Advanced Gene Therapy Strategies for the Repair of ACL Injuries
Abstract
1. Introduction
2. ACL: Basic Science, Clinical Questions
2.1. ACL Function and Structure
2.2. Clinical Aspects: Pathology, Natural Healing, and Current Treatments
2.3. Experimental Treatments: Tissue Engineering and Biological Augmentation
3. Classical Gene Therapy for the Repair of ACL Injuries
3.1. Gene Transfer Vectors
3.2. Candidate Therapeutic Factors
3.3. Applications of Classical Gene Therapy for ACL Repair
3.4. Limitations of Classical Gene Therapy for ACL Repair
4. Biomaterial-Guided Gene Therapy for the Repair of ACL Injuries
4.1. Principles
4.2. Applications of Biomaterial-Guided Gene Therapy for ACL Repair
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaeding, C.C.; Léger-St-Jean, B.; Magnussen, R.A. Epidemiology and Diagnosis of Anterior Cruciate Ligament Injuries. Clin. Sport. Med. 2016, 36, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kiapour, A.M.; Murray, M.M. Basic science of anterior cruciate ligament injury and repair. Bone Jt. Res. 2014, 3, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Prodromos, C.C.; Han, Y.; Rogowski, J.; Joyce, B.; Shi, K. A Meta-analysis of the Incidence of Anterior Cruciate Ligament Tears as a Function of Gender, Sport, and a Knee Injury–Reduction Regimen. Arthrosc. J. Arthrosc. Relat. Surg. 2007, 23, 1320–1325.e6. [Google Scholar] [CrossRef] [PubMed]
- Fetto, J.F.; Marshall, J.L. The natural history and diagnosis of anterior cruciate ligament insufficiency. Clin. Orthop. Relat. Res. 1980, 147, 29–38. [Google Scholar] [CrossRef]
- Murray, M.M.; Martin, S.D.; Martin, T.L.; Spector, M. Histological Changes in the Human Anterior Cruciate Ligament After Rupture. J. Bone Jt. Surg. 2000, 82, 1387–1397. [Google Scholar] [CrossRef]
- Woo, S.L.-Y.; Vogrin, T.M.; Abramowitch, S.D. Healing and Repair of Ligament Injuries in the Knee. J. Am. Acad. Orthop. Surg. 2000, 8, 364–372. [Google Scholar] [CrossRef]
- Duthon, V.L.A.; Barea, C.; Abrassart, S.; Fasel, J.H.; Fritschy, D.; Menetrey, J. Anatomy of the anterior cruciate ligament. Knee Surg. Sport. Traumatol. Arthrosc. 2006, 14, 204–213. [Google Scholar] [CrossRef]
- Murray, M.M. Current Status and Potential of Primary ACL Repair. Clin. Sport. Med. 2009, 28, 51–61. [Google Scholar] [CrossRef]
- Jung, H.-J.; Fisher, M.B.; Woo, S.L.-Y. Role of biomechanics in the understanding of normal, injured, and healing ligaments and tendons. BMC Sport. Sci. Med. Rehabil. 2009, 1, 9–17. [Google Scholar] [CrossRef]
- Murray, M.M.; Fleming, B.C. Biology of anterior cruciate ligament injury and repair: Kappa delta ann doner vaughn award paper 2013. J. Orthop. Res. 2013, 31, 1501–1506. [Google Scholar] [CrossRef]
- Yang, G.; Rothrauff, B.B.; Tuan, R.S. Tendon and ligament regeneration and repair: Clinical relevance and developmental paradigm. Birth Defects Res. Part C Embryo Today Rev. 2013, 99, 203–222. [Google Scholar] [CrossRef]
- Kannus, P.; Jarvinen, M. Nonoperative treatment of acute knee ligament injuries. A review with special reference to indications and methods. Sports Med. 1990, 9, 244–260. [Google Scholar] [CrossRef]
- Jokl, P.; Kaplan, N.; Stovell, P.; Keggi, K. Non-operative treatment of severe injuries to the medial and anterior cruciate ligaments of the knee. J. Bone Jt. Surg. 1984, 66, 741–744. [Google Scholar] [CrossRef]
- Buss, D.D.; Min, R.; Skyhar, M.; Galinat, B.; Warren, R.F.; Wickiewicz, T.L. Nonoperative Treatment of Acute Anterior Cruciate Ligament Injuries in a Selected Group of Patients. Am. J. Sport. Med. 1995, 23, 160–165. [Google Scholar] [CrossRef]
- Ciccotti, M.G.; Lombardo, S.J.; Nonweiler, B.; Pink, M. Non-operative treatment of ruptures of the anterior cruciate ligament in middle-aged patients. Results after long-term follow-up. J. Bone Jt. Surg. 1994, 76, 1315–1321. [Google Scholar] [CrossRef]
- Maffulli, N. Rehabilitation of an anterior cruciate ligament. Clin. Orthop. Relat. Res. 1997, 343, 253–255. [Google Scholar] [CrossRef]
- Frank, C.B.; Jackson, D.W. Current Concepts Review—The Science of Reconstruction of the Anterior Cruciate Ligament. J. Bone Jt. Surg. 1997, 79, 1556–1576. [Google Scholar] [CrossRef]
- Fu, F.H.; Bennett, C.H.; Lattermann, C.; Ma, C.B. Current trends in anterior cruciate ligament reconstruction. Part I. Biology and biomechanics of reconstruction. Am. J. Sport. Med. 1999, 27, 821–830. [Google Scholar] [CrossRef]
- Fu, F.H.; Bennett, C.H.; Ma, C.B.; Menetrey, J.; Lattermann, C. Current trends in anterior cruciate ligament reconstruction. Part II. Operative procedures and clinical correlations. Am. J. Sport. Med. 2000, 28, 124–130. [Google Scholar] [CrossRef]
- Andersson, C.; Odensten, M.; Gillquist, J. Knee function after surgical or nonsurgical treatment of acute rupture of the anterior cruciate ligament: A randomized study with a long-term follow-up period. Clin. Orthop. Relat. Res. 1991, 264, 255–263. [Google Scholar] [CrossRef]
- Delincé, P.; Ghafil, D. Anterior cruciate ligament tears: Conservative or surgical treatment? A critical review of the literature. Knee Surg. Sport. Traumatol. Arthrosc. 2012, 20, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Paschos, N.K.; Howell, S.M. Anterior cruciate ligament reconstruction: Principles of treatment. EFORT Open Rev. 2016, 1, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Noyes, F.R.; Butler, D.L.; Grood, E.S.; Zernicke, R.F.; Hefzy, M.S. Biomechanical analysis of human ligament grafts used in knee-ligament repairs and reconstructions. J. Bone Jt. Surg. 1984, 66, 344–352. [Google Scholar] [CrossRef]
- Silver, F.H.; Tria, A.J.; Zawadsky, J.P.; Dunn, M. Anterior cruciate ligament replacement: A review. J. Long-Term Eff. Med. Implant. 1991, 1, 135–154. [Google Scholar]
- Chang, S.K.; Egami, D.K.; Shaieb, M.D.; Kan, D.M.; Richardson, A.B. Anterior cruciate ligament reconstruction: Allograft versus autograft. Arthrosc. J. Arthrosc. Relat. Surg. 2003, 19, 453–462. [Google Scholar] [CrossRef]
- Mascarenhas, R.; MacDonald, P.B. Anterior cruciate ligament reconstruction: A look at prosthetics--past, present and possible future. Mcgill. J. Med. 2008, 11, 29–37. [Google Scholar] [CrossRef]
- Chen, J.; Xu, J.; Wang, A.; Zheng, M. Scaffolds for tendon and ligament repair: Review of the efficacy of commercial products. Expert Rev. Med. Devices 2009, 6, 61–73. [Google Scholar] [CrossRef]
- Legnani, C.; Ventura, A.; Terzaghi, C.; Borgo, E.; Albisetti, W. Anterior cruciate ligament reconstruction with synthetic grafts. A review of literature. Int. Orthop. 2010, 34, 465–471. [Google Scholar] [CrossRef]
- Viateau, V.; Manassero, M.; Anagnostou, F.; Guérard, S.; Mitton, D.; Migonney, V. Biological and Biomechanical Evaluation of the Ligament Advanced Reinforcement System (LARS AC) in a Sheep Model of Anterior Cruciate Ligament Replacement: A 3-Month and 12-Month Study. Arthrosc. J. Arthrosc. Relat. Surg. 2013, 29, 1079–1088. [Google Scholar] [CrossRef]
- Ritchie, J.R.; Parker, R.D. Graft Selection in Anterior Cruciate Ligament Revision Surgery. Clin. Orthop. Relat. Res. 1996, 325, 65–77. [Google Scholar] [CrossRef]
- Aglietti, P.; Buzzi, R.; Giron, F.; Simeone, A.J.V.; Zaccherotti, G. Arthroscopic-assisted anterior cruciate ligament reconstruction with the central third patellar tendon. Knee Surg. Sport. Traumatol. Arthrosc. 1997, 5, 138–144. [Google Scholar] [CrossRef]
- Bach, B.R.; Tradonsky, S.; Bojchuk, J.; Levy, M.E.; Bush-Joseph, C.A.; Khan, N.H. Arthroscopically Assisted Anterior Cruciate Ligament Reconstruction Using Patellar Tendon Autograft. Am. J. Sport. Med. 1998, 26, 20–29. [Google Scholar] [CrossRef]
- Jomha, N.M.; Borton, D.C.; Clingeleffer, A.J.; Pinczewski, L. Long Term Osteoarthritic Changes in Anterior Cruciate Ligament Reconstructed Knees. Clin. Orthop. Relat. Res. 1999, 358, 188–193. [Google Scholar] [CrossRef]
- Jomha, N.M.; A Pinczewski, L.; Clingeleffer, A.; Otto, D.D. Arthroscopic reconstruction of the anterior cruciate ligament with patellar-tendon autograft and interference screw fixation. The results at seven years. J. Bone Jt. Surg. Br. 1999, 81, 775–779. [Google Scholar] [CrossRef]
- Segawa, H.; Omori, G.; Koga, Y. Long-term results of non-operative treatment of anterior cruciate ligament injury. Knee 2001, 8, 5–11. [Google Scholar] [CrossRef]
- Anderson, A.F.; Snyder, R.B.; Lipscomb, A.B., Jr. Anterior cruciate ligament reconstruction. A prospective randomized study of three surgical methods. Am. J. Sports Med. 2001, 29, 272–279. [Google Scholar] [CrossRef]
- Weitzel, P.P.; Richmond, J.C.; Altman, G.H.; Calabro, T.; Kaplan, D.L. Future direction of the treatment of ACL ruptures. Orthop. Clin. N. Am. 2002, 33, 653–661. [Google Scholar] [CrossRef]
- Von Porat, A.; Roos, E.; Roos, H. High prevalence of osteoarthritis 14 years after an anterior cruciate ligament tear in male soccer players: A study of radiographic and patient relevant outcomes. Ann. Rheum. Dis. 2004, 63, 269–273. [Google Scholar] [CrossRef]
- Kaeding, C.C.; Aros, B.; Pedroza, A.; Pifel, E.; Amendola, A.; Andrish, J.T.; Dunn, W.R.; Marx, R.G.; McCarty, E.C.; Parker, R.D.; et al. Allograft versus autograft anterior cruciate ligament reconstruction: Predictors of failure from a MOON prospective longitudinal cohort. Sports Health 2011, 3, 73–81. [Google Scholar] [CrossRef]
- Paterno, M.V.; Rauh, M.J.; Schmitt, L.C.; Ford, K.; Hewett, T.E. Incidence of Second ACL Injuries 2 Years After Primary ACL Reconstruction and Return to Sport. Am. J. Sport. Med. 2014, 42, 1567–1573. [Google Scholar] [CrossRef]
- Ahmed, I.; Salmon, L.; Roe, J.; Pinczewski, L. The long-term clinical and radiological outcomes in patients who suffer recurrent injuries to the anterior cruciate ligament after reconstruction. Bone Jt. J. 2017, 99-B, 337–343. [Google Scholar] [CrossRef]
- Laurencin, C.; Attawia, M.; Botchwey, E.; Warren, R.; Attia, E. Cell-material systems for anterior cruciate ligament regeneration. In Vitro Cell Dev. Biol. Anim. 1998, 34, 90–92. [Google Scholar] [CrossRef]
- Laurencin, C.T.; Ambrosio, A.M.A.; Borden, M.D.; Cooper, J.A. Tissue Engineering: Orthopedic Applications. Annu. Rev. Biomed. Eng. 1999, 1, 19–46. [Google Scholar] [CrossRef]
- Altman, G.H.; Horan, R.L.; Martin, I.; Farhadi, J.; Stark, P.R.H.; Volloch, V.; Richmond, J.C.; Vunjak-Novakovic, G.; Kaplan, D.L. Cell differentiation by mechanical stress. FASEB J. 2002, 16, 270–272. [Google Scholar] [CrossRef]
- Vunjak-Novakovic, G.; Altman, G.; Horan, R.; Kaplan, D.L. Tissue Engineering of Ligaments. Annu. Rev. Biomed. Eng. 2004, 6, 131–156. [Google Scholar] [CrossRef]
- Laurencin, C.T.; Freeman, J.W. Ligament tissue engineering: An evolutionary materials science approach. Biomaterials 2005, 26, 7530–7536. [Google Scholar] [CrossRef]
- Petrigliano, F.A.; McAllister, D.R.; Wu, B.M. Tissue Engineering for Anterior Cruciate Ligament Reconstruction: A Review of Current Strategies. Arthrosc. J. Arthrosc. Relat. Surg. 2006, 22, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Angel, M.J.; Sgaglione, N.A.; Grande, D.A. Clinical applications of bioactive factors in sports medicine: Current concepts and future trends. Sports Med. Arthrosc. Rev. 2006, 14, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.L.Y.; Fisher, M.B.; Feola, A. Contribution of biomechanics to management of ligament and tendon injuries. Mol. Cell Biomech. 2008, 5, 49–68. [Google Scholar] [PubMed]
- Kahn, C.J.; Vaquette, C.; Rahouadj, R.; Wang, X. A novel bioreactor for ligament tissue engineering. Biomed. Mater. Eng. 2008, 18, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Benhardt, H.A.; Cosgriff-Hernandez, E.M. The Role of Mechanical Loading in Ligament Tissue Engineering. Tissue Eng. Part B Rev. 2009, 15, 467–475. [Google Scholar] [CrossRef]
- Hall, M.P.; Band, P.A.; Meislin, R.J.; Jazrawi, L.M.; Cardone, D.A. Platelet-rich Plasma: Current Concepts and Application in Sports Medicine. J. Am. Acad. Orthop. Surg. 2009, 17, 602–608. [Google Scholar] [CrossRef]
- Taylor, D.W.; Petrera, M.; Hendry, M.; Theodoropoulos, J. A Systematic Review of the Use of Platelet-Rich Plasma in Sports Medicine as a New Treatment for Tendon and Ligament Injuries. Clin. J. Sport Med. 2011, 21, 344–352. [Google Scholar] [CrossRef]
- Rodrigues, M.T.; Reis, R.L.; Gomes, M.E. Engineering tendon and ligament tissues: Present developments towards successful clinical products. J. Tissue Eng. Regen. Med. 2013, 7, 673–686. [Google Scholar] [CrossRef]
- Wang, T.; Gardiner, B.S.; Lin, Z.; Rubenson, J.; Kirk, T.B.; Wang, A.; Xu, J.; Smith, D.W.; Lloyd, D.G.; Zheng, M.H. Bioreactor Design for Tendon/Ligament Engineering. Tissue Eng. Part B Rev. 2013, 19, 133–146. [Google Scholar] [CrossRef]
- Leong, N.L.; Petrigliano, F.A.; McAllister, D.R. Current tissue engineering strategies in anterior cruciate ligament reconstruction. J. Biomed. Mater. Res. Part A 2014, 102, 1614–1624. [Google Scholar] [CrossRef]
- Yuan, T.; Zhang, C.-Q.; Wang, J.H.-C. Augmenting tendon and ligament repair with platelet-rich plasma (PRP). Muscle Ligaments Tendons J. 2013, 3, 139–149. [Google Scholar] [CrossRef]
- Moraes, V.Y.; Lenza, M.; Tamaoki, M.J.S.; Faloppa, F.; Belloti, J.C. Platelet-rich therapies for musculoskeletal soft tissue injuries. Cochrane Database Syst. Rev. 2014, 2014, CD010071. [Google Scholar] [CrossRef]
- Hogan, M.; Kawakami, Y.; Murawski, C.D.; Fu, F.H. Tissue Engineering of Ligaments for Reconstructive Surgery. Arthrosc. J. Arthrosc. Relat. Surg. 2015, 31, 971–979. [Google Scholar] [CrossRef]
- Nau, T.; Teuschl, A. Regeneration of the anterior cruciate ligament: Current strategies in tissue engineering. World J. Orthop. 2015, 6, 127–136. [Google Scholar] [CrossRef]
- Andriolo, L.; Di Matteo, B.; Kon, E.; Filardo, G.; Venieri, G.; Marcacci, M. PRP Augmentation for ACL Reconstruction. BioMed Res. Int. 2015, 2015, 371746. [Google Scholar] [CrossRef] [PubMed]
