Blocking Muscarinic Receptor 3 Attenuates Tumor Growth and Decreases Immunosuppressive and Cholinergic Markers in an Orthotopic Mouse Model of Colorectal Cancer
Abstract
1. Introduction
2. Results
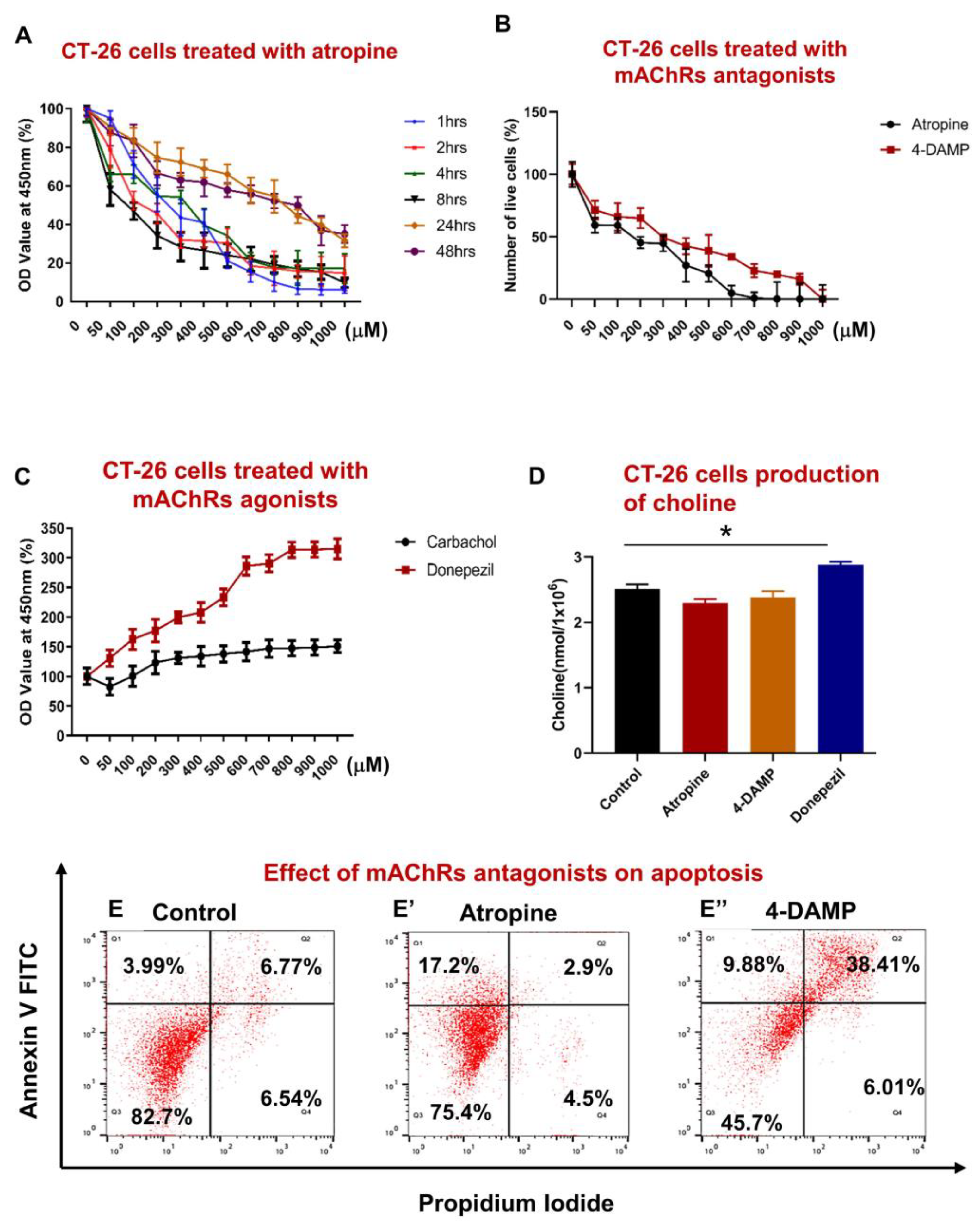
2.1. Effect of Blocking Muscarinic Receptors on Proliferation, Apoptosis, and Choline Production in CT-26 Cells
2.1.1. Atropine Decreases CT-26 Cells Proliferation in a Dose Dependent Manner
2.1.2. CT-26 Cells Can Produce the Choline Required for ACh Synthesis
2.1.3. Atropine and 4-DAMP Induce Apoptosis in CT-26 Cells
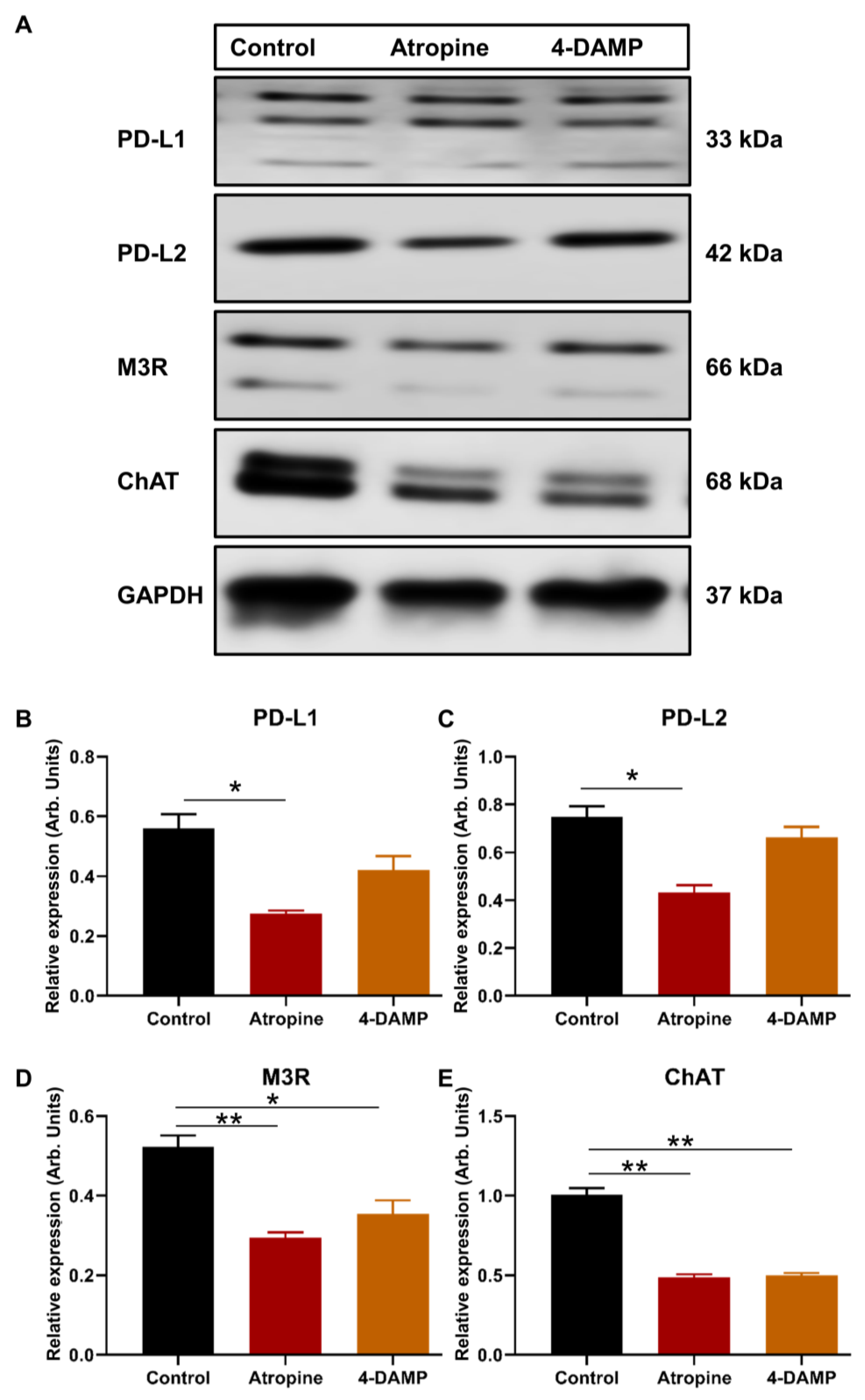
2.2. Effect of Atropine and 4-DAMP on the Expression of Immunosuppressive and Cholinergic Markers in an In Vitro Model
2.2.1. Atropine Decreases PD-L1 and PD-L2 Expression in CT-26 Cells
2.2.2. Atropine and 4-DAMP Attenuate M3R and ChAT Expression in CT-26 Cells
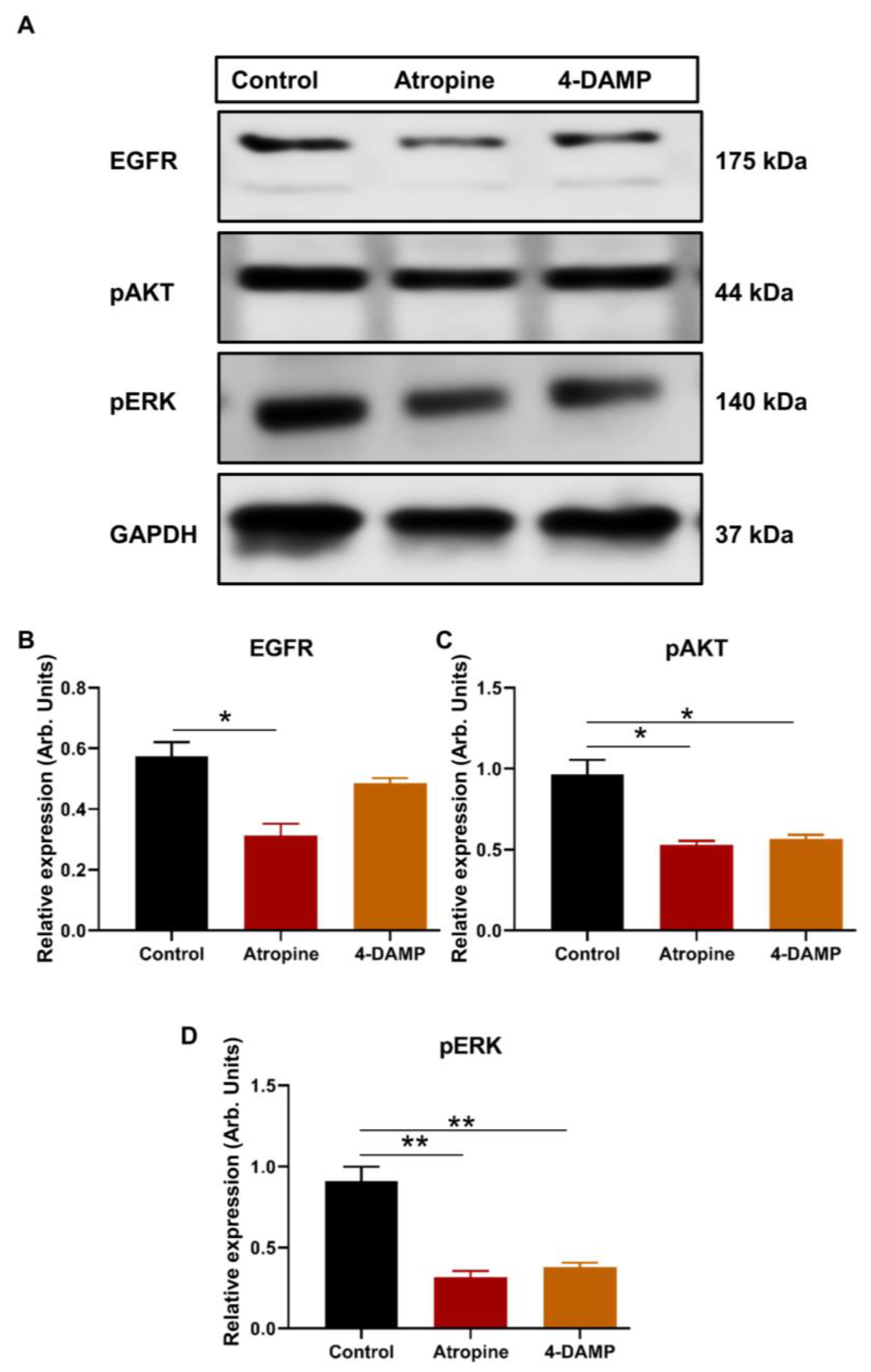
2.2.3. Atropine and 4-DAMP Inhibit Phosphorylation of Kinases and Activation of EGFR in CT-26 Cells
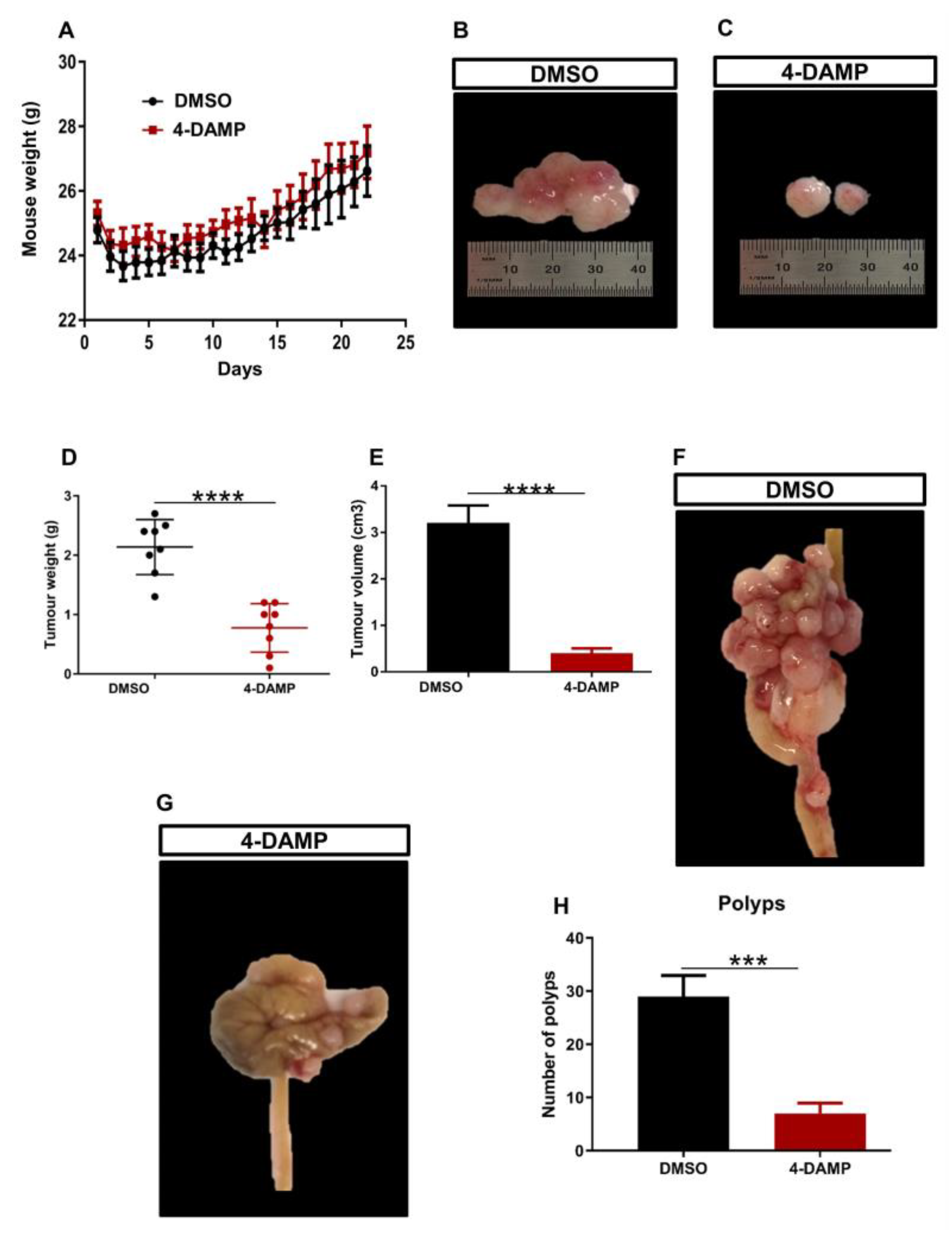
2.3. Effect of 4-DAMP on the Tumor Growth, Expression of Immunosuppressive, Cholinergic, Angiogenic Markers and Tumor-Infiltrating Immune Cells in an In Vivo Model
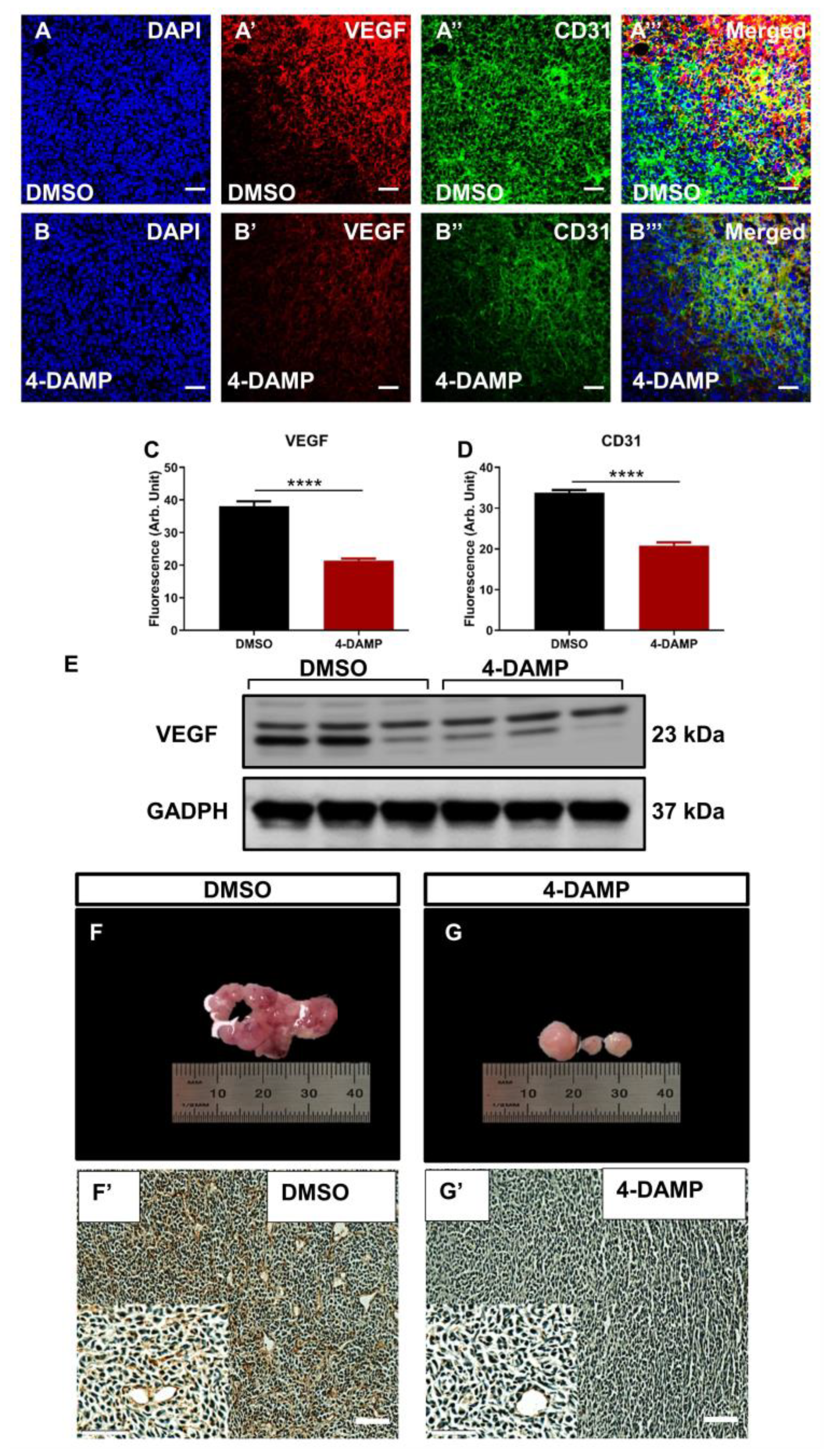
2.3.1. 4-DAMP Decreases Tumor Growth in an Orthotopic Mouse Model CRC
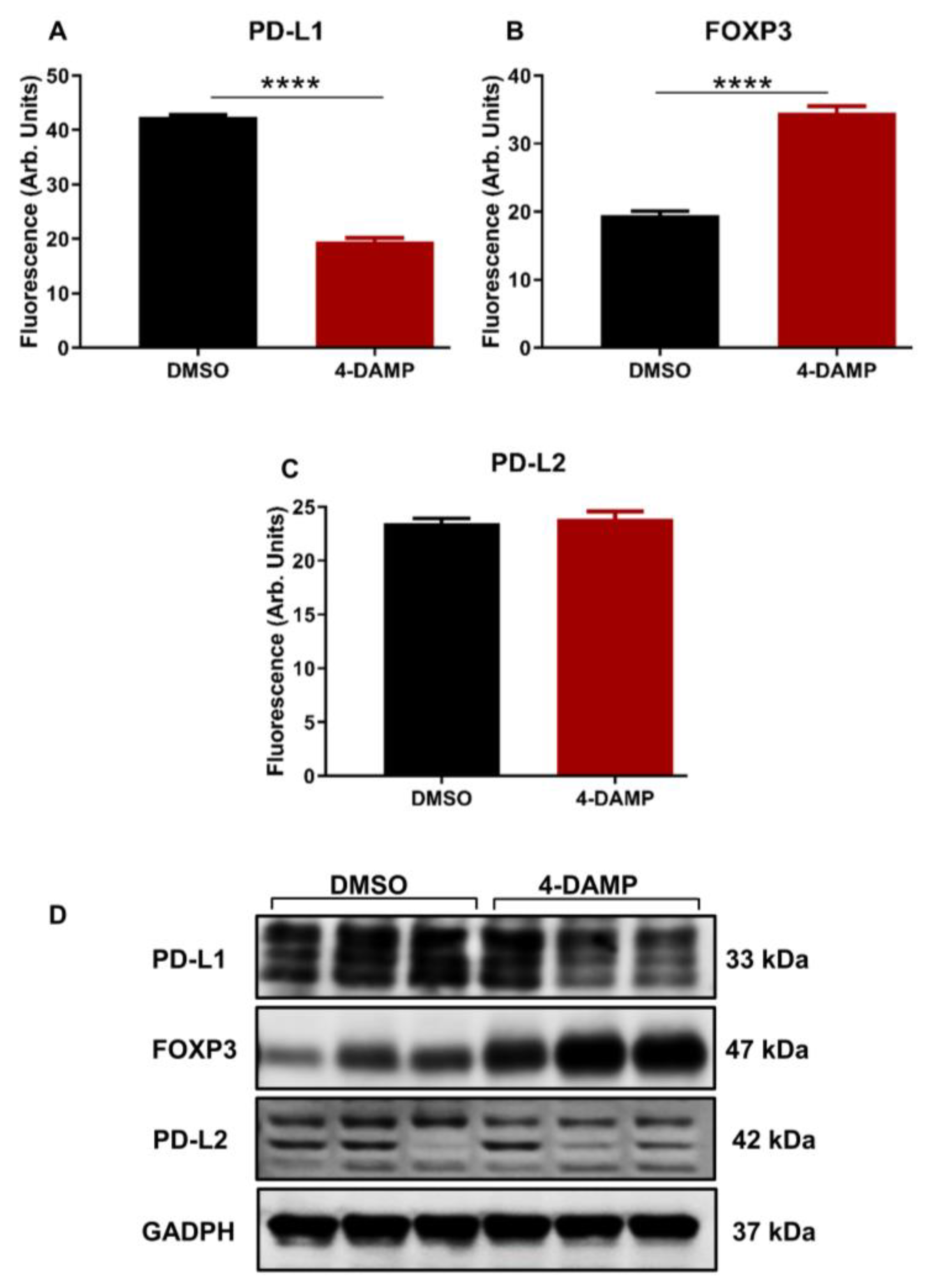
2.3.2. 4-DAMP Attenuates PD-L1 and Increases FOXP3 In Vivo
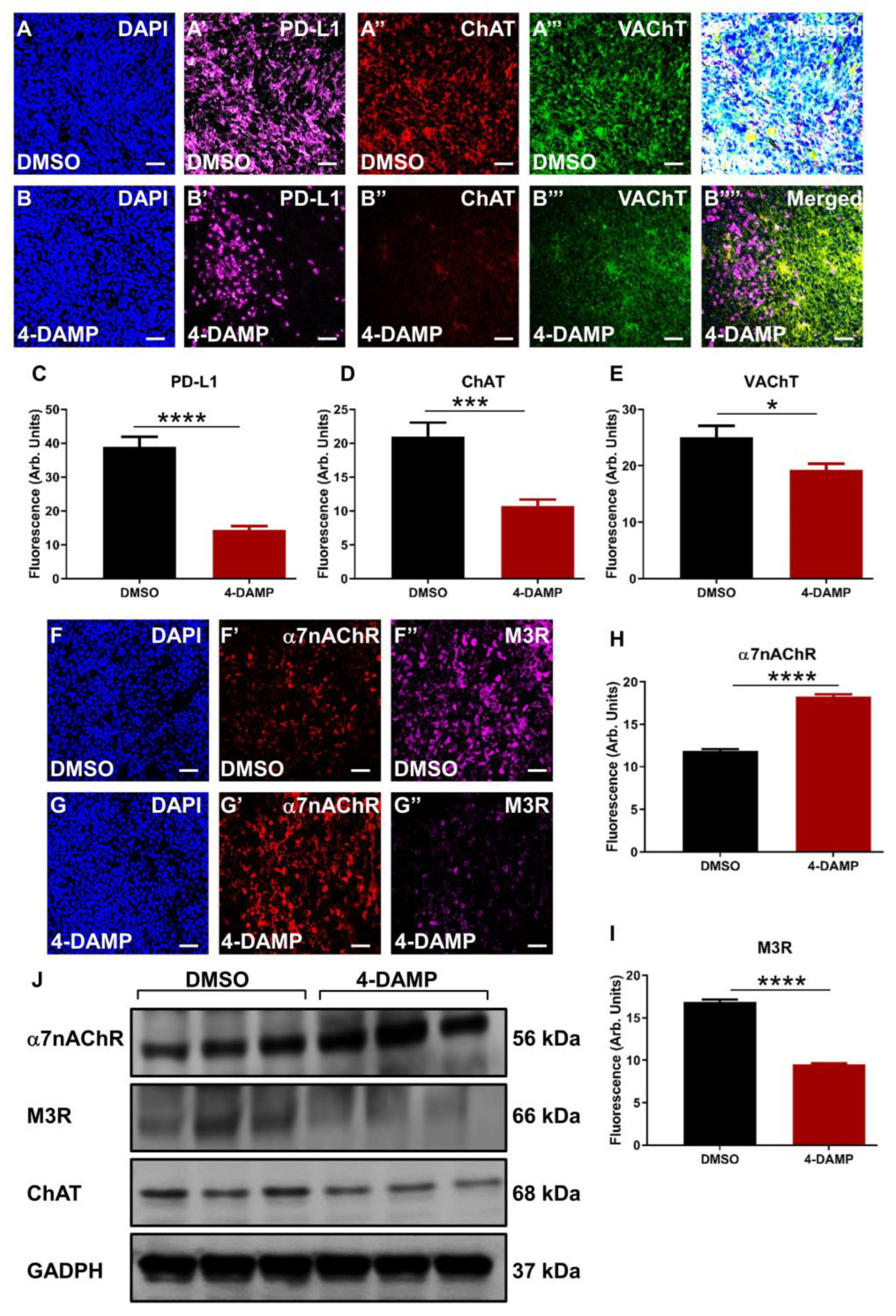
2.3.3. Correlation of PD-L1 Expression with Cholinergic Markers
2.3.4. 4-DAMP Treatment Augments α7nAChR and Attenuates M3R and ChAT Expression In Vivo
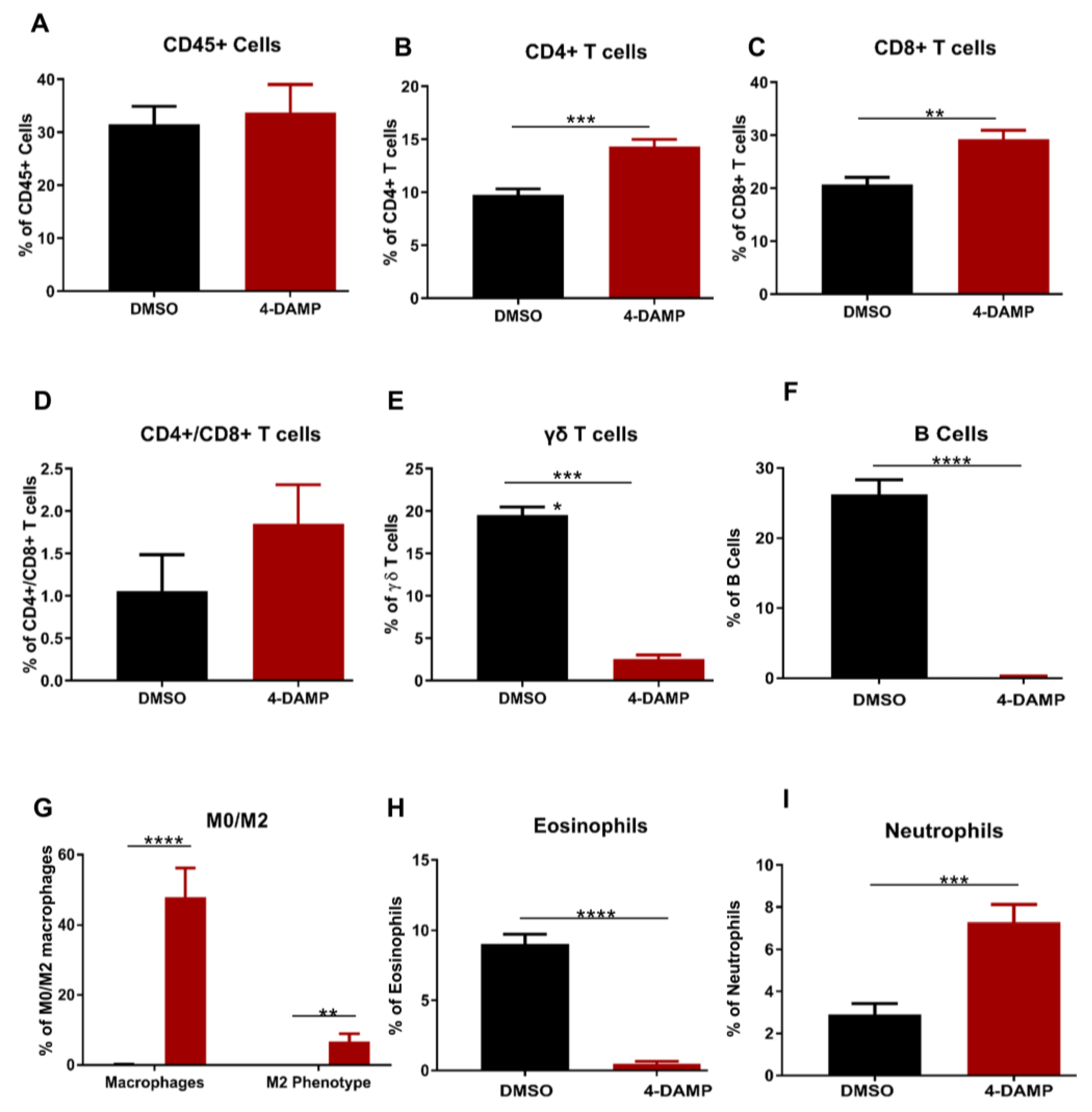
2.3.5. Effect of 4-DAMP Treatment on Tumor-Infiltrating Immune Cells
2.3.6. Blocking M3R Decreases Tumor Angiogenic Markers
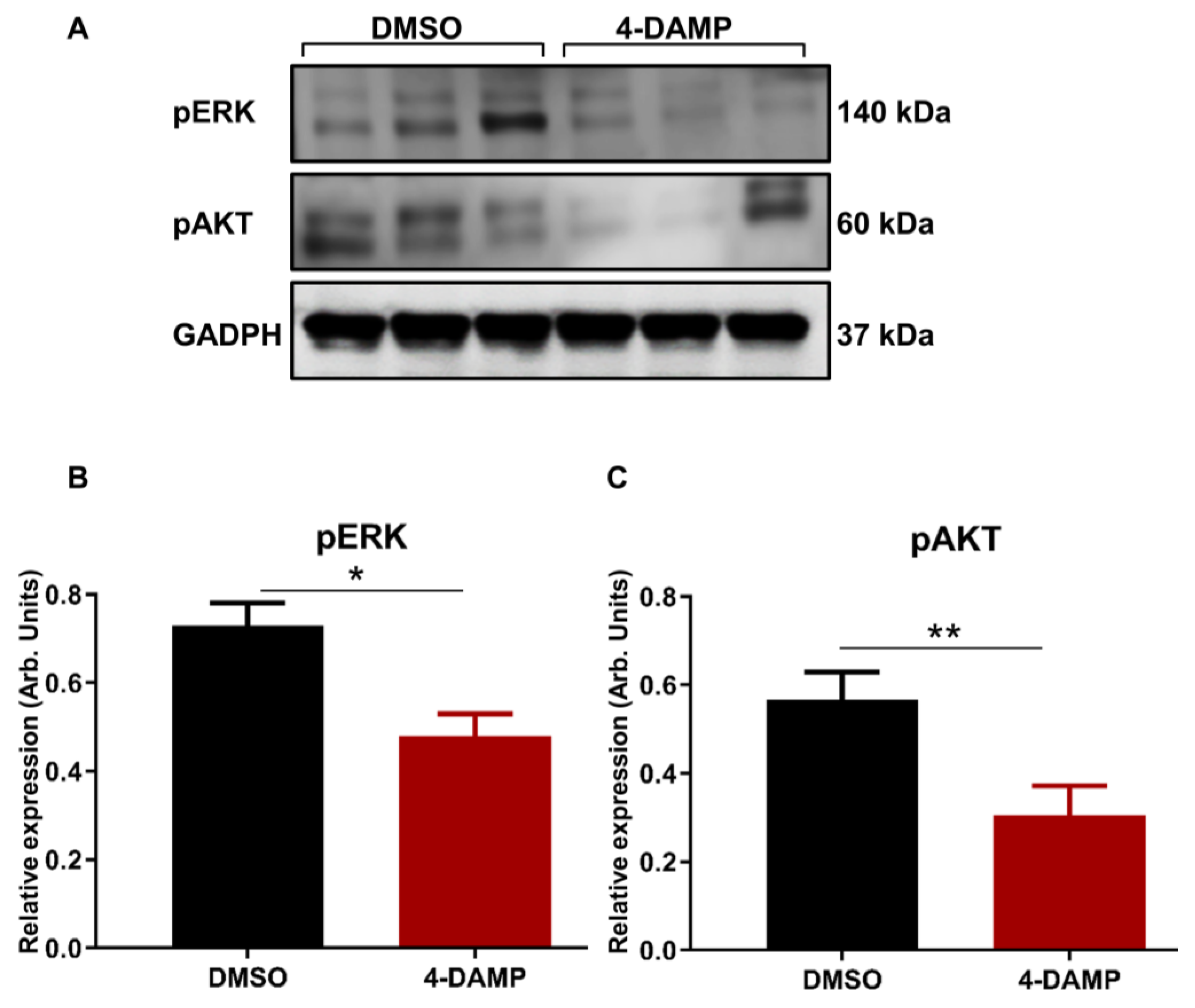
2.3.7. 4-DAMP Treatment Inhibits Phosphorylation of AKT and ERK In Vivo
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Cell Culture
4.3. Cell Viability
4.4. Annexin V Apoptosis Assay
4.5. Choline/Acetylcholine Assay
4.6. Orthotopic Implantation of CT-26 Tumor Cells
4.7. Intraperitoneal Injections and Tissue Collection
4.8. Immunohistochemistry in Cross-Sections
4.9. Immunoperoxidase Staining
4.10. Western Blot
4.11. Proteome Profiler Mouse Phospho-RTK Array Kit
4.12. Flow Cytometric Cell Staining
