Improvement of RNA In Situ Hybridisation for Grapevine Fruits and Ovules
Abstract
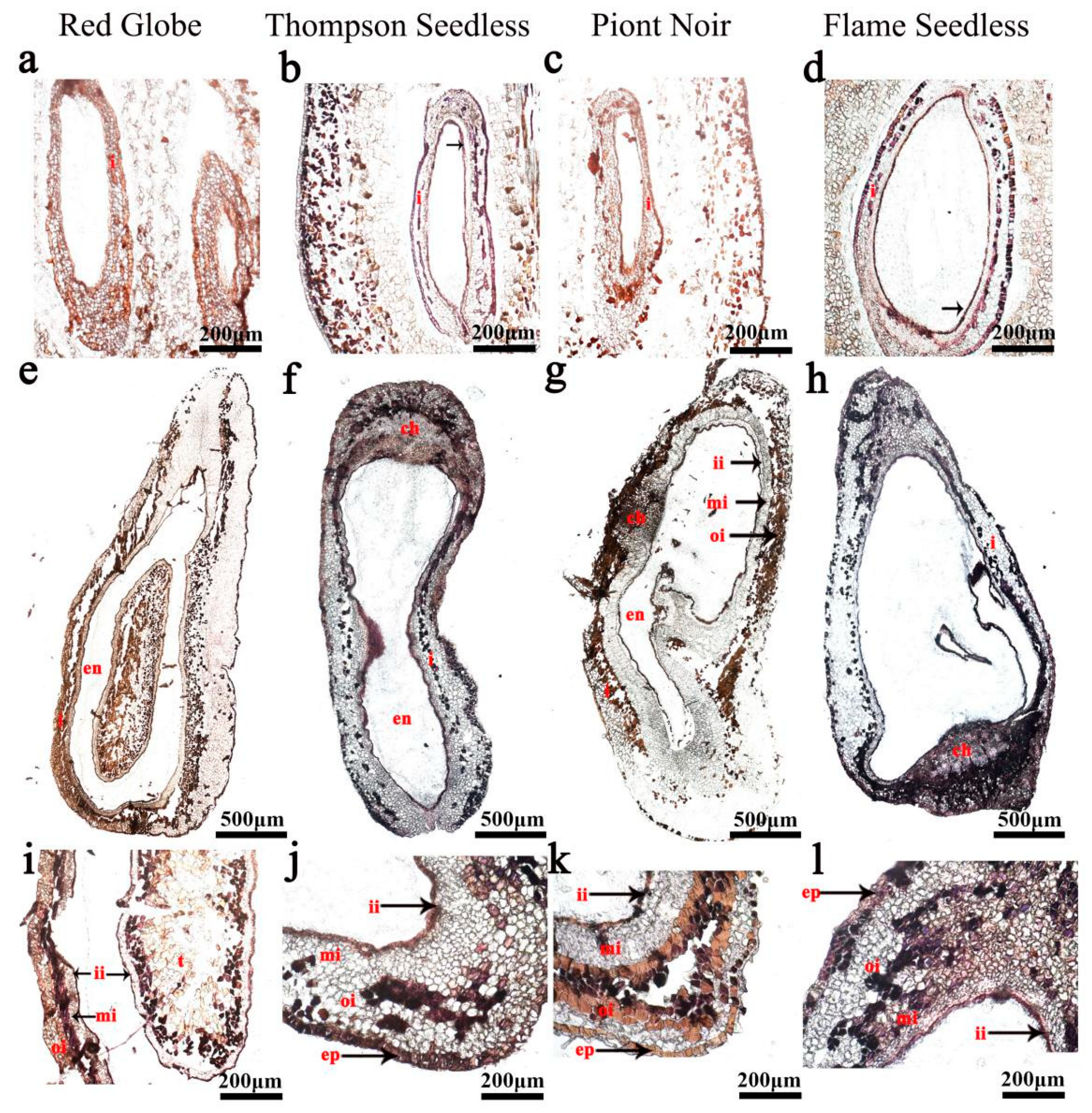
1. Introduction
2. Results
2.1. Proteinase K Treatment Time
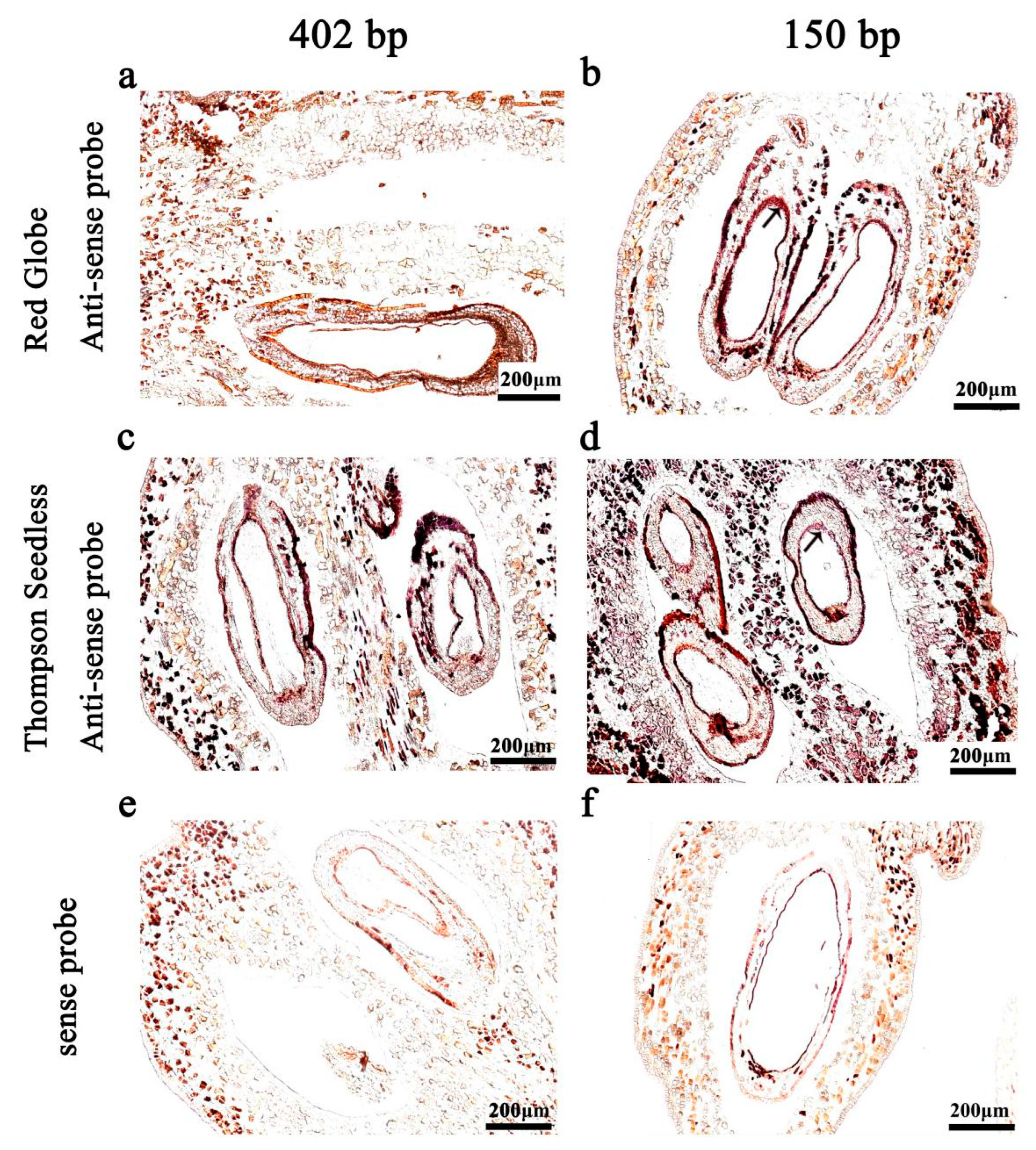
2.2. Probe Length
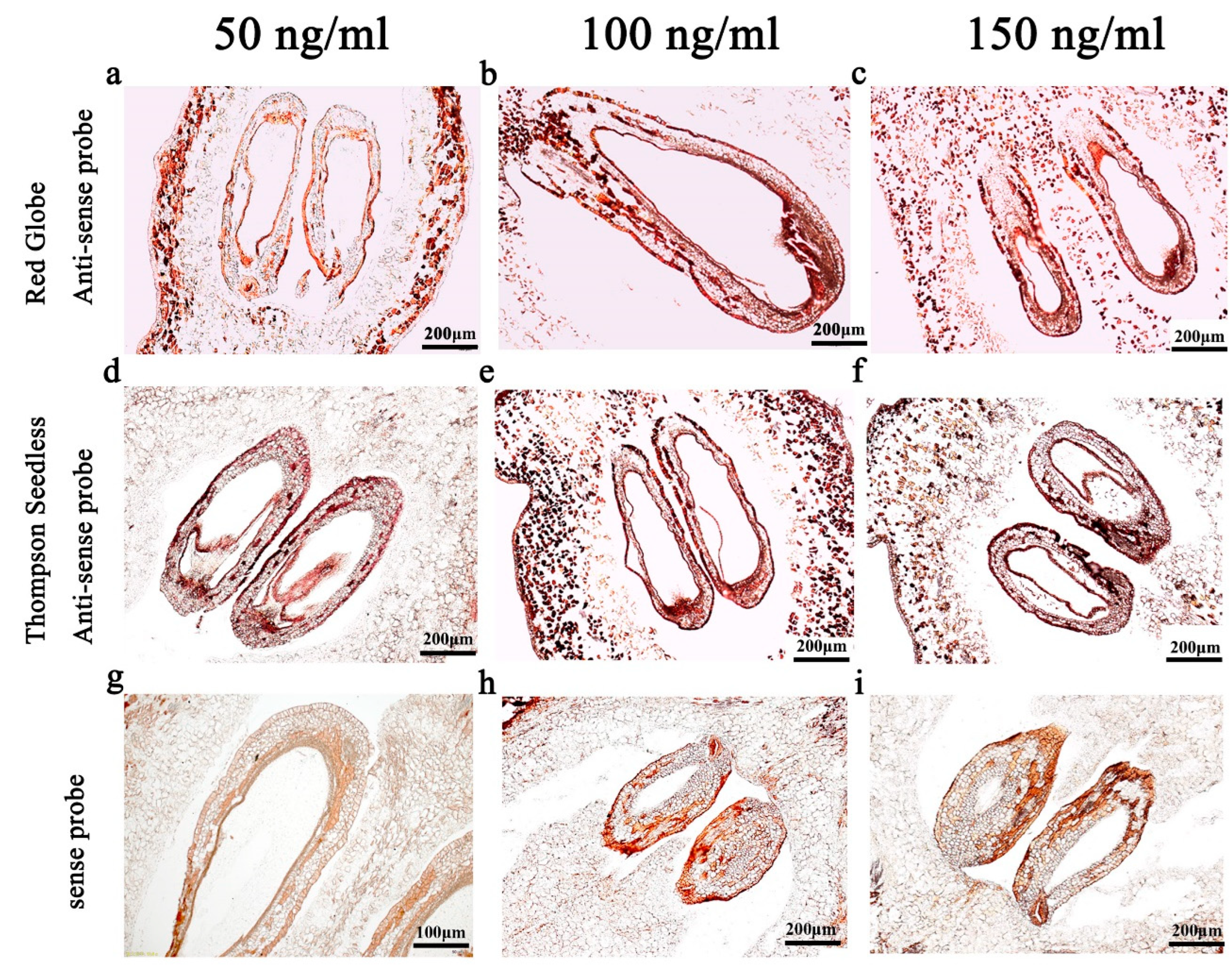
2.3. Probe Concentration
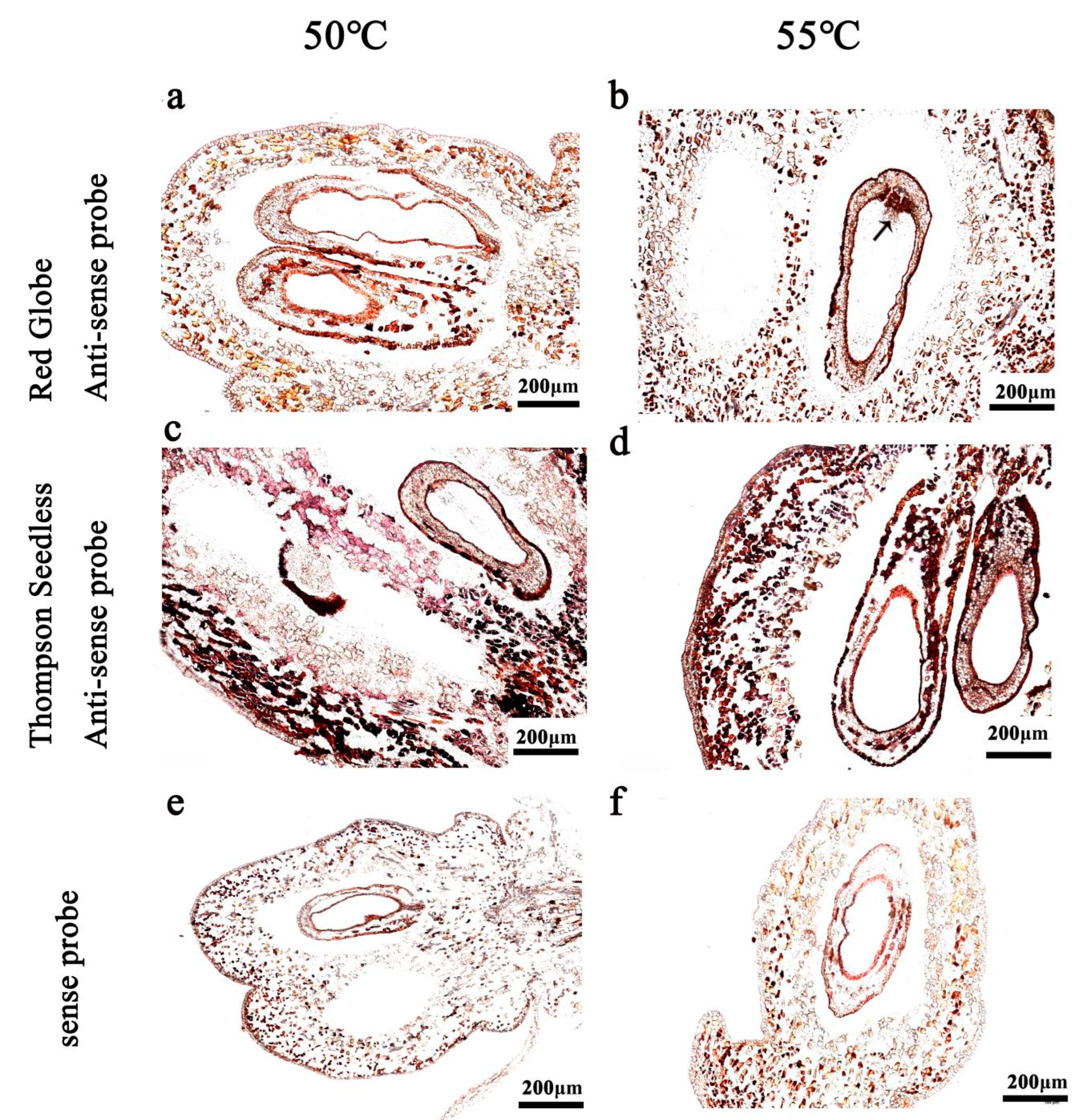
2.4. Hybridisation Temperature
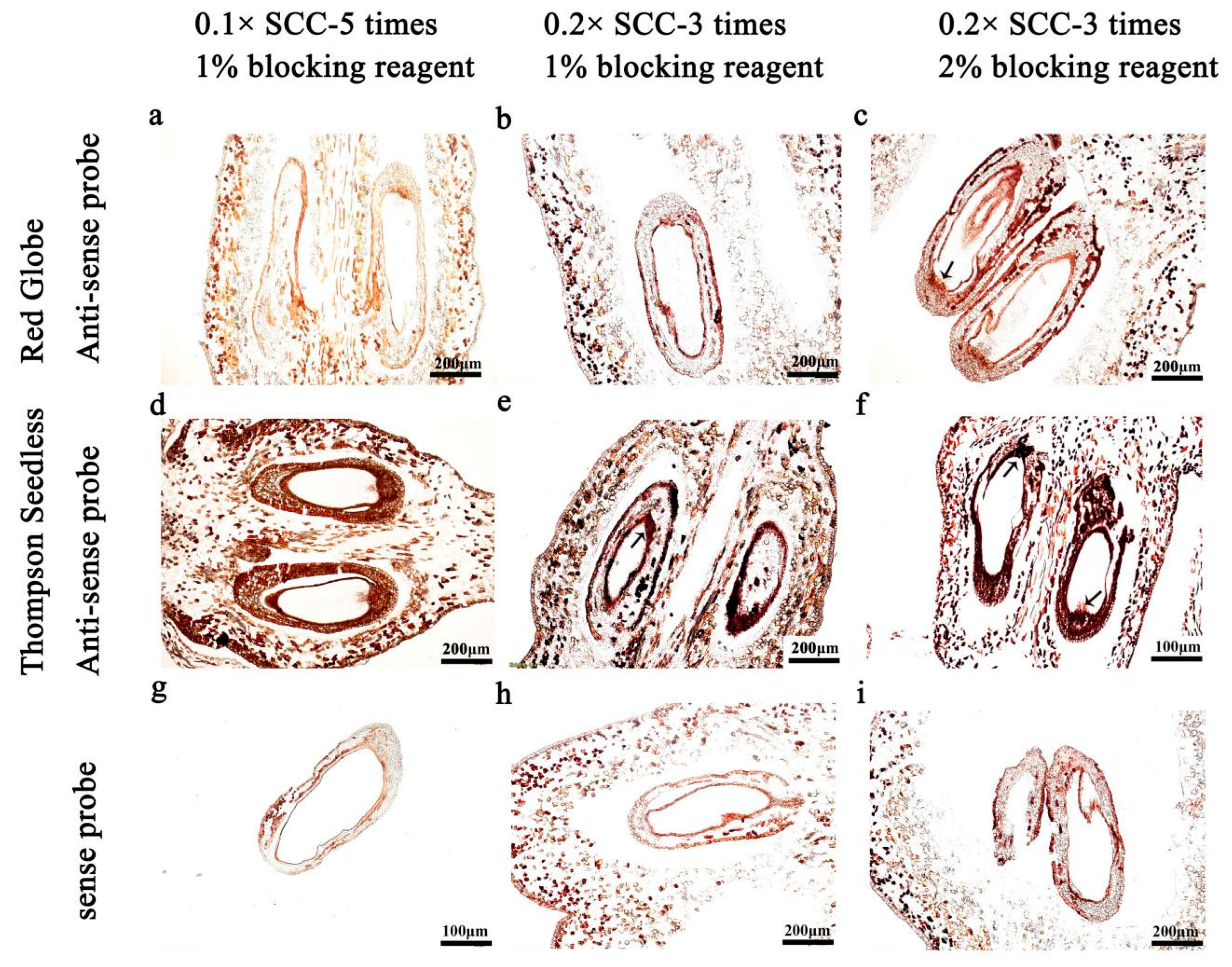
2.5. Washing Conditions
2.6. Use of Improved RNA ISH Protocol for Detection of Genes Associated with Seed Development, VvTAU and VvHB63
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Sample Collection
4.2. Preparation of Transcription Template
4.3. In Vitro Transcription Reaction
4.4. Probe Hydrolysis (Optional)
4.5. The In Situ Hybridisation
4.6. Sectioning
4.7. Deproteinisation
4.8. Hybridisation
4.9. Post-Hybridisation
4.10. Detection
5. Conclusions
- (1)
- Pre-hybridisation—proteinase K treatment time of 30 min;
- (2)
- Hybridisation—probe concentration of 100 ng/mL, probe length of 150 bp and temperature of 50 °C;
- (3)
- Post-hybridisation washing—three washes with 0.2× SSC solution and blocking with 1% blocking reagent for 45 min.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pardue, M.; Gall, J. Formation and detection of RNA-DNA hybrid Molecules in cytological preparations. Proc. Natl. Acad. Sci. USA 1969, 63, 378–383. [Google Scholar] [CrossRef]
