambrogio, M.; Rozier, F.; Fobis-Loisy, I.; Miege, C.; Gaude, T. The retromer protein VPS29 links cell polarity and organ initiation in plants. Cell 2007, 130, 1057–1070. [Google Scholar] [CrossRef] [PubMed]
- Jaillais, Y.; Fobis-Loisy, I.; Miege, C.; Rollin, C.; Gaude, T. AtSNX1 defines an endosome for auxin-carrier trafficking in Arabidopsis. Nature 2006, 443, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Munnik, T.; Sato, M.H. Phosphatidylinositol 3-phosphate 5-kinase, FAB1/PIKfyve kinase mediates endosome maturation to establish endosome-cortical microtubule interaction in Arabidopsis. Plant Physiol. 2015, 169, 1961–1974. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Li, X.; Chen, Q.; He, X.; Yang, Q.; Zhang, A.; Yu, X.; Chen, H.; Liu, N.; Xie, Q.; et al. BLOS1, a putative BLOC-1 subunit, interacts with SNX1 and modulates root growth in Arabidopsis. J. Cell Sci. 2010, 123 Pt 21, 3727–3733. [Google Scholar] [CrossRef] [PubMed]
- Hanzawa, T.; Shibasaki, K.; Numata, T.; Kawamura, Y.; Gaude, T.; Rahman, A. Cellular auxin homeostasis under high temperature is regulated through a sorting NEXIN1-dependent endosomal trafficking pathway. Plant Cell 2013, 25, 3424–3433. [Google Scholar] [CrossRef]
- Chu, Y.J.; Chen, X.; Xue, H.W. Ins(1, 4, 5) P3 suppresses protein degradation in plant vacuoles by regulating SNX-mediated protein sorting. Mol. Plant 2016, 9, 1440–1443. [Google Scholar] [CrossRef]
- Bonifacino, J.S.; Rojas, R. Retrograde transport from endosomes to the trans-Golgi network. Nat. Rev. Mol. Cell Biol. 2006, 7, 568–579. [Google Scholar] [CrossRef]
- Pahari, S.; Cormark, R.D.; Blackshaw, M.T.; Liu, C.; Erickson, J.L.; Schultz, E.A. Arabidopsis UNHINGED encodes a VPS51 homolog and reveals a role for the GARP complex in leaf shape and vein patterning. Development 2014, 141, 1894–1905. [Google Scholar] [CrossRef]
- Henne, W.M.; Buchkovich, N.J.; Emr, S.D. The ESCRT pathway. Dev. Cell 2011, 21, 77–91. [Google Scholar] [CrossRef]
- Gao, C.; Zhuang, X.; Shen, J.; Jiang, L. Plant ESCRT complexes: Moving beyond endosomal sorting. Trends Plant Sci. 2017, 22, 986–998. [Google Scholar] [CrossRef]
- Korbei, B.; Moulinier-Anzola, J.; De-Araujo, L.; Lucyshyn, D.; Retzer, K.; Khan, M.A.; Luschnig, C. Arabidopsis TOL proteins act as gatekeepers for vacuolar sorting of PIN2 plasma membrane protein. Curr. Biol. 2013, 23, 2500–2505. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Luo, M.; Zhao, Q.; Yang, R.; Cui, Y.; Zeng, Y.; Xia, J.; Jiang, L. A unique plant ESCRT component, FREE1, regulates multivesicular body protein sorting and plant growth. Curr. Biol. 2014, 24, 2556–2563. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Zhuang, X.; Cui, Y.; Fu, X.; He, Y.; Zhao, Q.; Zeng, Y.; Shen, J.; Luo, M.; Jiang, L. Dual roles of an Arabidopsis ESCRT component FREE1 in regulating vacuolar protein transport and autophagic degradation. Proc. Natl. Acad. Sci. USA 2015, 112, 1886–1891. [Google Scholar] [CrossRef]
- Spitzer, C.; Schellmann, S.; Sabovljevic, A.; Shahriari, M.; Keshavaiah, C.; Bechtold, N.; Herzog, M.; Muller, S.; Hanisch, F.G.; Hulskamp, M. The Arabidopsis elch mutant reveals functions of an ESCRT component in cytokinesis. Development 2006, 133, 4679–4689. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, Y.; Cheng, Y. The ESCRT-I components VPS28A and VPS28B are essential for auxin-mediated plant development. Plant J. 2020, 104, 1617–1634. [Google Scholar] [CrossRef]
