Abstract
Staphylococcus aureus is a major human pathogen whose characteristics support its success in various clinical settings including Cystic Fibrosis (CF). In CF, S. aureus is indeed the most commonly identified opportunistic pathogen in children and the overall population. S. aureus colonization/infection, either by methicillin-susceptible or methicillin-resistant strains, will become chronic in about one third of CF patients. The persistence of S. aureus in CF patients’ lungs, despite various eradication strategies, is favored by several traits in both host and pathogen. Among the latter, living in biofilm is a highly protective way to survive despite deleterious environmental conditions, and is a common characteristic shared by the main pathogens identified in CF. This is why CF has earned the status of a biofilm-associated disease for several years now. Biofilm formation by S. aureus, and the molecular mechanisms governing and regulating it, have been extensively studied but have received less attention in the specific context of CF lungs. Here, we review the current knowledge on S. aureus biofilm in this very context, i.e., the importance, study methods, molecular data published on mono- and multi-species biofilm and anti-biofilm strategies. This focus on studies including clinical isolates from CF patients shows that they are still under-represented in the literature compared with studies based on reference strains, and underlines the need for such studies. Indeed, CF clinical strains display specific characteristics that may not be extrapolated from results obtained on laboratory strains.
1. S. aureus, a Major Pathogen in the Biofilm-Associated Disease, Cystic Fibrosis
Staphylococcus aureus is a major human pathogen whose characteristics support its success in diverse clinical settings like bacteremia, skin and soft tissue infections, necrotizing pneumonia, device-associated infections like catheter-related infections and ventilator-associated pneumonia, and Cystic Fibrosis (CF) [1]. Besides a wide panel of secreted virulence factors and its multidrug-resistant capacities, biofilm formation is a significant feature that protects S. aureus against host defenses and eradication measures and, consequently, allows it to persist in the host [2,3]. Biofilm is defined as a more or less complex, diverse, three-dimensional structured microbial community embedded in a matrix formed by extracellular polymeric substances (EPS) whose major components are extracellular DNA (eDNA), extracellular polysaccharides and structural proteins. Biofilms were long considered as being attached to a surface. However, more recently, biofilms have also been found unattached to any surface. presenting as three-dimensional aggregates, a form that may be closer to the in vivo situation, particularly in mucosal infections like CF [4,5]. Biofilm formation is a dynamic, coordinated, cyclic process, classically described as involving several stages. It begins with the initial reversible, then irreversible, attachment of planktonic bacteria to a surface, secretion of various matrix-forming EPS and bacterial proliferation with microcolony formation leading to a mature, multilayered biofilm of sessile bacteria. The final stage in the biofilm lifecycle is dispersion, controlled through interbacterial communication such as quorum sensing, during which bacteria detach from the biofilm, return from a sessile to a planktonic stage, and may colonize new sites [1,6]. Biofilm formation is not just a protected way of life: it also represents an additional factor favoring the emergence of resistance towards antimicrobial treatments. Indeed, sessile cells are exposed to sub-inhibitory concentrations of antibiotics due to penetration defects and their close proximity in biofilms facilitated horizontal transfer of resistance-encoding genes. Finally, biofilms also contain a subpopulation of quiescent bacterial cells named persisters that are transiently tolerant to antimicrobial stresses [7]. Living in biofilm thus represents a major advantage for persistence during chronic diseases like CF and this must be considered in the management of biofilm-associated diseases like CF.
Cystic Fibrosis is an inherited disease affecting the CFTR (cystic fibrosis transmembrane conductance regulator) protein, marked by a vicious cycle of infection and inflammation which is highly deleterious for the patient’s lung function. Pseudomonas aeruginosa and S. aureus are the two major pathogens in CF. Although P. aeruginosa is dominant in the adult population, S. aureus is the most commonly identified opportunistic pathogen in children and the overall CF population [8] with 60 to 80% of CF patients under 20 years old being colonized according to French and American cystic fibrosis registries [9,10,11]. After initial colonization, a subset of patients, representing 36% of patients according to data from the European Cystic Fibrosis Society patient registry, will become chronically colonized [11]. This bacterial persistence is favored by local impairment in host defenses (for example, altered function of macrophages and antimicrobial peptides) as well as the adaptive faculties of the pathogen to the CF lung, a stressful environment wherein pathogens are subjected to diverse and fluctuant abiotic and biotic selective pressures such as hyperinflammation, oxidative stress, limitations in and competition for nutrients and space, anaerobiosis, increased acidity in airway surface liquid and exposure to antibiotics [12,13,14,15]. Biofilm formation is one of the bacterial adaptive responses to environmental stress and, despite administering suitable antibiotics to CF patients, pathogens may elicit persistent colonization that can be attributable to biofilm formation. Nowadays, CF is considered as a biofilm-associated disease but, due to the unique characteristics of CF airways, the particularities of biofilm formation by the major opportunistic pathogens identified in CF patients warrant further consideration. This will enable us to more precisely decipher the pathogenesis of persistent infections in this particular context, especially as biofilm control is the goal of many antimicrobial strategies [16,17]. However, as far as S. aureus is concerned, biofilm formation and the molecular mechanisms governing and regulating it have been extensively studied but have received less attention in the unique context of CF lungs.
2. Delineation of Information Gathered from a Review of the Literature and Scope of the Review
A literature search using “Staphylococcus aureus”, “Biofilm” and “Cystic Fibrosis” in the PubMed database found 152 publications and a similar search for “Pseudomonas aeruginosa” found 1200 results and a combined search for “Staphylococcus aureus” and “Pseudomonas aeruginosa” gave 108 publications (8 October 2022) (Figure 1). This highlighted the fact that studies on S. aureus biofilm formation in CF frequently included the two major pathogens, studied either independently or considered through the prism of interactions in dual-species biofilms, a situation closer to the conditions of the CF lung compared to mono-species biofilms. The proportion of patients co-infected by S. aureus and P. aeruginosa is indeed estimated to be around 30% [18].
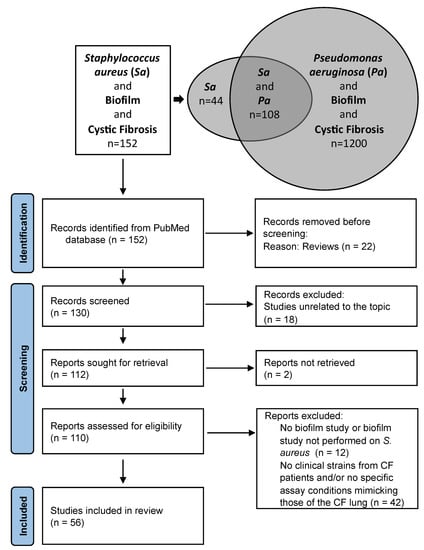
Figure 1.
Results of the literature search on S. aureus biofilm in the context of Cystic Fibrosis. (PubMed database, search date: 8 October 2022) (top) and flow diagram of study selection (bottom). Sa: Staphylococcus aureus; Pa: Pseudomonas aeruginosa; n: number of publications (not to scale); CF: Cystic Fibrosis.
The main limitation was identified after reviewing the sections ‘Materials and Methods’ sections of the manuscripts selected, as many articles did not include any clinical strains from CF patients and only studied reference laboratory strains as material to support the observations reported (Figure 1). This was notably observed when reviewing the literature on the consequences of bacterial interactions with S. aureus on biofilm formation in CF. Among the S. aureus strains most often used in the literature we reviewed were: the community-acquired methicillin-resistant (MRSA) strain USA300 LAC [19,20] and its derivative strain JE2 [18,21] from skin and soft tissue infection; the hospital-acquired MRSA USA100 Tokyo clone [19]; strain 15,981, a strong biofilm-forming strain from an otitis infection [22]; strain ATCC 29,213 (=LMG 10,147) from a wound [23]; and the Newman strain isolated in 1952 from a human infection [24,25]. Some surprising choices of S. aureus reference strains were also noted in certain studies. These included strain ATCC 6538, a standard strain for biocide susceptibility testing [26,27] and strain 502A [19], a strain isolated in 1963 from a nurse working in a newborn nursery and further used to colonize infants to prevent colonization by more invasive S. aureus strains [28].
The use of these reference strains may not reflect the behavior of clinical CF strains as laboratory maintenance may affect their genomic integrity and alter phenotypic traits over the years [29,30]. Currently there is increasing evidence that clinical and reference strains do not behave identically [31,32,33]. Although studies on reference strains and those including clinical strains provide complementary results, our particular aim was to summarize the knowledge obtained in the specific context of CF. We therefore voluntarily limited the data presented hereafter to those originating from studies on clinical strains isolated from CF patients, with the exception of studies based on reference strains when the latter were studied under specific experimental conditions mimicking those of CF airways (such as growth in a cystic fibrosis mucus-mimicking medium), to more faithfully reflect the in vivo conditions found in CF patients.
3. The Challenge of Studying Biofilm Formation in the Context of Cystic Fibrosis
A few of the studies we reviewed reported direct visualization of biofilm by scanning electron microscopy (SEM) and further viability evaluation by confocal laser scanning microscopy (CLSM) combined with Live/Dead stain. These approaches were applied to the toothbrushes of CF children and helped demonstrate that sessile S. aureus colonize the toothbrushes of colonized patients and may represent a reservoir for patient reinfection [34]. Interestingly, SEM that allows the study of the spatial structure of biofilm and detects the presence of EPS based on surface scattering and absorption of electrons, showed modifications of the biofilm structuration on toothbrushes of children who had received antibiotic treatment with no more surface bacteria attached to EPS and viable bacteria encased inside thick EPS [34]. Similarly, CLSM that provides three-dimensional images of biofilms showed clear differences in bacterial aggregation, depending on the type of biomaterial commonly used in dentistry [35]. Fluorescence electron in situ hybridization using a specific peptide nucleic acid (PNA-FISH) probe revealed the presence of sessile S. aureus on the sinus mucosa of CF patients supporting the fact that both upper and lower airways were the sites of S. aureus biofilm formation. In CF patients suffering from chronic rhinosinusitis, the sinuses may thus represent a reservoir for S. aureus, predisposing them to recurrent lung infection [36].
However, the majority of currently available studies are based on in vitro approaches to studying S. aureus biofilm whereas fewer, more recent, studies used ex vivo lung models. In both conditions, CF mucus-mimicking media, also called artificial sputum medium (ASM) or synthetic CF sputum medium (SCFM), were used in some studies to provide results more relevant to the CF lung than those obtained by using the media normally recommended, i.e., Trypticase Soja (TS) supplemented or not with glucose and NaCl, Brain Heart Infusion (BHI) or Luria Broth (LB) due to rheological properties closer to those of the CF mucus [37,38,39,40,41]. These studies showed that the results depend on the medium in which the biofilm was developed and, broadly speaking, on the overall culture conditions (atmosphere, biofilm substrate, etc.) affecting biofilm growth, metabolic activity and resistance to antimicrobials [37,38]. In particular, Haley et al. using SEM and CLSM to evaluate biovolume, biomass, thickness and roughness of the biofilm showed that these structural parameters varied according to biofilm growing conditions [37]. However, nine artificial mucus medium formulations were successively described that may not provide similar results due to variable composition, either containing mucin, egg emulsion, glucose, or not [37,41]. Similarly, another study used protein-conditioned surfaces, i.e., polystyrene microplates coated with mucin or elastin with the aim to reproduce conditions closer to those found in vivo in CF patients [42]. Finally, one interesting study used experiments based on the timeline of lung colonization, i.e., more generally, a first colonization by S. aureus followed by P. aeruginosa in CF patients, and demonstrated that introducing the two species according to a timeline, and at various inoculation strengths, affects the formation of biofilm [27].
3.1. Biofilms In Vitro Assay Approaches
The ability to form biofilm was evaluated by Bernardy et al. through qualitative phenotypic characterization of exopolysaccharide (EPS) production using Congo red agar plates on a collection of S. aureus from CF patients showing strains that did not produce EPS to EPS-overproducing strains, even in the same patient [43]. Besides this screening method, a large panel of methods is available for biofilm growth, characterization and visualization allowing the evaluation of bacterial adhesion, biofilm biomass, viability and/or matrix composition. In addition, the development of fluorescence imaging techniques either after live/dead staining, differential fluorescent staining of species in multispecies biofilms or staining of the biofilm components by specific probes (cells, EPS, proteins, …) allowed high resolution study of the biofilm spacial structure. For a review of all these biofilm study methods and their respective advantages and limitations, see Azeredo et al. [44]. They can globally be classified into static (usually using microplates) or dynamic (under flow or microfluidic) methods. Among them, biofilm biomass quantification based on Crystal Violet (CV) staining in microtiter plates was the most commonly used technique for studying S. aureus strains from CF patients (Supplementary Table S1). However, this method has limitations, such as the absence of a standardized protocol and a lack of both reproducibility and sensitivity. Considering these limitations, several works aimed to apply more standardized approaches to studying CF clinical isolates.
Boudet et al. used both the Biofilm Ring Test® (BRT®) (BioFilm Control, Saint-Beauzire, France) and the BioFluxTM 200 to study biofilm formation in the presence or absence of antibiotics by MRSA from CF patients [40]. BRT® evaluates bacterial adhesion and early biofilm formation by immobilizing magnetic beads throughout the formation of biofilm. It is also dedicated to studying the capacity of antibiotics to inhibit biofilm formation through an approach called the Antibiofilmogram® [45] whereas with the microfluidics system BioFlux™ 200, biofilm formation can be studied under dynamic conditions [46]. Both approaches were adapted for use with the mucin-containing synthetic growth medium, ASM [47,48], particularly the ASM had to be a modified (0.22 μm filtration) for use with the BRT® which did not accept opaque media. They showed that adhesion was enhanced in ASM compared with BHI medium. They also brought new insights into CF MRSA’s ability to form biofilm, previoulsy little studied, showing that biofilm formation was strain-dependent, even for clonally related strains isolated from a same sputum sample, and differentially influenced by antibiotics. The importance of the patient’s colonization history was also highlighted as two MRSA strains, isolated three years apart in a chronically colonized patient, showed distinct patterns of biofilm formation (increased biofilm formation for the latest isolated strain) in the BioFlux™ 200 assay. More recently, Cheng et al., considering that in vivo biofilm may be formed in the absence of any surface for attachment, developed a surface-independent method to study biofilm formation based on a hanging-drop biofilm culture model with the aim of reducing the gap between in vitro predictions and in vivo responses [4]. The method appeared highly attractive for studying CF clinical isolates as, in CF, biofilm consists of unattached aggregates dispersed in the mucus [49]. Using this original, 96-well plate hanging-drop technology coupled with gene expression quantification and CLSM with differential fluorescent staining of biofilm components, the authors showed that the biofilm formed by a CF MRSA isolate displayed specific characteristics compared with that of an MRSA strain from a central catheter-related infection [4]. Indeed, this biofilm was less rich in matrix and had a lower viable cell count but was formed with double-sized micro-colonies and had a 50% higher metabolic activity. Studying the expression of three representative genes that are known to be upregulated in surface-attached S. aureus biofilms: sdrC (Ser-Asp-Arg-rich fibrinogen-binding protein) encoding proteins for fibrinogen mediated cell adhesion, arcB (ornithine transcarbamylase) involved in extraction and catabolism of arginine, and ureC (urease accessory protein C) involved in metabolism of urea, showed that these three genes were differentially upregulated over time in hanging-drop S. aureus biofilms compared with surface-attached ones supporting that hanging-drop biofilm maturation occurred earlier than that of surface-attached biofilm. In addition, sdrC expression levels were unchanged in hanging-drop model but dramatically upregulated in surface-attached biofilm model suggesting that the biofilm formation in the hanging-drop formation may not depend on fibrinogen-mediated adhesion [4]. Again, these observations highlighted the importance of using clinical isolates and testing methods that mimic CF lung conditions as closely as possible.
