Silver Nanoparticles Modified by Carbosilane Dendrons and PEG as Delivery Vectors of Small Interfering RNA
Abstract
1. Introduction
2. Results
2.1. AgNP-siRNA Complexation
2.1.1. Changes in the Secondary Structure of RNA in the Presence of AgNP
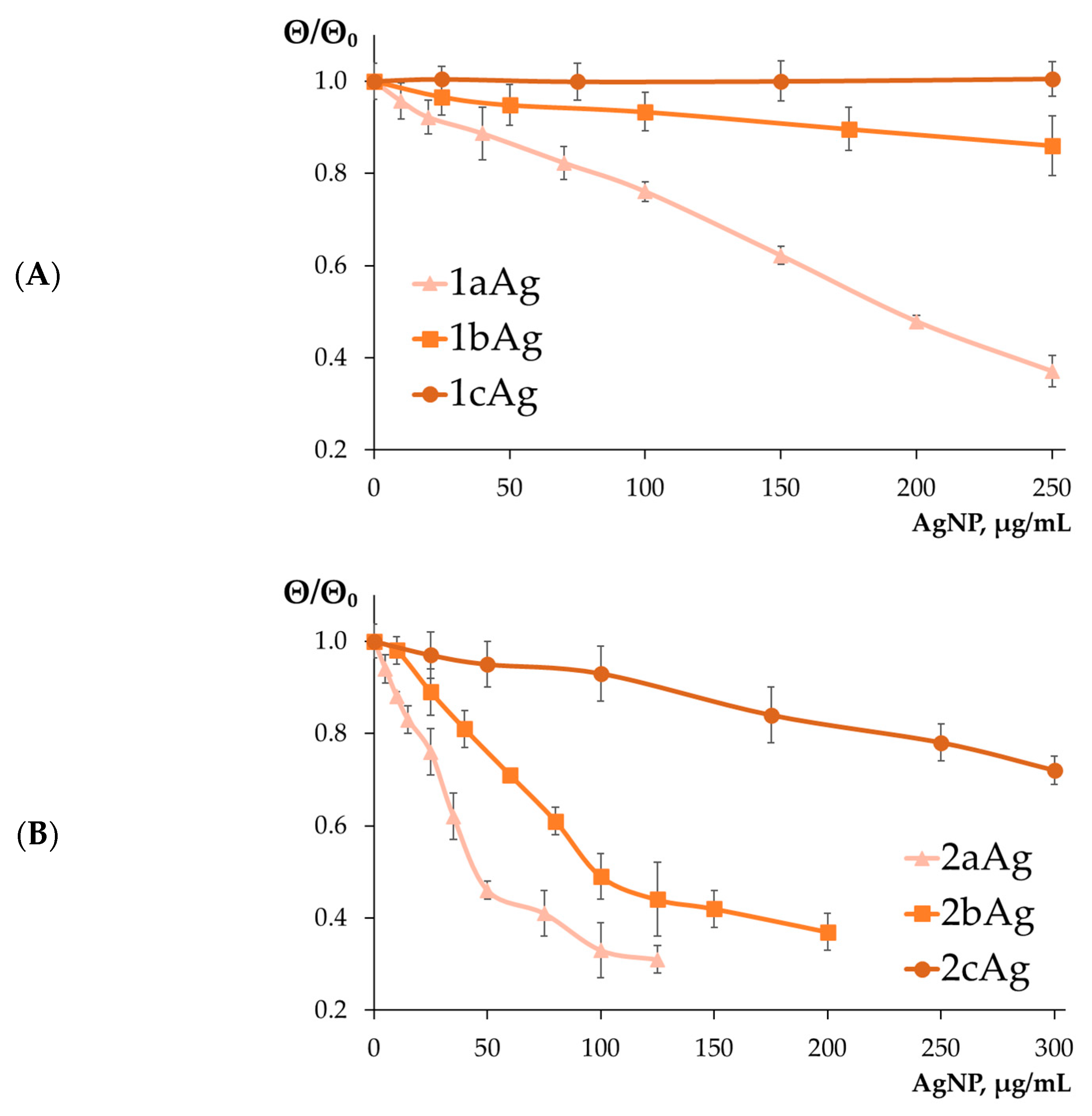
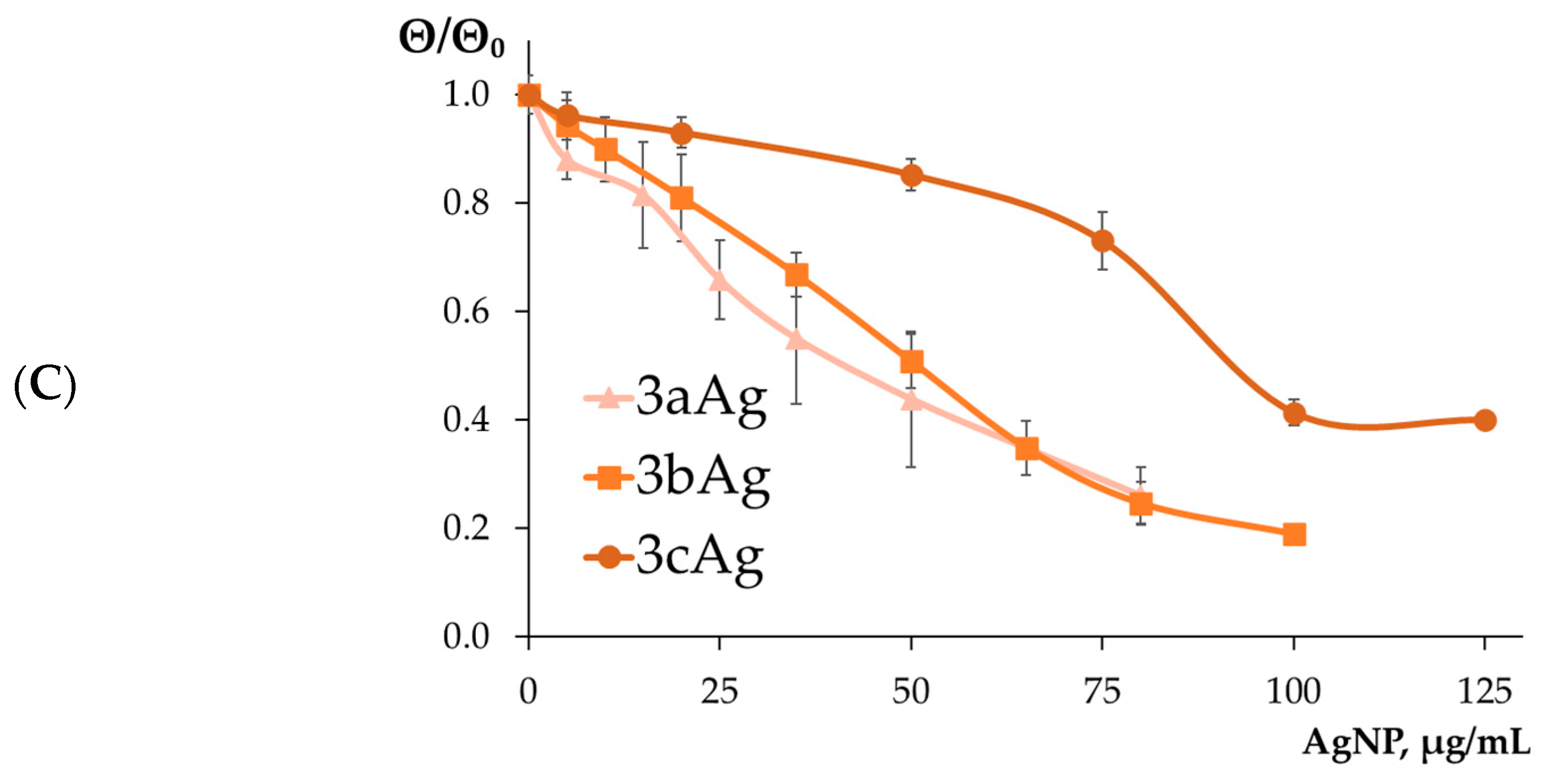
2.1.2. Determination of the Formation of AgNP-siRNA Complexes
2.1.3. Estimation of the Surface Charge of AgNP-siRNA Complexes
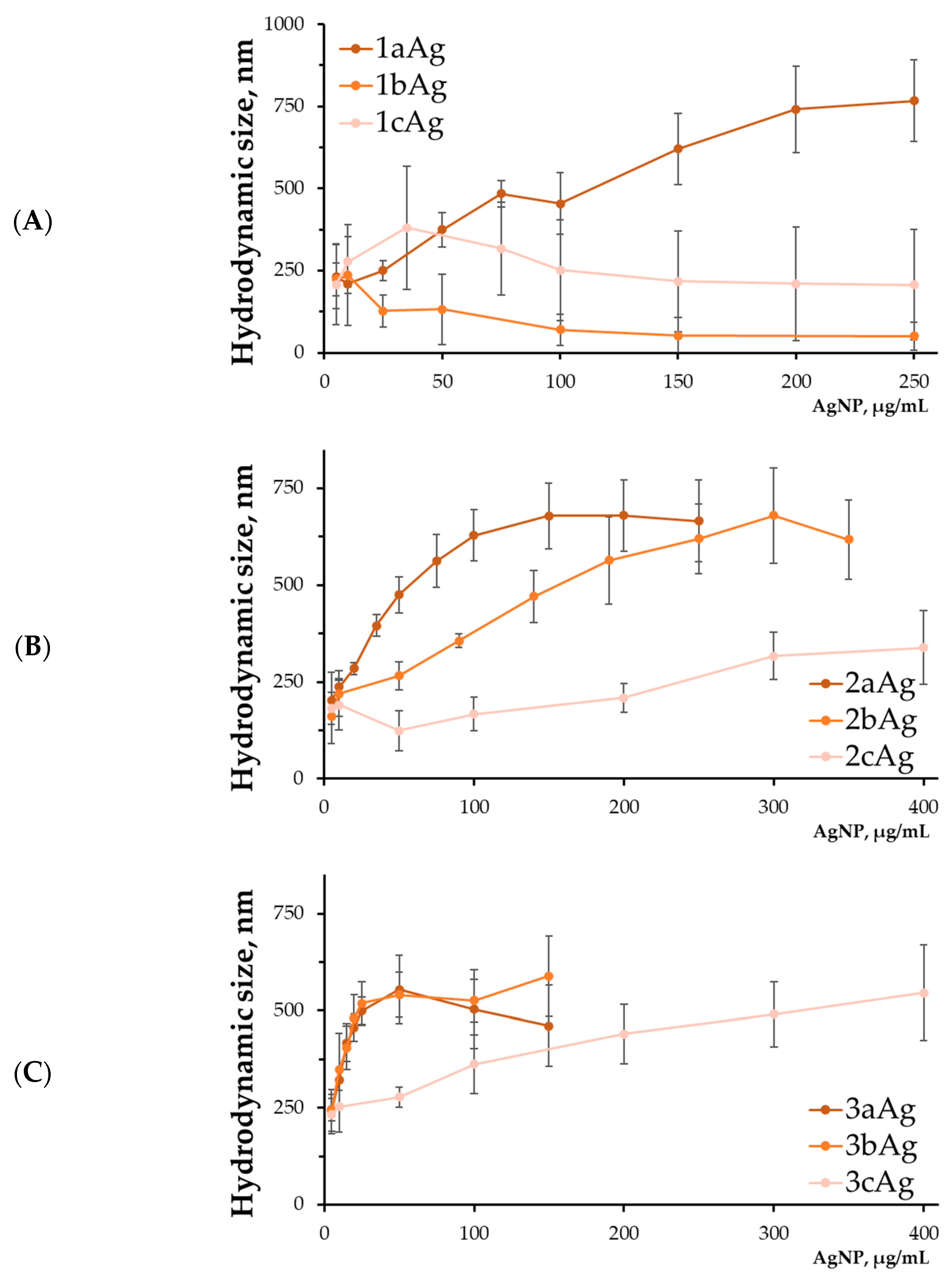
2.1.4. Hydrodynamic Size of AgNP-siRNA Complexes
2.2. Toxicity of Silver Nanoparticles for Blood Cells
2.2.1. Hemotoxicity
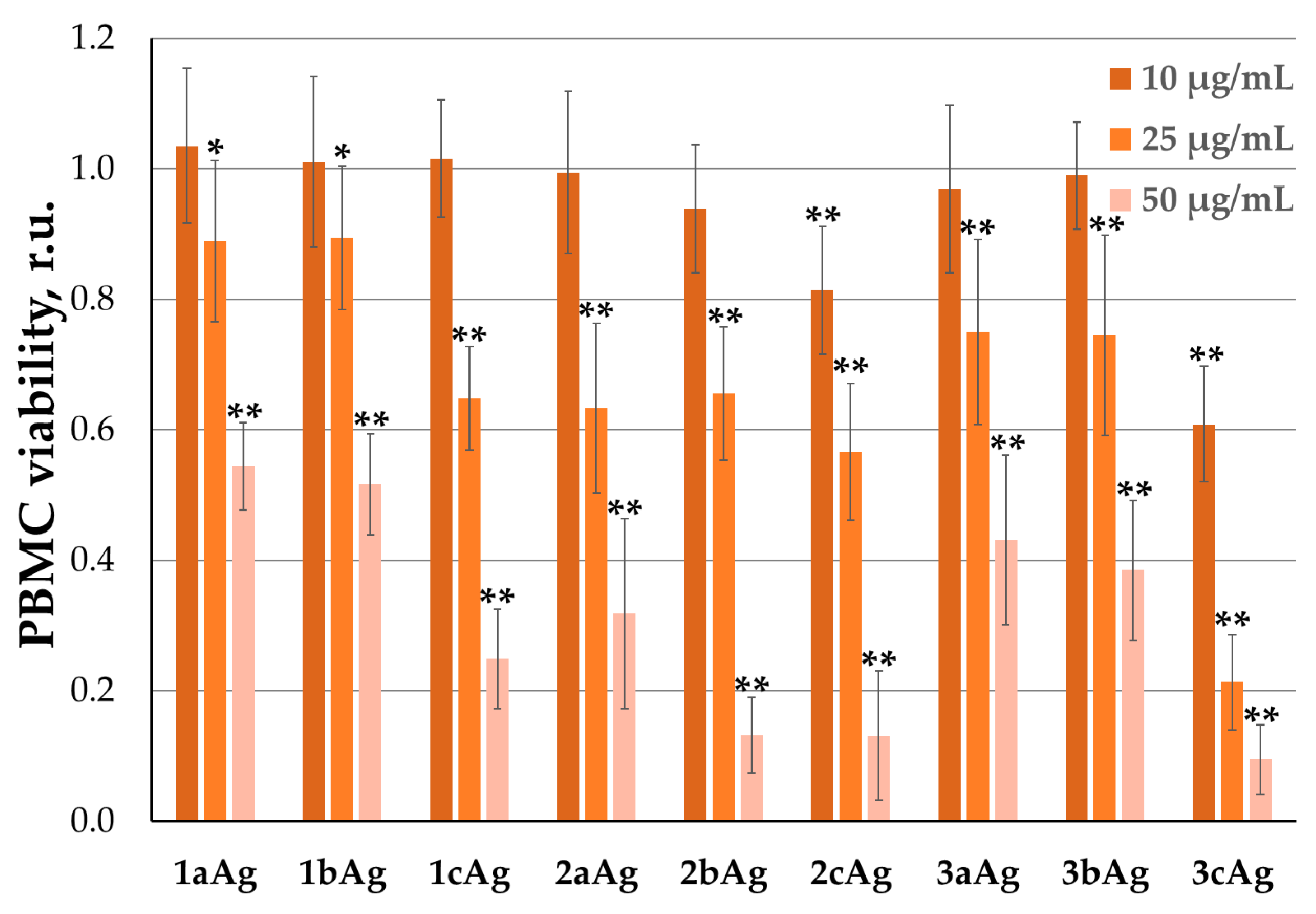
2.2.2. PBMC Inhibition
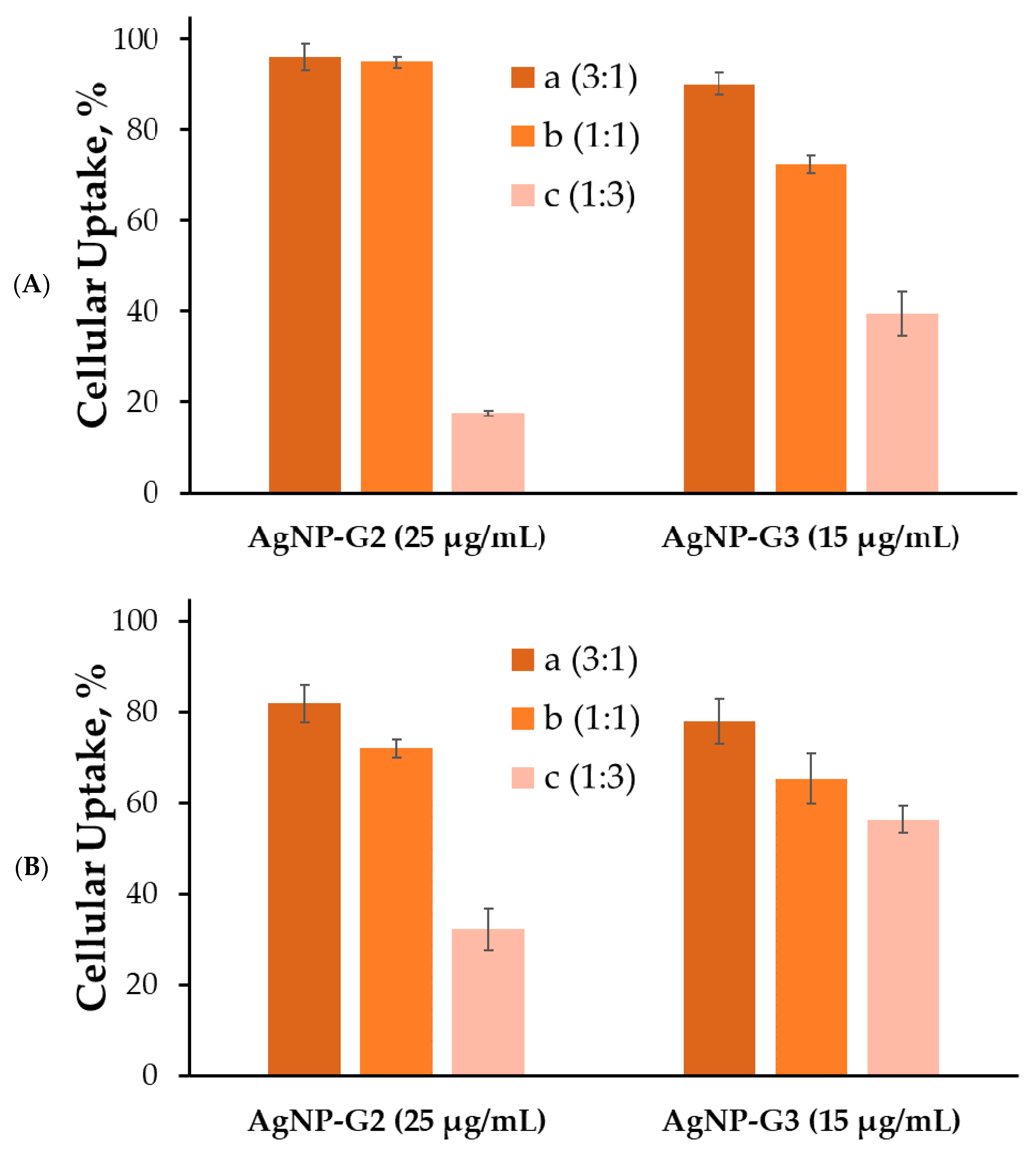
2.3. Cellular Uptake of Nanoparticle Complex
2.4. Cytotoxicity Effects of AgNPs and Their Complexes with siMCL-1
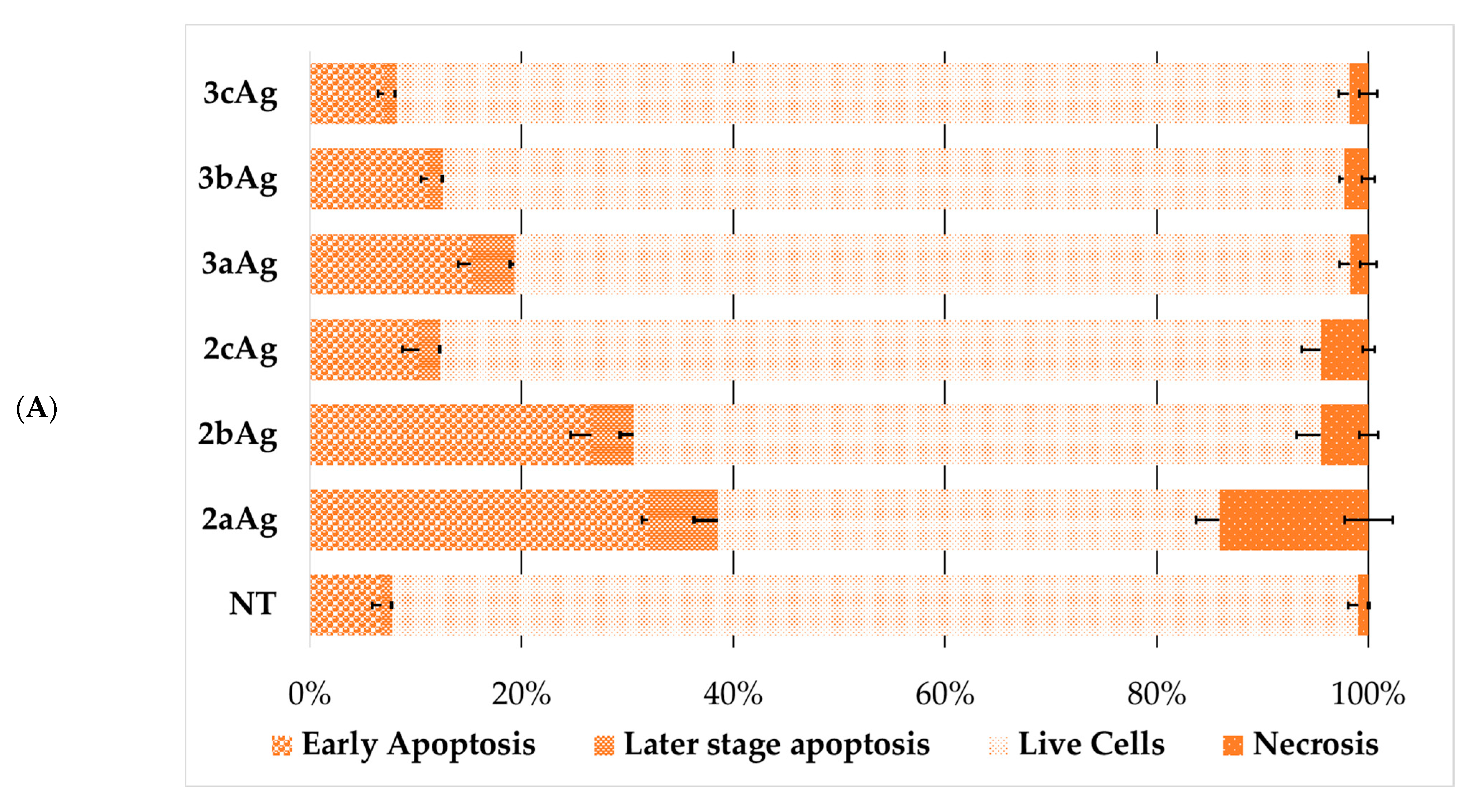
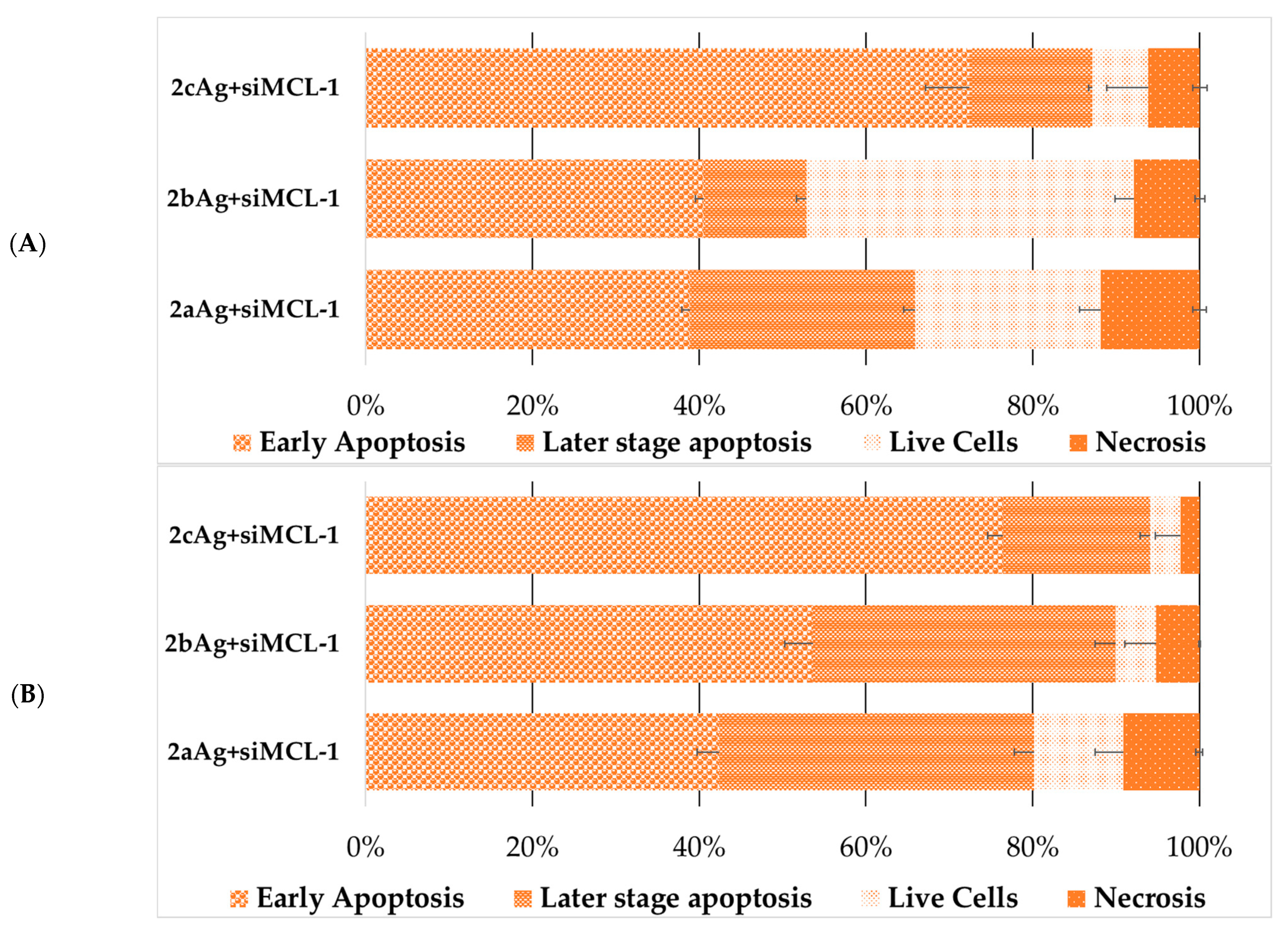
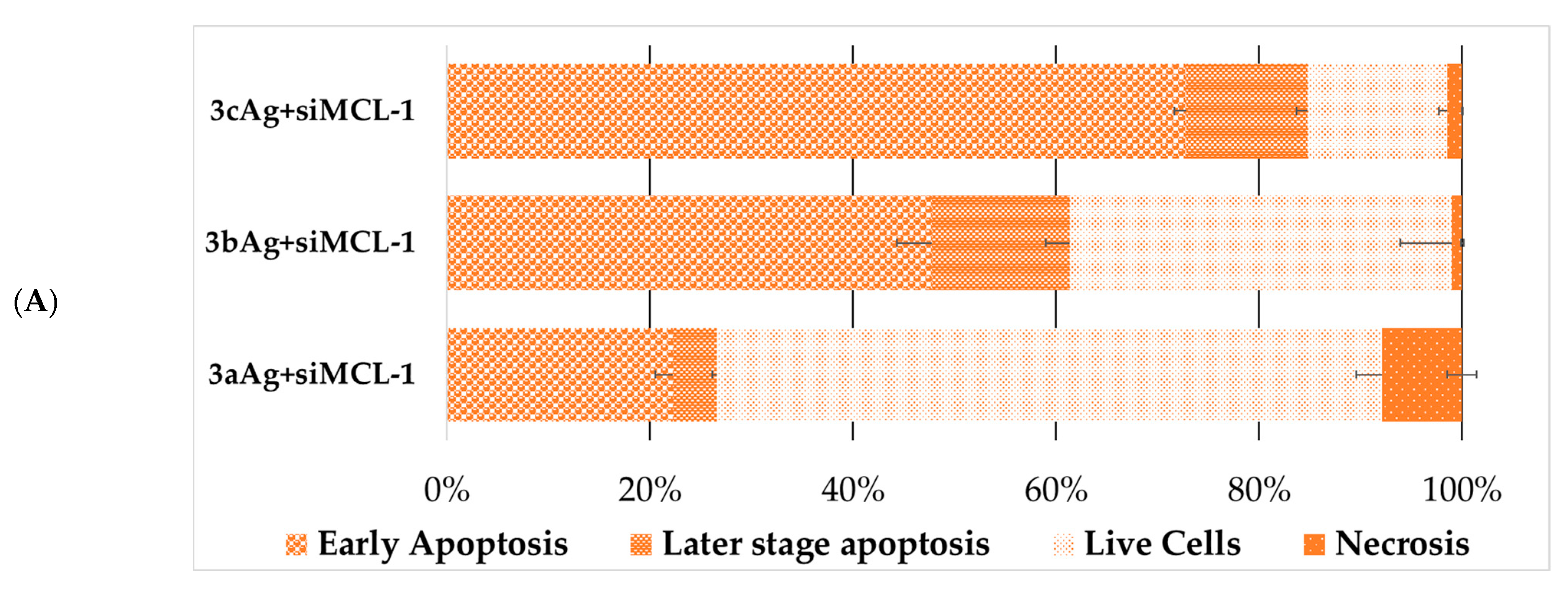
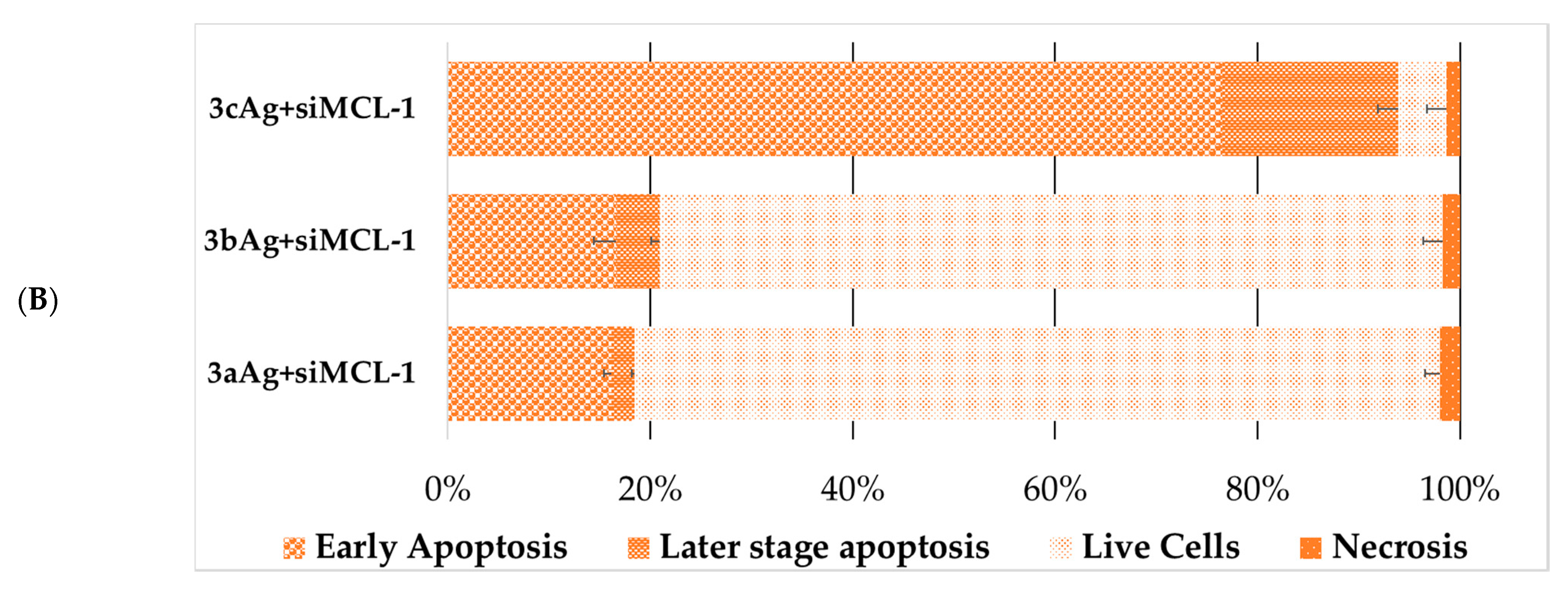
2.5. Assessment of Cell Death Due to Exposure to AgNP-siRNA Complexes
3. Discussion
4. Materials and Methods
4.1. Small Interfering RNA
4.2. Silver Nanoparticles
4.3. Analysis of Complexes
4.3.1. Gel-Electrophoresis
4.3.2. Circular Dichroism
4.3.3. Zeta-Potential and Dynamic Light Scattering
4.4. Evaluation of Toxicity of Nanoparticles for Blood Cells
4.4.1. Hemolytic Activity of Silver Nanoparticles
4.4.2. PBMC Inhibition
4.5. Cell Line Cultures
4.6. Cellular Uptake
4.7. Cytotoxicity Studies
4.8. Apoptosis-Necrosis Test
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Davidson, B.L.; McCray, P.B. Current prospects for RNA interference-based therapies. Nat. Rev. Genet. 2011, 12, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Cech, T.R.; Steitz, J.A.; Altuvia, S.; Zhang, A.; Argaman, L.; Tiwari, A.; Storz, G.; Ameres, S.L.; Zamore, P.D.; Ban, N.; et al. The noncoding RNA revolution-trashing old rules to forge new ones. Cell 2014, 157, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Aravin, A.; Tuschl, T. Identification and characterization of small RNAs involved in RNA silencing. FEBS Lett. 2005, 579, 5830–5840. [Google Scholar] [CrossRef]
