Effects of Immobilization and Swimming on the Progression of Osteoarthritis in Mice
Abstract
1. Introduction
2. Results
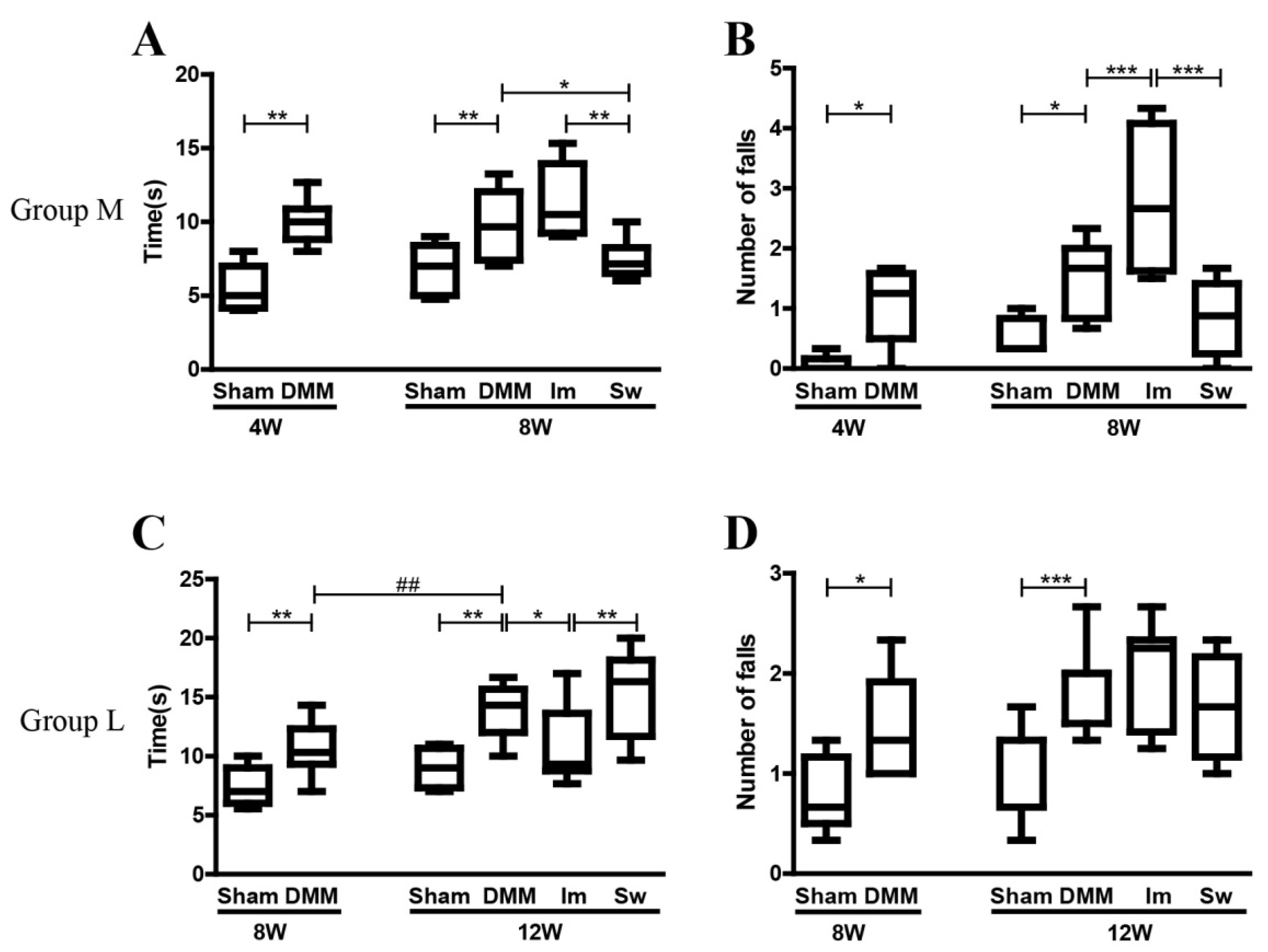
2.1. Immobilization Partly Rescues the Effects of DMM on Gait but Aggravates the Disadvantages of DMM on the Balance Ability at the Middle Stage of OA
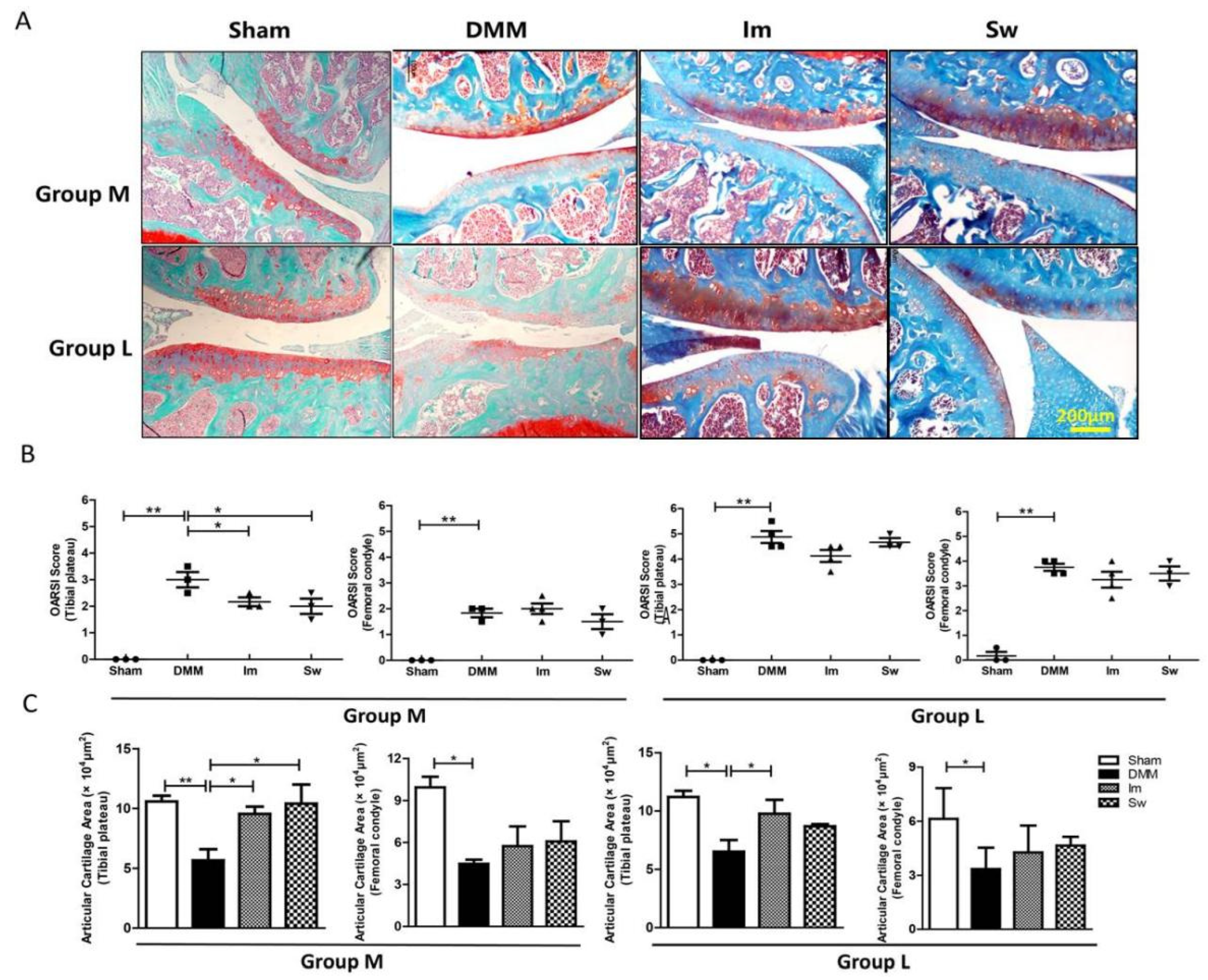
2.2. Immobilization and Swimming Both Play Positive Roles in Histopathological Changes in OA at the Middle Stage but Not the Late Stage
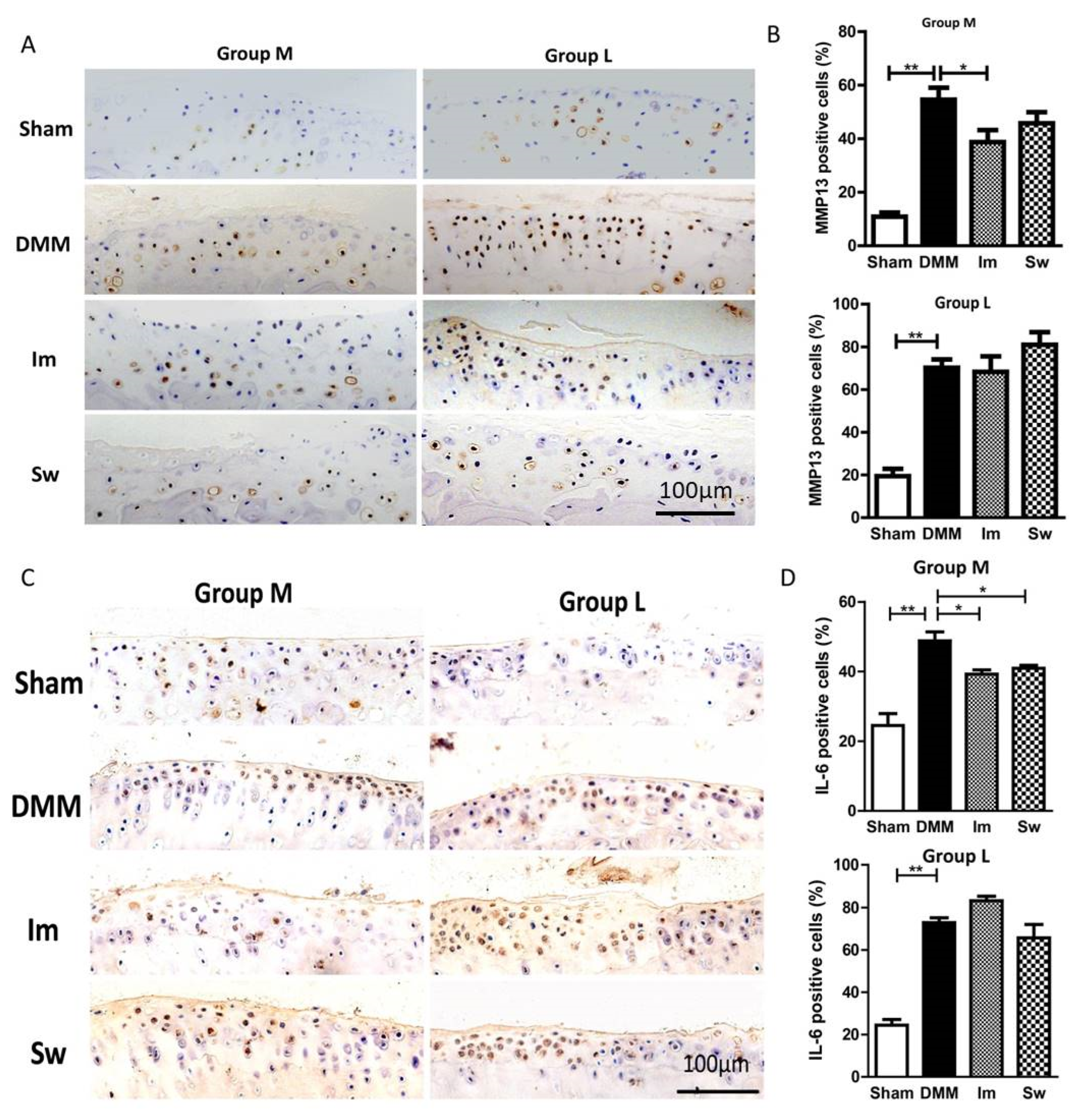
2.3. Swimming Slightly Relieves the Alteration in Subchondral Bone Only at the Middle Stage of OA, Not the Late Stage
3. Discussion
4. Materials and Methods
4.1. Experimental Animals and DMM Operation
4.2. Immobilization and Swimming Treatment
4.3. Ink Blot Analysis
4.4. Beam Walking Test
4.5. Histological Analysis
4.6. Immunohistochemistry (IHC)
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Man, G.; Mologhianu, G. Osteoarthritis pathogenesis—A complex process that involves the entire joint. J. Med. Life 2014, 7, 37–41. [Google Scholar] [PubMed]
- Badley, E.M.; Wang, P.P. Arthritis and the aging population: Projections of arthritis prevalence in Canada 1991 to 2031. J. Rheumatol. 1998, 25, 138–144. [Google Scholar] [PubMed]
- Allman-Farinelli, M.A.; Aitken, R.J.; King, L.A.; Bauman, A.E. Osteoarthritis—The forgotten obesity-related epidemic with worse to come. Med. J. Aust. 2008, 188, 317. [Google Scholar] [CrossRef] [PubMed]
- Guermazi, A.; Roemer, F.W.; Felson, D.T.; Brandt, K.D. Motion for debate: Osteoarthritis clinical trials have not identified efficacious therapies because traditional imaging outcome measures are inadequate. Arthritis Rheum. 2013, 65, 2748–2758. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Ma, Y.; Yao, X.; Zhou, W.; Wang, X.; Li, C.; Lin, J.; He, Q.; Leptihn, S.; Ouyang, H. Advanced hydrogels for the repair of cartilage defects and regeneration. Bioact. Mater. 2021, 6, 998–1011. [Google Scholar] [CrossRef]