- Wang, H.J.; Hsu, Y.W.; Guo, C.L.; Jane, W.N.; Wang, H.; Jiang, L.; Jauh, G.Y. VPS36-dependent multivesicular bodies are critical for plasmamembrane protein turnover and vacuolar biogenesis. Plant Physiol. 2017, 173, 566–581. [Google Scholar] [CrossRef]
- Spitzer, C.; Reyes, F.C.; Buono, R.; Sliwinski, M.K.; Haas, T.J.; Otegui, M.S. The ESCRT-related CHMP1A and B proteins mediate multivesicular body sorting of auxin carriers in Arabidopsis and are required for plant development. Plant Cell 2009, 21, 749–766. [Google Scholar] [CrossRef]
- Buono, R.A.; Paez-Valencia, J.; Miller, N.D.; Goodman, K.; Spitzer, C.; Spalding, E.P.; Otegui, M.S. Role of SKD1 regulators LIP5 and IST1-LIKE1 in endosomal sorting and plant development. Plant Physiol. 2016, 171, 251–264. [Google Scholar] [CrossRef]
- Dhonukshe, P.; Grigoriev, I.; Fischer, R.; Tominaga, M.; Robinson, D.G.; Hašek, J.; Paciorek, T.; Petrášek, J.; Seifertová, D.; Tejos, R.; et al. Auxin transport inhibitors impair vesicle motility and actin cytoskeleton dynamics in diverse eukaryotes. Proc. Natl. Acad. Sci. USA 2008, 105, 4489–4494. [Google Scholar] [CrossRef]
- Zhu, J.; Bailly, A.; Zwiewka, M.; Sovero, V.; Di Donato, M.; Ge, P.; Oehri, J.; Aryal, B.; Hao, P.; Linnert, M.; et al. TWISTED DWARF1 mediates the action of auxin transport inhibitors on actin cytoskeleton dynamics. Plant Cell 2016, 28, 930–948. [Google Scholar] [CrossRef]
- Ambrose, C.; Ruan, Y.; Gardiner, J.; Tamblyn, L.M.; Catching, A.; Kirik, V.; Marc, J.; Overall, R.; Wasteneys, G.O. CLASP interacts with sorting nexin 1 to link microtubules and auxin transport via PIN2 recycling in Arabidopsis thaliana. Dev. Cell 2013, 24, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Sieberer, T.; Seifert, G.J.; Hauser, M.-T.; Grisafi, P.; Fink, G.R.; Luschnig, C. Post-transcriptional control of the Arabidopsis auxin efflux carrier EIR1 requires AXR1. Curr. Biol. 2000, 10, 1595–1598. [Google Scholar] [CrossRef] [PubMed]
- Paciorek, T.; Zazimalova, E.; Ruthardt, N.; Petrasek, J.; Stierhof, Y.-D.; Kleine-Vehn, J.; Morris, D.A.; Emans, N.; Jurgens, G.; Geldner, N.; et al. Auxin inhibits endocytosis and promotes its own efflux from cells. Nature 2005, 435, 1251–1256. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Fujioka, S.; Peng, J.; Chen, J.; Li, G.; Chen, R. The E3 ubiquitin ligase SCFTIR1/AFB and membrane sterols play key roles in auxin regulation of endocytosis, recycling, and plasma membrane accumulation of the auxin efflux transporter PIN2 in Arabidopsis thaliana. Plant Cell 2009, 21, 568–580. [Google Scholar] [CrossRef] [PubMed]
- Jásik, J.; Bokor, B.; Stuchlík, S.; Mičieta, K.; Turňa, J.; Schmelzer, E. Effects of auxins on PIN-FORMED2 (PIN2) dynamics are not mediated by inhibiting PIN2 endocytosis. Plant Physiol. 2016, 172, 1019–1031. [Google Scholar] [CrossRef] [PubMed]
- Willige, B.C.; Isono, E.; Richter, R.; Zourelidou, M.; Schwechheimer, C. Gibberellin regulates PIN-FORMED abundance and is required for auxin transport–dependent growth and development in Arabidopsis thaliana. Plant Cell 2011, 23, 2184–2195. [Google Scholar] [CrossRef] [PubMed]
- Lofke, C.; Zwiewka, M.; Heilmann, I.; Van Montagu, M.C.; Teichmann, T.; Friml, J. Asymmetric gibberellin signaling regulates vacuolar trafficking of PIN auxin transporters during root gravitropism. Proc. Natl. Acad. Sci. USA 2013, 110, 3627–3632. [Google Scholar] [CrossRef]
