Existing and Novel Biomaterials for Bone Tissue Engineering
Abstract
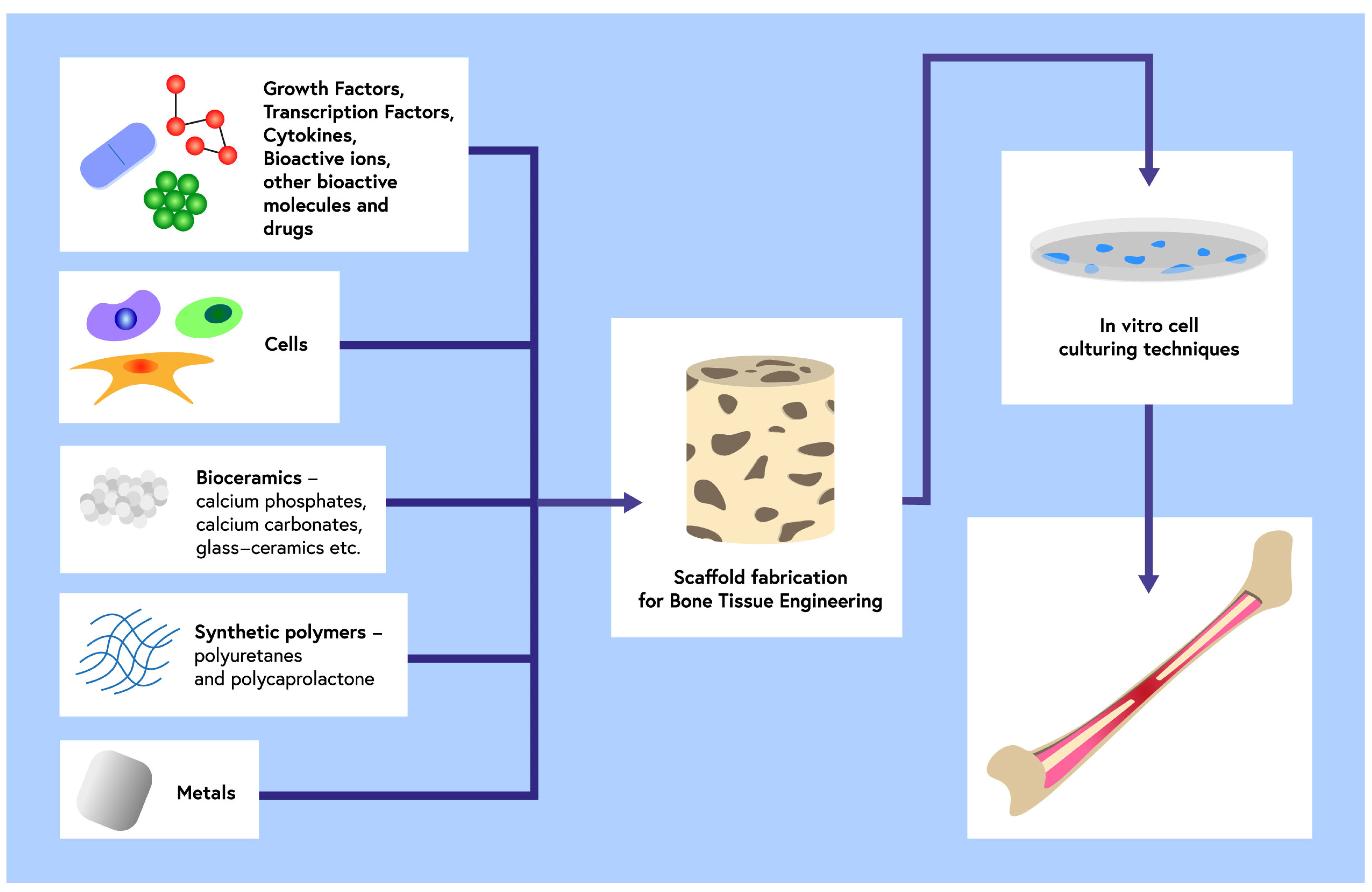
1. Introduction
2. Inorganic Materials
3. Natural Biomaterials
4. New Research Directions in the Search for Biomaterials for Bone Tissue Engineering
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Presbítero, G.; Gutiérrez, D.; Lemus-Martínez, W.R.; Vilchez, J.F.; García, P.; Arizmendi-Morquecho, A. Assessment of Quality in Osteoporotic Human Trabecular Bone and Its Relationship to Mechanical Properties. Appl. Sci. 2021, 11, 5479. [Google Scholar] [CrossRef]
- Pant, A.; Paul, E.; Niebur, G.L.; Vahdati, A. Integration of mechanics and biology in computer simulation of bone remodeling. Prog. Biophys. Mol. Biol. 2021, 164, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Merchán, E.C. Bone Healing Materials in the Treatment of Recalcitrant Nonunions and Bone Defects. Int. J. Mol. Sci. 2022, 23, 3352. [Google Scholar] [CrossRef] [PubMed]
- Schemitsch, E.H. Size Matters: Defining Critical in Bone Defect Size! J. Orthop. Trauma 2017, 31, S20–S22. [Google Scholar] [CrossRef] [PubMed]
- Bezstarosti, H.; Metsemakers, W.J.; van Lieshout, E.M.M.; Voskamp, L.W.; Kortram, K.; McNally, M.A.; Marais, L.C.; Verhofstad, M.H.J. Management of critical-sized bone defects in the treatment of fracture-related infection: A systematic review and pooled analysis. Arch. Orthop. Trauma Surg. 2021, 141, 1215–1230. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Lee, Y.-W.; Feng, L.; Wang, B.; Pan, Q.; Meng, X.; Cao, H.; Li, L.; Wang, H.; et al. Bioactive Scaffold Fabricated by 3D Printing for Enhancing Osteoporotic Bone Regeneration. Bioengineering 2022, 9, 525. [Google Scholar] [CrossRef]
- De Wildt, B.W.M.; Cramer, E.E.A.; de Silva, L.S.; Ito, K.; Gawlitta, D.; Hofmann, S. Evaluating material-driven regeneration in a tissue engineered human in vitro bone defect model. Bone 2022, 166, 116597. [Google Scholar] [CrossRef]
- Zhang, N.; Zhao, D.; Liu, N.; Wu, Y.; Yang, J.; Wang, Y.; Xie, H.; Ji, Y.; Zhou, C.; Zhuang, J.; et al. Assessment of the degradation rates and effectiveness of different coated Mg-Zn-Ca alloy scaffolds for in vivo repair of critical-size bone defects. J. Mater. Sci. Mater. Med. 2018, 29, 138. [Google Scholar] [CrossRef]
- Woodard, L.N.; Kmetz, K.T.; Roth, A.A.; Page, V.M.; Grunlan, M.A. Porous Poly(ε-caprolactone)-Poly(l-lactic acid) Semi-Interpenetrating Networks as Superior, Defect-Specific Scaffolds with Potential for Cranial Bone Defect Repair. Biomacromolecules 2017, 18, 4075–4083. [Google Scholar] [CrossRef]
- El-Rashidy, A.A.; Roether, J.A.; Harhaus, L.; Kneser, U.; Boccaccini, A.R. Regenerating bone with bioactive glass scaffolds: A review of in vivo studies in bone defect models. Acta Biomater. 2017, 62, 1–28. [Google Scholar] [CrossRef]
- Sovova, S.; Abalymov, A.; Pekar, M.; Skirtach, A.G.; Parakhonskiy, B. Calcium carbonate particles: Synthesis, temperature and time influence on the size, shape, phase, and their impact on cell hydroxyapatite formation. J. Mater. Chem. B 2021, 9, 8308–8320. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Singh, K. Review on titanium and titanium based alloys as biomaterials for orthopaedic applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 102, 844–862. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.; McFadden, R.; Chan, C.W.; Carson, L. Titanium for Orthopedic Applications: An Overview of Surface Modification to Improve Biocompatibility and Prevent Bacterial Biofilm Formation. iScience 2020, 23, 101745. [Google Scholar] [CrossRef]
