The γ-Core Motif Peptides of Plant AMPs as Novel Antimicrobials for Medicine and Agriculture
Abstract
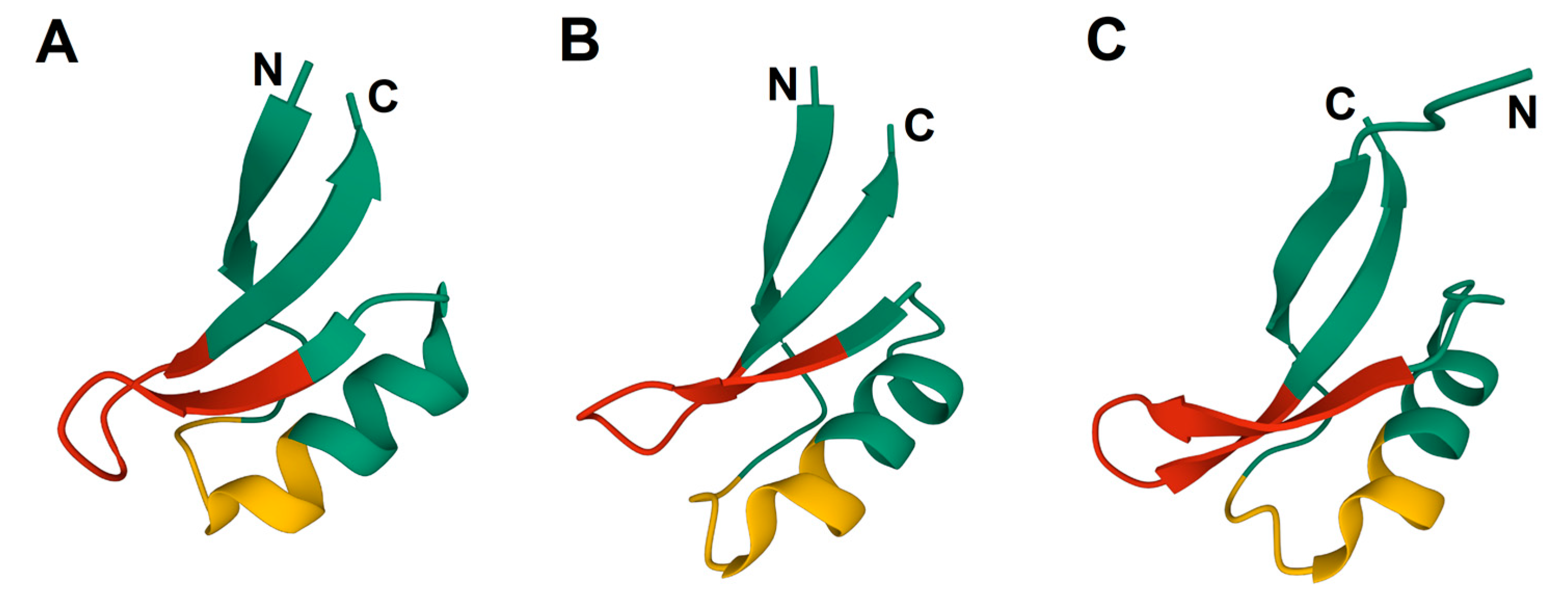
1. Introduction
2. γ-Core Motifs of Defensins
2.1. Radish Defensins Rs-AFP1 and Rs-AFP2 (Brassicaceae)
2.2. Brassica Hybrid Defensins BhDef1 and BhDef2 (Brassicaceae)
2.3. MsDef1 and MtDef4 (Fabaceae)
2.4. MtDef5 (Fabaceae)
2.5. PvD1 (Fabaceae)
2.6. VuDef1 (Fabaceae)
2.7. DefSm2-D (Asteraceae)
2.8. Atr-DEF2 (Amaranthaceae)
2.9. Spinach So-D2 (Amaranthaceae)
2.10. BcDef (Solanaceae)
2.11. Tomato SolyC07g007760 (Solanaceae)
2.12. Tomato SlDEFL2 and SlDEFL4 (Solanaceae)
2.13. HsAFP1 (Saxifragaceae)
2.14. Rice OsAFP1 (Poaceae)
2.15. Maize ZmESs (Poaceae)
2.16. Wheat TkDEFLs (Poaceae)
2.17. Olive tree OefDef1.1 (Oleaceae)
3. Non-Defensin γ-Cores
4. Discussion
4.1. Residues within γ-Core Sequences Responsible for Antimicrobial Activity
4.2. The Role of Net Charge in Antimicrobial Activity of γ-Core Peptides
4.3. The Role of Peptide’s Length and 3D Structure in Antimicrobial Activity
4.4. The Role of Cysteine Residues in Activity
4.5. The Mode of Action
4.6. Synergism with Antimycotics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Bosch, T.C.G.; Zasloff, M. Antimicrobial Peptides-or how our ancestors learned to control the microbiome. mBio 2021, 12, e0184721. [Google Scholar] [CrossRef] [PubMed]
- Lazzaro, B.P.; Zasloff, M.; Rolff, J. Antimicrobial peptides: Application informed by evolution. Science 2020, 368, eaau5480. [Google Scholar] [CrossRef]
- Sarkar, T.; Chetia, M.; Chatterjee, S. Antimicrobial peptides and proteins: From nature’s reservoir to the laboratory and beyond. Front. Chem. 2021, 9, 691532. [Google Scholar] [CrossRef] [PubMed]
- Park, S.C.; Park, Y.; Hahm, K.S. The role of antimicrobial peptides in preventing multidrug-resistant bacterial infections and biofilm formation. Int. J. Mol. Sci. 2011, 12, 5971–5992. [Google Scholar] [CrossRef] [PubMed]
- Feurstein, C.; Meyer, V.; Jung, S. Structure–activity predictions from computational mining of protein databases to assist modular design of antimicrobial peptides. Front. Microbiol. 2022, 13, 812903. [Google Scholar] [CrossRef]
- Tam, J.P.; Wang, S.; Wong, K.H.; Tan, W.L. Antimicrobial peptides from plants. Pharmaceuticals 2015, 8, 711–757. [Google Scholar] [CrossRef]
- Yount, N.Y.; Yeaman, M.R. Multidimensional signatures in antimicrobial peptides. Proc. Natl. Acad. Sci. USA 2004, 101, 7363–7368. [Google Scholar] [CrossRef]
- Sagaram, U.S.; Pandurangi, R.; Kaur, J.; Smith, T.J.; Shah, D.M. Structure-activity determinants in antifungal plant defensins MsDef1 and MtDef4 with different modes of action against Fusarium graminearum. PLoS ONE 2011, 6, e18550. [Google Scholar] [CrossRef]
- Li, H.; Velivelli, S.L.S.; Shah, D.M. Antifungal potency and modes of action of a novel olive tree defensin against closely related ascomycete fungal pathogens. Mol. Plant. Microbe Interact. 2019, 32, 1649–1664. [Google Scholar] [CrossRef]
- Parisi, K.; Shafee, T.M.A.; Quimbar, P.; van der Weerden, N.L.; Bleackley, M.R.; Anderson, M. The evolution, function and mechanisms of action for plant defensins. Semin. Cell Dev. Biol. 2019, 88, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Odintsova, T.I.; Slezina, M.P.; Istomina, E.A. Defensins of grasses: A systematic review. Biomolecules 2020, 10, 1029. [Google Scholar] [CrossRef] [PubMed]
- Thomma, B.P.; Cammue, B.P.; Thevissen, K. Plant defensins. Planta 2002, 216, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Lay, F.T.; Anderson, M.A. Defensins-components of the innate immune system in plants. Curr. Protein Pept. Sci. 2005, 6, 85–101. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.O.; Gomes, V.M. Plant defensins–prospects for the biological functions and biotechnological properties. Peptides 2009, 30, 1007–1020. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.O.; Gomes, V.M. Plant defensins and defensin-like peptides - biological activities and biotechnological applications. Curr. Pharm. Des. 2011, 17, 4270–4293. [Google Scholar] [CrossRef] [PubMed]
- Terras, F.R.; Schoofs, H.M.; De Bolle, M.F.; Van Leuven, F.; Rees, S.B.; Vanderleyden, J.; Cammue, B.P.; Broekaert, W.F. Analysis of two novel classes of plant antifungal proteins from radish (Raphanus sativus L.) seeds. J. Biol. Chem. 1992, 267, 15301–15309. [Google Scholar] [CrossRef]
- Thevissen, K.; de Mello Tavares, P.; Xu, D.; Blankenship, J.; Vandenbosch, D.; Idkowiak-Baldys, J.; Govaert, G.; Bink, A.; Rozental, S.; de Groot, P.W.; et al. The plant defensin RsAFP2 induces cell wall stress, septin mislocalization and accumulation of ceramides in Candida albicans. Mol. Microbiol. 2012, 84, 166–180. [Google Scholar] [CrossRef]
- Vriens, K.; Cools, T.L.; Harvey, P.J.; Craik, D.J.; Braem, A.; Vleugels, J.; De Coninck, B.; Cammue, B.P.; Thevissen, K. The radish defensins RsAFP1 and RsAFP2 act synergistically with caspofungin against Candida albicans biofilms. Peptides 2016, 75, 71–79. [Google Scholar] [CrossRef]
- De Samblanx, G.W.; Goderis, I.J.; Thevissen, K.; Raemaekers, R.; Fant, F.; Borremans, F.; Acland, D.P.; Osborn, R.W.; Patel, S.; Broekaert, W.F. Mutational analysis of a plant defensin from radish (Raphanus sativus L.) reveals two adjacent sites important for antifungal activity. J. Biol. Chem. 1997, 272, 1171–1179. [Google Scholar] [CrossRef]
- De Samblanx, G.W.; Fernandez del Carmen, A.; Sijtsma, L.; Plasman, H.H.; Schaaper, W.M.; Posthuma, G.A.; Fant, F.; Meloen, R.H.; Broekaert, W.F.; van Amerongen, A. Antifungal activity of synthetic 15-mer peptides based on the Rs-AFP2 (Raphanus sativus antifungal protein 2) sequence. Pept. Res. 1996, 9, 262–268. [Google Scholar] [PubMed]
- Schaaper, W.M.; Posthuma, G.A.; Plasman, H.H.; Sijtsma, L.; Fant, F.; Borremans, F.A.; Thevissen, K.; Broekaert, W.F.; Meloen, R.H.; van Amerongen, A. Synthetic peptides derived from the β2-β3 loop of Raphanus sativus antifungal protein 2 that mimic the active site. J. Pept. Res. 2001, 57, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Sathoff, A.E.; Velivelli, S.; Shah, D.M.; Samac, D.A. Plant Defensin peptides have antifungal and antibacterial activity against human and plant pathogens. Phytopathology 2019, 109, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Pandey, B.; Tyagi, C.; Prajapati, G.K.; Mishra, A.K.; Hashem, A.; Alqarawi, A.A.; Abd Allah, E.F.; Mohanta, T.K. Analysis of mutations of defensin protein using accelerated molecular dynamics simulations. PLoS ONE 2020, 15, e0241679. [Google Scholar] [CrossRef] [PubMed]
- Kaewklom, S.; Euanorasetr, J.; Intra, B.; Panbangred, W.; and Aunpad, R. Antimicrobial activities of novel peptides derived from defensin genes of Brassica hybrid cv Pule. Int. J. Pept. Res. Ther. 2016, 22, 93–100. [Google Scholar] [CrossRef]
- Muñoz, A.; Chu, M.; Marris, P.I.; Sagaram, U.S.; Kaur, J.; Shah, D.M.; Read, N.D. Specific domains of plant defensins differentially disrupt colony initiation, cell fusion and calcium homeostasis in Neurospora crassa. Mol. Microbiol. 2014, 92, 1357–1374. [Google Scholar] [CrossRef]
- Sathoff, A.E.; Lewenza, S.; Samac, D.A. Plant defensin antibacterial mode of action against Pseudomonas species. BMC Microbiol. 2020, 20, 173. [Google Scholar] [CrossRef]
- Sagaram, U.S.; El-Mounadi, K.; Buchko, G.W.; Berg, H.R.; Kaur, J.; Pandurangi, R.S.; Smith, T.J.; Shah, D.M. Structural and functional studies of a phosphatidic acid-binding antifungal plant defensin MtDef4: Identification of an RGFRRR motif governing fungal cell entry. PLoS ONE 2013, 8, e82485. [Google Scholar] [CrossRef]
- Islam, K.T.; Velivelli, S.L.S.; Berg, R.H.; Oakley, B.; Shah, D.M. A novel bi-domain plant defensin MtDef5 with potent broad-spectrum antifungal activity binds to multiple phospholipids and forms oligomers. Sci. Rep. 2017, 7, 16157. [Google Scholar] [CrossRef]
- Velivelli, S.L.S.; Islam, K.T.; Hobson, E.; Shah, D.M. Modes of action of a bi-domain plant defensin MtDef5 against a bacterial pathogen Xanthomonas campestris. Front. Microbiol. 2018, 9, 934. [Google Scholar] [CrossRef]
- Mello, É.O.; Taveira, G.B.; Carvalho, A.O.; Gomes, V.M. Improved smallest peptides based on positive charge increase of the γ-core motif from PvD1 and their mechanism of action against Candida species. Int. J. Nanomed. 2019, 14, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Toledo, E.B.; Lucas, D.R.; Simão, T.L.B.V.; Calixto, S.D.; Lassounskaia, E.; Muzitano, M.F.; Damica, F.Z.; Gomes, V.M.; de Oliveira Carvalho, A. Design of improved synthetic antifungal peptides with targeted variations in charge, hydrophobicity and chirality based on a correlation study between biological activity and primary structure of plant defensin γ-cores. Amino Acids 2021, 53, 219–237. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.O.; Machado, O.L.T.; Da Cunha, M.; Santos, I.S.; Gomes, V.M. Antimicrobial peptides and immunolocalization of a LTP in Vigna unguiculata seeds. Plant Physiol. Biochem. 2001, 39, 137–146. [Google Scholar] [CrossRef]
