The Normobaric Oxygen Paradox—Hyperoxic Hypoxic Paradox: A Novel Expedient Strategy in Hematopoiesis Clinical Issues
Abstract
1. Introduction
2. Hypoxia
2.1. Biological Cellular Response: Hypoxia Inducible Factors (HIFs)
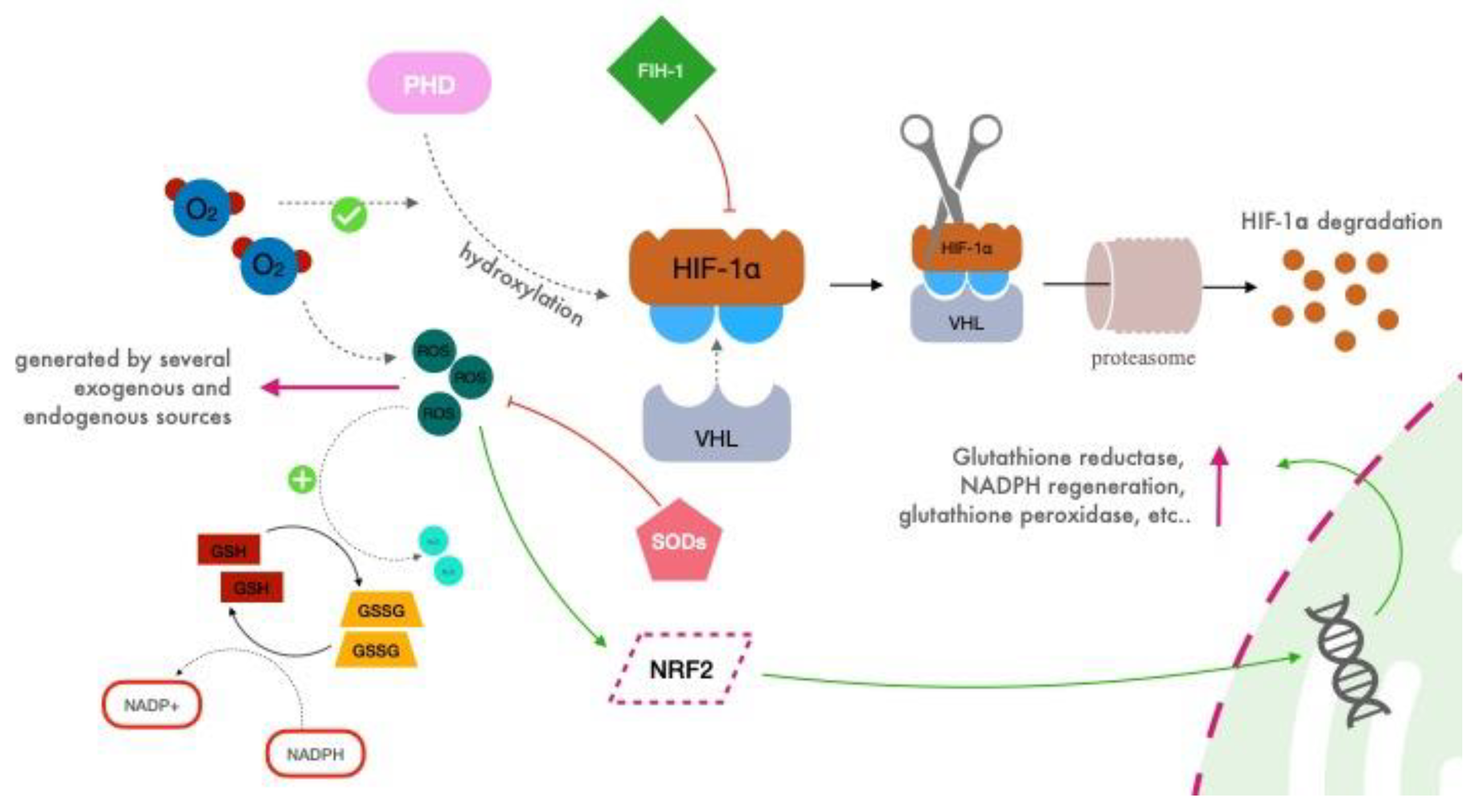
2.2. HIF Regulation
2.3. Reactive Oxygen Species (ROS)
3. The Normobaric Oxygen Paradox
3.1. Background
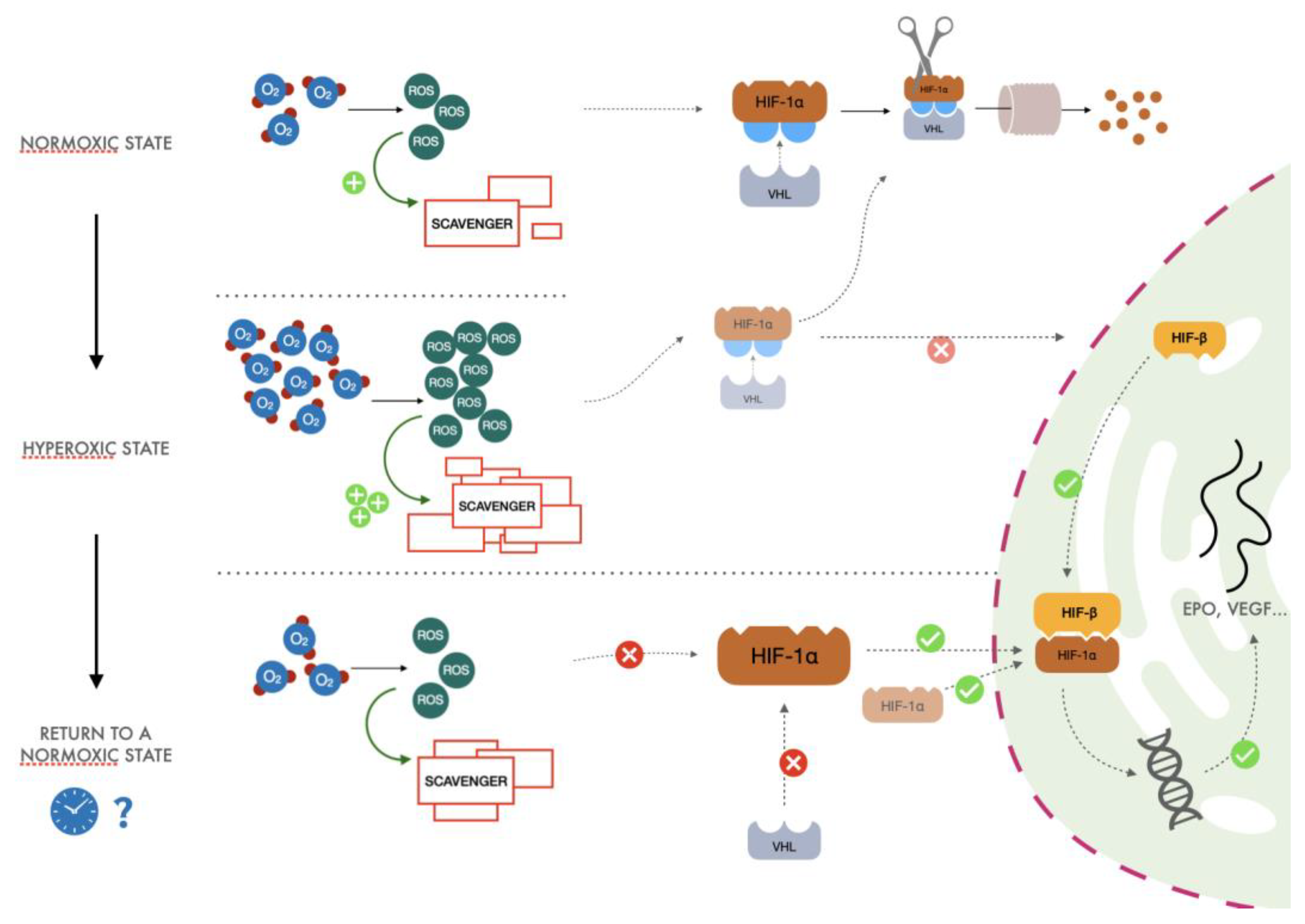
3.2. The Proposed Mechanism
- (1)
- After exposure to hyperoxia, the increased presence of ROS causes an increase in activity of the glutathione synthetase enzyme. This increase in scavenger molecules keeps the oxidative conditions of the cells under control, thus preventing the potential harm caused by reactive species to DNA and other pivotal cellular processes.
- (2)
- When returning to normoxia, normalization of oxygen levels and therefore of ROS is rapidly established, but activity of the scavenger power of the cells remains high for a longer period, exceeding the amount of ROS normally produced in the presence of a physiological concentration of oxygen. When the hyperoxic stimulus is interrupted, the more significant scavenger presence than ROS could drive a hypoxia-like cellular response as lower reactive oxygen species molecules are available. Therefore, less HIF undergoes proteasomal degradation, promoting the transcription of EPO, vascular endothelial growth factor (VEGF), and all the other genes linked to the HIF cascade.
- (3)
- Cyclical hyperoxic exposure causes a decrease in the ROS/scavenger ratio until this gradually becomes similar to the balance present under hypoxic conditions. From a molecular point of view, a reduction of hyperoxia generates a hypoxia-mimicking state by decreasing the percentage of ROS/scavenging capacity.