3.2. Ex Vivo Models
Using both immortalized human CF airway epithelial cells and primary CF human bronchial epithelial cells (obtained from the explanted lungs of CF patients), Kiedrowski et al. quantified biofilm biomass produced by S. aureus during co-infection with Respiratory Syncytial Virus (RSV) in live-cell imaging chambers. They observed that S. aureus biofilm growth was enhanced when cells were coinfected with RSV and that factors secreted during viral infection benefitted S. aureus biofilms. This observation obtained with RSV and S. aureus reference strains was also observed with human Rhinovirus 14 and confirmed with CF chronic rhinosinusitis clinical isolates [19]. Two other publications from the same team studied S. aureus biofilm formation after histological staining in an ex vivo model of CF infection comprising pig bronchiolar tissue (ex vivo pig lung, EVPL) and the synthetic mucus SCFM in the presence or absence of antibiotics [50,51]. They confirmed that a greater proportion of S. aureus localized as aggregates in the mucus (unattached biofilm) rather than associated with tissue. Sweeney et al. also provided interesting comparative results for (i) a pair of S. aureus strains isolated during lung exacerbation and stable clinical status showing differences in both growth and the location of bacterial cells and (ii) three lungs showing that growth of the strains depended on the lung tissue inoculated and thus on the host [50]. Regarding the effects of antibiotic treatment on biofilm formed on EVPL, clinical S. aureus isolates displayed an increased tolerance to antibiotics (linezolid, flucloxacillin) with effects not correlated with the Minimum Inhibitory Concentration (MIC). Thus, the EVPL model was reported as a host-mimicking model suitable for accurate antimicrobial susceptibility testing of CF pathogens [50,51].
All these methodological development efforts underline the challenge of studying biofilm formation in the context of CF and it probably remains illusory to mimic all the environmental stresses and conditions found in CF airways (acidity, anaerobiosis, inflammation and antimicrobial conditions—peptides, antibiotics; etc.). They also underline the different behavior of S. aureus strains according to the patient’s colonization history (because during chronic colonization, adapted strains are isolated) or clinical status (stable or with pulmonary exacerbation). This should be borne in mind when analyzing the literature and trying to compare results from different studies.
4. Biofilm Formation by S. aureus in the Unique Context of Cystic Fibrosis
4.1. Observational Studies
The studies reviewed in this part of the manuscript are summarized in Table 1 and complementary data are given in Supplementary Table S1. When detailed, strains forming biofilm are usually classified into different categories: non biofilm-producers, weak/minor, moderate/medium/intermediate and strong biofilm-producers. However, different criteria for biofilm production classification may be used according to the study. Unless otherwise specified, the studies investigated biofilm biomass formed by CF S. aureus strains through indirect measurement via CV staining.
4.1.1. Overall Ability of S. aureus to Form Biofilm in CF
First of all, we addressed the question of whether CF strains may have specific capacities for biofilm formation. We found two observational studies that compared the biofilm-forming ability of S. aureus from CF and non-CF patients with divergent results. In the study by Molina et al., comparing 17 strains collected from CF respiratory samples and 20 strains from blood cultures of non-CF patients, nearly all strains formed biofilm and no significant difference in biofilm formation between strain categories was observed (16 out of the CF strains, 94.1%, versus all the non-CF strains were biofilm-formers) [52]. By contrast, the study by Cakir Aktas et al. showed that biofilm production was significantly more often observed among 31 CF methicillin-susceptible S. aureus (MSSA) compared with 57 non-CF S. aureus isolates collected from hospitalized patients with lower respiratory tract infection (53.4% MRSA, 46.6% MSSA). Biofilm production was indeed detected in 96.8% of CF strains versus 47.4% of non-CF strains, suggesting that S. aureus may have a particular ability to form biofilm in the context of CF [53].
We then examined other studies reporting the overall proportion of CF strains displaying any ability to form biofilm. Taken together, three studies using the same classification criteria [54] examined a total of 86 CF S. aureus strains including 16 MRSA and 70 MSSA. Biofilm production was detected in 74.4% of the strains distributed in 25.6% of weak, 41.9% moderate and 7% strong biofilm-producers [35,55,56]. Other studies each applied specific criteria for strain classification according to their biofilm formation. Among the strains forming biofilm in the Cakir Aktas et al. study (96.8% of 31 MSSA), 32.3% exhibited strong, 38.7% moderate and 25.8% weak positive phenotype [53]. Wieneke et al. showed that most S. aureus isolates were minor biofilm-forming strains (including non-biofilm-producers, weak and moderate biofilm-producers in that study) (1773/2319 isolates, 76.5%), while 546 isolates (23.5%) were strong biofilm-producers [57]. Biofilm formation was detected for all the 14 CF MRSA included in another study with 71.4% of strains being strong biofilm-producers [58] and in 80% of the S. aureus strains examined by Pompilio et al. [59]. Finally, using the BRT® and ASM, 55.6% of 63 CF MRSA were shown to form early biofilm and were classified according to the biofilm formation index value generated by the device in 28.6% of intermediate and 27% of strong biofilm-producers [40].
We finally examined studies evaluating biofilm formation over time during chronic colonization of CF airways by comparing biofilm formation of S. aureus strains collected early in the course of CF airway infection (early strains) and long-term adapted S. aureus strains (late strains). The seven studies retrieved showed different results, as four studies including few strains found a positive association between biofilm production and the persistence of S. aureus over time in CF patients [40,60,61,62] whereas, for studies including a larger number of S. aureus strains, one reported no change [63] and two others highlighted the variable evolution of biofilm formation over time according to the patient [57,64]. These studies are presented successively hereafter and in Table 1.

Table 1.
Summarized presentation of studies on biofilm formation by clinical strains of S. aureus isolated from patients with Cystic Fibrosis.
Table 1.
Summarized presentation of studies on biofilm formation by clinical strains of S. aureus isolated from patients with Cystic Fibrosis.
| CF Patients | S. aureus Strains | Biofilm Formation | Ref. | ||
|---|---|---|---|---|---|
| n | Country | n (MSSA/MRSA) | CF Host-Adapted Strains | Biofilm-Producing Strains (%) & Evolution Weak (W)/Moderate (M)/Strong-Producers (S) (%) | |
| 18 | Spain | 93 (0/93) | Persistence of a single MRSA clone: 77.8% of patients | 14/15 CF MRSA pulsotypes | [52] |
| 31 | Turkey | 31 (31/0) | NA | 96.8% W (25.8%)/M (38.7%)/S (32.3%) | [53] |
| 183 | Iran | 24 (20/4) | NA | 66.6% W (37.5%)/% (20.8%)/S (8.3%) | [55] |
| NA | Poland | 33 (30/3) | NA | 90.9% W (24.2%)/M (60.6%)/S (6.1%) | [35] |
| 15 | Italy | 15 (8/7)) | NA | 80% | [59] |
| 42 | Italy | SCV: 28 (21/7) Non-SCV: 29 (20/9) | Patients chronically colonized | SCV positive strains: 100% W (25%)/M (53.6%)/S (21.4%) Non-SCV (normal phenotype) strains: 62% W (17.2%)/M (37.9%)/S (6.9%) | [56] |
| 14 | Germany | 2319 (unk.) 501 mucoid | Mean persistence: 15.6 y (range: 10–21 y) | No + W + M (76.5%)/S (23.5%) Evolution: unchanged: 8/:4/:2 patients | [57] |
| 5 | Italy | 14 (0/14) | Persistance: 5; chronic colonization: 2 patients | 100% (M and S) | [58] |
| 35 | France | 63 (0/63) | Chronic colonization: 16/35 | No (44.4%)/M (28.6%)/S (27%) | [40] |
| 2 | France | 2 (unk.) | Chronic colonization: 1 patient | 100% Late isolate: 5 x more biofilm than early isolate (from another patient) | [60] |
| 3 | France | 6 (2/4) 3 early, 3 late | Late isolates: 3/6 (interval early/late: 2.8–9 y) | 100% More biofilm formed by late isolates | [61] |
| 9 | Germany | 18 (6/12) | Late isolates: 9/18 (interval early/late: 3–13 y) | 11.1% (although all carried icaA, C and D genes) | [62] |
| 49 | U.S.A. | 98 (0/98) | Late isolates: 49/98 (interval early/late: ≥ 2 y) | 100% No differences between incident/chronic isolates | [63] |
| 29 | Germany | 58 (56/2) | Mean persistence: 8.25 y (range: 5.1–13.6 y) | W: 66% Evolution: unchanged: 17/:7/:5 patients | [64] |
| 8 | Germany | 425 (unk.) 115 mucoid (all carried the 5 bp-deletion) | Mean persistence of 29 m (range: 1–126 m) | All mucoid isolates: enhanced biofilm production, Non-mucoid strains: almost no biofilm formation | [65] |
| 81 | Germany | 1050 (unk.) 37 mucoid (25 carried the 5 bp-deletion) | NA | 6/7 patients: mucoid isolates formed significant higher amounts of biofilm than non-mucoid isolates; 1/7 patients: no biofilm formed by Sa with mucoid phenotype (no 5 bp-deletion) | [66] |
| NA | U.S.A. | 50 (unk.) | NA | 86% (including CFSa36 strain, see text) | [67] |
| 2 | Ireland | 2 (unk.) | NA | 100% when stimulated by bile or bile acids | [68] |
| 3 | NA | 12 (0/12) | 12/12 | 100% | [69] |
MSSA, methicillin-susceptible Staphylococcus aureus (Sa); MRSA, methicillin-resistant S. aureus; SCV, small colony variant; unk., unknown; y, years; m, months; NA, not available; No: non-biofilm formers.
Ciornei et al. compared two S. aureus clinical isolates, one isolated at the beginning of infection and one at a chronic stage in another patient. Despite the limitation that the two strains were not isolated from the same patient, the late isolate produced almost five times more biofilm than the early isolate with the continuous-flow culture bioreactors [60]. Congruent observations were made for strains successively isolated in the same patient. Two paired MRSA strains isolated three years apart from the sputum of the same CF patient showed that the late strain systematically displayed higher biofilm formation than the early strain using the BioFluxTM 200 system, whatever the experimental condition (BHI medium or ASM) and measured time point (5, 12, 24 or 36 h) [40]. Similarly, Tan et al. investigating one pair of MSSA and two pairs of MRSA demonstrated that late isolates had greater ability to form biofilm than early paired isolates, with a mean time between the collection of early and late isolates estimated at 6.2 years [61]. Of the nine early/late strain pairs investigated by Treffon et al., two of the late isolates became biofilm-producers, one livestock-associated MRSA and one MSSA, after eight and 13 years of colonization, respectively, whereas all other strains were non-biofilm formers [62]. By studying a larger number of S. aureus strains (49 pairs of CF MRSA), Gilpin et al. did not observe any significant differences in biofilm production between early and late isolates collected at least two years apart, although all strains showed the capacity to form biofilm [63]. Finally, two studies showed that S. aureus persistence was associated with variable evolution in biofilm formation. In a one-year observational prospective study, Wieneke et al. examined a total of 2319 CF S. aureus strains (methicillin-resistance not specified) collected from the sputum of 14 CF patients with long-term persistent infections (range: 10–21 years of persistence) [57]. They observed that biofilm formation had increased in four (29%), was unchanged with a large percentage of high biofilm-forming isolates in three (21%), unchanged with no biofilm-positive isolates in five (36%), and had decreased in two (14%) patients over time. Hirschhausen et al. investigated 29 pairs of S. aureus (including 3.4% MRSA and 96.6% MSSA) collected from the sputum of CF patients with a mean persistence of 8.25 years (range: 5.1–13.6 years) [64]. Biofilm formation had increased in seven (24%), was unchanged in 17 (59%) and had decreased in five (17%) late isolates compared with early isolates.
The variability observed in biofilm production both between and within studies might be explained by numerous confounding factors like the length time of observations and also the timeline of strain isolation regarding the patient’s colonization history (early strain are not necessarily isolated during the initial or first episodes of infection). The influence of co-infecting pathogens such as P. aeruginosa is also a major factor of variability of S. aureus biofilm formation (see also part 6) [57]. Finally, Hirschhausen et al. hypothesized that it might be more appropriate for S. aureus survival to increase biofilm formation at the onset of chronic colonization to protect itself against phagocytosis and antibiotic treatment whereas, during long-term persistence, it might be more suitable to form less biofilm to be able to disseminate in the airways of CF patients [64].
Altogether, biofilm formation was a common trait of both MRSA and MSSA strains in CF patients ranging from 55.6% to 100% of the strains studied according to the study with a proportion of strong biofilm-producing strains varying from 7 to 71.4%. As indicated, such variability may be associated with distinct methods of biofilm formation study or criteria applied for interpretation, to distinct studied strains and patient colonization history (MRSA vs. MSSA, strains from early episodes of infection vs. adapted strains from chronically colonized patients, strains from patients co-colonized by other pathogens or not). The clinical impact of biofilm formation has yet to be investigated but Wieneke et al. showed that high biofilm-forming S. aureus isolates were associated with fewer pulmonary exacerbations in CF patients and, conversely, that exacerbations had a negative impact on biofilm formation [57].
4.1.2. Biofilm Formation in Specific Subpopulations of CF S. aureus Strains
To date, we only found two studies that have compared MRSA and MSSA in their ability to form biofilm but only one included CF strains. In the latter study, Pompilio et al. showed a significantly higher median biofilm amount produced by MRSA compared with MSSA but this study was based on a few CF strains (three MRSA and three MSSA) [70]. These results were congruent with those obtained by Kadkhoda et al. on S. aureus collected from children with clinical symptoms of infection admitted to Children’s Medical Center Hospital in Tehran showing an association between biofilm formation and MRSA [71]. It thus remains interesting to study this association on a larger number of CF strains, particularly because studies suggested that mechanisms governing biofilm formation might be distinct in MRSA compared with MSSA. Indeed, intercellular adherence (ica) operon was shown to mediate biofilm formation in MSSA through the production of polysaccharide intercellular adhesin (PIA), also known as poly-N-acetyl-β-(1-6)-glucosamine (PNAG) whereas an ica-independent mechanism involving the fibronectin binding proteins, FnBPA and FnBPB, and the major autolysin Atl appears to play an important role in MRSA biofilm development [72,73].
Regarding small colony variants (SCV), which are slow-growing auxotrophic subpopulations of bacteria that play an important role in persistence in the CF lung, Morelli et al. observed that 28 SCV strains (7 MRSA and 21 MSSA collected from CF patients) showed a significantly higher ability to form biofilm than 29 strains with normal phenotypes (100% versus 62%) but also comprised a higher proportion of strong biofilm-producers (21.4% versus 6.9%) [56]. In that study, strong hypermutators also showed a greater ability to form biofilm than non-mutating strains, although the difference observed did not reach statistical significance.