- John, H.A.; Birnstiel, M.L.; Jones, K.W. RNA-DNA hybrids at the cytological level. Nature 1969, 223, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.G.; Hinchee, M.; Horsch, R. Cell and tissue specific expression localized by in situ RNA hybridisation in floral tissues. Plant Mol. Rep. 1987, 5, 237–241. [Google Scholar] [CrossRef]
- Singer, R.; Ward, D. Actin gene expression visualized in chicken muscle tissue culture by using in situ hybridisation with a biotinated nucleotide analog. Proc. Natl. Acad. Sci. USA 1982, 79, 7331–7335. [Google Scholar] [CrossRef]
- Brahic, M.; Haase, A.T. Detection of viral sequences of low reiteration frequency by in situ hybridisation. Proc. Natl. Acad. Sci. USA 1979, 75, 6125–6129. [Google Scholar] [CrossRef] [PubMed]
- Meyerowitz, E. In situ hybridisation to RNA in plant tissue. Plant Mol. Rep. 1987, 5, 242–250. [Google Scholar] [CrossRef]
- Jackson, D. In-situ hybridisation in plants. Mol. Plant Pathol. 1992, 1, 163–174. [Google Scholar]
- Wu, M.; Wagner, D. RNA in situ hybridisation in Arabidopsis. Methods Mol. Biol. 2012, 883, 75–86. [Google Scholar] [CrossRef]
- Ding, L.; Yan, S.; Jiang, L.; Liu, M.; Zhang, J.; Zhao, J.; Zhao, W.; Han, Y.; Wang, Q.; Zhang, X. HANABA TARANU regulates the shoot apical meristem and leaf development in cucumber (Cucumis sativus L.). J. Exp. Bot. 2015, 66, 7075–7087. [Google Scholar] [CrossRef]
