Response to Brigatinib Targeted Therapy in Non-Small Cell Lung Cancer Harboring Epidermal Growth Factor Receptor Exon 19 Deletion, T790M, and cis-C797S Triple Mutations: A Case Report
Abstract
1. Introduction
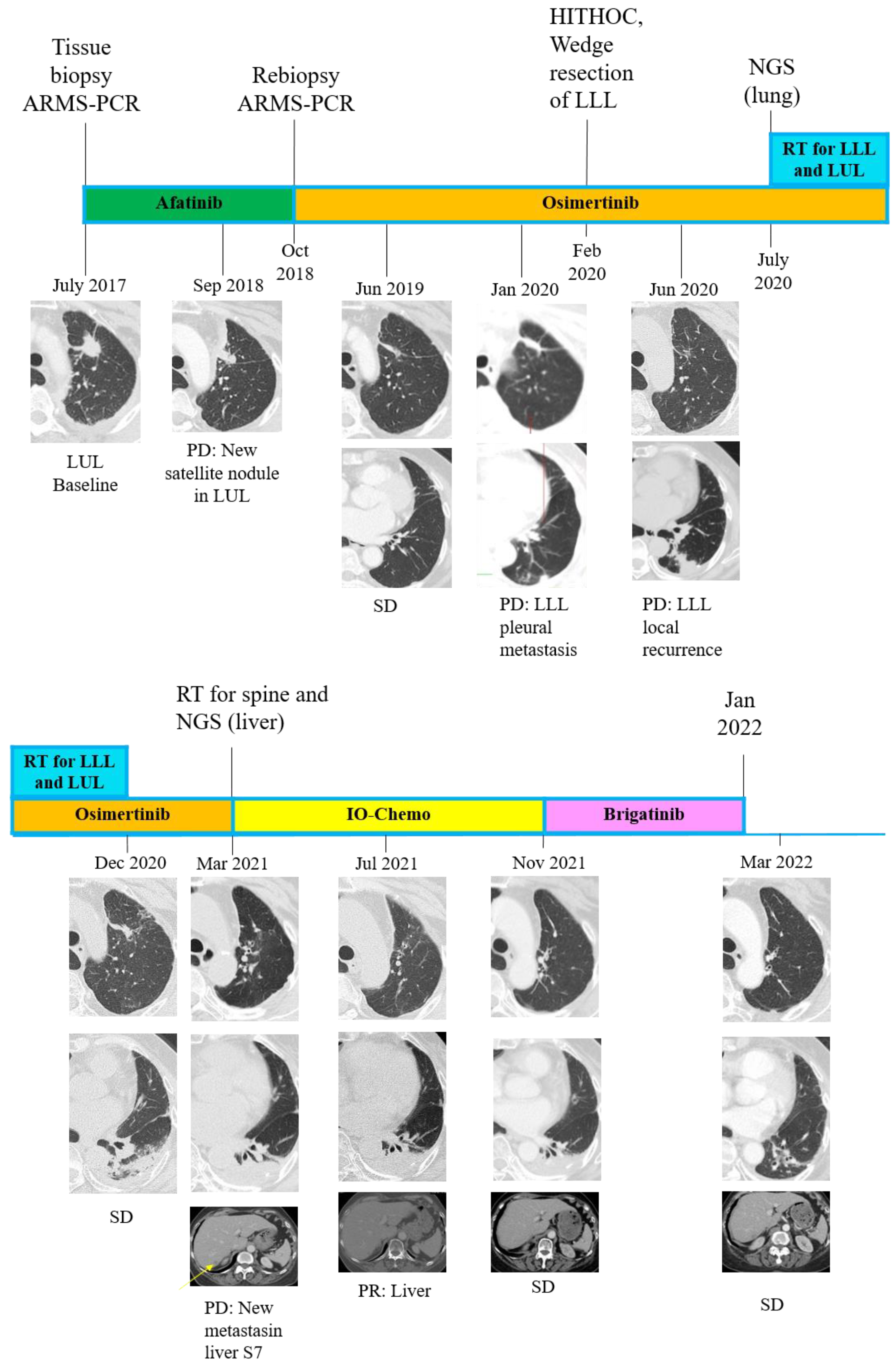
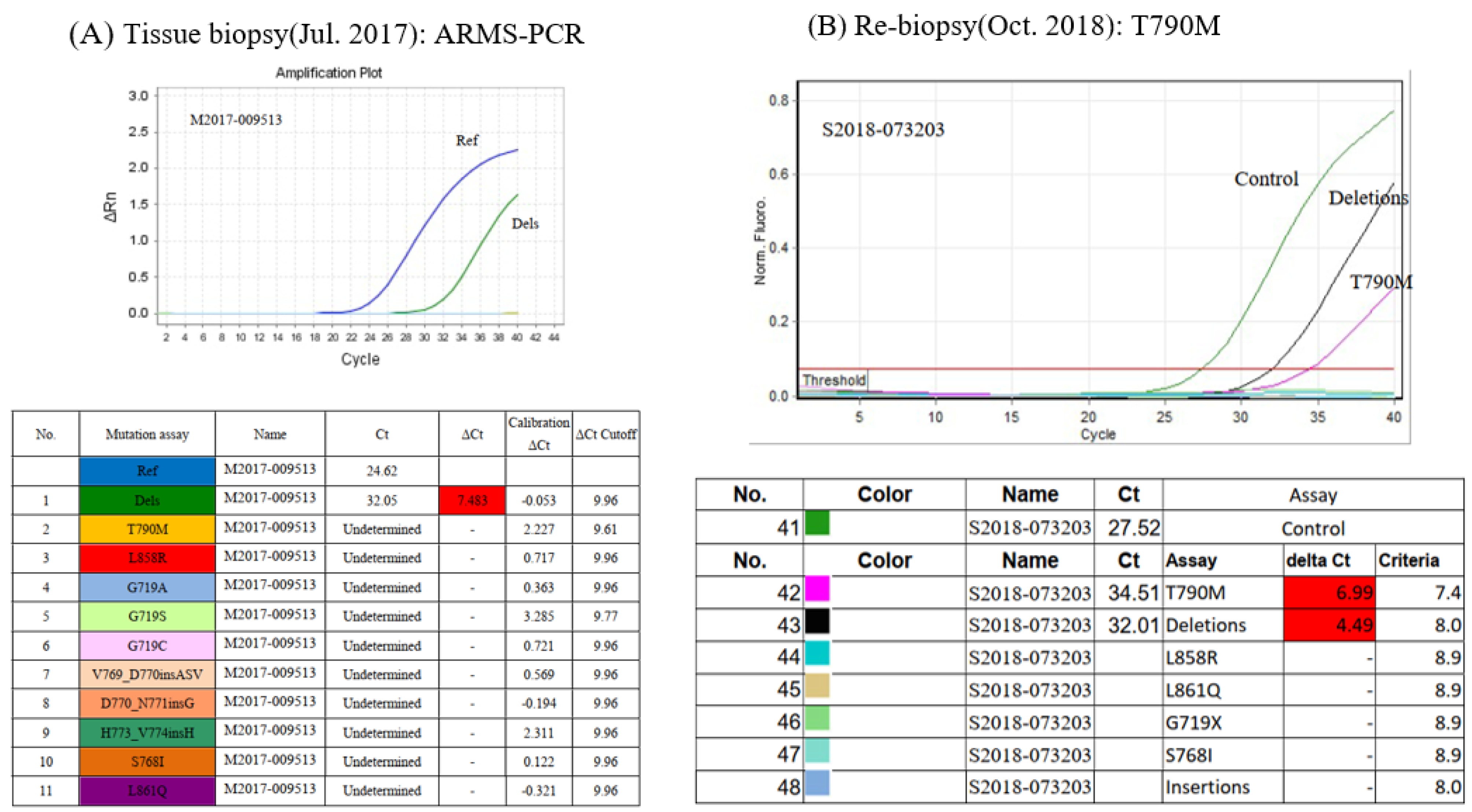
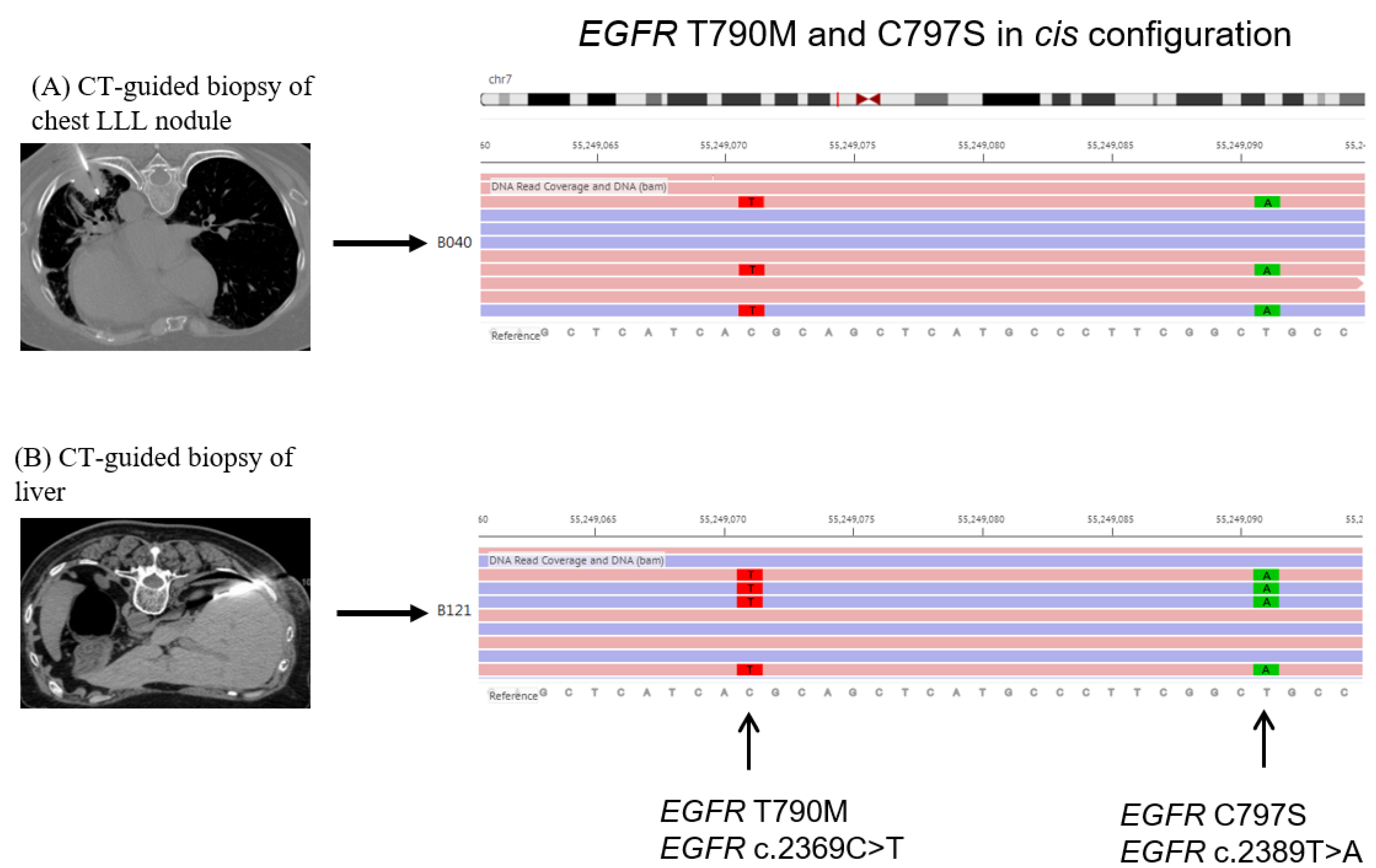
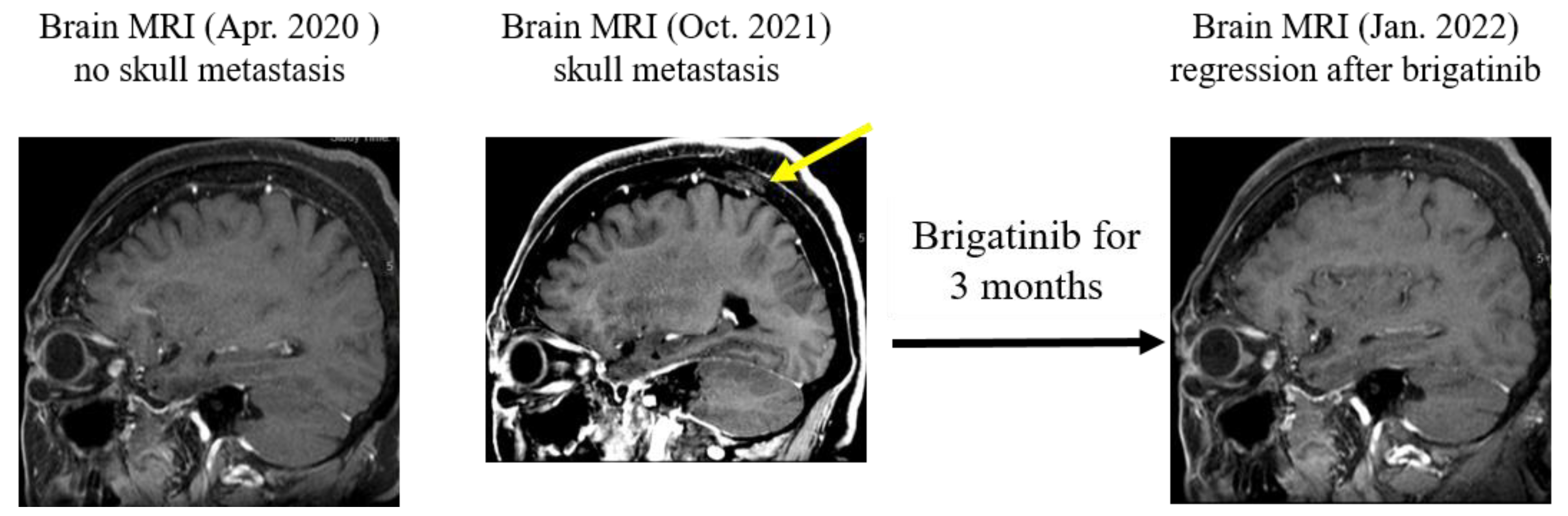
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, S.V.; Bell, D.W.; Settleman, J.; Haber, D.A. Epidermal growth factor receptor mutations in lung cancer. Nat. Rev. Cancer 2007, 7, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.C.H.; Wu, Y.L.; Schuler, M.; Sebastian, M.; Popat, S.; Yamamoto, N.; Zhou, C.; Hu, C.P.; O’Byrne, K.; Feng, J.; et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): Analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol. 2015, 16, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; Zhou, C.; Hu, C.P.; Feng, J.; Lu, S.; Huang, Y.; Li, W.; Hou, M.; Shi, J.H.; Lee, K.Y.; et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): An open-label, randomised phase 3 trial. Lancet Oncol. 2014, 15, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Camidge, D.R.; Pao, W.; Sequist, L.V. Acquired resistance to TKIs in solid tumours: Learning from lung cancer. Nat. Rev. Clin. Oncol. 