4.13. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA A Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Haggar, F.A.; Boushey, R.P. Colorectal cancer epidemiology: Incidence, mortality, survival, and risk factors. Clin. Colon Rectal Surg. 2009, 22, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Farhood, B.; Raei, B.; Malekzadeh, R.; Shirvani, M.; Najafi, M.; Mortezazadeh, T. A review of incidence and mortality of colorectal, lung, liver, thyroid, and bladder cancers in Iran and compared to other countries. Contemp. Oncol. 2019, 23, 7–15. [Google Scholar] [CrossRef]
- Weitz, J.; Koch, M.; Debus, J.; Hohler, T.; Galle, P.R.; Buchler, M.W. Colorectal cancer. Lancet 2005, 365, 153–165. [Google Scholar] [CrossRef]
- Shen, Z.; Gu, L.; Mao, D.; Chen, M.; Jin, R. Clinicopathological and prognostic significance of PD-L1 expression in colorectal cancer: A systematic review and meta-analysis. World J. Surg. Oncol. 2019, 17, 4. [Google Scholar] [CrossRef]
- Barriga, V.; Kuol, N.; Nurgali, K.; Apostolopoulos, V. The Complex Interaction between the Tumor Micro-Environment and Immune Checkpoints in Breast Cancer. Cancers 2019, 11, 1205. [Google Scholar] [CrossRef]
- Kuol, N.; Stojanovska, L.; Apostolopoulos, V.; Nurgali, K. Role of the nervous system in cancer metastasis. J. Exp. Clin. Cancer Res. 2018, 37, 5. [Google Scholar] [CrossRef]
- Kuol, N.; Stojanovska, L.; Apostolopoulos, V.; Nurgali, K. Role of the Nervous System in Tumor Angiogenesis. Cancer Microenviron. Off. J. Int. Cancer Microenviron. Soc. 2018, 11, 1–11. [Google Scholar] [CrossRef]
- Kuol, N.; Stojanovska, L.; Apostolopoulos, V.; Nurgali, K. Crosstalk between cancer and the neuro-immune system. J. Neuroimmunol. 2018, 315, 15–23. [Google Scholar] [CrossRef]
- Kuol, N.; Stojanovska, L.; Nurgali, K.; Apostolopoulos, V. The mechanisms tumor cells utilize to evade the host’s immune system. Maturitas 2017, 105, 8–15. [Google Scholar] [CrossRef]
- Mandai, M. PD-1/PD-L1 blockage in cancer treatment-from basic research to clinical application. Int. J. Clin. Oncol. 2016, 21, 447. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Azuma, T.; Yao, S.; Zhu, G.; Flies, A.S.; Flies, S.J.; Chen, L. B7-H1 is a ubiquitous antiapoptotic receptor on cancer cells. Blood 2008, 111, 3635–3643. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.J.; Wang, L.J.; Wang, G.D.; Guo, Z.Y.; Wei, M.; Meng, Y.L.; Yang, A.G.; Wen, W.H. B7-H1 expression is associated with poor prognosis in colorectal carcinoma and regulates the proliferation and invasion of HCT116 colorectal cancer cells. PLoS ONE 2013, 8, e76012. [Google Scholar] [CrossRef]
- Zhao, L.W.; Li, C.; Zhang, R.L.; Xue, H.G.; Zhang, F.X.; Zhang, F.; Gai, X.D. B7-H1 and B7-H4 expression in colorectal carcinoma: Correlation with tumor FOXP3(+) regulatory T-cell infiltration. Acta Histochem. 2014, 116, 1163–1168. [Google Scholar] [CrossRef] [PubMed]