- Scholz, C.; Wagner, E. Therapeutic plasmid DNA versus siRNA delivery: Common and different tasks for synthetic carriers. J. Control. Release 2012, 161, 554–565. [Google Scholar] [CrossRef]
- Juin, P.; Geneste, O.; Gautier, F.; Depil, S.; Campone, M. Decoding and unlocking the BCL-2 dependency of cancer cells. Nat. Rev. Cancer 2013, 13, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Quinn, B.A.; Das, S.K.; Dash, R.; Emdad, L.; Dasgupta, S.; Wang, X.Y.; Dent, P.; Reed, J.C.; Pellecchia, M.; et al. Targeting the Bcl-2 family for cancer therapy. Expert Opin. Ther. Targets 2013, 17, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, K.A.; Langer, R.; Anderson, D.G. Knocking down barriers: Advances in siRNA delivery. Nat. Rev. Drug Discov. 2009, 8, 129–138. [Google Scholar] [CrossRef]
- Kalaivani, R.; Maruthupandy, M.; Muneeswaran, T.; Hameedha Beevi, A.; Anand, M.; Ramakritinan, C.M.; Kumaraguru, A.K. Synthesis of chitosan mediated silver nanoparticles (Ag NPs) for potential antimicrobial applications. Front. Lab. Med. 2018, 2, 30–35. [Google Scholar] [CrossRef]
- Rai, M.; Kon, K.; Ingle, A.; Duran, N.; Galdiero, S.; Galdiero, M. Broad-spectrum bioactivities of silver nanoparticles: The emerging trends and future prospects. Appl. Microbiol. Biotechnol. 2014, 98, 1951–1961. [Google Scholar] [CrossRef]
- Thapa, R.K.; Kim, J.H.; Jeong, J.H.; Shin, B.S.; Choi, H.G.; Yong, C.S.; Kim, J.O. Silver nanoparticle-embedded graphene oxide-methotrexate for targeted cancer treatment. Colloids Surf. B Biointerfaces 2017, 153, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Rajeshkumar, S.; Malarkodi, C.; Vanaja, M.; Annadurai, G. Anticancer and enhanced antimicrobial activity of biosynthesizd silver nanoparticles against clinical pathogens. J. Mol. Struct. 2016, 1116, 165–173. [Google Scholar] [CrossRef]