2014, 11, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Chang, J.W.C.; Chang, C.F.; Huang, C.Y.; Yang, C.T.; Kuo, C.H.S.; Fang, Y.F.; Hsu, P.C.; Wu, C.E. Real-world Afatinib Outcomes in Advanced Non-small Cell Lung Cancer Harboring EGFR Mutations. Anticancer Res. 2022, 42, 2145–2157. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.J.; Chang, J.W.C.; Chang, C.F.; Huang, C.Y.; Yang, C.T.; Kuo, C.H.S.; Fang, Y.F.; Hsu, P.C.; Wu, C.E. Impact of T790M Mutation Status on Later-Line Osimertinib Treatment in Non-Small Cell Lung Cancer Patients. Cancers 2022, 14, 5095. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.G.; Chiang, C.L.; Liu, C.Y.; Wang, C.C.; Su, P.L.; Hsia, T.C.; Shih, J.Y.; Chang, G.C. An Observational Study of Acquired EGFR T790M-Dependent Resistance to EGFR-TKI Treatment in Lung Adenocarcinoma Patients in Taiwan. Front. Oncol. 2020, 10, 1481. [Google Scholar] [CrossRef] [PubMed]
- Chang, Z.-T.; Chan, T.-M.; Wu, C.-E. EGFR T751_I759delinsN Mutation in Exon19 Detected by NGS but Not by Real-Time PCR in a Heavily-Treated Patient with NSCLC. Int. J. Mol. Sci. 2022, 23, 13451. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.C.; Chang, J.W.C.; Chang, C.F.; Huang, C.Y.; Yang, C.T.; Kuo, C.H.S.; Fang, Y.F.; Wu, C.E. Sequential treatment in advanced non-small cell lung cancer harboring EGFR mutations. Ther. Adv. Respir. Dis. 2022, 16, 17534666221132731. [Google Scholar] [CrossRef] [PubMed]
- Jänne, P.A.; Yang, J.C.H.; Kim, D.W.; Planchard, D.; Ohe, Y.; Ramalingam, S.S.; Ahn, M.J.; Kim, S.W.; Su, W.C.; Horn, L.; et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N. Engl. J. Med. 2015, 372, 1689–1699. [Google Scholar] [CrossRef] [PubMed]
- Leonetti, A.; Sharma, S.; Minari, R.; Perego, P.; Giovannetti, E.; Tiseo, M. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br. J. Cancer 2019, 121, 725–737. [Google Scholar] [CrossRef]
- Uchibori, K.; Inase, N.; Araki, M.; Kamada, M.; Sato, S.; Okuno, Y.; Fujita, N.; Katayama, R. Brigatinib combined with anti-EGFR antibody overcomes osimertinib resistance in EGFR-mutated non-small-cell lung cancer. Nat. Commun. 2017, 8, 14768. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Han, R.; Zhu, M.; He, T.; He, Y. Case Report: Durable Response to the Combination of Brigatinib and Cetuximab Plus Icotinib in a NSCLC Patient Harboring EGFR L858R-T790M-cis-G796S and L718Q Resistance Mutations Following Progression With Osimertinib. Front. Oncol. 