- Souza, G.S.; de Carvalho, L.P.; de Melo, E.J.T.; Gomes, V.M.; Carvalho, A.O. The toxic effect of Vu-Defr, a defensin from Vigna unguiculata seeds, on Leishmania amazonensis is associated with reactive oxygen species production, mitochondrial dysfunction, and plasma membrane perturbation. Can. J. Microbiol. 2018, 64, 455–464. [Google Scholar] [CrossRef]
- Dos Santos, I.S.; Carvalho, A.O.; de Souza-Filho, G.A.; do Nascimento, V.V.; Machado, O.L.; Gomes, V.M. Purification of a defensin isolated from Vigna unguiculata seeds, its functional expression in Escherichia coli, and assessment of its insect α-amylase inhibitory activity. Protein Expr. Purif. 2010, 71, 8–15. [Google Scholar] [CrossRef]
- Bozelli, J.C., Jr.; Yune, J.; Dang, X.; Narayana, J.L.; Wang, G.; Epand, R.M. Membrane activity of two short Trp-rich amphipathic peptides. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183280. [Google Scholar] [CrossRef]
- Souza, G.S.; de Carvalho, L.P.; de Melo, E.J.T.; da Silva, F.C.V.; Machado, O.L.T.; Gomes, V.M.; Carvalho, A.O. A synthetic peptide derived of the β2-β3 loop of the plant defensin from Vigna unguiculata seeds induces Leishmania amazonensis apoptosis-like cell death. Amino Acids 2019, 51, 1633–1648. [Google Scholar] [CrossRef]
- Fernández, A.; Colombo, M.L.; Curto, L.M.; Gómez, G.E.; Delfino, J.M.; Guzmán, F.; Bakás, L.; Malbrán, I.; Vairo-Cavalli, S.E. Peptides derived from the α-core and γ-core regions of a putative Silybum marianum flower defensin show antifungal activity against Fusarium graminearum. Front. Microbiol. 2021, 12, 632008. [Google Scholar] [CrossRef]
- Osborn, R.W.; De Samblanx, G.W.; Thevissen, K.; Goderis, I.; Torrekens, S.; Van Leuven, F.; Attenborough, S.; Rees, S.B.; Broekaert, W.F. Isolation and characterisation of plant defensins from seeds of Asteraceae, Fabaceae, Hippocastanaceae and Saxifragaceae. FEBS Lett. 1995, 368, 257–262. [Google Scholar] [CrossRef]
- Moyer, T.B.; Purvis, A.L.; Wommack, A.J.; Hicks, L.M. Proteomic response of Escherichia coli to a membrane lytic and iron chelating truncated Amaranthus tricolor defensin. BMC Microbiol. 2021, 21, 110. [Google Scholar] [CrossRef]
- Segura, A.; Moreno, M.; Molina, A.; García-Olmedo, F. Novel defensin subfamily from spinach (Spinacia oleracea). FEBS Lett. 1998, 435, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Gbala, I.D.; Macharia, R.W.; Bargul, J.L.; Magoma, G. Membrane permeabilization and antimicrobial activity of recombinant defensin-d2 and actifensin against multidrug-resistant Pseudomonas aeruginosa and Candida albicans. Molecules 2022, 27, 4325. [Google Scholar] [CrossRef] [PubMed]
- Kaewklom, S.; Wongchai, M.; Petvises, S.; Hanpithakphong, W.; Aunpad, R. Structural and biological features of a novel plant defensin from Brugmansia x candida. PLoS ONE 2018, 13, e0201668. [Google Scholar] [CrossRef] [PubMed]
- Rigano, M.M.; Romanelli, A.; Fulgione, A.; Nocerino, N.; D’Agostino, N.; Avitabile, C.; Frusciante, L.; Barone, A.; Capuano, F.; Capparelli, R. A novel synthetic peptide from a tomato defensin exhibits antibacterial activities against Helicobacter pylori. J. Pept. Sci. 2012, 18, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Avitabile, C.; Capparelli, R.; Rigano, M.M.; Fulgione, A.; Barone, A.; Pedone, C.; Romanelli, A. Antimicrobial peptides from plants: Stabilization of the γ core of a tomato defensin by intramolecular disulfide bond. J. Pept. Sci. 2013, 19, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Slezina, M.P.; Istomina, E.A.; Kulakovskaya, E.V.; Abashina, T.N.; Odintsova, T.I. Synthetic oligopeptides mimicking γ-core regions of cysteine-rich peptides of Solanum lycopersicum possess antimicrobial activity against human and plant pathogens. Curr. Issues Mol. Biol. 2021, 43, 1226–1242. [Google Scholar] [CrossRef]
- Slezina, M.P.; Istomina, E.A.; Korostyleva, T.V.; Kovtun, A.S.; Kasianov, A.S.; Konopkin, A.A.; Shcherbakova, L.A.; Odintsova, T.I. Molecular insights into the role of cysteine-rich peptides in induced resistance to Fusarium oxysporum infection in tomato based on transcriptome profiling. Int. J. Mol. Sci. 2021, 22, 5741. [Google Scholar] [CrossRef]
- Vriens, K.; Cools, T.L.; Harvey, P.J.; Craik, D.J.; Spincemaille, P.; Cassiman, D.; Braem, A.; Vleugels, J.; Nibbering, P.H.; Drijfhout, J.W.; et al. synergistic activity of the plant defensin HsAFP1 and caspofungin against Candida albicans biofilms and planktonic cultures. PLoS ONE 2015, 10, e0132701. [Google Scholar] [CrossRef]
- Sagehashi, Y.; Takaku, H.; Yatou, O.J. Partial peptides from rice defensin OsAFP1 exhibited antifungal activity against the rice blast pathogen Pyricularia oryzae. J. Pestic. Sci. 2017, 42, 172–175. [Google Scholar] [CrossRef]
- Ochiai, A.; Ogawa, K.; Fukuda, M.; Ohori, M.; Kanaoka, T.; Tanaka, T.; Taniguchi, M.; Sagehashi, Y. Rice defensin OsAFP1 is a new drug candidate against human pathogenic fungi. Sci. Rep. 2018, 8, 11434. [Google Scholar] [CrossRef]
- Cordts, S.; Bantin, J.; Wittich, P.E.; Kranz, E.; Lörz, H.; Dresselhaus, T. ZmES genes encode peptides with structural homology to defensins and are specifically expressed in the female gametophyte of maize. Plant J. 2001, 25, 103–114. [Google Scholar] [CrossRef]
- Woriedh, M.; Merkl, R.; Dresselhaus, T. Maize EMBRYO SAC family peptides interact differentially with pollen tubes and fungal cells. J. Exp. Bot. 2015, 66, 5205–5216. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Slezina, M.P.; Istomina, E.A.; Kulakovskaya, E.V.; Korostyleva, T.V.; Odintsova, T.I. The γ-core motif peptides of AMPs from grasses display inhibitory activity against human and plant pathogens. Int. J. Mol. Sci. 2022, 23, 8383. [Google Scholar] [CrossRef] [PubMed]
- Odintsova, T.; Shcherbakova, L.; Slezina, M.; Pasechnik, T.; Kartabaeva, B.; Istomina, E.; Dzhavakhiya, V. Hevein-like antimicrobial peptides WAMPs: Structure–function relationship in antifungal activity and sensitization of plant pathogenic fungi to tebuconazole by WAMP-2-derived peptides. Int. J. Mol. Sci. 2020, 21, 7912. [Google Scholar] [CrossRef] [PubMed]
- Shcherbakova, L.; Odintsova, T.; Pasechnik, T.; Arslanova, L.; Smetanina, T.; Kartashov, M.; Slezina, M.; Dzhavakhiya, V. Fragments of a wheat hevein-like antimicrobial peptide augment the inhibitory effect of a triazole fungicide on Fusarium oxysporum and Alternaria solani spore germination. Antibiotics 2020, 9, 870. [Google Scholar] [CrossRef]
| Peptide | Amino Acid Sequence | Net Charge at pH 7.0 |
|---|---|---|
| Antimicrobial Activity | Reference | |
| Rs-AFP2 | QKLCQRPSGTWSGVCGNNNACKNQCIRLEKARHGSCNYVFPAHKCICYFPC | +6 |
| Inhibited the growth of Fusarium culmorum with MIC = 3 µg/mL (IC50 = 2.9 (2.1) * µg/mL in a low ionic strength medium SMF− and IC50 = 8.1 (4.6)* µg/mL in the same medium with salts SMF+); Alternaria brassicola with MIC = 3 µg/mL (IC50 = 3.2 µg/mL in a low ionic strength medium SMF− and IC50 > 50 µg/mL in the same medium with salts SMF+); Ascochyta pisi with MIC = 6 µg/mL (IC50 = 1.9 µg/mL in a low ionic strength medium SMF− and IC50 > 50 µg/mL in the same medium with salts SMF+); Botrytis cinerea with MIC = 12 µg/mL (IC50 = 1.8 µg/mL in a low ionic strength medium SMF− and IC50 > 50 µg/mL in the same medium with salts SMF+); Verticillium dahliae with MIC = 3 µg/mL (IC50 = 1 µg/mL in a low ionic strength medium SMF− and IC50 = 11 µg/mL in the same medium with salts SMF+). No activity at 400 µg/mL against bacterial species Aeromonas hydrophila ssp. proteoytica, Pseudomonas fluorescens, Escherichia coli, Bacillus cereus, B. subtilis, Staphylococcus aureus. Inhibited the growth of Nectria hematococca with IC50 = 2 µg/mL in a low ionic strength medium SMF− and IC50 = 48 µg/mL in the same medium with salts SMF+ against; Inhibited the growth of Phoma betae with IC50 = 0.9 µg/mL in a low ionic strength medium SMF− and IC50 = 14 µg/mL in the same medium with salts SMF+ against; Inhibited the growth of F. culmorum with IC50 = 5 µg/mL/. | [20,21,22] | |
| Peptide 1 | QKLCQRPSGTWSGVC | +2 |
| Inhibited the growth of F. culmorum and A. brassicola with MIC = 250 µg/mL; V. dahliae with MIC = 250 µg/mL; A. pisi and B. cinerea with MIC > 500 µg/mL. No activity at 400 µg/mL against bacterial species A. hydrophila ssp. proteoytica, P. fluorescens, E. coli, B. cereus, B. subtilis, S. aureus. | [21] | |
| Peptide 2 | QRPSGTWSGVCGNNN | +1 |
