Structure-Based Function and Regulation of NCX Variants: Updates and Challenges
Abstract
1. Introduction
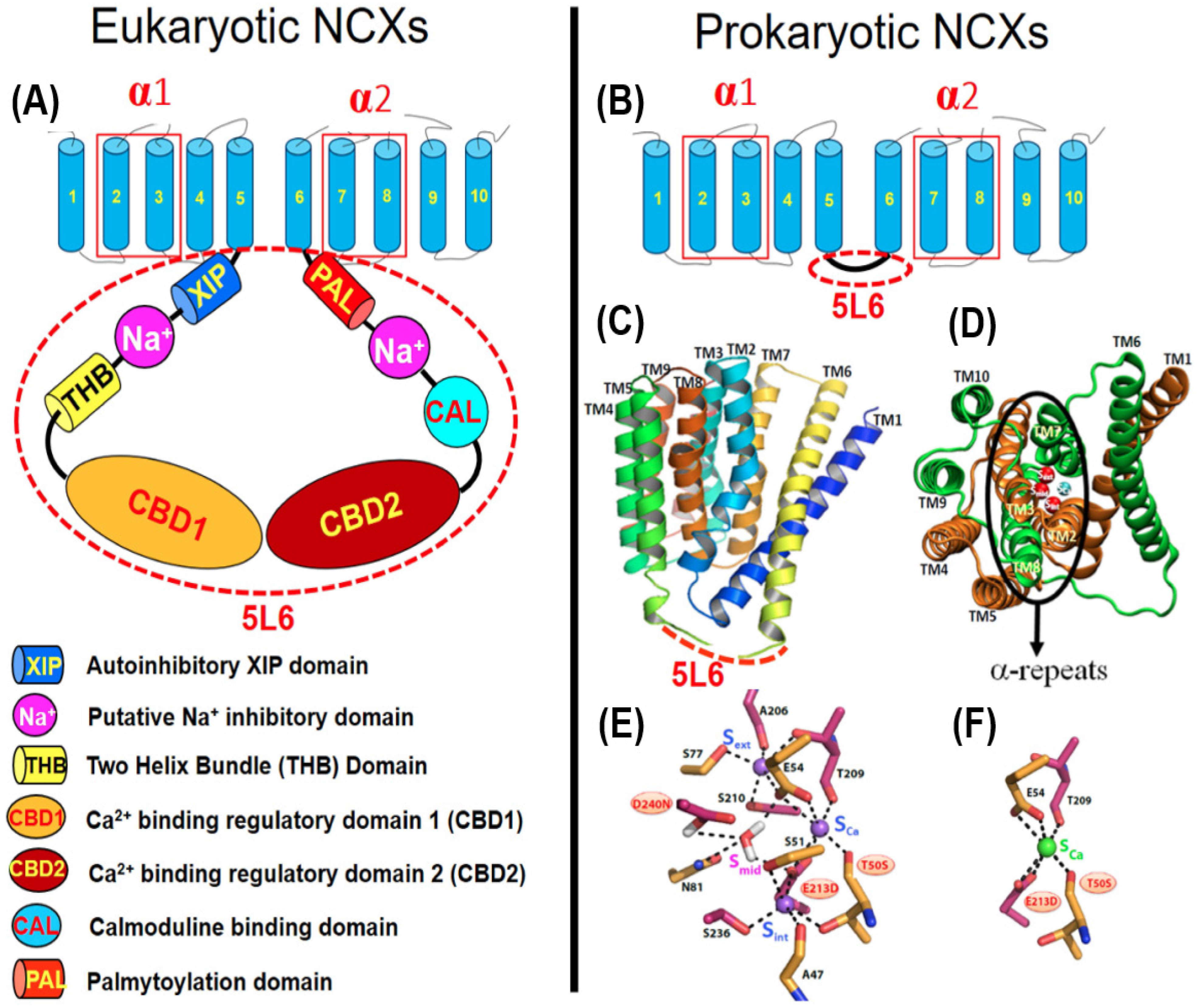
2. Molecular Hallmarks of Prokaryotic and Eukaryotic NCXs
2.1. NCX Proteins Share Some Basic Structural Motifs with Ca2+/CA Proteins
2.2. NCXs Share Ion-Exchange Stoichiometry While Owing Very Different Transport Rates
2.3. NCX Can Mediate Either the Ca2+-Exit (Forward) or Ca2+-Entry (Reverse) Mode
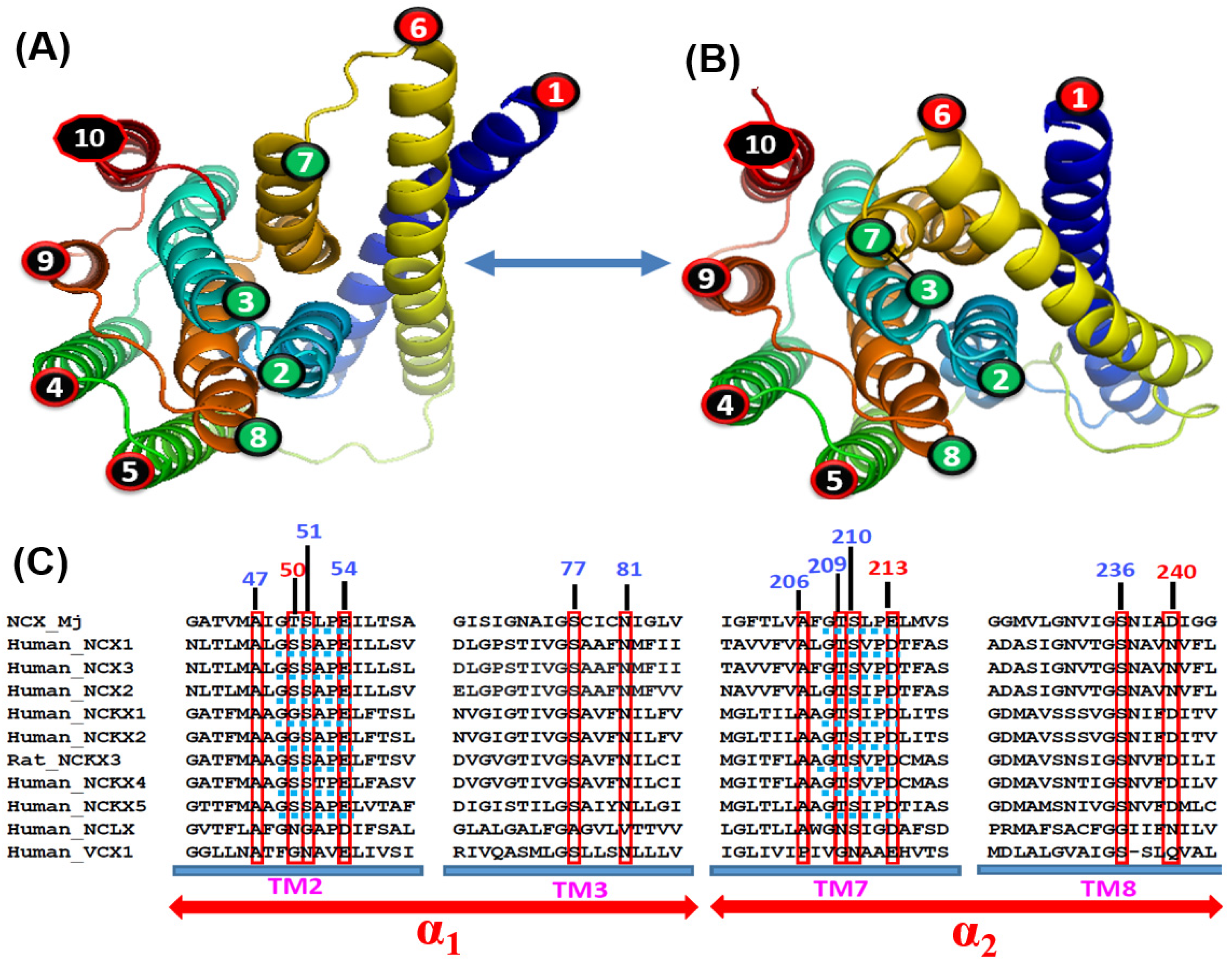
2.4. Bidirectional Ion Access/Transport Is Asymmetric in NCX_Mj and Eukaryotic NCX
2.5. Structure-Based Divergence of NCX Regulation Is Critical for Cell-Specific Functions
3. The Ca2+/CA Antiporters Translocate Ca2+ in Exchange for Different Counter-Ions
3.1. The NCX_Mj Structure as a Prototype Model for Studying Ion-Transport Mechanisms
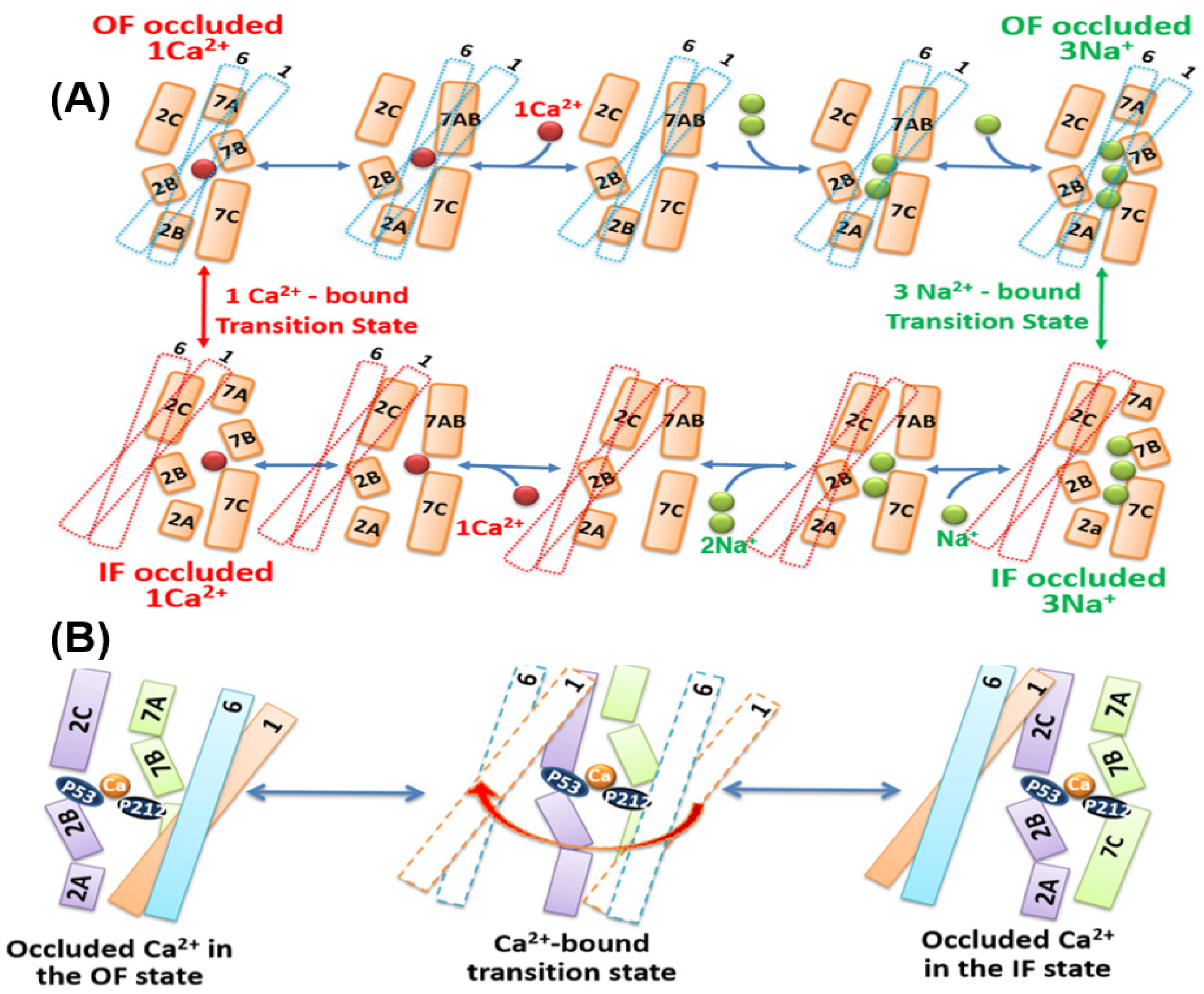
3.2. Structural Bases of Ion Transport Stoichiometry, Selectivity, and Alternating Access
3.3. The Ca2+/CA Proteins Might Share a Common Mechanism of Alternating Access
3.3.1. Ion Binding Sites of CAX
3.3.2. Ion Binding Sites of NCKX
3.3.3. Ion Binding Sites of NCLX
3.4. Structure-Dynamic Basis of Functional Asymmetry in NCX_Mj and Similar Proteins
3.5. Structure-Dynamic Specificities Associated with Ion Occlusion in NCX_Mj
3.6. Structural Elements Associated with the Unifying Mechanism of Alternating Access
3.7. Structure-Dynamic Causes of Kinetic Variances Remain to Be Resolved
3.8. Charge-Carrying Features of Ion-Bound Species Are Alike among NCXs
4. Regulatory Divergence of NCXs Is Required to Match Cell-Specific Ca2+ Signaling
4.1. NCX Coupling with Other Ion Transport Systems Requires a Further Resolution
4.2. Eukaryotic NCXs Exhibit Different Modes of Ion-Dependent Regulation
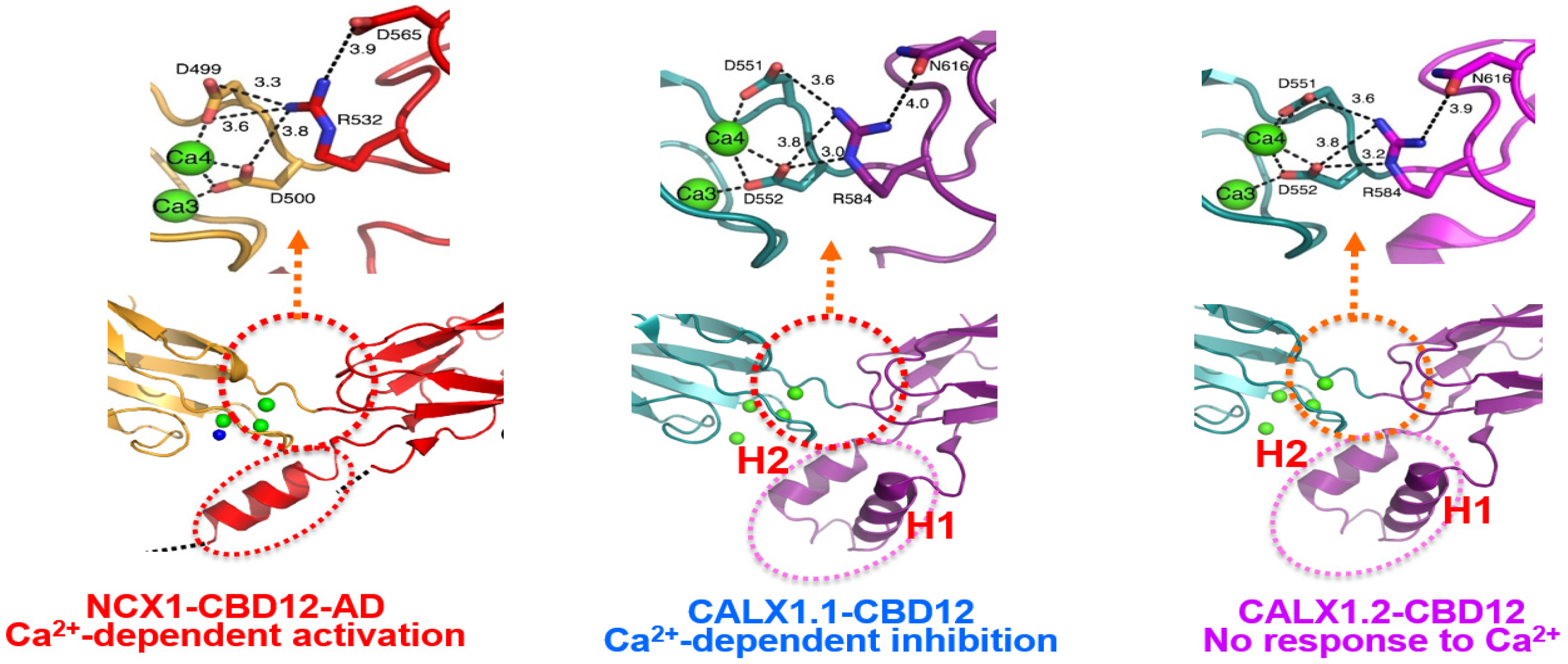
4.2.1. NCX and CALX Exhibit Positive, Negative, or No Response to Regulatory Ca2+
4.2.2. Na+-Induced Inactivation: Where Does the Na+ Site Locate and How Does It Operate?
4.2.3. Multiple Proton Sensors May Contribute to the Proton-Dependent NCX Inactivation
5. Structure-Dynamic Determinants of Regulatory Divergence in Eukaryotic NCXs
5.1. Structure-Functional Specificities of High-Affinity Ca2+ Binding Sites at CBD1
5.2. Varying Compositions of Exons Control the Affinity and Number of Ca2+ Sites at CBD2
5.3. The CBD1-CBD2 Linker and Dynamic Coupling of Ca2+-Dependent Tethering of CBDs
5.4. The Structure of the Two-Domain Interface Predefines the Dynamic Coupling of CBDs
5.5. Dynamic Features Might Predefine the Opposing Responses of NCX and CALX to Regulatory Ca2+
5.6. Population Shift Mechanism Can Account for Opposite Responses to Regulatory Ca2+
5.7. Allosteric Signal Transfer from CBD1 to CBD2 May Modulate the TM1/TM6 Sliding
5.8. Mutually Exclusive and Cassette Exons Operate through Different Regulatory Modules
6. Lipids Modulate Mammalian NCXs through Unknown Mechanisms
7. Palmitoylation of Mammalian NCX: A Coupling Unit for Functional Integration?
8. Conclusions and Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Philipson, K.D.; Nicoll, D.A. Sodium-calcium exchange: A molecular perspective. Annu. Rev. Physiol. 2000, 62, 111–133. [Google Scholar] [CrossRef]
- Blaustein, M.P.; Lederer, W.J. Sodium/calcium exchange: Its physiological implications. Physiol. Rev. 1999, 79, 763–854. [Google Scholar] [CrossRef] [PubMed]
- Khananshvili, D. Basic and editing mechanisms underlying ion transport and regulation in NCX variants. Cell Calcium 2019, 85, 102131. [Google Scholar] [CrossRef] [PubMed]
- Khananshvili, D. The Archaeal Na+/Ca2+ Exchanger (NCX_Mj) as a Model of Ion Transport for the Superfamily of Ca2+/CA Antiporters. Front. Chem. 2021, 9, 722336. [Google Scholar] [CrossRef] [PubMed]
- Lytton, J. Na+/Ca2+ exchangers: Three mammalian gene families control Ca2+-transport. Biochem. J. 2007, 406, 365–382. [Google Scholar] [CrossRef]
- Ottolia, M.; John, S.; Hazan, A.; Goldhaber, J.I. The Cardiac Na+-Ca2+ Exchanger: From Structure to Function. Compr. Physiol. 2021, 12, 2681–2717. [Google Scholar] [CrossRef]
- Khananshvili, D. The SLC8 gene family of sodium-calcium exchangers (NCX)—Structure, function, and regulation in health and disease. Mol. Aspects Med. 2013, 34, 220–235. [Google Scholar] [CrossRef]
- Khananshvili, D. Sodium-Calcium Exchangers (NCX): Molecular Hallmarks Underlying the Tissue-Specific and Systemic Functions. Plügers Arch. 2014, 466, 43–60. [Google Scholar] [CrossRef]
- Song, S.; Luo, L.; Sun, B.; Sun, D. Roles of glial ion transporters in brain diseases. Glia 2019, 68, 472–494. [Google Scholar] [CrossRef]
- Molinaro, P.; Natale, S.; Serani, A.; Calabrese, L.; Secondo, A.; Tedeschi, V.; Valsecchi, V.; Pannaccione, A.; Scorziello, A.; Annunziato, L. Genetically modified mice to unravel physiological and pathophysiological roles played by NCX isoforms. Cell Calcium 2020, 87, 102189. [Google Scholar] [CrossRef]
- Luo, L.; Song, S.; Ezenwukwa, C.C.; Jalali, S.; Sun, B.; Sun, D. Ion channels and transporters in microglial function in physiology and brain diseases. Neurochem. Int. 2020, 142, 104925. [Google Scholar] [CrossRef] [PubMed]
- Rose, C.R.; Ziemens, D.; Verkhratsky, A. On the special role of NCX in astrocytes: Translating Na+-transients into intracellular Ca2+ signals. Cell Calcium 2019, 86, 102154. [Google Scholar] [CrossRef] [PubMed]
- Boscia, F.; de Rosa, V.; Cammarota, M.; Secondo, A.; Pannaccione, A.; Annunziato, L. The Na+/Ca2+ exchangers in demyelinating diseases. Cell Calcium 2019, 85, 102130. [Google Scholar] [CrossRef] [PubMed]
- Rose, C.R.; Verkhratsky, A. Principles of sodium homeostasis and sodium signaling in astroglia. Glia 2016, 64, 1611–1627. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.; Piccirillo, S.; Magi, S.; Preziuso, A.; Ramos, V.d.S.; Serfilippi, T.; Orciani, M.; Alvarez, M.M.P.; Tersariol, I.L.D.S.; Amoroso, S.; et al. Control of Ca2+ and metabolic homeostasis by the Na+/Ca2+ exchangers (NCXs) in health and disease. Biochem. Pharmacol. 2022, 203, 115163. [Google Scholar] [CrossRef]