- Masugi, Y.; Nishihara, R.; Yang, J.; Mima, K.; da Silva, A.; Shi, Y.; Inamura, K.; Cao, Y.; Song, M.; Nowak, J.A.; et al. Tumour CD274 (PD-L1) expression and T cells in colorectal cancer. Gut 2017, 66, 1463–1473. [Google Scholar] [CrossRef]
- Rosenbaum, M.W.; Bledsoe, J.R.; Morales-Oyarvide, V.; Huynh, T.G.; Mino-Kenudson, M. PD-L1 expression in colorectal cancer is associated with microsatellite instability, BRAF mutation, medullary morphology and cytotoxic tumor-infiltrating lymphocytes. Mod. Pathol. 2016, 29, 1104–1112. [Google Scholar] [CrossRef]
- Schalper, K.A. PD-L1 expression and tumor-infiltrating lymphocytes: Revisiting the antitumor immune response potential in breast cancer. Oncoimmunology 2014, 3, e29288. [Google Scholar] [CrossRef]
- Yang, C.Y.; Lin, M.W.; Chang, Y.L.; Wu, C.T.; Yang, P.C. Programmed cell death-ligand 1 expression in surgically resected stage I pulmonary adenocarcinoma and its correlation with driver mutations and clinical outcomes. Eur. J. Cancer 2014, 50, 1361–1369. [Google Scholar] [CrossRef]
- Baptista, M.Z.; Sarian, L.O.; Derchain, S.F.; Pinto, G.A.; Vassallo, J. Prognostic significance of PD-L1 and PD-L2 in breast cancer. Hum. Pathol. 2016, 47, 78–84. [Google Scholar] [CrossRef]
- Ghiotto, M.; Gauthier, L.; Serriari, N.; Pastor, S.; Truneh, A.; Nunes, J.A.; Olive, D. PD-L1 and PD-L2 differ in their molecular mechanisms of interaction with PD-1. Int. Immunol. 2010, 22, 651–660. [Google Scholar] [CrossRef]
- Wang, H.; Yao, H.; Li, C.; Liang, L.; Zhang, Y.; Shi, H.; Zhou, C.; Chen, Y.; Fang, J.-Y.; Xu, J. PD-L2 expression in colorectal cancer: Independent prognostic effect and targetability by deglycosylation. Oncoimmunology 2017, 6, e1327494. [Google Scholar] [CrossRef] [PubMed]
- Mancino, M.; Ametller, E.; Gascon, P.; Almendro, V. The neuronal influence on tumor progression. Biochim. Biophys. Acta 2011, 1816, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Shang, A.C.; Drachenberg, C.B.; Zhan, M.; Raufman, J.-P. Differential expression of M3 muscarinic receptors in progressive colon neoplasia and metastasis. Oncotarget 2017, 8, 21106–21114. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Samimi, R.; Xie, G.; Shant, J.; Drachenberg, C.; Wade, M.; Davis, R.J.; Nomikos, G.; Raufman, J.P. Acetylcholine release by human colon cancer cells mediates autocrine stimulation of cell proliferation. Am. J. Physiology. Gastrointest. Liver Physiol. 2008, 295, G591–G597. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.J.; Ho, Y.S.; Guo, H.R.; Wang, Y.J. Rapid activation of Stat3 and ERK1/2 by nicotine modulates cell proliferation in human bladder cancer cells. Toxicol. Sci. Off. J. Soc. Toxicol. 2008, 104, 283–293. [Google Scholar] [CrossRef]