- Wu, X.; McSteen, P. The role of auxin transport during inflorescence development in maize (Zea mays, Poaceae). Am. J. Bot. 2007, 94, 1745–1755. [Google Scholar] [CrossRef]
- Cornish, E.; Pettitt, J.; Bonig, I.; Clarke, A. Developmentally controlled expression of a gene associated with self-incompatibility in Nicotiana alata. Nature 1987, 326, 99–102. [Google Scholar] [CrossRef]
- Minsung, K.; Thinh, P.; Ashley, H.; Sheila, M.; Robert, K.; Neelima, S. Reduced leaf complexity in tomato wiry mutants suggests a role for PHAN and KNOX genes in generating compound leaves. Development 2003, 130, 4405–4415. [Google Scholar] [CrossRef]
- Li, M.; Tang, D.; Wang, K.; Wu, X.; Lu, L.; Yu, H.; Gu, M.; Yan, C.; Cheng, Z. Mutations in the F-box gene LARGER PANICLE improve the panicle architecture and enhance the grain yield in rice. Plant Biotech. J. 2011, 9, 1002–1013. [Google Scholar] [CrossRef] [PubMed]
- Salemme, M.; Sica, M.; Iazzetti, G.; Gaudio, L.; Aceto, S. The AP2-Like gene OitaAP2 is alternatively spliced and differentially expressed in inflorescence and vegetative tissues of the Orchid Orchis italica. PLoS ONE. 2013, 8, e77454. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gambino, G.; Minuto, M.; Boccacci, P.; Perrone, I.; Vallania, R.; Gribaudo, I. Characterization of expression dynamics of WOX homeodomain transcription factors during somaticembryogenesis in Vitis vinifera. J. Exp. Bot. 2011, 62, 1089–1101. [Google Scholar] [CrossRef]