- Vedelago, J.; Gomez, C.G.; Valente, M.; Mattea, F. Green synthesis of silver nanoparticles aimed at improving theranostics. Radiat. Phys. Chem. 2018, 146, 55–67. [Google Scholar] [CrossRef]
- Natsuki, J. A Review of Silver Nanoparticles: Synthesis Methods, Properties and Applications. Int. J. Mater. Sci. Appl. 2015, 4, 325. [Google Scholar] [CrossRef]
- Ma, H.; Yin, B.; Wang, S.; Jiao, Y.; Pan, W.; Huang, S.; Chen, S.; Meng, F. Synthesis of Silver and Gold Nanoparticles by a Novel Electrochemical Method. ChemPhysChem 2004, 5, 68–75. [Google Scholar] [CrossRef]
- Huy, T.Q.; Huyen, P.T.M.; Le, A.-T.; Tonezzer, M. Recent Advances of Silver Nanoparticles in Cancer Diagnosis and Treatment. Anticancer Agents Med. Chem. 2019, 20, 1276–1287. [Google Scholar] [CrossRef]
- Shanmuganathan, R.; Karuppusamy, I.; Saravanan, M.; Muthukumar, H.; Ponnuchamy, K.; Ramkumar, V.S.; Pugazhendhi, A. Synthesis of Silver Nanoparticles and their Biomedical Applications—A Comprehensive Review. Curr. Pharm. Des. 2019, 25, 2650–2660. [Google Scholar] [CrossRef]
- Burdușel, A.C.; Gherasim, O.; Grumezescu, A.M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical applications of silver nanoparticles: An up-to-date overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef]
- Sarkar, K.; Banerjee, S.L.; Kundu, P.P.; Madras, G.; Chatterjee, K. Biofunctionalized surface-modified silver nanoparticles for gene delivery. J. Mater. Chem. B 2015, 3, 5266–5276. [Google Scholar] [CrossRef]
- Ivanova, N.; Gugleva, V.; Dobreva, M.; Pehlivanov, I.; Stefanov, S.; Andonova, V. Silver Nanoparticles as Multi-Functional Drug Delivery Systems. In Nanomedicines; Intech Open: London, UK, 2019. [Google Scholar]
- Telford, W.G. Multiparametric analysis of apoptosis by flow cytometry. In Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2018; Volume 1678, pp. 167–202. [Google Scholar]
- Pietkiewicz, S.; Schmidt, J.H.; Lavrik, I.N. Quantification of apoptosis and necroptosis at the single cell level by a combination of Imaging Flow Cytometry with classical Annexin V/propidium iodide staining. J. Immunol. Methods 2015, 423, 99–103. [Google Scholar] [CrossRef]