- Djouad, F.; Bouffi, C.; Ghannam, S.; Noël, D.; Jorgensen, C. Mesenchymal stem cells: Innovative therapeutic tools for rheumatic diseases. Nat. Rev. Rheumatol. 2009, 5, 392–399. [Google Scholar] [CrossRef]
- Huey, D.J.; Hu, J.C.; Athanasiou, K.A. Unlike bone, cartilage regeneration remains elusive. Science 2012, 338, 917–921. [Google Scholar] [CrossRef]
- Wiggers, T.G.; Winters, M.; Van den Boom, N.A.; Haisma, H.J.; Moen, M.H. Autologous stem cell therapy in knee osteoarthritis: A systematic review of randomised controlled trials. Br. J. Sports Med. 2021, 55, 1161–1169. [Google Scholar] [CrossRef]
- Kirkley, A.; Birmingham, T.B.; Litchfield, R.B.; Giffin, J.R.; Willits, K.R.; Wong, C.J.; Feagan, B.G.; Donner, A.; Griffin, S.H.; D’Ascanio, L.M.; et al. A randomized trial of arthroscopic surgery for osteoarthritis of the knee. N. Engl. J. Med. 2008, 359, 1097–1107. [Google Scholar] [CrossRef]
- Bannuru, R.R.; Osani, M.C.; Vaysbrot, E.E.; Arden, N.K.; Bennell, K.; Bierma-Zeinstra, S.M.A.; Kraus, V.B.; Lohmander, L.S.; Abbott, J.H.; Bhandari, M.; et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1578–1589. [Google Scholar] [CrossRef]
- Sun, H.B. Mechanical loading, cartilage degradation, and arthritis. Ann. N. Y. Acad. Sci. 2010, 1211, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Summary for Patients: A Stepped Exercise Program for Patients With Knee Osteoarthritis. Ann. Intern. Med. 2021, 174, I15. [CrossRef] [PubMed]
- de Rooij, M.; van der Leeden, M.; Cheung, J.; van der Esch, M.; Häkkinen, A.; Haverkamp, D.; Roorda, L.D.; Twisk, J.; Vollebregt, J.; Lems, W.F.; et al. Efficacy of Tailored Exercise Therapy on Physical Functioning in Patients With Knee Osteoarthritis and Comorbidity: A Randomized Controlled Trial. Arthritis Care Res. 2017, 69, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Wellsandt, E.; Golightly, Y. Exercise in the management of knee and hip osteoarthritis. Curr. Opin. Rheumatol. 