| Inhibited the growth of F. culmorum, A. brassicola, A. pisi, B. cinerea, V. dahliae with MIC > 500 µg/mL. No activity at 400 µg/mL against bacterial species A. hydrophila ssp. proteoytica, P. fluorescens, E. coli, B. cereus, B. subtilis, S. aureus. | [21] | |
| Peptide 3 | GTWSGVCGNNNACKN | +1 |
| Inhibited the growth of F. culmorum and A. brassicola with MIC = 250 µg/mL; V. dahliae with MIC = 250 µg/mL. No activity at 500 µg/mL against A. pisi and B. cinerea. No activity at 400 µg/mL against bacterial species A. hydrophila ssp. proteoytica, P. fluorescens. | [21] | |
| Peptide 4 | GVCGNNNACKNQCIR | +2 |
| Inhibited the growth of F. culmorum with MIC = 250−500 µg/mL; A. brassicola with MIC = 500 µg/mL; B. cinerea with MIC = 250 µg/mL; V. dahliae with MIC = 125 µg/mL. No activity at 500 µg/mL against A. pisi. No activity at 400 µg/mL against bacterial species A. hydrophila ssp. proteoytica, P. fluorescens, E. coli, B. cereus, B. subtilis, S. aureus. | [21] | |
| Peptide 5 | NNNACKNQCIRLEKA | +2 |
| Inhibited the growth of F. culmorum with MIC = 250 µg/mL; A. brassicola with MIC = 125 µg/mL; A. pisi with MIC = 500 µg/mL; B. cinerea with MIC = 500 µg/mL; V. dahliae with MIC = 250 µg/mL. No activity at 400 µg/mL against bacterial species A. hydrophila ssp. proteoytica, E. coli, B. cereus, S. aureus; Inhibited the growth of P. fluorescens, B. subtilis with MIC = 400 µg/mL. | [21] | |
| Peptide 6 | CKNQCIRLEKARHGS | +3 |
| Inhibited the growth of F. culmorum with MIC = 45 µg/mL; A. brassicola with MIC = 60 µg/mL; A. pisi with MIC = 125 µg/mL; B. cinerea with MIC = 250 µg/mL; V. dahliae with MIC = 30 µg/mL. No activity at 400 µg/mL against bacterial species A. hydrophila ssp. proteoytica, E. coli, B. cereus, S. aureus; Inhibited the growth of P. fluorescens, B. subtilis with MIC = 100 µg/mL. | [21] | |
| Peptide 7 | CIRLEKARHGSCNYV | +2 |
| Inhibited the growth of F. culmorum with MIC = 60 µg/mL; A. brassicola with MIC = 60 µg/mL; A. pisi with MIC = 250 µg/mL; B. cinerea with MIC = 250 µg/mL; V. dahlia with MIC = 30 µg/mL. No activity at 400 µg/mL against bacterial species A. hydrophila ssp. proteoytica, P. fluorescens, E. coli, B. cereus, B. subtilis, S. aureus. | [21] | |
| Peptide 8 | EKARHGSCNYVFPAH | +1 |
| Inhibited the growth of F. culmorum with MIC = 45 µg/mL; A. brassicola with MIC = 30 µg/mL; A. pisi with MIC = 125 µg/mL; B. cinerea with MIC = 250 µg/mL; V. dahliae MIC = 30 µg/mL. No activity at 400 µg/mL against bacterial species A. hydrophila ssp. proteoytica, P. fluorescens, E. coli, B. cereus, B. subtilis, S. aureus. | [21] | |
| Peptide 9 | HGSCNYVFPAHKCIC | +1 |
| Inhibited the growth of F. culmorum with MIC = 30 µg/mL; A. brassicola with MIC = 60 µg/mL; A. pisi with MIC = 250 µg/mL; B. cinerea with MIC = 250 µg/mL; V. dahliae MIC = 30 µg/mL. No activity at 400 µg/mL against bacterial species A. hydrophila ssp. proteoytica, P. fluorescens, E. coli, B. cereus, B. subtilis, S. aureus. | [21] | |
| Peptide 10 | NYVFPAHKCICYFPC | +1 |
| Inhibited the growth of F. culmorum and V. dahliae with MIC = 500 µg/mL; A. brassicola, A. pisi and B. cinerea with MIC > 500 µg/mL. No activity at 400 µg/mL against bacterial species A. hydrophila ssp. proteoytica, P. fluorescens, E. coli, B. cereus, B. subtilis, S. aureus. | [21] | |
| C36-C45 | CNYVFPAHKC | +1 |
| Inhibited the growth of F. culmorum with MIC = 150 µg/mL. | [21] | |
| C36-C45(Y38A) | CNAVFPAHKC | +1 |
| Inhibited the growth of F. culmorum with MIC > 400 µg/mL. | [21] | |
| Rs-AFP2 (γ-core) | GSCNYVFPAHKCICYFP | +1 |
| IC50 = 5.3 μM against Fusarium tricinctum. No activity at 30 μg/mL against Phoma medicaginis STC and WS-2 strains, F. oxysporum f. sp. medicaginis 7F-3 and 31F-3 strains, Colletotrichum trifolli FG-1 and WS-5 strains, Aphanomyces euteiches, F. redolens, F. incarnatum, F.solani; Inhibited the growth of E. coli, Pseudomonas syringae pv. syringae, Sinorhizobium meliloti. | [23] | |
| Rs-AFP2(Q5M) | QKLCMRPSGTWSGVCGNNNACKNQCIRLEKARHGSCNYVFPAHKCICYFPC | +6 |
| IC50 = 4.1 µg/mL in a low ionic strength medium SMF− and IC50 = 5.4 µg/mL in the same medium with salts SMF+ against F. culmorum. | [20] | |
| Rs-AFP2(T10G) | QKLCQRPSGGWSGVCGNNNACKNQCIRLEKARHGSCNYVFPAHKCICYFPC | +6 |
| IC50 = 11 µg/mL in a low ionic strength medium SMF− and IC50 > 100 µg/mL in the same medium with salts SMF+ against F. culmorum. | [20] | |
| Rs-AFP2(G16M) | QKLCQRPSGTWSGVCMNNNACKNQCIRLEKARHGSCNYVFPAHKCICYFPC | +6 |
| IC50 = 2.2 µg/mL in a low ionic strength medium SMF− and IC50 = 5.0 µg/mL in the same medium with salts SMF+ against F. culmorum. | [20] | |
| Rs-AFP2(A31W) | QKLCQRPSGTWSGVCGNNNACKNQCIRLEKWRHGSCNYVFPAHKCICYFPC | +6 |
| IC50 = 30 µg/mL in a low ionic strength medium SMF− and IC50 > 100 µg/mL in the same medium with salts SMF+ against F. culmorum. | [20] | |
| Rs-AFP2(Y38G) | QKLCQRPSGTWSGVCGNNNACKNQCIRLEKARHGSCNGVFPAHKCICYFPC | +6 |
| IC50 = 42 µg/mL in a low ionic strength medium SMF− and IC50 > 200 µg/mL in the same medium with salts SMF+ against F. culmorum. | [20] | |
| Rs-AFP2(F40M) | QKLCQRPSGTWSGVCGNNNACKNQCIRLEKARHGSCNYVMPAHKCICYFPC | +6 |
| IC50 = 16 µg/mL in a low ionic strength medium SMF− and IC50 = 54 µg/mL in the same medium with salts SMF+ against F. culmorum. | [20] | |
| Rs-AFP2(P41∆) | QKLCQRPSGTWSGVCGNNNACKNQCIRLEKARHGSCNYVF∆AHKCICYFPC | +6 |
| IC50 = 100 µg/mL in a low ionic strength medium SMF− and IC50 > 200 µg/mL in the same medium with salts SMF+ against F. culmorum. | [20] | |
| Rs-AFP2(K44Q) | QKLCQRPSGWSGVCGNNNACKNQCIRLEKARHGSCNYVFPAHQCICYFPC | +5 |
| IC50 = 3.6 µg/mL in a low ionic strength medium SMF− and IC50 = 36 µg/mL in the same medium with salts SMF+ against F. culmorum. | [20] | |
| Rs-AFP2(Y48I) | QKLCQRPSGTWSGVCGNNNACKNQCIRLEKARHGSCNYVFPAHKCICIFPC | +6 |
| IC50 = 9.3 µg/mL in a low ionic strength medium SMF− and IC50 = 11 µg/mL in the same medium with salts SMF+ against F. culmorum. | [20] | |
| Rs-AFP2(P7R) | QKLCQRRSGTWSGVCGNNNACKNQCIRLEKARHGSCNYVFPAHKCICYFPC | +7 |
| IC50 = 6.8 µg/mL in a low ionic strength medium SMF− and IC50 = 8.8 µg/mL in the same medium with salts SMF+ against F. culmorum. | [20] | |
| Rs-AFP2(G9R) | QKLCQRPSRTWSGVCGNNNACKNQCIRLEKARHGSCNYVFPAHKCICYFPC | +7 |
| IC50 = 3 µg/mL in a low ionic strength medium SMF− and IC50 = 3.3 µg/mL in the same medium with salts SMF+ against F. culmorum. | [20] | |
| Rs-AFP2(S12R) | QKLCQRPSGTWRGVCGNNNACKNQCIRLEKARHGSCNYVFPAHKCICYFPC | +7 |
| IC50 = 3.5 µg/mL in a low ionic strength medium SMF− and IC50 = 20 µg/mL in the same medium with salts SMF+ against F. culmorum. | [20] | |
| Rs-AFP2(I26R) | QKLCQRPSGTWSGVCGNNNACKNQCRRLEKARHGSCNYVFPAHKCICYFPC | +7 |
| IC50 = 7.2 µg/mL in a low ionic strength medium SMF− and IC50 = 9.6 µg/mL in the same medium with salts SMF+ against F. culmorum. | [20] | |
| Rs-AFP2(L28R) | QKLCQRPSGTWSGVCGNNNACKNQCIRREKARHGSCNYVFPAHKCICYFPC | +7 |
| IC50 = 6.4 µg/mL in a low ionic strength medium SMF− and IC50 >100 µg/mL in the same medium with salts SMF+ against F. culmorum. | [20] | |
| Rs-AFP2(N37R) | QKLCQRPSGTWSGVCGNNNACKNQCIRLEKARHGSCRYVFPAHKCICYFPC | +7 |
| IC50 = 2.8 µg/mL in a low ionic strength medium SMF− and IC50 = 7.0 µg/mL in the same medium with salts SMF+ against F. culmorum. | [20] | |
| Rs-AFP2(V39R) | QKLCQRPSGTWSGVCGNNNACKNQCIRLEKARHGSCNYRFPAHKCICYFPC | +7 |
| IC50 = 4.0 (2.2)* µg/mL in a low ionic strength medium SMF− and IC50 = 3.2 (2.3)* µg/mL in the same medium with salts SMF+ against F. culmorum; IC50 = 2.5 µg/mL in a low ionic strength medium SMF− and IC50 = 50 µg/mL in the same medium with salts SMF+ against A. brassicicola; IC50 = 2.0 µg/mL in a low ionic strength medium SMF− and IC50 > 50 µg/mL in the same medium with salts SMF+ against A. pisi; IC50 = 1.6 µg/mL in a low ionic strength medium SMF− and IC50 > 50 µg/mL in the same medium with salts SMF+ against B. cinerea; IC50 = 0.4 µg/mL in a low ionic strength medium SMF− and IC50 = 2.3 µg/mL in the same medium with salts SMF+ against V. dahlia; IC50 = 2.1 µg/mL in a low ionic strength medium SMF− and IC50 = 9 µg/mL in the same medium with salts SMF+ against N. hematococca; IC50 = 1.4 µg/mL in a low ionic strength medium SMF− and IC50 = 40 µg/mL in the same medium with salts SMF+ against P. betae. | [20] | |
| Rs-AFP2(A42R) | QKLCQRPSGTWSGVCGNNNACKNQCIRLEKARHGSCNYVFPRHKCICYFPC | +7 |
| IC50 = 4.2 µg/mL in a low ionic strength medium SMF− and IC50 = 18 µg/mL in the same medium with salts SMF+ against F. culmorum. | [20] | |
| Rs-AFP2(I46R) | QKLCQRPSGTWSGVCGNNNACKNQCIRLEKARHGSCNYVFPAHKCRCYFPC | +7 |
| IC50 = 12 µg/mL in a low ionic strength medium SMF− and IC50 > 40 µg/mL in the same medium with salts SMF+ against F. culmorum. | [20] | |
| Rs-AFP2(F49R) | QKLCQRPSGTWSGVCGNNNACKNQCIRLEKARHGSCNYVFPAHKCICYRPC | +7 |