- Li, Y.; Chang, Y.; Lian, X.; Zhou, L.; Yu, Z.; Wang, H.; An, F. Silver nanoparticles for enhanced cancer theranostics: In Vitro and In Vivo perspectives. J. Biomed. Nanotechnol. 2018, 14, 1515–1542. [Google Scholar] [CrossRef] [PubMed]
- Gomes, H.I.O.; Martins, C.S.M.; Prior, J.A.V. Silver nanoparticles as carriers of anticancer drugs for efficient target treatment of cancer cells. Nanomaterials 2021, 11, 964. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.J.; Liu, Y.J.; Wang, H.B.; Wang, Y.Y. A sensitive electrochemical DNA biosensor based on silver nanoparticles-polydopamine@graphene composite. Electrochim. Acta 2014, 118, 130–137. [Google Scholar] [CrossRef]
- Jeong, H.H.; Choi, E.; Ellis, E.; Lee, T.C. Recent advances in gold nanoparticles for biomedical applications: From hybrid structures to multi-functionality. J. Mater. Chem. B 2019, 7, 3480–3496. [Google Scholar] [CrossRef]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver nanoparticles and their antibacterial applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef]
- Behnam, M.A.; Emami, F.; Sobhani, Z.; Koohi-Hosseinabadi, O.; Dehghanian, A.R.; Zebarjad, S.M.; Moghim, M.H.; Oryan, A. Novel combination of silver nanoparticles and carbon nanotubes for plasmonic photo thermal therapy in melanoma cancer model. Adv. Pharm. Bull. 2018, 8, 49–55. [Google Scholar] [CrossRef]
- Park, T.; Lee, S.; Amatya, R.; Cheong, H.; Moon, C.; Kwak, H.D.; Min, K.A.; Shin, M.C. ICG-loaded pegylated BSA-silver nanoparticles for effective photothermal cancer therapy. Int. J. Nanomed. 2020, 15, 5459–5471. [Google Scholar] [CrossRef]
- Boca, S.C.; Potara, M.; Gabudean, A.M.; Juhem, A.; Baldeck, P.L.; Astilean, S. Chitosan-coated triangular silver nanoparticles as a novel class of biocompatible, highly effective photothermal transducers for In Vitro cancer cell therapy. Cancer Lett. 2011, 311, 131–140. [Google Scholar] [CrossRef]