4. Methods
5. Human Studies
6. Discussion
Future Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| ASA | American Society of Anesthesiologists physical status classification system |
| ARE | Antioxidant Response Elements |
| ATA | Atmosphere Absolute |
| EPO | Erythropoietin |
| FIH | Factor Inhibiting HIF |
| FiO2 | Inspired Fraction of Oxygen |
| GSH | Glutathione Reduced |
| GSSG | Glutathione Oxidized |
| HIF | Hypoxia Inducible Factor |
| HRE | Hypoxia Responsive Element |
| LOS | Length of Stay |
| NF-κB | Nuclear Factor-kappa B |
| NOP | Normobaric Oxygen Paradox |
| NRF2 | Nuclear Factor Erythroid 2 Related—Factor 2 |
| PaO2 | Oxygen Arterial Partial Pressure |
| PHD | Prolyl Hydroxylase domain |
| POD | Post-Operative Day |
| RBC | Red Blood Cells |
| ROS | Reactive Oxygen Species |
| SOD | Superoxide Dismutase |
| VHL | Von Hippen Lindau protein |
References
- Bhutta, B.S.; Alghoula, F.; Berim, I. Hypoxia; StatPearls: Tampa, FL, USA, 2022. [Google Scholar]
- Mallat, J.; Rahman, N.; Hamed, F.; Hernandez, G.; Fischer, M.O. Pathophysiology, Mechanisms, and Managements of Tissue Hypoxia. Anaesth. Crit. Care Pain Med. 2022, 41, 101087. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Hypoxia-Inducible Factors in Physiology and Medicine. Cell 2012, 148, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Angus, D.C. Oxygen Therapy for the Critically Ill. N. Engl. J. Med. 2020, 382, 1054–1056. [Google Scholar] [CrossRef] [PubMed]
- Helmerhorst, H.J.F.; Roos-Blom, M.J.; van Westerloo, D.J.; de Jonge, E. Association Between Arterial Hyperoxia and Outcome in Subsets of Critical Illness: A Systematic Review, Meta-Analysis, and Meta-Regression of Cohort Studies. Crit. Care Med. 2015, 43, 1508–1519. [Google Scholar] [CrossRef] [PubMed]
- Damiani, E.; Donati, A.; Girardis, M. Oxygen in the Critically Ill: Friend or Foe? Curr. Opin. Anaesthesiol. 2018, 31, 129–135. [Google Scholar] [CrossRef]
- Ariyaratnam, P.; Loubani, M.; Bennett, R.; Griffin, S.; Chaudhry, M.A.; Cowen, M.E.; Guvendik, L.; Cale, A.R.J.; Morice, A.H. Hyperoxic Vasoconstriction of Human Pulmonary Arteries: A Novel Insight into Acute Ventricular Septal Defects. ISRN Cardiol. 2013, 2013, 685735. [Google Scholar] [CrossRef]
- Brueckl, C.; Kaestle, S.; Kerem, A.; Habazettl, H.; Krombach, F.; Kuppe, H.; Kuebler, W.M. Hyperoxia-Induced Reactive Oxygen Species Formation in Pulmonary Capillary Endothelial Cells in Situ. Am. J. Respir. Cell Mol. Biol. 2006, 34, 453–463. [Google Scholar] [CrossRef]
- Johnson, N.J.; Dodampahala, K.; Rosselot, B.; Perman, S.M.; Mikkelsen, M.E.; Goyal, M.; Gaieski, D.F.; Grossestreuer, A.V. The Association Between Arterial Oxygen Tension and Neurological Outcome After Cardiac Arrest. Ther. Hypothermia Temp. Manag. 2017, 7, 36–41. [Google Scholar] [CrossRef]
- Shah, J. Hyperbaric Oxygen Therapy. J. Am. Coll. Certif. Wound Spec. 2010, 2, 9–13. [Google Scholar] [CrossRef]
- Pelaia, C.; Bruni, A.; Garofalo, E.; Rovida, S.; Arrighi, E.; Cammarota, G.; Navalesi, P.; Pelaia, G.; Longhini, F. Oxygenation Strategies during Flexible Bronchoscopy: A Review of the Literature. Respir. Res. 2021, 22, 253. [Google Scholar] [CrossRef]
- Balestra, C.; Kot, J. Oxygen: A Stimulus, Not “Only” a Drug. Medicina 2021, 57, 1161. [Google Scholar] [CrossRef]
- Fu, Q.; Duan, R.; Sun, Y.; Li, Q. Hyperbaric Oxygen Therapy for Healthy Aging: From Mechanisms to Therapeutics. Redox Biol. 2022, 53, 102352. [Google Scholar] [CrossRef]
- Cimino, F.; Balestra, C.; Germonpré, P.; de Bels, D.; Tillmans, F.; Saija, A.; Speciale, A.; Virgili, F. Pulsed High Oxygen Induces a Hypoxic-like Response in Human Umbilical Endothelial Cells and in Humans. J. Appl. Physiol. 2012, 113, 1684–1689. [Google Scholar] [CrossRef]
- Balestra, C.; Germonpré, P.; Poortmans, J.R.; Marroni, A. Serum Erythropoietin Levels in Healthy Humans after a Short Period of Normobaric and Hyperbaric Oxygen Breathing: The “Normobaric Oxygen Paradox”. J. Appl. Physiol. 2006, 100, 512–518. [Google Scholar] [CrossRef]
- Balestra, C.; Lambrechts, K.; Mrakic-Sposta, S.; Vezzoli, A.; Levenez, M.; Germonpré, P.; Virgili, F.; Bosco, G.; Lafère, P. Hypoxic and Hyperoxic Breathing as a Complement to Low-Intensity Physical Exercise Programs: A Proof-of-Principle Study. Int. J. Mol. Sci. 2021, 22, 9600. [Google Scholar] [CrossRef]
- Fratantonio, D.; Virgili, F.; Zucchi, A.; Lambrechts, K.; Latronico, T.; Lafère, P.; Germonpré, P.; Balestra, C. Increasing Oxygen Partial Pressures Induce a Distinct Transcriptional Response in Human PBMC: A Pilot Study on the “Normobaric Oxygen Paradox”. Int. J. Mol. Sci. 2021, 22, 458. [Google Scholar] [CrossRef]
- Khalife, M.; ben Aziz, M.; Balestra, C.; Valsamis, J.; Sosnowski, M. Physiological and Clinical Impact of Repeated Inhaled Oxygen Variation on Erythropoietin Levels in Patients After Surgery. Front. Physiol. 2021, 12, 744074. [Google Scholar] [CrossRef]
- Donati, A.; Damiani, E.; Zuccari, S.; Domizi, R.; Scorcella, C.; Girardis, M.; Giulietti, A.; Vignini, A.; Adrario, E.; Romano, R.; et al. Effects of Short-Term Hyperoxia on Erythropoietin Levels and Microcirculation in Critically Ill Patients: A Prospective Observational Pilot Study. BMC Anesthesiol. 2017, 17, 49. [Google Scholar] [CrossRef]
- Balestra, C.; Arya, A.K.; Leveque, C.; Virgili, F.; Germonpré, P.; Lambrechts, K.; Lafère, P.; Thom, S.R. Varying Oxygen Partial Pressure Elicits Blood-Borne Microparticles Expressing Different Cell-Specific Proteins—Toward a Targeted Use of Oxygen? Int. J. Mol. Sci. 2022, 23, 7888. [Google Scholar] [CrossRef]
- Malacrida, S.; Giannella, A.; Ceolotto, G.; Reggiani, C.; Vezzoli, A.; Mrakic-Sposta, S.; Moretti, S.; Turner, R.; Falla, M.; Brugger, H.; et al. Transcription Factors Regulation in Human Peripheral White Blood Cells during Hypobaric Hypoxia Exposure: An in-Vivo Experimental Study. Sci. Rep. 2019, 9, 9901. [Google Scholar] [CrossRef] [PubMed]
- Majmundar, A.J.; Wong, W.J.; Simon, M.C. Hypoxia-Inducible Factors and the Response to Hypoxic Stress. Mol. Cell 2010, 40, 294–309. [Google Scholar] [CrossRef] [PubMed]
- The Nobel Prize in Physiology or Medicine 2019—Advanced Information—NobelPrize.Org. Available online: https://www.nobelprize.org/prizes/medicine/2019/advanced-information/ (accessed on 7 November 2022).