| IC50 = 22 µg/mL in a low ionic strength medium SMF− and IC50 = 23 µg/mL in the same medium with salts SMF+ against F. culmorum. | [20] | |
| MBG01 | ARHGSCNYVFPAHKCICYF | +2 |
| Inhibited the growth of F. culmorum with IC50 = 33 µg/mL. | [22] | |
| MBG02 | ARHGSBNYVFPAHKBIBYF | nd |
| Inhibited the growth of F. culmorum with IC50 = 8 µg/mL. | [22] | |
| MBG03 | CRHGSBNYVFPAHKBIBYC | nd |
| Inhibited the growth of F. culmorum with IC50 = 17 µg/mL. | [22] | |
| MBG04 | CRHGScNYVFPAHKcIBYC | nd |
| Inhibited the growth of F. culmorum with IC50 = 15 µg/mL. | [22] | |
| MAT09 | HGSCNYVFPAHKCIC | +1 |
| Inhibited the growth of F. culmorum with IC50 = 41 µg/mL. | [22] | |
| MBG05 | HGSBNYVFPAHKBIB | nd |
| Inhibited the growth of F. culmorum with IC50 > 400 µg/mL. | [22] | |
| MBG06 | ARHGSC | +1 |
| Inhibited the growth of F. culmorum with IC50 = 159 µg/mL. | [22] | |
| MBG08 | ARHGSB | nd |
| Inhibited the growth of F. culmorum with IC50 > 400 µg/mL. | [22] | |
| MBG07 | HKCICY | +1 |
| Inhibited the growth of F. culmorum with IC50 = 16µg/mL. | [22] | |
| MBG09 | HKBIBY | nd |
| Inhibited the growth of F. culmorum with IC50 > 400 µg/mL. | [22] | |
| MAT02 | QRPSGTWSGVCGNNN | +1 |
| Inhibited the growth of F. culmorum with IC50 > 400 µg/mL. | [22] | |
| overlapping 13- to 15-mer peptides derived from Rs-AFP2(26−49) | IRLEKARHGSBNYVFPAHKBIBYF | nd |
| 15-mer peptide 26−40 had the lowest IC50 = 51 µg/mL against F. culmorum. | [22] | |
| overlapping 16- to 20-mer peptides derived from Rs-AFP2(26–49) | IRLEKARHGSBNYVFPAHKBIBYF | nd |
| Peptide 30–49: IC50 = 5 µg/mL against F. culmorum; Peptide 31–49: IC50 = 6 µg/mL against F. culmorum; Peptide 30–48: IC50 = 10 µg/mL against F. culmorum; Peptide 32–49: IC50 = 9 µg/mL against F. culmorum. | [22] |
| Peptide | Amino Acid Sequence | Net Charge at pH 7.0 |
|---|---|---|
| Antimicrobial Activity | Reference | |
| BhDef1 | --------KLCERGRG--TWSGVCGNNNACKNQCIRLEGAQHGSCNNVFPAHKCICYFPC | +4 |
| − | [25] | |
| BhDef11 | KLCERGRG--TWSGVCG | +2 |
| BhDef11 showed inhibitory activity against Colletotrichum gloeosporioides DoA c1060 at a concentration of 1.024 mg/mL with inhibition zone around 4 mm, and no activity against C. gloeosporioides DoA d0762 and C. capsici DoA c1511 at 1.024 mg/mL; it inhibited the growth of methicillin resistant Staphylococcus aureus (MRSA, ATCC 43300) with MIC99 = 2.23 mg/mL and the growth of Salmonella Typhi (ATCC 13311) with MIC99 = 2.02 mg/mL. | [25] | |
| BhDef12 | GVCGNNNAC | 0 |
| No activity against C. gloeosporioides DoA c1060 at a concentration of 0.512 mg/mL and against C. gloeosporioides DoA d0762 and C. capsici DoA c1511 at 1.024 mg/mL. | [25] | |
| BhDef12M | GVCGRRRAC | +3 |
| BhDef12M showed inhibitory activity against C. gloeosporioides DoA c1060 at a concentration of 0.52 mM (0.512mg/mL) with inhibition zone around 4 mm; no activity against C. gloeosporioides DoA d0762 and C. capsici DoA c1511 at 1.024 mg/mL; inhibited the growth of S. aureus (MRSA) with MIC99 = 0.93 mg/mL; inhibited the growth of Salmonella Typhi with MIC99 = 2.00 mg/mL | [25] | |
| BhDef13 | RLEGAQHGSCNNVFPAHKC | +1 |
| BhDef13 showed inhibitory activity against C. gloeosporioides DoA c1060 at a concentration of 0.50 mM (1.024 mg/mL) with inhibition zone around 7 mm; no activity against C. gloeosporioides DoA d0762 and C. capsici DoA c1511 at 1.024 mg/mL, inhibited the growth of S. aureus (MRSA) with MIC99 = 2.26 mg/mL; inhibited the growth of Salmonella Typhi with MIC99 = 1.71 mg/mL. | [25] | |
| BhDef14 | KLCERGRG--TWSGVCGNNNACKNQCIR | +4 |
| BhDef14 showed inhibitory activity against C. gloeosporioides DoA c1060 at a concentration of 0.36 mM (1.024 mg/mL) with inhibition zone around 3 mm. No activity against C. gloeosporioides DoA d0762 and C. capsici DoA c1511 at 1.024 mg/mL, inhibited the growth of S. aureus (MRSA) with MIC99 = 2.40 mg/mL; inhibited the growth of Salmonella Typhi with MIC99 = 1.88 mg/mL. | [25] | |
| BhDef2 | EASALRGGKRCEKRNSSTSFSGVCQYDNACMNQCINLEGAQDGKCNNAVPTPKCICYFPC | +2 |
| − | [25] | |
| BhDef21 | KRCEKRNSSTSFSGVCQ | +3 |
| No activity against C. gloeosporioides DoA c1060 at a concentration of 0.512 mg/mL; no activity against C. gloeosporioides DoA d0762 and C. capsici DoA c1511 at 1.024 mg/mL; inhibited the growth of S. aureus (MRSA) with MIC99 = 2.14 mg/mL; inhibited the growth of Salmonella Typhi with MIC99 = 1.93 mg/mL. | [25] | |
| BhDef22 | NLEGAQDGKCNNAVPTPKC | 0 |
| No activity against C. gloeosporioides DoA c1060 at a concentration of 0.512 mg/mL; no activity against C. gloeosporioides DoA d0762 and C. capsici DoA c1511 at 1.024 mg/mL. | [25] | |
| BhDef23 | GVCQYDNAC | −1 |
| BhDef23 showed inhibitory activity against C. gloeosporioides DoA c1060 at a concentration of 1.05 mM (1.024 mg/mL) with inhibition zone around 10 mm; no activity against C. gloeosporioides DoA d0762 and C. capsici DoA c1511 at 1.024 mg/mL; inhibited the growth of S. aureus (MRSA) with MIC99 = 2.93 mg/mL; inhibited the growth of Salmonella Typhi with MIC99 = 1.77 mg/mL. | [25] |
| Peptide | Amino Acid Sequence | Net Charge at pH 7.0 |
|---|---|---|
| Antimicrobial Activity | Reference | |
| MsDef1 | RTCENLADKYRGPCFS-G-CDTHCTTKENAVSGRCR-DDFRCWCTKRC | +3 |
| IC50 = 2−4 μM against F. graminearum; MsDef1 caused 41 ± 5% growth inhibition of F. verticillioides at 24 μM; 90% growth inhibition of Aspergillus flavus at concentrations of 12 to 48 µM; IC50 (germination) > 24.50 µM, IC50(CAT (conidial anastomosis tube) fusion) = 7.62 µM and IC50 (cell death) = 1.62 µM against Neurospora crassa. | [9,26] | |
| MsDef1-R38Q | RTCENLADKYRGPCFS-G-CDTHCTTKENAVSGRCR-DDFQCWCTKRC | +2 |
| IC50 > 6 μM against F. graminearum. | [9] | |
| MsDef1-γ4 | RTCENLADKYRGPCFS-G-CDTHCTTKENAVSGRCRGFRRRCWCTKRC | +7 |
| IC50 = 1.3−1.5 μM against F. graminearum. | [9] | |
| GMA1-C | GRCR-DDFRCWCTKRC | +3 |
| IC50 = 14 μM against F. graminearum; GMA1-C inhibited more than 90% of F. verticillioides growth at 24 µM; 90% growth inhibition of A. flavus at concentrations of 24 µM; IC50 = 12.7 μM against P. medicaginis STC and IC50 = 14.8 μM against P. medicaginis WS-2; No activity at 30 μg/mL against F. oxysporum f. sp. medicaginis 7F-3 and 31F-3 strains, C. trifolli FG-1 and WS-5 strains, A. euteiches, F. solani, F. tricinctum, F. redolens, F. incarnatum; IC50 = 7.9 μM against Xanthomonas alfalfae subsp. alfalfae; IC50 = 8.8 μM against P. syringae pv. syringae; IC50(germination) = 8.54 µM, IC50(CAT fusion) = 6.46 µM and IC50 (cell death) = 24.15 µM against N. crassa. | [9,23,26] | |
| GMA1 | GRCR-DDFRC | +1 |
| IC50 > 192 μM against F. graminearum; IC50 (germination) > 80 µM, IC50 (CAT fusion) >80 µM and IC50 (cell death) > 80 µM against N. crassa. | [9,26] | |
| GMA1-L | R-DDFR | 0 |
| IC50 >96 μM against F. graminearum; IC50 (germination) > 80 µM, IC50 (CAT fusion) >80 µM and IC50 (cell death) > 80 µM against N. crassa. | [9,26] | |
| ALP1 | GPCFS-G-C | 0 |
| IC50 >48 μM against F. graminearum. | [9] | |
| MtDef4 | RTCESQSHKFKGPCASDHNCASVCQT-ERFSGGRCRGFRRRCFCTTHC | +6 |
| IC50 = 0.75−1 μM against F. graminearum; MtDef4 inhibited more than 90% of F. verticillioides growth at 12 µM; A. flavus growth inhibition of approximately 75% at 48 µM; IC50 = 0.3 μM against P. medicaginis STC and IC50 = 2.6 μM against P. medicaginis WS-2; IC50 = 0.7 μM against against F. oxysporum f. sp. medicaginis 7F-3 and IC50 = 1.9 μM against 31F-3 strain; no activity at 30 μg/mL against A. euteiches, C. trifolli FG-1 and WS-5 strains; IC50 = 0.6 μM against X. alfalfae subsp. alfalfae; IC50 = 0.4 μM against P. syringae pv. syringae; IC50 = 0.1 μM against Clavibacter insidiosus; IC50 (germination) = 0.65 µM, IC50 (CAT fusion) = 0.52 µM and IC50 (cell death) = 0.83 µM against N. crassa. | [9,23,26] | |
| GMA4-C | GRCRGFRRRCFCTTHC | +5 |
| IC50 = 3 μM against F. graminearum; GMA4-C inhibited more than 90% of F. verticillioides growth at 3 µM; 90% growth inhibition of A. flavus at concentrations of 6 µM; IC50 = 7.3 μM against P. medicaginis STC and IC50 = 5.3 μM against P. medicaginis WS-2; IC50 = 7.1 μM against F. oxysporum f. sp. medicaginis 7F-3 and IC50 = 6.9 μM against 31F-3 strain. No activity at 30 μg/mL against C. trifolli FG-1 and WS-5 strains, A. euteiches, F. redolens, F. incarnatum; IC50 = 6.0 μM against F. solani; IC50 = 14.7 μM against F. tricinctum; IC50 = 11.4 μM against X. alfalfae subsp. alfalfae; IC50 = 3.4 μM against P. syringae pv. syringae; IC50 = 8.4 μM against Serratia marcescens; IC50 = 2.3 μM against Enterobacter aerogenes; IC50 = 2.7 μM against Pseudomonas aeruginosa; no activity at 30 μg/mL against Enterococcus casseliflavus; IC50 = 1.7-4.2 μM against P. aeruginosa strains; IC50 (germination) = 1.6 µM, IC50 (CAT fusion) = 1.37 µM and IC50 (cell death) = 2.21 µM against N. crassa. | [9,23,26,27] | |