- Malabarba, J.; Buffon, V.; Mariath, J.; Gaeta, M.; Dornelas, M.; Margis-Pinheiro, M.; Pasquali, G.; Revers, L. The MADS-box gene Agamous-like 11 is essential for seed morphogenesis in grapevine. J. Exp. Bot. 2017, 68, 1493–1506. [Google Scholar] [CrossRef]
- Amato, A.; Cardone, M.; Ocarez, N.; Alagna, F.; Ruperti, B.; Fattorini, C.; Velasco, R.; Mejía, N.; Zenoni, S.; Bergamini, C. VviAGL11 self-regulates and targets hormone- andsecondary metabolism-related genes during seeddevelopment. Hortic. Res. 2022, 9, uhac133. [Google Scholar] [CrossRef]
- Wang, L.; Yin, X.; Cheng, C.; Wang, H.; Guo, R.; Xu, X.; Zhao, J.; Zheng, Y.; Wang, X. Evolutionary and expression analysis of a MADS-box gene superfamily involved in ovule development of seeded and seedless grapevines. Mol. Genet. Genom. 2015, 290, 825–846. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, S.; Li, X.; Zhang, X.; Wang, X.; Wang, L.; Li, Z.; Wang, X. A MADS-box transcription factor from grapevine, VvMADS45, influences seed development. Plant Cell Tissue Organ Cult. (PCTOC) 2020, 141, 105–118. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, Y.; Yao, J.; Zhang, S.; Wang, L.; Guo, C.; Steve, N.; Wang, X. Genome-wide identification and expression analyses of the homeobox transcription factor family during ovule development in seedless and seeded grapes. Sci. Rep. 2017, 7, 12638. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, L.; Sun, X.; Li, Y.; Yao, J.; Steve, N.; Wang, X. Genome-Wide Analysis of the YABBY Gene Family in Grapevine and Functional Characterization of VvYABBY4. Front. Plant Sci. 2019, 10, 1207. [Google Scholar] [CrossRef] [PubMed]
- Diakou, P.; Carde, J.P. In situ fixation of grape berries. Protoplasma 2001, 218, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Coito, J.; Silva, H.; Ramos, M.; Montez, M.; Cunha, J.; Amâncio, S.; Costa, M.; Rocheta, M. Vitis Flower Sex Specification Acts Downstream and Independently of the ABCDE Model Genes. Front. Plant Sci. 2018, 9, 1029–1048. [Google Scholar] [CrossRef] [PubMed]
- Fouquet, R.; Léon, C.; Ollat, N.; Barrieu, F. Identification of grapevine aquaporins and expression analysis in developing berries. Plant Cell Rep. 2008, 27, 1541–1550. [Google Scholar] [CrossRef] [PubMed]
- Villette, J.; Cuellar, T.; Zimmermann, S.D.; Verdeil, J.L.; Gaillard, I. Unique features of the grapevine VvK5.1 channel support novel functions for outward K+ channels in plants. J. Exp. Bot. 2019, 70, 6181–6193. [Google Scholar] [CrossRef]
- Li, C.; Hu, S.; Lei, Q.; Wang, C.; Yang, Y.; Yang, Y.P.; Sun, X. Establishment and optimization of mRNA in situ hybridisation system in turnip (Brassica rapa var. rapa). Plant Methods 2019, 15, 115. [Google Scholar] [CrossRef]