2022, 12, 875313. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yang, N.; Ou, Q.; Xiang, Y.; Jiang, T.; Wu, X.; Bao, H.; Tong, X.; Wang, X.; Shao, Y.W.; et al. Investigating Novel Resistance Mechanisms to Third-Generation EGFR Tyrosine Kinase Inhibitor Osimertinib in Non–Small Cell Lung Cancer PatientsNovel Resistance Mechanisms to Osimertinib in NSCLC Patients. Clin. Cancer Res. 2018, 24, 3097–3107. [Google Scholar] [CrossRef]
- Oxnard, G.R.; Hu, Y.; Mileham, K.F.; Husain, H.; Costa, D.B.; Tracy, P.; Feeney, N.; Sholl, L.M.; Dahlberg, S.E.; Redig, A.J.; et al. Assessment of Resistance Mechanisms and Clinical Implications in Patients With EGFR T790M-Positive Lung Cancer and Acquired Resistance to Osimertinib. JAMA Oncol. 2018, 4, 1527–1534. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, N.; Zhang, Y.; Li, L.; Han, R.; Zhu, M.; Feng, M.; Chen, H.; Lizaso, A.; Qin, T.; et al. Effective Treatment of Lung Adenocarcinoma Harboring EGFR-Activating Mutation, T790M, and cis-C797S Triple Mutations by Brigatinib and Cetuximab Combination Therapy. J. Thorac. Oncol. 2020, 15, 1369–1375. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.-W.; Lin, G.; Shih, H.-J.; Wu, C.