- Liu, Z.; Cheng, Q.; Ma, X.; Song, M. Suppressing Effect of Na+/Ca2+ Exchanger (NCX) Inhibitors on the Growth of Melanoma Cells. Int. J. Mol. Sci. 2022, 23, 901. [Google Scholar] [CrossRef]
- Magli, E.; Fattorusso, C.; Persico, M.; Corvino, A.; Esposito, G.; Fiorino, F.; Luciano, P.; Perissutti, E.; Santagada, V.; Severino, B.; et al. New Insights into the Structure–Activity Relationship and Neuroprotective Profile of Benzodiazepinone Derivatives of Neurounina-1 as Modulators of the Na+/Ca2+ Exchanger Isoforms. J. Med. Chem. 2021, 64, 17901–17919. [Google Scholar] [CrossRef]
- Ward, P.A.; Fattahi, F. New strategies for treatment of infectious sepsis. J. Leukoc. Biol. 2019, 106, 187–192. [Google Scholar] [CrossRef]
- Szlovák, J.; Tomek, J.; Zhou, X.; Tóth, N.; Veress, R.; Horváth, B.; Szentandrássy, N.; Levijoki, J.; Papp, J.G.; Herring, N.; et al. Blockade of sodium-calcium exchanger via ORM-10962 attenuates cardiac alternans. J. Mol. Cell. Cardiol. 2020, 153, 111–122. [Google Scholar] [CrossRef]
- Tóth, A.; Kiss, L.; Varró, A.; Nánási, P.P. Potential therapeutic effects of Na+/Ca2+ exchanger inhibition in cardiac diseases. Curr. Med. Chem. 2009, 16, 3294–3321. [Google Scholar] [CrossRef]
- Pignataro, G.; Sirabella, R.; Anzilotti, S.; Di Renzo, G.; Annunziato, L. Does Na+/Ca2+ Exchanger, NCX, Represent a New Druggable Target in Stroke Intervention? Transl. Stroke Res. 2013, 5, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Chovancova, B.; Liskova, V.; Babula, P.; Krizanova, O. Role of Sodium/Calcium Exchangers in Tumors. Biomolecules 2020, 10, 1257. [Google Scholar] [CrossRef] [PubMed]
- Annunziato, L.; Secondo, A.; Pignataro, G.; Scorziello, A.; Molinaro, P. New perspectives for selective NCX activators in neurodegenerative diseases. Cell Calcium 2020, 87, 102170. [Google Scholar] [CrossRef] [PubMed]
- Giladi, M.; Shor, R.; Lisnyansky, M.; Khananshvili, D. Structure-Functional Basis of Ion Transport in Sodium–Calcium Exchanger (NCX) Proteins. Int. J. Mol. Sci. 2016, 17, 1949. [Google Scholar] [CrossRef]
- Giladi, M.; Tal, I.; Khananshvili, D. Structural Features of Ion Transport and Allosteric Regulation in Sodium-Calcium Exchanger (NCX) Proteins. Front. Physiol. 2016, 7, 30. [Google Scholar] [CrossRef]
- Khananshvili, D. Structure-Dynamic and Regulatory Specificities of Epithelial Na+/Ca2+ Exchangers (NCX). In Ion Channels and Transporters of Epithelia in Heatllh and Disease; Hamilton, K., Devor, C.D., Eds.; Springer International Publishing AG: Cham, Switzerland, 2020; Chapter 8; pp. 325–381. [Google Scholar]
- Liao, J.; Li, H.; Zeng, W.; Sauer, D.B.; Belmares, R.; Jiang, Y. Structural Insight into the Ion-Exchange Mechanism of the Sodium/Calcium Exchanger. Science 2012, 335, 686–690. [Google Scholar] [CrossRef]
- Liao, J.; Marinelli, F.; Lee, C.; Huang, Y.; Faraldo-Gómez, J.D.; Jiang, Y. Mechanism of extracellular ion exchange and binding-site occlusion in a sodium/calcium exchanger. Nat. Struct. Mol. Biol. 2016, 23, 590–599. [Google Scholar] [CrossRef]
- DiPolo, R.; Beaugé, L. Sodium/calcium exchanger: Influence of metabolic regulation on ion carrier interactions. Physiol. Rev. 2006, 86, 155–203. [Google Scholar] [CrossRef]
- Kaback, H.R.; Smirnova, I.; Kasho, V.; Nie, Y.; Zhou, Y. The Alternating Access Transport Mechanism in LacY. J. Membr. Biol. 2010, 239, 85–93. [Google Scholar] [CrossRef]
- Forrest, L.R.; Krämer, R.; Ziegler, C. The structural basis of secondary active transport mechanisms. Biochim. Biophys. Acta 2011, 1807, 167–188. [Google Scholar] [CrossRef]
- Drew, D.; Boudker, O. Shared molecular mechanisms of membrane transporters. Annu. Rev. Biochem. 2016, 85, 543–572. [Google Scholar] [CrossRef] [PubMed]
- Roux, B.; Bernèche, S.; Egwolf, B.; Lev, B.; Noskov, S.; Rowley, C.; Yu, H. Ion selectivity in channels and transporters. J. Gen. Physiol. 2011, 137, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Waight, A.B.; Pedersen, B.P.; Schlessinger, A.; Bonomi, M.; Chau, B.H.; Roe-Zurz, Z.; Risenmay, A.J.; Sali, A.; Stroud, R.M. Structural basis for alternating access of a eukaryotic calcium/proton exchanger. Nature 2013, 499, 107–110. [Google Scholar] [CrossRef]
- Nishizawa, T.; Kita, S.; Maturana, A.D.; Furuya, N.; Hirata, K.; Kasuya, G.; Ogasawara, S.; Dohmae, N.; Iwamoto, T.; Ishitani, R.; et al. Structural Basis for the Counter-Transport Mechanism of a H+/Ca2+ Exchanger. Science 2013, 341, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Tong, S.; Waltersperger, S.; Diederichs, K.; Wang, M.; Zheng, L. Crystal structure of Ca2+/H+ antiporter protein YfkE reveals the mechanisms of Ca2+ efflux and its pH regulation. Proc. Natl. Acad. Sci. USA 2013, 110, 11367–11372. [Google Scholar] [CrossRef]
- Hilge, M.; Aelen, J.; Vuister, G.W. Ca2+ regulation in the Na+/Ca2+ exchanger involves two markedly different Ca2+ sensors. Mol. Cell 2006, 22, 15–25. [Google Scholar] [CrossRef]
- Hilge, M.; Aelen, J.; Foarce, A.; Perrakis, A.; Vuister, G.W. Ca2+ regulation in the Na+/Ca2+ exchanger features a dual electrostatic switch mechanism. Proc. Natl. Acad. Sci. USA 2009, 106, 14333–14338. [Google Scholar] [CrossRef]
- Nicoll, D.A.; Sawaya, M.R.; Kwon, S.; Cascio, D.; Philipson, K.D.; Abramson, J. The crystal structure of the primary Ca2+ sensor of the Na+/Ca2+ exchanger reveals a novel Ca2+ binding motif. J. Biol. Chem. 2006, 281, 21577–21581. [Google Scholar] [CrossRef]
- Besserer, G.M.; Ottolia, M.; Nicoll, D.A.; Chaptal, V.; Cascio, D.; Philipson, K.D.; Abramson, J. The second Ca2+-binding domain of the Na+-Ca2+ exchanger is essential for regulation: Crystal structures and mutational analysis. Proc. Natl. Acad. Sci. USA 2007, 104, 18467–18472. [Google Scholar] [CrossRef]
- Wu, M.; Tong, S.; Gonzalez, J.; Jayaraman, V.; Spudich, J.L.; Zheng, L. Structural Basis of the Ca2+ Inhibitory Mechanism of Drosophila Na+/Ca2+ Exchanger CALX and Its Modification by Alternative Splicing. Structure 2011, 19, 1509–1517. [Google Scholar] [CrossRef]