- Wang, G.L.; Semenza, G.L. Purification and Characterization of Hypoxia-Inducible Factor 1. J. Biol. Chem. 1995, 270, 1230–1237. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.L.; Jiang, B.H.; Rue, E.A.; Semenza, G.L. Hypoxia-Inducible Factor 1 Is a Basic-Helix-Loop-Helix-PAS Heterodimer Regulated by Cellular O2 Tension. Proc. Natl. Acad. Sci. USA 1995, 92, 5510. [Google Scholar] [CrossRef] [PubMed]
- Zhe, N.; Chen, S.; Zhou, Z.; Liu, P.; Lin, X.; Yu, M.; Cheng, B.; Zhang, Y.; Wang, J. HIF-1α Inhibition by 2-Methoxyestradiol Induces Cell Death via Activation of the Mitochondrial Apoptotic Pathway in Acute Myeloid Leukemia. Cancer Biol. Ther. 2016, 17, 625. [Google Scholar] [CrossRef] [PubMed]
- Wiesener, M.S.; Jürgensen, J.S.; Rosenberger, C.; Scholze, C.; Hörstrup, J.H.; Warnecke, C.; Mandriota, S.; Bechmann, I.; Frei, U.A.; Pugh, C.W.; et al. Widespread, Hypoxia-Inducible Expression of HIF-2α in Distinct Cell Populations of Different Organs. FASEB J. 2003, 17, 271–273. [Google Scholar] [CrossRef]
- Shomento, S.H.; Wan, C.; Cao, X.; Faugere, M.C.; Bouxsein, M.L.; Clemens, T.L.; Riddle, R.C. Hypoxia-Inducible Factors 1α and 2α Exert Both Distinct and Overlapping Functions in Long Bone Development. J. Cell. Biochem. 2010, 109, 196–204. [Google Scholar] [CrossRef]
- Choudhry, H.; Harris, A.L. Advances in Hypoxia-Inducible Factor Biology. Cell Metab. 2018, 27, 281–298. [Google Scholar] [CrossRef]
- Simon, M.C. The Hypoxia Response Pathways—Hats Off! N. Eng. J. Med. 2016, 375, 1687–1689. [Google Scholar] [CrossRef]
- Briggs, K.J.J.; Koivunen, P.; Cao, S.; Backus, K.M.M.; Olenchock, B.A.A.; Patel, H.; Zhang, Q.; Signoretti, S.; Gerfen, G.J.J.; Richardson, A.L.L.; et al. Paracrine Induction of HIF by Glutamate in Breast Cancer: EglN1 Senses Cysteine. Cell 2016, 166, 126–139. [Google Scholar] [CrossRef]
- Fujita, N.; Markova, D.; Greg Anderson, D.; Chiba, K.; Toyama, Y.; Shapiro, I.M.; Risbud, M.V. Expression of Prolyl Hydroxylases (PHDs) Is Selectively Controlled by HIF-1 and HIF-2 Proteins in Nucleus Pulposus Cells of the Intervertebral Disc. J. Biol. Chem. 2012, 287, 16975–16986. [Google Scholar] [CrossRef]
- Hadanny, A.; Efrati, S. The Hyperoxic-Hypoxic Paradox. Biomolecules 2020, 10, 958. [Google Scholar] [CrossRef]
- Mahon, P.C.; Hirota, K.; Semenza, G.L. FIH-1: A Novel Protein That Interacts with HIF-1alpha and VHL to Mediate Repression of HIF-1 Transcriptional Activity. Genes Dev. 2001, 15, 2675–2686. [Google Scholar] [CrossRef]
- Wenger, R.H.; Stiehl, D.P.; Camenisch, G. Integration of Oxygen Signaling at the Consensus HRE. Sci. STKE 2005, 2005, re12. [Google Scholar] [CrossRef]
- Xia, O.; Lemieux, M.E.; Li, W.; Carroll, J.S.; Brown, M.; Shirley Liu, X.; Kung, A.L. Integrative Analysis of HIF Binding and Transactivation Reveals Its Role in Maintaining Histone Methylation Homeostasis. Proc. Natl. Acad. Sci. USA 2009, 106, 4260–4265. [Google Scholar] [CrossRef]
- Fratantonio, D.; Cimino, F.; Speciale, A.; Virgili, F. Need (More than) Two to Tango: Multiple Tools to Adapt to Changes in Oxygen Availability. BioFactors 2018, 44, 207–218. [Google Scholar] [CrossRef]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal. 2018, 29, 1727–1745. [Google Scholar] [CrossRef]
- Green, D.E.; Tzagoloff, A. The Mitochondrial Electron Transfer Chain. Arch. Biochem. Biophys. 1966, 116, 293–304. [Google Scholar] [CrossRef]
- Auten, R.L.; Davis, J.M. Oxygen Toxicity and Reactive Oxygen Species: The Devil Is in the Details. Pediatr. Res. 2009, 66, 121–127. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive Oxygen Species (ROS) and Response of Antioxidants as ROS-Scavengers during Environmental Stress in Plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Wu, G.; Fang, Y.Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Glutathione Metabolism and Its Implications for Health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [CrossRef]