- Salanenka, Y.; Verstraeten, I.; Lofke, C.; Tabata, K.; Naramoto, S.; Glanc, M.; Friml, J. Gibberellin DELLA signaling targets the retromer complex to redirect protein trafficking to the plasma membrane. Proc. Natl. Acad. Sci. USA 2018, 115, 3716–3721. [Google Scholar] [CrossRef]
- Cucinotta, M.; Manrique, S.; Guazzotti, A.; Quadrelli, N.E.; Mendes, M.A.; Benkova, E.; Colombo, L. Cytokinin response factors integrate auxin and cytokinin pathways for female reproductive organ development. Development 2016, 143, 4419–4424. [Google Scholar] [CrossRef]
- Marhavý, P.; Bielach, A.; Abas, L.; Abuzeineh, A.; Duclercq, J.; Tanaka, H.; Pařezová, M.; Petrášek, J.; Friml, J.; Kleine-Vehn, J.; et al. Cytokinin modulates endocytic trafficking of PIN1 auxin efflux carrier to control plant organogenesis. Dev. Cell 2011, 21, 796–804. [Google Scholar] [CrossRef]
- Chen, P.; Ge, Y.; Chen, L.; Yan, F.; Cai, L.; Zhao, H.; Lei, D.; Jiang, J.; Wang, M.; Tao, Y. SAV4 is required for ethylene-induced root hair growth through stabilizing PIN2 auxin transporter in Arabidopsis. New Phytol. 2022, 234, 1735–1752. [Google Scholar] [CrossRef]
- Ge, Y.; Yan, F.; Zourelidou, M.; Wang, M.; Ljung, K.; Fastner, A.; Hammes, U.Z.; Di Donato, M.; Geisler, M.; Schwechheimer, C.; et al. SHADE AVOIDANCE 4 is required for proper auxin distribution in the hypocotyl. Plant Physiol. 2017, 173, 788–800. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Deng, M.; Liu, H.; Li, Y.; Chen, Y.; Jia, M.; Xue, H.; Shao, J.; Zhao, J.; Qi, Y.; et al. ABNORMAL SHOOT 6 interacts with KATANIN 1 and SHADE AVOIDANCE 4 to promote cortical microtubule severing and ordering in Arabidopsis. J. Integr. Plant Biol. 2021, 63, 646–661. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Chen, Q.; Qi, L.; Jiang, H.; Li, S.; Xu, Y.; Liu, F.; Zhou, W.; Pan, J.; Li, X.; et al. Jasmonate modulates endocytosis and plasma membrane accumulation of the Arabidopsis PIN2 protein. New Phytol. 2011, 191, 360–375. [Google Scholar] [CrossRef] [PubMed]
- Jásik, J.; Boggetti, B.; Baluška, F.; Volkmann, D.; Gensch, T.; Rutten, T.; Altmann, T.; Schmelzer, E. PIN2 turnover in Arabidopsis root epidermal cells explored by the photoconvertible protein Dendra2. PLoS ONE 2013, 8, e61403. [Google Scholar] [CrossRef]
- Wu, Y.; Chang, Y.; Luo, L.; Tian, W.; Gong, Q.; Liu, X. Abscisic acid employs NRP-dependent PIN2 vacuolar degradation to suppress auxin-mediated primary root elongation in Arabidopsis. New Phytol. 2022, 233, 297–312. [Google Scholar] [CrossRef]
- Kiss, J.Z. Mechanisms of the early phases of plant gravitropism. Crit. Rev. Plant Sci. 2000, 19, 551–573. [Google Scholar] [CrossRef]
- Vandenbrink, J.P.; Kiss, J.Z. Plant responses to gravity. Semin. Cell Dev. Biol. 2019, 92, 122–125. [Google Scholar]
- Li, Y.; Li, Y.; Liu, Y.; Wu, Y.; Xie, Q. The sHSP22 heat shock protein requires the ABI1 protein phosphatase to modulate polar auxin transport and downstream responses. Plant Physiol. 2018, 176, 2406–2425. [Google Scholar] [CrossRef]
- Lin, D.L.; Yao, H.Y.; Jia, L.H.; Tan, J.F.; Xu, Z.H.; Zheng, W.M.; Xue, H.W. Phospholipase D-derived phosphatidic acid promotes root hair development under phosphorus deficiency by suppressing vacuolar degradation of PIN-FORMED2. New Phytol. 2020, 226, 142–155. [Google Scholar] [CrossRef]