2018, 30, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Messier, S.P.; Mihalko, S.L.; Beavers, D.P.; Nicklas, B.J.; DeVita, P.; Carr, J.J.; Hunter, D.J.; Lyles, M.; Guermazi, A.; Bennell, K.L.; et al. Effect of High-Intensity Strength Training on Knee Pain and Knee Joint Compressive Forces Among Adults With Knee Osteoarthritis: The START Randomized Clinical Trial. JAMA 2021, 325, 646–657. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, I.; Matsuzaki, T.; Kuroki, H.; Hoso, M. Joint unloading inhibits articular cartilage degeneration in knee joints of a monosodium iodoacetate-induced rat model of osteoarthritis. Osteoarthr Cartilage 2019, 27, 1084–1093. [Google Scholar] [CrossRef]
- Cantero-Téllez, R.; Villafañe, J.H.; Valdes, K.; Berjano, P. Effect of immobilization of metacarpophalangeal joint in thumb carpometacarpal osteoarthritis on pain and function. A quasi-experimental trial. J. Hand Ther. 2018, 31, 68–73. [Google Scholar] [CrossRef]
- Cantero-Téllez, R.; Valdes, K.; Schwartz, D.A.; Medina-Porqueres, I.; Arias, J.C.; Villafañe, J.H. Necessity of Immobilizing the Metacarpophalangeal Joint in Carpometacarpal Osteoarthritis: Short-term Effect. Hand 2018, 13, 412–417. [Google Scholar] [CrossRef]
- Allen, K.D.; Woolson, S.; Hoenig, H.M.; Bongiorni, D.; Byrd, J.; Caves, K.; Hall, K.S.; Heiderscheit, B.; Hodges, N.J.; Huffman, K.M.; et al. Stepped Exercise Program for Patients With Knee Osteoarthritis: A Randomized Controlled Trial. Ann. Intern. Med. 2021, 174, 298–307. [Google Scholar] [CrossRef]
- Nelligan, R.K.; Hinman, R.S.; Kasza, J.; Crofts, S.J.C.; Bennell, K.L. Effects of a Self-directed Web-Based Strengthening Exercise and Physical Activity Program Supported by Automated Text Messages for People With Knee Osteoarthritis: A Randomized Clinical Trial. JAMA Intern. Med. 2021, 181, 776–785. [Google Scholar] [CrossRef]
- Videman, T. Experimental osteoarthritis in the rabbit: Comparison of different periods of repeated immobilization. Acta Orthop. Scand. 1982, 53, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Franco, M.R.; Morelhão, P.K.; de Carvalho, A.; Pinto, R.Z. Aquatic Exercise for the Treatment of Hip and Knee Osteoarthritis. Phys. Ther. 2017, 97, 693–697. [Google Scholar] [CrossRef] [PubMed]