- Giladi, M.; Sasson, Y.; Fang, X.; Hiller, R.; Buki, T.; Wang, Y.-X.; Hirsch, J.A.; Khananshvili, D. Ca2+-Driven Interdomain Switch of NCX: Structural and Biochemical Studies of the Two-Domain Ca2+ Sensor. PLoS ONE 2012, 7, e39985. [Google Scholar]
- Emery, L.; Whelan, S.; Hirschi, K.D.; Pittman, J.K. Protein Phylogenetic analysis of Ca2+/CA antiporters and insights into their evolution in plants. Front. Plant Sci. 2012, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Pittman, J.K.; Hirschi, K.D. CAX-ing a wide net: Cation/H+ transporters in metal remediation and abiotic stress signalling. Plant Biol. 2016, 18, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Schnetkamp, P.P. The SLC24 gene family of Na+/Ca2+–K+ exchangers: From sight and smell to memory consolidation and skin pigmentation. Mol. Asp. Med. 2013, 34, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Jalloul, A.H.; Szerencsei, R.T.; Rogasevskaia, T.P.; Schnetkamp, P.P.M. Structure-function relationships of K+-dependent Na+/Ca2+ exchangers (NCKX). Cell Calcium 2020, 86, 102153. [Google Scholar] [CrossRef] [PubMed]
- Zhekova, H.; Zhao, C.; Schnetkamp, P.P.M.; Noskov, S.Y. Characterization of the Cation Binding Sites in the NCKX2 Na+/Ca2+-K+ Exchanger. Biochemistry 2016, 55, 6445–6455. [Google Scholar] [CrossRef] [PubMed]
- Assali, E.A.; Sekler, I. Sprinkling salt on mitochondria: The metabolic and pathophysiological roles of mitochondrial Na+ signaling mediated by NCLX. Cell Calcium 2021, 97, 102416. [Google Scholar] [CrossRef]
- Forrest, L.R. Structural Symmetry in Membrane Proteins. Annu. Rev. Biophys. 2015, 44, 311–337. [Google Scholar] [CrossRef]
- Duran, A.M.; Meiler, J. Inverted Topologies in Membrane Proteins: A Mini-Review. Comput. Struct. Biotechnol. J. 2013, 8, e201308004. [Google Scholar] [CrossRef][Green Version]
- Reeves, J.P.; Hale, C.C. The stoichiometry of the cardiac sodium-calcium exchange system. J. Biol. Chem. 1984, 259, 7733–7739. [Google Scholar] [CrossRef]
- Bers, N.M.; Ginsburg, K.S. Na:Ca stoichiometry and cytosolic Ca-dependent activation of NCX in intact cardiomyocytes. In Annals of the New York Academy of Sciences; Blackwell Publishing Inc.: Hoboken, NJ, USA, 2007; pp. 326–338. [Google Scholar] [CrossRef]
- Shlosman, I.; Marinelli, F.; Faraldo-Gómez, J.D.; Mindell, J.A. The prokaryotic Na+/Ca2+ exchanger NCX_Mj transports Na+ and Ca2+ in a 3:1 stoichiometry. J. Gen. Physiol. 2017, 150, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Khananshvili, D. Distinction between the two basic mechanisms of cation transport in the cardiac Na+-Ca2+ exchange system. Biochemistry 1990, 29, 2437–2442. [Google Scholar] [CrossRef]
- Hilgemann, D.W.; Nicoll, D.A.; Philipson, K.D. Charge movement during Na+ translocation by native and cloned cardiac Na+/Ca2+ exchanger. Nature 1991, 352, 715–718. [Google Scholar] [CrossRef] [PubMed]
- Niggli, E.; Lederer, W. Molecular operations of the sodium–calcium exchanger revealed by conformation currents. Nature 1991, 349, 621–624. [Google Scholar] [CrossRef]
- Bers, D.M. (2002) Cardiac excitation-contraction coupling. Nature 2002, 415, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Hilgemann, D. Unitary cardiac Na+, Ca2+ exchange current magnitudes determined from channel-like noise and charge movements of ion transport. Biophys. J. 1996, 71, 759–768. [Google Scholar] [CrossRef]
- Baazov, D.; Wang, X.; Khananshvili, D. Time-resolved monitoring of electrogenic Na+-Ca2+ exchange in the isolated cardiac sarcolemma vesicles by using a rapid-response fluorescent probe. Biochemistry 1999, 38, 1435–1445. [Google Scholar] [CrossRef]
- Almagor, L.; Giladi, M.; van Dijk, L.; Buki, T.; Hiller, R.; Khananshvili, D. Functional asymmetry of bidirectional Ca2+-movements in an archaeal sodium–calcium exchanger (NCX_Mj). Cell Calcium 2014, 56, 276–284. [Google Scholar] [CrossRef]
- Cheon, J.; Reeves, J.P. Site density of the sodium-calcium exchange carrier in reconstituted vesicles from bovine cardiac sarcolemma. J. Biol. Chem. 1988, 263, 2309–2315. [Google Scholar] [CrossRef]
- Durkin, J.T.; Ahrens, D.C.; Aceto, J.F.; Condrescu, M.; Reeves, J.P. Molecular and Functional Studies of the Cardiac Sodium-Calcium Exchanger. Ann. N. Y. Acad. Sci. 1991, 639, 189–201. [Google Scholar] [CrossRef]
- Doering, A.E.; Eisner, D.A.; Lederer, W.J. Cardiac Na-Ca exchange and pH. In Annals of the New York Academy of Sciences; Blackwell Publishing Inc.: Hoboken, NJ, USA, 1996; pp. 182–198. [Google Scholar] [CrossRef]
- Dipolo, R. Calcium influx in internally dialyzed squid giant axons. J. Gen. Physiol. 1979, 73, 91–113. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, E.M.; Benzer, S. Calx, a Na-Ca exchanger gene of Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 1997, 94, 10249–10254. [Google Scholar] [CrossRef] [PubMed]
- An, D.; Husovic, A.; Ali, L.; Weddle, E.; Nagle, L.; Ahearn, G.A. Ocean acidification: Synergistic inhibitory effects of protons and heavy metals on 45Ca uptake by lobster branchiostegite membrane vesicles. J. Comp. Physiol. B 2019, 189, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Refaeli, B.; Hiller, R.; Khananshvili, D. Characteristic attributes limiting the transport rates in NCX orthologs. Biochim. Biophys. Acta (BBA)-Biomembr. 2021, 1864, 183792. [Google Scholar] [CrossRef]
- Giladi, M.; Almagor, L.; van Dijk, L.; Hiller, R.; Man, P.; Forest, E.; Khananshvili, D. Asymmetric Preorganization of Inverted Pair Residues in the Sodium-Calcium Exchanger. Sci. Rep. 2016, 6, 20753. [Google Scholar] [CrossRef]
- Giladi, M.; van Dijk, L.; Refaeli, B.; Almagor, L.; Hiller, R.; Man, P.; Forest, E.; Khananshvili, D. Dynamic distinctions in the sodium-calcium exchanger adopting the inward- and outward-facing conformational states. J. Biol. Chem. 2017, 292, 12311–12323. [Google Scholar] [CrossRef]
- Iwaki, M.; Refaeli, B.; van Dijk, L.; Hiller, R.; Giladi, M.; Kandori, H.; Khananshvili, D. Structure-affinity insights into the Na+ and Ca2+ interactions with multiple sites of a sodium-calcium exchanger. FEBS J. 2020, 287, 4678–4695. [Google Scholar] [CrossRef]