- Malenica, N.; Abas, L.; Benjamins, R.; Kitakura, S.; Sigmund, H.F.; Jun, K.S.; Hauser, M.-T.; Friml, J.; Luschnig, C. MODULATOR OF PIN genes control steady-state levels of Arabidopsis PIN proteins. Plant J. 2007, 51, 537–550. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, M.; Poitout, A.; Otegui, M.S. Arabidopsis vascular complexity and connectivity controls PIN-FORMED1 dynamics and lateral vein patterning during embryogenesis. Development 2021, 148, dev197210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, C.; Lin, Q.; Liu, A.; Wang, T.; Feng, X.; Liu, J.; Han, H.; Ma, Y.; Bonea, D.; et al. A tetratricopeptide repeat domain-containing protein SSR1 located in mitochondria is involved in root development and auxin polar transport in Arabidopsis. Plant J. 2015, 83, 582–599. [Google Scholar] [CrossRef] [PubMed]
- Ohbayashi, I.; Huang, S.; Fukaki, H.; Song, X.; Sun, S.; Morita, M.T.; Tasaka, M.; Millar, A.H.; Furutani, M. Mitochondrial pyruvate dehydrogenase contributes to auxin-regulated organ development. Plant Physiol. 2019, 180, 896–909. [Google Scholar] [CrossRef]
- Huang, J.B.; Liu, H.; Chen, M.; Li, X.; Wang, M.; Yang, Y.; Wang, C.; Huang, J.; Liu, G.; Liu, Y.; et al. ROP3 GTPase contributes to polar auxin transport and auxin responses and is important for embryogenesis and seedling growth in Arabidopsis. Plant Cell 2014, 26, 3501–3518. [Google Scholar] [CrossRef]
- Nagawa, S.; Xu, T.; Lin, D.; Dhonukshe, P.; Zhang, X.; Friml, J.; Scheres, B.; Fu, Y.; Yang, Z. ROP GTPase-dependent actin microfilaments promote PIN1 polarization by localized inhibition of clathrin-dependent endocytosis. PLoS Biol. 2012, 10, e1001299. [Google Scholar] [CrossRef]
- Chen, X.; Naramoto, S.; Robert, S.; Tejos, R.; Löfke, C.; Lin, D.; Yang, Z.; Friml, J. ABP1 and ROP6 GTPase signaling regulate clathrin-mediated endocytosis in Arabidopsis roots. Curr. Biol. 2012, 22, 1326–1332. [Google Scholar] [CrossRef]
- Lin, D.; Nagawa, S.; Chen, J.; Cao, L.; Chen, X.; Xu, T.; Li, H.; Dhonukshe, P.; Yamamuro, C.; Friml, J.; et al. A ROP GTPase-dependent auxin signaling pathway regulates the subcellular distribution of PIN2 in Arabidopsis roots. Curr. Biol. 2012, 22, 1319–1325. [Google Scholar] [CrossRef]
- Platre, M.P.; Bayle, V.; Armengot, L.; Bareille, J.; Marquès-Bueno, M.D.M.; Creff, A.; Maneta-Peyret, L.; Fiche, J.B.; Nollmann, M.; Miège, C.; et al. Developmental control of plant Rho GTPase nano-organization by the lipid phosphatidylserine. Science 2019, 364, 57–62. [Google Scholar] [CrossRef]
- Marques-Bueno, M.M.; Armengot, L.; Noack, L.C.; Bareille, J.; Rodriguez, L.; Platre, M.P.; Bayle, V.; Liu, M.; Opdenacker, D.; Vanneste, S.; et al. Auxin-regulated reversible inhibition of TMK1 signaling by MAKR2 modulates the dynamics of root gravitropism. Curr. Biol. 2021, 31, 228–237.e10. [Google Scholar] [CrossRef]
- Xi, J.; Zeng, J.; Fu, X.; Zhang, L.; Li, G.; Li, B.; Yan, X.; Chu, Q.; Xiao, Y.; Pei, Y.; et al. GhROP6 GTPase promotes cell-specific GhPIN3a degradation in cotton fibers by regulating GhPIN3a localization. J. Exp. Bot. 2022, 74, 265–282. [Google Scholar]
| Gene | Complex | Process | Reference |
|---|---|---|---|
| TOLs | ESCRT-0 | Recognition of ubiquitinated PIN2, PIN2 vacuolar targeting, root gravitropism, development of cotyledons, inflorescences and meristems | Korbei et al., 2013 [71] |
| FREE1 | ESCRT-0 | Recognition of ubiquitinated PIN2, PIN2 targeting to the tonoplast | Gao et al., 2014 [72]; Gao et al., 2015 [73] |