- Reed, M.C.; Thomas, R.L.; Pavisic, J.; James, S.J.; Ulrich, C.M.; Nijhout, H.F. A Mathematical Model of Glutathione Metabolism. Theor. Biol. Med. Model. 2008, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Buettner, G.R. Superoxide Dismutase in Redox Biology: The Roles of Superoxide and Hydrogen Peroxide. Anticancer Agents Med. Chem. 2011, 11, 341. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmann, D.C.; Brüne, B. Mitochondrial Composition and Function under the Control of Hypoxia. Redox Biol. 2017, 12, 208–215. [Google Scholar] [CrossRef]
- Jones, D.P.; Sies, H. The Redox Code. Antioxid. Redox Signal. 2015, 23, 734–746. [Google Scholar] [CrossRef] [PubMed]
- Cyran, A.M.; Zhitkovich, A. HIF1, HSF1, and NRF2: Oxidant-Responsive Trio Raising Cellular Defenses and Engaging Immune System. Chem. Res. Toxicol. 2022, 35, 1690–1700. [Google Scholar] [CrossRef]
- Lee, P.; Chandel, N.S.; Simon, M.C. Cellular Adaptation to Hypoxia through Hypoxia Inducible Factors and Beyond. Nat. Rev. Mol. Cell Biol. 2020, 21, 268–283. [Google Scholar] [CrossRef]
- Rocco, M.; D’Itri, L.; de Bels, D.; Corazza, F.; Balestra, C. The “Normobaric Oxygen Paradox”: A New Tool for the Anesthetist? Minerva Anestesiol. 2014, 80, 366–372. [Google Scholar]
- Corrado, C.; Fontana, S. Hypoxia and HIF Signaling: One Axis with Divergent Effects. Int. J. Mol. Sci. 2020, 21, 5611. [Google Scholar] [CrossRef]
- Greer, S.N.; Metcalf, J.L.; Wang, Y.; Ohh, M. The Updated Biology of Hypoxia-Inducible Factor. EMBO J. 2012, 31, 2448–2460. [Google Scholar] [CrossRef]
- Shvetsova, A.N.; Mennerich, D.; Kerätär, J.M.; Hiltunen, J.K.; Kietzmann, T. Non-Electron Transfer Chain Mitochondrial Defects Differently Regulate HIF-1α Degradation and Transcription. Redox Biol. 2017, 12, 1052. [Google Scholar] [CrossRef]
- Keramidas, M.E.; Kounalakis, S.N.; Debevec, T.; Norman, B.; Gustafsson, T.; Eiken, O.; Mekjavic, I.B. Acute Normobaric Hyperoxia Transiently Attenuates Plasma Erythropoietin Concentration in Healthy Males: Evidence against the “normobaric Oxygen Paradox” Theory. Acta Physiol. 2011, 202, 91–98. [Google Scholar] [CrossRef]
- Ciccarella, Y.; Balestra, C.; Valsamis, J.; van der Linden, P. Increase in Endogenous Erythropoietin Synthesis through the Normobaric Oxygen Paradox in Cardiac Surgery Patients. Br. J. Anaesth. 2011, 106, 752–753. [Google Scholar] [CrossRef][Green Version]
- Debevec, T.; Keramidas, M.E.; Norman, B.; Gustafsson, T.; Eiken, O.; Mekjavic, I.B.; Ferretti, G. Acute Short-Term Hyperoxia Followed by Mild Hypoxia Does Not Increase EPO Production: Resolving the “Normobaric Oxygen Paradox”. Eur. J. Appl. Physiol. 2012, 112, 1059–1065. [Google Scholar] [CrossRef]
- Lafère, P.; Schubert, T.; de Bels, D.; Germonpré, P.; Balestra, C. Can the Normobaric Oxygen Paradox (NOP) Increase Reticulocyte Count after Traumatic Hip Surgery? J. Clin. Anesth. 2013, 25, 129–134. [Google Scholar] [CrossRef][Green Version]
- Revelli, L.; Vagnoni, S.; D’Amore, A.; di Stasio, E.; Lombardi, C.P.; Storti, G.; Proietti, R.; Balestra, C.; Ricerca, B.M. EPO Modulation in a 14-Days Undersea Scuba Dive. Int. J. Sports Med. 2013, 34, 856–860. [Google Scholar] [CrossRef][Green Version]
- Kiboub, F.Z.; Balestra, C.; Loennechen, Ø.; Eftedal, I. Hemoglobin and Erythropoietin After Commercial Saturation Diving. Front. Physiol. 2018, 9, 1176. [Google Scholar] [CrossRef]
- Perović, A.; Žarak, M.; Bratičević, M.N.; Dumić, J. Effects of Recreational Scuba Diving on Erythropoiesis-“normobaric Oxygen Paradox” or “Plasma Volume Regulation” as a Trigger for Erythropoietin? Eur. J. Appl. Physiol. 2020, 120, 1689–1697. [Google Scholar] [CrossRef]
- Christoulas, K.; Karamouzis, M.; Mandroukas, K. “Living High—Training Low” vs. “Living High—Training High”: Erythropoietic Responses and Performance of Adolescent Cross-Country Skiers. J. Sports Med. Phys. Fitness 2011, 51, 74–81. [Google Scholar]