- Boudenot, A.; Presle, N.; Uzbekov, R.; Toumi, H.; Pallu, S.; Lespessailles, E. Effect of interval-training exercise on subchondral bone in a chemically-induced osteoarthritis model. Osteoarthr. Cartil. 2014, 22, 1176–1185. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.M.; Brandt, K.D. Immobilization ameliorates chemically-induced articular cartilage damage. Arthritis Rheum. 1984, 27, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Lafeber, F.P.; Intema, F.; Van Roermund, P.M.; Marijnissen, A.C. Unloading joints to treat osteoarthritis, including joint distraction. Curr. Opin. Rheumatol. 2006, 18, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Tsai, L.C.; McLean, S.; Colletti, P.M.; Powers, C.M. Greater muscle co-contraction results in increased tibiofemoral compressive forces in females who have undergone anterior cruciate ligament reconstruction. J. Orthop. Res. 2012, 30, 2007–2014. [Google Scholar] [CrossRef] [PubMed]
- Boettger, M.K.; Leuchtweis, J.; Schaible, H.G.; Schmidt, M. Videoradiographic analysis of the range of motion in unilateral experimental knee joint arthritis in rats. Arthritis Res. Ther. 2011, 13, R79. [Google Scholar] [CrossRef]
- Cunha, J.E.; Barbosa, G.M.; Castro, P.; Luiz, B.L.F.; Silva, A.C.A.; Russo, T.L.; Vasilceac, F.A.; Cunha, T.M.; Cunha, F.Q.; Salvini, T.F. Knee osteoarthritis induces atrophy and neuromuscular junction remodeling in the quadriceps and tibialis anterior muscles of rats. Sci. Rep. 2019, 9, 6366. [Google Scholar] [CrossRef]
- Hsia, A.W.; Jbeily, E.H.; Mendez, M.E.; Cunningham, H.C.; Biris, K.K.; Bang, H.; Lee, C.A.; Loots, G.G.; Christiansen, B.A. Post-traumatic osteoarthritis progression is diminished by early mechanical unloading and anti-inflammatory treatment in mice. Osteoarthr. Cartil. 2021, 29, 1709–1719. [Google Scholar] [CrossRef]