- Mace, J.; Wheelton, A.; Khan, W.S.; Anand, S. The Role of Bioreactors in Ligament and Tendon Tissue Engineering. Curr. Stem Cell Res. Ther. 2016, 11, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Negahi Shirazi, A.; Chrzanowski, W.; Khademhosseini, A.; Dehghani, F. Anterior cruciate ligament structure, injuries and regenerative treatments. Adv. Exp. Med. Biol. 2015, 881, 161–186. [Google Scholar] [PubMed]
- Hexter, A.T.; Thangarajah, T.; Blunn, G.; Haddad, F.S. Biological augmentation of graft healing in anterior cruciate ligament reconstruction: A systematic review. Bone Jt. J. 2018, 100-B, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Chahla, J.; Kennedy, M.I.; Aman, Z.S.; LaPrade, R.F. Ortho-Biologics for Ligament Repair and Reconstruction. Clin. Sport. Med. 2019, 38, 97–107. [Google Scholar] [CrossRef]
- Riediger, M.D.; Stride, D.; Coke, S.E.; Kurz, A.Z.; Duong, A.; Ayeni, O.R. ACL Reconstruction with Augmentation: A Scoping Review. Curr. Rev. Musculoskelet. Med. 2019, 12, 166–172. [Google Scholar] [CrossRef]
- Hevesi, M.; LaPrade, M.; Saris, D.B.F.; Krych, A.J. Stem Cell Treatment for Ligament Repair and Reconstruction. Curr. Rev. Musculoskelet. Med. 2019, 12, 446–450. [Google Scholar] [CrossRef]
- Lim, W.L.; Liau, L.L.; Ng, M.H.; Chowdhury, S.R.; Law, J.X. Current Progress in Tendon and Ligament Tissue Engineering. Tissue Eng. Regen. Med. 2019, 16, 549–571. [Google Scholar] [CrossRef]
- Uchida, R.; Jacob, G.; Shimomura, K.; Horibe, S.; Nakamura, N. Biological Augmentation of ACL Repair and Reconstruction: Current Status and Future Perspective. Sport. Med. Arthrosc. Rev. 2020, 28, 49–55. [Google Scholar] [CrossRef]
- Looney, A.M.; Leider, J.D.; Horn, A.R.; Bodendorfer, B.M. Bioaugmentation in the surgical treatment of anterior cruciate ligament injuries: A review of current concepts and emerging techniques. SAGE Open Med. 2020, 8, 2050312120921057. [Google Scholar] [CrossRef]
- Kon, E.; Di Matteo, B.; Altomare, D.; Iacono, F.; Kurpyakov, A.; Lychagin, A.; Timashev, P.; Kalinsky, E.; Lipina, M. Biologic agents to optimize outcomes following ACL repair and reconstruction: A systematic review of clinical evidence. J. Orthop. Res. 2022, 40, 10–28. [Google Scholar] [CrossRef]
- Su, C.A.; Jildeh, T.R.; Vopat, M.L.; Waltz, R.A.; Millett, P.J.; Provencher, M.T.; Philippon, M.J.; Huard, J. Current State of Platelet-Rich Plasma and Cell-Based Therapies for the Treatment of Osteoarthritis and Tendon and Ligament Injuries. J. Bone Jt. Surg. 2022, 104, 1406–1414. [Google Scholar] [CrossRef]
- Gerich, T.G.; Fu, F.H.; Robbins, P.D.; Evans, C.H. Prospects for gene therapy in sports medicine. Knee Surg. Sport. Traumatol. Arthrosc. 1996, 4, 180–187. [Google Scholar] [CrossRef]
- Evans, C.H.; Ghivizzani, S.C.; Robbins, P.D. Potential Applications of Gene Therapy in Sports Medicine. Phys. Med. Rehabil. Clin. North Am. 2000, 11, 405–416. [Google Scholar] [CrossRef]
- Wu, D.; Razzano, P.; Grande, D.A. Gene therapy and tissue engineering in repair of the musculoskeletal system. J. Cell. Biochem. 2003, 88, 467–481. [Google Scholar] [CrossRef]
- Huard, J.; Li, Y.; Peng, H.; Fu, F.H. Gene therapy and tissue engineering for sports medicine. J. Gene Med. 2003, 5, 93–108. [Google Scholar] [CrossRef]
- Hildebrand, K.; Frank, C.; Hart, D. Gene intervention in ligament and tendon: Current status, challenges, future directions. Gene Ther. 2004, 11, 368–378. [Google Scholar] [CrossRef]
- A Eming, S.; Krieg, T.; Davidson, J.M. Gene transfer in tissue repair: Status, challenges and future directions. Expert Opin. Biol. Ther. 2004, 4, 1373–1386. [Google Scholar] [CrossRef]
- Kofron, M.D.; Laurencin, C.T. Orthopaedic applications of gene therapy. Curr. Gene Ther. 2005, 5, 37–61. [Google Scholar] [CrossRef]
- Nixon, A.J.; Goodrich, L.R.; Scimeca, M.S.; Witte, T.; Schnabel, L.V.; Watts, A.E.; Robbins, P.D. Gene Therapy in Musculoskeletal Repair. Ann. N. Y. Acad. Sci. 2007, 1117, 310–327. [Google Scholar] [CrossRef]
- Docheva, D.; Müller, S.A.; Majewski, M.; Evans, C.H. Biologics for tendon repair. Adv. Drug Deliv. Rev. 2015, 84, 222–239. [Google Scholar] [CrossRef] [PubMed]
- Rey-Rico, A.; Cucchiarini, M. Controlled release strategies for rAAV-mediated gene delivery. Acta Biomater. 2016, 29, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cucchiarini, M. Human gene therapy: Novel approaches to improve the current gene delivery systems. Discov. Med. 2016, 21, 495–506. [Google Scholar] [PubMed]
- Dunbar, C.E.; High, K.A.; Joung, J.K.; Kohn, D.B.; Ozawa, K.; Sadelain, M. Gene therapy comes of age. Science 2018, 359, eaan4672. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.K.; Falentin-Daudré, C.; Leroux, A.; Migonney, V.; Cucchiarini, M. Controlled release of gene therapy constructs from solid scaffolds for therapeutic applications in orthopedics. Discov. Med. 2018, 25, 195–203. [Google Scholar] [PubMed]
- Balmayor, E.R.; Evans, C.H. RNA Therapeutics for Tissue Engineering. Tissue Eng. Part A 2019, 25, 9–11. [Google Scholar] [CrossRef]
- Cucchiarini, M.; Madry, H. Biomaterial-guided delivery of gene vectors for targeted articular cartilage repair. Nat. Rev. Rheumatol. 2019, 15, 18–29. [Google Scholar] [CrossRef]
- Evans, C.; De la Vega, R.E.; Evans, C.H.; van Griensven, M.; Balmayor, E.R. Healing with RNA. Injury 2019, 50, 625–626. [Google Scholar] [CrossRef]
- High, K.A.; Roncarolo, M.G. Gene therapy. N. Engl. J. Med. 2019, 381, 455–464. [Google Scholar] [CrossRef]
- Venkatesan, J.K.; Rey-Rico, A.; Cucchiarini, M. Current Trends in Viral Gene Therapy for Human Orthopaedic Regenerative Medicine. Tissue Eng. Regen. Med. 2019, 16, 345–355. [Google Scholar] [CrossRef]
- Giordano, L.; Della Porta, G.; Peretti, G.M.; Maffulli, N. Therapeutic potential of microRNA in tendon injuries. Br. Med. Bull. 2020, 133, 79–94. [Google Scholar] [CrossRef]
- Evans, C.H.; Ghivizzani, S.C.; Robbins, P.D. Orthopaedic gene therapy: Twenty-five years on. JBJS Rev. 2021, 9, e20. [Google Scholar] [CrossRef]
- Balmayor, E.R. Synthetic mRNA—Emerging new class of drug for tissue regeneration. Curr. Opin. Biotechnol. 2022, 74, 8–14. [Google Scholar] [CrossRef]
- Lechardeur, D.; Lukacs, G.L. Intracellular barriers to non-viral gene transfer. Curr. Gene Ther. 2002, 2, 183–194. [Google Scholar] [CrossRef]
- Huang, H.-Y.; Huang, Y.-Y. Intracellular Trafficking, Metabolism and Toxicity of Current Gene Carriers. Curr. Drug Metab. 2009, 10, 885–894. [Google Scholar]
- Kay, M.A. State-of-the-art gene-based therapies: The road ahead. Nat. Rev. Genet. 2011, 12, 316–328. [Google Scholar] [CrossRef]
- Kaufmann, K.B.; Büning, H.; Galy, A.; Schambach, A.; Grez, M. Gene therapy on the move. EMBO Mol. Med. 2013, 5, 1642–1661. [Google Scholar] [CrossRef]
- Shirley, J.L.; de Jong, Y.P.; Terhorst, C.; Herzog, R.W. Immune Responses to Viral Gene Therapy Vectors. Mol. Ther. 2020, 28, 709–722. [Google Scholar] [CrossRef]
- Shea, L.D.; Smiley, E.; Bonadio, J.; Mooney, D.J. DNA delivery from polymer matrices for tissue engineering. Nat. Biotechnol. 1999, 17, 551–554. [Google Scholar] [CrossRef]
- Pannier, A.K.; Shea, L.D. Controlled release systems for DNA delivery. Mol. Ther. 2004, 10, 19–26. [Google Scholar] [CrossRef]
- Jang, J.-H.; Schaffer, D.V.; Shea, L.D. Engineering Biomaterial Systems to Enhance Viral Vector Gene Delivery. Mol. Ther. 2011, 19, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Raftery, R.; Walsh, D.; Castaño, I.M.; Heise, A.; Duffy, G.; Cryan, S.-A.; O’Brien, F.J. Delivering Nucleic-Acid Based Nanomedicines on Biomaterial Scaffolds for Orthopedic Tissue Repair: Challenges, Progress and Future Perspectives. Adv. Mater. 2016, 28, 5447–5469. [Google Scholar] [CrossRef] [PubMed]
- Curtin, C.; Castaño, I.M.; O’Brien, F.J. Scaffold-Based microRNA Therapies in Regenerative Medicine and Cancer. Adv. Health Mater. 2018, 7, 1700695. [Google Scholar] [CrossRef]
- Kelly, D.C.; Raftery, R.M.; Curtin, C.M.; O’Driscoll, C.M.; O’Brien, F.J. Scaffold-Based Delivery of Nucleic Acid Therapeutics for Enhanced Bone and Cartilage Repair. J. Orthop. Res. 2019, 37, 1671–1680. [Google Scholar] [CrossRef] [PubMed]
- Arnoczky, S.P. Anatomy of the anterior cruciate ligament. Clin. Orthop. Relat. Res. 1983, 172, 19–25. [Google Scholar] [CrossRef]
- Sakane, M.; Fox, R.J.; Glen, S.L.-Y.W.; Livesay, A.; Li, G.; Fu, F.H. In situ forces in the anterior cruciate ligament and its bundles in response to anterior tibial loads. J. Orthop. Res. 1997, 15, 285–293. [Google Scholar] [CrossRef]
- Beynnon, B.D.; Johnson, R.J.; Fleming, B.C.; Peura, G.D.; Renstrom, P.A.; Nichols, C.E.; Pope, M.H. The Effect of Functional Knee Bracing on the Anterior Cruciate Ligament in the Weightbearing and Nonweightbearing Knee. Am. J. Sport. Med. 1997, 25, 353–359. [Google Scholar] [CrossRef]
- Noyes, F.R.; Grood, E.S. The strength of the anterior cruciate ligament in humans and Rhesus monkeys. J. Bone Jt. Surg. 1976, 58, 1074–1082. [Google Scholar] [CrossRef]
- Guarino, V.; Causa, F.; Ambrosio, L. Bioactive scaffolds for bone and ligament tissue. Expert Rev. Med. Devices 2007, 4, 405–418. [Google Scholar] [CrossRef]
- Gomez-Rodriguez, J.; Readinger, J.A.; Viorritto, I.C.; Mueller, K.L.; Houghtling, R.A.; Schwartzberg, P.L. Tec kinases, actin, and cell adhesion. Immunol. Rev. 2007, 218, 45–64. [Google Scholar] [CrossRef]
- Dourte, L.M.; Kuntz, A.F.; Soslowsky, L.J. Twenty-five years of tendon and ligament research. J. Orthop. Res. 2008, 26, 1297–1305. [Google Scholar] [CrossRef]
- Connizzo, B.K.; Yannascoli, S.M.; Soslowsky, L.J. Structure–function relationships of postnatal tendon development: A parallel to healing. Matrix Biol. 2013, 32, 106–116. [Google Scholar] [CrossRef]
- Cserjesi, P.; Brown, D.; Ligon, K.L.; E Lyons, G.; Copeland, N.G.; Gilbert, D.J.; A Jenkins, N.; Olson, E.N. Scleraxis: A basic helix-loop-helix protein that prefigures skeletal formation during mouse embryogenesis. Development 1995, 121, 1099–1110. [Google Scholar] [CrossRef]
- Schweitzer, R.; Chyung, J.H.; Murtaugh, L.C.; E Brent, A.; Rosen, V.; Olson, E.N.; Lassar, A.; Tabin, C.J. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development 2001, 128, 3855–3866. [Google Scholar] [CrossRef]
- Tozer, S.; Duprez, D. Tendon and ligament: Development, repair and disease. Birth Defects Res. C Embryo. Today 2005, 75, 226–236. [Google Scholar] [CrossRef]
- Anderson, D.M.; Arredondo, J.; Hahn, K.; Valente, G.; Martin, J.F.; Wilson-Rawls, J.; Rawls, A. Mohawk is a novel homeobox gene expressed in the developing mouse embryo. Dev. Dyn. 2006, 235, 792–801. [Google Scholar] [CrossRef]
- Shukunami, C.; Takimoto, A.; Oro, M.; Hiraki, Y. Scleraxis positively regulates the expression of tenomodulin, a differentiation marker of tenocytes. Dev. Biol. 2006, 298, 234–247. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, S.; Zhang, C.; Lu, P.; Hu, J.; Yin, Z.; Ma, Y.; Chen, X.; OuYang, H. Crucial transcription factors in tendon development and differentiation: Their potential for tendon regeneration. Cell Tissue Res. 2014, 356, 287–298. [Google Scholar] [CrossRef]
- Asahara, H.; Inui, M.; Lotz, M.K. Tendons and Ligaments: Connecting Developmental Biology to Musculoskeletal Disease Pathogenesis. J. Bone Miner. Res. 2017, 32, 1773–1782. [Google Scholar] [CrossRef]
- Bobzin, L.; Roberts, R.R.; Chen, H.-J.; Crump, J.G.; Merrill, A.E. Development and maintenance of tendons and ligaments. Development 2021, 148, dev186916. [Google Scholar] [CrossRef]
- Zantop, T.; Petersen, W.; Sekiya, J.K.; Musahl, V.; Fu, F.H. Anterior cruciate ligament anatomy and function relating to anatomical reconstruction. Knee Surg. Sport. Traumatol. Arthrosc. 2006, 14, 982–992. [Google Scholar] [CrossRef] [PubMed]
- Lessim, S.; Migonney, V.; Thoreux, P.; Lutomski, D.; Changotade, S. PolyNaSS bioactivation of LARS artificial ligament promotes human ligament fibroblast colonisation in vitro. Bio-Med. Mater. Eng. 2013, 23, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Brodsky, J.W.; Schneidler, C. Diabetic foot infections. Orthop. Clin. N. Am. 1991, 22, 473–489. [Google Scholar] [CrossRef]
- Carpenter, J.E.; Thomopoulos, S.; Soslowsky, L.J. Animal Models of Tendon and Ligament Injuries for Tissue Engineering Applications. Clin. Orthop. Relat. Res. 1999, 367, S296–S311. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.L.-Y.; Hildebrand, K.; Watanabe, N.; Fenwick, J.A.; Papageorgiou, C.D.; Wang, J.H.-C. Tissue Engineering of Ligament and Tendon Healing. Clin. Orthop. Relat. Res. 1999, 367, S312–S323. [Google Scholar] [CrossRef] [PubMed]
- Goh, J.C.-H.; Ouyang, H.-W.; Teoh, S.-H.; Chan, C.K.; Lee, E.-H. Tissue-Engineering Approach to the Repair and Regeneration of Tendons and Ligaments. Tissue Eng. 2003, 9, 31–44. [Google Scholar] [CrossRef]
- Laurencin, C.T.; Khan, Y.; Kofron, M.; El-Amin, S.; Botchwey, E.; Yu, X.; Cooper, J.A., Jr. The ABJS Nicolas Andry Award: Tissue engineering of bone and ligament: A 15-year perspective. Clin. Orthop. Relat. Res. 2006, 447, 221–236. [Google Scholar]
- Doroski, D.M.; Brink, K.S.; Temenoff, J.S. Techniques for biological characterization of tissue-engineered tendon and ligament. Biomaterials 2007, 28, 187–202. [Google Scholar] [CrossRef]
- Freed, L.E.; Guilak, F.; Guo, X.E.; Gray, M.L.; Tranquillo, R.; Holmes, J.W.; Radisic, M.; Sefton, M.V.; Kaplan, D.; Vunjak-Novakovic, G. Advanced Tools for Tissue Engineering: Scaffolds, Bioreactors, and Signaling. Tissue Eng. 2006, 12, 3285–3305. [Google Scholar] [CrossRef]
- Mikos, A.G.; Herring, S.W.; Ochareon, P.; Elisseeff, J.; Lu, H.H.; Kandel, R.; Schoen, F.J.; Toner, M.; Mooney, D.; Atala, A.; et al. Engineering Complex Tissues. Tissue Eng. 2006, 12, 3307–3339. [Google Scholar] [CrossRef]