- Behnam, M.A.; Emami, F.; Sobhani, Z. Pegylated carbon nanotubes decorated with silver nanoparticles: Fabrication, cell cytotoxicity and application in photo thermal therapy. Iran. J. Pharm. Res. 2021, 20, 91–104. [Google Scholar] [CrossRef]
- Pędziwiatr-Werbicka, E.; Gorzkiewicz, M.; Horodecka, K.; Abashkin, V.; Klajnert-Maculewicz, B.; Peña-González, C.E.; Sánchez-Nieves, J.; Gómez, R.; Javier de la Mata, F.; Bryszewska, M. Silver nanoparticles surface-modified with carbosilane dendrons as carriers of anticancer siRNA. Int. J. Mol. Sci. 2020, 21, 4647. [Google Scholar] [CrossRef]
- Vermeulen, L.M.P.; De Smedt, S.C.; Remaut, K.; Braeckmans, K. The proton sponge hypothesis: Fable or fact? Eur. J. Pharm. Biopharm. 2018, 129, 184–190. [Google Scholar] [CrossRef]
- Barrios-Gumiel, A.; Sánchez-Nieves, J.; Pedziwiatr-Werbicka, E.; Abashkin, V.; Shcharbina, N.; Shcharbin, D.; Glińska, S.; Ciepluch, K.; Kuc-Ciepluch, D.; Lach, D.; et al. Effect of PEGylation on the biological properties of cationic carbosilane dendronized gold nanoparticles. Int. J. Pharm. 2020, 573, 118867. [Google Scholar] [CrossRef] [PubMed]
- Pędziwiatr-Werbicka, E.; Gorzkiewicz, M.; Michlewska, S.; Ionov, M.; Shcharbin, D.; Klajnert-Maculewicz, B.; Peña-González, C.E.; Sánchez-Nieves, J.; Gómez, R.; de la Mata, F.J.; et al. Evaluation of dendronized gold nanoparticles as siRNAs carriers into cancer cells. J. Mol. Liq. 2021, 324, 114726. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Li, W.; Jiang, X.; Ji, Y.; Wu, X.; Xu, L.; Qiu, Y.; Zhao, K.; Wei, T.; et al. Selective targeting of gold nanorods at the mitochondria of cancer cells: Implications for cancer therapy. Nano Lett. 2011, 11, 772–780. [Google Scholar] [CrossRef]
- Chithrani, B.D.; Chan, W.C.W. Elucidating the mechanism of cellular uptake and removal of protein-coated gold nanoparticles of different sizes and shapes. Nano Lett. 2007, 7, 1542–1550. [Google Scholar] [CrossRef]
- Huang, J.G.; Leshuk, T.; Gu, F.X. Emerging nanomaterials for targeting subcellular organelles. Nano Today 2011, 6, 478–492. [Google Scholar] [CrossRef]