- Venglat, S.P.; Dumonceaux, T.; Rozwadowski, K.; Parnell, L.; Babic, V.; Keller, W.; Martienssen, R.; Selvaraj, G.; Datla, R. The homeobox gene BREVIPEDICELLUS is a key regulator of inflorescence architecture in Arabidopsis. Proc. Natl. Acad. Sci. USA 2002, 99, 4730–4735. [Google Scholar] [CrossRef]
- Wang, L.; Hu, X.; Jiao, C.; Li, Z.; Fei, Z.; Yan, X.; Liu, C.; Wang, Y.; Wang, X. Transcriptome analyses of seed development in grape hybrids reveals a possible mechanism influencing seed size. BMC Genom. 2016, 17, 898. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, Z. Fluorescence in situ hybridisation on Rice Chromosomes. Plant Cell Div. 2016, 1370, 105–112. [Google Scholar] [CrossRef]
- Hou, A.; Peffley, E.B. Recombinant chromosomes of advanced backcross plants between Allium cepa L. and A. fistulosum L. revealed by in situ hybridisation. Theor. Appl. Genet. 2000, 100, 1190–1196. [Google Scholar] [CrossRef]
- Kitazawa, S.; Kitazawa, R.; Maeda, S. In situhybridisation with polymerase chain reaction-derivedsingle-stranded DNA probe and S1 nuclease. Histochem. Cell Biol. 1999, 111, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Erdtmann-Vourliotis, M.; Peter, M.; Riechert, U.; Handel, M.; Kriebitzsch, J.; Hollt, V. Rational design of oligonucleotide probes to avoid optimization steps in in situ hybridisation. Brain Res. Protoc. 1999, 4, 82–91. [Google Scholar] [CrossRef]