- Asri, R.I.M.; Harun, W.S.W.; Samykano, M.; Lah, N.A.C.; Ghani, S.A.C.; Tarlochan, F.; Raza, M.R. Corrosion and surface modification on biocompatible metals: A review. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 77, 1261–1274. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef] [PubMed]
- Venkatraman, S.K.; Swamiappan, S. Review on calcium- and magnesium-based silicates for bone tissue engineering applications. J. Biomed. Mater. Res. A 2020, 108, 1546–1562. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Yang, Z.Y.; Tan, L.L.; Li, H.; Zhang, Y.Z. An animal experimental study of porous magnesium scaffold degradation and osteogenesis. Braz. J. Med. Biol. Res. 2014, 47, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Komaki, H.; Chazono, M.; Kitasato, S.; Kakuta, A.; Akiyama, S.; Marumo, K. Basic research and clinical application of beta-tricalcium phosphate (β-TCP). Morphologie 2017, 101, 164–172. [Google Scholar] [CrossRef]
- Qin, H.; Wei, Y.; Han, J.; Jiang, X.; Yang, X.; Wu, Y.; Gou, Z.; Chen, L. 3D printed bioceramic scaffolds: Adjusting pore dimension is beneficial for mandibular bone defects repair. J. Tissue Eng. Regen. Med. 2022, 16, 409–421. [Google Scholar] [CrossRef]
- Saidak, Z.; Marie, P.J. Strontium signaling: Molecular mechanisms and therapeutic implications in osteoporosis. Pharmacol. Ther. 2012, 136, 216–226. [Google Scholar] [CrossRef]
- Marx, D.; Yazdi, A.R.; Papini, M.; Towler, M. A review of the latest insights into the mechanism of action of strontium in bone. Bone Rep. 2020, 12, 100273. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Gong, W.; Li, F.; Xie, M. Pilose antler aqueous extract promotes the proliferation and osteogenic differentiation of bone marrow mesenchymal stem cells by stimulating the BMP-2/Smad1, 5/Runx2 signaling pathway. Chin. J. Nat. Med. 2019, 17, 756–767. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Feng, K.; Liu, X.; Zhu, Z.; Chen, H.; Chang, Y.; Sun, Z.; Wang, H.; Zhang, J.; Yu, D.; et al. Quercetin inhibits macrophage polarization through the p-38α/β signalling pathway and regulates OPG/RANKL balance in a mouse skull model. J. Cell Mol. Med. 2020, 24, 3203–3216. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.W.; Hsu, Y.W.; Pan, W.L.; Hsu, F.Y. The Effect of Strontium-Substituted Hydroxyapatite Nanofibrous Matrix on Osteoblast Proliferation and Differentiation. Membranes 2021, 11, 624. [Google Scholar] [CrossRef]
- Kuang, G.; Yau, W.P.; Wu, J.; Yeung, K.W.K.; Pan, H.; Lam, W.M.; Lu, W.W.; Chiu, K.Y. Strontium exerts dual effects on calcium phosphate cement: Accelerating the degradation and enhancing the osteoconductivity both in vitro and in vivo. J. Biomed. Mater. Res. A. 2015, 103, 1613–1621. [Google Scholar] [CrossRef]