- Altman, G.H.; Horan, R.L.; Weitzel, P.; Richmond, J.C. The Use of Long-term Bioresorbable Scaffolds for Anterior Cruciate Ligament Repair. J. Am. Acad. Orthop. Surg. 2008, 16, 177–187. [Google Scholar] [CrossRef]
- Vieira, A.; Guedes, R.; Marques, A. Development of ligament tissue biodegradable devices: A review. J. Biomech. 2009, 42, 2421–2430. [Google Scholar] [CrossRef]
- Vavken, P.; Murray, M.M. Translational studies in anterior cruciate ligament repair. Tissue Eng. Part B Rev. 2010, 16, 5–11. [Google Scholar] [CrossRef]
- Silva, S.S.; Mano, J.F.; Reis, R.L. Potential applications of natural origin polymer-based systems in soft tissue regeneration. Crit. Rev. Biotechnol. 2010, 30, 200–221. [Google Scholar] [CrossRef]
- Atala, A.; Kasper, F.K.; Mikos, A.G. Engineering Complex Tissues. Sci. Transl. Med. 2012, 4, 160rv12. [Google Scholar] [CrossRef]
- Muller, B.; Bowman, K.F.; Bedi, A. ACL Graft Healing and Biologics. Clin. Sport. Med. 2013, 32, 93–109. [Google Scholar] [CrossRef]
- Middleton, K.K.; Barro, V.; Muller, B.; Terada, S.; Fu, F.H. Evaluation of the effects of platelet-rich plasma (PRP) therapy involved in the healing of sports-related soft tissue injuries. Iowa Orthop. J. 2012, 32, 150–163. [Google Scholar]
- Hutchinson, I.D.; Rodeo, S.A.; Perrone, G.S.; Murray, M.M. Can platelet-rich plasma enhance anterior cruciate ligament and meniscal repair? J. Knee Surg. 2015, 28, 19–28. [Google Scholar] [CrossRef]
- Teh, T.K.H.; Goh, J.C.H.; Toh, S.L. Controlled Bioactive Molecules Delivery Strategies for Tendon and Ligament Tissue Engineering using Polymeric Nanofibers. Curr. Pharm. Des. 2015, 21, 1991–2005. [Google Scholar] [CrossRef]
- Mengsteab, P.Y.; Nair, L.S.; Laurencin, C.T. The past, present and future of ligament regenerative engineering. Regen. Med. 2016, 11, 871–881. [Google Scholar] [CrossRef]
- Cengiz, I.F.; Pereira, H.; De Girolamo, L.; Cucchiarini, M.; Espregueira-Mendes, J.; Reis, R.L.; Oliveira, J.M. Orthopaedic regenerative tissue engineering en route to the holy grail: Disequilibrium between the demand and the supply in the operating room. J. Exp. Orthop. 2018, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, P.; Horriat, S.; Anand, B.S. Anterior cruciate ligament repair—Past, present and future. J. Exp. Orthop. 2018, 5, 20. [Google Scholar] [CrossRef] [PubMed]
- Cengiz, I.F.; Pereira, H.; Espregueira-Mendes, J.; Reis, R.L.; Oliveira, J.M. The clinical use of biologics in the knee lesions: Does the patient benefit? Curr. Rev. Musculoskelet. Med. 2019, 12, 406–414. [Google Scholar] [CrossRef] [PubMed]
- No, Y.J.; Castilho, M.; Ramaswamy, Y.; Zreiqat, H. Role of Biomaterials and Controlled Architecture on Tendon/Ligament Repair and Regeneration. Adv. Mater. 2020, 32, e1904511. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, S.; Chen, X. Injectable hydrogels for tendon and ligament tissue engineering. J. Tissue Eng. Regen. Med. 2020, 14, 1333–1348. [Google Scholar] [CrossRef]
- Dyment, N.A.; Barrett, J.G.; Awad, H.A.; Bautista, C.A.; Banes, A.J.; Butler, D.L. A brief history of tendon and ligament bioreactors: Impact and future prospects. J. Orthop. Res. 2020, 38, 2318–2330. [Google Scholar] [CrossRef]
- Rinoldi, C.; Kijeńska-Gawrońska, E.; Khademhosseini, A.; Tamayol, A.; Swieszkowski, W. Fibrous Systems as Potential Solutions for Tendon and Ligament Repair, Healing, and Regeneration. Adv. Health Mater. 2021, 10, e2001305. [Google Scholar] [CrossRef]
- McRobb, J.; Kamil, K.H.; Ahmed, I.; Dhaif, F.; Metcalfe, A. Influence of platelet-rich plasma (PRP) analogues on healing and clinical outcomes following anterior cruciate ligament (ACL) reconstructive surgery: A systematic review. Eur. J. Orthop. Surg. Traumatol. 2022, 1–29. [Google Scholar] [CrossRef]
- Heidari, B.S.; Ruan, R.; Vahabli, E.; Chen, P.; De-Juan-Pardo, E.M.; Zheng, M.; Doyle, B. Natural, synthetic and commercially-available biopolymers used to regenerate tendons and ligaments. Bioact. Mater. 2023, 19, 179–197. [Google Scholar] [CrossRef]
- Hensler, D.; Illingworth, K.D.; Musahl, V.; Working, Z.M.; Kobayashi, T.; Miyawaki, M.; Lorenz, S.; Witt, M.; Irrgang, J.J.; Huard, J.; et al. Does fibrin clot really enhance graft healing after double-bundle ACL reconstruction in a caprine model? Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 669–679. [Google Scholar] [CrossRef]
- Sun, P.; Chen, S.; Liu, L.; Gao, X. Enhancement of Anterior cruciate ligament injury repairing using connective tissue growth factor in a rabbit model. Pak. J. Pharm. Sci. 2018, 31, 2873–2878. [Google Scholar]
- Wiig, M.E.; Amiel, D.; Vandeberg, J.; Kitabayashi, L.; Harwood, F.L.; Arfors, K.E. The early effect of high molecular weight hyaluronan (hyaluronic acid) on anterior cruciate ligament healing: An experimental study in rabbits. J. Orthop. Res. 1990, 8, 425–434. [Google Scholar] [CrossRef]
- Cristino, S.; Grassi, F.; Toneguzzi, S.; Piacentini, A.; Grigolo, B.; Santi, S.; Riccio, M.; Tognana, E.; Facchini, A.; Lisignoli, G. Analysis of mesenchymal stem cells grown on a three-dimensional HYAFF 11®-based prototype ligament scaffold. J. Biomed. Mater. Res. Part A 2005, 73A, 275–283. [Google Scholar] [CrossRef]
- Berry, S.M.; Green, M.H.; Amiel, D. Hyaluronan: A potential carrier for growth factors for the healing of ligamentous tissues. Wound Repair Regen. 1997, 5, 33–38. [Google Scholar] [CrossRef]
- Wang, Y.; Shimmin, A.; Ghosh, P.; Marks, P.; Linklater, J.; Connell, D.; Hall, S.; Skerrett, D.; Itescu, S.; Cicuttini, F.M. Safety, tolerability, clinical, and joint structural outcomes of a single intra-articular injection of allogeneic mesenchymal precursor cells in patients following anterior cruciate ligament reconstruction: A controlled double-blind randomised trial. Arthritis Res. Ther. 2017, 19, 180. [Google Scholar] [CrossRef]
- Masuko, T.; Iwasaki, N.; Yamane, S.; Funakoshi, T.; Majima, T.; Minami, A.; Ohsuga, N.; Ohta, T.; Nishimura, S.-I. Chitosan–RGDSGGC conjugate as a scaffold material for musculoskeletal tissue engineering. Biomaterials 2005, 26, 5339–5347. [Google Scholar] [CrossRef]
- Dunn, M.; Tria, A.J.; Kato, Y.P.; Bechler, J.R.; Ochner, R.S.; Zawadsky, J.P.; Silver, F.H. Anterior cruciate ligament reconstruction using a composite collagenous prosthesis. Am. J. Sport. Med. 1992, 20, 507–515. [Google Scholar] [CrossRef]
- Chvapil, M.; Speer, D.P.; Holubec, H.; Chvapil, T.A.; King, D.H. Collagen fibers as a temporary scaffold for replacement of ACL in goats. J. Biomed. Mater. Res. 1993, 27, 313–325. [Google Scholar] [CrossRef]
- Dunn, M.G.; Liesch, J.B.; Tiku, M.L.; Zawadsky, J.P. Development of fibroblast-seeded ligament analogs for ACL reconstruction. J. Biomed. Mater. Res. 1995, 29, 1363–1371. [Google Scholar] [CrossRef]
- Bellincampi, L.D.; Closkey, R.F.; Prasad, R.; Zawadsky, J.P.; Dunn, M.G. Viability of fibroblast-seeded ligament analogs after autogenous implantation. J. Orthop. Res. 1998, 16, 414–420. [Google Scholar] [CrossRef]
- Gentleman, E.; Lay, A.N.; Dickerson, D.A.; Nauman, E.A.; Livesay, G.A.; Dee, K.C. Mechanical characterization of collagen fibers and scaffolds for tissue engineering. Biomaterials 2003, 24, 3805–3813. [Google Scholar] [CrossRef]
- Caruso, A.B.; Dunn, M.G. Changes in mechanical properties and cellularity during long-term culture of collagen fiber ACL reconstruction scaffolds. J. Biomed. Mater. Res. Part A 2005, 73A, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Nöth, U.; Schupp, K.; Heymer, A.; Kall, S.; Jakob, F.; Schütze, N.; Baumann, B.; Barthel, T.; Eulert, J.; Hendrich, C. Anterior cruciate ligament constructs fabricated from human mesenchymal stem cells in a collagen type I hydrogel. Cytotherapy 2005, 7, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.M.; Spindler, K.P.; Abreu, E.; Muller, J.A.; Nedder, A.; Kelly, M.; Frino, J.; Zurakowski, D.; Valenza, M.; Snyder, B.D.; et al. Collagen-platelet rich plasma hydrogel enhances primary repair of the porcine anterior cruciate ligament. J. Orthop. Res. 2006, 25, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Little, D.; Guilak, F.; Ruch, D.S. Ligament-Derived Matrix Stimulates a Ligamentous Phenotype in Human Adipose-Derived Stem Cells. Tissue Eng. Part A 2010, 16, 2307–2319. [Google Scholar] [CrossRef]
- Robayo, L.M.; Moulin, V.; Tremblay, P.; Cloutier, R.; Lamontagne, J.; Larkin, A.-M.; Chabaud, S.; Simon, F.; Islam, N.; Goulet, F. New ligament healing model based on tissue-engineered collagen scaffolds. Wound Repair Regen. 2011, 19, 38–48. [Google Scholar] [CrossRef]
- Figueroa, D.; Espinosa, M.; Calvo, R.; Scheu, M.; Vaisman, A.; Gallegos, M.; Conget, P. Anterior cruciate ligament regeneration using mesenchymal stem cells and collagen type I scaffold in a rabbit model. Knee Surg. Sport. Traumatol. Arthrosc. 2014, 22, 1196–1202. [Google Scholar] [CrossRef]
- Full, S.M.; Delman, C.; Gluck, J.; Abdmaulen, R.; Shemin, R.J.; Heydarkhan-Hagvall, S. Effect of fiber orientation of collagen-based electrospun meshes on human fibroblasts for ligament tissue engineering applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 39–46. [Google Scholar] [CrossRef]
- Altman, G.H.; Horan, R.L.; Lu, H.H.; Moreau, J.; Martin, I.; Richmond, J.C.; Kaplan, D.L. Silk matrix for tissue engineered anterior cruciate ligaments. Biomaterials 2002, 23, 4131–4141. [Google Scholar] [CrossRef]
- Altman, G.H.; Lu, H.H.; Horan, R.L.; Calabro, T.; Ryder, D.; Kaplan, D.L.; Stark, P.; Martin, I.; Richmond, J.C.; Vunjak-Novakovic, G. Advanced Bioreactor with Controlled Application of Multi-Dimensional Strain for Tissue Engineering. J. Biomech. Eng. 2002, 124, 742–749. [Google Scholar] [CrossRef]
- Chen, J.; Altman, G.H.; Karageorgiou, V.; Horan, R.; Collette, A.; Volloch, V.; Colabro, T.; Kaplan, D.L. Human bone marrow stromal cell and ligament fibroblast responses on RGD-modified silk fibers. J. Biomed. Mater. Res. 2003, 67A, 559–570. [Google Scholar] [CrossRef]
- Moreau, J.E.; Chen, J.; Horan, R.L.; Kaplan, D.L.; Altman, G.H. Sequential Growth Factor Application in Bone Marrow Stromal Cell Ligament Engineering. Tissue Eng. 2005, 11, 1887–1897. [Google Scholar] [CrossRef]
- Liu, H.; Ge, Z.; Wang, Y.; Toh, S.L.; Sutthikhum, V.; Goh, J.C.H. Modification of sericin-free silk fibers for ligament tissue engineering application. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 82B, 129–138. [Google Scholar] [CrossRef]
- Chen, J.; Horan, R.L.; Bramono, D.; Moreau, J.E.; Wang, Y.; Geuss, L.R.; Collette, A.L.; Volloch, V.; Altman, G.H. Monitoring Mesenchymal Stromal Cell Developmental Stage to Apply On-Time Mechanical Stimulation for Ligament Tissue Engineering. Tissue Eng. 2006, 12, 3085–3095. [Google Scholar] [CrossRef]
- Liu, H.; Fan, H.; Wang, Y.; Toh, S.L.; Goh, J.C. The interaction between a combined knitted silk scaffold and microporous silk sponge with human mesenchymal stem cells for ligament tissue engineering. Biomaterials 2008, 29, 662–674. [Google Scholar] [CrossRef]
- Liu, H.; Fan, H.; Toh, S.L.; Goh, J.C. A comparison of rabbit mesenchymal stem cells and anterior cruciate ligament fibroblasts responses on combined silk scaffolds. Biomaterials 2008, 29, 1443–1453. [Google Scholar] [CrossRef]
- Fan, H.; Liu, H.; Wong, E.J.; Toh, S.L.; Goh, J.C. In vivo study of anterior cruciate ligament regeneration using mesenchymal stem cells and silk scaffold. Biomaterials 2008, 29, 3324–3337. [Google Scholar] [CrossRef]
- Seo, Y.-K.; Yoon, H.-H.; Song, K.-Y.; Kwon, S.-Y.; Lee, H.-S.; Park, Y.-S.; Park, J.-K. Increase in cell migration and angiogenesis in a composite silk scaffold for tissue-engineered ligaments. J. Orthop. Res. 2009, 27, 495–503. [Google Scholar] [CrossRef]
- Horan, R.; Toponarski, I.; Boepple, H.; Weitzel, P.; Richmond, J.; Altman, G. Design and Characterization of a Scaffold for Anterior Cruciate Ligament Engineering. J. Knee Surg. 2009, 22, 82–92. [Google Scholar] [CrossRef]
- Fan, H.; Liu, H.; Toh, S.L.; Goh, J.C. Anterior cruciate ligament regeneration using mesenchymal stem cells and silk scaffold in large animal model. Biomaterials 2009, 30, 4967–4977. [Google Scholar] [CrossRef]
- Teh, T.K.; Toh, S.L.; Goh, J.C. Aligned hybrid silk scaffold for enhanced differentiation of mesenchymal stem cells into ligament fibroblasts. Tissue Eng. Part C Methods 2011, 17, 687–703. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Snedeker, J. Wired silk architectures provide a biomimetic ACL tissue engineering scaffold. J. Mech. Behav. Biomed. Mater. 2013, 22, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Teuschl, A.; Heimel, P.; Nurnberger, S.; van Griensven, M.; Redl, H.; Nau, T. A novel silk fiber-based scaffold for regeneration of the anterior cruciate ligament: Histological results from a study in sheep. Am. J. Sports Med. 2016, 44, 1547–1557. [Google Scholar] [CrossRef] [PubMed]
- Lin, V.S.; Lee, M.C.; O’Neal, S.; McKean, J.; Sung, K.-L.P. Ligament Tissue Engineering Using Synthetic Biodegradable Fiber Scaffolds. Tissue Eng. 1999, 5, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Cabaud, H.E.; Feagin, J.A.; Rodkey, W.G. Acute anterior cruciate ligament injury and repair reinforced with a biodegradable intraarticular ligament. Am. J. Sport. Med. 1982, 10, 259–265. [Google Scholar] [CrossRef]
- Lu, H.H.; Cooper, J.A.; Manuel, S.; Freeman, J.W.; Attawia, M.A.; Ko, F.K.; Laurencin, C.T. Anterior cruciate ligament regeneration using braided biodegradable scaffolds: In vitro optimization studies. Biomaterials 2005, 26, 4805–4816. [Google Scholar] [CrossRef]
- Dürselen, L.; Dauner, M.; Hierlemann, H.; Planck, H.; Claes, L.E.; Ignatius, A. Resorbable polymer fibers for ligament augmentation. J. Biomed. Mater. Res. 2001, 58, 666–672. [Google Scholar] [CrossRef]
