Application of Antimicrobial Photodynamic Therapy for Inactivation of Acinetobacter baumannii Biofilms
Abstract
1. Introduction
2. Results
2.1. Inactivation of Planktonic A. baumannii Cells with aPDT
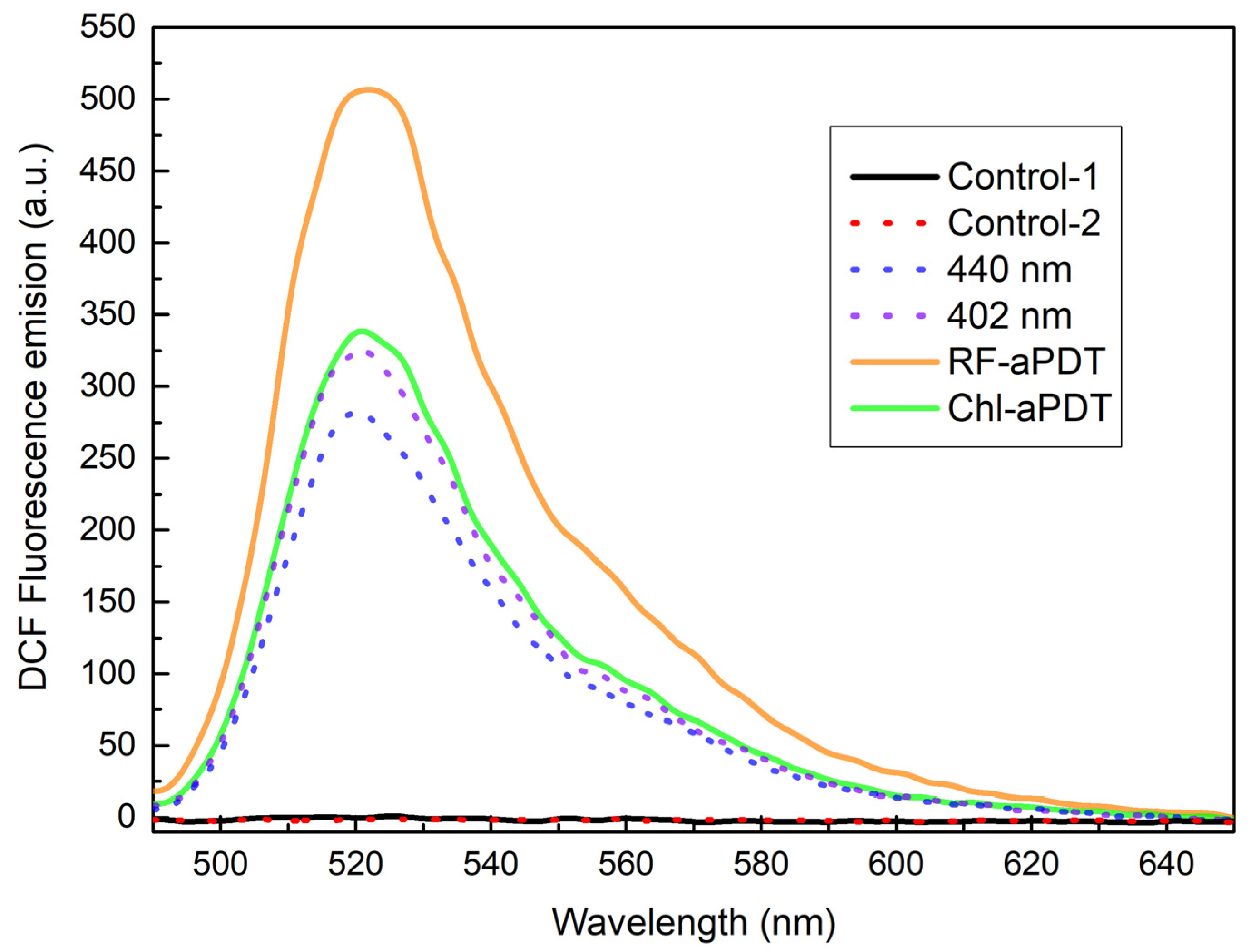
2.2. Intracellular ROS Generation
2.3. A. baumannii Biofilm Formation after aPDT Treatment
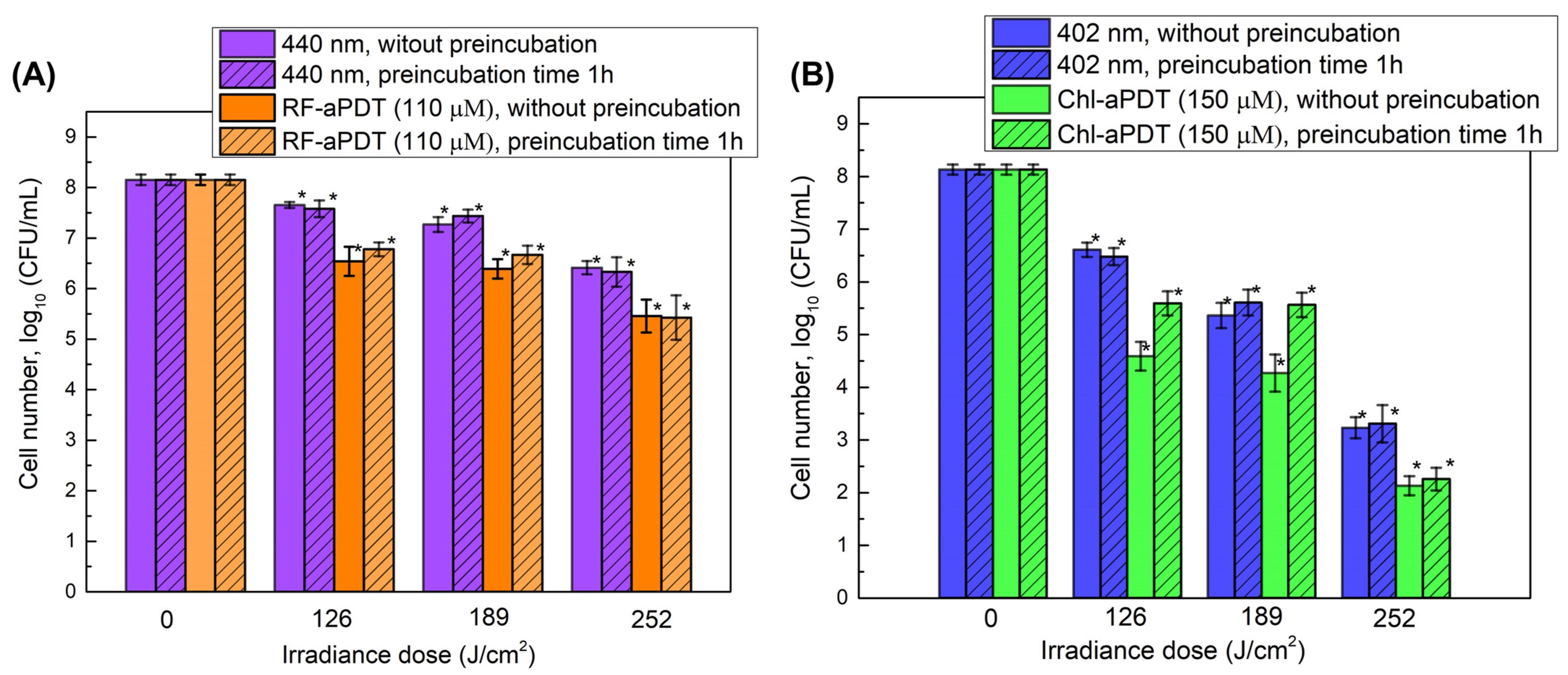
2.4. Inactivation of A. baumannii Biofilms with RF-aPDT and Chl-PDT
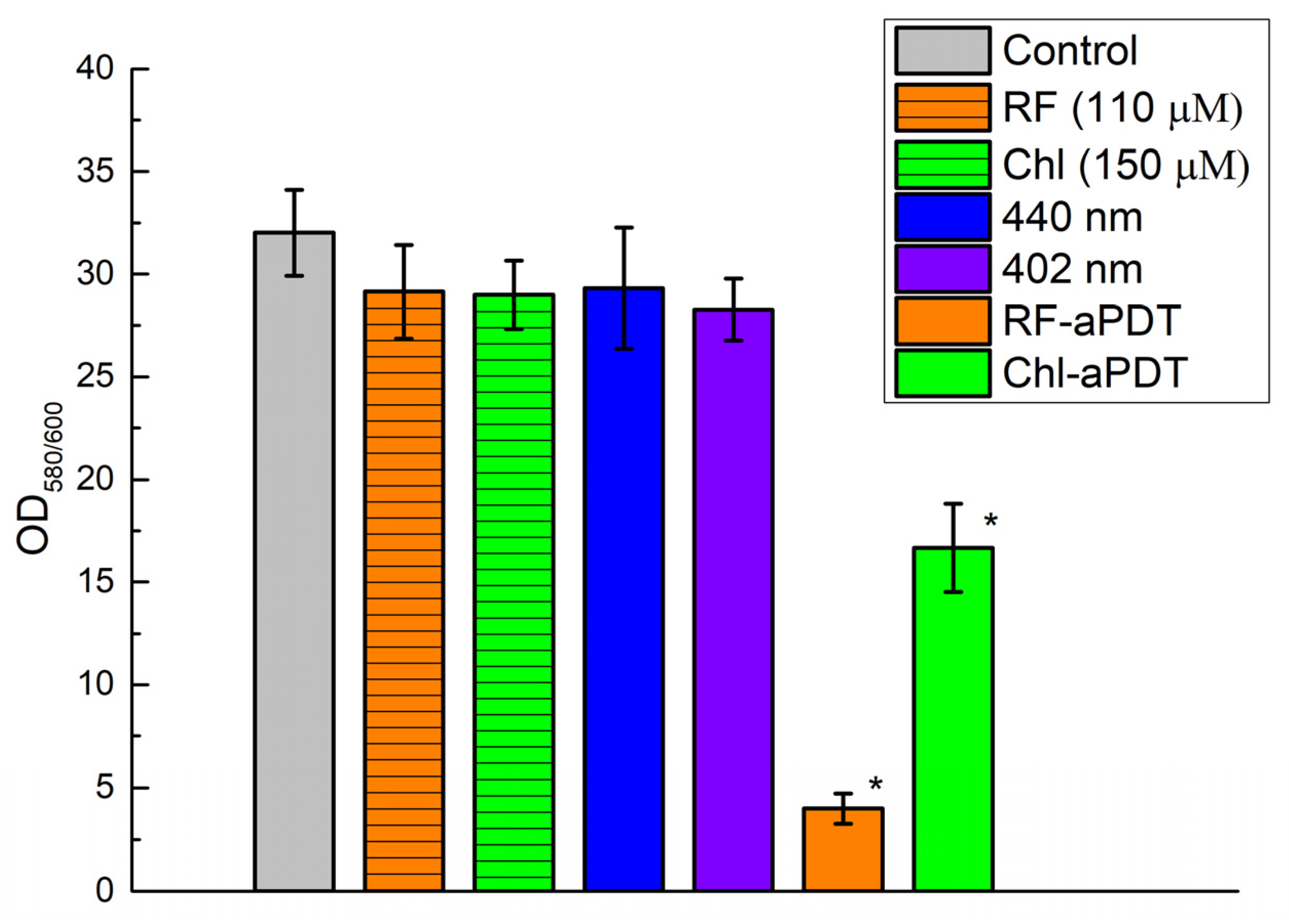
2.5. Quantification of Biofilms after aPDT
3. Discussion
4. Materials and Methods
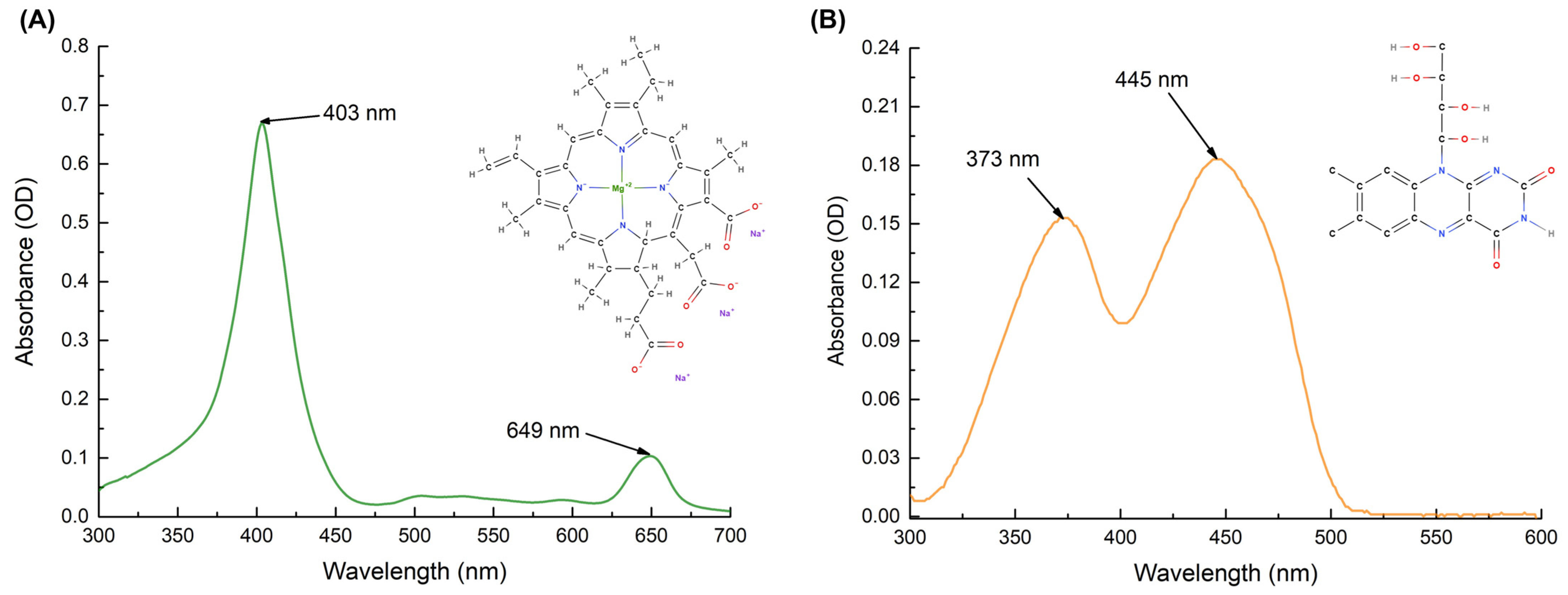
4.1. Preparation of Photosensitizers
4.2. Bacterial Strain
4.3. Light Source for aPDT
4.4. aPDT of A. baumannii Planktonic Cells
4.5. Detection of Intracellular ROS
4.6. A. baumannii Biofilm Formation after aPDT
4.7. aPDT for Inactivation of A. baumannii Biofilms
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Dadgostar, P. Antimicrobial resistance: Implications and costs. Infect. Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. The Antibiotic Resistance Crisis Part 1: Causes and Threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Mulani, M.S.; Kamble, E.E.; Kumkar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging Strategies to Combat ESKAPE Pathogens in the Era of Antimicrobial Resistance: A Review. Front. Microbiol. 2019, 10, 539. [Google Scholar] [CrossRef]