- Refaeli, B.; Liu, S.; Hiller, R.; Giladi, M.; Baiz, C.R.; Khananshvili, D. Proton-modulated interactions of ions with transport sites of prokaryotic and eukaryotic NCX prototypes. Cell Calcium 2021, 99, 102476. [Google Scholar] [CrossRef]
- van Dijk, L.; Giladi, M.; Refaeli, B.; Hiller, R.; Cheng, M.H.; Bahar, I.; Khananshvili, D. Key residues controlling bidirectional ion movements in Na+/Ca2+ exchanger. Cell Calcium 2018, 76, 10–22. [Google Scholar] [CrossRef]
- Nicoll, D.A.; Longoni, S.; Philipson, K.D. Molecular cloning and functional expression of the cardiac sarcolemmal Na+-Ca2+ exchanger. Science 1990, 250, 562–565. [Google Scholar] [CrossRef]
- Li, Z.; Matsuoka, S.; Hryshko, L.V.; Nicoll, D.A.; Bersohn, M.M.; Burke, E.P.; Lifton, R.P.; Philipson, K.D. Cloning of the NCX2 isoform of the plasma membrane Na+-Ca2+ exchanger. J. Biol. Chem. 1994, 269, 17434–17439. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.L.; Yu, A.S.; Lytton, J. Tissue-specific expression of Na+-Ca2+ exchanger isoforms. J. Biol. Chem. 1994, 269, 14849–14852. [Google Scholar] [CrossRef] [PubMed]
- Kofuji, P.; Lederer, W.J.; Schulze, D.H. Mutually exclusive and cassette exons underlie alternatively spliced isoforms of the Na/Ca exchanger. J. Biol. Chem. 1994, 269, 5145–5149. [Google Scholar] [CrossRef] [PubMed]
- Linck, B.; Qiu, Z.; He, Z.; Tong, Q.; Hilgemann, D.W.; Philipson, K.D. Functional comparison of the three isoforms of the Na+/Ca2+ exchanger (NCX1, NCX2, NCX3). Am. J. Physiol. Cell Physiol. 1998, 274, C415–C423. [Google Scholar] [CrossRef]
- Michel, L.Y.M.; Hoenderop, J.G.J.; Bindels, R.J.M. Towards Understanding the Role of the Na⁺-Ca²⁺ Exchanger Isoform 3. Rev. Physiol. Biochem. Pharmacol. 2015, 168, 31–57. [Google Scholar] [CrossRef] [PubMed]
- Sosnoski, D.M.; Gay, C.V. NCX3 is a major functional isoform of the sodium–calcium exchanger in osteoblasts. J. Cell. Biochem. 2007, 103, 1101–1110. [Google Scholar] [CrossRef]
- Morales, A.; Lachuer, J.; Bilbaut, A.; Georges, B.; Andrieu, J.-L.; Diez, J.; Ojeda, C. Characterization of a Na+-Ca2+ exchanger NCX1 isoform in bovine fasciculata cells of adrenal gland. Mol. Cell. Biochem. 2001, 218, 41–45. [Google Scholar] [CrossRef]
- Lariccia, V.; Amoroso, S. Calcium- and ATP-dependent regulation of Na/Ca exchange function in BHK cells: Comparison of NCX1 and NCX3 exchangers. Cell Calcium 2018, 73, 95–103. [Google Scholar] [CrossRef]
- Marinelli, F.; Almagor, L.; Hiller, R.; Giladi, M.; Khananshvili, D.; Faraldo-Gómez, J.D. Sodium recognition by the Na+/Ca2+ exchanger in the outward-facing conformation. Proc. Natl. Acad. Sci. USA 2014, 111, E5354–E5362. [Google Scholar] [CrossRef]
- Giladi, M.; Khananshvili, D. Hydrogen-deuterium exchange mass-spectrometry (HDX-MS) of transporters: From structural dynamics to molecular mechanisms. Front. Pharmacol. 2020, 11, 70. [Google Scholar] [CrossRef]
- Giladi, M.; Mitra, S.; Simhaev, L.; Hiller, R.; Refaeli, B.; Strauss, T.; Baiz, C.R.; Khananshvili, D. Exploring the Li+ transporting mutant of NCX_Mj for assigning ion binding sites of mitochondrial NCLX. Cell Calcium 2022, 107, 102651. [Google Scholar] [CrossRef]
- Carafoli, E. The release of calcium from heart mitochondria by sodium. J. Mol. Cell. Cardiol. 1974, 6, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Palty, R.; Silverman, W.F.; Hershfinkel, M.; Caporale, T.; Sensi, S.L.; Parnis, J.; Nolte, C.; Fishman, D.; Shoshan-Barmatz, V.; Herrmann, S.; et al. NCLX is an essential component of mitochondrial Na+/Ca2+ exchange. Proc. Natl. Acad. Sci. USA 2009, 107, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Boyman, L.; Williams, G.S.; Khananshvili, D.; Sekler, I.; Lederer, W. NCLX: The mitochondrial sodium calcium exchanger. J. Mol. Cell. Cardiol. 2013, 59, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Garbincius, J.F.; Elrod, J.W. Mitochondrial calcium exchange in physiology and disease. Physiol. Rev. 2022, 102, 893–992. [Google Scholar] [CrossRef] [PubMed]
- Refaeli, B.; Giladi, M.; Hiller, R.; Khananshvili, D. Structure-Based Engineering of Lithium-Transport Capacity in an Archaeal Sodium–Calcium Exchanger. Biochemistry 2016, 55, 1673–1676. [Google Scholar] [CrossRef]
- Giladi, M.; Lee, S.Y.; Refaeli, B.; Hiller, R.; Chung, K.Y.; Khananshvili, D. Structure-dynamic and functional relationships in a Li+-transporting sodium-calcium exchanger mutant. Biochim. Biophys. Acta Bioenerg. 2018, 1860, 189–200. [Google Scholar] [CrossRef]
- Adhikary, S.; Deredge, D.J.; Nagarajan, A.; Forrest, L.R.; Wintrode, P.L.; Singh, S.K. Conformational dynamics of a neurotransmitter:sodium symporter in a lipid bilayer. Proc. Natl. Acad. Sci. USA 2017, 114, E1786–E1795. [Google Scholar] [CrossRef]
- Wallant, K.C.T.; Martens, C. Hydrogen-deuterium exchange coupled to mass spectrometry: A multifaceted tool to decipher the molecular mechanism of transporters. Biochimie 2022, S0300-9084(22)00218-8. [Google Scholar] [CrossRef]
- Stariha, J.T.B.; Hoffmann, R.M.; Hamelin, D.J.; Burke, J.E. Probing Protein–Membrane Interactions and Dynamics Using Hydrogen–Deuterium Exchange Mass Spectrometry (HDX-MS). Methods Mol. Biol. 2021, 2263, 465–485. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, X.; Lv, G.; Razavi, A.M.; Huysmans, G.H.M.; Weinstein, H.; Bracken, C.; Eliezer, D.; Boudker, O. Use of paramagnetic 19F NMR to monitor domain movement in a glutamate transporter homolog. Nat. Chem. Biol. 2020, 16, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.K.; Pandey, A.; Tran, D.P.; Villanueva, N.L.; Kitao, A.; Sunahara, R.K.; Sljoka, A.; Prosser, R.S. Delineating the conformational landscape of the adenosine A2A receptor during G protein coupling. Cell 2021, 184, 1884–1894.e14. [Google Scholar] [CrossRef] [PubMed]
- Puthenveetil, R.; Christenson, E.T.; Vinogradova, O. New Horizons in Structural Biology of Membrane Proteins: Experimental Evaluation of the Role of Conformational Dynamics and Intrinsic Flexibility. Membranes 2022, 12, 227. [Google Scholar] [CrossRef] [PubMed]
- Khananshvili, D. Voltage-dependent modulation of ion-binding and translocation in the cardiac Na+-Ca2+ exchanger. J. Biol. Chem. 1991, 266, 13764–13769. [Google Scholar] [CrossRef] [PubMed]