- Navya, P.N.; Kaphle, A.; Srinivas, S.P.; Bhargava, S.K.; Rotello, V.M.; Daima, H.K. Current trends and challenges in cancer management and therapy using designer nanomaterials. Nano Converg. 2019, 6, 23. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Cheng, Y. Structure-activity relationships of fluorinated dendrimers in DNA and siRNA delivery. Acta Biomater. 2016, 46, 204–210. [Google Scholar] [CrossRef]
- Rahme, K.; Guo, J.; Holmes, J.D. Bioconjugated gold nanoparticles enhance siRNA delivery in prostate cancer cells. In Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2019; Volume 1974, pp. 291–301. [Google Scholar]
- Juling, S.; Niedzwiecka, A.; Böhmert, L.; Lichtenstein, D.; Selve, S.; Braeuning, A.; Thünemann, A.F.; Krause, E.; Lampen, A. Protein Corona Analysis of Silver Nanoparticles Links to Their Cellular Effects. J. Proteome Res. 2017, 16, 4020–4034. [Google Scholar] [CrossRef]
- Pederzoli, F.; Tosi, G.; Vandelli, M.A.; Belletti, D.; Forni, F.; Ruozi, B. Protein corona and nanoparticles: How can we investigate on? Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1467. [Google Scholar] [CrossRef]
- Spagnoletti, F.N.; Kronberg, F.; Spedalieri, C.; Munarriz, E.; Giacometti, R. Protein corona on biogenic silver nanoparticles provides higher stability and protects cells from toxicity in comparison to chemical nanoparticles. J. Environ. Manag. 2021, 297, 113434. [Google Scholar] [CrossRef] [PubMed]
- Yeung, K.T.; Yang, J. Epithelial-mesenchymal transition in tumor metastasis. Mol. Oncol. 2017, 11, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Nakkala, J.R.; Mata, R.; Raja, K.; Khub Chandra, V.; Sadras, S.R. Green synthesized silver nanoparticles: Catalytic dye degradation, in vitro anticancer activity and in vivo toxicity in rats. Mater. Sci. Eng. C 2018, 91, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Pongrac, I.M.; Ahmed, L.B.; Mlinarić, H.; Jurašin, D.D.; Pavičić, I.; Marjanović Čermak, A.M.; Milić, M.; Gajović, S.; Vinković