- Harding, C.M.; Hennon, S.W.; Feldman, M.F. Uncovering the mechanisms of Acinetobacter baumannii virulence. Nat. Rev. Microbiol. 2017, 16, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Ayobami, O.; Willrich, N.; Harder, T.; Okeke, I.N.; Eckmanns, T.; Markwart, R. The incidence and prevalence of hospital-acquired (carbapenem-resistant) Acinetobacter baumannii in Europe, Eastern Mediterranean and Africa: A systematic review and meta-analysis. Emerg. Microbes Infect. 2019, 8, 1747–1759. [Google Scholar] [CrossRef]
- Roy, S.; Chowdhury, G.; Mukhopadhyay, A.K.; Dutta, S.; Basu, S. Convergence of Biofilm Formation and Antibiotic Resistance in Acinetobacter baumannii Infection. Front. Med. 2022, 9, 793615. [Google Scholar] [CrossRef] [PubMed]
- Asif, M.; Alvi, I.A.; Rehman, S.U. Insight into Acinetobacter baumannii: Pathogenesis, global resistance, mechanisms of resistance, treatment options, and alternative modalities. Infect. Drug Resist. 2018, 11, 1249–1260. [Google Scholar] [CrossRef]
- Songca, S.P.; Adjei, Y. Applications of Antimicrobial Photodynamic Therapy against Bacterial Biofilms. Int. J. Mol. Sci. 2022, 23, 3209. [Google Scholar] [CrossRef]
- Hu, X.; Huang, Y.Y.; Wang, Y.; Wang, X.; Hamblin, M.R. Antimicrobial Photodynamic Therapy to Control Clinically Relevant Bio-film Infections. Front. Microbiol. 2018, 9, 1299. [Google Scholar] [CrossRef]
- Hamblin, M.R. Antimicrobial photodynamic inactivation: A bright new technique to kill resistant microbes. Curr. Opin. Microbiol. 2016, 33, 67–73. [Google Scholar] [CrossRef]
- Wainwright, M.; Maisch, T.; Nonell, S.; Plaetzer, K.; Almeida, A.; Tegos, G.P.; Hamblin, M.R. Photoantimicrobials—Are we afraid of the light? Lancet Infect. Dis. 2017, 17, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Gilaberte, Y.; Rezusta, A.; Juarranz, A.; Hamblin, M.R. Editorial: Antimicrobial Photodynamic Therapy: A New Paradigm in the Fight against Infections. Front. Med 2021, 8, 788888. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.; Gomes, I.B.; Saavedra, M.J.; Simões, M. Photodynamic therapy and combinatory treatments for the control of biofilm-associated infections. Lett. Appl. Microbiol. 2022, 75, 548–564. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Misba, L.; Khan, A.U. Antibiotics versus biofilm: An emerging battleground in microbial communities. Antimicrob. Resist. Infect. Control 2019, 8, 76. [Google Scholar] [CrossRef]
- Goulart, R.D.; Bolean, M.; Paulino, T.D.; Thedei, G., Jr.; Souza, S.L.; Tedesco, A.C.; Ciancaglini, P. Photodynamic therapy in planktonic and biofilm cultures of Aggregatibacter actinomycetemcomitans. Photomed. Laser Surg. 2010, 28, 53–60. [Google Scholar] [CrossRef] [PubMed]
- García, I.; Ballesta, S.; Gilaberte, Y.; Rezusta, A.; Pascual, Á. Antimicrobial photodynamic activity of hypericin against methicillin-susceptible and resistant Staphylococcus aureus biofilms. Future Microbiol. 2015, 10, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Diogo, P.; Fernandes, C.; Caramelo, F.; Mota, M.; Miranda, I.M.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Uliana, M.P.; de Oliveira, K.T.; Santos, J.M.; et al. Antimicrobial Photodynamic Therapy against Endodontic Enterococcus faecalis and Candida albicans Mono and Mixed Biofilms in the Presence of Photosensitizers: A Comparative Study with Classical Endodontic Irrigants. Front. Microbiol. 2017, 8, 498. [Google Scholar] [CrossRef] [PubMed]