- Khananshvili, D.; Weil-Maslansky, E. The Cardiac Na+-Ca2+ exchanger: Relative rates of calcium and sodium movements and their modulation by protonation-deprotonation of the carrier. Biochemistry 1994, 33, 312–319. [Google Scholar] [CrossRef]
- Khananshvili, D.; Shaulov, G.; Weil-Maslansky, E. Rate-limiting mechanisms of exchange reactions in the cardiac sarcolemma Na+-Ca2+ exchanger. Biochemistry 1995, 33, 10290–10297. [Google Scholar] [CrossRef]
- Vemuri, R.; Philipson, K.D. Phospholipid composition modulates the Na+-Ca2+ exchange activity of cardiac sarcolemma in reconstituted vesicles. Biochim. Biophys. Acta 1988, 937, 258–268. [Google Scholar] [CrossRef]
- Vemuri, R.; Philipson, K.D. Influence of sterols and phospholipids on sarcolemmal and sarcoplasmic reticular cation transporters. J. Biol. Chem. 1989, 264, 8680–8685. [Google Scholar] [CrossRef]
- Habeck, M.; Kapri-Pardes, E.; Sharon, M.; Karlish, S.J.D. Specific phospholipid binding to Na, K-ATPase at two distinct sites. Proc. Natl. Acad. Sci. USA 2017, 114, 2904–2909. [Google Scholar] [CrossRef]
- Ander, B.P.; Hurtado, C.; Raposo, C.S.; Maddaford, T.G.; Deniset, J.F.; Hryshko, L.V.; Pierce, G.N.; Lukas, A. Differential sensitivities of the NCX1.1 and NCX1.3 isoforms of the Na+-Ca2+ exchanger to alpha-linolenic acid. Cardiovasc. Res. 2007, 73, 395–403. [Google Scholar] [CrossRef]
- Läuger, P. Voltage dependence of sodium-calcium exchange: Predictions from kinetic models. J. Membr. Biol. 1987, 99, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hilgemann, D.W. Regulation of ion transport from within ion transit pathways. J. Gen. Physiol. 2019, 152, e201912455. [Google Scholar] [CrossRef] [PubMed]
- Hilgemann, D.W. Control of cardiac contraction by sodium: Promises, reckonings, and new beginnings. Cell Calcium 2019, 85, 102129. [Google Scholar] [CrossRef] [PubMed]
- Hilgemann, D.W.; Matsuoka, S.; Nagel, G.; Collins, A. Steady-state and dynamic properties of cardiac sodium-calcium exchange. Sodium-dependent inactivation. J. Gen. Physiol. 1992, 100, 905–932. [Google Scholar] [CrossRef]
- Hilgemann, D.W. Regulation and deregulation of cardiac Na+-Ca2+ exchange in giant excised sarcolemmal membrane patches. Nature 1990, 344, 242–245. [Google Scholar] [CrossRef]
- Hilgemann, D.W.; Ball, R. Regulation of cardiac Na+/Ca2+ exchange and MgATP potassium channels by PIP2. Science 1996, 273, 956–959. [Google Scholar] [CrossRef]
- Hilgemann, D.W. Local PIP2 signals: When, where, and how? Pflugers. Arch. 2007, 455, 55–67. [Google Scholar] [CrossRef]
- Hilgemann, D.W.; Feng, S.; Nasuhoglu, C. The Complex and Intriguing Lives of PIP2 with Ion Channels and Transporters. Sci. Signal. 2001, 2001, re19. [Google Scholar] [CrossRef]
- Lariccia, V.; Piccirillo, S.; Preziuso, A.; Amoroso, S.; Magi, S. Cracking the code of sodium/calcium exchanger (NCX) gating: Old and new complexities surfacing from the deep web of secondary regulations. Cell Calcium 2020, 87, 102169. [Google Scholar] [CrossRef]
- Shen, C.; Lin, M.J.; Yaradanakul, A.; Lariccia, V.; Hill, J.A.; Hilgemann, D.W. Dual control of cardiac Na+-Ca2+ exchange by PIP2: Analysis of the surface membrane fraction by extracellular cysteine PEGylation. J. Physiol. 2007, 582, 1011–1026. [Google Scholar] [CrossRef]
- John, S.A.; Ribalet, B.; Weiss, J.N.; Philipson, K.D.; Ottolia, M. Ca2+-dependent structural rearrangements within Na+-Ca2+ exchanger dimers. Proc. Natl. Acad. Sci. USA 2011, 108, 1699–1704. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Ottolia, M.; John, S.A.; Chen, J.-N.; Philipson, K.D. Conformational changes of a Ca2+-binding domain of the Na+/Ca2+ exchanger monitored by FRET in transgenic zebrafish heart. Am. J. Physiol. Cell Physiol. 2008, 295, C388–C393. [Google Scholar] [CrossRef]
- Giladi, M.; Boyman, L.; Mikhasenko, H.; Hiller, R.; Khananshvili, D. Essential Role of the CBD1-CBD2 Linker in Slow Dissociation of Ca2+ from the Regulatory Two-domain Tandem of NCX1. J. Biol. Chem. 2010, 285, 28117–28125. [Google Scholar] [CrossRef] [PubMed]
- Giladi, M.; Bohbot, H.; Buki, T.; Schulze, D.H.; Hiller, R.; Khananshvili, D. Dynamic features of allosteric Ca2+ sensor in tissue-specific NCX variants. Cell Calcium 2012, 51, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Giladi, M.; Hiller, R.; Hirsch, J.A.; Khananshvili, D. Population Shift Underlies Ca2+-induced Regulatory Transitions in the Sodium-Calcium Exchanger (NCX). J. Biol. Chem. 2013, 288, 23141–23149. [Google Scholar] [CrossRef] [PubMed]
- Boyman, L.; Mikhasenko, H.; Hiller, R.; Khananshvili, D. Kinetic and equilibrium properties of regulatory calcium sensors of NCX1 protein. J. Biol. Chem. 2009, 284, 6185–6193. [Google Scholar] [CrossRef] [PubMed]
- Boyman, L.; Hagen, B.M.; Giladi, M.; Hiller, R.; Lederer, W.J.; Khananshvili, D. Proton-Sensing Ca2+ Binding Domains Regulate the Cardiac Na+/Ca2+ Exchanger. J. Biol. Chem. 2011, 286, 28811–28820. [Google Scholar] [CrossRef]
- Giladi, M.; Khananshvili, D. Molecular Determinants of Allosteric Regulation in NCX Proteins. Adv. Exp. Med. Biol. 2012, 961, 35–48. [Google Scholar] [CrossRef]
- Giladi, M.; Lee, S.Y.; Hiller, R.; Chung, K.Y.; Khananshvili, D. Structure-dynamic determinants governing a mode of regulatory response and propagation of allosteric signal in splice variants of Na+/Ca2+ exchange (NCX) proteins. Biochem. J. 2015, 465, 489–501. [Google Scholar] [CrossRef]
- Lee, S.Y.; Giladi, M.; Bohbot, H.; Hiller, R.; Chung, K.Y.; Khananshvili, D. Structure-dynamic basis of splicing dependent regulation in tissue-specific variants of the sodium-calcium exchanger (NCX1). FASEB J. 2016, 30, 1356–1366. [Google Scholar] [CrossRef]
- Giladi, M.; Lee, S.Y.; Ariely, Y.; Teldan, Y.; Granit, R.; Strulevich, R.; Haitin, Y.; Chung, K.Y.; Khananshvili, D. Structure-based dynamic arrays in regulatory domains of sodium-calcium exchanger (NCX) isoforms. Sci. Rep. 2017, 7, 993. [Google Scholar] [CrossRef] [PubMed]