- Boanini, E.; Torricelli, P.; Fini, M.; Bigi, A. Osteopenic bone cell response to strontium-substituted hydroxyapatite. J. Mater. Sci. Mater. Med. 2011, 22, 2079–2088. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Jang, Y.S.; Kim, Y.K.; Kim, S.Y.; Lee, M.H.; Bae, T.S. Osteogenesis-Related Gene Expression and Guided Bone Regeneration of a Strontium-Doped Calcium-Phosphate-Coated Titanium Mesh. ACS Biomater. Sci. Eng. 2019, 5, 6715–6724. [Google Scholar] [CrossRef]
- Kołodziejska, B.; Stępień, N.; Kolmas, J. The Influence of Strontium on Bone Tissue Metabolism and Its Application in Osteoporosis Treatment. Int. J. Mol. Sci. 2021, 22, 6564. [Google Scholar] [CrossRef]
- Geng, Z.; Wang, X.; Zhao, J.; Li, Z.; Ma, L.; Zhu, S.; Liang, Y.; Cui, Z.; He, H.; Yang, X. The synergistic effect of strontium-substituted hydroxyapatite and microRNA-21 on improving bone remodeling and osseointegration. Biomater. Sci. 2018, 6, 2694–2703. [Google Scholar] [CrossRef]
- Tao, Z.-S.; Zhou, W.-S.; He, X.-W.; Liu, W.; Bai, B.-L.; Zhou, Q.; Huang, Z.-L.; Tu, K.-K.; Li, H.; Sun, T.; et al. A comparative study of zinc, magnesium, strontium-incorporated hydroxyapatite-coated titanium implants for osseointegration of osteopenic rats. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 62, 226–232. [Google Scholar] [CrossRef]
- Liu, C.; Fu, X.; Pan, H.; Wan, P.; Wang, L.; Tan, L.; Wang, K.; Zhao, Y.; Yang, K.; Chu, P.K. Biodegradable Mg-Cu alloys with enhanced osteogenesis, angiogenesis, and long-lasting antibacterial effects. Sci. Rep. 2016, 6, 27374. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Yang, Y.; Yu, L.; Liu, K.; Fei, Y.; Guo, C.; Zhou, Y.; Hu, J.; Shi, L.; Ji, H. Inhibition of Inflammatory Response and Promotion of Osteogenic Activity of Zinc-Doped Micro-Arc Titanium Oxide Coatings. ACS Omega 2022, 7, 14920–14932. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhou, C.; Xie, Y.; Xu, A.; Guan, Y. Zinc- and strontium- co-incorporated nanorods on titanium surfaces with favorable material property, osteogenesis, and enhanced antibacterial activity. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 1754–1767. [Google Scholar] [CrossRef] [PubMed]
- Thian, E.S.; Konishi, T.; Kawanobe, Y.; Lim, P.N.; Choong, C.; Ho, B.; Aizawa, M. Zinc-substituted hydroxyapatite: A biomaterial with enhanced bioactivity and antibacterial properties. J. Mater. Sci. Mater. Med. 2013, 24, 437–445. [Google Scholar] [CrossRef]
- Andersen, O.Z.; Offermanns, V.; Sillassen, M.; Almtoft, K.P.; Andersen, I.H.; Sørensen, S.; Jeppesen, C.S.; Kraft, D.C.; Bøttiger, J.; Rasse, M.; et al. Accelerated bone ingrowth by local delivery of strontium from surface functionalized titanium implants. Biomaterials 2013, 34, 5883–5890. [Google Scholar] [CrossRef]
- Lee, M.; Arikawa, K.; Nagahama, F. Micromolar Levels of Sodium Fluoride Promote Osteoblast Differentiation through Runx2 Signaling. Biol. Trace Elem. Res. 2017, 178, 283–291. [Google Scholar] [CrossRef]
- Keppler, A.M.; Saller, M.M.; Alberton, P.; Westphal, I.; Heidenau, F.; Schönitzer, V.; Böcker, W.; Kammerlander, C.; Schieker, M.; Aszodi, A. Bone defect reconstruction with a novel biomaterial containing calcium phosphate and aluminum oxide reinforcement. J. Orthop. Surg. Res. 2020, 15, 287. [Google Scholar] [CrossRef]