- da Fonseca, A.D.S.; Mencalha, A.L.; de Paoli, F. Antimicrobial photodynamic therapy against Acinetobacter baumannii. Photodiagnosis Photodyn. Ther. 2021, 35, 102430. [Google Scholar] [CrossRef]
- Buchovec, I.; Gricajeva, A.; Kalėdienė, L.; Vitta, P. Antimicrobial Photoinactivation Approach Based on Natural Agents for Control of Bacteria Biofilms in Spacecraft. Int. J. Mol. Sci. 2020, 21, 6932. [Google Scholar] [CrossRef]
- Polat, E.; Kang, K. Natural Photosensitizers in Antimicrobial Photodynamic Therapy. Biomedicines 2021, 9, 584. [Google Scholar] [CrossRef]
- Buchovec, I.; Klimkaitė, L.; Sužiedėlienė, E.; Bagdonas, S. Inactivation of opportunistic pathogens Acinetobacter baumannii and Stenotrophomonas maltophilia by antimicrobial photodynamic therapy. Microorganisms 2022, 10, 506. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, Y.; Gupta, A.; Huang, Y.; Murray, C.K.; Vrahas, M.S.; Sherwood, M.E.; Baer, D.G.; Hamblin, M.R.; Dai, T. Antimicrobial blue light therapy for multidrug-resistant Acinetobacter baumannii infection in a mouse burn model: Implications for prophylaxis and treatment of combat-related wound infections. J. Infect. Dis. 2014, 209, 1963–1971. [Google Scholar] [CrossRef]
- Hadi, J.; Wu, S.; Soni, A.; Gardner, A.; Brightwell, G. Genetic Factors Affect the Survival and Behaviors of Selected Bacteria during Antimicrobial Blue Light Treatment. Int. J. Mol. Sci. 2021, 22, 10452. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.; Fernandes, E.; Lima, J.L. Fluorescence probes used for detection of reactive oxygen species. J. Biochem. Biophys. Methods 2005, 65, 45–80. [Google Scholar] [CrossRef] [PubMed]