- Rey, F.; Balsari, A.; Giallongo, T.; Ottolenghi, S.; di Giulio, A.M.; Samaja, M.; Carelli, S. Erythropoietin as a Neuroprotective Molecule: An Overview of Its Therapeutic Potential in Neurodegenerative Diseases. ASN Neuro 2019, 11, 1759091419871420. [Google Scholar] [CrossRef]
- Teng, R.; Calvert, J.W.; Sibmooh, N.; Piknova, B.; Suzuki, N.; Sun, J.; Martinez, K.; Yamamoto, M.; Schechter, A.N.; Lefer, D.J.; et al. Acute Erythropoietin Cardioprotection Is Mediated by Endothelial Response. Basic Res. Cardiol 2011, 106, 343–354. [Google Scholar] [CrossRef]
- Vogel, V.; Kramer, H.J.; Bäcker, A.; Meyer-Lehnert, H.; Jelkmann, W.; Fandrey, J. Effects of Erythropoietin on Endothelin-1 Synthesis and the Cellular Calcium Messenger System in Vascular Endothelial Cells. Am. J. Hypertens. 1997, 10, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Momeni, M.; de Kock, M.; Devuyst, O.; Liistro, G. Effect of N-Acetyl-Cysteine and Hyperoxia on Erythropoietin Production. Eur. J. Appl. Physiol. 2011, 111, 2681–2686. [Google Scholar] [CrossRef] [PubMed]
- Zembron-Lacny, A.; Slowinska-Lisowska, M.; Szygula, Z.; Witkowski, K.; Szyszka, K. The Comparison of Antioxidant and Hematological Properties of N-Acetylcysteine and Alpha-Lipoic Acid in Physically Active Males. Physiol. Res. 2009, 58, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, V.; Afsharian, P.; Shahhoseini, M.; Kalantar, S.M.; Moini, A. A Review on Various Uses of N-Acetyl Cysteine. Cell J. (Yakhteh) 2017, 19, 11. [Google Scholar] [CrossRef]
- Medved, I.; Brown, M.J.; Bjorksten, A.R.; Leppik, J.A.; Sostaric, S.; McKenna, M.J. N-Acetylcysteine Infusion Alters Blood Redox Status but Not Time to Fatigue during Intense Exercise in Humans. J. Appl. Physiol. 2003, 94, 1572–1582. [Google Scholar] [CrossRef]
- Haddad, J.J.E.; Olver, R.E.; Land, S.C. Antioxidant/pro-Oxidant Equilibrium Regulates HIF-1alpha and NF-Kappa B Redox Sensitivity. Evidence for Inhibition by Glutathione Oxidation in Alveolar Epithelial Cells. J. Biol. Chem. 2000, 275, 21130–21139. [Google Scholar] [CrossRef]
- Zhang, Z.; Yan, J.; Taheri, S.; Liu, K.J.; Shi, H. Hypoxia-Inducible Factor 1 Contributes to N-Acetylcysteine’s Protection in Stroke. Free Radic. Biol. Med. 2014, 68, 8–21. [Google Scholar] [CrossRef]
- Viikinkoski, E.; Jalkanen, J.; Gunn, J.; Vasankari, T.; Lehto, J.; Valtonen, M.; Biancari, F.; Jalkanen, S.; Airaksinen, K.E.J.; Hollmén, M.; et al. Red Blood Cell Transfusion Induces Abnormal HIF-1α Response to Cytokine Storm after Adult Cardiac Surgery. Sci. Rep. 2021, 11, 22230. [Google Scholar] [CrossRef]
- Calzia, E.; Asfar, P.; Hauser, B.; Matejovic, M.; Ballestra, C.; Radermacher, P.; Georgieff, M. Hyperoxia May Be Beneficial. Crit. Care Med. 2010, 38, S559–S568. [Google Scholar] [CrossRef]
- Smeyne, M.; Sladen, P.; Jiao, Y.; Dragatsis, I.; Smeyne, R.J. HIF1α Is Necessary for Exercise-Induced Neuroprotection While HIF2α Is Needed for Dopaminergic Neuron Survival in the Substantia Nigra Pars Compacta. Neuroscience 2015, 295, 23–38. [Google Scholar] [CrossRef]
- Brugniaux, J.V.; Coombs, G.B.; Barak, O.F.; Dujic, Z.; Sekhon, M.S.; Ainslie, P.N. Highs and Lows of Hyperoxia: Physiological, Performance, and Clinical Aspects. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R1–R27. [Google Scholar] [CrossRef]
- Zhu, T.; Zhan, L.; Liang, D.; Hu, J.; Lu, Z.; Zhu, X.; Sun, W.; Liu, L.; Xu, E. Hypoxia-Inducible Factor 1α Mediates Neuroprotection of Hypoxic Postconditioning against Global Cerebral Ischemia. J. Neuropathol. Exp. Neurol. 2014, 73, 975–986. [Google Scholar] [CrossRef]
- Tekin, D.; Dursun, A.D.; Xi, L. Hypoxia Inducible Factor 1 (HIF-1) and Cardioprotection. Acta Pharmacol. Sin. 2010, 31, 1085–1094. [Google Scholar] [CrossRef]
- D’Anselmi, F.; Valerio, M.; Cucina, A.; Galli, L.; Proietti, S.; Dinicola, S.; Pasqualato, A.; Manetti, C.; Ricci, G.; Giuliani, A.; et al. Metabolism and Cell Shape in Cancer: A Fractal Analysis. Int. J. Biochem. Cell Biol. 2011, 43, 1052–1058. [Google Scholar] [CrossRef]