Finally, an unusual mucoid phenotype was described for S. aureus [74] and further identified in 2.5% (8/313), 8.6% (7/81) and up to 71% (10/14) of CF patients with positive S. aureus cultures, either MRSA or MSSA [57,65,66]. The higher prevalence of mucoid isolates in the study by Wieneke et al. [57] compared to the two earlier studies [65,66] might be explained by the lower number of patients included, the patient selection criteria implying a long period of S. aureus persistence (mean duration of 15.6 years) during which the number of SCVs increased longitudinally and the deep culturing conditions. Nevertheless, congruent results were observed in all three studies showing that mucoid S. aureus strains produced significantly higher amounts of biofilm compared to non-mucoid strains [57,65,66]. Conversely biofilm formation was also associated with the mucoid phenotype [57]. Mucoidy associated with high biofilm formation thus represents survival advantages in the CF lung thereby certainly contributing to the prolonged persistence of S. aureus in patients’ airways.
4.2. Factors Influencing Biofilm Formation in CF and the Molecular Mechanisms Governing It
S. aureus biofilm formation, as well as the genes and proteins involved in biofilm production have been widely described in contexts other than CF [3]: FnBPA and FnBPB [72,75], Sa G5-E repeat protein SasG, the Clf-Sdr family consisting of clumping factor A/B (ClfA/B) and the serine-aspartate repeat family (Sdr) proteins, ica operon (icaADBC) transcription resulting in the expression of PIA/PNAG. Similarly, different regulators were characterized such as the intracellular adhesin locus regulator (icaR), the teicoplanin-associated locus regulator (tcaR), the staphylococcal accessory regulator (sarA) and the accessory gene regulator (agr) [67,76,77,78] (for a recent review, see Schilcher & Horswill [79]).
In the following subsections, we describe the different factors that were shown to influence biofilm formation of S. aureus strains from CF patients, i.e., host response, environmental factors (metal ions, anaerobic conditions, bile acids), mutations and altered gene expression, and mucoidy, as well as the genes and molecular mechanisms that are critical for biofilm formation by S. aureus in the unique context of CF. These factors remain poorly explored in the literature and are promising targets for fighting persistent S. aureus infections especially in patients with CF.
4.2.1. Biofilm Formation and Host Response
Sadowska et al. studied two CF S. aureus strains (methicillin resistance not specified) trying to understand “frustrated phagocytosis”, i.e., the weak activity of phagocytic cells against microbial biofilm [80]. They showed that S. aureus cell wall components, such as peptidoglycan and lipoteichoic acids, could influence the stimulation of leukocytes resulting in various amounts of cytokine production, including interleukin (IL)-10, IL-6 and tumor necrosis factor-α. However, although these immunomodulatory properties were observed for biofilm-forming CF S. aureus strains, they were not specific to these strains and also present in their planktonic counterparts, as already shown by Ciornei et al. [60].
4.2.2. Biofilm Formation and Environmental Factors
Biofilm formation by S. aureus in CF is affected by environmental factors including metal ions. Indeed, in an MRSA strain called CFSa36 (sequence-type not determined), Liu et al. identified a putative cobalt transporter ATP binding domain (CbiO) that was required for biofilm formation. They showed that the copper ions (Cu2+) entirely complemented the capacity of the cbiO knockout mutant to form biofilm in a dose-dependent manner without having any impact on bacterial growth. Conversely, iron ions (Fe3+) significantly decreased the ability of MRSA to form biofilms in a dose-dependent manner. Thus, they hypothesized that CbiO might mediate S. aureus biofilm formation by affecting the transport of copper ions [67,81].
Anaerobic conditions, typically observed in the CF lung due to mucus obstruction, can also influence S. aureus biofilm production. By studying 98 CF MRSA, Gilpin et al. showed that biofilm formation was significantly lower under anaerobic conditions than under aerobic conditions [63]. It should be noted that this finding on clinical MRSA strains differed from that reported by Cramton et al. and Ulrich et al. on reference MSSA strains, who demonstrated that anaerobic in vitro growth conditions triggered increased ica gene transcription and PIA/PNAG expression by S. aureus and consequently, biofilm formation [82,83]. Because of these opposite observations between studies including strains of distinct origins and methicillin-susceptibility, additional investigations are still needed to decipher the influence of anaerobic conditions on biofilm formation by S. aureus.
Bile acids are another environmental factors affecting biofilm formation. On two S. aureus strains from CF children (specimen collection site and methicillin resistance not specified), Ulluwishewa et al. demonstrated that physiologically relevant concentrations of bile and, more precisely, sodium cholate and sodium deoxycholate bile acids enhanced biofilm formation [68]. Regulatory mutations involved in cell wall teichoic acid synthesis (surface-exposed anionic glycopolymers bound to the peptidoglycan layer) apparently induce greater sensitivity to bile causing increased cell wall stress. Thus, in the presence of sub-inhibitory concentrations of bile, S. aureus develops an adaptive response by increasing biofilm production. Although bile-stimulated biofilm formation was also observed in two community-acquired MRSA strains from non-CF patients, suggesting that it is not a CF-specific phenomenon, it may be more prevalent in CF patients as up to 40% of CF children and 80% of CF adults can suffer from gastro-oesophageal reflux disease resulting in the presence of bile acids into the lungs [68,84].
4.2.3. Biofilm Formation, Mutations and Altered Gene Expression
Tan et al. compared the genomes of paired early and late S. aureus strains (one pair of MSSA and two pairs of MRSA) collected from chronically infected CF patients. Mutations and altered gene expression occurred over time, some of which seems to give CF S. aureus strains greater ability to produce biofilm, i.e., mutations in the fakA (fatty acid kinase A) gene and mutations in the saeR (Staphylococcus exoprotein expression protein R) gene causing an up-regulation of SdrD adhesin involved in biofilm production, down-regulation of the agr regulon resulting in an overexpression of adhesins [61]. Indeed, apart from the fakA gene currently little described in S. aureus, these genes have already been reported as being involved in S. aureus biofilm production [69,85,86]. Gabryszewski et al. documented genomic and transcriptional changes in some, but not all, CF host-adapted MRSA strains. First, they observed that mutations and altered expression of dacA and gpdP genes correlated with increased biofilm formation over time. The dacA gene encodes a deadenylate cyclase required for the synthesis of cyclic-di-AMP (c-di-AMP), a multifunctional secondary metabolite involved in biofilm formation whereas gpdP encodes a phosphodiesterase responsible for c-di-AMP hydrolysis [69]. These mutations had already been shown to affect Sa biofilm formation by Corrigan et al., and are not specific to CF strains [87]. Moreover, as also described by Tan et al., they observed mutations in the saeR gene [61,69]. However, Gabryszewski et al. also found changes in the expression of certain genes, so far little described in the literature, which may be specific to biofilm formation by S. aureus in CF patients: increased expression of specific metabolic genes including gapR (which encodes a central glycolytic regulator), zwf (involved in the pentose phosphate pathway) and oxidative tricarboxylic acid (TCA) cycle genes like sdh and mqo1 as well as decreased expression of gltA, all of which lead to biofilm production [69]. Interestingly, in all CF host-adapted MRSA isolates, a 10,000-fold increase was observed in the fumC gene expression that functions primarily as a malate dehydratase increasing fumarate concentrations not significantly assimilated by MRSA strains. Thus, in CF patients, MRSA strains undergo metabolic reprogramming with minimal consumption of TCA cycle substrates (which limit pro-oxidant generation) and conversely increased assimilation of pyruvate and glucose polymers which promote the production of extracellular polysaccharide-composing biofilm, such as PIA/PNAG (inherently anti-oxidant and acting as an intercellular adhesin [4]). By protecting bacteria from oxidative stress and promoting biofilm formation, all these mechanisms allow MRSA isolates to persist within the CF lung and develop chronic infections [69].
4.2.4. Biofilm Formation and Mucoidy
Schwartbeck et al. not only showed that mucoid S. aureus strains isolated from CF patients were hyper-producers of PIA-associated biofilms but also described the underlying molecular mechanism for mucoidy and its impact on biofilm formation [65]. The 115 mucoid strains investigated displayed a diversity of genetic background (Sequence type (ST)25, ST188, ST5, ST30, ST7 and ST1909) but all of them carried the same 5 bp-deletion (TATTT), in the same intergenic region between icaR and icaA. Deletion affected binding of the repressor of biofilm “rob” [88], resulting in increased expression of PIA/PNAG. In addition, in two CF patients persistently infected by mucoid S. aureus strains, Schwartbeck et al. identified non-mucoid and non-biofilm-forming late isolates without PIA/PNAG hyperexpression, and carrying the 5 bp-deletion. They demonstrated that various compensatory mutations in icaA, icaD and icaC caused the abrogation of biofilm formation and a non-mucoid phenotype. Thanks to whole genome sequencing, they confirmed that mucoid isolates evolved from the non-mucoid S. aureus clonal strain and that the isolates with both 5 bp-deletion and compensatory mutations evolved from the mucoid isolates. For some, but not all mucoid isolates, mucoidy might thus confer a short-term advantage but, due to mucoidy-associated fitness loss, not a long-term advantage for persistence, resulting in the emergence of compensatory mutations. This corroborated certain observations of a decrease in biofilm formation over time and the hypothesis drawn by Hirschhausen et al. that, after an initial increase in biofilm formation to enhance protection during establishment in the CF lungs, decreasing biofilm formation could be more appropriate for dissemination and persistence [64].
4.2.5. Biofilm Formation and Bacterial Interactions
Pulmonary infections in CF patients are considered as polymicrobial [89,90,91] and studying a single pathogen has limitations with results that probably do not reflect the bacterial behavior of the CF lung. With the diversity of bacterial species and abundance of the community present in the airways of CF patients, interactions between bacteria and competition for space and nutrients can indeed be established through physical proximity and chemical communication pathways [92,93]. The close relationships within multi-species biofilms facilitate these bacterial interactions and examining the interspecies interactions affecting biofilm formation and multi-species biofilms is certainly more representative of the conditions in the CF lungs, although it is probably impossible to fully grasp the complexity of this environment through laboratory experiments [94,95,96,97].
Diverse associations of pathogens may be observed in CF patients [98], the most studied of which being the S. aureus-P. aeruginosa as this is the co-infection most frequently observed in CF patients. Although co-infections with Haemophilus influenzae are observed before adulthood (less than 10% of patients colonized by Sa), the majority of co-infections are indeed those observed with P. aeruginosa. A study on 134 patients from 2004 to 2017 managed to identify S. aureus-P. aeruginosa co-infections in 30–50% of patients [99]. More generally, co-infection by these two species has been identified in 28.3% of CF patients (8 studies and 1432 patients) [100]. A similar observation was made as far as dual-species biofilms are concerned, S. aureus being mostly studied in association with P. aeruginosa. Nevertheless, among all these studies, we noted that (i) most studies on the P. aeruginosa-S. aureus combination focused on P. aeruginosa with few or no results on S. aureus [101] and (ii) P. aeruginosa co-studied strains were mostly reference strains, i.e., PA14, originating from a wound on a burns patient [102,103], PAO1, a moderately virulent strain isolated from a wound over 50 years ago [22,39] and strain ATCC 27,853 from blood culture [96]. As in other parts of this review, we voluntarily focus hereafter on data obtained from CF S. aureus and P. aeruginosa clinical strains, with the aim of reporting results as close as possible to the specific clinical context of CF. A single exception was made for the publication by Barraza & Whiteley that studied laboratory strains of P. aeruginosa (PA14) and S. aureus (LAC) but used synthetic CF sputum medium (SCFM2), to be as close as possible to CF lung conditions [39]. Regarding S. aureus-P. aeruginosa studies, we noted a major lack of studies on clinical strains from CF patients as the majority of studies—even the most recently published ones—only included reference strains. The most relevant study among all those we selected is probably the one by Fugère et al. which included S. aureus and P. aeruginosa clinical strains co-isolated from the same patient. It showed that some results were specifically obtained when studying these pairs of strains, hence the need for such studies which are extremely rare in the literature [104].
First, regarding the overall architecture of biofilm, results obtained for laboratory strains in SCFM2 highlighted a modification in the structural frame of biofilm formed by S. aureus co-cultured with P. aeruginosa with (i) fewer S. aureus aggregates, S. aureus planktonic cells being more numerous, and (ii) S. aureus aggregates of smaller size [39].
On a molecular level, S. aureus produces various exoproducts that can be sensed by P. aeruginosa [101,105]. Those linked to modifications in biofilm formation were: staphylopine (StP), a broad-spectrum metallophore with a central role in metal acquisition and S. aureus virulence, and the Staphylococcal protein A of S. aureus (SpA), a secreted virulence factor that affects host immune response and inhibits phagocytosis (Figure 2). Both exoproducts significantly decreased the biofilm biomass formed by clinical CF P. aeruginosa isolates. StP was shown to inhibit biofilm formation by P. aeruginosa by reducing the availability of zinc [101]. Interestingly, the study included strains co-isolated from three different CF patients showing that the interaction was consistently observed despite strain-to-strain variability. These observations were congruent with those of Menetrey et al. who tested S. aureus (chronic colonization) and P. aeruginosa (sporadic colonization) from the same CF patient via the CV-based method and showed a decrease in P. aeruginosa biofilm formation in S. aureus-P. aeruginosa co-culture compared with P. aeruginosa monoculture [106]. So far, this recently published observation on the implication of StP in the S. aureus-P. aeruginosa interplay has only been reported for strains originating from CF patients. On the other hand, SpA interacts with two important determinants of P. aeruginosa biofilm formation: i) exopolysaccharide, encoded by the polysaccharide synthesis locus (psl), which is the predominant polysaccharide of the extracellular matrix of P. aeruginosa biofilm and a crucial component for its formation and ii) PilA, a component of the type IV pili of P. aeruginosa [107]. SpA was also shown to lead to bacterial aggregation in P. aeruginosa biofilms and the interaction between SpA and Psl was associated with an increase of P. aeruginosa tolerance towards tobramycin, an antibiotic commonly used against this pathogen in aerosol therapy [108].
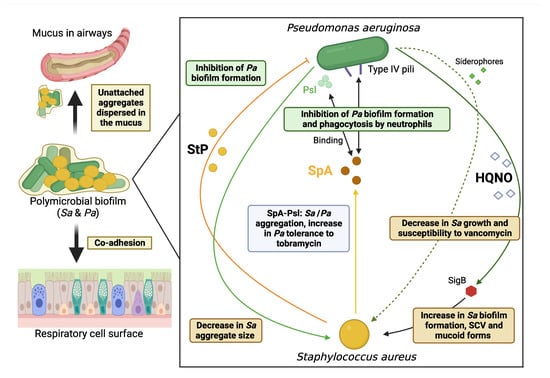
Figure 2.
Diagram showing the major mechanisms involved in the interaction between CF Staphylococcus aureus (Sa, in orange) and Pseudomonas aeruginosa (Pa, in green) strains in dual-species biofilm. The text boxes specify the role associated with each molecule/mechanism. StP: Staphylopine; SpA: Staphylococcal protein A; HQNO: 2-heptyl-4-hydroxyquinoline N-oxide; Psl: Polysaccharide synthesis locus; SigB: Sigma Factor B; SVC: Small Colony Variant (figure created with Biorender.com (accessed on 31 October 2022)).