| GMA4 | GRCRGFRRRC | +5 |
| IC50 = 3 μM against F. graminearum; IC50 (germination) = 2.20 µM, IC50 (CAT fusion) = 1.68 µM and IC50 (cell death) = 2.64 µM against N. crassa. | [9,26] | |
| GMA4-L | RGFRRR | +4 |
| IC50 = 4 μM against F. graminearum; IC50 (germination) = 55.38 µM, IC50(CAT fusion) = 4.10 µM and IC50 (cell death) > 80 µM against N. crassa. | [9,26] | |
| GMA4-L1 | RGARRR | +4 |
| IC50 > 96 μM against F. graminearum. | [9] | |
| GMA4-L2 | RGFARR | +3 |
| IC50 > 96 μM against F. graminearum. | [9] | |
| ALP4 | GPCASDHNC | −1 |
| IC50 > 48 μM against F. graminearum. | [9] | |
| MtDef4RGFRRR/AAAARR | RTCESQSHKFKGPCASDHNCASVCQT-ERFSGGRCAAAARRCFCTTHC | +4 |
| Growth inhibition of F. graminearum by less than 10% at 3 µM. | [28] | |
| MtDef4RGFRRR/RGAARR | RTCESQSHKFKGPCASDHNCASVCQT-ERFSGGRCRGAARRCFCTTHC | +5 |
| More than 90% growth inhibition of F. graminearum at 3 μM. | [28] | |
| MtDef4RGFRRR/RGFRAA | RTCESQSHKFKGPCASDHNCASVCQT-ERFSGGRCRGFRAACFCTTHC | +4 |
| About 30% growth inhibition of F. graminearum at 3 μM. | [28] |
| Peptide | Amino Acid Sequence | Net Charge at pH 7.0 |
|---|---|---|
| Antimicrobial Activity | Reference | |
| MtDef5 |
KLCQKRSTTWSGPCLNTGNCKRQCINVEHATFGACHRQGFGFACFCYKKCAPKKVEP KLCERRSKTWSGPCLISGNCKRQCINVEHATSGACHRQGIGFACFCKKKC | +16 |
| MtDef5 inhibited the growth of F. graminearum and N. crassa with an IC50 value of 0.25–0.3 µM (MIC of 0.70–0.75 µM). It also inhibited the growth of several other filamentous plant fungal pathogens including F. verticilloides, F. thapsinum, Alternaria brassicicola, Colletotrichum higginsianum, and Botrytis cinerea, C. higginsianum was the least sensitive pathogen among this group of fungal pathogens with an IC50 value of 1.75 µM (MIC of 3.0 µM); MtDef5 inhibited the growth of Xanthomonas campestris with MIC of 12 µM and Clavibacter michiganensis with MIC > 12 µM; IC50 = 1.5 μM against P. medicaginis STC and IC50 = 1.6 μM against P. medicaginis WS-2; IC50 = 0.8 μM against agaisnt F. oxysporum f. sp. medicaginis 7F-3 and IC50 = 1.3 μM against 31F-3 strain; no activity at 30 μg/mL against C. trifolli FG-1 and WS-5 strains, A. euteiches; IC50 = 0.1 μM against P. syringae pv. syringae; no activity at 30 μg/mL against X. alfalfae subsp. alfalfae and Clavibacter insidiosus. | [23,29,30] | |
| MtDef5A | KLCQKRSTTWSGPCLNTGNCKRQCINVEHATFGACHRQGFGFACFCYKKC | +7 |
| MtDef5A inhibited the growth of F. graminearum with an IC50 value of 0.75–1.0 µM (MIC of 1.5-3.0 µM); inhibited the growth of X. campestris with MIC of 12 µM and C. michiganensis with MIC > 12 µM. | [29,30] | |
| MtDef5B | KLCERRSKTWSGPCLISGNCKRQCINVEHATSGACHRQGIGFACFCKKKC | +8 |
| MtDef5B inhibited the growth of F. graminearum with an IC50 value of 0.5–0.75 µM (MIC of 1.0–1.5 µM); inhibited the growth of X. campestris with MIC of 6 µM and C. michiganensis with MIC > 12 µM. | [29,30] | |
| MtDef5H36A, R37A/H93A, R94A (MtDef5_V1) |
KLCQKRSTTWSGPCLNTGNCKRQCINVEHATFGACAAQGFGFACFCYKKCAPKKVEP KLCERRSKTWSGPCLISGNCKRQCINVEHATSGACAAQGIGFACFCKKKC | +14 |
| MtDef5_V1 almost completely lost its antifungal activity against F. graminearum; inhibited the growth of X. campestris and C. michiganensis with MIC > 12 µM. | [29,30] | |
| MtDef5Q38A, G39A/Q95A, G96A (MtDef5_V2) |
KLCQKRSTTWSGPCLNTGNCKRQCINVEHATFGACHRAAFGFACFCYKKCAPKKVEP KLCERRSKTWSGPCLISGNCKRQCINVEHATSGACHRAAIGFACFCKKKC | +16 |
| MtDef5_V2 showed 2-fold reduction in its antifungal activity against F. graminearum relative to that of MtDef5. | [29] | |
| MtDef5F40A, G41A/I97A, G98A (MtDef5_V3) |
KLCQKRSTTWSGPCLNTGNCKRQCINVEHATFGACHRQGAAFACFCYKKCAPKKVEP KLCERRSKTWSGPCLISGNCKRQCINVEHATSGACHRQGAAFACFCKKKC | +16 |
| MtDef5_V3 retained MtDef5 antifungal activity against F. graminearum. | [29] | |
| MtDef5F42A/F99A (MtDef5_V4) |
KLCQKRSTTWSGPCLNTGNCKRQCINVEHATFGACHRQGFGAACFCYKKCAPKKVEP KLCERRSKTWSGPCLISGNCKRQCINVEHATSGACHRQGIGAACFCKKKC | +16 |
| MtDef5_V4 retained MtDef5 antifungal activity against F. graminearum. | [29] | |
| MtDef5A (γ-core) | GACHRQGFGFACFCYKKC | +3 |
| IC50 = 19.5 μM against P. medicaginis STC and IC50 = 8.5 μM against P. medicaginis WS-2; IC50 = 4.1 μM against F. solani; no activity at 30 μg/mL agaisnt F. oxysporum f. sp. medicaginis 7F-3 and 31F-3 strains, C. trifolli FG-1 and WS-5 strains, A. euteiches, F. tricinctum, F. redolens, F. incarnatum; IC50 = 4.5 μM against P. syringae pv. syringae; no activity at 30 μg/mL against X. alfalfae subsp. alfalfae; IC50 = 6.0 μM against S. marcescens; IC50 = 2.8 μM against E. aerogenes; IC50 = 11.8 μM against P. aeruginosa; no activity at 30 μg/mL against Enterococcus casseliflavus; IC50 = 8.5–14.6 μM against P. aeruginosa strains. | [23,27] |
| Peptide | Amino Acid Sequence | Net Charge at pH 7.0 |
|---|---|---|
| Antimicrobial Activity | Reference | |
| PvD1 | KTCENLADTYKGPCFTTGSCDDHCKNKEHLRSGRCRDDFRCWCTKNC | +2 |
| Inhibited the growth of the yeasts, C. albicans, C. parapsilosis, C. tropicalis, C. guilliermondii, Kluyveromyces marxiannus and Saccharomyces cerevisiae and phytopathogenic fungi including F. oxysporum, F. solani, F. lateritium and Rizoctonia solani. | [31] | |
| γ31-45PvD1 | RSGRARDDFRAWATK | +3 |
| 40% growth inhibition of C. albicans at 293.6 μM; 100% growth inhibition of C. buinensis at 293.6 μM. | [31] | |
| γ31-45PvD1++ | RSGRARRRFRAWATK | +7 |
| 100% growth inhibition of C. albicans at 73.4 μM; 100% growth inhibition of C. buinensis at 18.35 μM. | [31] | |
| γ33-41PvD1 | GRARDDFRA | +1 |
| No activity at 293.6 μM against C. albicans; 17% growth inhibition of C. buinensis at 293.6 μM. | [31] | |
| γ33-41PvD1++ | GRARRRFRA | +5 |
| 63% growth inhibition of C. albicans at 293.6 μM; 100% growth inhibition of C. buinensis at 36.7 μM. | [31] |
| Peptide | Amino Acid Sequence | Net Charge at pH 7.0 |
|---|---|---|
| Antimicrobial Activity | Reference | |
| VuDef1 | KTCENLADTYRGPCFTTGSCDDHCKNKEHLLSGRCRDDVRCWCTRNC | +1 |
| 51% growth inhibition of Leishmania amazonensis at 18.5 μM. | [32] | |
| A36,42,44γ32-46VuDef (DD) | LSGRARDDVRAWATR | +2 |
| 41% growth inhibition of L. amazonensis at 18.5 μM; no activity at 18.5 μM against C. albicans, C. buinensis, C. tropicalis, Saccharomices cerevisiae, C. parapsilosis and C. pelliculosa. | [32] | |
| A36,42,44R37,38γ32-46VuDef (RR) | LSGRARRRVRAWATR | +6 |
| 47.5, 100 and 72.1% growth inhibition of C. albicans, C. buinensis, and C. tropicalis, respectively, at 18.5 μM; for C. tropicalis, MIC100 and LD100 = 27.5 μM; no activity at 18.5 μM against S. cerevisiae, C. parapsilosis and C. pelliculosa. | [32] | |
| D-A36,42,44R37,38γ32-46VuDef (D-RR) | LSGRARRRVRAWATR | +6 |
| 84.9, 99.7, and 100% growth inhibition of C. albicans, C. buinensis, and C. tropicalis, respectively, at 18.5 μM; for C. albicans, MIC100 = 23 μM and LD100 = 36.5 μM; for C. tropicalis, MIC100 = 14 μM and LD100 = 23 μM; no activity at 18.5 μM against S. cerevisiae, C. parapsilosis and C. pelliculosa. | [32] | |
| A42,44R37,38W36,39γ32-46VuDef (WR) | LSGRWRRRWRAWATR | +6 |
| 26.1, 96.2, 98.5, and 58.2% growth inhibition of S. cerevisiae, C. albicans, C. buinensis, and C. tropicalis, respectively, at 18.5 μM; for C. albicans, MIC100 = 18.5 μM and LD100 = 27.5 μM; no activity at 18.5 μM against C. parapsilosis and C. pelliculosa. | [32] |
| Peptide | Amino Acid Sequence | Net Charge at pH 7.0 |
|---|---|---|
| Antimicrobial Activity | Reference | |
| DefSm2-D | -----KLCEKPSKTWFGNCGNPRHCGDQCKSWEGAVHGACHVRNGKHMCFCYFNCPQAE | +3 |
| − | [38] | |
| SmAPγ27-44 | WEGAVHGACHVRNGKHMC | +1 |
| Inhibited the growth of F. graminearum with MIC = 20 µM. | [38] | |
| SmAPγ29-35 | GAVHGAC | 0 |
| No activity at 100 μM against F. graminearum. | [38] | |
| SmAPα1-21 | KLCEKPSKTWFGNCGNPRHCG | +3 |
| Inhibited the growth of F. graminearum with MIC = 32 µM. | [38] | |
| SmAPα10-21 | WFGNCGNPRHCG | +1 |
| Inhibited the growth of F. graminearum with MIC = 70 µM. | [38] | |
| Atr-DEF2 | -----RICESASYRFKGICVSRTNCANVCKT-EGFPGGRCRG--FRRRCFCYKHCA | +9 |
| − | [40] | |
| Atr-DEF2(G39-C54) | GRCRG--FRRRCFCYKHC | +6 |
| IC50 = 9 μM against E. coli; IC50 = 68 μM against Klebsiella pneumoniae. | [40] | |
| So-D2 | GIFSSRKCKTPSKTFKGICTRDSNCDTSCR-YEGYPAGDCKG--IRRRCMCSKPC | +8 |
| EC50 (effective concentration for 50% inhibition) = 1 μM against C. michiganensis; EC50 = 2 μM against Ralstonia solanacearum; EC50 = 0.2 μM against F. culmorum; EC50 = 11 μM against F. solani; MIC = 7.5 μg/mL against P. aeruginosa ATCC 27853; MIC = 7.5 μg/mL against C. albicans ATCC 64124. It also inhibited E. coli ATCC 25922 (MIC = 30 μg/mL) and K. pneumoniae ATCC 700603 (MIC = 30 μg/mL). | [41,42] | |
| So-D2 (γ-core) | GDCKG--IRRRCMCSKPL | +4 |