- Salinas, R.K.; Bruschweiler-Li, L.; Johnson, E.; Brüschweiler, R. Ca2+ Binding Alters the Interdomain Flexibility between the Two Cytoplasmic Calcium-binding Domains in the Na+/Ca2+ Exchanger. J. Biol. Chem. 2011, 286, 32123–32131. [Google Scholar] [CrossRef] [PubMed]
- Ottolia, M.; Nicoll, D.A.; John, S.; Philipson, K. Interactions between Ca2+ binding domains of the Na+-Ca2+ exchanger and secondary regulation. Channels 2010, 4, 159–162. [Google Scholar] [CrossRef]
- Eisenrauch, A.; Juhaszova, M.; Blaustein, M. Regulatory Processes on the Cytoplasmic Surface of the Na+/Ca2+ Exchanger from Lobster Exoskeletal Muscle. J. Membr. Biol. 2000, 174, 225–235. [Google Scholar] [CrossRef]
- Tedeschi, V.; Sisalli, M.J.; Pannaccione, A.; Piccialli, I.; Molinaro, P.; Annunziato, L.; Secondo, A. Na+/Ca2+ exchanger isoform 1 (NCX1) and canonical transient receptor potential channel 6 (TRPC6) are recruited by STIM1 to mediate Store-Operated Calcium Entry in primary cortical neurons. Cell Calcium 2021, 101, 102525. [Google Scholar] [CrossRef] [PubMed]
- Pobarko, D.; Fameli, N.; Kuo, K.H.; van Breemen, C. Ca2+ signaling in smooth muscle: TRPC6, NCX, and Na+ in nanodomains. Channels 2008, 2, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Cantero-Recasens, G.; Butnaru, C.M.; Brouwers, N.; Mitrovic, S.; Valverde, M.A.; Malhotra, V. Sodium channel TRPM4 and sodium/calcium exchangers (NCX) cooperate in the control of Ca2+-induced mucin secretion from goblet cells. J. Biol. Chem. 2019, 294, 816–826. [Google Scholar] [CrossRef] [PubMed]
- Lemos, V.S.; Poburko, D.; Liao, C.-H.; Cole, W.C.; van Breemen, C. Na+ entry via TRPC6 causes Ca2+ entry via NCX reversal in ATP stimulated smooth muscle cells. Biochem. Biophys. Res. Commun. 2007, 352, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Andrikopoulos, P.; Eccles, S.A.; Yaqoob, M.M. Coupling between the TRPC3 ion channel and the NCX1 transporter contributed to VEGF-induced ERK1/2 activation and angiogenesis in human primary endothelial cells. Cell. Signal. 2017, 37, 12–30. [Google Scholar] [CrossRef]
- Harper, A.G.S.; Sage, S.O. TRP-Na+/Ca2+ Exchanger Coupling. Adv. Exp. Med. Biol. 2016, 898, 67–85. [Google Scholar]
- Doleschal, B.; Primessnig, U.; Wolkart, G.; Wolf, S.; Schernthaner, M.; Lichtenegger, M.; Glasnov, T.N.; Kappe, C.O.; Mayer, B.; Antoons, G.; et al. TRPC3 contributes to regulation of cardiac contractility and arrhythmogenesis by dynamic interaction with NCX1. Cardiovasc. Res. 2015, 106, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J. New Insights into the Contribution of Arterial NCX to the Regulation of Myogenic Tone and Blood Pressure. Adv. Exp. Med. Biol. 2012, 961, 329–343. [Google Scholar] [CrossRef]
- Iwamoto, T.; Kita, S.; Zhang, J.; Blaustein, M.P.; Arai, Y.; Yoshida, S.; Wakimoto, K.; Komuro, I.; Katsuragi, T. Salt-sensitive hypertension is triggered by Ca2+ entry via Na+/Ca2+ exchanger type-1 in vascular smooth muscle. Nat. Med. 2004, 10, 1193–1199. [Google Scholar] [CrossRef] [PubMed]
- Pulina, M.V.; Zulian, A.; Baryshnikov, S.G.; Linde, C.I.; Karashima, E.; Hamlyn, J.M.; Ferrari, P.; Blaustein, M.P.; Golovina, V.A. Cross Talk between Plasma Membrane Na+/Ca2+ Exchanger-1 and TRPC/Orai-Containing Channels: Key Players in Arterial Hypertension. Adv. Exp. Med. Biol. 2012, 961, 365–374. [Google Scholar] [CrossRef]
- Lee, M.Y.; Song, H.; Nakai, J.; Ohkura, M.; Kotlikoff, M.I.; Kinsey, S.P.; Golovina, V.A.; Blaustein, M.P. Local subplasma membrane Ca2+ signals detected by a tethered Ca2+ sensor. Proc. Natl. Acad. Sci. USA 2006, 103, 13232–13237. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Zhao, L.; Clapham, D.E. Genome-wide RNAi screen identifies Letm1 as a mitochondrial Ca2+/H+ antiporter. Science. 2009, 326, 144–147. [Google Scholar] [CrossRef]
- Tsai, M.F.; Jiang, D.; Zhao, L.; Clapham, D.; Miller, C. Functional reconstitution of the mitochondrial Ca2+/H+ antiporter Letm1. J. Gen. Physiol. 2014, 143, 67–73. [Google Scholar] [CrossRef]
- Austin, S.; Mekis, R.; Mohammed, S.E.M.; Scalise, M.; Wang, W.A.; Galluccio, M.; Pfeiffer, C.; Borovec, T.; Parapatics, K.; Vitko, D.; et al. TMBIM5 is the Ca2+/H+ antiporter of mammalian mitochondria. EMBO Rep. 2022, 23, e54978. [Google Scholar] [CrossRef]
- Patron, M.; Tarasenko, D.; Nolte, H.; Kroczek, L.; Ghosh, M.; Ohba, Y.; Lasarzewski, Y.; Ahmadi, Z.A.; Cabrera-Orefice, A.; Eyiama, A.; et al. Regulation of mitochondrial proteostasis by the proton gradient. EMBO J. 2022, 2022 41, e110476. [Google Scholar] [CrossRef]
- Ren, M.; Schlame, M. The mystery of mitochondrial plasticity: TMBIM5 integrates metabolic state and proteostasis. EMBO J. 2022, 41, e111834. [Google Scholar] [CrossRef]
- Tanwar, J.; Singh, J.B.; Motiani, R.K. Molecular machinery regulating mitochondrial calcium levels: The nuts and bolts of mitochondrial calcium dynamics. Mitochondrion 2021, 57, 9–22. [Google Scholar] [CrossRef]
- Natarajan, G.K.; Mishra, J.; Camara, A.K.S.; Kwok, W.M. LETM1: A Single Entity With Diverse Impact on Mitochondrial Metabolism and Cellular Signaling. Front Physiol. 2021, 12, 637852. [Google Scholar] [CrossRef]
- Takeuchi, A.; Matsuoka, S. Physiological and Pathophysiological Roles of Mitochondrial Na+-Ca2+ Exchanger, NCLX, in Hearts. Biomolecules 2021, 11, 1876. [Google Scholar] [CrossRef] [PubMed]
- Pathak, T.; Trebak, M. Mitochondrial Ca2+ signaling. Pharmacol. Ther. 2018, 192, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Katoshevski, T.; Ben-Kasus Nissim, T.; Sekler, I. Recent studies on NCLX in health and diseases. Cell Calcium. 2021, 94, 102345. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, P.; Castaldo, P.; Minelli, A.; Salucci, S.; Magi, S.; Corcione, E.; Amoroso, S. Mitochondrial localization of Na+/Ca2+ exchangers NCX1-3 in neurons and astrocytes of adult rat brain in situ. Pharmacol Res. 2007, 56, 556–565. [Google Scholar] [CrossRef]
- Pannaccione, A.; Piccialli, I.; Secondo, A.; Ciccone, R.; Molinaro, P.; Boscia, F.; Annunziato, L. The Na+/Ca2+ exchanger in Alzheimer’s disease. Cell Calcium. 2020, 87, 102190. [Google Scholar] [CrossRef]