- Davies, J.M.S.; Cillard, J.; Friguet, B.; Cadenas, E.; Cadet, J.; Cayce, R.; Fishmann, A.; Liao, D.; Bulteau, A.L.; Derbré, F.; et al. The Oxygen Paradox, the French Paradox, and Age-Related Diseases. Geroscience 2017, 39, 499–550. [Google Scholar] [CrossRef]
- Yang, K.; Yu, G.; Tian, R.; Zhou, Z.; Deng, H.; Li, L.; Yang, Z.; Zhang, G.; Liu, D.; Wei, J.; et al. Oxygen-Evolving Manganese Ferrite Nanovesicles for Hypoxia-Responsive Drug Delivery and Enhanced Cancer Chemoimmunotherapy. Adv. Funct. Mater. 2021, 31, 2008078. [Google Scholar] [CrossRef]
- Höckel, M.; Vaupel, P. Tumor Hypoxia: Definitions and Current Clinical, Biologic, and Molecular Aspects. J. Natl. Cancer Inst. 2001, 93, 266–276. [Google Scholar] [CrossRef]
- Herrera-Campos, A.B.; Zamudio-Martinez, E.; Delgado-Bellido, D.; Fernández-Cortés, M.; Montuenga, L.M.; Oliver, F.J.; Garcia-Diaz, A. Implications of Hyperoxia over the Tumor Microenvironment: An Overview Highlighting the Importance of the Immune System. Cancers 2022, 14, 2740. [Google Scholar] [CrossRef]
- Terraneo, L.; Virgili, E.; Caretti, A.; Bianciardi, P.; Samaja, M. In Vivo Hyperoxia Induces Hypoxia-Inducible Factor-1α Overexpression in LNCaP Tumors without Affecting the Tumor Growth Rate. Int. J. Biochem. Cell Biol. 2014, 51, 65–74. [Google Scholar] [CrossRef] [PubMed]
- de Bels, D.; Tillmans, F.; Corazza, F.; Bizzari, M.; Germonpre, P.; Radermacher, P.; Orman, K.G.; Balestra, C. Hyperoxia Alters Ultrastructure and Induces Apoptosis in Leukemia Cell Lines. Biomolecules 2020, 10, 282. [Google Scholar] [CrossRef] [PubMed]
- Raa, A.; Stansberg, C.; Steen, V.M.; Bjerkvig, R.; Reed, R.K.; Stuhr, L.E.B. Hyperoxia Retards Growth and Induces Apoptosis and Loss of Glands and Blood Vessels in DMBA-Induced Rat Mammary Tumors. BMC Cancer 2007, 7, 23. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, H.Y.; Kim, I.K.; Lee, H.I.; Lee, H.Y.; Kang, H.S.; Yeo, C.D.; Kang, H.H.; Moon, H.S.; Lee, S.H. Combination of Carboplatin and Intermittent Normobaric Hyperoxia Synergistically Suppresses Benzo[a]Pyrene-Induced Lung Cancer. Korean J. Intern. Med. 2018, 33, 541–551. [Google Scholar] [CrossRef]
- Hatfield, S.M.; Kjaergaard, J.; Lukashev, D.; Schreiber, T.H.; Belikoff, B.; Abbott, R.; Sethumadhavan, S.; Philbrook, P.; Ko, K.; Cannici, R.; et al. Immunological Mechanisms of the Antitumor Effects of Supplemental Oxygenation. Sci. Transl. Med. 2015, 7, 277ra30. [Google Scholar] [CrossRef]
- de Bels, D.; Corazza, F.; Germonpré, P.; Balestra, C. The Normobaric Oxygen Paradox: A Novel Way to Administer Oxygen as an Adjuvant Treatment for Cancer? Med. Hypotheses 2011, 76, 467–470. [Google Scholar] [CrossRef]
| Author (Year) | Type of Study | No. of Patients | Intervention | Main Results |
|---|---|---|---|---|
| Balestra (2006) [15] |
Human experimental study | 16 healthy adults | Exposure to normobaric oxygen at FiO2 1.0 for 2 h vs. exposure to hyperbaric oxygen 2.5 ATA FiO2 1.0 for 1.5 h |
Increase in EPO after normobaric oxygen exposure and decrease in EPO after hyperbaric oxygen exposure |
| Keramidas (2011) [53] |
Single-blinded experimental study | 10 healthy males | Exposure to normobaric oxygen at FiO2 1 for 2 h × 7 d | Decrease in EPO levels after hyperoxic exposure compared with control group |
| Ciccarella (2011) [54] |
Double-blind prospective pilot study | 20 post cardiac surgery patients who had intraoperative CPB and MV |
Exposure to normobaric oxygen at FiO2 1.0 for 2 h vs. FiO2 0.5 for 2 h |
Increase in EPO in both groups but slope of the increase in the EPO plasma level significantly higher in those exposed to hyperoxia and relative hypoxia |
| Debevec (2011) [55] |
Human experimental study | 18 healthy male adults |
Single exposure to 1 h normobaric oxygen FiO2 1.0 followed by 1 h normobaric FiO2 0.15 |
Exposure to hyperoxia followed by mild hypoxia led to temporary decrease in EPO levels. No difference in late time points for EPO levels Compared to control group |