| IC50 = 6.4 μM against P. medicaginis STC and IC50 = 6.1 μM against P. medicaginis WS-2; IC50 = 33.1 μM against F. oxysporum f. sp. medicaginis 7F-3; IC50 = 13.8 μM against F. solani; No activity at 30 μg/mL against F. oxysporum f. sp. medicaginis 31F-3, F. redolens, F. incarnatum, F. tricinctum, C. trifolli FG-1 and WS-5 strains, A. euteiches; IC50 = 25.9 μM against P. syringae pv. syringae; IC50 = 19.3 μM against X. alfalfae subsp. alfalfae; No activity at 30 μg/mL against C. insidiosus | [23] | |
| BcDef | -----RHCESQSQRFKGTCLSEKNCASVCE-TEGFSGGDCRG--LRRRCFCTRPC | +4 |
| − | [43] | |
| BcDef1 | FSGGDCRG--LRRRCFCTR | +4 |
| Inhibited the growth of Gram-negative E. coli ATCC25922 with MIC > 251.21 µM; E. coli O157 with MIC = 229.09 µM; P. aeruginosa with MIC > 251.21 µM; Vibrio cholerae with MIC = 125.61 µM; Shigella sonnei with MIC = 125.61 µM; Staphylococcus typhimurium with MIC = 31.40 µM; Gram-positive Enterococcus faecalis with MIC = 251.21 µM; B. cereus with MIC = 251.21 µM; Staphylococcus aureus ATCC25923 and MRSA with MIC >251.21 µM; Staphylococcus epidermidis with MIC = 15.70 µM. | [43] |
| Peptide | Amino Acid Sequence | Net Charge at pH 7.0 |
|---|---|---|
| Antimicrobial Activity | Reference | |
| Solyc07g007760 | RHCESLSHRFKGPCVSDKNCASVCETERFSGGNCRGFRRRCFCTKPC | +6 |
| − | [44] | |
| SolyC | FSGGNCRGFRRRCFCTK | +5 |
| Inhibited the growth of Gram-negative E. coli ATCC25922 and Salmonella enterica serovar Parathyphi with MIC = 15 µg/mL; Helicobacter pylori with MIC = 10−15 µg/mL; inhibited the growth of Gram-positive Staphylococcus aureus A170, S. epidermidis and Listeria monocytogenes with MIC = 40 µg/mL; 15% and 13% growth inhibition of Lactobacillum plantarum and L. paracasei at 50 μg/mL, respectively. | [44] | |
| SolyC-t | GNCRGFRRRCFCTK | +5 |
| Inhibited the growth of Gram-negative S. enterica serovar Parathyphi B and H. pylori with MIC = 15 µg/mL; inhibited the growth of Gram-positive S. aureus with MIC = 50 µg/mL; S. epidermidis and L. monocytogenes with MIC = 80 µg/mL. | [45] | |
| SolyC1 | FSGGNCRGFRRRCFSTK | +5 |
| Inhibited the growth of Gram-negative S. enterica serovar Parathyphi B with MIC = 40 µg/mL; H. pylori with MIC = 15 µg/mL; Inhibited the growth of Gram-positive S. aureus with MIC = 50 µg/mL; S. epidermidis with MIC = 100 µg/mL; L. monocytogenes with MIC = 80 µg/mL. | [45] | |
| SolyC1-t | GNCRGFRRRCFSTK | +5 |
| Inhibited the growth of Gram-negative S. enterica serovar Parathyphi B with MIC = 80 µg/mL; H. pylori with MIC = 15 µg/mL; inhibited the growth of Gram-positive S. aureus with MIC = 50 µg/mL; S. epidermidis with MIC = 100 µg/mL; L. monocytogenes with MIC = 80 µg/mL. | [45] | |
| SolyC2 | FSGGNCRGFRRRSFCTK | +5 |
| Inhibited the growth of Gram-negative S. enterica serovar Parathyphi B and H. pylori with MIC = 15 µg/mL; inhibited the growth of Gram-positive S. aureus with MIC = 80 µg/mL; S. epidermidis with MIC = 100 µg/mL; L. monocytogenes with MIC = 80 µg/mL. | [45] | |
| SolyC2-t | GNCRGFRRRSFCTK | +5 |
| Inhibited the growth of Gram-negative S. enterica serovar Parathyphi B with MIC = 40 µg/mL; H. pylori with MIC = 20 µg/mL; inhibited the growth of Gram-positive S. aureus with MIC = 80 µg/mL; S. epidermidis with MIC = 100 µg/mL; L. monocytogenes with MIC = 80 µg/mL | [45] | |
| SolyC1-ox (C6–C13) | FSGGNCRGFRRRCFSTK | +5 |
| Inhibited the growth of Gram-negative S. enterica serovar Parathyphi B and H. pylori with MIC = 15 µg/mL; inhibited the growth of Gram-positive S. aureus with MIC = 80 µg/mL; S. epidermidis with MIC = 100 µg/mL; L. monocytogenes with MIC = 80 µg/mL. | [45] | |
| SolyC1-t-ox (C6–C13) | GNCRGFRRRCFSTK | +5 |
| Inhibited the growth of Gram-negative S. enterica serovar Parathyphi B and H. pylori with MIC = 15 µg/mL; inhibited the growth of Gram-positive S. aureus with MIC = 50 µg/mL; S. epidermidis with MIC = 100 µg/mL; L. monocytogenes with MIC = 80 µg/mL. | [45] | |
| SolyC2-ox (C6–C15) | FSGGNCRGFRRRSFCTK | +5 |
| Inhibited the growth of Gram-negative S. enterica serovar Parathyphi B with MIC = 10 µg/mL; H. pylori with MIC = 20 µg/mL; inhibited the growth of Gram-positive S. aureus and S. epidermidis with MIC = 100 µg/mL; L. monocytogenes with MIC = 80 µg/mL. | [45] | |
| SolyC2-t-ox (C6–C15) | GNCRGFRRRSFCTK | +5 |
| Inhibited the growth of Gram-negative S. enterica serovar Parathyphi B and H. pylori with MIC = 20 µg/mL; inhibited the growth of Gram-positive S. aureus with MIC = 80 µg/mL; S. epidermidis with MIC = 100 µg/mL; L. monocytogenes with MIC = 80 µg/mL. | [45] | |
| SlDEFL2 | RTCESQSHRFKGPCVSEKNCASVCETEGFSGGDCRGFRRRCFCTRPC | +4 |
| − | [46] | |
| γ58-74SlDEFL2 | FSGGDCRGFRRRCFCTR | +4 |
| IC50 = 11.5 μM against Cryptococcus neoformans; IC50 = 19.8 μM against Clavibacter michiganensis; IC50 = 44.8 μM against F. culmorum; IC50 = 165.8 μM against F. oxysporum; 97% growth inhibition of C. albicans at 300 μM; 85% growth inhibition of Pseudomonas savastanoi at 300 μM; 48% growth inhibition of Pectobacterium carotovorum at 300 μM; 31% growth inhibition of Botrytis cinerea at 300 μM; no activity against F. solani, F. verticillioides, Bipolaris sorokiniana at 300 μM. | [46] | |
| SlDEFL4 | RTCESQSHHFKGNCLSDTNCGSVCRTEGFTGGNCRGFRRRCFCTRNC | +5 |
| − | [46] | |
| γ58-74SlDEFL4 | FTGGNCRGFRRRCFCTR | +5 |
| IC50 = 8.1 μM against C. neoformans; IC50 = 21.5 μM against C. michiganensis; IC50 = 42.3 μM against F. culmorum; IC50 = 124.8 μM against F. oxysporum; 97% growth inhibition of C. albicans at 300 μM; 100% growth inhibition of P. savastanoi at 300 μM; 81% growth inhibition of P. carotovorum at 300 μM; 45% growth inhibition of B. cinerea at 300 μM; No activity against F. solani, F. verticillioides, B. sorokiniana at 300 μM. | [46] |
| Peptide | Amino Acid Sequence | Net Charge at pH 7.0 |
|---|---|---|
| Antimicrobial Activity | Reference | |
| HsAFP1 | DGVKLCDVPSGTWSGHCGSSSKCSQQCKDREHFAYGGACHYQFPSVKCFCKRQC | +3 |
| IC50 = 0.45 μM against F. culmorum; MIC50 = 18.00 μM against planktonic C. albicans cultures; BIC50 (biofilm formation) = 11.00 μM against C. albicans. | [48] | |
| HsLin01 | DGVKLBDVPSGTWSGHBGSSSKBS | nd |
| BIC50 > 175 μM against C. albicans. | [48] | |
| HsLin02 | DVPSGTWSGHBGSSSKBSQQBKDR | nd |
| BIC50 > 175 μM against C. albicans. | [48] | |
| HsLin03 | WSGHBGSSSKBSQQBKDREHFAYG | nd |
| BIC50 = 96.78 μM against C. albicans. | [48] | |
| HsLin04 | SSSKBSQQBKDREHFAYGGABHYQ | nd |
| BIC50 > 175 μM against C. albicans. | [48] | |
| HsLin05 | QQBKDREHFAYGGABHYQFPSVKB | nd |
| BIC50 = 160.00 μM against C. albicans. | [48] |
| Peptide | Amino Acid Sequence | Net Charge at pH 7.0 |
|---|---|---|
| Antimicrobial Activity | Reference | |
| OsAFP1 | RHCLSQSHRFKGMCVSSNNCANVCRTESFPDGECKSHGLERKCFCKKVC | +5 |
| IC50 = 2 μM against C. albicans (MIC = 4 μM); MIC = 4 μM and 16 μM against Saccharomyces cerevisiae By4742 and S288C strains, respectively; MIC > 32 μM against Gram-negative bacteria Porphyromonas gingivalis and E. coli; MIC > 32 μM against Gram-positive bacteria Streptococcus mutans, Staphylococcus aureus, Propionibacterium acnes; IC50 = 0.99 μg/mL against Pyricularia oryzae; IC50 = 1.48 μg/mL against Rhizoctonia solani; IC50 = 3.75 μg/mL against Gibberella fujikuroi; IC50 > 30 μg/mL against bacteria Burkholderia plantari, B. glumae and Acidovorax evenae. | [49,50] | |
| OsAFP1 (K35A) | RHCLSQSHRFKGMCVSSNNCANVCRTESFPDGECASHGLERKCFCKKVC | +4 |
| IC50 = 15 μM against C. albicans. | [50] | |
| OsAFP1 (H37A) | RHCLSQSHRFKGMCVSSNNCANVCRTESFPDGECKSAGLERKCFCKKVC | +5 |
| IC50 = 17 μM against C. albicans. | [50] | |
| OsAFP1 (L39A) | RHCLSQSHRFKGMCVSSNNCANVCRTESFPDGECKSHGAERKCFCKKVC | +5 |
| IC50 > 32 μM against C. albicans. | [50] | |
| OsAFP1 (R41A) | RHCLSQSHRFKGMCVSSNNCANVCRTESFPDGECKSHGLEAKCFCKKVC | +4 |
| IC50 > 32 μM against C. albicans. | [50] | |
| OsAFP1 (K42A) | RHCLSQSHRFKGMCVSSNNCANVCRTESFPDGECKSHGLERACFCKKVC | +4 |
| IC50 = 20 μM against C. albicans. | [50] | |
| Peptide 1 | RHCLSQSHRF | +2 |
| IC50 = 6 μM against C. albicans; IC50 = 0.41 μg/mL against P. oryzae. | [49,50] | |
| Peptide 2 | SHRFKGMCVS | +2 |
| IC50 = 19 μM against C. albicans; IC50 = 0.87 μg/mL against P. oryzae. | [49,50] | |
| Peptide 3 | VSSNNCANV | 0 |
| IC50 > 25 μM against C. albicans; IC50 > 30 μg/mL against P. oryzae. | [49,50] | |
| Peptide 4 | SNNCANVCRTE | 0 |
| IC50 > 25 μM against C. albicans; IC50 > 30 μg/mL against P. oryzae. | [49,50] | |
| Peptide 5 | RTESFPDGE | −2 |
| IC50 > 25 μM against C. albicans; IC50 > 30 μg/mL against P. oryzae. | [49,50] | |
| Peptide 6 | FPDGECKSHG | −1 |
| IC50 > 25 μM against C. albicans; IC50 > 30 μg/mL against P. oryzae. | [49,50] | |
| Peptide 7 | KSHGLERKCF | +2 |
| IC50 = 10 μM against C. albicans; IC50 = 0.84 μg/mL against P. oryzae. | [49,50] | |
| Peptide 8 | ERKCFCKKVC | +3 |
| IC50 = 13 μM against C. albicans; IC50 = 1.42 μg/mL against P. oryzae. | [49,50] |