- Cooper, J.A.; Lu, H.H.; Ko, F.K.; Freeman, J.W.; Laurencin, C.T. Fiber-based tissue-engineered scaffold for ligament replacement: Design considerations and in vitro evaluation. Biomaterials 2005, 26, 1523–1532. [Google Scholar] [CrossRef]
- Cooper, J.A.; Bailey, L.O.; Carter, J.N.; Castiglioni, C.E.; Kofron, M.D.; Ko, F.K.; Laurencin, C.T. Evaluation of the anterior cruciate ligament, medial collateral ligament, achilles tendon and patellar tendon as cell sources for tissue-engineered ligament. Biomaterials 2006, 27, 2747–2754. [Google Scholar] [CrossRef]
- Heckmann, L.; Schlenker, H.-J.; Fiedler, J.; Brenner, R.; Dauner, M.; Bergenthal, G.; Mattes, T.; Claes, L.; Ignatius, A. Human mesenchymal progenitor cell responses to a novel textured poly(L-lactide) scaffold for ligament tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 81B, 82–90. [Google Scholar] [CrossRef]
- Freeman, J.W.; Woods, M.D.; Laurencin, C.T. Tissue engineering of the anterior cruciate ligament using a braid–twist scaffold design. J. Biomech. 2007, 40, 2029–2036. [Google Scholar] [CrossRef]
- Freeman, J.W.; Woods, M.D.; Cromer, D.A.; Wright, L.D.; Laurencin, C.T. Tissue Engineering of the Anterior Cruciate Ligament: The Viscoelastic Behavior and Cell Viability of a Novel Braid–Twist Scaffold. J. Biomater. Sci. Polym. Ed. 2009, 20, 1709–1728. [Google Scholar] [CrossRef]
- Freeman, J.W.; Woods, M.D.; Cromer, D.A.; Ekwueme, E.C.; Andric, T.; Atiemo, E.A.; Bijoux, C.H.; Laurencin, C.T. Evaluation of a hydrogel–fiber composite for ACL tissue engineering. J. Biomech. 2011, 44, 694–699. [Google Scholar] [CrossRef]
- Kreja, L.; Liedert, A.; Schlenker, H.; Brenner, R.E.; Fiedler, J.; Friemert, B.; Dürselen, L.; Ignatius, A. Effects of mechanical strain on human mesenchymal stem cells and ligament fibroblasts in a textured poly(l-lactide) scaffold for ligament tissue engineering. J. Mater. Sci. Mater. Med. 2012, 23, 2575–2582. [Google Scholar] [CrossRef]
- Araque-Monrós, M.C.; Gamboa-Martínez, T.C.; Gil Santos, L.; Bernabé, S.G.; Pradas, M.M.; Estellés, J.M. New concept for a regenerative and resorbable prosthesis for tendon and ligament: Physicochemical and biological characterization of PLA-braided biomaterial. J. Biomed. Mater. Res. Part A 2013, 101, 3228–3237. [Google Scholar] [CrossRef]
- Van Eijk, F.; Saris, D.; Riesle, J.; Willems, W.; Van Blitterswijk, C.; Verbout, A.; Dhert, W.; Van Blitterswijk, C. Tissue Engineering of Ligaments: A Comparison of Bone Marrow Stromal Cells, Anterior Cruciate Ligament, and Skin Fibroblasts as Cell Source. Tissue Eng. 2004, 10, 893–903. [Google Scholar] [CrossRef]
- Sahoo, S.; Ouyang, H.; Goh, J.C.-H.; Tay, T.; Toh, S. Characterization of a Novel Polymeric Scaffold for Potential Application in Tendon/Ligament Tissue Engineering. Tissue Eng. 2006, 12, 91–99. [Google Scholar] [CrossRef]
- Jenner, J.; Van Eijk, F.; Saris, D.; Willems, W.; Dhert, W.; Creemers, L. Effect of Transforming Growth Factor-Beta and Growth Differentiation Factor-5 on Proliferation and Matrix Production by Human Bone Marrow Stromal Cells Cultured on Braided Poly Lactic-Co-Glycolic Acid Scaffolds for Ligament Tissue Engineering. Tissue Eng. 2007, 13, 1573–1582. [Google Scholar] [CrossRef]
- Petrigliano, F.A.; English, C.S.; Barba, D.; Esmende, S.; Wu, B.M.; McAllister, D.R. The Effects of Local bFGF Release and Uniaxial Strain on Cellular Adaptation and Gene Expression in a 3D Environment: Implications for Ligament Tissue Engineering. Tissue Eng. 2007, 13, 2721–2731. [Google Scholar] [CrossRef]
- Shao, H.J.; Chen, C.S.; Lee, Y.T.; Wang, J.H.; Young, T.H. The phenotypic responses of human anterior cruciate ligament cells cultured on poly(epsilon-caprolactone) and chitosan. J. Biomed. Mater. Res. A 2010, 93, 1297–1305. [Google Scholar]
- Peach, M.S.; Kumbar, S.G.; James, R.; Toti, U.S.; Balasubramaniam, D.; Deng, M.; Ulery, B.; Mazzocca, A.D.; McCarthy, M.B.; Morozowich, N.L.; et al. Design and optimization of polyphosphazene functionalized fiber matrices for soft tissue regeneration. J. Biomed. Nanotechnol. 2012, 8, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Huot, S.; Rohman, G.; Riffault, M.; Pinzano, A.; Grossin, L.; Migonney, V. Increasing the bioactivity of elastomeric poly(epsilon-caprolactone) scaffolds for use in tissue engineering. Biomed Mater. Eng. 2013, 23, 281–288. [Google Scholar] [PubMed]
- Leong, N.L.; Kabir, N.; Arshi, A.; Nazemi, A.; Wu, B.; Petrigliano, F.A.; McAllister, D.R. Evaluation of Polycaprolactone Scaffold with Basic Fibroblast Growth Factor and Fibroblasts in an Athymic Rat Model for Anterior Cruciate Ligament Reconstruction. Tissue Eng. Part A 2015, 21, 1859–1868. [Google Scholar] [CrossRef] [PubMed]
- Leroux, A.; Egles, C.; Migonney, V. Impact of chemical and physical treatments on the mechanical properties of poly(epsilon-caprolactone) fibers bundles for the anterior cruciate ligament reconstruction. PLoS ONE 2018, 13, e0205722. [Google Scholar] [CrossRef] [PubMed]
- Leroux, A.; Venkatesan, J.K.; Castner, D.G.; Cucchiarini, M.; Migonney, V. Analysis of early cellular responses of anterior cruciate ligament fibroblasts seeded on different molecular weight polycaprolactone films functionalized by a bioactive poly(sodium styrene sulfonate) polymer. Biointerphases 2019, 14, 041004. [Google Scholar] [CrossRef] [PubMed]
- Leroux, A.; Nguyen, T.N.; Rangel, A.; Cacciapuoti, I.; Duprez, D.; Castner, D.G.; Migonney, V. Long-term hydrolytic degradation study of polycaprolactone films and fibers grafted with poly(sodium styrene sulfonate): Mechanism study and cell response. Biointerphases 2020, 15, 061006. [Google Scholar] [CrossRef]
- Doroski, D.M.; Levenston, M.; Temenoff, J.S. Cyclic Tensile Culture Promotes Fibroblastic Differentiation of Marrow Stromal Cells Encapsulated in Poly(Ethylene Glycol)-Based Hydrogels. Tissue Eng. Part A 2010, 16, 3457–3466. [Google Scholar] [CrossRef]
- Ciobanu, M.; Siove, A.; Gueguen, V.; Gamble, L.J.; Castner, A.D.G.; Migonney, V. Radical Graft Polymerization of Styrene Sulfonate on Poly(ethylene terephthalate) Films for ACL Applications: “Grafting From” and Chemical Characterization. Biomacromolecules 2006, 7, 755–760. [Google Scholar] [CrossRef]
- Pavon-Djavid, G.; Gamble, L.J.; Ciobanu, M.; Gueguen, V.; Castner, A.D.G.; Migonney, V. Bioactive Poly(ethylene terephthalate) Fibers and Fabrics: Grafting, Chemical Characterization, and Biological Assessment. Biomacromolecules 2007, 8, 3317–3325. [Google Scholar] [CrossRef]
- Zhou, J.; Ciobanu, M.; Pavon-Djavid, G.; Gueguen, V.; Migonney, V. Morphology and adhesion of human fibroblast cells cultured on bioactive polymer grafted ligament prosthesis. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2007, 2007, 5115–5118. [Google Scholar]
- Vaquette, C.; Viateau, V.; Guérard, S.; Anagnostou, F.; Manassero, M.; Castner, D.G.; Migonney, V. The effect of polystyrene sodium sulfonate grafting on polyethylene terephthalate artificial ligaments on in vitro mineralisation and in vivo bone tissue integration. Biomaterials 2013, 34, 7048–7063. [Google Scholar] [CrossRef]
- Nguyen, T.N.; Rangel, A.; Grainger, D.W.; Migonney, V. Influence of spin finish on degradation, functionalization and long-term storage of polyethylene terephthalate fabrics dedicated to ligament prostheses. Sci. Rep. 2021, 11, 4258. [Google Scholar] [CrossRef]
- Murray, M.M.; Martin, S.D.; Spector, M. Migration of cells from human anterior cruciate ligament explants into collagen-glycosaminoglycan scaffolds. J. Orthop. Res. 2000, 18, 557–564. [Google Scholar] [CrossRef]
- Murray, M.M.; Spector, M. The migration of cells from the ruptured human anterior cruciate ligament into collagen-glycosaminoglycan regeneration templates in vitro. Biomaterials 2001, 22, 2393–2402. [Google Scholar] [CrossRef]
- Panas-Perez, E.; Gatt, C.J.; Dunn, M.G. Development of a silk and collagen fiber scaffold for anterior cruciate ligament reconstruction. J. Mater. Sci. Mater. Electron. 2013, 24, 257–265. [Google Scholar] [CrossRef]
- Shen, W.; Chen, X.; Hu, Y.; Yin, Z.; Zhu, T.; Hu, J.; Chen, J.; Zheng, Z.; Zhang, W.; Ran, J.; et al. Long-term effects of knitted silk–collagen sponge scaffold on anterior cruciate ligament reconstruction and osteoarthritis prevention. Biomaterials 2014, 35, 8154–8163. [Google Scholar] [CrossRef]
- Bi, F.; Shi, Z.; Liu, A.; Guo, P.; Yan, S. Anterior Cruciate Ligament Reconstruction in a Rabbit Model Using Silk-Collagen Scaffold and Comparison with Autograft. PLoS ONE 2015, 10, e0125900. [Google Scholar] [CrossRef]
- Tovar, N.; Murthy, N.S.; Kohn, J.; Gatt, C.; Dunn, M. ACL reconstruction using a novel hybrid scaffold composed of polyarylate fibers and collagen fibers. J. Biomed. Mater. Res. Part A 2012, 100A, 2913–2920. [Google Scholar] [CrossRef]
- Sahoo, S.; Toh, S.L.; Goh, J.C. A bFGF-releasing silk/PLGA-based biohybrid scaffold for ligament/tendon tissue engineering using mesenchymal progenitor cells. Biomaterials 2010, 31, 2990–2998. [Google Scholar] [CrossRef]
- Majima, T.; Irie, T.; Sawaguchi, N.; Funakoshi, T.; Iwasaki, N.; Harada, K.; Minami, A.; Nishimura, S.-I. Chitosan-based hyaluronan hybrid polymer fibre scaffold for ligament and tendon tissue engineering. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2007, 221, 537–546. [Google Scholar] [CrossRef]
- Sarukawa, J.; Takahashi, M.; Abe, M.; Suzuki, D.; Tokura, S.; Furuike, T.; Tamura, H. Effects of Chitosan-Coated Fibers as a Scaffold for Three-Dimensional Cultures of Rabbit Fibroblasts for Ligament Tissue Engineering. J. Biomater. Sci. Polym. Ed. 2011, 22, 717–732. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.-J.; Lee, Y.-T.; Chen, C.-S.; Wang, J.-H.; Young, T.-H. Modulation of gene expression and collagen production of anterior cruciate ligament cells through cell shape changes on polycaprolactone/chitosan blends. Biomaterials 2010, 31, 4695–4705. [Google Scholar] [CrossRef] [PubMed]
- Vaquette, C.; Kahn, C.; Frochot, C.; Nouvel, C.; Six, J.-L.; De Isla, N.; Luo, L.-H.; Cooper-White, J.; Rahouadj, R.; Wang, X. Aligned poly(L-lactic-co-e-caprolactone) electrospun microfibers and knitted structure: A novel composite scaffold for ligament tissue engineering. J. Biomed. Mater. Res. Part A 2010, 94A, 1270–1282. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.; Goh, J.C.H.; Lee, E.H. The Effects of Bone Marrow-Derived Mesenchymal Stem Cells and Fascia Wrap Application to Anterior Cruciate Ligament Tissue Engineering. Cell Transplant. 2005, 14, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.M.; Spindler, K.P.; Devin, C.; Snyder, B.S.; Muller, J.; Takahashi, M.; Ballard, P.; Nanney, L.B.; Zurakowski, D. Use of a collagen-platelet rich plasma scaffold to stimulate healing of a central defect in the canine ACL. J. Orthop. Res. 2006, 24, 820–830. [Google Scholar] [CrossRef]
- Murray, M.M.; Spindler, K.P.; Ballard, P.; Welch, T.P.; Zurakowski, D.; Nanney, L.B. Enhanced histologic repair in a central wound in the anterior cruciate ligament with a collagen–platelet-rich plasma scaffold. J. Orthop. Res. 2007, 25, 1007–1017. [Google Scholar] [CrossRef]
- Joshi, S.M.; Mastrangelo, A.N.; Magarian, E.M.; Fleming, B.C.; Murray, M.M. Collagen-Platelet Composite Enhances Biomechanical and Histologic Healing of the Porcine Anterior Cruciate Ligament. Am. J. Sport. Med. 2009, 37, 2401–2410. [Google Scholar] [CrossRef]
- Vavken, P.; Fleming, B.C.; Mastrangelo, A.N.; Machan, J.; Murray, M.M. Biomechanical Outcomes After Bioenhanced Anterior Cruciate Ligament Repair and Anterior Cruciate Ligament Reconstruction Are Equal in a Porcine Model. Arthrosc. J. Arthrosc. Relat. Surg. 2012, 28, 672–680. [Google Scholar] [CrossRef]
- Cheng, M.; Wang, H.; Yoshida, R.; Murray, M.M. Platelets and Plasma Proteins Are Both Required to Stimulate Collagen Gene Expression by Anterior Cruciate Ligament Cells in Three-Dimensional Culture. Tissue Eng. Part A 2010, 16, 1479–1489. [Google Scholar] [CrossRef]
- Xu, J.; Ye, Z.; Han, K.; Zheng, T.; Zhang, T.; Dong, S.; Jiang, J.; Yan, X.; Cai, J.; Zhao, J. Infrapatellar Fat Pad Mesenchymal Stromal Cell–Derived Exosomes Accelerate Tendon-Bone Healing and Intra-articular Graft Remodeling After Anterior Cruciate Ligament Reconstruction. Am. J. Sport. Med. 2022, 50, 662–673. [Google Scholar] [CrossRef]
- Li, Z.; Li, Q.; Tong, K.; Zhu, J.; Wang, H.; Chen, B.; Chen, L. BMSC-derived exosomes promote tendon-bone healing after anterior cruciate ligament reconstruction by regulating M1/M2 macrophage polarization in rats. Stem. Cell Res. Ther. 2022, 13, 295. [Google Scholar] [CrossRef]
- Fallouh, L.; Nakagawa, K.; Sasho, T.; Arai, M.; Kitahara, S.; Wada, Y.; Moriya, H.; Takahashi, K. Effects of Autologous Platelet-Rich Plasma on Cell Viability and Collagen Synthesis in Injured Human Anterior Cruciate Ligament. J. Bone Jt. Surg. 2010, 92, 2909–2916. [Google Scholar] [CrossRef]
- Vavken, P.; Sadoghi, P.; Murray, M.M. The Effect of Platelet Concentrates on Graft Maturation and Graft-Bone Interface Healing in Anterior Cruciate Ligament Reconstruction in Human Patients: A Systematic Review of Controlled Trials. Arthrosc. J. Arthrosc. Relat. Surg. 2011, 27, 1573–1583. [Google Scholar] [CrossRef]
- Xie, X.; Zhao, S.; Wu, H.; Xie, G.; Huangfu, X.; He, Y.; Zhao, J. Platelet-rich plasma enhances autograft revascularization and reinnervation in a dog model of anterior cruciate ligament reconstruction. J. Surg. Res. 2013, 183, 214–222. [Google Scholar] [CrossRef]
- Scull, G.; Fisher, M.B.; Brown, A.C. Fibrin-based biomaterial systems to enhance anterior cruciate ligament healing. Med. Devices Sensors 2021, 4, e10147. [Google Scholar] [CrossRef]
- Evans, C.H. Cytokines and the Role They Play in the Healing of Ligaments and Tendons. Sport. Med. 1999, 28, 71–76. [Google Scholar] [CrossRef]