- Echave, M.; Erezuma, I.; Golafshan, N.; Castilho, M.; Kadumudi, F.B.; Pimenta-Lopes, C.; Ventura, F.; Pujol, A.; Jimenez, J.; Camara, J.; et al. Bioinspired gelatin/bioceramic composites loaded with bone morphogenetic protein-2 (BMP-2) promote osteoporotic bone repair. Biomater. Adv. 2022, 134, 112539. [Google Scholar] [CrossRef]
- Vavken, J.; Mameghani, A.; Vavken, P.; Schaeren, S. Complications and cancer rates in spine fusion with recombinant human bone morphogenetic protein-2 (rhBMP-2). Eur. Spine J. 2016, 25, 3979–3989. [Google Scholar] [CrossRef]
- Köse, S.; Kankilic, B.; Gizer, M.; Dede, E.C.; Bayramli, E.; Korkusuz, P.; Korkusuz, F. Stem Cell and Advanced Nano Bioceramic Interactions. Adv. Exp. Med. Biol. 2018, 1077, 317–342. [Google Scholar]
- Xia, L.; Lin, K.; Jiang, X.; Fang, B.; Xu, Y.; Liu, J.; Zeng, D.; Zhang, M.; Zhang, X.; Chang, J.; et al. Effect of nano-structured bioceramic surface on osteogenic differentiation of adipose derived stem cells. Biomaterials 2014, 35, 8514–8527. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wu, C.; Lin, K.; Fan, W.; Chen, L.; Xiao, Y.; Chang, J. Biological responses of human bone marrow mesenchymal stem cells to Sr-M-Si (M = Zn, Mg) silicate bioceramics. J. Biomed. Mater. Res. A 2012, 100, 2979–2990. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Xia, L.; Chang, J.; Liu, J.; Jiang, L.; Wu, C.; Fang, B. The synergistic effects of Sr and Si bioactive ions on osteogenesis, osteoclastogenesis and angiogenesis for osteoporotic bone regeneration. Acta Biomater. 2017, 61, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Gromadzka, G.; Tarnacka, B.; Flaga, A.; Adamczyk, A. Copper Dyshomeostasis in Neurodegenerative Diseases-Therapeutic Implications. Int. J. Mol. Sci. 2020, 21, 9259. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Gao, S.; Zhang, Y.W.; Zhou, R.B.; Zhou, F. Antibacterial biomaterials in bone tissue engineering. J. Mater. Chem. B 2021, 9, 2594–2612. [Google Scholar] [CrossRef]
- Prajapati, N.; Karan, A.; Khezerlou, E.; DeCoster, M.A. The Immunomodulatory Potential of Copper and Silver Based Self-Assembled Metal Organic Biohybrids Nanomaterials in Cancer Theranostics. Front. Chem. 2021, 8, 629835. [Google Scholar] [CrossRef]
- Fiore, M.; Bruschi, A.; Giannini, C.; Morante, L.; Rondinella, C.; Filippini, M.; Sambri, A.; De Paolis, M. Is Silver the New Gold? A Systematic Review of the Preclinical Evidence of Its Use in Bone Substitutes as Antiseptic. Antibiotics 2022, 11, 995. [Google Scholar] [CrossRef]
- Kim, Y.J.; Saiki, C.E.T.; Silva, K.; Massuda, C.K.M.; De Souza Faloni, A.P.; Braz-Silva, P.H.; Pallos, D.; Sendyk, W.R. Bone Formation in Grafts with Bio-Oss and Autogenous Bone at Different Proportions in Rabbit Calvaria. Int. J. Dent. 2020, 2020, 2494128. [Google Scholar] [CrossRef]
- Ma, X.J.; Bian, Y.F.; Wu, D.; Chen, N.; Wang, L.J. A comparative study of two kinds of artificial bone powder for tooth extraction site preservation. Oral Biomed. 2019, 10, 139–142. [Google Scholar]
- Xu, M.Y.; Liu, Y.H.; Wang, P.S.; Hu, Y.C. Research progress of bioactive glass-based bone repair materials. Chin. J. Orthop. 2019, 12, 440–448. [Google Scholar]