- Langenskiöld, A.; Michelsson, J.E.; Videman, T. Osteoarthritis of the knee in the rabbit produced by immobilization. Attempts to achieve a reproducible model for studies on pathogenesis and therapy. Acta Orthop. Scand. 1979, 50, 1–14. [Google Scholar] [CrossRef]
- Ni, G.X.; Zhou, Y.Z.; Chen, W.; Xu, L.; Li, Z.; Liu, S.Y.; Lei, L.; Zhan, L.Q. Different responses of articular cartilage to strenuous running and joint immobilization. Connect. Tissue Res. 2016, 57, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, I.; Matsuzaki, T.; Kuroki, H.; Hoso, M. Disuse Atrophy of Articular Cartilage Induced by Unloading Condition Accelerates Histological Progression of Osteoarthritis in a Post-traumatic Rat Model. Cartilage 2021, 13, 1522S–1529S. [Google Scholar] [CrossRef] [PubMed]
- Holyoak, D.T.; Chlebek, C.; Kim, M.J.; Wright, T.M.; Otero, M.; van der Meulen, M.C.H. Low-level cyclic tibial compression attenuates early osteoarthritis progression after joint injury in mice. Osteoarthr. Cartil. 2019, 27, 1526–1536. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Li, X.; Li, J.; Wang, X.; Liu, D.; Zhai, L.; Ding, B.; Li, G.; Sun, Y.; Yokota, H.; et al. Mechanical loading mitigates osteoarthritis symptoms by regulating the inflammatory microenvironment in a mouse model. Ann. N. Y. Acad. Sci. 2022, 1512, 141–153. [Google Scholar] [CrossRef]
- Kijowski, R.; Demehri, S.; Roemer, F.; Guermazi, A. Osteoarthritis year in review 2019: Imaging. Osteoarthr. Cartil. 2020, 28, 285–295. [Google Scholar] [CrossRef]
- Chu, L.; Liu, X.; He, Z.; Han, X.; Yan, M.; Qu, X.; Li, X.; Yu, Z. Articular Cartilage Degradation and Aberrant Subchondral Bone Remodeling in Patients with Osteoarthritis and Osteoporosis. J. Bone Miner. Res. 2020, 35, 505–515. [Google Scholar] [CrossRef]
- Fahlgren, A.; Messner, K.; Aspenberg, P. Meniscectomy leads to an early increase in subchondral bone plate thickness in the rabbit knee. Acta Orthop. Scand. 2003, 74, 437–441. [Google Scholar] [CrossRef]