- Woo, S.L.-Y.; Jia, F.; Zou, L.; Gabriel, M.T. Functional tissue engineering for ligament healing: Potential of antisense gene therapy. Ann. Biomed. Eng. 2004, 32, 342–351. [Google Scholar] [CrossRef]
- Gantenbein, B.; Tang, S.; Guerrero, J.; Higuita-Castro, N.; Salazar-Puerta, A.I.; Croft, A.S.; Gazdhar, A.; Purmessur, D. Non-viral Gene Delivery Methods for Bone and Joints. Front. Bioeng. Biotechnol. 2020, 8, 598466. [Google Scholar] [CrossRef]
- Zu, H.; Gao, D. Non-viral Vectors in Gene Therapy: Recent Development, Challenges, and Prospects. AAPS J. 2021, 23, 78. [Google Scholar] [CrossRef]
- Goins, W.F.; Hall, B.; Cohen, J.B.; Glorioso, J.C. Retargeting of herpes simplex virus (HSV) vectors. Curr. Opin. Virol. 2016, 21, 93–101. [Google Scholar] [CrossRef]
- Greber, U.F.; Gomez-Gonzalez, A. Adenovirus—A blueprint for gene delivery. Curr. Opin. Virol. 2021, 48, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Nishikawaji, Y.; Kawakami, H.; Kosai, K.-I. Adenovirus Biology, Recombinant Adenovirus, and Adenovirus Usage in Gene Therapy. Viruses 2021, 13, 2502. [Google Scholar] [CrossRef] [PubMed]
- Elsner, C.; Bohne, J. The retroviral vector family: Something for everyone. Virus Genes 2017, 53, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Milone, M.C.; O’Doherty, U. Clinical use of lentiviral vectors. Leukemia 2018, 32, 1529–1541. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Samulski, R.J. Engineering adeno-associated virus vectors for gene therapy. Nat. Rev. Genet. 2020, 21, 255–272. [Google Scholar] [CrossRef] [PubMed]
- Maurer, A.C.; Weitzman, M.D. Adeno-Associated Virus Genome Interactions Important for Vector Production and Transduction. Hum. Gene Ther. 2020, 31, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Cottard, V.; Valvason, C.; Falgarone, G.; Lutomski, D.; Boissier, M.-C.; Bessis, N. Immune Response Against Gene Therapy Vectors: Influence of Synovial Fluid on Adeno-Associated Virus Mediated Gene Transfer to Chondrocytes. J. Clin. Immunol. 2004, 24, 162–169. [Google Scholar] [CrossRef]
- Mingozzi, F.; Chen, Y.; Edmonson, S.; Zhou, S.; Thurlings, R.M.; Tak, P.P.; High, K.; Vervoordeldonk, M.J. Prevalence and pharmacological modulation of humoral immunity to AAV vectors in gene transfer to synovial tissue. Gene Ther. 2013, 20, 417–424. [Google Scholar] [CrossRef]
- Verdera, H.C.; Kuranda, K.; Mingozzi, F. AAV Vector Immunogenicity in Humans: A Long Journey to Successful Gene Transfer. Mol. Ther. 2020, 28, 723–746. [Google Scholar] [CrossRef]
- Murphy, P.G.; Loitz, B.J.; Frank, C.B.; Hart, D.A. Influence of exogenous growth factors on the synthesis and secretion of collagen types I and III by explants of normal and healing rabbit ligaments. Biochem. Cell Biol. 1994, 72, 403–409. [Google Scholar] [CrossRef]
- Schmidt, C.C.; Georgescu, H.I.; Kwoh, C.K.; Blomstrom, G.L.; Engle, C.P.; Larkin, L.A.; Evans, C.H.; Woo, S.L.-Y. Effect of growth factors on the proliferation of fibroblasts from the medial collateral and anterior cruciate ligaments. J. Orthop. Res. 1995, 13, 184–190. [Google Scholar] [CrossRef]
- Desrosiers, E.A.; Yahia, L.; Rivard, C.-H. Proliferative and matrix synthesis response of canine anterior cruciate ligament fibroblasts submitted to combined growth factors. J. Orthop. Res. 1996, 14, 200–208. [Google Scholar] [CrossRef]
- Spindler, K.P.; Imro, A.K.; Mayes, C.E.; Davidson, J.M. Patellar tendon and anterior cruciate ligament have different mitogenic responses to platelet-derived growth factor and transforming growth factor beta. J. Orthop. Res. 1996, 14, 542–546. [Google Scholar] [CrossRef]
- Marui, T.; Niyibizi, C.; Georgescu, H.I.; Cao, M.; Kavalkovich, K.W.; Levine, R.E.; Woo, S.L.-Y. Effect of growth factors on matrix synthesis by ligament fibroblasts. J. Orthop. Res. 1997, 15, 18–23. [Google Scholar] [CrossRef]
- Yoshida, M.; Fujii, K. Differences in cellular properties and responses to growth factors between human ACL and MCL cells. J. Orthop. Sci. 1999, 4, 293–298. [Google Scholar] [CrossRef]
- Murray, M.M.; Rice, K.; Wright, R.J.; Spector, M. The effect of selected growth factors on human anterior cruciate ligament cell interactions with a three-dimensional collagen-GAG scaffold. J. Orthop. Res. 2003, 21, 238–244. [Google Scholar] [CrossRef]
- Okuizumi, T.; Tohyama, H.; Kondo, E.; Yasuda, K. The effect of cell-based therapy with autologous synovial fibroblasts activated by exogenous TGF-beta1 on the in situ frozen-thawed anterior cruciate ligament. J. Orthop. Sci. 2004, 9, 488–494. [Google Scholar] [CrossRef]
- Kondo, E.; Yasuda, K.; Yamanaka, M.; Minami, A.; Tohyama, H. Effects of Administration of Exogenous Growth Factors on Biomechanical Properties of the Elongation-type Anterior Cruciate Ligament Injury with Partial Laceration. Am. J. Sport. Med. 2005, 33, 188–196. [Google Scholar] [CrossRef]
- Wolfman, N.M.; Hattersley, G.; Cox, K.; Celeste, A.J.; Nelson, R.; Yamaji, N.; Dube, J.L.; DiBlasio-Smith, E.; Nove, J.; Song, J.J.; et al. Ectopic induction of tendon and ligament in rats by growth and differentiation factors 5, 6, and 7, members of the TGF-beta gene family. J. Clin. Investig. 1997, 100, 321–330. [Google Scholar] [CrossRef]
- Hannafin, J.A.; Attia, E.T.; Warren, R.F.; Bhargava, M.M. Characterization of chemotactic migration and growth kinetics of canine knee ligament fibroblasts. J. Orthop. Res. 1999, 17, 398–404. [Google Scholar] [CrossRef]
- Tashiro, T.; Hiraoka, H.; Ikeda, Y.; Ohnuki, T.; Suzuki, R.; Ochi, T.; Nakamura, K.; Fukui, N. Effect of GDF-5 on ligament healing. J. Orthop. Res. 2006, 24, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Date, H.; Furumatsu, T.; Sakoma, Y.; Yoshida, A.; Hayashi, Y.; Abe, N.; Ozaki, T. GDF-5/7 and bFGF activate integrin alpha2-mediated cellular migration in rabbit ligament fibroblasts. J. Orthop. Res. 2010, 28, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Amiel, D.; Nagineni, C.N.; Choi, S.H.; Lee, J. Intrinsic properties of ACL and MCL cells and their responses to growth factors. Med. Sci. Sport. Exerc. 1995, 27, 844–851. [Google Scholar] [CrossRef]
- Letson, A.K.; E Dahners, L. The effect of combinations of growth factors on ligament healing. Clin. Orthop. Relat. Res. 1994, 308, 207–212. [Google Scholar] [CrossRef]
- Scherping, S.C.; Schmidt, C.C.; Georgescu, H.I.; Kwoh, C.K.; Evans, C.H.; Woo, S.L.-Y. Effect of Growth Factors on the Proliferation of Ligament Fibroblasts from Skeletally Mature Rabbits. Connect. Tissue Res. 1997, 36, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, D.; Kurosaka, M.; Yoshiya, S.; Mizuno, K. Effect of basic fibroblast growth factor on the healing of defects in the canine anterior cruciate ligament. Knee Surg. Sport. Traumatol. Arthrosc. 1997, 5, 189–194. [Google Scholar] [CrossRef]
- Lee, J.; Green, M.H.; Amiel, D. Synergistic effect of growth factors on cell outgrowth from explants of rabbit anterior cruciate and medial collateral ligaments. J. Orthop. Res. 1995, 13, 435–441. [Google Scholar] [CrossRef]
- Weiler, A.; Förster, C.; Hunt, P.; Falk, R.; Jung, T.; Unterhauser, F.N.; Bergmann, V.; Schmidmaier, G.; Haas, N.P. The Influence of Locally Applied Platelet-Derived Growth Factor-BB on Free Tendon Graft Remodeling after Anterior Cruciate Ligament Reconstruction. Am. J. Sport. Med. 2004, 32, 881–891. [Google Scholar] [CrossRef]
- Mckean, J.M.; Hsieh, A.H.; Sung, K.L.P. Epidermal growth factor differentially affects integrin-mediated adhesion and proliferation of ACL and MCL fibroblasts. Biorheology 2004, 41, 139–152. [Google Scholar]
- Woo, Y.K.; Kwon, S.Y.; Lee, H.S.; Park, Y.S. Proliferation of anterior cruciate ligament cells in vitro by photo-immobilized epidermal growth factor. J. Orthop. Res. 2007, 25, 73–80. [Google Scholar] [CrossRef]
- Ju, Y.-J.; Tohyama, H.; Kondo, E.; Yoshikawa, T.; Muneta, T.; Shinomiya, K.; Yasuda, K. Effects of Local Administration of Vascular Endothelial Growth Factor on Properties of the in Situ Frozen-Thawed Anterior Cruciate Ligament in Rabbits. Am. J. Sport. Med. 2006, 34, 84–91. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Tohyama, H.; Katsura, T.; Kondo, E.; Kotani, Y.; Matsumoto, H.; Toyama, Y.; Yasuda, K. Effects of Local Administration of Vascular Endothelial Growth Factor on Mechanical Characteristics of the Semitendinosus Tendon Graft after Anterior Cruciate Ligament Reconstruction in Sheep. Am. J. Sport. Med. 2006, 34, 1918–1925. [Google Scholar] [CrossRef]
- Caceres, M.D.; Pfeifer, C.G.; Docheva, D. Understanding Tendons: Lessons from Transgenic Mouse Models. Stem Cells Dev. 2018, 27, 1161–1174. [Google Scholar] [CrossRef]
- Murchison, N.D.; Price, B.A.; Conner, D.A.; Keene, D.R.; Olson, E.N.; Tabin, C.J.; Schweitzer, R. Regulation of tendon differentiation by scleraxis distinguishes force-transmitting tendons from muscle-anchoring tendons. Development 2007, 134, 2697–2708. [Google Scholar] [CrossRef]
- Alberton, P.; Popov, C.; Prägert, M.; Kohler, J.; Shukunami, C.; Schieker, M.; Docheva, D. Conversion of Human Bone Marrow-Derived Mesenchymal Stem Cells into Tendon Progenitor Cells by Ectopic Expression of Scleraxis. Stem Cells Dev. 2012, 21, 846–858. [Google Scholar] [CrossRef]
- Chen, X.; Yin, Z.; Chen, J.-L.; Shen, W.-L.; Liu, H.-H.; Tang, Q.-M.; Fang, Z.; Lu, L.-R.; Ji, J.; Ouyang, H.-W. Force and scleraxis synergistically promote the commitment of human ES cells derived MSCs to tenocytes. Sci. Rep. 2012, 2, 977. [Google Scholar] [CrossRef]
- Dyment, N.A.; Liu, C.-F.; Kazemi, N.; Aschbacher-Smith, L.E.; Kenter, K.; Breidenbach, A.P.; Shearn, J.T.; Wylie, C.; Rowe, D.W.; Butler, D.L. The Paratenon Contributes to Scleraxis-Expressing Cells during Patellar Tendon Healing. PLoS ONE 2013, 8, e59944. [Google Scholar] [CrossRef]
- Nichols, A.E.; Werre, S.R.; Dahlgren, L.A. Transient Scleraxis Overexpression Combined with Cyclic Strain Enhances Ligament Cell Differentiation. Tissue Eng. Part A 2018, 24, 1444–1455. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, Z.; Wu, S.; Li, Y.; Xiong, H.; Zou, G.; Jin, Y.; Yang, J.; You, Q.; Zhang, J.; et al. Enhanced tenogenic differentiation and tendon-like tissue formation by Scleraxis overexpression in human amniotic mesenchymal stem cells. Histochem. J. 2020, 51, 209–220. [Google Scholar] [CrossRef]
- Ito, Y.; Toriuchi, N.; Yoshitaka, T.; Ueno-Kudoh, H.; Sato, T.; Yokoyama, S.; Nishida, K.; Akimoto, T.; Takahashi, M.; Miyaki, S.; et al. The Mohawk homeobox gene is a critical regulator of tendon differentiation. Proc. Natl. Acad. Sci. USA 2010, 107, 10538–10542. [Google Scholar] [CrossRef]
- Nakahara, H.; Hasegawa, A.; Otabe, K.; Ayabe, F.; Matsukawa, T.; Onizuka, N.; Ito, Y.; Ozaki, T.; Lotz, M.K.; Asahara, H. Transcription Factor Mohawk and the Pathogenesis of Human Anterior Cruciate Ligament Degradation. Arthritis Care Res. 2013, 65, 2081–2089. [Google Scholar] [CrossRef] [PubMed]
- Otabe, K.; Nakahara, H.; Hasegawa, A.; Matsukawa, T.; Ayabe, F.; Onizuka, N.; Inui, M.; Takada, S.; Ito, Y.; Sekiya, I.; et al. Transcription factor Mohawk controls tenogenic differentiation of bone marrow mesenchymal stem cells in vitro and in vivo. J. Orthop. Res. 2015, 33, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, K.; Kurimoto, R.; Tsutsumi, H.; Chiba, T.; Kato, T.; Shishido, K.; Kato, M.; Ito, Y.; Cho, Y.; Hoshi, O.; et al. In vitro Neo-Genesis of Tendon/Ligament-Like Tissue by Combination of Mohawk and a Three-Dimensional Cyclic Mechanical Stretch Culture System. Front. Cell Dev. Biol. 2020, 8, 307. [Google Scholar] [CrossRef]
- Cameron, M.L.; Fu, F.H.; Paessler, H.H.; Schneider, M.; Evans, C.H. Synovial fluid cytokine concentrations as possible prognostic indicators in the ACL-deficient knee. Knee Surg. Sport. Traumatol. Arthrosc. 1994, 2, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Lohmander, L.S.; Roos, H.; Dahlberg, L.; Hoerrner, L.A.; Lark, M.W. Temporal patterns of stromelysin-1, tissue inhibitor, and proteoglycan fragments in human knee joint fluid after injury to the cruciate ligament or meniscus. J. Orthop. Res. 1994, 12, 21–28. [Google Scholar] [CrossRef]
- Irie, K.; Uchiyama, E.; Iwaso, H. Intraarticular inflammatory cytokines in acute anterior cruciate ligament injured knee. Knee 2003, 10, 93–96. [Google Scholar] [CrossRef]
- Higuchi, H.; Shirakura, K.; Kimura, M.; Terauchi, M.; Shinozaki, T.; Watanabe, H.; Takagishi, K. Changes in biochemical parameters after anterior cruciate ligament injury. Int. Orthop. 2006, 30, 43–47. [Google Scholar] [CrossRef]
- Murakami, H.; Shinomiya, N.; Kikuchi, T.; Yoshihara, Y.; Nemoto, K. Upregulated expression of inducible nitric oxide synthase plays a key role in early apoptosis after anterior cruciate ligament injury. J. Orthop. Res. 2006, 24, 1521–1534. [Google Scholar] [CrossRef]
- Tang, Z.; Yang, L.; Xue, R.; Zhang, J.; Wang, Y.; Chen, P.C.; Sung, K.L. Differential expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in anterior cruciate ligament and medial collateral ligament fibroblasts after a mechanical injury: Involvement of the p65 subunit of NF-kappaB. Wound Repair Regen. 2009, 17, 709–716. [Google Scholar] [CrossRef]
- Xie, J.; Wang, C.; Yin, L.; Xu, C.; Zhang, Y.; Sung, K.-L.P. Interleukin-1 beta influences on lysyl oxidases and matrix metalloproteinases profile of injured anterior cruciate ligament and medial collateral ligament fibroblasts. Int. Orthop. 2013, 37, 495–505. [Google Scholar] [CrossRef]
- Bigoni, M.; Sacerdote, P.; Turati, M.; Franchi, S.; Gandolla, M.; Gaddi, D.; Moretti, S.; Munegato, D.; Augusti, C.A.; Bresciani, E.; et al. Acute and late changes in intraarticular cytokine levels following anterior cruciate ligament injury. J. Orthop. Res. 2013, 31, 315–321. [Google Scholar] [CrossRef]
- Xie, J.; Jiang, J.; Huang, W.; Zhang, Y.; Xu, C.; Wang, C.; Yin, L.; Chen, P.C.; Sung, K.P. TNF-α induced down-regulation of lysyl oxidase family in anterior cruciate ligament and medial collateral ligament fibroblasts. Knee 2014, 21, 47–53. [Google Scholar] [CrossRef]