- Jin, S.Y.; Kim, S.G.; Oh, J.S.; You, J.S.; Lim, S.C.; Jeong, M.A.; Kim, J.S. Histomorphometric Analysis of Contaminated Autogenous Tooth Graft Materials after Various Sterilization. Implant Dent. 2016, 25, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S. Autogenous fresh demineralized tooth graft prepared at chairside for dental implant. Maxillofac. Plast. Reconstr. Surg. 2015, 37, 8. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yadav, N.; Srivastava, P. Osteoblast studied on gelatin based biomaterials in rabbit Bone Bioengineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109892. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Mi, H.Y.; Peng, J.; Peng, X.F.; Turng, L.S. Electrospun aligned poly(propylene carbonate) microfibers with chitosan nanofibers as tissue engineering scaffolds. Carbohydr. Polym. 2015, 117, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Pangon, A.; Saesoo, S.; Saengkrit, N.; Ruktanonchai, U.; Intasanta, V. Multicarboxylic acids as environment-friendly solvents and in situ crosslinkers for chitosan/PVA nanofibers with tunable physicochemical properties and biocompatibility. Carbohydr. Polym. 2016, 138, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Januariyasa, I.K.; Ana, I.D.; Yusuf, Y. Nanofibrous poly(vinyl alcohol)/chitosan contained carbonated hydroxyapatite nanoparticles scaffold for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 107, 110347. [Google Scholar] [CrossRef] [PubMed]
- Adamski, R.; Siuta, D. Mechanical, Structural, and Biological Properties of Chitosan/Hydroxyapatite/Silica Composites for Bone Tissue Engineering. Molecules 2021, 26, 1976. [Google Scholar] [CrossRef]
- Saric, M.; Scheibel, T. Engineering of silk proteins for materials applications. Curr. Opin. Biotechnol. 2019, 60, 213–220. [Google Scholar] [CrossRef]
- Sofi, H.S.; Ashraf, R.; Beigh, M.A.; Sheikh, F.A. Scaffolds Fabricated from Natural Polymers/Composites by Electrospinning for Bone Tissue Regeneration. Adv. Exp. Med. Biol. 2018, 1078, 49–78. [Google Scholar]
- Belbéoch, C.; Lejeune, J.; Vroman, P.; Salaün, F. Silkworm and spider silk electrospinning: A review. Environ. Chem. Lett. 2021, 19, 1737–1763. [Google Scholar] [CrossRef]
- Wittmer, C.R.; Hu, X.; Gauthier, P.C.; Weisman, S.; Kaplan, D.L.; Sutherland, T.D. Production, structure and in vitro degradation of electrospun honeybee silk nanofibers. Acta Biomater. 2011, 7, 3789–3795. [Google Scholar] [CrossRef] [PubMed]
- Unalan, I.; Colpankan, O.; Albayrak, A.Z.; Gorgun, C.; Urkmez, A.S. Biocompatibility of plasma-treated poly(3-hydroxybutyrate-co-3-hydroxyvalerate) nanofiber mats modified by silk fibroin for bone tissue regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 68, 842–850. [Google Scholar] [CrossRef]
- Bhattacharjee, P.; Naskar, D.; Maiti, T.K.; Bhattacharya, D.; Kundu, S.C. Non-mulberry silk fibroin grafted poly (ɛ-caprolactone)/nano hydroxyapatite nanofibrous scaffold for dual growth factor delivery to promote bone regeneration. J. Colloid Interface Sci. 2016, 472, 16–33. [Google Scholar] [CrossRef] [PubMed]
- Zulkifli, F.H.; Hussain, F.S.J.; Harun, W.S.W.; Yusoff, M.M. Highly porous of hydroxyethyl cellulose biocomposite scaffolds for tissue engineering. Int. J Biol. Macromol. 2019, 122, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Ao, C.; Niu, Y.; Zhang, X.; He, X.; Zhang, W.; Lu, C. Fabrication and characterization of electrospun cellulose/nano-hydroxyapatite nanofibers for bone tissue engineering. Int. J. Biol. Macromol. 2017, 97, 568–573. [Google Scholar] [CrossRef]