- Lin, G.; Sun, L.; Wang, R.; Guo, Y.; Xie, C. Overexpression of muscarinic receptor 3 promotes metastasis and predicts poor prognosis in non-small-cell lung cancer. J. Thorac. Oncol. 2014, 9, 170–178. [Google Scholar] [PubMed]
- Yu, H.; Xia, H.; Tang, Q.; Xu, H.; Wei, G.; Chen, Y.; Dai, X.; Gong, Q.; Bi, F. Acetylcholine acts through M3 muscarinic receptor to activate the EGFR signaling and promotes gastric cancer cell proliferation. Sci. Rep. 2017, 7, 40802. [Google Scholar] [PubMed]
- Xu, R.; Shang, C.; Zhao, J.; Han, Y.; Liu, J.; Chen, K.; Shi, W. Activation of M3 muscarinic receptor by acetylcholine promotes non-small cell lung cancer cell proliferation and invasion via EGFR/PI3K/AKT pathway. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2015, 36, 4091–4100. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.H.; Li, D.L.; Bi, X.Y.; Sun, L.; Yu, X.J.; Fang, H.L.; Miao, Y.; Zhao, M.; He, X.; Liu, J.J.; et al. Acetylcholine Inhibits LPS-Induced MMP-9 Production and Cell Migration via the alpha7 nAChR-JAK2/STAT3 Pathway in RAW264.7 Cells. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2015, 36, 2025–2038. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.-X.; Wang, B.; Yao, Y.-N.; Pan, Z.-J.; Shen, Q.; Zhou, J.-Y. Autonomic nervous infiltration positively correlates with pathological risk grading and poor prognosis in patients with lung adenocarcinoma. Thorac. Cancer 2016, 7, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.P.S.; Yu, L.; Lam, E.K.Y.; Tai, E.K.K.; Wu, W.K.K.; Cho, C.-H. Nicotine promotes colon tumor growth and angiogenesis through β-adrenergic activation. Toxicol. Sci. 2007, 97, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Lombardi Mí, G.; Negroni, M.; Pelegrina, L.T.; Castro Mí, E.; Fiszman, G.L.; Azar Mí, E.; Morgado, C.C.; Sales Mí, E. Autoantibodies against muscarinic receptors in breast cancer: Their role in tumor angiogenesis. PLoS ONE 2013, 8, e57572. [Google Scholar] [CrossRef] [PubMed]
- de la Torre, E.; Davel, L.; Jasnis, M.A.; Gotoh, T.; de Lustig, E.S.; Sales, M.E. Muscarinic receptors participation in angiogenic response induced by macrophages from mammary adenocarcinoma-bearing mice. Breast Cancer Res. 2005, 7, R345–R352. [Google Scholar] [CrossRef] [PubMed]