- Allen, J.; Imbert, I.; Havelin, J.; Henderson, T.; Stevenson, G.; Liaw, L.; King, T. Effects of Treadmill Exercise on Advanced Osteoarthritis Pain in Rats. Arthritis Rheumatol. 2017, 69, 1407–1417. [Google Scholar] [CrossRef]
- Zhang, J.; Hao, X.; Chi, R.; Qi, J.; Xu, T. Moderate mechanical stress suppresses the IL-1β-induced chondrocyte apoptosis by regulating mitochondrial dynamics. J. Cell. Physiol. 2021, 236, 7504–7515. [Google Scholar] [CrossRef]
- Zheng, W.; Ding, B.; Li, X.; Liu, D.; Yokota, H.; Zhang, P. Knee loading repairs osteoporotic osteoarthritis by relieving abnormal remodeling of subchondral bone via Wnt/β-catenin signaling. FASEB J. 2020, 34, 3399–3412. [Google Scholar] [CrossRef]
- Zhang, H.; Cai, D.; Bai, X. Macrophages regulate the progression of osteoarthritis. Osteoarthr. Cartil. 2020, 28, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Irie, K.; Uchiyama, E.; Iwaso, H. Intraarticular inflammatory cytokines in acute anterior cruciate ligament injured knee. Knee 2003, 10, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Kurz, B.; Lemke, A.K.; Fay, J.; Pufe, T.; Grodzinsky, A.J.; Schünke, M. Pathomechanisms of cartilage destruction by mechanical injury. Ann. Anat.-Anat. Anz. 2005, 187, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Yang, Y.X.; Li, J.; Fei, Y.; Guo, H.; Sun, Z.; Lu, J.; Xu, X.; Jiang, Q.; Ikegawa, S.; et al. Molecular Classification of Knee Osteoarthritis. Front. Cell Dev. Biol. 2021, 9, 725568. [Google Scholar] [CrossRef]
- Villafañe, J.H.; Valdes, K.; Pedersini, P.; Berjano, P. Osteoarthritis: A call for research on central pain mechanism and personalized prevention strategies. Clin. Rheumatol. 2019, 38, 583–584. [Google Scholar] [CrossRef]
- Andrew, T.W.; Koepke, L.S.; Wang, Y.; Lopez, M.; Steininger, H.; Struck, D.; Boyko, T.; Ambrosi, T.H.; Tong, X.; Sun, Y.; et al. Sexually dimorphic estrogen sensing in skeletal stem cells controls skeletal regeneration. Nat. Commun. 2022, 13, 1–13. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, B.; Liu, C.-j. Establishment of a Surgically-induced Model in Mice to Investigate the Protective Role of Progranulin in Osteoarthritis. J. Vis. Exp. 2014, 84, e50924. [Google Scholar] [CrossRef]