On the S. aureus side, interacting with P. aeruginosa led to several modifications in characteristics, presented hereafter, all favoring the persistence of clinical CF S. aureus isolates (Figure 2).
Several studies have observed a correlation between HQNO (2-heptyl-4-hydroxyquinoline N-oxide) molecules of P. aeruginosa whose production is under the control of the Pseudomonas Quinolone Signal (PQS) quorum sensing system of P. aeruginosa and increased biofilm formation by S. aureus strains [104,109]. The role of these molecules was confirmed with supernatants from P. aeruginosa isogenic mutants deficient in PQS and HQNO production which significantly stimulated less biofilm formation by S. aureus [104]. Adding P. aeruginosa supernatants to S. aureus biofilms grown either on epithelial cells or on plastic also significantly decreased the susceptibility of clinical CF S. aureus strains to vancomycin [110]. HQNO molecules were again shown to be involved in this phenomenon [110], just as the two P. aeruginosa siderophores, pyoverdine and pyochelin, which also induce a decrease in S. aureus growth correlated with the decrease in susceptibility to certain antibiotics observed [109,110]. The observations made by studying S. aureus and P. aeruginosa strains co-isolated from the same CF patient were of great interest, showing that results vary depending on the origin of the strains, with less HQNO production by P. aeruginosa and a less stimulated S. aureus biofilm formation for co-isolated strains. These results highlighted the within-host co-evolution of S. aureus and P. aeruginosa adapting to the specific abiotic conditions of CF lungs and also to other pathogens present in the niche [104].
HQNO molecules also induce SCVs of S. aureus, a form adapted to intracellular survival [109]. Interestingly, CF patients with SCVs of S. aureus were significantly more frequently co-colonized with P. aeruginosa than patients colonized by S. aureus of normal phenotype (75% vs. 37.9%) [56]. Both the stimulation of biofilm production and the switch to SCV under HQNO exposure were shown to be dependent on the activity of the global S. aureus regulator Sigma factor B (SigB), a crucial factor for adaptation in chronic infections, which was previously linked to an increased expression of both the FnBPA-encoding gene and the biofilm-associated sarA gene [109,111]. Additionally, co-infection with P. aeruginosa selected for mucoid phenotype of S. aureus that was associated with high biofilm formation and this represented another adaptive characteristic of S. aureus for protectin and survival against attacks by small molecules produced by P. aeruginosa such as HQNO [57].
Finally, in the dual-species biofilm with P. aeruginosa, S. aureus may also undergo a switch to a Viable But-Non-Cultivable (VBNC) state that favors its survival and persistence in the co-colonized host. Molecular investigations revealed that several S. aureus genes involved in virulence (Quorum Sensing genes sarA and hld, biofilm formation gene icaA, cytotoxicity gene cplP, and stress response genes sodA and uspA) were overexpressed during this switch [112], suggesting that the phenotypic switching to VBNC state might account for S. aureus pathogenicity and be involved in the clinical outcome of the co-infection [112].
Introducing a third partner add complexity to the interactions. We found only one study on three species—the study by Tavernier et al.—in which S. aureus was studied in mixed biofilm assays with Streptococcus anginosus in the presence or absence of P. aeruginosa in a mucin-containing medium [113]. The community composition was shown to influence the antimicrobial susceptibility of partners in such multispecies biofilms. Changes in antimicrobial susceptibility were shown to depend on the antibiotic, the species and the strain involved, but S. aureus secreted compound(s) protected sessile S. anginosus from antibiotic killing whereas the antibiotic killing of P. aeruginosa was not influenced by the presence of S. aureus or S. anginosus and more S. aureus cells were killed by antibiotic treatment when grown together with S. anginosus and P. aeruginosa [113].
Despite growing interest in characterizing species interactions, their potential implications in the progression of polymicrobial pathologies are still poorly understood [100,114]. However, co-infection with S. aureus and P. aeruginosa correlates with a decline in lung function and a higher number of exacerbations and intravenous antibiotic treatments compared to infection with S. aureus or P. aeruginosa alone [100]. Particularly, co-infection with P. aeruginosa and MRSA is associated with the most severe clinical pictures compared to co-infections with P. aeruginosa and MSSA [100]. Furthermore, in young children co-infected with P. aeruginosa and MRSA, an increase in markers of lower airway inflammation was observed [115]. These studies all showed that there is an important need to further understand polymicrobial interactions [116], particularly in the context of multi-species biofilm, which have an impact on patients’ health. Altogether, reviewing this part of literature revealed reciprocal, complex interactions between S. aureus and P. aeruginosa affecting biofilm formation during Cystic Fibrosis but also that CF clinical isolates may display distinctively different traits from reference laboratory strains, thereby supporting the need for additional studies including clinically documented S. aureus strains from CF patients. These observations also warrant the need for studies of multispecies biofilms formed by S. aureus and other clinically relevant species in CF, either bacterial species such as members of the Burkholderia cepacia complex or emerging pathogens Stenotrophomonas maltophilia and Achromobacter spp., or fungal species. Indeed, interactions highlighted between S. aureus and these species in planktonic cultures [106,117,118,119] or in dual-species biofilms in contexts other than CF [120] should be explored further in biofilm conditions using CF clinical strains.
5. Anti-Biofilm Strategies Targeting S. aureus in CF
Limiting biofilm formation has thus become an important tactic considered in the development of new antimicrobial strategies. Regarding S. aureus and CF, several compounds including natural ones and antibiotics were shown to display anti-biofilm activity, whether limiting the formation of biofilm or affecting preformed biofilm.
5.1. Antimicrobial Peptides and Proteins of the Innate Immune System
Antimicrobial peptides (AMPs) are natural products of the immune system of particular interest with their broad-spectrum antimicrobial activity due to their disruptive mode of action against bacterial membranes occurring after electrostatic interaction between AMPs and bacterial cells [121]. Three cationic α-helical AMPs, i.e., two cathelicidin-derived peptides of bovine origin (bovine myeloid antimicrobial peptide (BMAP)-27, BMAP-28) and an artificial peptide P19(9/B) were tested on 15 clinical strains from CF patients [59]. Biofilm assays were performed using polystyrene plates and CV staining under reduced oxygen concentration, at acidic pH and in SCFM with the aim of simulating CF lung conditions. BMAP-28 and P19(9/B) at sub-inhibitory concentrations (1/2x MIC) were the most active with a significant decrease in biofilm formation (at least 25%) observed for 70% and 60% of the tested strains, respectively. At 1/4x MIC, BMAP-28 was significantly more active than the other two AMPs, still showing significant biofilm reduction in 50% of the strains compared to non-exposed controls. However, in all conditions, AMPs were shown to be less active than tobramycin in limiting biofilm formation. Testing combinations of AMP and tobramycin, the three AMPs showed either a synergistic effect with tobramycin or indifference to it [59].
More recently, Japonicin-2LF, an AMP secreted by the skin of the amphibian Limnonectes fujianensis, was shown to be particularly effective against two sessile MRSA CF strains through membrane permeabilization [122]. Interestingly, in vitro experiments showing biofilm disintegration under Japonicin-2LF challenge were completed by in vivo investigations showing that using this AMP was associated with a significant decrease in mortality of Galleria mellonella larvae infected by MRSA whereas no death of larvae were observed after injection of two doses of Japonicin-2LF. This AMP appeared to be a potential candidate for further evaluations as cytotoxicity, haemolytic activity and lactate deshydrogenase release were observed with high concentrations of 64 μM of Japonicin-2LF alone [122].
Human SPLUNC1 (short palate lung and nasal epithelial clone 1) is a protein of the innate immune system secreted in the human respiratory tract. It acts as a surfactant and regulates the epithelial sodium channel (ENaC) whose deregulation in CF worsens the mucus dehydration and ion imbalance due to a defect in CFTR [123]. Although human SPLUNC1 proteins were ineffective against biofilm formation by S. aureus, unlike the observations made for P. aeruginosa and B. cepacia, proteins modified by the addition of negatively charged residues in the α1−α4 region showed increased anti-biofilm activity against S. aureus. Despite the origin of the clinical S. aureus strains used in this study is unclear, the study showed the importance of this region of the protein in anti-biofilm activity against S. aureus. A further study addressed the antibiofilm activities of SPLUNC1-modified proteins and SPLUNC1-derived peptides against six MRSA strains isolated from CF patients [124]. A focus was made on modifications of the α4 helix that shared a similar structure with cationic AMPs and one peptide named α4M1 with enhanced amphipathicity was shown to reduce biofilm formation to 1 to 20% of the initial value after 24 h of incubation according to the strain tested. Cytotoxic evaluation did not show any hemolytic activity, even at high peptide concentrations up to 100μM allowing the pursuit of investigations on this peptide in a perspective of use in diverse biofilm-associated diseases.
5.2. Natural Compounds
Essential oils are complex mixtures derived from plants and may display certain antimicrobial properties. Papa et al. studied the anti-biofilm effect of 61 essential oils (EOs) on three CF clinical isolates 4S, 5S and 19S (including two MRSA and one MSSA) compared with two reference laboratory strains using microtiter plate biofilm assay and CV staining [125]. All EOs were tested for biofilm growth inhibition at 1.00% v/v concentration and it was found that several EOs had the ability to inhibit biofilm formation by one or several S. aureus CF strains. EOs from Piper nigrum (black pepper) (EO45) and Mentha suaveolens (sweet mint) (EO58) were selected for an in-depth study based on their constant activity on the studied S. aureus strains and biofilm reduction of up to 40% or more of its initial value, despite their different compositions. We may note that neither EO45 nor EO58 had any antibacterial activity on the CF clinical isolates showing an antibiofilm effect unrelated to the inhibition of bacterial growth. SEM analyses made it possible to visualize the highly disruptive action of both EOs on S. aureus biofilm showing a deconstructed surface and EPS disintegration. However, the dose-dependent effect of EOs was also highlighted as lower concentrations (0.05% v/v) mostly ended up abolishing the inhibition effect on biofilm formation and even enhanced biofilm growth in certain cases. Finally, chemical analysis led to the identification of the EO constituents related to the most effective biofilm growth inhibition as being eugenol, β-caryophyllene and, partially, β-pinene. Because of their various distinct components, essential oils probably have a multi-target action as each of the EO compounds may exhibit a different mechanism of action against biofilm formation by S. aureus strains from CF patients [125]. In a general manner, EOs have been shown to disrupt membrane integrity and metabolic pathways of the targeted pathogens [126]. Other phytochemicals, namely, combination of borneol and citral, and Pickering emulsions—stabilized by solid particles—of these compounds, were recently tested for anti-biofilm activity on the CF clinical isolate P8-AE1 in SCFM2. They showed inhibitory activity on biofilm formation and eradication of established biofilm (24-h-old S. aureus P8-AE1 biofilms) associated with G. mellonella larvae protection from S. aureus-induced killing [127]. Encapsulation increased their anti-biofilm activity while confering reduced toxicity and enhanced stability. Pickering emulsions thus represent attractive formulations to improve the efficacy of phytochemicals against biofilms.
Among the numerous complementary, alternative medicine practices, CF patients may use herbal therapy. In this context, the phenolic-rich fraction of an extract of the aerial parts of Pulmonaria officinalis was investigated for anti-adhesion and anti-biofilm properties on 20 clinical isolates from the sputum of children (1.5–19 years) with CF and chronic respiratory tract infection. The assay used inert polystyrene surfaces conditioned or not with mucin and elastin to mimic respiratory tract mucosa [42]. Adhesion was reduced by about 54% on the inert surface, 20–36% on mucin-coated surfaces and 14–45% on elastin-coated surfaces. Effects that were strain- and extract concentration-dependent without associated loss of S. aureus viability were suggestive of variable reduction in surface adhesin expression. However, subsequent observation of biofilm reduction did not reach significance and biofilm may even be increased under certain conditions; the extract was not effective in eradicating preformed biofilm either [42]. However, other observations like a significant reduction in α-toxin and sortase A (SrtA) activities warrant further exploration, as both α-toxin and SrtA were previously related to biofilm formation in other settings. SrtA is a transpeptidase involved in the cell wall anchoring of the MSCRAMMs. These surface-exposed molecules recognize extracellular host proteins such as fibrinogen and collagen, and are therefore essential in host-bacteria interactions, the first step of adhesion, biofilm formation and invasion. Reducing SrtA production will therefore limit bacterial adhesion to the host tissues leading to a decrease in biofilm production [128] making SrtA inhibitors promising anti-biofilm and anti-virulence compounds [129]. Alpha-toxin/alpha-hemolysin (HlA) production was also shown to be required for biofilm formation by S. aureus in several studies [130,131,132]. This transmembrane pore-forming multimeric toxin appeared required for cell-to-cell interactions during biofilm formation [130]. Others suggested that the lysis of underlying host cells by HlA may provide a nutrient source for S. aureus enhancing adhesion and inducing the production of biofilm components [132]. Again, anti-HlA compounds represent an alternative anti-S. aureus strategy as they might reduce not only epithelial toxicity but also biofilm formation on host tissues.
Secondary metabolites from lichens, more precisely, the fungal component of lichens, were first challenged for antibiofilm activity on CF S. aureus strains by Pompilio et al., 2013. Usnic acid and atranorin, which displayed antimicrobial activity on six S. aureus strains from CF patients (three MSSA, three MRSA), were evaluated for their activity against adhesiveness, biofilm formation and preformed biofilm using CV staining. Both secondary metabolites at 1/2x MIC affected adhesion of all strains and biofilm formation in all strains except one (unaffected by usnic acid only). Atranorin also decreased bacterial adhesion at lower concentrations (1/4x and 1/8x MIC) (75% reduction of adhesiveness compared with control for most conditions). At these subinhibitory concentrations, usnic acid and atranorin displayed variable results on biofilm formation depending on the S. aureus strain and metabolite concentration, with the highest activity for atranorin on MRSA and usnic acid on MSSA. Both metabolites were also significantly active on biofilm preformed by the two strongest biofilm-producing strains in this study (Sa3 and Sa15 MRSA) whatever the concentration tested (1x MIC and bactericidal concentrations 5x and 10x MIC) [70]. The effects of usnic acid on biofilm formation were further investigated on Sa3; ultrastructural and proteomic observations showed bacterial cell wall alterations and a decrease in the biosynthesis of amino acids and proteins essential to bacterial viability [133]. Regarding the anti-adhesion and anti-biofilm effects previously observed, usnic acid was shown to significantly reduce the transcription of the genes encoding the host matrix-binding proteins (elastin, laminin, fibronectin), as well as genes encoding lipase and thermonuclease. A dose-dependent effect was also observed on agrA expression. These modifications led to the inhibition of the first stage of biofilm formation (adhesion) which further contributes to biofilm formation reduction [133]. However, despite the clinical relevance of the anti-biofilm effects of usnic acid, its use is still under investigations as suitable formulations need to be developed due to toxicity issues [70].