- Struglics, A.; Larsson, S.; Kumahashi, N.; Frobell, R.; Lohmander, L.S. Changes in cytokines and aggrecan ARGS neoepitope in synovial fluid and serum and in C-terminal crosslinking telopeptide of type II collagen and N-terminal crosslinking telopeptide of type I collagen in urine over five years after anterior cruciate ligament rupture: An exploratory analysis in the knee anterior cruciate ligament, nonsurgical versus surgical treatment trial. Arthritis Rheumatol. 2015, 67, 1816–1825. [Google Scholar]
- Chamberlain, C.S.; Leiferman, E.M.; Frisch, K.E.; Kuehl, S.; Brickson, S.L.; Murphy, W.L.; Baer, G.S.; Vanderby, R. Interleukin-1 receptor antagonist modulates inflammation and scarring after ligament injury. Connect. Tissue Res. 2014, 55, 177–186. [Google Scholar] [CrossRef][Green Version]
- Chamberlain, C.S.; Leiferman, E.M.; Frisch, K.E.; Brickson, S.L.; Murphy, W.L.; Baer, G.S.; Vanderby, R. Interleukin Expression after Injury and the Effects of Interleukin-1 Receptor Antagonist. PLoS ONE 2013, 8, e71631. [Google Scholar] [CrossRef]
- Lawrence, J.T.R.; Birmingham, J.; Toth, A.P. Emerging Ideas: Prevention of Posttraumatic Arthritis Through Interleukin-1 and Tumor Necrosis Factor-alpha Inhibition. Clin. Orthop. Relat. Res. 2011, 469, 3522–3526. [Google Scholar] [CrossRef]
- Kraus, V.B.; Birmingham, J.; Stabler, T.V.; Feng, S.; Taylor, D.C.; Moorman, C.T., 3rd; Garrett, W.E.; Toth, A.P. Effects of intraarticular IL1-Ra for acute anterior cruciate ligament knee injury: A randomized controlled pilot trial (NCT00332254). Osteoarthr. Cartil. 2012, 20, 271–278. [Google Scholar] [CrossRef]
- Ricchetti, E.T.; Reddy, S.C.; Ansorge, H.L.; Zgonis, M.H.; Van Kleunen, J.P.; Liechty, K.W.; Soslowsky, L.J.; Beredjiklian, P.K. Effect of Interleukin-10 Overexpression on the Properties of Healing Tendon in a Murine Patellar Tendon Model. J. Hand Surg. 2008, 33, 1843–1852. [Google Scholar] [CrossRef]
- Bigoni, M.; Zanchi, N.; Omeljaniuk, R.J.; Zatti, G.; Locatelli, V.; Torsello, A.; Turati, M. Role of interleukin-10 in the synovial fluid of the anterior cruciate ligament injured knee. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 932–940. [Google Scholar]
- Wang, C.; Sha, Y.; Wang, S.; Chi, Q.; Sung, K.P.; Xu, K.; Yang, L. Lysyl oxidase suppresses the inflammatory response in anterior cruciate ligament fibroblasts and promotes tissue regeneration by targeting myotrophin via the nuclear factor-kappa B pathway. J. Tissue Eng. Regen. Med. 2020, 14, 1063–1076. [Google Scholar] [CrossRef]
- Sun, M.; Connizzo, B.K.; Adams, S.M.; Freedman, B.R.; Wenstrup, R.J.; Soslowsky, L.J.; Birk, D.E. Targeted Deletion of Collagen V in Tendons and Ligaments Results in a Classic Ehlers-Danlos Syndrome Joint Phenotype. Am. J. Pathol. 2015, 185, 1436–1447. [Google Scholar] [CrossRef] [PubMed]
- Evangelopoulos, D.S.; Kohl, S.; Schwienbacher, S.; Gantenbein, B.; Exadaktylos, A.; Ahmad, S.S. Collagen application reduces complication rates of mid-substance ACL tears treated with dynamic intraligamentary stabilization. Knee Surg. Sport. Traumatol. Arthrosc. 2017, 25, 2414–2419. [Google Scholar] [CrossRef] [PubMed]
- Page, R.L.; Wharton, J.M.; E Wilkinson, W.; Friedman, I.M.; Claypool, W.D.; Karim, A.; Ms, K.G.K.; BS, S.J.M.; Gardiner, P.; Pritchett, E.L.C.; et al. Bidisomide (SC-40230), a new antiarrhythmic agent: Initial study of tolerability and pharmacokinetics. Clin. Pharmacol. Ther. 1992, 51, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Alberton, P.; Dex, S.; Popov, C.; Shukunami, C.; Schieker, M.; Docheva, D. Loss of Tenomodulin Results in Reduced Self-Renewal and Augmented Senescence of Tendon Stem/Progenitor Cells. Stem Cells Dev. 2015, 24, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Dex, S.; Alberton, P.; Willkomm, L.; Söllradl, T.; Bago, S.; Milz, S.; Shakibaei, M.; Ignatius, A.; Bloch, W.; Clausen-Schaumann, H.; et al. Tenomodulin is Required for Tendon Endurance Running and Collagen I Fibril Adaptation to Mechanical Load. eBioMedicine 2017, 20, 240–254. [Google Scholar] [CrossRef]
- Yin, H.; Caceres, M.D.; Yan, Z.; Schieker, M.; Nerlich, M.; Docheva, D. Tenomodulin regulates matrix remodeling of mouse tendon stem/progenitor cells in an ex vivo collagen I gel model. Biochem. Biophys. Res. Commun. 2019, 512, 691–697. [Google Scholar] [CrossRef]
- Lewis, J.L.; Krawczak, D.A.; Oegema, T.R.; Westendorf, J.J. Effect of Decorin and Dermatan Sulfate on the Mechanical Properties of a Neocartilage. Connect. Tissue Res. 2010, 51, 159–170. [Google Scholar] [CrossRef]
- Dunkman, A.A.; Buckley, M.R.; Mienaltowski, M.J.; Adams, S.M.; Thomas, S.J.; Kumar, A.; Beason, D.P.; Iozzo, R.V.; Birk, D.E.; Soslowsky, L.J. The injury response of aged tendons in the absence of biglycan and decorin. Matrix Biol. 2014, 35, 232–238. [Google Scholar] [CrossRef]
- Robinson, K.A.; Sun, M.; Barnum, C.E.; Weiss, S.N.; Huegel, J.; Shetye, S.S.; Lin, L.; Saez, D.; Adams, S.M.; Iozzo, R.V.; et al. Decorin and biglycan are necessary for maintaining collagen fibril structure, fiber realignment, and mechanical properties of mature tendons. Matrix Biol. 2017, 64, 81–93. [Google Scholar] [CrossRef]
- Delalande, A.; Gosselin, M.-P.; Suwalski, A.; Guilmain, W.; Leduc, C.; Berchel, M.; Jaffrès, P.-A.; Baril, P.; Midoux, P.; Pichon, C. Enhanced Achilles tendon healing by fibromodulin gene transfer. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1735–1744. [Google Scholar] [CrossRef]
- Nourissat, G.; Berenbaum, F.; Duprez, D. Tendon injury: From biology to tendon repair. Nat. Rev. Rheumatol. 2015, 11, 223–233. [Google Scholar] [CrossRef]
- Nakamichi, R.; Asahara, H. Regulation of tendon and ligament differentiation. Bone 2021, 143, 115609. [Google Scholar] [CrossRef]
- Goomer, R.S.; Maris, T.M.; Gelberman, R.; Boyer, M.; Silva, M.; Amiel, D. Nonviral In Vivo Gene Therapy for Tissue Engineering of Articular Cartilage and Tendon Repair. Clin. Orthop. Relat. Res. 2000, 379, S189–S200. [Google Scholar] [CrossRef]
- Gerich, T.G.; Kang, R.; Fu, F.H.; Robbins, P.D.; Evans, C.H. Gene transfer to the rabbit patellar tendon: Potential for genetic enhancement of tendon and ligament healing. Gene Ther. 1996, 3, 1089–1093. [Google Scholar]
- Gerich, T.G.; Kang, R.; Fu, F.H.; Robbins, P.D.; Evans, C.H. Gene transfer to the patellar tendon. Knee Surg. Sports Traumatol. Arthrosc. 1997, 5, 118–123. [Google Scholar] [CrossRef]
- Lou, J.; Tu, Y.; Ludwig, F.J.; Zhang, J.; Manske, P.R. Effect of Bone Morphogenetic Protein-12 Gene Transfer on Mesenchymal Progenitor Cells. Clin. Orthop. Relat. Res. 1999, 369, 333–339. [Google Scholar] [CrossRef]
- Pascher, A.; Steinert, A.F.; Palmer, G.D.; Betz, O.; Gouze, J.-N.; Gouze, E.; Pilapil, C.; Ghivizzani, S.C.; Evans, C.H.; Murray, M.M. Enhanced Repair of the Anterior Cruciate Ligament by in Situ Gene Transfer: Evaluation in an in Vitro Model. Mol. Ther. 2004, 10, 327–336. [Google Scholar] [CrossRef]
- Steinert, A.F.; Weber, M.; Kunz, M.; Palmer, G.D.; Nöth, U.; Evans, C.H.; Murray, M.M. In situ IGF-1 gene delivery to cells emerging from the injured anterior cruciate ligament. Biomaterials 2008, 29, 904–916. [Google Scholar] [CrossRef]
- Majewski, M.; Betz, O.; E Ochsner, P.; Liu, F.; Porter, R.M.; Evans, C.H. Ex vivo adenoviral transfer of bone morphogenetic protein 12 (BMP-12) cDNA improves Achilles tendon healing in a rat model. Gene Ther. 2008, 15, 1139–1146. [Google Scholar] [CrossRef]
- Wei, X.-L.; Lin, L.; Hou, Y.; Fu, X.; Zhang, J.-Y.; Mao, Z.-B.; Yu, C.-L. Construction of recombinant adenovirus co-expression vector carrying the human transforming growth factor-beta1 and vascular endothelial growth factor genes and its effect on anterior cruciate ligament fibroblasts. Chin. Med. J. 2008, 121, 1426–1432. [Google Scholar] [CrossRef]
- Hou, Y.; Mao, Z.; Wei, X.; Lin, L.; Chen, L.; Wang, H.; Fu, X.; Zhang, J.; Yu, C. Effects of transforming growth factor-beta1 and vascular endothelial growth factor 165 gene transfer on Achilles tendon healing. Matrix Biol. 2009, 28, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Haddad-Weber, M.; Prager, P.; Kunz, M.; Seefried, L.; Jakob, F.; Murray, M.M.; Evans, C.H.; Nöth, U.; Steinert, A.F. BMP12 and BMP13 gene transfer induce ligamentogenic differentiation in mesenchymal progenitor and anterior cruciate ligament cells. Cytotherapy 2010, 12, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Gulotta, L.V.; Kovacevic, D.; Packer, J.D.; Deng, X.H.; Rodeo, S.A. Bone Marrow–Derived Mesenchymal Stem Cells Transduced with Scleraxis Improve Rotator Cuff Healing in a Rat Model. Am. J. Sport. Med. 2011, 39, 1282–1289. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Kunz, M.; Stehle, J.; Noth, U.; Steinert, A. BMP-12 transduced MSCs in collagen hydrogel for ligament reconstruction. J. Stem Cells Regen. Med. 2007, 2, 72–73. [Google Scholar] [PubMed]
- Takayama, K.; Kawakami, Y.; Mifune, Y.; Matsumoto, T.; Tang, Y.; Cummins, J.H.; Greco, N.; Kuroda, R.; Kurosaka, M.; Wang, B.; et al. The effect of blocking angiogenesis on anterior cruciate ligament healing following stem cell transplantation. Biomaterials 2015, 60, 9–19. [Google Scholar] [CrossRef]
- Kawakami, Y.; Takayama, K.; Matsumoto, T.; Tang, Y.; Wang, B.; Mifune, Y.; Cummins, J.H.; Warth, R.J.; Kuroda, R.; Kurosaka, M.; et al. Anterior Cruciate Ligament–Derived Stem Cells Transduced with BMP2 Accelerate Graft-Bone Integration After ACL Reconstruction. Am. J. Sport. Med. 2017, 45, 584–597. [Google Scholar] [CrossRef]
- Tsutsumi, H.; Kurimoto, R.; Nakamichi, R.; Chiba, T.; Matsushima, T.; Fujii, Y.; Sanada, R.; Kato, T.; Shishido, K.; Sakamaki, Y.; et al. Generation of a tendon-like tissue from human iPS cells. J. Tissue Eng. 2022, 13, 20417314221074018. [Google Scholar] [CrossRef]
- Madry, H.; Kohn, D.; Cucchiarini, M. Direct FGF-2 Gene Transfer via Recombinant Adeno-Associated Virus Vectors Stimulates Cell Proliferation, Collagen Production, and the Repair of Experimental Lesions in the Human ACL. Am. J. Sport. Med. 2012, 41, 194–202. [Google Scholar] [CrossRef]
- Nakamura, N.; Hart, D.A.; Frank, C.B.; Marchuk, L.L.; Shrive, N.G.; Ota, N.; Taira, K.; Yoshikawa, H.; Kaneda, Y. Efficient Transfer of Intact Oligonucleotides into the Nucleus of Ligament Scar Fibroblasts by HVJ-Cationic Liposomes Is Correlated with Effective Antisense Gene Inhibition. J. Biochem. 2001, 129, 755–759. [Google Scholar] [CrossRef]
- Shimomura, T.; Jia, F.; Niyibizi, C.; Woo, S.L. Antisense oligonucleotides reduce synthesis of procollagen alpha1 (V) chain in human patellar tendon fibroblasts: Potential application in healing ligaments and tendons. Connect. Tissue Res. 2003, 44, 167–172. [Google Scholar] [CrossRef]
- Groth, K.; Berezhanskyy, T.; Aneja, M.K.; Geiger, J.; Schweizer, M.; Maucksch, L.; Pasewald, T.; Brill, T.; Tigani, B.; Weber, E.; et al. Tendon healing induced by chemically modified mRNAs. Eur. Cells Mater. 2017, 33, 294–307. [Google Scholar] [CrossRef]
- Chen, X.-H.; Tong, Y.; Xu, W.-L.; Jin, J.; Qian, W.-B. Expression of telomere binding factor 2 (TRF2) on leukemia cell lines and primary leukemia cells. Zhejiang Da Xue Xue Bao Yi Xue Ban 2008, 37, 170–175. [Google Scholar]
- Li, F.; Jia, H.; Yu, C. ACL reconstruction in a rabbit model using irradiated Achilles allograft seeded with mesenchymal stem cells or PDGF-B gene-transfected mesenchymal stem cells. Knee Surg. Sport. Traumatol. Arthrosc. 2007, 15, 1219–1227. [Google Scholar] [CrossRef]
- Lu, Y.F.; Liu, Y.; Fu, W.M.; Xu, J.; Wang, B.; Sun, Y.X.; Wu, T.Y.; Xu, L.L.; Chan, K.M.; Zhang, J.F.; et al. Long noncoding RNA H19 accelerates tenogenic differentiation and promotes tendon healing through targeting miR-29b-3p and activating TGF-beta1 signaling. FASEB J. 2017, 31, 954–964. [Google Scholar] [CrossRef]
- Lu, P.; Zhang, G.-R.; Cai, Y.-Z.; Heng, B.C.; Ren, H.; Wang, L.-L.; Ji, J.; Zou, X.-H.; Ouyang, H.-W. Lentiviral-Encoded shRNA Silencing of Proteoglycan Decorin Enhances Tendon Repair and Regeneration within a Rat Model. Cell Transplant. 2013, 22, 1507–1517. [Google Scholar] [CrossRef]
- Chen, L.; Wang, G.-D.; Liu, J.-P.; Wang, H.-S.; Liu, X.-M.; Wang, Q.; Cai, X.-H. miR-135a modulates tendon stem/progenitor cell senescence via suppressing ROCK1. Bone 2015, 71, 210–216. [Google Scholar] [CrossRef]
- Nakamura, N.; Horibe, S.; Matsumoto, N.; Tomita, T.; Natsuume, T.; Kaneda, Y.; Shino, K.; Ochi, T. Transient introduction of a foreign gene into healing rat patellar ligament. J. Clin. Investig. 1996, 97, 226–231. [Google Scholar] [CrossRef][Green Version]
- Nakamura, N.; Shino, K.; Natsuume, T.; Horibe, S.; Matsumoto, N.; Kaneda, Y.; Ochi, T. Early biological effect of in vivo gene transfer of platelet-derived growth factor (PDGF)-B into healing patellar ligament. Gene Ther. 1998, 5, 1165–1170. [Google Scholar] [CrossRef]
- Ozkan, I.; Shino, K.; Nakamura, N.; Natsuume, T.; Matsumoto, N.; Horibe, S.; Tomita, T.; Kaneda, Y.; Ochi, T. Direct in vivo gene transfer to healing rat patellar ligament by intra-arterial delivery of haemagglutinating virus of Japan liposomes. Eur. J. Clin. Invest. 1999, 29, 63–67. [Google Scholar] [CrossRef]
- Hildebrand, K.A.; Deie, M.; Allen, C.R.; Smith, D.W.; Georgescu, H.I.; Evans, C.H.; Robbins, P.D.; Woo, S.L.-Y. Early expression of marker genes in the rabbit medial collateral and anterior cruciate ligaments: The use of different viral vectors and the effects of injury. J. Orthop. Res. 1999, 17, 37–42. [Google Scholar] [CrossRef]