- Castro-Alférez, M.; Polo-López, M.I.; Fernández-Ibáñez, P. Intracellular mechanisms of solar water disinfection. Sci. Rep. 2016, 6, 38145. [Google Scholar] [CrossRef]
- Khan, S.; Rayis, M.; Rizvi, A.; Alam, M.M.; Rizvi, M.; Naseem, I. ROS mediated antibacterial activity of photoilluminated riboflavin: A photodynamic mechanism against nosocomial infections. Toxicol. Rep. 2019, 6, 136–142. [Google Scholar] [CrossRef]
- Banerjee, S.; Ghosh, D.; Vishakha, K.; Das, S.; Mondal, S.; Ganguli, A. Photodynamic antimicrobial chemotherapy (PACT) using riboflavin inhibits the mono and dual species biofilm produced by antibiotic resistant Staphylococcus aureus and Escherichia coli. Photodiagnosis Photodyn. Ther. 2020, 32, 102002. [Google Scholar] [CrossRef]
- Kim, D.; Shin, M.; Kim, H.; Kang, D. Inactivation efficacy of combination treatment of blue light-emitting diodes (LEDs) and riboflavin to control E. coli O157:H7 and S. typhimurium in apple juice. Innov. Food Sci. Emerg. Technol. 2022, 78, 103014. [Google Scholar] [CrossRef]
- Buchovec, I.; Lukseviciūtė, V.; Kokstaite, R.; Labeikyte, D.; Kaziukonyte, L.; Luksiene, Z. Inactivation of Gram (−) bacteria Salmonella enterica by chlorophyllin-based photosensitization: Mechanism of action and new strategies to enhance the inactivation efficiency. J. Photochem. Photobiol. B 2017, 172, 1–10. [Google Scholar] [CrossRef]
- Demidova, T.N.; Hamblin, M.R. Effect of cell-photosensitizer binding and cell density on microbial photoinactivation. Antimicrob. Agents Chemother. 2005, 49, 2329–2335. [Google Scholar] [CrossRef]