| Peptide | Amino Acid Sequence | Net Charge at pH 7.0 |
|---|---|---|
| Antimicrobial Activity | Reference | |
| ZmES1 | RDCLTQSTRLPGHLCVRSDYCAIGCRAEGKGYTGGRCLISPIPLDGILCYCVKPCPSNTTT | +3 |
| 66.7% germination inhibition of F. graminearum at 90 μM; 55.9% germination inhibition of Ustilago maydis at 90 μM. | [52] | |
| ZmES4 | RDCLTQSTRLPGHLCVRSDYCAIGCRAEGKGYTGGRCLISPITLDGILCYCVKPCTSTTTK | +4 |
| 67.8% germination inhibition of F. graminearum at 90 μM; 56.4% germination inhibition of U. maydis at 90 μM. | [52] | |
| mES4 | RDCLTQSTRLPGHLCVRSDYCAIGCRAEGKGYTGGRCQISPITLAVIQCYCVKPCTSTTTK | +5 |
| Significant inhibition was not observed. | [52] | |
| ES-a | RDCLTQSTRLPGHLCV | +1 |
| Significant inhibition was not observed. | [52] | |
| ES-b | LCVRSDYCAIGCR | +1 |
| Significant inhibition was not observed. | [52] | |
| ES-c | GCRAEGKGYTGGRCL | +2 |
| 77.1% germination inhibition of F. graminearum at 90 μM; 80.9% germination inhibition of U. maydis at 90 μM. | [52] | |
| ES-d | RCLISPITLDGILCY | 0 |
| 79.3% germination inhibition of F. graminearum at 90 μM; 78.7% germination inhibition of U. maydis at 90 μM. | [52] | |
| mES-d1 | RCQISPITLAVIQCY | +1 |
| Significant inhibition was not observed. | [52] | |
| mES-d2 | RCLAAAATLDGILCY | 0 |
| Significant inhibition was not observed. | [52] | |
| ES-e | LCYCVKPCTSTTTK | +2 |
| Significant inhibition was not observed. | [52] |
| Peptide | Amino Acid Sequence | Net Charge at pH 7.0 |
|---|---|---|
| Antimicrobial Activity | Reference | |
| TkDEFL1-11 | RICTGKSQHHSFPCISDKSCTKTCLGEHGAKWTAGYCK--ISR-CTCQREC | +5 |
| − | [53] | |
| TkDEFL1-1155-68 | GYCK---ISR-CTCQREC | +2 |
| 11% germination inhibition C. neoformans at 300 μM; 17% germination inhibition of C. albicans at 300 μM; 11% germination inhibition of P. carotovorum at 300 μM; No activity at 300 μM against F. culmorum, F. oxysporum, F. solani, F. verticillioides, C. michiganensis, P. savastanoi. | [53] | |
| TkDEFL1-32 | RICTGKSQHHSFPCISDKSCTKTCLGEHGAKWTAGYCK---FRR-CTCQREC | +6 |
| − | [53] | |
| TkDEFL1-3255-68 | GYCK---FRR-CTCQREC | +3 |
| IC50 = 42.9 μM against C. neoformans (100% germination inhibition at 80 μM); 92% germination inhibition of C. albicans at 300 μM; IC50 = 38.5 μM against C. michiganensis (76% germination inhibition at 300 μM); IC50 = 97.8 μM against F. culmorum (79% germination inhibition at 150 μM); IC50 = 89.3 μM against F. oxysporum (71% germination inhibition at 300 μM); 39% germination inhibition of P. savastanoi at 300 μM; 56% germination inhibition of P. carotovorum at 300 μM; no activity at 300 μM against F. solani, F. verticillioides. | [53] | |
| TkDEFL1-12 | KICRQRSAGFKGPCLSDKNCAQVCLQER---WGGGNCDG--PFRRCKCIRQC | +7 |
| − | [53] | |
| TkDEFL1-1262-77 | GNCDG--PFRRCKCIRQC | +3 |
| IC50 = 37.3 μM against C. neoformans (97% germination inhibition at 100 μM); 5% germination inhibition of C. albicans at 300 μM; IC50 = 69.1 μM against C. michiganensis (89% germination inhibition at 300 μM); 71% germination inhibition of F. culmorum at 300 μM; 62% germination inhibition of F. oxysporum at 300 μM; 45% germination inhibition of F. solani at 300 μM; 6% germination inhibition of P. savastanoi at 300 μM; 30% germination inhibition of P. carotovorum at 300 μM; no activity against F. verticillioides. | [53] | |
| TkDEFL1-16 | RTCESRSHRFRGPCVRRSNCANVCKTEG-FP--DGKCRG--FRRRCFCTTHCHH | +9 |
| − | [53] | |
| TkDEFL1-1665-82 | GKCRG--FRRRCFCTTHCHH | +5 |
| IC50 = 4.4 μM against C. neoformans (100% germination inhibition at 20 μM); IC50 = 14.6 μM against C. albicans (96% germination inhibition at 20 μM); IC50 = 14.6 μM against C. michiganensis (91% germination inhibition at 20 μM); IC50 = 20.7 μM against F. culmorum (82% germination inhibition at 30 μM); IC50 = 12.1 μM against F. oxysporum (81% germination inhibition at 30 μM); IC50 = 52.5 μM against F. solani (74% germination inhibition at 150 μM); IC50 = 48.4 μM against F. verticillioides (59% germination inhibition at 150 μM); IC50 = 56.2 μM against P. savastanoi (100% germination inhibition at 100 μM); IC50 = 70.7 μM against P. carotovorum (100% germination inhibition at 100 μM). | [53] | |
| TkDEFL1-20 | RTCLSQSHKFKGTCLSNSNCAGVCRTEN-FP--DGECNTHLVERKCYCKRTC | +4 |
| − | [53] | |
| TkDEFL1-2065-82 | GECNTHLVERKCYCKRTC | +2 |
| 74% germination inhibition of C. neoformans at 300 μM; 14% germination inhibition of C. albicans at 300 μM; 94% germination inhibition of C. michiganensis at 300 μM; 48% germination inhibition of F. culmorum at 300 μM; 53% germination inhibition of F. oxysporum at 300 μM; 31% germination inhibition of P. carotovorum at 300 μM; no activity at 300 μM against F. solani, F. verticillioides, P. savastanoi. | [53] | |
| TkDEFL1-23 | RTCLSQSHKFKGTCLSNSNCAGVCRTEN-FP--DGECNSHRLERKCYCKRTC | +5 |
| − | [53] | |
| TkDEFL1-2365-82 | GECNSHRLERKCYCKRTC | +3 |
| IC50 = 39.8 μM against C. neoformans (98% germination inhibition at 100 μM); IC50 = 57.1 μM against C. michiganensis (93% germination inhibition at 300 μM); IC50 = 46 μM against F. culmorum (75% germination inhibition at 75 μM); IC50 = 56.7 μM against F. oxysporum (70% germination inhibition at 150 μM); 28% germination inhibition of P. savastanoi at 300 μM; 29% germination inhibition of P. carotovorum at 100 μM; No activity at 300 μM against C. albicans, F. solani, F. verticillioides. | [53] | |
| TkDEFL1-40 | RTCLSQSHKFKGTCLSNSNCAGVCRTEN-FP--DGECNSHRLERKCFCKRTC | +5 |
| − | [53] | |
| TkDEFL1-4065-82 | GECNSHRLERKCFCKRTC | +3 |
| IC50 = 39.0 μM against C. neoformans (98% germination inhibition at 100 μM); IC50 = 51.6 μM against C. michiganensis (88% germination inhibition at 300 μM); IC50 = 59.3 μM against F. culmorum (78% germination inhibition at 150 μM); IC50 = 49.9 μM against F. oxysporum (78% germination inhibition at 150 μM); 32% germination inhibition of P. savastanoi at 300 μM; 19% germination inhibition of P. carotovorum at 300 μM; No activity at 300 μM against C. albicans, F. solani, F. verticillioides. | [53] | |
| TkDEFL1-36 | RDCLSQSHKFKGACISSSNCAGVCRTEN-FP--DGECHTHNFARKCFCKRAC | +4 |
| − | [53] | |
| TkDEFL1-3665-82 | GECHTHNFARKCFCKRAC | +3 |
| IC50 = 16.8 μM against C. neoformans (100% germination inhibition at 50 μM); 97% germination inhibition of C. albicans at 300 μM; IC50 = 48.6 μM against C. michiganensis (100% germination inhibition at 100 μM); IC50 = 38.3 μM against F. culmorum (80% germination inhibition at 50 μM); IC50 = 52.4 μM against F. oxysporum (80% germination inhibition at 70 μM);45% germination inhibition of F. solani at 300 μM; 55% germination inhibition of F. verticillioides at 180 μM; 54% germination inhibition of P. carotovorum at 300 μM; No activity against P. savastanoi at 300 μM. | [53] | |
| TkDEFL4-4 | EQSISYKSLDQAHQACPKHGTCAPRGFSYTRGAKCIFYNGECLG | +2 |
| − | [53] | |
| TkDEFL4-446-57 | APRGFSYTRGAK | +3 |
| 40% germination inhibition of C. neoformans at 300 μM; 18% germination inhibition of C. albicans at 300 μM; 19% germination inhibition of F. culmorum at 300 μM; 8% germination inhibition of F. oxysporum at 300 μM; 24% germination inhibition of F. solani at 300 μM; 28% germination inhibition of F. verticillioides at 300 μM; 21% germination inhibition of P. savastanoi at 300 μM; 12% germination inhibition of P. carotovorum at 300 μM; no activity at 300 μM against C. michiganensis. | [53] | |
| TkDEFL4-8 | -----------GTTHAIPVPTLRGIEDDDVGFAEREE--AAYP-RRRV LYGDQYISYKGVQASRPACS--GSCAGRGQPYT-GSGCQAIFG-CHGR | +1 |
| − | [53] | |
| TkDEFL4-882-94 | CAGRGQPYT-GSGC | +1 |
| 8% germination inhibition of C. neoformans at 300 μM; 17% germination inhibition of C. albicans at 300 μM; 14% germination inhibition of C. michiganensis at 300 μM; 10% germination inhibition of F. oxysporum at 300 μM; 15% germination inhibition of F. solani at 300 μM; 26% germination inhibition of F. verticillioides at 300 μM; 19% germination inhibition of P. savastanoi at 300 μM; 42% germination inhibition of P. carotovorum at 300 μM; no activity at 300 μM against F. culmorum. | [53] | |
| TkDEFL4-883-93 | AGRGQPYT-GSG | +1 |
| 3% germination inhibition of C. neoformans at 300 μM; 26% germination inhibition of C. albicans at 300 μM; 11% germination inhibition of C. michiganensis at 300 μM; 17% germination inhibition of F. oxysporum at 300 μM; 35% germination inhibition of F. solani at 300 μM; 13% germination inhibition of F. verticillioides at 300 μM; 5% germination inhibition of P. savastanoi at 300 μM; 12% germination inhibition of P. carotovorum at 300 μM; no activity at 300 μM against F. culmorum. | [53] | |
| TkDEFL4-37 | AALARIDAAAAVMPTSSATWMKLEDGVAPELLGSTA---VDLEGHRRV LAS-TSITASSLNPNKAACTRT--CPARGRPYT-GRACLRRYQ-CRQGQ | +6 |
| − | [53] | |
| TkDEFL4-3790-102 | CPARGRPYT-GRAC | +3 |