Vrček, I. Surface coating affects uptake of silver nanoparticles in neural stem cells. J. Trace Elem. Med. Biol. 2018, 50, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Guo, H.; Liu, L.; Liu, Y.; Xie, L. Size-dependent cellular uptake and localization profiles of silver nanoparticles. Int. J. Nanomed. 2019, 14, 4247–4259. [Google Scholar] [CrossRef]
- Malysheva, A.; Ivask, A.; Doolette, C.L.; Voelcker, N.H.; Lombi, E. Cellular binding, uptake and biotransformation of silver nanoparticles in human T lymphocytes. Nat. Nanotechnol. 2021, 16, 926–932. [Google Scholar] [CrossRef]
- Nguyen, K.C.; Seligy, V.L.; Massarsky, A.; Moon, T.W.; Rippstein, P.; Tan, J.; Tayabali, A.F. Comparison of toxicity of uncoated and coated silver nanoparticles. J. Phys. Conf. Ser. 2013, 429, 012025. [Google Scholar] [CrossRef]
- Jabeen, S.; Qureshi, R.; Munazir, M.; Maqsood, M.; Munir, M.; Shah, S.S.H.; Rahim, B.Z. Application of green synthesized silver nanoparticles in cancer treatment—A critical review. Mater. Res. Express 2021, 8, 092001. [Google Scholar] [CrossRef]
- Brkić Ahmed, L.; Milić, M.; Pongrac, I.M.; Marjanović, A.M.; Mlinarić, H.; Pavičić, I.; Gajović, S.; Vinković Vrček, I. Impact of surface functionalization on the uptake mechanism and toxicity effects of silver nanoparticles in HepG2 cells. Food Chem. Toxicol. 2017, 107, 349–361. [Google Scholar] [CrossRef]
- Kvach, M.V.; Tsybulsky, D.A.; Ustinov, A.V.; Stepanova, I.A.; Bondarev, S.L.; Gontarev, S.V.; Korshun, V.A.; Shmanai, V.V. 5(6)-Carboxyfluorescein revisited: New protecting group, separation of isomers, and their spectral properties on oligonucleotides. Bioconjug. Chem. 2007, 18, 1691–1696. [Google Scholar] [CrossRef]
- Ulashchik, E.A.; Martynenko-Makaev, Y.V.; Akhlamionok, T.P.; Melnik, D.M.; Shmanai, V.V.; Zatsepin, T.S. Synthesis of galNAc-oligonucleotide conjugates using galnac phosphoramidite and triple-galNAc CPG solid support. In Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2021; Volume 2282, pp. 101–118. [Google Scholar]