- Patil, P.P.; Reagan, M.R.; Bohara, R.A. Silk fibroin and silk-based biomaterial derivatives for ideal wound dressings. Int. J. Biol. Macromol. 2020, 164, 4613–4627. [Google Scholar] [CrossRef]
- Janoušková, O. Synthetic polymer scaffolds for soft tissue engineering. Physiol. Res. 2018, 67, S335–S348. [Google Scholar] [CrossRef]
- Kalteis, T.; Lüring, C.; Gugler, G.; Zysk, S.; Caro, W.; Handel, M.; Grifka, J. Acute tissue toxicity of PMMA bone cements. Z. Orthop. Ihre Grenzgeb. 2004, 142, 666–672. [Google Scholar] [CrossRef]
- Hoess, A.; López, A.; Engqvist, H.; Ott, M.K.; Persson, C. Comparison of a quasi-dynamic and a static extraction method for the cytotoxic evaluation of acrylic bone cements. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 62, 274–282. [Google Scholar] [CrossRef]
- Prabha, R.D.; Kraft, D.C.E.; Harkness, L.; Melsen, B.; Varma, H.; Nair, P.D.; Kjems, J.; Kassem, M. Bioactive nano-fibrous scaffold for vascularized craniofacial bone regeneration. J. Tissue Eng. Regen. Med. 2018, 12, e1537–e1548. [Google Scholar] [CrossRef]
- Teodorescu, M.; Bercea, M.; Morariu, S. Biomaterials of PVA and PVP in medical and pharmaceutical applications: Perspectives and challenges. Biotechnol. Adv. 2019, 37, 109–131. [Google Scholar] [CrossRef] [PubMed]
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Markel, D.C.; Wang, S.; Shi, T.; Mao, G.; Ren, W. Electrospun polyvinyl alcohol-collagen-hydroxyapatite nanofibers: A biomimetic extracellular matrix for osteoblastic cells. Nanotechnology 2012, 23, 115101. [Google Scholar] [CrossRef] [PubMed]
- Kwak, S.; Haider, A.; Gupta, K.C.; Kim, S.; Kang, I.K. Micro/Nano Multilayered Scaffolds of PLGA and Collagen by Alternately Electrospinning for Bone Tissue Engineering. Nanoscale Res. Lett. 2016, 11, 323. [Google Scholar] [CrossRef]
- Liao, H.; Qi, R.; Shen, M.; Cao, X.; Guo, R.; Zhang, Y.; Shi, X. Improved cellular response on multiwalled carbon nanotube-incorporated electrospun polyvinyl alcohol/chitosan nanofibrous scaffolds. Colloids Surf. B Biointerfaces 2011, 84, 528–535. [Google Scholar] [CrossRef]
- Talebian, S.; Foroughi, J.; Wade, S.J.; Cao, X.; Guo, R.; Zhang, Y.; Shi, X. Biopolymers for Antitumor Implantable Drug Delivery Systems: Recent Advances and Future Outlook. Adv. Mater. 2018, 30, e1706665. [Google Scholar] [CrossRef]
- Dias, J.R.; Sousa, A.; Augusto, A.; Bártolo, P.J.; Granja, P.L. Electrospun Polycaprolactone (PCL) Degradation: An In Vitro and In Vivo Study. Polymers 2022, 14, 3397. [Google Scholar] [CrossRef]
- Bazgir, M.; Zhang, W.; Zhang, X.; Elies, J.; Saeinasab, M.; Coates, P.; Youseffi, M.; Sefat, F. Degradation and Characterisation of Electrospun Polycaprolactone (PCL) and Poly(lactic-co-glycolic acid) (PLGA) Scaffolds for Vascular Tissue Engineering. Materials 2021, 14, 4773. [Google Scholar] [CrossRef]
- Ren, K.; Wang, Y.; Sun, T.; Yue, W.; Zhang, H. Electrospun PCL/gelatin composite nanofiber structures for effective guided bone regeneration membranes. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 324–332. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, B.; Sun, R.; Liu, W.; Zhu, Q.; Zhang, X.; Wang, R.; Chen, C. PLGA-based biodegradable microspheres in drug delivery: Recent advances in research and application. Drug Deliv. 2021, 28, 1397–1418. [Google Scholar] [CrossRef]