- Belo, A.; Cheng, K.; Chahdi, A.; Shant, J.; Xie, G.; Khurana, S.; Raufman, J.-P. Muscarinic receptor agonists stimulate human colon cancer cell migration and invasion. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G749–G760. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Cheng, K.; Shant, J.; Raufman, J.P. Acetylcholine-induced activation of M3 muscarinic receptors stimulates robust matrix metalloproteinase gene expression in human colon cancer cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G755–G763. [Google Scholar] [CrossRef] [PubMed]
- Tseng, W.; Leong, X.; Engleman, E. Orthotopic mouse model of colorectal cancer. J. Vis. Exp. 2007, 10, 484. [Google Scholar] [CrossRef]
- Mu, C.Y.; Huang, J.A.; Chen, Y.; Chen, C.; Zhang, X.G. High expression of PD-L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation. Med. Oncol. 2011, 28, 682–688. [Google Scholar] [CrossRef]
- Wang, X.; Teng, F.; Kong, L.; Yu, J. PD-L1 expression in human cancers and its association with clinical outcomes. OncoTargets Ther. 2016, 9, 5023. [Google Scholar]
- Kim, M.; Grimmig, T.; Grimm, M.; Lazariotou, M.; Meier, E.; Rosenwald, A.; Tsaur, I.; Blaheta, R.; Heemann, U.; Germer, C.T.; et al. Expression of Foxp3 in colorectal cancer but not in Treg cells correlates with disease progression in patients with colorectal cancer. PLoS ONE 2013, 8, e53630. [Google Scholar] [CrossRef]
- Kuol Nyanbol, G.J.; Kmiec, Z.; Vogrin, S.; Fraser, S.; Apostolopoulos, V.; Nurgali, K. Cholinergic signaling influences the expression of immune checkpoint inhibitors, PD-L1 and PD-L2, in human colorectal cancer tissues and cell lines. BMC Cancer 2022. [Google Scholar] [CrossRef]
- Wong, H.P.; Yu, L.; Lam, E.K.; Tai, E.K.; Wu, W.K.; Cho, C.H. Nicotine promotes cell proliferation via alpha7-nicotinic acetylcholine receptor and catecholamine-synthesizing enzymes-mediated pathway in human colon adenocarcinoma HT-29 cells. Toxicol. Appl. Pharmacol. 2007, 221, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Bielenberg, D.R.; Zetter, B.R. The Contribution of Angiogenesis to the Process of Metastasis. Cancer J. 2015, 21, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Calon, A.; Lonardo, E.; Berenguer-Llergo, A.; Espinet, E.; Hernando-Momblona, X.; Iglesias, M.; Sevillano, M.; Palomo-Ponce, S.; Tauriello, D.V.; Byrom, D.; et al. Stromal gene expression defines poor-prognosis subtypes in colorectal cancer. Nat. Genet. 2015, 47, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Calon, A.; Espinet, E.; Palomo-Ponce, S.; Tauriello, D.V.; Iglesias, M.; Cespedes, M.V.; Sevillano, M.; Nadal, C.; Jung, P.; Zhang, X.H.; et al. Dependency of colorectal cancer on a TGF-beta-driven program in stromal cells for metastasis initiation. Cancer Cell 2012, 22, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Konishi, M.; Hayakawa, Y.; Koike, K. Role of Muscarinic Acetylcholine Signaling in Gastrointestinal Cancers. Biomedicines 2019, 7, 58. [Google Scholar] [CrossRef]