5.3. Antibiotics
Despite antimicrobial resistance may be drastically increased in biofilm [7,50,51,134,135,136], antibiotics remain the therapeutic of choice for CF patients. Besides their antibacterial growth properties, antibiotics may also have an anti-biofilm effect. As seen in previous parts of this review, the anti-biofilm activities of antibiotics on clinical strains isolated from CF patients were mostly studied against P. aeruginosa. For S. aureus, an antibiofilm effect was observed with tobramycin, the comparator used to evaluate AMP’s anti-biofilm activity against S. aureus CF strains [59]. In this study, tobramycin showed the ability to significantly reduce biofilm formation (at least 25% for the three CF S. aureus strains tested), up to the lowest sub-inhibitory concentration evaluated (1/8x MIC). The Antibiofilmogram® approach by BRT® in filtered ASM was also used to study the effect of five antibiotics (trimethoprim, rifampicin, linezolid, ceftobiprole and ceftaroline) on biofilm formation by 17 strongly adherent/strong biofilm producers MRSA strains from CF patients [40]. Trimethoprim was totally ineffective at limiting adhesion and early biofilm formation, rifampicin was active on a highly limited number of strains (18%) whereas linezolid, ceftobiprole and ceftaroline were able to inhibit biofilm formation (biofilm MICs under the corresponding resistance threshold) of 65%, 70.5% and 76.5% of the strains, respectively. Biofilm formation by MRSA strains adapted to the CF lung after up to nine years of colonization was also affected by linezolid, ceftaroline and ceftobiprole. The dynamic analysis using the BioFluxTM 200 system and ASM confirmed the activity of these three antimicrobial agents in limiting biofilm formation and showed that ceftaroline and ceftobiprole had a significantly greater effect than linezolid [40]. Finally, the activity of micronized tobramycin (4 μg/mL) and clarithromycin (200 μg/mL), alone or in combination, was also evaluated on 24-h-old and 12-day-old biofilms formed by six biofilm-forming strains of S. aureus (two MRSA and four MSSA) isolated from CF patients [137]. The main results obtained on these preformed biofilms were that: i) 12-day-old biofilms were systematically more resistant to antibiotics alone or in combination than 24-h-old biofilms; ii) the logarithmic decrease in colony-forming units (CFU) from antibiotic-treated biofilms was systematically greater with tobramycin (mean 1.07–5.31 and 0.31–4.44 CFU log10 decreases in 24-h-old and 12-day-old biofilms, respectively) than with clarithromycin (mean 0.52–3.74 and 0.17–1.22 CFU log10 decreases in 24-h-old and 12-day-old biofilms, respectively); iii) there was no influence from combining tobramycin with clarithromycin [137].
As not all anti-S. aureus therapeutic options have been yet evaluated for their anti-biofilm activity, such investigations have to be pursued to completely characterize the anti-biofilm potential and mechanisms of the different treatments against S. aureus since results might be helpful to guide the choice of the most effective therapy against CF airway infection.
5.4. Microbial Interaction
Among the bacterial genera described as having predatorial activity, the Bdellovibrio bacteriovorus species is a member of the Oligoflexia class found in the human gut microbiota and known to prey on Gram-negative bacteria. Iebba et al. showed that the B. bacteriovorus strain HD100 was also able to prey on a S. aureus isolate from a chronically mono-colonized CF patient. However, its mode of attack was however specific compared with Gram-negative prey as direct contact was observed during the whole predation process and three non-released bacteriolytic enzymes that remain to be characterized appeared to have a role in this predator-prey interaction [138]. S. aureus biofilms preformed during 24 h either statically (microtiter plate CV-straining) or under flow (BioFlux microfluidics system and electron microscopy biofilm vizualisation) were exposed to B. bacteriovorus in predatory assays. Epibiotic predation led to decreases in the amounts of preformed biofilm observed, both in static and dynamic settings, with a significant reduction in biofilm of 74% after 24 h of contact with the predator in the static assay and a 33% reduction after 14 h of contact, increasing to 46% after 20 h in the dynamic assay. The destruction of S. aureus cells seemed to occur by breaking down the bacterial wall and releasing the intracytoplasmic content of S. aureus. B. bacteriovorus was also shown to be a predator for CF P. aeruginosa, with an even higher reduction observed for preformed P. aeruginosa biofilm than S. aureus. Considering its presence in healthy human gut microbiota and its inability to infect mammalian cells, its ability to reduce established biofilms appeared of interest for in vivo applications during CF [138].
Surprisingly, our search criteria did not find any publications on bacteriophage anti-biofilm activity on CF S. aureus strains despite the growing interest in bacteriophage-mediated control of biofilm, based on the ability of these bacteriophages to penetrate existing biofilm and eliminate its structure [139]. This topic of interest warrants further investigations as the well-characterized staphylococcal bacteriophage, Sb-1, previously proposed as a promising tool to remove biofilms in other settings [140], was successfully used to treat a CF patient with chronic S. aureus colonization [141].
5.5. Miscellaneous
Ahonen et al. also studied the development of nitric oxide (NO)-releasing alginate oligosaccharides, considering NO’s anti-bacterial activity both on planktonic bacteria and biofilms and the need to decrease its toxicity through finely-controlled release [142]. Alginate oligosaccharides also have the ability to decrease mucus viscoelasticity. The study used two reference S. aureus strains only (MSSA ATCC 29,213 and MRSA ATCC 33,591) studied in ASM supplemented with 0.25% glucose and in two oxygen conditions for biofilm eradication assays with the aim of mimicking CF conditions more accurately. Biofilms grown in ASM for 48 h were challenged with NO-releasing alginates for 24 h under aerobic and anaerobic conditions and the results were compared to those obtained with tobramycin and vancomycin. The anti-biofilm effect of NO-releasing alginates was clearly demonstrated, particularly for Alg5-PAPA-DPTA/NO, with a 5-log reduction in biofilm viability observed after 24 h and alginates performed more efficiently than tobramycin and vancomycin in both oxygen conditions. As NO disrupts vital bacterial cell functions and structures through protein, DNA, and metabolic enzyme alterations, NO-releasing alginates display a broad-spectrum activity, including P. aeruginosa and B. cepacia in addition to S. aureus, making them promising candidates for future therapeutic options [142].
Pompilio et al. evaluated electrochemically synthesized silver nanoparticles (AgNP) formulation on a strong biofilm-forming S. aureus strain (Sa2) from a CF patient, showing the effectiveness of AgNPs against biofilm viability, with a dose-dependent effect and a maximum biofilm-killing rate of 98.2 ± 0.5% at 2x MIC [143]. These new formulations are active on biofilm formed by other pathogens like P. aeruginosa and shown to be non-toxic in in vivo studies on G. mellonella larvae. They require further investigation to elucidate their mode of action on S. aureus biofilms and their therapeutic potential for CF patients.
Physical stimulations, either electrical or magnetic, have also been applied to biofilms formed by S. aureus strains from CF patients [144,145]. In a context of increasing interest in the oral health of CF patients, Minkiewicz-Zochniak et al. analyzed the influence of low-intensity current on the ability of three CF S. aureus strains (one each among weak, moderate and strong biofilm formers) to form biofilm on titane (Ti-6Al-4V) and zirconium oxide biomaterials commonly used in dental implants [145]. Beside the implications on implant life cycle and potential local inflammation/infection processes, these biofilms are of importance as they may also represent a reservoir for both upper and lower airway infections in CF patients. Marked effects were noticed with zirconium compared with titanium. A low-amperage electrical current of 10 mA led to significant S. aureus biofilm reduction, affecting both adhesion (CV-staining evaluation) and S. aureus survival (live/dead fluorescence microscopy) as well as detaching biofilm-forming S. aureus from the biomaterial. Indeed, biofilm structure damage was visible after 10 min and attributed to an increase in the repulsive electrostatic forces between S. aureus and the biomaterial [145].
Altering the electrostatic interactions that might reduce S. aureus adhesiveness and the subsequent formation of biofilm was also the hypothesis drawn from the observation that applying a magnetic field of extremely low frequency to three S. aureus strains from CF patients (strong biofilm formers and multidrug resistant) significantly decreased biofilm formation and viability [144]. Indeed, selecting for a specific ionic channel (Ca2+, Cu2+, Fe2+/Fe3+, K+, Mg2+, Na+, Zn2+) showed that stimulating nearly all ions caused a significant reduction in S. aureus biofilm biomass formation (at least 25% compared with control) and in viability of the biofilm formed, suggesting that there were modifications in the attraction between the S. aureus surface, normally negatively-charged, and the positively-charged polystyrene surface used in this study. The high potential of such an intervention has been suggested to prevent biofilm formation or to eradicate biofilm on medical devices like nebulizers used by CF patients.
A summary of the strategies limiting biofilm formation by S. aureus clinical isolates from CF patients is given in Figure 3 with respect to the stage of biofilm lifecycle under evaluation in the selected studies.
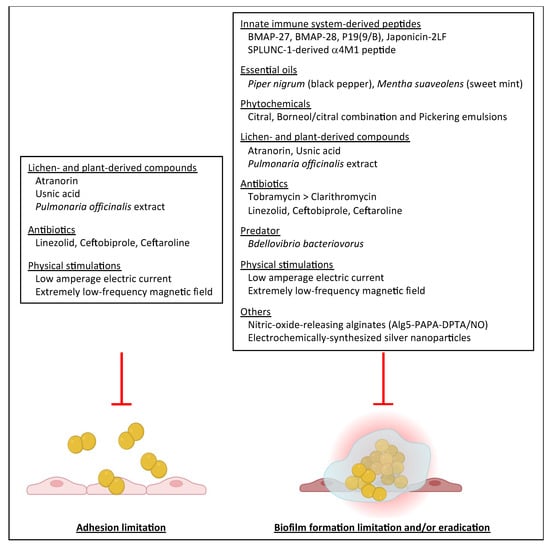
Figure 3.
Presentation of anti-biofilm strategies shown to affect biofilm formation by S. aureus strains isolated from CF patients according to the two stages of biofilm formation specifically under study in the literature reviewed, i.e., adhesion and maturation/development (including biofilm formation and biofilm eradication).
In our review of the literature, we found no strategies with a specific action on the dispersal stage of biofilm but it is obvious that each affected stage of biofilm formation (adhesion, maturation and dispersal) will impact the subsequent ones (Figure 3). When available, mechanisms, as well as biological targets supporting the anti-biofilm activity of the strategies summarized in Figure 3 on clinical S. aureus strains isolated from patients with CF, were reported in the text. However, observational studies are the main studies performed in the CF context and affected targets by the anti-biofilm strategies reviewed in our manuscript remain largely unidentified in this context but also more generally in the literature.
6. Concluding Remarks
S. aureus is a major opportunistic pathogen in CF patients and biofilm production is a determining factor in the onset of persistent S. aureus respiratory infections in these patients. During CF, a broad panel of biotic and abiotic factors influences the different stages of biofilm development by S. aureus due to the specific, stressful conditions found in CF lungs. In this unique environment, S. aureus strains display specific features driven by bacterial adaptation and within-host evolution during persistence. Biofilm formation is one of these important traits representing a major obstacle, protecting S. aureus from host defenses and eradication attempts. Greater knowledge of the specific characteristics of biofilm formation by well documented S. aureus strains originating from CF patients is required as modulating or inhibiting biofilm formation is an important strategy for infection control and a target for the development of new therapeutic agents [125]. Although some of the current anti-biofilm approaches reviewed herein may be promising in the fight against S. aureus in CF patients, none of them has currently been currently evaluated during clinical trials. However, yet available results pave the way to further research that should address the more promising strategies for managing S. aureus infections in these patients but also their advantages and limitations. Indeed, biofilm eradicating strategies that kill bacteria independently of their physiological stage and are thus also active on persister cells within biofilm are of great interest [146]. The potential of combining diverse anti-biofilm strategies, i.e., biofilm eradicating agents, anti-adhesion agents, biofilm growth inhibitors, has also to be studied with special attention to be paid to synergy or antagonism that could establish between anti-biofilm agents but also between these agents and antibiotics. For some of the anti-biofilm agents under consideration herein like EOs, formulation development is also still required to enhance their efficacy and limit the impact of their usual dose-dependent effect on biofilm formation and, sometimes, toxicity before they could be considered as therapeutic options against biofilm-associated infections like CF [147]. Finally, several other approaches not yet investigated during CF may also be considered to enlarge the possibilities to counteract biofilm consequences [79,148].
Supplementary Materials
The following supporting information can be downloaded from: https://www.mdpi.com/article/10.3390/ijms24010597/s1, Table S1: Data complementary to those given in Table 1 on patients, strains and biofilm evaluation for studies reviewed on biofilm formation by clinical strains of S. aureus isolated from patients with Cystic Fibrosis.
Author Contributions
Conceptualization, H.M.; writing, preparation of the original draft, V.J.-P., A.B., P.S., Q.M. and H.M.; writing, review and editing, R.C., J.-P.L. and H.M.; supervision, H.M.; funding acquisition, J.-P.L. and H.M. All authors have read and agreed to the published version of the manuscript.
Funding
This review was written with a grant for research by Nîmes University Hospital (NîmAO 2018.02).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors gratefully acknowledge Teresa Sawyers, English medical writer at Nîmes University Hospital, for editing this paper. We also thank Nîmes University Hospital for its structural, human, and financial support through the award obtained by our team during the internal call for tenders “Thématiques phares” (Flagship topics). All authors except Q.M. are members of the FHU InCh (Fédération Hospitalo-Universitaire Infections Chroniques, Aviesan).
Conflicts of Interest
The authors have no conflicts of interest to declare and the funder had no role in writing of this review.
References
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial biofilm and its role in the pathogenesis of disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef]
- Olsen, I. Biofilm-specific antibiotic tolerance and resistance. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 877–886. [Google Scholar] [CrossRef]
- Paharik, A.E.; Horswill, A.R. The Staphylococcal biofilm: Adhesins, regulation, and host response. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef]
- Cheng, T.; Torres, N.S.; Chen, P.; Srinivasan, A.; Cardona, S.; Lee, G.C.; Leung, K.P.; Lopez-Ribot, J.L.; Ramasubramanian, A.K. A facile high-throughput model of surface-independent Staphylococcus aureus biofilms by spontaneous aggregation. mSphere 2021, 6, e00186-e21. [Google Scholar] [CrossRef]
- Sauer, K.; Stoodley, P.; Goeres, D.M.; Hall-Stoodley, L.; Burmølle, M.; Stewart, P.S.; Bjarnsholt, T. The biofilm life cycle: Expanding the conceptual model of biofilm formation. Nat. Rev. Microbiol. 2022, 20, 608–620. [Google Scholar] [CrossRef]
- Rumbaugh, K.P.; Sauer, K. Biofilm dispersion. Nat. Rev. Microbiol. 2020, 18, 571–586. [Google Scholar] [CrossRef]
- Cascioferro, S.; Carbone, D.; Parrino, B.; Pecoraro, C.; Giovannetti, E.; Cirrincione, G.; Diana, P. Therapeutic strategies to counteract antibiotic resistance in MRSA biofilm-associated infections. ChemMedChem 2021, 16, 65–80. [Google Scholar] [CrossRef]
- Rajan, S.; Saiman, L. Pulmonary infections in patients with cystic fibrosis. Semin. Respir. Infect. 2002, 17, 47–56. [Google Scholar] [CrossRef]
- Registre Français de la Mucoviscidose—Bilan des Données 2021. Vaincre la Mucoviscidose. Paris, Août. 2022. Available online: https://www.vaincrelamuco.org/sites/default/files/registre_francais_de_la_mucoviscidose_bilan_2021_0.pdf (accessed on 16 October 2022).