- Zoltan, T.; Vargas, F.; López, V.; Chávez, V.; Rivas, C.; Ramírez, Á.H. Influence of charge and metal coordination of meso-substituted porphyrins on bacterial photoinactivation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 135, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Anane, Y.A.; Apalata, T.; Vasaikar, S.; Okuthe., G.E.; Songca, S.P. In vitro antimicrobial photodynamic inactivation of multidrug-resistant Acinetobacter baumannii biofilm using Protoporphyrin IX and Methylene blue. Photodiagnosis Photodyn. Ther. 2020, 30, 101752. [Google Scholar] [CrossRef] [PubMed]
- Galo, I.D.C.; Carvalho, J.A.; Santos, J.L.M.C.; Braoios, A.; Prado, R.P. The ineffectiveness of antimicrobial photodynamic therapy in the absence of preincubation of the microorganisms in the photosensitizer. Fisioter. Mov. 2020, 33, e003304. [Google Scholar] [CrossRef]
- Stefanelli, M.; Mandoj, F.; Magna, G.; Lettieri, R.; Venanzi, M.; Paolesse, R.; Monti, D. The Self-Aggregation of Porphyrins with Multiple Chiral Centers in Organic/Aqueous Media: The Case of Sugar- and Steroid-Porphyrin Conjugates. Molecules 2020, 25, 4544. [Google Scholar] [CrossRef]
- Skerniškytė, J.; Krasauskas, R.; Péchoux, C.; Kulakauskas, S.; Armalytė, J.; Sužiedėlienė, E. Surface-related features and virulence among Acinetobacter baumannii clinical isolates belonging to international clones I and II. Front. Microbiol. 2019, 9, 3116. [Google Scholar] [CrossRef]
- Naghili, H.; Tajik, H.; Mardani, K.; Razavi Rouhani, S.M.; Ehsani, A.; Zare, P. Validation of drop plate technique for bacterial enumeration by parametric and nonparametric tests. Vet. Res. Forum 2013, 4, 179–183. [Google Scholar]