- Scorziello, A.; Savoia, C.; Sisalli, M.J.; Adornetto, A.; Secondo, A.; Boscia, F.; Esposito, A.; Polishchuk, E.V.; Polishchuk, R.S.; Molinaro, P.; et al. NCX3 regulates mitochondrial Ca2+ handling through the AKAP121-anchored signaling complex and prevents hypoxia-induced neuronal death. J. Cell. Sci. 2013, 126, 5566–5577. [Google Scholar]
- Wood-Kaczmar, A.; Deas, E.; Wood, N.W.; Abramov, A.Y. The role of the mitochondrial NCX in the mechanism of neurodegeneration in Parkinson’s disease. Adv. Exp. Med Biol. 2013, 961, 241–249. [Google Scholar] [CrossRef]
- Sisalli, M.J.; Ianniello, G.; Savoia, C.; Cuomo, O.; Annunziato, L.; Scorziello, A. Knocking-out the Siah2 E3 ubiquitin ligase prevents mitochondrial NCX3 degradation, regulates mitochondrial fission and fusion, and restores mitochondrial function in hypoxic neurons. Cell Commun. Signal. 2020, 18, 42. [Google Scholar] [CrossRef]
- Plain, F.; Congreve, S.D.; Yee, R.S.Z.; Kennedy, J.; Howie, J.; Kuo, C.-W.; Fraser, N.J.; Fuller, W. An amphipathic α-helix directs palmitoylation of the large intracellular loop of the sodium/calcium exchanger. J. Biol. Chem. 2017, 292, 10745–10752. [Google Scholar] [CrossRef] [PubMed]
- Reilly, L.; Howie, J.; Wypijewski, K.; Ashford, M.L.; Hilgemann, D.W.; Fuller, W. Palmitoylation of the Na/Ca exchanger cytoplasmic loop controls its inactivation and internalization during stress signaling. FASEB J. 2015, 29, 4532–4543. [Google Scholar] [CrossRef] [PubMed]
- Fuller, W.; Reilly, L.; Hilgemann, D.W. S-palmitoylation and the regulation of NCX1. Channels 2015, 10, 75–77. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gök, C.; Main, A.; Gao, X.; Kerekes, Z.; Plain, F.; Kuo, C.-W.; Robertson, A.D.; Fraser, N.J.; Fuller, W. Insights into the molecular basis of the palmitoylation and depalmitoylation of NCX1. Cell Calcium 2021, 97, 102408. [Google Scholar] [CrossRef]
- Main, A.; Fuller, W. Protein S-Palmitoylation: Advances and challenges in studying a therapeutically important lipid modification. FEBS J. 2021, 289, 861–882. [Google Scholar] [CrossRef]
- Qu, M.; Zhou, X.; Wang, X.; Li, H. Lipid-induced S-palmitoylation as a Vital Regulator of Cell Signaling and Disease Development. Int. J. Biol. Sci. 2021, 17, 4223–4237. [Google Scholar] [CrossRef]
- Sardar, A.; Dewangan, N.; Panda, B.; Bhowmick, D.; Tarafdar, P.K. Lipid and Lipidation in Membrane Fusion. J. Membr. Biol. 2022, 255, 691–703. [Google Scholar] [CrossRef]
- Cassinelli, S.; Viñola-Renart, C.; Benavente-Garcia, A.; Navarro-Pérez, M.; Capera, J.; Felipe, A. Palmitoylation of Voltage-Gated Ion Channels. Int. J. Mol. Sci. 2022, 23, 9357. [Google Scholar] [CrossRef]
- Gök, C.; Fuller, W. Regulation of NCX1 by palmitoylation. Cell Calcium 2020, 86, 102158. [Google Scholar] [CrossRef]
- Lim, D.; Dematteis, G.; Tapella, L.; Genazzani, A.A.; Calì, T.; Brini, M.; Verkhratsky, A. Ca2+ handling at the mitochondria-ER contact sites in neurodegeneration. Cell Calcium 2021, 98, 102453. [Google Scholar] [CrossRef]
- Csordás, G.; Weaver, D.; Hajnóczky, G. Endoplasmic reticulum–mitochondrial contactology: Structure and signaling functions. Trends Cell Biol. 2018, 28, 523–540. [Google Scholar] [CrossRef] [PubMed]
- Scorrano, L.; De Matteis, M.A.; Emr, S.; Giordano, F.; Hajnóczky, G.; Kornmann, B.; Lackner, L.L.; Levine, T.P.; Pellegrini, L.; Reinisch, K.; et al. Coming together to define membrane contact sites. Nat. Commun. 2019, 10, 1287. [Google Scholar] [CrossRef] [PubMed]
- Prinz, W.A.; Toulmay, A.; Balla, T. The functional universe of membrane contact sites. Nat. Rev. Mol. Cell Biol. 2020, 21, 7–24. [Google Scholar] [CrossRef]
- Dunn, J.; Elias, C.L.; Le, H.D.; Omelchenko, A.; Hryshko, L.V.; Lytton, J. The molecular determinants of ionic regulatory differences between brain and kidney Na+/Ca2+ exchanger (NCX1) isoforms. J. Biol. Chem. 2002, 277, 33957–33962. [Google Scholar] [CrossRef] [PubMed]
- Dyck, C.; Omelchenko, A.; Elias, C.L.; Quednau, B.D.; Philipson, K.D.; Hnatowich, M.; Hryshko, L.V. Ionic Regulatory Properties of Brain and Kidney Splice Variants of the Ncx1 Na+-Ca2+ Exchanger. J. Gen. Physiol. 1999, 114, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Hryshko, L. What regulates Na+/Ca2+ exchange? Focus on “Sodium-dependent inactivation of sodium/calcium exchange in transfected Chinese hamster ovary cells”. Am. J. Physiol. Cell Physiol. 2008, 295, C869–C871. [Google Scholar] [CrossRef]
- Levitsky, D.O.; Nicoll, D.A.; Philipson, K.D. Identification of the high affinity Ca2+-binding domain of the cardiac Na+-Ca2+ exchanger. J. Biol. Chem. 1994, 269, 22847–22852. [Google Scholar] [CrossRef]
- Hryshko, L.V.; Matsuoka, S.; Nicoll, D.A.; Weiss, J.N.; Schwarz, E.M.; Benzer, S.; Philipson, K.D. Anomalous regulation of the Drosophila Na+-Ca2+ exchanger by Ca2+. J. Gen. Physiol. 1996, 108, 67–74. [Google Scholar] [CrossRef]
- Wu, M.; Wang, M.; Nix, J.; Hryshko, L.V.; Zheng, L. Crystal structure of CBD2 from the Drosophila Na+/Ca2+ exchanger: Diversity of Ca2+ regulation and its alternative splicing modification. J. Mol. Biol. 2009, 387, 104–112. [Google Scholar] [CrossRef]
- Wu, M.; Le, H.D.; Wang, M.; Yurkov, V.; Omelchenko, A.; Hnatowich, M.; Nix, J.; Hryshko, L.V.; Zheng, L. Crystal structures of progressive Ca2+ binding states of the Ca2+ sensor Ca2+ binding domain 1 (CBD1) from the CALX Na+/Ca2+ exchanger reveal incremental conformational transitions. J. Biol. Chem. 2020, 285, 2554–2561. [Google Scholar] [CrossRef]
- Reeves, J.P.; Condrescu, M. Ionic regulation of the cardiac sodium-calcium exchanger. Channels 2008, 2, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Scranton, K.; John, S.; Escobar, A.; Goldhaber, J.I.; Ottolia, M. Modulation of the cardiac Na+-Ca2+ exchanger by cytoplasmic protons: Molecular mechanisms and physiological implications. Cell Calcium 2019, 87, 102140. [Google Scholar] [CrossRef] [PubMed]