- Wang, L.; Zhi, X.; Zhang, Q.; Wei, S.; Li, Z.; Zhou, J.; Jiang, J.; Zhu, Y.; Yang, L.; Xu, H.; et al. Muscarinic receptor M3 mediates cell proliferation induced by acetylcholine and contributes to apoptosis in gastric cancer. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2016, 37, 2105–2117. [Google Scholar] [CrossRef]
- Xiang, T.; Fei, R.; Wang, Z.; Shen, Z.; Qian, J.; Chen, W. Nicotine enhances invasion and metastasis of human colorectal cancer cells through the nicotinic acetylcholine receptor downstream p38 MAPK signaling pathway. Oncol. Rep. 2016, 35, 205–210. [Google Scholar] [CrossRef]
- Hung, C.-S.; Peng, Y.-J.; Wei, P.-L.; Lee, C.-H.; Su, H.-Y.; Ho, Y.-S.; Lin, S.-Y.; Wu, C.-H.; Chang, Y.-J. The alpha9 Nicotinic Acetylcholine Receptor is the Key Mediator in Nicotine-enhanced Cancer Metastasis in Breast Cancer Cells. J. Exp. Clin. Med. 2011, 3, 283–292. [Google Scholar] [CrossRef]
- Nie, H.; Cao, Q.; Zhu, L.; Gong, Y.; Gu, J.; He, Z. Acetylcholine acts on androgen receptor to promote the migration and invasion but inhibit the apoptosis of human hepatocarcinoma. PLoS ONE 2013, 8, e61678. [Google Scholar] [CrossRef]
- Kamiya, A.; Hayama, Y.; Kato, S.; Shimomura, A.; Shimomura, T.; Irie, K.; Kaneko, R.; Yanagawa, Y.; Kobayashi, K.; Ochiya, T. Genetic manipulation of autonomic nerve fiber innervation and activity and its effect on breast cancer progression. Nat. Neurosci. 2019, 22, 1289–1305. [Google Scholar] [CrossRef] [PubMed]
- Fukumura, D.; Kloepper, J.; Amoozgar, Z.; Duda, D.G.; Jain, R.K. Enhancing cancer immunotherapy using antiangiogenics: Opportunities and challenges. Nat. Rev. Clin. Oncol. 2018, 15, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhao, J.; Zhang, L.; Wei, F.; Lian, Y.; Wu, Y.; Gong, Z.; Zhang, S.; Zhou, J.; Cao, K.; et al. Role of tumor microenvironment in tumorigenesis. J. Cancer 2017, 8, 761–773. [Google Scholar] [CrossRef] [PubMed]
- Magnon, C. Role of the autonomic nervous system in tumorigenesis and metastasis. Mol. Cell. Oncol. 2015, 2, e975643. [Google Scholar] [CrossRef]
- Goon, P.K.; Lip, G.Y.; Boos, C.J.; Stonelake, P.S.; Blann, A.D. Circulating endothelial cells, endothelial progenitor cells, and endothelial microparticles in cancer. Neoplasia 2006, 8, 79–88. [Google Scholar] [CrossRef]
- Samame Perez-Vargas, J.C.; Biondani, P.; Maggi, C.; Gariboldi, M.; Gloghini, A.; Inno, A.; Volpi, C.C.; Gualeni, A.V.; di Bartolomeo, M.; de Braud, F.; et al. Role of cMET in the development and progression of colorectal cancer. Int. J. Mol. Sci. 2013, 14, 18056–18077. [Google Scholar] [CrossRef]