- Peña-González, C.E.; Pedziwiatr-Werbicka, E.; Martín-Pérez, T.; Szewczyk, E.M.; Copa-Patiño, J.L.; Soliveri, J.; Pérez-Serrano, J.; Gómez, R.; Bryszewska, M.; Sánchez-Nieves, J.; et al. Antibacterial and antifungal properties of dendronized silver and gold nanoparticles with cationic carbosilane dendrons. Int. J. Pharm. 2017, 528, 55–61. [Google Scholar] [CrossRef] [PubMed]










| Dendron Generation | Group Name | Dendron/PEG Molar Ratio | ||
|---|---|---|---|---|
| 3:1 | 1:1 | 1:3 | ||
| G1 | AgNP-G1 | 1aAg | 1bAg | 1cAg |
| G2 | AgNP-G2 | 2aAg | 2bAg | 2cAg |
| G3 | AgNP-G3 | 3aAg | 3bAg | 3cAg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abashkin, V.; Pędziwiatr-Werbicka, E.; Horodecka, K.; Zhogla, V.; Ulashchik, E.; Shmanai, V.; Shcharbin, D.; Bryszewska, M. Silver Nanoparticles Modified by Carbosilane Dendrons and PEG as Delivery Vectors of Small Interfering RNA. Int. J. Mol. Sci. 2023, 24, 840. https://doi.org/10.3390/ijms24010840
Abashkin V, Pędziwiatr-Werbicka E, Horodecka K, Zhogla V, Ulashchik E, Shmanai V, Shcharbin D, Bryszewska M. Silver Nanoparticles Modified by Carbosilane Dendrons and PEG as Delivery Vectors of Small Interfering RNA. International Journal of Molecular Sciences. 2023; 24(1):840. https://doi.org/10.3390/ijms24010840
Chicago/Turabian StyleAbashkin, Viktar, Elżbieta Pędziwiatr-Werbicka, Katarzyna Horodecka, Victoriya Zhogla, Egor Ulashchik, Vadim Shmanai, Dzmitry Shcharbin, and Maria Bryszewska. 2023. "Silver Nanoparticles Modified by Carbosilane Dendrons and PEG as Delivery Vectors of Small Interfering RNA" International Journal of Molecular Sciences 24, no. 1: 840. https://doi.org/10.3390/ijms24010840
APA StyleAbashkin, V., Pędziwiatr-Werbicka, E., Horodecka, K., Zhogla, V., Ulashchik, E., Shmanai, V., Shcharbin, D., & Bryszewska, M. (2023). Silver Nanoparticles Modified by Carbosilane Dendrons and PEG as Delivery Vectors of Small Interfering RNA. International Journal of Molecular Sciences, 24(1), 840. https://doi.org/10.3390/ijms24010840