- Beenken, K.E.; Campbell, M.J.; Ramirez, A.M.; Alghazali, K.; Walker, C.M.; Jackson, B.; Griffin, C.; King, W.; Bourdo, S.E.; Rifkin, R.; et al. Evaluation of a bone filler scaffold for local antibiotic delivery to prevent Staphylococcus aureus infection in a contaminated bone defect. Sci. Rep. 2021, 11, 10254. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chen, C.; Liu, W.; He, X.; Zhou, N.; Zhang, D.; Gu, H.; Li, J.; Jiang, J.; Huang, W. Levofloxacin loaded mesoporous silica microspheres/nano-hydroxyapatite/polyurethane composite scaffold for the treatment of chronic osteomyelitis with bone defects. Sci. Rep. 2017, 7, 41808. [Google Scholar] [CrossRef] [PubMed]
- Lawrie, C.M.; Jo, S.; Barrack, T.; Roper, S.; Wright, R.W.; Nunley, R.M.; Barrack, R.L. Local delivery of tobramycin and vancomycin in primary total knee arthroplasty achieves minimum inhibitory concentrations for common bacteria causing acute prosthetic joint infection. Bone Jt. J. 2020, 102, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Masters, E.A.; Ricciardi, B.F.; Bentley, K.L.D.M.; Moriarty, T.F.; Schwarz, E.M.; Muthukrishnan, G. Skeletal infections: Microbial pathogenesis, immunity and clinical management. Nat. Rev. Microbiol. 2022, 20, 385–400. [Google Scholar] [CrossRef] [PubMed]
- Maffulli, N.; Papalia, R.; Zampogna, B.; Torre, G.; Albo, E.; Denaro, V. The management of osteomyelitis in the adult. Surgeon 2016, 14, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.; Kumar, A. Biobased technologies for the efficient extraction of biopolymers from waste biomass. Bioprocess Biosyst. Eng. 2019, 42, 1893–1901. [Google Scholar] [CrossRef]
- Bharadwaz, A.; Jayasuriya, A.C. Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110698. [Google Scholar] [CrossRef]
- Shen, R.; Xu, W.; Xue, Y.; Chen, L.; Ye, H.; Zhong, E.; Ye, Z.; Gao, J.; Yan, Y. The use of chitosan/PLA nano-fibers by emulsion eletrospinning for periodontal tissue engineering. Artif. Cells Nanomed. Biotechnol. 2018, 46, 419–430. [Google Scholar] [CrossRef]
- Chae, T.; Yang, H.; Ko, F.; Troczynski, T. Bio-inspired dicalcium phosphate anhydrate/poly(lactic acid) nanocomposite fibrous scaffolds for hard tissue regeneration: In situ synthesis and electrospinning. J. Biomed. Mater. Res. A 2014, 102, 514–522. [Google Scholar] [CrossRef]
- Qiao, S.; Wu, D.; Li, Z.; Zhu, Y.; Zhan, F.; Lai, H.; Gu, Y. The combination of multi-functional ingredients-loaded hydrogels and three-dimensional printed porous titanium alloys for infective bone defect treatment. J. Tissue Eng. 2020, 11, 2041731420965797. [Google Scholar] [CrossRef]
- Kankala, R.K.; Xu, X.-M.; Liu, C.-G.; Chen, A.-Z.; Wang, S.-B. 3D-Printing of Microfibrous Porous Scaffolds Based on Hybrid Approaches for Bone Tissue Engineering. Polymers 2018, 10, 807. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Liu, Z.; Liu, Y.; Yu, J.; Wang, X.; Tan, Z.; Ye, X. Recent Trends in the Development of Bone Regenerative Biomaterials. Front. Cell Dev. Biol. 2021, 9, 665813. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Wang, X.; Xiao, G.; Zhao, Z.; Zou, S.; Li, M.; Richardson, J.J.; Tardy, B.L.; Xie, L.; Komasa, S.; et al. Hierarchical assembly of nanostructured coating for siRNA-based dual therapy of bone regeneration and revascularization. Biomaterials 2020, 235, 119784. [Google Scholar] [CrossRef] [PubMed]