- John, S.; Kim, B.; Olcese, R.; Goldhaber, J.I.; Ottolia, M. Molecular determinants of pH regulation in the cardiac Na+–Ca2+ exchanger. J. Gen. Physiol. 2018, 150, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Philipson, K.D.; Bersohn, M.M.; Nishimoto, A.Y. Effects of pH on Na+-Ca2+ exchange in canine cardiac sarcolemmal vesicles. Circ. Res. 1982, 50, 287–293. [Google Scholar] [CrossRef]
- Philipson, K.D.; Nishimoto, A.Y. Stimulation of Na+-Ca2+ exchange in cardiac sarcolemmal vesicles by phospholipase D. J. Biol. Chem. 1984, 259, 16–19. [Google Scholar] [CrossRef]
- Abiko, L.A.; Vitale, P.M.; Favaro, D.C.; Hauk, P.; Li, D.-W.; Yuan, J.; Bruschweiler-Li, L.; Salinas, R.K.; Brüschweiler, R. Model for the allosteric regulation of the Na+/Ca2+ exchanger NCX. Proteins Struct. Funct. Bioinform. 2016, 84, 580–590. [Google Scholar] [CrossRef]
- Degenhardt, M.F.d.S.; Vitale, P.A.; Abiko, L.A.; Zacharias, M.; Sattler, M.; Oliveira, C.L.; Salinas, R.K. Molecular insights on CALX-CBD12 interdomain dynamics from MD simulations, RDCs, and SAXS. Biophys. J. 2021, 120, 3664–3675. [Google Scholar] [CrossRef]
- Cardoso, M.V.; Rivera, J.D.; Vitale, P.A.; Degenhardt, M.F.; Abiko, L.A.; Oliveira, C.L.; Salinas, R.K. CALX-CBD1 Ca2+-Binding Cooperativity Studied by NMR Spectroscopy and ITC with Bayesian Statistics. Biophys. J. 2020, 119, 337–348. [Google Scholar] [CrossRef]
- Ma, B.; Nussinov, R. Enzyme dynamics point to stepwise conformational selection in catalysis. Curr. Opin. Chem. Biol. 2010, 14, 652–659. [Google Scholar] [CrossRef]
- Ma, B.; Tsai, C.-J.; Haliloğlu, T.; Nussinov, R. Dynamic Allostery: Linkers are not merely flexible. Structure 2011, 19, 907–917. [Google Scholar] [CrossRef]
- Cafiso, D.S. Identifying and Quantitating Conformational Exchange in Membrane Proteins Using Site-Directed Spin Labeling. Accounts Chem. Res. 2014, 47, 3102–3109. [Google Scholar] [CrossRef] [PubMed]
- Ricci, F.; Vallée-Bélisle, A.; Simon, A.J.; Porchetta, A.; Plaxco, K.W. Using Nature’s “Tricks” To Rationally Tune the Binding Properties of Biomolecular Receptors. Accounts Chem. Res. 2016, 49, 1884–1892. [Google Scholar] [CrossRef] [PubMed]
- Babu, M.M.; Kriwacki, R.W.; Pappu, R.V. Versatility from protein disorder. Science 2012, 337, 1460–1464. [Google Scholar] [CrossRef] [PubMed]
- Nussinov, R.; Zhang, M.; Liu, Y.; Jang, H. AlphaFold, Artificial Intelligence (AI), and Allostery. J. Phys. Chem. B 2022, 126, 6372–6383. [Google Scholar] [CrossRef]
- Uversky, V.N. Protein intrinsic disorder and structure-function continuum. Prog. Mol. Biol. Transl. Sci. 2019, 166, 1–17. [Google Scholar] [CrossRef]
- Sharma, R.; Raduly, Z.; Miskei, M.; Fuxreiter, M. Fuzzy complexes: Specific binding without complete folding. FEBS Lett. 2015, 589 Pt 19, 2533–2542. [Google Scholar] [CrossRef]
- Babu, M.M. The contribution of intrinsically disordered regions to protein function, cellular complexity, and human disease. Biochem. Soc. Trans. 2016, 44, 1185–1200. [Google Scholar] [CrossRef]
- Buljan, M.; Chalancon, G.; Dunker, A.K.; Bateman, A.; Balaji, S.; Fuxreiter, M.; Babu, M.M. Alternative splicing of intrinsically disordered regions and rewiring of protein interactions. Curr. Opin. Struct. Biol. 2013, 23, 443–450. [Google Scholar] [CrossRef]
- Philipson, K.D.; Nishimoto, A.Y. Na+-Ca2+ exchange in inside-out cardiac sarcolemmal vesicles. J. Biol. Chem. 1982, 257, 5111–5117. [Google Scholar] [CrossRef]
- Veseli, E.; Soboloff, J. Palmitoylation: A new mechanism for control of NCX1 function. Cell Calcium 2020, 91, 102254. [Google Scholar] [CrossRef]
- Philipson, K.D. Interaction of charged amphiphiles with Na+-Ca2+ exchange in cardiac sarcolemmal vesicles. J. Biol. Chem. 1984, 259, 13999–14002. [Google Scholar] [CrossRef] [PubMed]
- Hilgemann, D.W.; Collins, A. Mechanism of cardiac Na+-Ca2+ exchange current stimulation by MgATP: Possible involvement of aminophospholipid translocase. J. Physiol. 1992, 454, 59–82. [Google Scholar] [CrossRef] [PubMed]
- Philipson, K.D.; Ward, R. Effects of fatty acids on Na+-Ca2+ exchange and Ca2+ permeability of cardiac sarcolemmal vesicles. J. Biol. Chem. 1985, 260, 9666–9671. [Google Scholar] [CrossRef] [PubMed]
- Riedel, M.J.; Baczko, I.; Searle, G.J.; Webster, N.; Fercho, M.; Jones, L.; Lang, J.; Lytton, J.; Dyck, J.R.; Light, P.E. Metabolic regulation of sodium-calcium exchange by intracellular acyl CoAs. EMBO J. 2006, 25, 4605–4614. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Feng, S.; Tong, Q.; Hilgemann, D.W.; Philipson, K.D. Interaction of PIP2 with the XIP region of the cardiac Na/Ca exchanger. Am. J. Physiol. Cell Physiol. 2000, 278, C661–C666. [Google Scholar] [CrossRef] [PubMed]
- Yaradanakul, A.; Feng, S.; Shen, C.; Lariccia, V.; Lin, M.J.; Yang, J.; Kang, T.M.; Dong, P.; Yin, H.L.; Albanesi, J.P.; et al. Dual control of cardiac Na+-Ca2+ exchange by PIP2: Electrophysiological analysis of direct and indirect mechanisms. J. Physiol. 2007, 582, 991–1010. [Google Scholar] [CrossRef]
- Khananshvili, D. How a helix imposes palmitoylation of membrane protein?—What one can learn from NCX. J. Biol. Chem. 2017, 292, 10753–10754. [Google Scholar] [CrossRef]
- Hedger, G.; Sansom, M.S. Lipid interaction sites on channels, transporters and receptors: Recent insights from molecular dynamics simulations. Biochim. Biophys. Acta 2016, 1858, 2390–2400. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khananshvili, D. Structure-Based Function and Regulation of NCX Variants: Updates and Challenges. Int. J. Mol. Sci. 2023, 24, 61. https://doi.org/10.3390/ijms24010061
Khananshvili D. Structure-Based Function and Regulation of NCX Variants: Updates and Challenges. International Journal of Molecular Sciences. 2023; 24(1):61. https://doi.org/10.3390/ijms24010061
Chicago/Turabian StyleKhananshvili, Daniel. 2023. "Structure-Based Function and Regulation of NCX Variants: Updates and Challenges" International Journal of Molecular Sciences 24, no. 1: 61. https://doi.org/10.3390/ijms24010061
APA StyleKhananshvili, D. (2023). Structure-Based Function and Regulation of NCX Variants: Updates and Challenges. International Journal of Molecular Sciences, 24(1), 61. https://doi.org/10.3390/ijms24010061