- De Smedt, L.; Lemahieu, J.; Palmans, S.; Govaere, O.; Tousseyn, T.; Van Cutsem, E.; Prenen, H.; Tejpar, S.; Spaepen, M.; Matthijs, G.; et al. Microsatellite instable vs stable colon carcinomas: Analysis of tumour heterogeneity, inflammation and angiogenesis. Br. J. Cancer 2015, 113, 500–509. [Google Scholar] [CrossRef]
- Albini, A.; Bruno, A.; Noonan, D.M.; Mortara, L. Contribution to Tumor Angiogenesis From Innate Immune Cells Within the Tumor Microenvironment: Implications for Immunotherapy. Front. Immunol. 2018, 9, 527. [Google Scholar] [CrossRef]
- Xue, S.; Hu, M.; Li, P.; Ma, J.; Xie, L.; Teng, F.; Zhu, Y.; Fan, B.; Mu, D.; Yu, J. Relationship between expression of PD-L1 and tumor angiogenesis, proliferation, and invasion in glioma. Oncotarget 2017, 8, 49702–49712. [Google Scholar] [CrossRef]
- Shin, S.J.; Jeon, Y.K.; Kim, P.J.; Cho, Y.M.; Koh, J.; Chung, D.H.; Go, H. Clinicopathologic Analysis of PD-L1 and PD-L2 Expression in Renal Cell Carcinoma: Association with Oncogenic Proteins Status. Ann. Surg. Oncol. 2016, 23, 694–702. [Google Scholar] [CrossRef]
- Mohamed, S.Y.; Mohammed, H.L.; Ibrahim, H.M.; Mohamed, E.M.; Salah, M. Role of VEGF, CD105, and CD31 in the Prognosis of Colorectal Cancer Cases. J. Gastrointest. Cancer 2019, 50, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Ma, Y.; Zhan, S.; Zhang, G.; Cao, L.; Zhang, X.; Shi, T.; Chen, W. B7-H3 promotes colorectal cancer angiogenesis through activating the NF-kappaB pathway to induce VEGFA expression. Cell Death Dis. 2020, 11, 55. [Google Scholar] [CrossRef] [PubMed]
- Lebrun, J.J. The Dual Role of TGFbeta in Human Cancer: From Tumor Suppression to Cancer Metastasis. ISRN Mol. Biol. 2012, 2012, 381428. [Google Scholar] [PubMed]
- Pak, K.H.; Park, K.C.; Cheong, J.H. VEGF-C induced by TGF- beta1 signaling in gastric cancer enhances tumor-induced lymphangiogenesis. BMC Cancer 2019, 19, 799. [Google Scholar] [CrossRef] [PubMed]
- Wehler, T.C.; Frerichs, K.; Graf, C.; Drescher, D.; Schimanski, K.; Biesterfeld, S.; Berger, M.R.; Kanzler, S.; Junginger, T.; Galle, P.R.; et al. PDGFRalpha/beta expression correlates with the metastatic behavior of human colorectal cancer: A possible rationale for a molecular targeting strategy. Oncol. Rep. 2008, 19, 697–704. [Google Scholar]
- Schimanski, C.; Wehler, T.; Galle, P.; Gockel, I.; Moehler, M. PDGFR-α/β expression correlates with the metastatic behavior of human colorectal cancer—A rationale for a molecular targeting strategy? J. Clin. Oncol. 2008, 26, 22019. [Google Scholar] [CrossRef]