-E. Response to Brigatinib Targeted Therapy in Non-Small Cell Lung Cancer Harboring Epidermal Growth Factor Receptor Exon 19 Deletion, T790M, and cis-C797S Triple Mutations: A Case Report. Int. J. Mol. Sci. 2023, 24, 602. https://doi.org/10.3390/ijms24010602
Chen Z-W, Lin G, Shih H-J, Wu C-E. Response to Brigatinib Targeted Therapy in Non-Small Cell Lung Cancer Harboring Epidermal Growth Factor Receptor Exon 19 Deletion, T790M, and cis-C797S Triple Mutations: A Case Report. International Journal of Molecular Sciences. 2023; 24(1):602. https://doi.org/10.3390/ijms24010602
Chicago/Turabian StyleChen, Zi-Wei, Gigin Lin, Hsuan-Jen Shih, and Chiao-En Wu. 2023. "Response to Brigatinib Targeted Therapy in Non-Small Cell Lung Cancer Harboring Epidermal Growth Factor Receptor Exon 19 Deletion, T790M, and cis-C797S Triple Mutations: A Case Report" International Journal of Molecular Sciences 24, no. 1: 602. https://doi.org/10.3390/ijms24010602
APA StyleChen, Z.-W., Lin, G., Shih, H.-J., & Wu, C.-E. (2023). Response to Brigatinib Targeted Therapy in Non-Small Cell Lung Cancer Harboring Epidermal Growth Factor Receptor Exon 19 Deletion, T790M, and cis-C797S Triple Mutations: A Case Report. International Journal of Molecular Sciences, 24(1), 602. https://doi.org/10.3390/ijms24010602