- Menetrey, J.; Kasemkijwattana, C.; Day, C.S.; Bosch, P.; Fu, F.H.; Moreland, M.S.; Huard, J. Direct-, Fibroblast- and Myoblast-Mediated Gene Transfer to the Anterior Cruciate Ligament. Tissue Eng. 1999, 5, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Helm, G.A.; Li, J.Z.; Alden, T.D.; Hudson, S.B.; Beres, E.J.; Cunningham, M.; Mikkelsen, M.M.; Pittman, D.D.; Kerns, K.M.; Kallmes, D.F. A light and electron microscopic study of ectopic tendon and ligament formation induced by bone morphogenetic protein—13 adenoviral gene therapy. J. Neurosurg. 2001, 95, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, N.; Hart, D.A.; Boorman, R.S.; Kaneda, Y.; Shrive, N.G.; Marchuk, L.L.; Shino, K.; Ochi, T.; Frank, C.B. Decorin antisense gene therapy improves functional healing of early rabbit ligament scar with enhanced collagen fibrillogenesis in vivo. J. Orthop. Res. 2000, 18, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Herbst, E.; Imhoff, F.B.; Foehr, P.; Milz, S.; Plank, C.; Rudolph, C.; Hasenpusch, G.; Geiger, J.P.; Aneja, M.K.; Groth, K.; et al. Chemically Modified Messenger RNA: Modified RNA Application for Treatment of Achilles Tendon Defects. Tissue Eng. Part A 2019, 25, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, N.; A Timmermann, S.; A Hart, D.; Kaneda, Y.; Shrive, N.G.; Shino, K.; Ochi, T.; Frank, C.B. A comparison of in vivo gene delivery methods for antisense therapy in ligament healing. Gene Ther. 1998, 5, 1455–1461. [Google Scholar] [CrossRef][Green Version]
- Bez, M.; Kremen, T.; Tawackoli, W.; Avalos, P.; Sheyn, D.; Shapiro, G.; Giaconi, J.C.; Ben David, S.; Snedeker, J.; Gazit, Z.; et al. Ultrasound-Mediated Gene Delivery Enhances Tendon Allograft Integration in Mini-Pig Ligament Reconstruction. Mol. Ther. 2018, 26, 1746–1755. [Google Scholar] [CrossRef]
- Martinek, V.; Latterman, C.; Usas, A.; Abramowitch, S.; Woo, S.L.; Fu, F.H.; Huard, J. Enhancement of tendon-bone integration of anterior cruciate ligament grafts with bone morphogenetic protein-2 gene transfer: A histological and biomechanical study. J. Bone Jt. Surg. Am. 2002, 84, 1123–1131. [Google Scholar] [CrossRef]
- Wei, X.; Mao, Z.; Hou, Y.; Lin, L.; Xue, T.; Chen, L.; Wang, H.; Yu, C. Local administration of TGFbeta-1/VEGF165 gene-transduced bone mesenchymal stem cells for Achilles allograft replacement of the anterior cruciate ligament in rabbits. Biochem. Biophys. Res. Commun. 2011, 406, 204–210. [Google Scholar] [CrossRef]
- Schuettrumpf, J.; Zou, J.; Zhang, Y.; Schlachterman, A.; Liu, Y.-L.; Edmonson, S.; Xiao, W.; Arruda, V.R. The inhibitory effects of anticoagulation on in vivo gene transfer by adeno-associated viral or adenoviral vectors. Mol. Ther. 2006, 13, 88–97. [Google Scholar] [CrossRef]
- Mangeat, B.; Trono, D. Lentiviral Vectors and Antiretroviral Intrinsic Immunity. Hum. Gene Ther. 2005, 16, 913–920. [Google Scholar] [CrossRef]
- Calcedo, R.; Wilson, J.M.M. Humoral Immune Response to AAV. Front. Immunol. 2013, 4, 341. [Google Scholar] [CrossRef]
- Fausther-Bovendo, H.; Kobinger, G.P. Pre-existing immunity against Ad vectors: Humoral, cellular, and innate response, what’s important? Hum. Vaccin. Immunother. 2014, 10, 2875–2884. [Google Scholar] [CrossRef]
- Ronzitti, G.; Gross, D.; Mingozzi, F. Human Immune Responses to Adeno-Associated Virus (AAV) Vectors. Front. Immunol. 2020, 11, 670. [Google Scholar] [CrossRef]
- Goff, S.P. Intracellular trafficking of retroviral genomes during the early phase of infection: Viral exploitation of cellular pathways. J. Gene Med. 2001, 3, 517–528. [Google Scholar] [CrossRef]
- Setten, R.L.; Rossi, J.J.; Han, S.-P. The current state and future directions of RNAi-based therapeutics. Nat. Rev. Drug Discov. 2019, 18, 421–446. [Google Scholar] [CrossRef]
- Riyad, J.M.; Weber, T. Intracellular trafficking of adeno-associated virus (AAV) vectors: Challenges and future directions. Gene Ther. 2021, 28, 683–696. [Google Scholar] [CrossRef]
- Schaffer, D.V.; Koerber, J.T.; Lim, K.-I. Molecular Engineering of Viral Gene Delivery Vehicles. Annu. Rev. Biomed. Eng. 2008, 10, 169–194. [Google Scholar] [CrossRef]
- Mitchell, A.M.; Nicolson, S.C.; Warischalk, J.K.; Samulski, R.J. AAV’s anatomy: Roadmap for optimizing vectors for translational success. Curr. Gene Ther. 2010, 10, 319–340. [Google Scholar] [CrossRef]
- Kotterman, M.A.; Schaffer, D.V. Engineering adeno-associated viruses for clinical gene therapy. Nat. Rev. Genet. 2014, 15, 445–451. [Google Scholar] [CrossRef]
- Kotterman, M.A.; Chalberg, T.W.; Schaffer, D.V. Viral Vectors for Gene Therapy: Translational and Clinical Outlook. Annu. Rev. Biomed. Eng. 2015, 17, 63–89. [Google Scholar] [CrossRef]
- Rabinowitz, J.; Chan, Y.K.; Samulski, R.J. Adeno-Associated Virus (AAV) Versus Immune Response. Viruses 2019, 11, 102. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Athirasala, A.; Menezes, P.P.; Ashwanikumar, N.; Zou, T.; Sahay, G.; Bertassoni, L.E. Messenger RNA Delivery for Tissue Engineering and Regenerative Medicine Applications. Tissue Eng. Part A 2019, 25, 91–112. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, E.W.; Green, J.J. Toward Gene Transfer Nanoparticles as Therapeutics. Adv. Health Mater. 2022, 11, e2102145. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.S.; Peppas, N.A. Present and future applications of biomaterials in controlled drug delivery systems. Biomaterials 1981, 2, 201–214. [Google Scholar] [CrossRef]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef]
- Weiser, J.R.; Saltzman, W.M. Controlled release for local delivery of drugs: Barriers and models. J. Control. Release 2014, 190, 664–673. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Concheiro, A. Smart drug delivery systems: From fundamentals to the clinic. Chem. Commun. 2014, 50, 7743–7765. [Google Scholar] [CrossRef]
- Mitragotri, S.; Burke, P.A.; Langer, R. Overcoming the challenges in administering biopharmaceuticals: Formulation and delivery strategies. Nat. Rev. Drug Discov. 2014, 13, 655–672. [Google Scholar] [CrossRef]
- Adepu, S.; Ramakrishna, S. Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef]
- Bonadio, J.; Smiley, E.; Patil, P.; Goldstein, S. Localized, direct plasmid gene delivery in vivo: Prolonged therapy results in reproducible tissue regeneration. Nat. Med. 1999, 5, 753–759. [Google Scholar] [CrossRef]
- Evans, C.H.; Robbins, P.D. Genetically Augmented Tissue Engineering of the Musculoskeletal System. Clin. Orthop. Relat. Res. 1999, 367, S410–S418. [Google Scholar] [CrossRef]
- Bonadio, J.; A Goldstein, S.; Levy, R.J. Gene therapy for tissue repair and regeneration. Adv. Drug Deliv. Rev. 1998, 33, 53–69. [Google Scholar] [CrossRef]
- Bonadio, J. Tissue engineering via local gene delivery. Klin. Wochenschr. 2000, 78, 303–311. [Google Scholar]
- Bonadio, J. Tissue engineering via local gene delivery: Update and future prospects for enhancing the technology. Adv. Drug Deliv. Rev. 2000, 44, 185–194. [Google Scholar] [CrossRef]
- Jang, J.-H.; Houchin, T.L.; Shea, L.D. Gene delivery from polymer scaffolds for tissue engineering. Expert Rev. Med. Devices 2004, 1, 127–138. [Google Scholar] [CrossRef]
- De Laporte, L.; Shea, L.D. Matrices and scaffolds for DNA delivery in tissue engineering. Adv. Drug Deliv. Rev. 2007, 59, 292–307. [Google Scholar] [CrossRef]
- Evans, C.H.; Ghivizzani, S.C.; Robbins, P.D. Orthopedic Gene Therapy in 2008. Mol. Ther. 2009, 17, 231–244. [Google Scholar] [CrossRef]
- Evans, C.H. Advances in Regenerative Orthopedics. Mayo Clin. Proc. 2013, 88, 1323–1339. [Google Scholar] [CrossRef]
- Gower, R.M.; Shea, L.D. Biomaterial Scaffolds for Controlled, Localized Gene Delivery of Regenerative Factors. Adv. Wound Care 2013, 2, 100–106. [Google Scholar] [CrossRef]
- Smith, B.D.; Grande, D.A. The current state of scaffolds for musculoskeletal regenerative applications. Nat. Rev. Rheumatol. 2015, 11, 213–222. [Google Scholar] [CrossRef]
- Evans, C.H.; Huard, J. Gene therapy approaches to regenerating the musculoskeletal system. Nat. Rev. Rheumatol. 2015, 11, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Silva, E.A.; Mooney, D.J. Growth factor delivery-based tissue engineering: General approaches and a review of recent developments. J. R. Soc. Interface 2011, 8, 153–170. [Google Scholar] [CrossRef] [PubMed]
- Liechty, W.B.; Kryscio, D.R.; Slaughter, B.V.; Peppas, N.A. Polymers for drug delivery systems. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 149–173. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhu, C.; Wu, Y.F.; Zhang, L.; Tang, J.B. Effective modulation of transforming growth factor-beta1 expression through engineered microRNA-based plasmid-loaded nanospheres. Cytotherapy 2015, 17, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Hou, Z.-Q.; Wang, H.; Wang, X.-L.; Liu, Y.-Y.; Gao, X.-H.; Lin, Y.-J.; Huang, B.-H. Effects of gene-activated matrix on autograft healing of anterior cruciate ligament. Mol. Med. Rep. 2013, 7, 679–683. [Google Scholar] [CrossRef]
- Wu, G.; Sun, B.; Zhao, C.; Wang, Z.; Teng, S.; Yang, M.; Cui, Z.; Zhu, G.; Yu, Y. Three-dimensional tendon scaffold loaded with TGF-beta1 gene silencing plasmid prevents tendon adhesion and promotes tendon repair. ACS Biomater. Sci. Eng. 2021, 7, 5739–5748. [Google Scholar] [CrossRef]
- Basile, P.; Dadali, T.; Jacobson, J.; Hasslund, S.; Ulrich-Vinther, M.; Søballe, K.; Nishio, Y.; Drissi, M.H.; Langstein, H.N.; Mitten, D.J.; et al. Freeze-dried Tendon Allografts as Tissue-engineering Scaffolds for Gdf5 Gene Delivery. Mol. Ther. 2008, 16, 466–473. [Google Scholar] [CrossRef]
- Hasslund, S.; Dadali, T.; Ulrich-Vinther, M.; Søballe, K.; Schwarz, E.M.; A Awad, H. Freeze-dried allograft-mediated gene or protein delivery of growth and differentiation factor 5 reduces reconstructed murine flexor tendon adhesions. J. Tissue Eng. 2014, 5, 2041731414528736. [Google Scholar] [CrossRef]
- Shoji, T.; Nakasa, T.; Yamasaki, K.; Kodama, A.; Miyaki, S.; Niimoto, T.; Okuhara, A.; Kamei, N.; Adachi, N.; Ochi, M. The Effect of Intra-articular Injection of MicroRNA-210 on Ligament Healing in a Rat Model. Am. J. Sport. Med. 2012, 40, 2470–2478. [Google Scholar] [CrossRef]
- Usman, M.A.; Nakasa, T.; Shoji, T.; Kato, T.; Kawanishi, Y.; Hamanishi, M.; Kamei, N.; Ochi, M. The effect of administration of double stranded MicroRNA-210 on acceleration of Achilles tendon healing in a rat model. J. Orthop. Sci. 2015, 20, 538–546. [Google Scholar] [CrossRef]
- Sakti, M.; Nakasa, T.; Shoji, T.; Usman, M.A.; Kawanishi, Y.; Hamanishi, M.; Yusuf, I.; Ochi, M. Acceleration of healing of the medial collateral ligament of the knee by local administration of synthetic microRNA-210 in a rat model. Asia-Pac. J. Sport. Med. Arthrosc. Rehabil. Technol. 2015, 2, 129–136. [Google Scholar] [CrossRef]
- Ginn, S.L.; Amaya, A.K.; Alexander, I.E.; Edelstein, M.; Abedi, M.R. Gene therapy clinical trials worldwide to 2017: An update. J. Gene Med. 2018, 20, e3015. [Google Scholar] [CrossRef]
- Evans, C.H.; Ghivizzani, S.C.; Robbins, P.D. Arthritis gene therapy is becoming a reality. Nat. Rev. Rheumatol. 2018, 14, 381–382. [Google Scholar] [CrossRef]
- Watson-Levings, R.S.; Palmer, G.D.; Levings, P.P.; Dacanay, E.A.; Evans, C.H.; Ghivizzani, S.C. Gene Therapy in Orthopaedics: Progress and Challenges in Pre-Clinical Development and Translation. Front. Bioeng. Biotechnol. 2022, 10, 901317. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Park, S.H.; Choi, Y.-J.; Moon, S.W.; Lee, B.H.; Shim, J.-H.; Cho, D.-W.; Wang, J.H. Three-Dimensional Bio-Printed Scaffold Sleeves with Mesenchymal Stem Cells for Enhancement of Tendon-to-Bone Healing in Anterior Cruciate Ligament Reconstruction Using Soft-Tissue Tendon Graft. Arthrosc. J. Arthrosc. Relat. Surg. 2018, 34, 166–179. [Google Scholar] [CrossRef]
- Cunniffe, G.M.; Gonzalez-Fernandez, T.; Daly, A.; Sathy, B.N.; Jeon, O.; Alsberg, E.; Kelly, D. Three-Dimensional Bioprinting of Polycaprolactone Reinforced Gene Activated Bioinks for Bone Tissue Engineering. Tissue Eng. Part A 2017, 23, 891–900. [Google Scholar] [CrossRef]
- Potyondy, T.; Uquillas, J.A.; Tebon, P.J.; Byambaa, B.; Hasan, A.; Tavafoghi, M.; Mary, H.; Aninwene, G.E., 2nd; Pountos, I.; Khademhosseini, A.; et al. Recent advances in 3D bioprinting of musculoskeletal tissues. Biofabrication 2021, 13, 022001. [Google Scholar] [CrossRef]
- Bakirci, E.; Guenat, O.; Ahmad, S.; Gantenbein, B. Tissue engineering approaches for the repair and regeneration of the anterior cruciate ligament: Towards 3D bioprinted ACL-on-chip. Eur. Cells Mater. 2022, 44, 21–42. [Google Scholar] [CrossRef]
- Perez-Pinera, P.; Kocak, D.D.; Vockley, C.M.; Adler, A.F.; Kabadi, A.M.; Polstein, L.R.; Thakore, P.I.; Glass, K.A.; Ousterout, D.G.; Leong, K.W.; et al. RNA-guided gene activation by CRISPR-Cas9–based transcription factors. Nat. Methods 2013, 10, 973–976. [Google Scholar] [CrossRef]
- Almarza, D.; Cucchiarini, M.; Loughlin, J. Genome editing for human osteoarthritis—A perspective. Osteoarthr. Cartil. 2017, 25, 1195–1198. [Google Scholar] [CrossRef] [PubMed]
- Brunger, J.M.; Zutshi, A.; Willard, V.P.; Gersbach, C.A.; Guilak, F. CRISPR/Cas9 Editing of Murine Induced Pluripotent Stem Cells for Engineering Inflammation-Resistant Tissues. Arthritis Rheumatol. 2017, 69, 1111–1121. [Google Scholar] [CrossRef] [PubMed]
- Brunger, J.M.; Zutshi, A.; Willard, V.P.; Gersbach, C.A.; Guilak, F. Genome Engineering of Stem Cells for Autonomously Regulated, Closed-Loop Delivery of Biologic Drugs. Stem Cell Rep. 2017, 8, 1202–1213. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-R.; Collins, K.H.; Lee, J.-W.; Kang, H.-J.; Guilak, F. Genome Engineering for Osteoarthritis: From Designer Cells to Disease-Modifying Drugs. Tissue Eng. Regen. Med. 2019, 16, 335–343. [Google Scholar] [CrossRef]
- Choi, Y.-R.; Collins, K.H.; Springer, L.E.; Pferdehirt, L.; Ross, A.K.; Wu, C.-L.; Moutos, F.T.; Harasymowicz, N.S.; Brunger, J.M.; Pham, C.T.N.; et al. A genome-engineered bioartificial implant for autoregulated anticytokine drug delivery. Sci. Adv. 2021, 7, eabj1414. [Google Scholar] [CrossRef]