- Carvalho, I.; Milanezi, F.; Martins, A.; Reis, R.M.; Schmitt, F. Overexpression of platelet-derived growth factor receptor alpha in breast cancer is associated with tumour progression. Breast Cancer Res. 2005, 7, R788–R795. [Google Scholar] [CrossRef]
- Zhu, C.; Wei, Y.; Wei, X. AXL receptor tyrosine kinase as a promising anti-cancer approach: Functions, molecular mechanisms and clinical applications. Mol. Cancer 2019, 18, 153. [Google Scholar] [CrossRef] [PubMed]
- Ruan, G.X.; Kazlauskas, A. Axl is essential for VEGF-A-dependent activation of PI3K/Akt. EMBO J. 2012, 31, 1692–1703. [Google Scholar] [CrossRef]
- Tanaka, M.; Siemann, D.W. Axl signaling is an important mediator of tumor angiogenesis. Oncotarget 2019, 10, 2887–2898. [Google Scholar] [CrossRef] [PubMed]
- Stavely, R.; Robinson, A.M.; Miller, S.; Boyd, R.; Sakkal, S.; Nurgali, K. Human adult stem cells derived from adipose tissue and bone marrow attenuate enteric neuropathy in the guinea-pig model of acute colitis. Stem Cell Res. Ther. 2015, 6, 244. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuol, N.; Davidson, M.; Karakkat, J.; Filippone, R.T.; Veale, M.; Luwor, R.; Fraser, S.; Apostolopoulos, V.; Nurgali, K. Blocking Muscarinic Receptor 3 Attenuates Tumor Growth and Decreases Immunosuppressive and Cholinergic Markers in an Orthotopic Mouse Model of Colorectal Cancer. Int. J. Mol. Sci. 2023, 24, 596. https://doi.org/10.3390/ijms24010596
Kuol N, Davidson M, Karakkat J, Filippone RT, Veale M, Luwor R, Fraser S, Apostolopoulos V, Nurgali K. Blocking Muscarinic Receptor 3 Attenuates Tumor Growth and Decreases Immunosuppressive and Cholinergic Markers in an Orthotopic Mouse Model of Colorectal Cancer. International Journal of Molecular Sciences. 2023; 24(1):596. https://doi.org/10.3390/ijms24010596
Chicago/Turabian StyleKuol, Nyanbol, Majid Davidson, Jimsheena Karakkat, Rhiannon T. Filippone, Margaret Veale, Rodney Luwor, Sarah Fraser, Vasso Apostolopoulos, and Kulmira Nurgali. 2023. "Blocking Muscarinic Receptor 3 Attenuates Tumor Growth and Decreases Immunosuppressive and Cholinergic Markers in an Orthotopic Mouse Model of Colorectal Cancer" International Journal of Molecular Sciences 24, no. 1: 596. https://doi.org/10.3390/ijms24010596
APA StyleKuol, N., Davidson, M., Karakkat, J., Filippone, R. T., Veale, M., Luwor, R., Fraser, S., Apostolopoulos, V., & Nurgali, K. (2023). Blocking Muscarinic Receptor 3 Attenuates Tumor Growth and Decreases Immunosuppressive and Cholinergic Markers in an Orthotopic Mouse Model of Colorectal Cancer. International Journal of Molecular Sciences, 24(1), 596. https://doi.org/10.3390/ijms24010596