- Fleming, K.; Evans, M.; Ryley, K.; Franklin, D.; Lovell-Badge, R.; Morey, A. Optimization of non-isotopic in situ hybridisation on formalin-fixed, paraffin-embedded material using digoxigenin-labelled probes and transgenic tissues. J. Pathol. 1992, 167, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wanner, I.B.; Roper, S.D.; Chaudhari, N. An Optimized Method for in situ hybridisation with Signal Amplification That Allows the Detection of Rare mRNAs. J. Histochem. Cytochem. 1999, 47, 431–445. [Google Scholar] [CrossRef] [PubMed]
- Guiot, Y. The effects of varying key steps in the non-radioactive in situ hybridisation protocol: A quantitative study. Histochem. J. 1995, 27, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Holland, M.S.; Mackenzie, C.D.; Bull, R.W.; Silva, R.F. A comparative study of histological conditions suitable for both immunofluorescence and in situ hybridisation in the detection of herpesvirus and its antigens in chicken tissues. Histochem. J. 1996, 44, 259–265. [Google Scholar] [CrossRef]
- Kuchen, S.; Seemayer, C.A.; Neidhart, M.; Gay, R.; Gay, S. In situ hybridisation of synovial tissue. Methods Mol. Med. 2007, 135, 65–75. [Google Scholar] [PubMed]
- Jaillon, O.; Aury, J.; Noel, B.; Policriti, A.; Clepet, C.; Casagrande, A.; Choisne, N.; Aubourg, S.; Vitulo, N.; Jubin, C.; et al. The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature 2007, 449, 463–467. [Google Scholar] [CrossRef]