- Doudna, J.A.; Charpentier, E. The new frontier of genome engineering with CRISPR-Cas9. Science 2014, 346, 1258096. [Google Scholar] [CrossRef]
- Farhang, N.; Brunger, J.M.; Stover, J.D.; Thakore, P.I.; Lawrence, B.; Guilak, F.; Gersbach, C.A.; Setton, L.A.; Bowles, R.D. CRISPR-Based Epigenome Editing of Cytokine Receptors for the Promotion of Cell Survival and Tissue Deposition in Inflammatory Environments. Tissue Eng. Part A 2017, 23, 738–749. [Google Scholar] [CrossRef]
- Guilak, F.; Pferdehirt, L.; Ross, A.K.; Choi, Y.; Collins, K.H.; Nims, R.J.; Katz, D.B.; Klimak, M.; Tabbaa, S.; Pham, C.T.N. Designer Stem Cells: Genome Engineering and the Next Generation of Cell-Based Therapies. J. Orthop. Res. 2019, 37, 1287–1293. [Google Scholar] [CrossRef]
- Klimak, M.; Nims, R.J.; Pferdehirt, L.; Collins, K.H.; Harasymowicz, N.S.; Oswald, S.J.; Setton, L.A.; Guilak, F. Immunoengineering the next generation of arthritis therapies. Acta Biomater. 2021, 133, 74–86. [Google Scholar] [CrossRef]
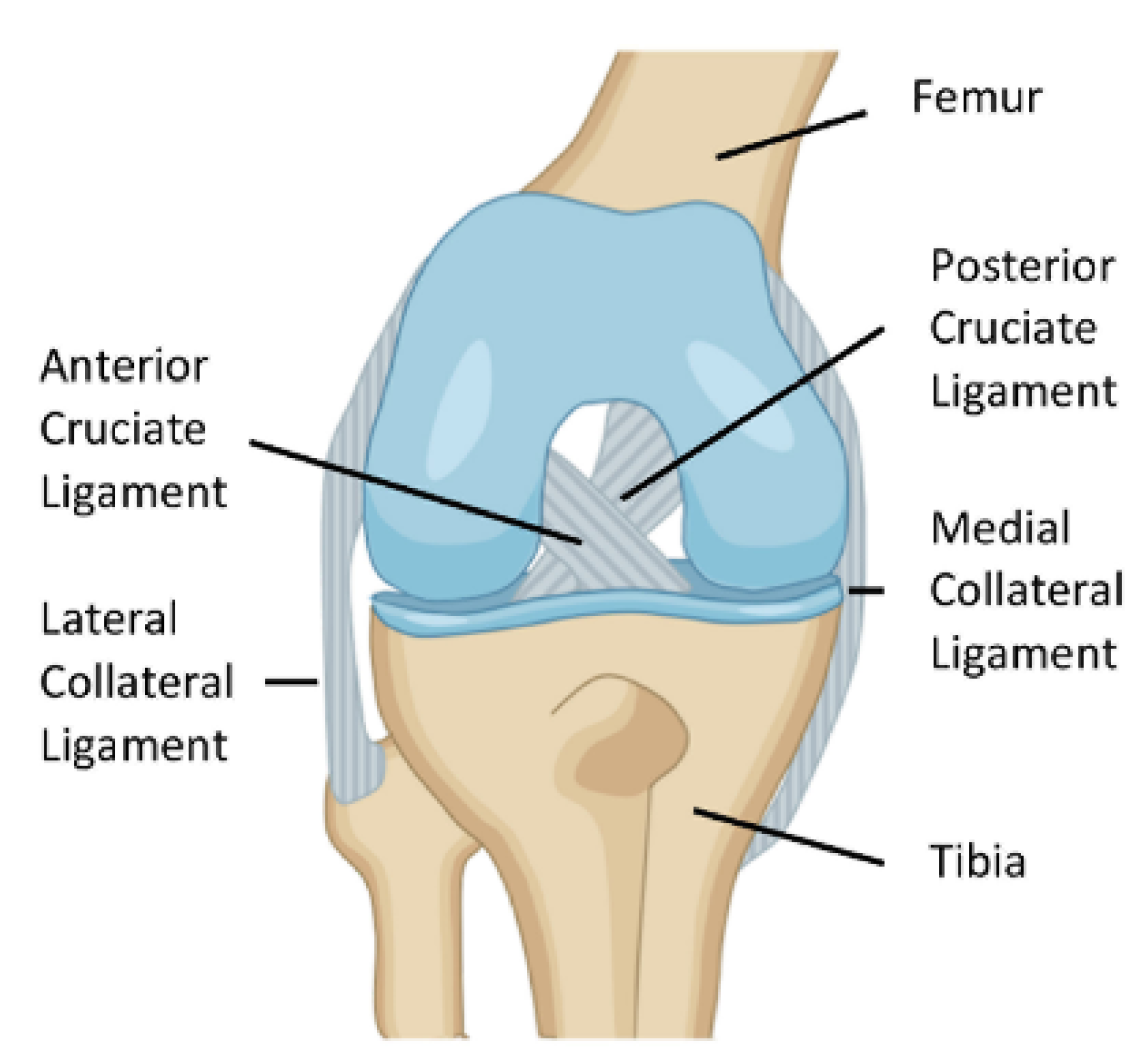

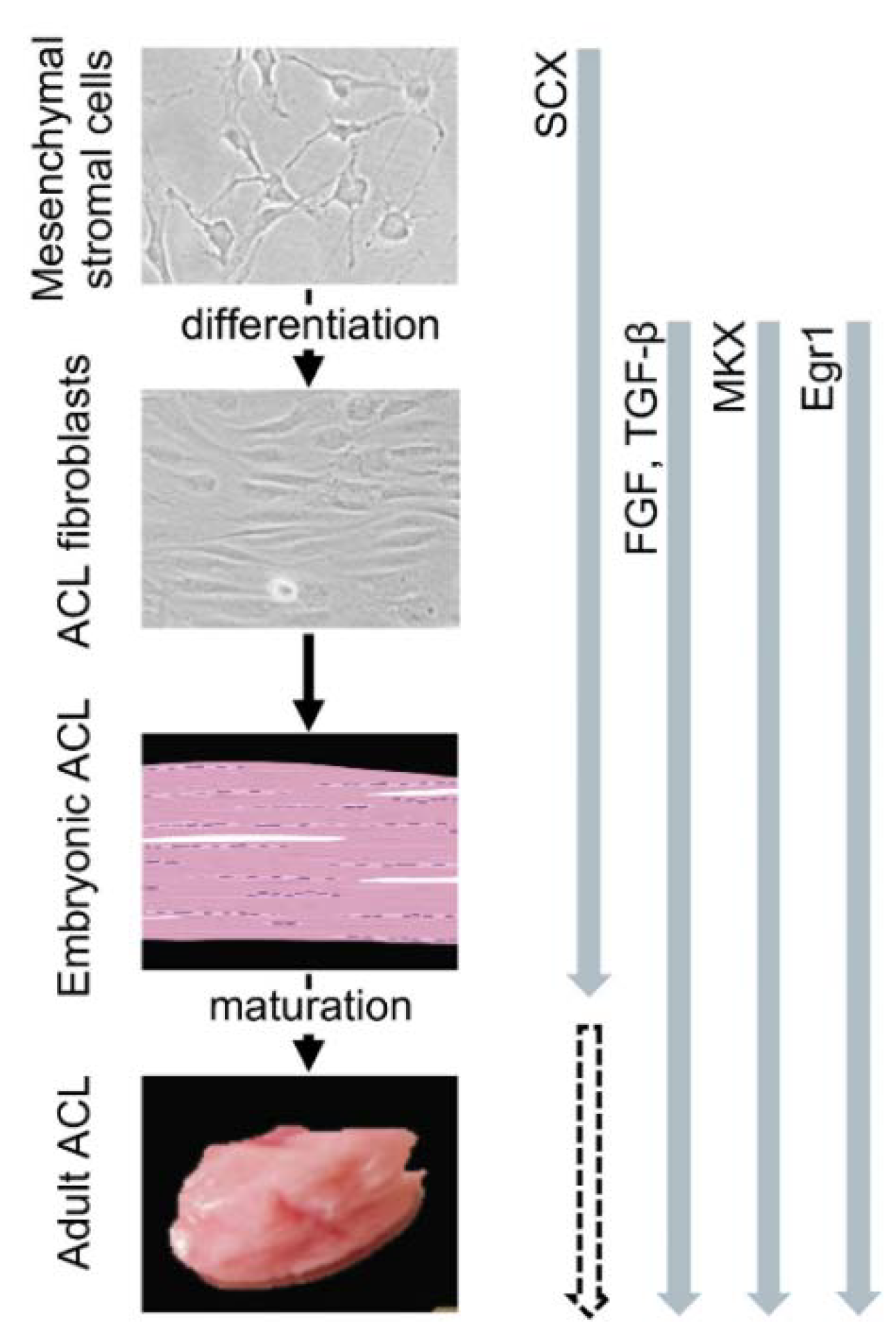
| Scaffolds | Cells | Factors | Bioreactors | Effects | Refs. |
|---|---|---|---|---|---|
| fibrin | - | - | - | improved ACL repair (goats) | [150] |
| CTGF | - | improved ACL repair (rabbits) | [151] | ||
| HA | - | - | - | improved ACL repair (rabbits, patients) | [152,155] |
| TGF-β, FGF-2, PDGF, insulin | - | higher cell outgrowth in ACL explants | [154] | ||
| MSCs | - | - | higher cell growth, differentiation | [153] | |
| chitosan | ACL fibroblasts | - | - | higher cell adhesion, differentiation | [156] |
| collagen | - | - | - | improved ACL repair (rabbits, pigs) | [157,158,161,162,164] |
| ACL, skin fibroblasts | - | - | higher cell growth, differentiation | [159,160,166,168] | |
| MSCs | - | + | higher cell growth, differentiation, improved ACL repair (rabbits) | [44,163,167] | |
| ASCs | - | - | higher cell growth, differentiation | [165] | |
| silk | - | - | - | improved ACL repair (pigs, goats) | [179,180] |
| ACL, skin fibroblasts | - | - | higher cell growth, differentiation, improved ACL repair (dogs) | [171,178,182] | |
| MSCs | - | - | higher cell growth, differentiation, improved ACL repair (sheep) | [169,171,173,174,175,176,177,179,181,183] | |
| + | higher cell growth, differentiation | [170] | |||
| TGF-β, FGF-2, EGF | - | higher cell growth, differentiation | [172] | ||
| PGA | - | - | - | improved ACL repair (dogs) | [185] |
| ACL fibroblasts | - | - | higher cell growth, differentiation | [184,186] | |
| PLA | ACL fibroblasts | - | - | higher cell growth, differentiation | [186,188,189,194] |
| MSCs | - | - | higher cell growth, differentiation | [190,194] | |
| PLGA | ACL, skin fibroblasts | - | - | higher cell growth, differentiation | [168,186,188,196] |
| MSCs | - | - | higher cell growth, differentiation | [196,197] | |
| TGF-β, GDF-5 | - | higher cell growth, differentiation | [198] | ||
| PCL | - | FGF-2 | - | improved ACL repair (rats) | [203] |
| ACL fibroblasts | - | - | higher cell growth, differentiation | [200,204,205,206] | |
| MSCs | - | + | higher cell growth, differentiation | [201] | |
| FGF-2 | + | higher cell growth, differentiation | [199] | ||
| PU | ACL fibroblasts | - | - | higher cell growth, differentiation | [168] |
| OPF | MSCs | + | higher cell growth, differentiation | [207] | |
| PET | - | - | - | tissue integration (sheep) | [211] |
| ACL fibroblasts | - | - | higher cell growth, differentiation | [209] | |
| collagen/GAG | ACL explants | - | - | higher cell migration, differentiation | [213,214] |
| collagen/silk | - | - | - | improved ACL repair (rabbits) | [215,216,217] |
| collagen/p(DTD DD) | - | - | - | improved ACL repair (sheep) | [218] |
| collagen/PRP | - | - | - | improved ACL repair (dogs, pigs) | [164,225,226,227,228] |
| ACL fibroblasts | - | - | higher cell growth, differentiation | [229] | |
| silk/PLGA | MSCs | FGF-2 | - | higher cell growth, differentiation | [219] |
| chitosan/HA | - | - | - | improved ACL repair (rats) | [220] |
| chitosan/PLA | ACL fibroblasts | - | - | higher cell growth, differentiation | [221] |
| chitosan/PCL | ACL fibroblasts | - | - | higher cell growth, differentiation | [222] |
| PLA/PCL | MSCs | - | - | higher cell growth, differentiation | [223] |
| PGA/PCL | ACL fibroblasts | - | - | higher cell growth, differentiation | [184] |
| PLA/PLGA | MSCs | - | - | improved ACL repair (rabbits) | [224] |
| Vectors | Integration | Advantages | Limitations | |
|---|---|---|---|---|
| Type | Class | |||
| nonviral vectors | no | . large capacity . cost effectiveness . no infectiosity/replication . no toxicity/immunogenicity |
| |
| viral vectors | adenoviral | no | . large capacity . high efficacy . dividing/quiescent cells | . short-term expression . toxicity/immunogenicity |
| HSV | no | . large capacity . high efficacy . dividing/quiescent cells | . short-term expression . toxicity/immunogenicity | |
| retroviral | yes | . large capacity . long-term expression | . low efficacy without selection . only dividing cells . toxicity/immunogenicity . potential insertional mutagenesis | |
| lentiviral | yes | . large capacity . dividing/quiescent cells . long-term expression | . low efficacy without selection . toxicity/immunogenicity . potential insertional mutagenesis . HIV material | |
| rAAV | no | . high efficacy . dividing/quiescent cells . long-term expression | . small capacity . possible immunogenicity . potential insertional mutagenesis | |
| Vectors | Cell Targets | Genes | Effects | Refs. |
|---|---|---|---|---|
| nonviral vectors | rabbit ligament cells | ODN (decorin) | suppression of decorin expression (one day) | [329] |
| human tendon cells | ODN (TVPα1) | suppression of TVPα1 expression (one day) | [330] | |
| horse, bovine, sheep, pig, rat tendons | mRNA (lacZ, luc, BMP-7) | effective gene expression (one day) | [331] | |
| rabbit perichondrial cells | lacZ, TGF-β | effective gene expression (2 days) | [313] | |
| adenoviral vectors | rabbit ACL cells | lacZ, TGF-β, VEGF | effective gene expression, high DNA, type-I/-III collagen, FN contents (3 days) | [314,315,320] |
| bovine ACL cells | GFP, luc, TGF-β (type-I collagen gel) | effective gene expression (6 days), high DNA and type-I/-III collagen contents (3 weeks) | [317] | |
| human ACL cells | GFP, luc, BMP-12, -13, IGF-I (type-I collagen gel) | effective gene expression, high DNA, type-I/-III collagen, tenascin, TNMD, elastin, vimentin, decorin, FN, biglycan, SCX contents (3 weeks) | [318,322] | |
| murine MSC line | BMP-12 | effective gene expression (5 days) | [316,332] | |
| rat MSCs | SCX | effective gene expression (one day) | [323] | |
| rabbit MSCs | TGF-β, VEGF | effective gene expression (2 days) | [321] | |
| human MSCs | BMP-12, -13 (type-I collagen gel) | effective gene expression (2 weeks), high DNA, type-III collagen, tenascin, TNMD, elastin, vimentin, decorin, FN, biglycan, SCX contents (3 weeks) | [322,324] | |
| SCX, MKX | effective gene expression, high type-I collagen, tenascin, TNMD contents (one week) | [282] | ||
| retroviral vectors | rabbit ACL cells | lacZ | effective gene expression (one week) | [314,315] |
| murine MSC line | MKX | high type-I collagen, decorin contents (5 days) | [283] | |
| rabbit MSCs | PDGF | effective gene expression (12 weeks) | [333] | |
| human tendon-derived stem cells | lncRNA (H19) | enhanced tenogenic differentiation (one week) | [334] | |
| lentiviral vectors | rat tendon cells | shRNA (decorin) | suppression of decorin expression (3 days) | [335] |
| rat ACL-derived stem cells | VEGF | effective gene expression (2 days) | [325] | |
| rat tendon-derived stem cells | miRNA (ROCK1) | high tenogenic differentiation (one week) | [336] | |
| human MSCs | SCX | effective gene expression, high type-I collagen, TNMD, decorin, FN, fibromodulin, lumican, α-SMA contents (cell selection) | [275] | |
| human ACL-derived stem cells | BMP-2 | effective gene expression (3 days) | [326] | |
| human iPSCs | MKX | high type-III collagen, decorin, fibromodulin, SCX contents (cell selection) | [327] | |
| rAAV vectors | human ACL cells, explants (normal, torn) | lacZ, FGF-2 | effective gene expression, high DNA, type-I/-III collagen, SCX, α-SMA, NF-κB contents (one month) | [328] |
| Vectors | Animal Models | Genes | Effects | Refs. |
|---|---|---|---|---|
| nonviral vectors | rat ligament lesion | lacZ, PDGF | effective gene expression, high type-I collagen deposition (4 weeks) | [337,338,339] |
| ODN (decorin) | suppression of decorin expression (4 weeks), high type-I collagen deposition with stronger mechanical properties (6 weeks) | [338,343] | ||
| rat, sheep tendon injury | mRNA (BMP-7) | effective gene expression, high type-III collagen deposition (one week) | [331] | |
| adenoviral vectors | rat ligament lesion | BMP-13 | high collagen deposition, neoligament formation (14 weeks) | [342] |
| rabbit ligament lesion | lacZ | effective gene expression (2 weeks; 6 weeks at very high vector dose) | [340,341] | |
| - | rat tendon injury | mRNA (FGF-2) | effective gene expression, stronger mechanical properties (2 weeks) | [344] |
| Vectors | Animal Models | Cells | Genes | Effects | Refs. |
|---|---|---|---|---|---|
| nonviral vectors | minipig ACL lesion | ACL graft | GFP, BMP-6 | effective gene expression (2 weeks), ACL repair with stronger mechanical properties (8 weeks) | [346] |
| adenoviral vectors | mouse ectopic injection (muscle) | murine MSC line | BMP-12 | high collagen deposition, neoligament formation (4 weeks) | [316] |
| rabbit ligament lesion | rabbit ACL cells | lacZ | effective gene expression (2 weeks) | [341] | |
| rabbit ACL lesion | rabbit MSCs | TGF-β, VEGF | ACL remodeling with stronger mechanical properties (24 weeks) | [348] | |
| rabbit tendon graft | lacZ, BMP-2 | effective gene expression (2 weeks), high collagen deposition with stronger mechanical properties (8 weeks) | [347] | ||
| retroviral vectors | rabbit ligament lesion | rabbit ACL cells | lacZ | effective gene expression (10 days) | [340] |
| rabbit MSCs | PDGF | high collagen deposition, ligament remodeling (12 weeks) | [333] | ||
| mouse tendon injury | human tendon-derived stem cells | lncRNA (H19) | high type-I collagen deposition, TNMD, decorin contents (4 weeks) | [334] | |
| lentiviral vectors | rat ACL lesion | human ACL-derived stem cells | BMP-2 | high type-I/-III collagen deposition, α-SMA contents, ACL repair (8 weeks) | [326] |
| rat ACL-derived stem cells | VEGF | high type-I collagen deposition, ligament remodeling with stronger mechanical properties (4 weeks) | [325] | ||
| rat tendon injury | rat tendon cells | shRNA (decorin) | high collagen deposition (4 weeks) | [335] |
| Animal Models | Vectors | Cell Targets | Genes | Biomaterials | Effects | Refs. |
|---|---|---|---|---|---|---|
| - | nonviral vectors | chicken tendon cells | GFP, miRNA (TGF-β) | PLGA nanospheres | effective gene expression (GFP), suppression of TGF-β expression (miRNA TGF-β) (3 weeks) | [384] |
| adenoviral vectors | bovine ACL cells | GFP | type-I collagen gel | effective gene expression (3 weeks) | [317] | |
| bovine ACL explants (torn) | TGF-β | type-I collagen gel | effective gene expression, high type-I/-III deposition, ACL repair (4 weeks) | [317] | ||
| human ACL explants (torn) | GFP, IGF-I | type-I collagen gel | effective gene expression, high DNA contents (IGF-I) (3 weeks) | [318] | ||
| rabbit ACL lesion | nonviral vectors | - | TGF-β | tendon graft | high collagen deposition, wound healing with stronger mechanical properties (6 months) | [385] |
| chicken tendon injury | nonviral vectors | - | miRNA (TGF-β) | PLGA nanospheres, 3DB composite (PCL, PDA NPs, gelatin, HA, alginate) | suppression of TGF-β expression, wound healing (6 weeks) | [384,386] |
| rat tendon injury | adenoviral vectors | - | BMP-12 | muscle graft | high collagen deposition, wound healing (4 weeks) | [319] |
| lentiviral vectors | - | shRNA (decorin) | tendon graft | high collagen deposition, wound healing (4 weeks) | [335] | |
| mouse tendon injury | rAAV vectors | - | luc, GDF-5 | tendon graft | effective gene expression, wound healing with stronger mechanical properties (2–3 weeks) | [387,388] |
| rat ACL lesion, tendon injury | - | - | miRNA (angiogenic miR-210) | type-I collagen gel | high type-I collagen deposition, VEGF expression, wound healing with stronger mechanical properties (4–12 weeks) | [389,390,391] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amini, M.; Venkatesan, J.K.; Liu, W.; Leroux, A.; Nguyen, T.N.; Madry, H.; Migonney, V.; Cucchiarini, M. Advanced Gene Therapy Strategies for the Repair of ACL Injuries. Int. J. Mol. Sci. 2022, 23, 14467. https://doi.org/10.3390/ijms232214467
Amini M, Venkatesan JK, Liu W, Leroux A, Nguyen TN, Madry H, Migonney V, Cucchiarini M. Advanced Gene Therapy Strategies for the Repair of ACL Injuries. International Journal of Molecular Sciences. 2022; 23(22):14467. https://doi.org/10.3390/ijms232214467
Chicago/Turabian StyleAmini, Mahnaz, Jagadeesh K. Venkatesan, Wei Liu, Amélie Leroux, Tuan Ngoc Nguyen, Henning Madry, Véronique Migonney, and Magali Cucchiarini. 2022. "Advanced Gene Therapy Strategies for the Repair of ACL Injuries" International Journal of Molecular Sciences 23, no. 22: 14467. https://doi.org/10.3390/ijms232214467
APA StyleAmini, M., Venkatesan, J. K., Liu, W., Leroux, A., Nguyen, T. N., Madry, H., Migonney, V., & Cucchiarini, M. (2022). Advanced Gene Therapy Strategies for the Repair of ACL Injuries. International Journal of Molecular Sciences, 23(22), 14467. https://doi.org/10.3390/ijms232214467