- Sutton, A.; Muir, K.; Mockett, S.; Fentem, P. A case-control study to investigate the relation between low and moderate levels of physical activity and osteoarthritis of the knee using data collected as part of the Allied Dunbar National Fitness Survey. Ann. Rheum. Dis. 2001, 60, 756–764. [Google Scholar] [CrossRef]
- You, J.S.; Anderson, G.B.; Dooley, M.S.; Hornberger, T.A. The role of mTOR signaling in the regulation of protein synthesis and muscle mass during immobilization in mice. Dis. Model. Mech. 2015, 8, 1059–1069. [Google Scholar] [CrossRef]
- Flis, D.J.; Dzik, K.; Kaczor, J.J.; Halon-Golabek, M.; Antosiewicz, J.; Wieckowski, M.R.; Ziolkowski, W. Swim Training Modulates Skeletal Muscle Energy Metabolism, Oxidative Stress, and Mitochondrial Cholesterol Content in Amyotrophic Lateral Sclerosis Mice. Oxidative Med. Cell. Longev. 2018, 2018, 5940748. [Google Scholar] [CrossRef]
- Walker, J.L.; Resig, P.; Guarnieri, S.; Sisken, B.F.; Evans, J.M. Improved footprint analysis using video recording to assess functional recovery following injury to the rat sciatic nerve. Restor. Neurol. Neurosci. 1994, 6, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Southwell, A.L.; Ko, J.; Patterson, P.H. Intrabody gene therapy ameliorates motor, cognitive, and neuropathological symptoms in multiple mouse models of Huntington’s disease. J. Neurosci. 2009, 29, 13589–13602. [Google Scholar] [CrossRef] [PubMed]
- Iijima, H.; Aoyama, T.; Ito, A.; Tajino, J.; Nagai, M.; Zhang, X.; Yamaguchi, S.; Akiyama, H.; Kuroki, H. Destabilization of the medial meniscus leads to subchondral bone defects and site-specific cartilage degeneration in an experimental rat model. Osteoarthr. Cartil. 2014, 22, 1036–1043. [Google Scholar] [CrossRef] [PubMed]