| Biopolymers | Metals | Bioceramics | |
|---|---|---|---|
| Type of connections | Covalent or van der Walls | Metallic | Ionic/covalent |
| Chemical stability | Weak | Good | Very good |
| Electrical conductivity | Very low | High | Very low |
| Thermal conductivity | Very low | High | Low |
| Characteristics and advantages | Degradable, similar density to soft tissue, and easy to process | High hardness and strength | Non-conductive and biologically inert; optimally imitate the properties of bone |
| Mechanical strength | Very high strength and plasticity (easy to shape and process) | Resistance to stretching | Brittle and fragile |
| Main disadvantages | Thermally unstable and low strength | Wear and corrosion | High density and brittleness |
| Biomedical applications | Soft tissue implants, drug delivery systems, and tissue engineering | Orthopaedic and dental implants | Tissue engineering |
| Yield Strength [MPa] | Compressive Strength [MPa] | Tensile Strength [MPa] | Young’s Modulus [GPa] | Flexural Strength [MPa] | Density [g/cm3] | |
|---|---|---|---|---|---|---|
| Cancellous bone | 2–20 | 01–2 | 15.8 | 1.0–1.4 | ||
| Cortical bone | 148 | 100–200 | 50–151 | 5–20 | ≥160 | 1.80–2.10 |
| Alloy TiAl6Nb7 | ≥800 | 1074–1086 | ≥900 | ≥105 | 895–905 | 4.51 |
| Alloy Ti6Al4V | ≥795 | 450–1850 | ≥860 | ≥100 | 4.05 | |
| Polyethylene | ≥21 | 38–49 | ≥3 | 1.6–1.8 | 0.942–0.965 | |
| Mg Alloys | 65–100 | 170–270 | 41–45 | 1.45–2.0 | ||
| Aluminum oxide ceramic Al2O3 | 150 | ≥500 | ≥380 | 400 | 3.94 | |
| Alloy Mg-Zn | 67–169.5 | 42.3–45.3 | 1.7–2.0 | |||
| Hydroxyapatite HaP | 500–1000 | 40–300 | 80–120 | 38–250 | 3.10 | |
| Porous hydroxyapatite 82–86% | 120–900 | 3 | 0.83–1.6 × 10−3 | 2–11 | ||
| Hydroxycellulose hydrogel | 32 | 3.5 | 2.23 | |||
| Bioglass | 800–1200 | 42 | 40–140 | 1.8–29 | ||
| PEEK | ≥90 | 80 | ≥100 | 3–4 | ≥150 | 1.3 |
| PLA | 4–25 | 50–70 | 2.4 | 1.25 | ||
| PLGA | 2.90–4.19 | 41.4–55.2 | 1.4–2.08 | |||
| PCL | 20.7–34.5 | 0.21–0.35 | 1.11 | |||
| Chitosan fibers | 9.0–16.0 | 250–380 | ||||
| PLA/chitosan | 47.1 | 44.5 | 156.9 | |||
| Silk | 500–740 | 5–17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dec, P.; Modrzejewski, A.; Pawlik, A. Existing and Novel Biomaterials for Bone Tissue Engineering. Int. J. Mol. Sci. 2023, 24, 529. https://doi.org/10.3390/ijms24010529
Dec P, Modrzejewski A, Pawlik A. Existing and Novel Biomaterials for Bone Tissue Engineering. International Journal of Molecular Sciences. 2023; 24(1):529. https://doi.org/10.3390/ijms24010529
Chicago/Turabian StyleDec, Paweł, Andrzej Modrzejewski, and Andrzej Pawlik. 2023. "Existing and Novel Biomaterials for Bone Tissue Engineering" International Journal of Molecular Sciences 24, no. 1: 529. https://doi.org/10.3390/ijms24010529
APA StyleDec, P., Modrzejewski, A., & Pawlik, A. (2023). Existing and Novel Biomaterials for Bone Tissue Engineering. International Journal of Molecular Sciences, 24(1), 529. https://doi.org/10.3390/ijms24010529