- Cystic Fibrosis Foundation Patient Registry 2020 Annual Data Report, Bethesda Maryland. 2021. Available online: https://www.cff.org/medical-professionals/patient-registry (accessed on 16 October 2022).
- Orenti, A.; Zolin, A.; Jung, A.; van Rens, J.; Fox, A.; Kransyk, M.; Daneau, G.; Hatziagorou, E.; Mei-Zahav, M.; Naehrlich, L.; et al. European Cystic Fibrosis Society Patient Registry (ECFSPR) Annual Report 2020. 2022, pp. 48–67. Available online: https://www.ecfs.eu/sites/default/files/ECFSPR_Report_2020_v1.0%20%2807Jun2022%29_website.pdf (accessed on 16 October 2022).
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef]
- Goerke, C.; Wolz, C. Adaptation of Staphylococcus aureus to the cystic fibrosis lung. Int. J. Med. Microbiol. 2010, 300, 520–525. [Google Scholar] [CrossRef]
- Akil, N.; Muhlebach, M.S. Biology and management of methicillin resistant Staphylococcus aureus in cystic fibrosis. Pediatr. Pulmonol. 2018, 53, S64–S74. [Google Scholar] [CrossRef]
- Jurado-Martín, I.; Sainz-Mejías, M.; McClean, S. Pseudomonas aeruginosa: An audacious pathogen with an adaptable arsenal of virulence factors. Int. J. Mol. Sci. 2021, 22, 3128. [Google Scholar] [CrossRef]
- Lebeaux, D.; Chauhan, A.; Rendueles, O.; Beloin, C. From in vitro to in vivo models of bacterial biofilm-related infections. Pathogens 2013, 2, 288–356. [Google Scholar] [CrossRef]
- Beloin, C.; Renard, S.; Ghigo, J.M.; Lebeaux, D. Novel approaches to combat bacterial biofilms. Curr. Opin. Pharmacol. 2014, 18, 61–68. [Google Scholar] [CrossRef]
- Price, C.E.; Brown, D.G.; Limoli, D.H.; Phelan, V.V.; O’Toole, G.A. Exogenous alginate protects Staphylococcus aureus from killing by Pseudomonas aeruginosa. J. Bacteriol. 2020, 202, e00559-19. [Google Scholar] [CrossRef]
- Kiedrowski, M.R.; Gaston, J.R.; Kocak, B.R.; Coburn, S.L.; Lee, S.; Pilewski, J.M.; Myerburg, M.M.; Bomberger, J.M. Staphylococcus aureus biofilm growth on cystic fibrosis airway epithelial cells is enhanced during respiratory syncytial virus coinfection. mSphere 2018, 3, e00341-18. [Google Scholar] [CrossRef]
- Alford, M.A.; Mann, S.; Akhoundsadegh, N.; Hancock, R.E.W. Competition between Pseudomonas aeruginosa and Staphylococcus aureus is dependent on intercellular signaling and regulated by the NtrBC two-component system. Sci. Rep. 2022, 12, 9027. [Google Scholar] [CrossRef]
- Wakeman, C.A.; Moore, J.L.; Noto, M.J.; Zhang, Y.; Singleton, M.D.; Prentice, B.M.; Gilston, B.A.; Doster, R.S.; Gaddy, J.A.; Chazin, W.J.; et al. The innate immune protein calprotectin promotes Pseudomonas aeruginosa and Staphylococcus aureus interaction. Nat. Commun. 2016, 7, 11951. [Google Scholar] [CrossRef] [PubMed]
- Chew, S.C.; Yam, J.K.H.; Matysik, A.; Seng, Z.J.; Klebensberger, J.; Givskov, M.; Doyle, P.; Rice, S.A.; Yang, L.; Kjelleberg, S. Matrix polysaccharides and SiaD diguanylate cyclase alter community structure and competitiveness of Pseudomonas aeruginosa during dual-species biofilm development with Staphylococcus aureus. mBio 2018, 9, e00585-18. [Google Scholar] [CrossRef]
- Tavernier, S.; Sass, A.; De Bruyne, M.; Baeke, F.; De Rycke, R.; Crabbé, A.; Vandecandelaere, I.; Van Nieuwerburgh, F.; Coenye, T. Decreased susceptibility of Streptococcus anginosus to vancomycin in a multispecies biofilm is due to increased thickness of the cell wall. J. Antimicrob. Chemother. 2018, 73, 2323–2330. [Google Scholar] [CrossRef]
- Baldan, R.; Cigana, C.; Testa, F.; Bianconi, I.; De Simone, M.; Pellin, D.; Di Serio, C.; Bragonzi, A.; Cirillo, D.M. Adaptation of Pseudomonas aeruginosa in cystic fibrosis airways influences virulence of Staphylococcus aureus in vitro and murine models of co-infection. PLoS ONE 2014, 9, e89614. [Google Scholar] [CrossRef] [PubMed]
- Orazi, G.; Jean-Pierre, F.; O’Toole, G.A. Pseudomonas aeruginosa PA14 enhances the efficacy of norfloxacin against Staphylococcus aureus Newman biofilms. J. Bacteriol. 2020, 202, e00159-20. [Google Scholar] [CrossRef] [PubMed]
- Pallett, R.; Leslie, L.J.; Lambert, P.A.; Milic, I.; Devitt, A.; Marshall, L.J. Anaerobiosis influences virulence properties of Pseudomonas aeruginosa cystic fibrosis isolates and the interaction with Staphylococcus aureus. Sci. Rep. 2019, 9, 6748. [Google Scholar] [CrossRef] [PubMed]
- Woods, P.W.; Haynes, Z.M.; Mina, E.G.; Marques, C.N.H. Maintenance of S. aureus in co-culture with P. aeruginosa while growing as biofilms. Front. Microbiol. 2019, 9, 3291. [Google Scholar] [CrossRef]
- Houck, P.W.; Nelson, J.D.; Kay, J.L. Fatal septicemia due to Staphylococcus aureus 502A. Report of a case and review of the infectious complications of bacterial interference programs. Am. J. Dis. Child. 1972, 123, 45–48. [Google Scholar] [CrossRef][Green Version]
- Klockgether, J.; Munder, A.; Neugebauer, J.; Davenport, C.F.; Stanke, F.; Larbig, K.D.; Heeb, S.; Schöck, U.; Pohl, T.M.; Wiehlmann, L.; et al. Genome diversity of Pseudomonas aeruginosa PAO1 laboratory strains. J. Bacteriol. 2010, 192, 1113–1121. [Google Scholar] [CrossRef]
- Chin, P.J.; Liao, H.M.; Li, B.; Hung, G.C.; Tsai, S.; Lo, S.C. Genomic alterations of Staphylococcus aureus ATCC 25923 after prolonged passage in the laboratory. Microbiol. Resour. Announc. 2018, 7, e01108-18. [Google Scholar] [CrossRef]
- Fux, C.A.; Shirtliff, M.; Stoodley, P.; Costerton, J.W. Can laboratory reference strains mirror “real-world” pathogenesis? Trends Microbiol. 2005, 13, 58–63. [Google Scholar] [CrossRef]
- Oliver, A.; Cantón, R.; Campo, P.; Baquero, F.; Blázquez, J. High frequency of hypermutable Pseudomonas aeruginosa in cystic fibrosis lung infection. Science 2000, 288, 1251–1254. [Google Scholar] [CrossRef]
- Bernardy, E.E.; Raghuram, V.; Goldberg, J.B. Staphylococcus aureus and Pseudomonas aeruginosa isolates from the same cystic fibrosis respiratory sample coexist in coculture. Microbiol. Spectr. 2022, 10, e0097622. [Google Scholar] [CrossRef]
- Hu, H.; Clothier, N.; Jacombs, A.; Mckay, K.; Deva, A.K.; Vickery, K. Biofilm on toothbrushes of children with cystic fibrosis: A potential source of lung re-infection after antibiotic treatment? Materials 2022, 15, 2139. [Google Scholar] [CrossRef] [PubMed]
- Minkiewicz-Zochniak, A.; Jarzynka, S.; Iwańska, A.; Strom, K.; Iwańczyk, B.; Bartel, M.; Mazur, M.; Pietruczuk-Padzik, A.; Konieczna, M.; Augustynowicz-Kopeć, E.; et al. Biofilm formation on dental implant biomaterials by Staphylococcus aureus strains isolated from patients with cystic fibrosis. Materials 2021, 14, 2030. [Google Scholar] [CrossRef] [PubMed]
- Aanaes, K.; Eickhardt, S.; Johansen, H.K.; von Buchwald, C.; Skov, M.; Høiby, N.; Bjarnsholt, T. Sinus biofilms in patients with cystic fibrosis: Is adjusted eradication therapy needed? Eur. Arch. Otorhinolaryngol. 2015, 272, 2291–2297. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Haley, C.L.; Colmer-Hamood, J.A.; Hamood, A.N. Characterization of biofilm-like structures formed by Pseudomonas aeruginosa in a synthetic mucus medium. BMC Microbiol. 2012, 12, 181. [Google Scholar] [CrossRef] [PubMed]
- Diaz Iglesias, Y.; Wilms, T.; Vanbever, R.; Van Bambeke, F. Activity of antibiotics against Staphylococcus aureus in an in vitro model of biofilms in the context of cystic fibrosis: Influence of the culture medium. Antimicrob. Agents Chemother. 2019, 63, e00602-19. [Google Scholar] [CrossRef]
- Barraza, J.P.; Whiteley, M. A Pseudomonas aeruginosa antimicrobial affects the biogeography but not fitness of Staphylococcus aureus during coculture. mBio 2021, 12, e00047-21. [Google Scholar] [CrossRef]
- Boudet, A.; Sorlin, P.; Pouget, C.; Chiron, R.; Lavigne, J.-P.; Dunyach-Remy, C.; Marchandin, H. Biofilm formation in methicillin-resistant Staphylococcus aureus isolated in cystic fibrosis patients is strain-dependent and differentially influenced by antibiotics. Front. Microbiol. 2021, 12, 750489. [Google Scholar] [CrossRef]
- Neve, R.L.; Carrillo, B.D.; Phelan, V.V. Impact of artificial sputum medium formulation on Pseudomonas aeruginosa secondary metabolite production. J. Bacteriol. 2021, 203, e0025021. [Google Scholar] [CrossRef]
- Sadowska, B.; Wójcik, U.; Krzyżanowska-Kowalczyk, J.; Kowalczyk, M.; Stochmal, A.; Rywaniak, J.; Burzyńska, J.; Różalska, B. The pros and cons of cystic fibrosis (CF) patient use of herbal supplements containing Pulmonaria officinalis L. extract: The evidence from an in vitro study on Staphylococcus aureus CF clinical isolates. Molecules 2019, 24, 1151. [Google Scholar] [CrossRef]
- Bernardy, E.E.; Petit, R.A., 3rd; Raghuram, V.; Alexander, A.M.; Read, T.D.; Goldberg, J.B. Genotypic and phenotypic diversity of Staphylococcus aureus isolates from cystic fibrosis patient lung infections and their interactions with Pseudomonas aeruginosa. mBio 2020, 11, e00735-20. [Google Scholar] [CrossRef]
- Azeredo, J.; Azevedo, N.F.; Briandet, R.; Cerca, N.; Coenye, T.; Costa, A.R.; Desvaux, M.; Di Bonaventura, G.; Hébraud, M.; Jaglic, Z.; et al. Critical review on biofilm methods. Crit. Rev. Microbiol. 2017, 43, 313–351. [Google Scholar] [CrossRef] [PubMed]
- Tasse, J.; Croisier, D.; Badel-Berchoux, S.; Chavanet, P.; Bernardi, T.; Provot, C.; Laurent, F. Preliminary results of a new antibiotic susceptibility test against biofilm installation in device-associated infections: The Antibiofilmogram®. Pathog. Dis. 2016, 74, ftw057. [Google Scholar] [CrossRef] [PubMed]
- Benoit, M.R.; Conant, C.G.; Ionescu-Zanetti, C.; Schwartz, M.; Matin, A. New device forhigh-throughput viability screening of flow biofilms. Appl. Environ. Microbiol. 2010, 76, 4136–4142. [Google Scholar] [CrossRef] [PubMed]
- Sriramulu, D.D.; Lünsdorf, H.; Lam, J.S.; Römling, U. Microcolony formation: A novel biofilm model of Pseudomonas aeruginosa for the cystic fibrosis lung. J. Med. Microbiol. 2005, 54, 667–676. [Google Scholar] [CrossRef]
- Diraviam Dinesh, S. Artificial sputum medium. Protoc. Exch. 2010. [Google Scholar] [CrossRef]
- Ulrich, M.; Herbert, S.; Berger, J.; Bellon, G.; Louis, D.; Münker, G.; Döring, G. Localization of Staphylococcus aureus in infected airways of patients with cystic fibrosis and in a cell culture model of S. aureus adherence. Am. J. Respir. Cell Mol. Biol. 1998, 19, 83–91. [Google Scholar] [CrossRef]
- Sweeney, E.; Harrington, N.E.; Harley Henriques, A.G.; Hassan, M.M.; Crealock-Ashurst, B.; Smyth, A.R.; Hurley, M.N.; Tormo-Mas, M.Á.; Harrison, F. An ex vivo cystic fibrosis model recapitulates key clinical aspects of chronic Staphylococcus aureus infection. Microbiology 2021, 167, 000987. [Google Scholar] [CrossRef]
- Harrington, N.E.; Sweeney, E.; Alav, I.; Allen, F.; Moat, J.; Harrison, F. Antibiotic efficacy testing in an ex vivo model of Pseudomonas aeruginosa and Staphylococcus aureus biofilms in the cystic fibrosis lung. J. Vis. Exp. 2021, 167, e62187. [Google Scholar] [CrossRef]
- Molina, A.; Del Campo, R.; Maiz, L.; Morosini, M.I.; Lamas, A.; Baquero, F.; Cantón, R. High prevalence in cystic fibrosis patients of multiresistant hospital-acquired methicillin-resistant Staphylococcus aureus ST228-SCCmecI capable of biofilm formation. J. Antimicrob. Chemother. 2008, 62, 961–967. [Google Scholar] [CrossRef]
- Cakir Aktas, N.; Erturan, Z.; Karatuna, O.; Karahasan Yagci, A. Panton-Valentine leukocidin and biofilm production of Staphylococcus aureus isolated from respiratory tract. J. Infect. Dev. Ctries. 2013, 7, 888–891. [Google Scholar] [CrossRef]
- Stepanović, S.; Vuković, D.; Hola, V.; Bonaventura, G.D.; Djukić, S.; Ćirković, I.; Ruzicka, F. Quantification of biofilm in microtiter plates: Overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS 2007, 115, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Kodori, M.; Nikmanesh, B.; Hakimi, H.; Ghalavand, Z. Antibiotic susceptibility and biofilm formation of bacterial isolates derived from pediatric patients with Cystic Fibrosis from Tehran, Iran. Arch. Razi Inst. 2021, 76, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Morelli, P.; Alessandri, A.D.; Manno, G.; Marchese, A.; Bandettini, R.; Bassi, M.; Lobello, R.; Minicucci, L.; Bandettini, R. Characterization of Staphylococcus aureus small colony variant strains isolated from Italian patients attending a regional cystic fibrosis care centre. New Microbiol. 2015, 38, 235–243. [Google Scholar] [PubMed]