- Glasson, S.S.; Chambers, M.G.; Van Den Berg, W.B.; Little, C.B. The OARSI histopathology initiative—recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthr. Cartil. 2010, 18 (Suppl. 3), S17–S23. [Google Scholar] [CrossRef]

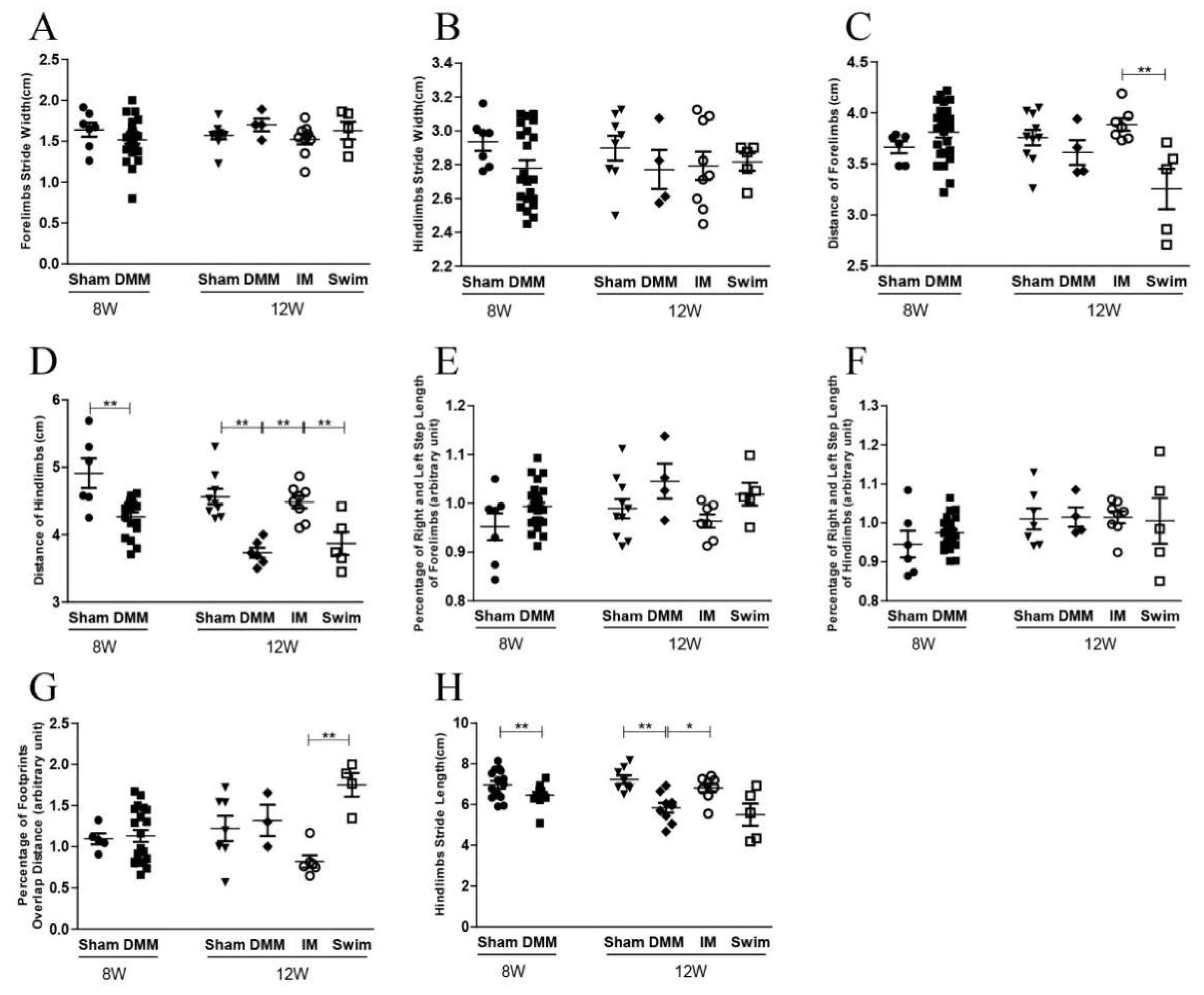

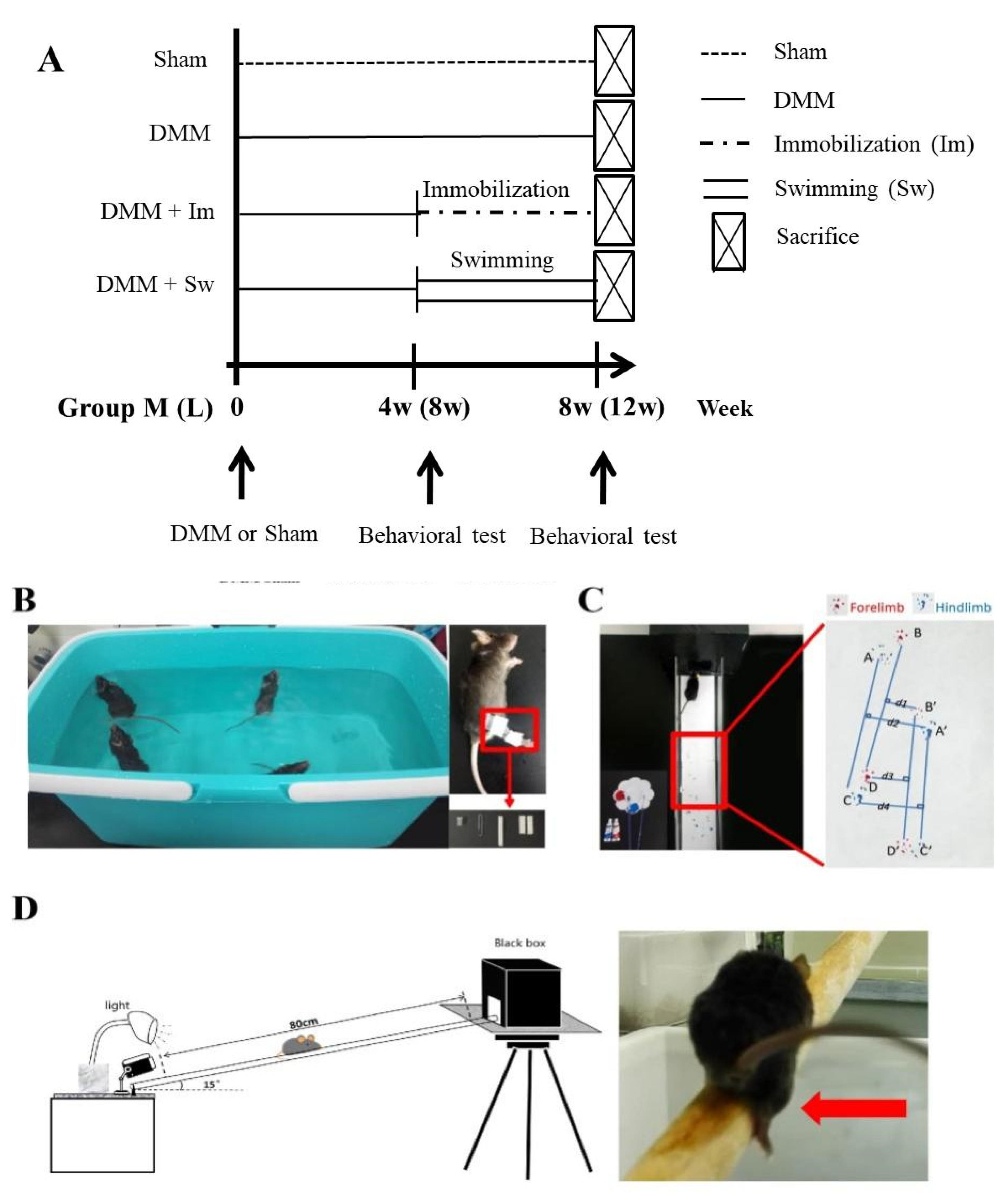
| CatWalk Parameter | Explanation |
|---|---|
| Stride length | Distance between the placement of a paw and the subsequent placement of the same paw, BD and B’D’ (Forelimb, F) or AC and A’C’ (Hindlimb, H) |
| Stride width | Vertical distance from the paw to the midperpendicular, (d1 + d3)/2 (F) or (d2 + d4)/2 (H) |
| Step distance | Distance between several continuous imprints, (BB’ + B’D + DD’)/2 (F) or (AA’ + A’C + CC’)/2 (H) |
| Percentage of right-to-left stride length | Percentage of average stride length of right to left paw, B’D’/BD × 100% (F) or A’C’/AC × 100% (H). If it was >1, right stride length was longer than left |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, T.; Ning, K.; Yang, B.; Dou, X.; Liu, S.; Wang, D.; Xu, H. Effects of Immobilization and Swimming on the Progression of Osteoarthritis in Mice. Int. J. Mol. Sci. 2023, 24, 535. https://doi.org/10.3390/ijms24010535
Xue T, Ning K, Yang B, Dou X, Liu S, Wang D, Xu H. Effects of Immobilization and Swimming on the Progression of Osteoarthritis in Mice. International Journal of Molecular Sciences. 2023; 24(1):535. https://doi.org/10.3390/ijms24010535
Chicago/Turabian StyleXue, Tong, Kaiting Ning, Baoqiang Yang, Xiangya Dou, Shuaiting Liu, Dongen Wang, and Huiyun Xu. 2023. "Effects of Immobilization and Swimming on the Progression of Osteoarthritis in Mice" International Journal of Molecular Sciences 24, no. 1: 535. https://doi.org/10.3390/ijms24010535
APA StyleXue, T., Ning, K., Yang, B., Dou, X., Liu, S., Wang, D., & Xu, H. (2023). Effects of Immobilization and Swimming on the Progression of Osteoarthritis in Mice. International Journal of Molecular Sciences, 24(1), 535. https://doi.org/10.3390/ijms24010535