| Lafere (2013) [56] |
Double-blind multicenter clinical study | 85 ASA 1 and 2 patients undergoing surgery for traumatic hip fracture |
Exposure to 30 min of FiO2 1.0 normobaric oxygen vs. Air from POD 1 until discharge |
Increase in reticulocytes count and reduction in hospital LOS and RBC transfusion in the experimental group. |
| Revelli (2013) [57] |
Human experimental study | 6 scuba divers |
14-days of diving (8–10 m) with air at 1.8–2 ATA |
Significant rise in serum EPO observed at 24 h post emersion |
| Donati (2017) [19] |
Prospective observational pilot study | 40 mechanical ventilated patients | Exposure to normobaric oxygen at FiO2 1.0 for 2 h |
ROS increase after 1 h and glutathione level after 2 h from hyperoxia exposure.
Reduction of microvascular density and perfusion during oxygen exposure rapidly normalized after returning to ambient air. EPO level rise after 48 h. |
| Kiboub (2018) [58] |
Human experimental study | 13 scuba divers | Decompression to surface pressure after long (from 25 to 27 days) professional saturation dive at 80–90 m depth | EPO markedly increased within 24 h after decompression |
| Perović (2020) [59] |
Human experimental study | 14 scuba divers |
One dive per week over 5 weeks at a depth of 20–30 m for 30 min | A significant EPO increase before and after the third and the fifth dive compared to the level before and after the first dive. |
| Fratantonio (2021) [17] |
Human experimental study | 12 healthy adults |
1 h exposure to normobaric oxygen FiO2 0.3 vs. normobaric oxygen FiO2 1.0 vs. hyperbaric oxygen 1.4 bar FiO2 1.4 | Exposure to lower level of FiO2 associated with a stronger response in HIF1-α synthesis and a lower level of inflammation and oxidative stress which was also less persistent than exposure to hyperbaric 1.4 FiO2 oxygen |
| Khalife (2021) [18] |
Prospective randomized clinical study | 26 female patients undergoing breast surgery |
1 h per day of normobaric oxygen FiO2 1.0 from POD 1 for 8 consecutive days | No difference in EPO or hemoglobin levels between the groups |
| Balestra (2022) [20] |
Human experimental study | 48 healthy adults |
Single 1 h exposure to FiO2 0.10, 0.15, 0.3 or 1.0 normobaric oxygen and 1.4, 2.5 ATA hyperbaric oxygen |
Significant elevation in microparticles from different cells was observed after exposure to every different oxygen concentration except after hyperbaric 1.4 ATA oxygen exposure. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvagno, M.; Coppalini, G.; Taccone, F.S.; Strapazzon, G.; Mrakic-Sposta, S.; Rocco, M.; Khalife, M.; Balestra, C. The Normobaric Oxygen Paradox—Hyperoxic Hypoxic Paradox: A Novel Expedient Strategy in Hematopoiesis Clinical Issues. Int. J. Mol. Sci. 2023, 24, 82. https://doi.org/10.3390/ijms24010082
Salvagno M, Coppalini G, Taccone FS, Strapazzon G, Mrakic-Sposta S, Rocco M, Khalife M, Balestra C. The Normobaric Oxygen Paradox—Hyperoxic Hypoxic Paradox: A Novel Expedient Strategy in Hematopoiesis Clinical Issues. International Journal of Molecular Sciences. 2023; 24(1):82. https://doi.org/10.3390/ijms24010082
Chicago/Turabian StyleSalvagno, Michele, Giacomo Coppalini, Fabio Silvio Taccone, Giacomo Strapazzon, Simona Mrakic-Sposta, Monica Rocco, Maher Khalife, and Costantino Balestra. 2023. "The Normobaric Oxygen Paradox—Hyperoxic Hypoxic Paradox: A Novel Expedient Strategy in Hematopoiesis Clinical Issues" International Journal of Molecular Sciences 24, no. 1: 82. https://doi.org/10.3390/ijms24010082
APA StyleSalvagno, M., Coppalini, G., Taccone, F. S., Strapazzon, G., Mrakic-Sposta, S., Rocco, M., Khalife, M., & Balestra, C. (2023). The Normobaric Oxygen Paradox—Hyperoxic Hypoxic Paradox: A Novel Expedient Strategy in Hematopoiesis Clinical Issues. International Journal of Molecular Sciences, 24(1), 82. https://doi.org/10.3390/ijms24010082