| VPS28A, VPS28B | ESCRT-Ⅰ | PIN1 polarity, vacuole formation, embryogenesis | Liu et al., 2020 [75] |
| VPS23/ELC | ESCRT-Ⅰ | Interaction with FREE1, interaction with ubiquitin, interaction with VPS28 and VPS37, cytokinesis, embryogenesis | Gao et al., 2014 [72]; Spitzer et al., 2006 [74] |
| VPS37 | ESCRT-Ⅰ | interaction with VPS28 and VPS23 | Spitzer et al., 2006 [74] |
| VPS36 | ESCRT-Ⅱ | Polarity of PIN1/2, interaction with ubiquitin, VPS22 and VPS25, embryogenesis | Wang et al., 2017 [76] |
| VPS22 | ESCRT-Ⅱ | Interaction with VPS25 and VPS36 | Wang et al., 2017 [76] |
| VPS25 | ESCRT-Ⅱ | Interaction with VPS22 and VPS36 | Wang et al., 2017 [76] |
| VPS2.1 | ESCRT-III | Interaction with AMSH3, intracellular trafficking, PIN vacuolar targeting | Isono et al., 2010 [34]; Katsiarimpa et al., 2014 [35] |
| VPS24.1 | ESCRT-III | Interaction with AMSH3, intracellular trafficking, PIN vacuolar targeting | Isono et al., 2010 [34]; Katsiarimpa et al., 2014 [35] |
| CHMP1A | ESCRT-III | MVB sorting of PIN1,2, embryogenesis | Spitzer et al., 2009 [77]; Buono et al., 2016 [78] |
| CHMP1B | ESCRT-III | MVB sorting of PIN1,2, embryogenesis | Spitzer et al., 2009 [77] |
| ISTL1 | ESCRT-III | Interaction with LIP5 to inhibit SKD1 activity | Buono et al., 2016 [78] |
| LIP5 | VPS4 complex | Interaction with CHMP1A and ISTL1, inhibition of SKD1 activity, cargo (PIN2,3) sequestration into ILVs, root gravitropism | Spitzer et al., 2009 [77]; Buono et al., 2016 [78] |
| SKD1 | VPS4 complex | interaction with CHMP1A, recycling of the ESCRT-III | Spitzer et al., 2009 [77]; Buono et al., 2016 [78] |
| VPS29 | Retromer | Vesicular trafficking from endosomes to the TGN and to PM, MVB morphology, PIN2 vacuolar sorting and stability, root responses to light, gravity and high temperature | Jaillais et al., 2007 [61]; Kleine-Vehn et al., 2008 [18]; Yuan et al., 2018 [47]. |
| SNX1 | Retromer | Jaillais et al., 2007 [61]; Kleine-Vehn et al., 2008 [18]; Hanzawa et al., 2013 [65]; | |
| VPS35 | Retromer | Seaman, 2012 [59]. | |
| VPS26 | Retromer | Seaman, 2012 [59]. | |
| UNH | GRAP | PIN1 targeting to the vacuole | Pahari et al., 2014 [68]. |
| VAM3/SYP22 | SNARE | PIN1 polarity, PIN1 vacuolar trafficking, leaf development | Shirakawa et al., 2009 [52] |
| SYP42, SYP43 | SNARE | PIN2 vacuolar transport | Sanderfoot, 2007 [51]; Uemura et al., 2012 [53] |
| VTI12 | SNARE | PIN vacuolar transport mediated by EpsinR2 and AP3 | Lee et al., 2007 [54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Guo, Y.; Zhang, Y.; Li, Y.; Pei, Y.; Zhang, M. Regulation of PIN-FORMED Protein Degradation. Int. J. Mol. Sci. 2023, 24, 843. https://doi.org/10.3390/ijms24010843
Zhang L, Guo Y, Zhang Y, Li Y, Pei Y, Zhang M. Regulation of PIN-FORMED Protein Degradation. International Journal of Molecular Sciences. 2023; 24(1):843. https://doi.org/10.3390/ijms24010843
Chicago/Turabian StyleZhang, Liuqin, Yifan Guo, Yujie Zhang, Yuxin Li, Yan Pei, and Mi Zhang. 2023. "Regulation of PIN-FORMED Protein Degradation" International Journal of Molecular Sciences 24, no. 1: 843. https://doi.org/10.3390/ijms24010843
APA StyleZhang, L., Guo, Y., Zhang, Y., Li, Y., Pei, Y., & Zhang, M. (2023). Regulation of PIN-FORMED Protein Degradation. International Journal of Molecular Sciences, 24(1), 843. https://doi.org/10.3390/ijms24010843