- Wieneke, M.K.; Dach, F.; Neumann, C.; Görlich, D.; Kaese, L.; Thißen, T.; Dübbers, A.; Kessler, C.; Große-Onnebrink, J.; Küster, P.; et al. Association of diverse Staphylococcus aureus populations with Pseudomonas aeruginosa coinfection and inflammation in cystic fibrosis airway infection. mSphere 2021, 6, e0035821. [Google Scholar] [CrossRef] [PubMed]
- Cafiso, V.; Bertuccio, T.; Spina, D.; Campanile, F.; Bongiorno, D.; Santagati, M.; Sciacca, A.; Sciuto, C.; Stefani, S. Methicillin resistance and vancomycin heteroresistance in Staphylococcus aureus in cystic fibrosis patients. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 1277–1285. [Google Scholar] [CrossRef] [PubMed]
- Pompilio, A.; Crocetta, V.; Scocchi, M.; Pomponio, S.; Di Vincenzo, V.; Mardirossian, M.; Gherardi, G.; Fiscarelli, E.; Dicuonzo, G.; Gennaro, R.; et al. Potential novel therapeutic strategies in cystic fibrosis: Antimicrobial and anti-biofilm activity of natural and designed α-helical peptides against Staphylococcus aureus, Pseudomonas aeruginosa, and Stenotrophomonas maltophilia. BMC Microbiol. 2012, 12, 145. [Google Scholar] [CrossRef] [PubMed]
- Ciornei, C.D.; Novikov, A.; Beloin, C.; Fitting, C.; Caroff, M.; Ghigo, J.M.; Cavaillon, J.M.; Adib-Conquy, M. Biofilm-forming Pseudomonas aeruginosa bacteria undergo lipopolysaccharide structural modifications and induce enhanced inflammatory cytokine response in human monocytes. Innate Immun. 2010, 16, 288–301. [Google Scholar] [CrossRef]
- Tan, X.; Coureuil, M.; Ramond, E.; Euphrasie, D.; Dupuis, M.; Tros, F.; Meyer, J.; Nemazanyy, I.; Chhuon, C.; Guerrera, I.C.; et al. Chronic Staphylococcus aureus lung infection correlates with proteogenomic and metabolic adaptations leading to an increased intracellular persistence. Clin. Infect. Dis. 2019, 69, 1937–1945. [Google Scholar] [CrossRef]
- Treffon, J.; Fotiadis, S.A.; van Alen, S.; Becker, K.; Kahl, B.C. The virulence potential of livestock-associated methicillin-resistant Staphylococcus aureus cultured from the airways of cystic fibrosis patients. Toxins 2020, 12, 360. [Google Scholar] [CrossRef]
- Gilpin, D.; Hoffman, L.R.; Ceppe, A.; Muhlebach, M.S. Phenotypic characteristics of incident and chronic MRSA isolates in cystic fibrosis. J. Cyst. Fibros. 2021, 20, 692–698. [Google Scholar] [CrossRef]
- Hirschhausen, N.; Block, D.; Bianconi, I.; Bragonzi, A.; Birtel, J.; Lee, J.C.; Dübbers, A.; Küster, P.; Kahl, J.; Peters, G.; et al. Extended Staphylococcus aureus persistence in cystic fibrosis is associated with bacterial adaptation. Int. J. Med. Microbiol. 2013, 303, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Schwartbeck, B.; Birtel, J.; Treffon, J.; Langhanki, L.; Mellmann, A.; Kale, D.; Kahl, J.; Hirschhausen, N.; Neumann, C.; Lee, J.C.; et al. Dynamic in vivo mutations within the ica operon during persistence of Staphylococcus aureus in the airways of cystic fibrosis patients. PLoS Pathog. 2016, 12, e1006024. [Google Scholar] [CrossRef] [PubMed]
- Lennartz, F.E.; Schwartbeck, B.; Dübbers, A.; Große-Onnebrink, J.; Kessler, C.; Küster, P.; Schültingkemper, H.; Peters, G.; Kahl, B.C. The prevalence of Staphylococcus aureus with mucoid phenotype in the airways of patients with cystic fibrosis—A prospective study. Int. J. Med. Microbiol. 2019, 309, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, J.; Ji, M.; Phillips, J.; Wylam, M.; Ji, Y. Identification of cbiO gene critical for biofilm formation by MRSA CFSa36 strain isolated from pediatric patient with cystic fibrosis. Pathogens 2021, 10, 1363. [Google Scholar] [CrossRef]
- Ulluwishewa, D.; Wang, L.; Pereira, C.; Flynn, S.; Cain, E.; Stick, S.; Reen, F.J.; Ramsay, J.P.; O’Gara, F. Dissecting the regulation of bile-induced biofilm formation in Staphylococcus aureus. Microbiology 2016, 162, 1398–1406. [Google Scholar] [CrossRef]
- Gabryszewski, S.J.; Wong Fok Lung, T.; Annavajhala, M.K.; Tomlinson, K.L.; Riquelme, S.A.; Khan, I.N.; Noguera, L.P.; Wickersham, M.; Zhao, A.; Mulenos, A.M.; et al. Metabolic adaptation in methicillin-resistant Staphylococcus aureus pneumonia. Am. J. Respir. Cell Mol. Biol. 2019, 61, 185–197. [Google Scholar] [CrossRef]
- Pompilio, A.; Pomponio, S.; Di Vincenzo, V.; Crocetta, V.; Nicoletti, M.; Piovano, M.; Garbarino, J.A.; Di Bonaventura, G. Antimicrobial and antibiofilm activity of secondary metabolites of lichens against methicillin-resistant Staphylococcus aureus strains from cystic fibrosis patients. Future Microbiol. 2013, 8, 281–292. [Google Scholar] [CrossRef]
- Kadkhoda, H.; Ghalavand, Z.; Nikmanesh, B.; Kodori, M.; Houri, H.; Taghizadeh Maleki, D.; Karimi Bavandpour, A.; Eslami, G. Characterization of biofilm formation and virulence factors of Staphylococcus aureus isolates from paediatric patients in Tehran, Iran. Iran. J. Basic Med. Sci. 2020, 23, 691–698. [Google Scholar] [CrossRef]
- O’Neill, E.; Pozzi, C.; Houston, P.; Humphreys, H.; Robinson, D.A.; Loughman, A.; Foster, T.J.; O’Gara, J.P. A novel Staphylococcus aureus biofilm phenotype mediated by the fibronectin-binding proteins, FnBPA and FnBPB. J. Bacteriol. 2008, 190, 3835–3850. [Google Scholar] [CrossRef]
- Houston, P.; Rowe, S.E.; Pozzi, C.; Waters, E.M.; O’Gara, J.P. Essential role for the major autolysin in the fibronectin-binding protein-mediated Staphylococcus aureus biofilm phenotype. Infect. Immun. 2011, 79, 1153–1165. [Google Scholar] [CrossRef]
- Jefferson, K.K.; Cramton, S.E.; Götz, F.; Pier, G.B. Identification of a 5-nucleotide sequence that controls expression of the ica locus in Staphylococcus aureus and characterization of the DNA-binding properties of IcaR. Mol. Microbiol. 2003, 48, 889–899. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, E.; Humphreys, H.; O’Gara, J.P. Carriage of both the fnbA and fnbB genes and growth at 37 °C promote FnBP-mediated biofilm development in meticillin-resistant Staphylococcus aureus clinical isolates. J. Med. Microbiol. 2009, 58, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.; Toledo-Arana, A.; Berasain, C.; Ghigo, J.M.; Amorena, B.; Penadés, J.R.; Lasa, I. SarA and not σB is essential for biofilm development by Staphylococcus aureus. Mol. Microbiol. 2003, 48, 1075–1087. [Google Scholar] [CrossRef] [PubMed]
- Beenken, K.E.; Blevins, J.S.; Smeltzer, M.S. Mutation of sarA in Staphylococcus aureus limits biofilm formation. Infect. Immun. 2003, 71, 4206–4211. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, K.K.; Pier, D.B.; Goldmann, D.A.; Pier, G.B. The teicoplanin-associated locus regulator (TcaR) and the intercellular adhesin locus regulator (IcaR) are transcriptional inhibitors of the ica locus in Staphylococcus aureus. J. Bacteriol. 2004, 186, 2449–2456. [Google Scholar] [CrossRef] [PubMed]
- Schilcher, K.; Horswill, A.R. Staphylococcal biofilm development: Structure, regulation, and treatment strategies. Microbiol. Mol. Biol. Rev. 2020, 84, e00026-19. [Google Scholar] [CrossRef] [PubMed]
- Sadowska, B.; Więckowska-Szakiel, M.; Paszkiewicz, M.; Różalska, B. The immunomodulatory activity of Staphylococcus aureus products derived from biofilm and planktonic cultures. Arch. Immunol. Ther. Exp. 2013, 61, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, J.; Zhong, D.; Ji, L.; Yang, J.; Phillips, J.; Ji, Y. Characterization of Staphylococcus aureus isolates from pediatric patients with cystic fibrosis. World J. Microbiol. Biotechnol. 2016, 32, 162. [Google Scholar] [CrossRef]
- Cramton, S.E.; Ulrich, M.; Götz, F.; Döring, G. Anaerobic conditions induce expression of polysaccharide intercellular adhesin in Staphylococcus aureus and Staphylococcus epidermidis. Infect. Immun. 2001, 69, 4079–4085. [Google Scholar] [CrossRef]
- Ulrich, M.; Bastian, M.; Cramton, S.E.; Ziegler, K.; Pragman, A.A.; Bragonzi, A.; Memmi, G.; Wolz, C.; Schlievert, P.M.; Cheung, A.; et al. The staphylococcal respiratory response regulator SrrAB induces ica gene transcription and polysaccharide intercellular adhesin expression, protecting Staphylococcus aureus from neutrophil killing under anaerobic growth conditions. Mol. Microbiol. 2007, 65, 1276–1287. [Google Scholar] [CrossRef]
- Reen, F.J.; Woods, D.F.; Mooij, M.J.; Adams, C.; O’Gara, F. Respiratory pathogens adopt a chronic lifestyle in response to bile. PLoS ONE 2012, 7, e45978. [Google Scholar] [CrossRef] [PubMed]
- Moormeier, D.E.; Bayles, K.W. Staphylococcus aureus biofilm: A complex developmental organism. Mol. Microbiol. 2017, 104, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Idrees, M.; Sawant, S.; Karodia, N.; Rahman, A. Staphylococcus aureus biofilm: Morphology, genetics, pathogenesis and treatment strategies. Int. J. Environ. Res. Public Health 2021, 18, 7602. [Google Scholar] [CrossRef] [PubMed]
- Corrigan, R.M.; Abbott, J.C.; Burhenne, H.; Kaever, V.; Gründling, A. c-di-AMP is a new second messenger in Staphylococcus aureus with a role in controlling cell size and envelope stress. PLoS Pathog. 2011, 7, e1002217. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Hisatsune, J.; Hayashi, I.; Tatsukawa, N.; Sato’o, Y.; Mizumachi, E.; Kato, F.; Hirakawa, H.; Pier, G.B.; Sugai, M. A novel repressor of the ica locus discovered in clinically isolated super-biofilm-elaborating Staphylococcus aureus. mBio 2017, 8, e02282-16. [Google Scholar] [CrossRef] [PubMed]
- Sibley, C.D.; Rabin, H.; Surette, M.G. Cystic fibrosis: A polymicrobial infectious disease. Future Microbiol. 2006, 1, 53–61. [Google Scholar] [CrossRef]
- Peters, B.M.; Jabra-Rizk, M.A.; O’May, G.A.; Costerton, J.W.; Shirtliff, M.E. Polymicrobial Interactions: Impact on pathogenesis and human disease. Clin. Microbiol. Rev. 2012, 25, 193–213. [Google Scholar] [CrossRef]
- O’Brien, T.J.; Welch, M. Recapitulation of polymicrobial communities associated with cystic fibrosis airway infections: A perspective. Future Microbiol. 2019, 14, 1437–1450. [Google Scholar] [CrossRef]
- Høiby, N.; Ciofu, O.; Bjarnsholt, T. Pseudomonas aeruginosa biofilms in cystic fibrosis. Future Microbiol. 2010, 5, 1663–1674. [Google Scholar] [CrossRef]
- Armbruster, C.R.; Coenye, T.; Touqui, L.; Bomberger, J.M. Interplay between host-microbe and microbe-microbe interactions in cystic fibrosis. J. Cyst. Fibros. 2020, 19, S47–S53. [Google Scholar] [CrossRef]
- Bjarnsholt, T.; Alhede, M.; Alhede, M.; Eickhardt-Sørensen, S.R.; Moser, C.; Kühl, M.; Jensen, P.Ø.; Høiby, N. The in vivo biofilm. Trends Microbiol. 2013, 21, 466–474. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, S.; Fothergill, J.L. The role of multispecies social interactions in shaping Pseudomonas aeruginosa pathogenicity in the cystic fibrosis lung. FEMS Microbiol. Lett. 2017, 364, fnx128. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.K.; Chae, S.-W.; Go, Y.Y.; Im, G.J.; Song, J.-J. In vitro multi-species biofilms of methicillin-resistant Staphylococcus aureus and Pseudomonas aeruginosa and their host interaction during in vivo colonization of an otitis media rat model. Front. Cell. Infect. Microbiol. 2017, 7, 125. [Google Scholar] [CrossRef] [PubMed]
- Vandeplassche, E.; Sass, A.; Ostyn, L.; Burmølle, M.; Kragh, K.N.; Bjarnsholt, T.; Coenye, T.; Crabbé, A. Antibiotic susceptibility of cystic fibrosis lung microbiome members in a multispecies biofilm. Biofilm 2020, 2, 100031. [Google Scholar] [CrossRef]
- Granchelli, A.M.; Adler, F.R.; Keogh, R.H.; Kartsonaki, C.; Cox, D.R.; Liou, T.G. Microbial Interactions in the cystic fibrosis airway. J. Clin. Microbiol. 2018, 56, e00354-18. [Google Scholar] [CrossRef]
- Fischer, A.J.; Singh, S.B.; LaMarche, M.M.; Maakestad, L.J.; Kienenberger, Z.E.; Peña, T.A.; Stoltz, D.A.; Limoli, D.H. Sustained coinfections with Staphylococcus aureus and Pseudomonas aeruginosa in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2021, 203, 328–338. [Google Scholar] [CrossRef]
- Limoli, D.H.; Hoffman, L.R. Help, hinder, hide and harm: What can we learn from the interactions between Pseudomonas aeruginosa and Staphylococcus aureus during respiratory infections? Thorax 2019, 74, 684–692. [Google Scholar] [CrossRef]
- Zarrella, T.M.; Khare, A. Systematic identification of molecular mediators of interspecies sensing in a community of two frequently coinfecting bacterial pathogens. PLoS Biol. 2022, 20, e3001679. [Google Scholar] [CrossRef]
- Mathee, K. Forensic investigation into the origin of Pseudomonas aeruginosa PA14—Old but not lost. J. Med. Microbiol. 2018, 67, 1019–1021. [Google Scholar] [CrossRef]
- Magalhães, A.P.; França, A.; Pereira, M.O.; Cerca, N. Unveiling co-infection in cystic fibrosis airways: Transcriptomic analysis of Pseudomonas aeruginosa and Staphylococcus aureus dual-species biofilms. Front. Genet. 2022, 13, 883199. [Google Scholar] [CrossRef]