- Drews, G.N.; Bowman, J.L.; Meyerowitz, E.M. Negative regulation of the Arabidopsis homeotic gene AGAMOUS by the APETALA2 product. Cell 1991, 6, 991–1002. [Google Scholar] [CrossRef]


| Gene | Forward Primer Sequence | Reverse Primer Sequence |
|---|---|---|
| VvTAU | 5′ ATTTTCATGGAGTTTTTCAGCAGAT 3′ | 5′ TGTACAGCATTCACATCTCATCTTA 3′ |
| VvHB63 | 5′ TGAACAAGCAGCAGGGAGGAATAG3′ | 5′ ATATTACACATGCTAGGAATTCCAA 3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, J.; Li, X.; Wu, N.; Zhang, S.; Gao, M.; Wang, X. Improvement of RNA In Situ Hybridisation for Grapevine Fruits and Ovules. Int. J. Mol. Sci. 2023, 24, 800. https://doi.org/10.3390/ijms24010800
Yao J, Li X, Wu N, Zhang S, Gao M, Wang X. Improvement of RNA In Situ Hybridisation for Grapevine Fruits and Ovules. International Journal of Molecular Sciences. 2023; 24(1):800. https://doi.org/10.3390/ijms24010800
Chicago/Turabian StyleYao, Jin, Xingmei Li, Na Wu, Songlin Zhang, Min Gao, and Xiping Wang. 2023. "Improvement of RNA In Situ Hybridisation for Grapevine Fruits and Ovules" International Journal of Molecular Sciences 24, no. 1: 800. https://doi.org/10.3390/ijms24010800
APA StyleYao, J., Li, X., Wu, N., Zhang, S., Gao, M., & Wang, X. (2023). Improvement of RNA In Situ Hybridisation for Grapevine Fruits and Ovules. International Journal of Molecular Sciences, 24(1), 800. https://doi.org/10.3390/ijms24010800