- Misba, L.; Zaidi, S.; Khan, A.U. Efficacy of photodynamic therapy against Streptococcus mutans biofilm: Role of singlet oxygen. J. Photochem. Photobiol. B Biol. 2018, 183, 16–21. [Google Scholar] [CrossRef]




| Planktonic Cells | Biofilms | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Time (min) | 10 | 15 | 20 | 25 | 30 | 40 | 60 | 90 | 120 |
| Irradiance dose (J/cm2) | 21 | 31.5 | 42 | 52.5 | 63 | 84 | 126 | 189 | 252 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buchovec, I.; Vyčaitė, E.; Badokas, K.; Sužiedelienė, E.; Bagdonas, S. Application of Antimicrobial Photodynamic Therapy for Inactivation of Acinetobacter baumannii Biofilms. Int. J. Mol. Sci. 2023, 24, 722. https://doi.org/10.3390/ijms24010722
Buchovec I, Vyčaitė E, Badokas K, Sužiedelienė E, Bagdonas S. Application of Antimicrobial Photodynamic Therapy for Inactivation of Acinetobacter baumannii Biofilms. International Journal of Molecular Sciences. 2023; 24(1):722. https://doi.org/10.3390/ijms24010722
Chicago/Turabian StyleBuchovec, Irina, Enrika Vyčaitė, Kazimieras Badokas, Edita Sužiedelienė, and Saulius Bagdonas. 2023. "Application of Antimicrobial Photodynamic Therapy for Inactivation of Acinetobacter baumannii Biofilms" International Journal of Molecular Sciences 24, no. 1: 722. https://doi.org/10.3390/ijms24010722
APA StyleBuchovec, I., Vyčaitė, E., Badokas, K., Sužiedelienė, E., & Bagdonas, S. (2023). Application of Antimicrobial Photodynamic Therapy for Inactivation of Acinetobacter baumannii Biofilms. International Journal of Molecular Sciences, 24(1), 722. https://doi.org/10.3390/ijms24010722