- Fugère, A.; Lalonde Séguin, D.; Mitchell, G.; Déziel, E.; Dekimpe, V.; Cantin, A.M.; Frost, E.; Malouin, F. Interspecific small molecule interactions between clinical isolates of Pseudomonas aeruginosa and Staphylococcus aureus from adult cystic fibrosis patients. PLoS ONE 2014, 9, e86705. [Google Scholar] [CrossRef] [PubMed]
- Camus, L.; Briaud, P.; Bastien, S.; Elsen, S.; Doléans-Jordheim, A.; Vandenesch, F.; Moreau, K. Trophic cooperation promotes bacterial survival of Staphylococcus aureus and Pseudomonas aeruginosa. ISME J. 2020, 14, 3093–3105. [Google Scholar] [CrossRef] [PubMed]
- Menetrey, Q.; Dupont, C.; Chiron, R.; Jumas-Bilak, E.; Marchandin, H. High occurrence of bacterial competition among clinically documented opportunistic pathogens including Achromobacter xylosoxidans in cystic fibrosis. Front. Microbiol. 2020, 11, 558160. [Google Scholar] [CrossRef] [PubMed]
- Armbruster, C.R.; Wolter, D.J.; Mishra, M.; Hayden, H.S.; Radey, M.C.; Merrihew, G.; MacCoss, M.J.; Burns, J.; Wozniak, D.J.; Parsek, M.R.; et al. Staphylococcus aureus protein A mediates interspecies interactions at the cell surface of Pseudomonas aeruginosa. mBio 2016, 7, e00538-16. [Google Scholar] [CrossRef] [PubMed]
- Beaudoin, T.; Yau, Y.C.W.; Stapleton, P.J.; Gong, Y.; Wang, P.W.; Guttman, D.S.; Waters, V. Staphylococcus aureus interaction with Pseudomonas aeruginosa biofilm enhances tobramycin resistance. NPJ Biofilms Microbiomes 2017, 13, 25. [Google Scholar] [CrossRef]
- Mitchell, G.; Séguin, D.L.; Asselin, A.E.; Déziel, E.; Cantin, A.M.; Frost, E.H.; Michaud, S.; Malouin, F. Staphylococcus aureus sigma B-dependent emergence of small-colony variants and biofilm production following exposure to Pseudomonas aeruginosa 4-hydroxy-2-heptylquinoline-N-oxide. BMC Microbiol. 2010, 10, 33. [Google Scholar] [CrossRef]
- Orazi, G.; O’Toole, G.A. Pseudomonas aeruginosa alters Staphylococcus aureus sensitivity to vancomycin in a biofilm model of cystic fibrosis Infection. mBio 2017, 8, e00873-17. [Google Scholar] [CrossRef]
- Tuchscherr, L.; Bischoff, M.; Lattar, S.M.; Noto Llana, M.; Pförtner, H.; Niemann, S.; Geraci, J.; Van de Vyver, H.; Fraunholz, M.J.; Cheung, A.L.; et al. Sigma factor SigB is crucial to mediate Staphylococcus aureus adaptation during chronic infections. PLoS Pathog. 2015, 11, e1004870. [Google Scholar] [CrossRef]
- Magalhães, A.P.; Grainha, T.; Sousa, A.M.; França, Â.; Cerca, N.; Pereira, M.O. Viable but non-cultivable state: A strategy for Staphylococcus aureus survivable in dual-species biofilms with Pseudomonas aeruginosa? Environ. Microbiol. 2021, 23, 5639–5649. [Google Scholar] [CrossRef]
- Tavernier, S.; Crabbé, A.; Hacioglu, M.; Stuer, L.; Henry, S.; Rigole, P.; Dhondt, I.; Coenye, T. Community composition determines activity of antibiotics against multispecies biofilms. Antimicrob. Agents Chemother. 2017, 61, e00302-17. [Google Scholar] [CrossRef]
- He, X.; Shi, W. Oral microbiology: Past, present and future. Int. J. Oral. Sci. 2009, 1, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Sagel, S.D.; Gibson, R.L.; Emerson, J.; McNamara, S.; Burns, J.L.; Wagener, J.S.; Ramsey, B.W. Inhaled tobramycin in young children study group; Cystic Fibrosis foundation therapeutics development network. Impact of Pseudomonas and Staphylococcus infection on inflammation and clinical status in young children with cystic fibrosis. J. Pediatr. 2009, 154, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Yung, D.B.Y.; Sircombe, K.J.; Pletzer, D. Friends or enemies? The complicated relationship between Pseudomonas aeruginosa and Staphylococcus aureus. Mol Microbiol. 2021, 116, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Nair, N.; Biswas, R.; Götz, F.; Biswas, L. Impact of Staphylococcus aureus on pathogenesis in polymicrobial infections. Infect. Immun. 2014, 82, 2162–2169. [Google Scholar] [CrossRef] [PubMed]
- Rüger, M.; Ackermann, M.; Reichl, U. Species-specific viability analysis of Pseudomonas aeruginosa, Burkholderia cepacia and Staphylococcus aureus in mixed culture by flow cytometry. BMC Microbiol. 2014, 14, 56. [Google Scholar] [CrossRef]
- Moriano, A.; Serra, D.O.; Hoard, A.; Montaña, S.; Degrossi, J.; Bonomo, R.A.; Papp-Wallace, K.M.; Ramirez, M.S. Staphylococcus aureus potentiates the hemolytic activity of Burkholderia cepacia complex (Bcc) bacteria. Curr. Microbiol. 2021, 78, 1864–1870. [Google Scholar] [CrossRef]
- Peters, B.M.; Jabra-Rizk, M.A.; Scheper, M.A.; Leid, J.G.; Costerton, J.W.; Shirtliff, M.E. Microbial interactions and differential protein expression in Staphylococcus aureus-Candida albicans dual-species biofilms. FEMS Immunol. Med. Microbiol. 2010, 59, 493–503. [Google Scholar] [CrossRef]
- Batoni, G.; Maisetta, G.; Esin, S. Therapeutic potential of antimicrobial peptides in polymicrobial biofilm-associated infections. Int. J. Mol. Sci. 2021, 22, 482. [Google Scholar] [CrossRef]
- Yuan, Y.; Zai, Y.; Xi, X.; Ma, C.; Wang, L.; Zhou, M.; Shaw, C.; Chen, T. A novel membrane-disruptive antimicrobial peptide from frog skin secretion against cystic fibrosis isolates and evaluation of anti-MRSA effect using Galleria mellonella model. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 849–856. [Google Scholar] [CrossRef]
- Walton, W.G.; Ahmad, S.; Little, M.S.; Kim, C.S.; Tyrrell, J.; Lin, Q.; Di, Y.P.; Tarran, R.; Redinbo, M.R. Structural features essential to the antimicrobial functions of human SPLUNC1. Biochemistry 2016, 55, 2979–2991. [Google Scholar] [CrossRef]
- Yu, Z.; Deslouches, B.; Walton, W.G.; Redinbo, M.R.; Di, Y.P. Enhanced biofilm prevention activity of a SPLUNC1-derived antimicrobial peptide against Staphylococcus aureus. PLoS ONE 2018, 13, e0203621. [Google Scholar] [CrossRef] [PubMed]
- Papa, R.; Garzoli, S.; Vrenna, G.; Sabatino, M.; Sapienza, F.; Relucenti, M.; Donfrancesco, O.; Fiscarelli, E.V.; Artini, M.; Selan, L.; et al. Essential oils biofilm modulation activity, chemical and machine learning analysis—Application on Staphylococcus aureus isolates from cystic fibrosis patients. Int. J. Mol. Sci. 2020, 21, 9258. [Google Scholar] [CrossRef] [PubMed]
- Swamy, M.K.; Akhtar, M.S.; Sinniah, U.R. Antimicrobial properties of plant essential oils against human pathogens and their mode of action: An updated review. Evid.-Based Complement. Alternat. Med. 2016, 2016, 3012462. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Bao, X.; Bové, M.; Rigole, P.; Meng, X.; Su, J.; Coenye, T. Antibiofilm activities of borneol-citral-loaded Pickering emulsions against Pseudomonas aeruginosa and Staphylococcus aureus in physiologically relevant chronic infection models. Microbiol. Spectr. 2022, 10, e0169622. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, P.; He, X.; Yuan, Z.W.; Yin, Z.Q.; Fu, H.; Lin, J.; He, C.; Liang, X.; Lv, C.; Shu, G.; et al. Erianin against Staphylococcus aureus infection via inhibiting sortase A. Toxins 2018, 10, 385. [Google Scholar] [CrossRef]
- Cascioferro, S.; Raffa, D.; Maggio, B.; Raimondi, M.V.; Schillaci, D.; Daidone, G. Sortase A inhibitors: Recent advances and future perspectives. J. Med. Chem. 2015, 58, 9108–9123. [Google Scholar] [CrossRef]
- Caiazza, N.C.; O’Toole, G.A. Alpha-toxin is required for biofilm formation by Staphylococcus aureus. J. Bacteriol. 2003, 185, 3214–3217. [Google Scholar] [CrossRef]
- Anderson, M.J.; Lin, Y.-C.; Gillman, A.N.; Parks, P.J.; Schlievert, P.M.; Peterson, M. Alpha-toxin promotes mucosal biofilm formation by Staphylococcus aureus. Front. Cell. Infect. Microbiol. 2012, 2, 64. [Google Scholar] [CrossRef]
- Anderson, M.J.; Schaaf, E.; Breshears, L.M.; Wallis, H.W.; Johnson, J.R.; Tkaczyk, C.; Sellman, B.R.; Sun, J.; Peterson, M.L. Alpha-toxin contributes to biofilm formation among Staphylococcus aureus wound isolates. Toxins 2018, 10, 157. [Google Scholar] [CrossRef]
- Pompilio, A.; Riviello, A.; Crocetta, V.; Di Giuseppe, F.; Pomponio, S.; Sulpizio, M.; Di Ilio, C.; Angelucci, S.; Barone, L.; Di Giulio, A.; et al. Evaluation of antibacterial and antibiofilm mechanisms by usnic acid against methicillin-resistant Staphylococcus aureus. Future Microbiol. 2016, 11, 1315–1338. [Google Scholar] [CrossRef]
- King, P.; Lomovskaya, O.; Griffith, D.C.; Burns, J.L.; Dudley, M.N. In vitro pharmacodynamics of levofloxacin and other aerosolized antibiotics under multiple conditions relevant to chronic pulmonary infection in cystic fibrosis. Antimicrob. Agents Chemother. 2010, 54, 143–148. [Google Scholar] [CrossRef] [PubMed]
- McCaughey, G.; McKevitt, M.; Elborn, J.S.; Tunney, M.M. Antimicrobial activity of fosfomycin and tobramycin in combination against cystic fibrosis pathogens under aerobic and anaerobic conditions. J. Cyst. Fibros. 2012, 11, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Britt, N.S.; Hazlett, D.S.; Horvat, R.T.; Liesman, R.M.; Steed, M.E. Activity of pulmonary vancomycin exposures versus planktonic and biofilm isolates of methicillin-resistant Staphylococcus aureus from cystic fibrosis sputum. Int. J. Antimicrob. Agents. 2020, 55, 105898. [Google Scholar] [CrossRef] [PubMed]
- Tré-Hardy, M.; Macé, C.; El Manssouri, N.; Vanderbist, F.; Traore, H.; Devleeschouwer, M.J. Effect of antibiotic co-administration on young and mature biofilms of cystic fibrosis clinical isolates: The importance of the biofilm model. Int. J. Antimicrob. Agents. 2009, 33, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Iebba, V.; Totino, V.; Santangelo, F.; Gagliardi, A.; Ciotoli, L.; Virga, A.; Ambrosi, C.; Pompili, M.; De Biase, R.V.; Selan, L.; et al. Bdellovibrio bacteriovorus directly attacks Pseudomonas aeruginosa and Staphylococcus aureus cystic fibrosis isolates. Front. Microbiol. 2014, 5, 280. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Yu, X.; Guo, W.; Guo, C.; Guo, X.; Li, Q.; Zhu, Y. Bacteriophage-mediated control of biofilm: A promising new dawn for the future. Front. Microbiol. 2022, 13, 825828. [Google Scholar] [CrossRef] [PubMed]
- Ferriol-González, C.; Domingo-Calap, P. Phages for biofilm removal. Antibiotics 2020, 9, 268. [Google Scholar] [CrossRef]
- Kvachadze, L.; Balarjishvili, N.; Meskhi, T.; Tevdoradze, E.; Skhirtladze, N.; Pataridze, T.; Adamia, R.; Topuria, T.; Kutter, E.; Rohde, C.; et al. Evaluation of lytic activity of staphylococcal bacteriophage Sb-1 against freshly isolated clinical pathogens. Microb. Biotechnol. 2011, 4, 643–650. [Google Scholar] [CrossRef]
- Ahonen, M.J.R.; Dorrier, J.M.; Schoenfisch, M.H. Antibiofilm efficacy of nitric oxide-releasing alginates against cystic fibrosis bacterial pathogens. ACS Infect. Dis. 2019, 5, 1327–1335. [Google Scholar] [CrossRef]
- Pompilio, A.; Geminiani, C.; Bosco, D.; Rana, R.; Aceto, A.; Bucciarelli, T.; Scotti, L.; Di Bonaventura, G. Electrochemically synthesized silver nanoparticles are active against planktonic and biofilm cells of Pseudomonas aeruginosa and other cystic fibrosis-associated bacterial pathogens. Front. Microbiol. 2018, 9, 1349. [Google Scholar] [CrossRef]
- Di Bonaventura, G.; Pompilio, A.; Crocetta, V.; De Nicola, S.; Barbaro, F.; Giuliani, L.; D’Emilia, E.; Fiscarelli, E.; Bellomo, R.G.; Saggini, R. Exposure to extremely low-frequency magnetic field affects biofilm formation by cystic fibrosis pathogens. Future Microbiol. 2014, 9, 1303–1317. [Google Scholar] [CrossRef] [PubMed]
- Minkiewicz-Zochniak, A.; Strom, K.; Jarzynka, S.; Iwańczyk, B.; Koryszewska-Bagińska, A.; Olędzka, G. Effect of low amperage electric current on Staphylococcus aureus—Strategy for combating bacterial biofilms formation on dental implants in cystic fibrosis patients, in vitro study. Materials 2021, 14, 6117. [Google Scholar] [CrossRef] [PubMed]
- Pecoraro, C.; Carbone, D.; Deng, D.; Cascioferro, S.M.; Diana, P.; Giovannetti, E. Biofilm formation as valuable target to fight against severe chronic infections. Curr. Med. Chem. 2022, 29, 4307–4310. [Google Scholar] [CrossRef] [PubMed]
- Bianchera, A.; Buttini, F.; Bettini, R. Micro/nanosystems and biomaterials for controlled delivery of antimicrobial and anti-biofilm agents. Expert Opin. Ther. Pat. 2020, 30, 983–1000. [Google Scholar] [CrossRef]
- Cascioferro, S.; Parrino, B.; Carbone, D.; Pecoraro, C.; Diana, P. Novel strategies in the war against antibiotic resistance. Future Med. Chem. 2021, 13, 529–531. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).