| 100% germination inhibition of C. neoformans at 300 μM; 38% germination inhibition of C. albicans at 300 μM; 24% germination inhibition of F. culmorum at 300 μM; 31% germination inhibition of F. oxysporum at 300 μM; 34% germination inhibition of F. verticillioides at 150 μM; 38% germination inhibition of P. savastanoi at 300 μM; 36% germination inhibition of P. carotovorum at 300 μM; no activity at 300 μM against C. michiganensis and F. solani. | [53] | |
| TkDEFL4-3791-101 | ARGRPYT-GRA | +3 |
| 10% germination inhibition of C. neoformans at 300 μM; 29% germination inhibition of C. albicans at 300 μM; 29% germination inhibition of F. culmorum at 300 μM; 18% germination inhibition of F. oxysporum at 300 μM; 25% germination inhibition of F. verticillioides at 300 μM; 14% germination inhibition of P. savastanoi at 300 μM; no activity at 300 μM against C. michiganensis, F. solani and P. carotovorum. | [53] | |
| TkDEFL4-20 | DISAGFAASGAAYSIDAAVRQLMSPSSMKLEDGVDPEFSVDLEVHRRV LAG---ISPGALSRNRPACP--GACPAPGGSYT-NRGCQKKYQ-CRG | +2 |
| − | [53] | |
| TkDEFL4-2086-110 | ACP--GACPAPGGSYT-NRGCQKKYQ-CR | +4 |
| 95% germination inhibition of C. neoformans at 300 μM; 11% germination inhibition of C. albicans at 300 μM; 91% germination inhibition of C. michiganensis at 300 μM; 60% germination inhibition of F. culmorum at 150 μM; 54% germination inhibition of F. oxysporum at 150 μM; 14% germination inhibition of P. carotovorum at 300 μM; no activity at 300 μM against F. solani, F. verticillioides and P. savastanoi. | [53] | |
| TkDEFL4-2092-102 | PAPGGSYT-NRG | +1 |
| 15% germination inhibition of C. neoformans at 300 μM; 16% germination inhibition of C. albicans at 300 μM; 9% germination inhibition of C. michiganensis at 300 μM; 6% germination inhibition of F. oxysporum at 300 μM; 14% germination inhibition of F. solani at 300 μM; 5% germination inhibition of F. verticillioides at 300 μM; no activity at 300 μM against F. culmorum, P. savastanoi and P. carotovorum. | [53] | |
| HvDEFL4-1 | ----------------------------AAFAGGTASIDMAAAVHRRI LAD-PGLGSGVYNANNAACGSQ--CAGHGKRYT-GRGCDSFYG-CRSKPP | +4 |
| − | [53] | |
| HvDEFL4-167-77 | AGHGKRYT-GRG | +3 |
| 100% germination inhibition of C. neoformans at 300 μM; 25% germination inhibition of C. albicans at 300 μM; 34% germination inhibition of F. culmorum at 300 μM; 32% germination inhibition of F. verticillioides at 300 μM; No activity at 300 μM against C. michiganensis, F. oxysporum, F. solani, P. savastanoi and P. carotovorum. | [53] |
| Peptide | Amino Acid Sequence | Net Charge at pH 7.0 |
|---|---|---|
| Antimicrobial Activity | Reference | |
| OefDef1.1 | KPCTKLSKGWRGLCAPHKCSSYCIHHEGAYHGACLKNRHSKHYGCYCYYRHCY | +8 |
| IC50 = 1.6 μM against F. graminearum (MIC = 3 μM); IC50 = 1.1 μM against F. virguliforme (MIC = 3 μM); IC50 = 0.4 μM against F. oxysporum (MIC = 1.5 μM); IC50 = 0.7 μM against B. cinerea (MIC = 1.5 μM). | [10] | |
| OefDef1.1_V1 | KPCTKLSKGWRGLCAPHKCSSYCIHHEGAYHGRCRG----FRRRCYCYYRHCY | +10 |
| IC50 = 2−3 μM against F. oxysporum; IC50 = 2−3 μM against B. cinerea. | [10] | |
| OefDef1.1_V2 | KPCTKLSKGWRGLCAPHKCSSYCIHHEGAYHGACHVRN-GKHM-CYCYYRHCY | +7 |
| IC50 = 0.3−0.75 μM against F. oxysporum; IC50 = 0.7−1.5 μM against B. cinerea. | [10] | |
| OefDef1.1_V3 | KPCTKLSKGWRGLCAPHKCSSYCIHHEGAYHGACAAARHSKHYGCYCYYRHCY | +7 |
| Displayed antifungal activity similar to that of the wild-type OefDef1.1. | [10] | |
| OefDef1.1_V4 | KPCTKLSKGWRGLCAPHKCSSYCIHHEGAYHGACLKNAAAKHYGCYCYYRHCY | +7 |
| Displayed antifungal activity similar to that of the wild-type OefDef1.1. | [10] | |
| OefDef1.1_V5 | KPCTKLSKGWRGLCAPHKCSSYCIHHEGAYHGACLKNRHSAAAACYCYYRHCY | +7 |
| Was even more potent than the wild-type OefDef1.1. | [10] |
| Peptide | Amino Acid Sequence | Net Charge at pH 7.0 |
|---|---|---|
| Antimicrobial Activity | Reference | |
| SlSN2 | ----------------------------------------IQTDQVSSNA---ISEGADSYK-KI DCGGACAARCRLSSRPR-LCHRACGTCCARCNCVPPGTSGNTETCP-CYASLTTHG--NKRKCP | +6 |
| − | [46] | |
| γ48-65SlSN2 | GACAARCRLSSRPR-LCHR | +5 |
| IC50 = 4.2 μM against C. neoformans; IC50 = 23.1 μM against C. michiganensis; IC50 = 42.1 μM against F. culmorum; IC50 = 57.1 μM against F. oxysporum; IC50 = 47.5 μM against F. solani; IC50 = 152.0 μM against F. verticilllioides; 96% growth inhibition of C. albicans at 300 μM; 16% growth inhibition of P. savastanoi at 300 μM; 66% growth inhibition of P. carotovorum at 300 μM; 46% growth inhibition of B. cinerea at 300 μM; 31% growth inhibition of B. sorokiniana at 300 μM. | [46] | |
| SlSN9 | QDSIIDLKEVEEDKQQHVGLSQALRVFTRGANRRLVQDIVLKVAKYLNNGDIALAPAPAPPPSPL DCGGLCKYRCSLHSRPN-VCFRACGTCCVRCKCVPPGTFGNREKCGKCYTEMTTHG--NKTKCP | +8 |
| − | [46] | |
| γ89-106SlSN9 | GLCKYRCSLHSRPN-VCFR | +4 |
| IC50 = 5.1 μM against C. neoformans; IC50 = 24.0 μM against C. michiganensis; IC50 = 42.4 μM against F. culmorum; IC50 = 138.8 μM against F. solani; IC50 = 99.8 μM against F. verticilllioides; 96% growth inhibition of C. albicans at 300 μM; 100% growth inhibition of P. savastanoi at 300 μM; 58% growth inhibition of P. carotovorum at 300 μM; 58% growth inhibition of F. oxysporum at 300 μM; 60% growth inhibition of B. cinerea at 300 μM; 33% growth inhibition of B. sorokiniana at 300 μM. | [46] | |
| SlSN10 | -----------------------------------------LQEVISGKP---PAPSPQPPK-PI DCTGSCKTRCSKSSRQN-LCNRACGSCCRTCHCVPPGTSGNYEACP-CYFNLTTHN--STRKCP | +7 |
| − | [46] | |
| γ47-64SlSN10 | GSCKTRCSKSSRQN-LCNR | +5 |
| IC50 = 126.7 μM against F. culmorum; IC50 = 43.8 μM against F. oxysporum; 96% growth inhibition of C. neoformans at 300 μM; 90% growth inhibition of C. albicans at 300 μM; 94% growth inhibition of C. michiganensis at 300 μM; 18% growth inhibition of P. savastanoi at 300 μM; 65% growth inhibition of P. carotovorum at 300 μM; 39% growth inhibition of B. cinerea at 300 μM; 22% growth inhibition of B. sorokiniana at 300 μM; no activity against F. verticillioides at 300 μM. | [46] | |
| TkSN1 | --------------------------------------------------------------ASG FCAGKCAVRCARSRAKRGACMKYCGLCCEECACVPTGRSGSRDECP-CYRDMLTAGPRKRPKCP | +9 |
| − | [53] | |
| TkSN139-57 | GKCAVRCARSRAKRGACMK | +7 |
| IC50 = 6.0 μM against C. neoformans (100% germination inhibition at 20 μM); 98% germination inhibition of C. albicans at 300 μM; IC50 = 12.0 μM against C. michiganensis (100% germination inhibition at 50 μM); IC50 = 27.5 μM against F. culmorum (80% germination inhibition at 150 μM); IC50 = 93.6 μM against F. oxysporum (68% germination inhibition at 150 μM); 54% germination inhibition of F. solani at 300 μM; 18% germination inhibition of P. savastanoi at 180 μM; 59% germination inhibition of P. carotovorum at 300 μM; no activity against F. verticillioides at 300 μM. | [53] |
| Peptide | Amino Acid Sequence | Net Charge at pH 7.0 |
|---|---|---|
| Antimicrobial Activity | Reference | |
| SlLTPg2.4 | -----------AQLSSDCTNVL-----VSMSPCLNYITGN-SSSSPSSGCCTQLGTVVKNNPECLCQVLNGGGS--NMGL NINQTQALALPNACKVQT-PSISKCNAGSPTSSPAGTPSSPNTGGSGSGSIPSSRDASNDASLTKMIDLPFFLILFISSYASAFMA | 0 |
| − | [46] | |
| γ56-72SlLTPg2.4 | SGCCTQLGTVVKNNPEC | 0 |
| 8% growth inhibition of C. albicans at 300 μM; 4% growth inhibition of C. michiganensis at 300 μM; 2% growth inhibition of P. savastanoi at 300 μM; 4% growth inhibition of P. carotovorum at 300 μM; no activity against C. neoformans, F. culmorum, F. oxysporum, F. solani, F. verticillioides, B. cinerea and B. sorokiniana at 300 μM. | [46] | |
| SlLTPg2.5 | ------------QESDDCTNVW-----VSMSPCLNYYVD--STSPQFSGCCTQLSTVVDEKSECLCQVLK------DLG LNINQTRLSALTTACKVQT-PPASNCNGRGSASQGGPNDATSTNMAAPFSFFFLLIASYASIINIT | −4 |
| − | [46] | |
| γ53-69SlLTPg2.5 | SGCCTQLSTVVDEKSEC | −2 |
| 19% growth inhibition of C. neoformans at 300 μM; 13% growth inhibition of C. albicans at 300 μM; 8% growth inhibition of C. michiganensis at 300 μM; 20% growth inhibition of P. savastanoi at 300 μM; 5% growth inhibition of P. carotovorum at 300 μM; no activity against F. culmorum, F. oxysporum, F. solani, F. verticillioides, B. cinerea and B. sorokiniana at 300 μM. | [46] | |
| SlLTPg2.8 | QDSPPAPEAPAPSPGVDCFRVL-----VNMSDCLAFVERGSNTTTPGKGCCPEIAGLLDSNPICLCHMLGRAHSGAKIG FNIDVDKALKLPSACSLEF-PPSTTCSDLGIPVGAPLPSEESPAPSPGKQTLAVFSVLF | −5 |
| − | [46] | |
| γ70-86SlLTPg2.8 | KGCCPEIAGLLDSNPIC | −1 |
| 54% growth inhibition of C. neoformans at 300 μM; 2% growth inhibition of C. albicans at 300 μM; 15% growth inhibition of P. savastanoi at 300 μM; 5% growth inhibition of P. carotovorum at 300 μM; 22% growth inhibition of B. sorokiniana at 300 μM; no activity against C. michiganensis, F. culmorum, F. oxysporum, F. solani, F. verticillioides, B. cinerea at 300 μM. | [46] | |
| TkLTP2.25 | --------------AAGCDASA-------LSPCVGAIMVG---GAVTPGCCARLRAQRA----CLCQYAREP----SYR GYVNSPRAQSVVAACGLPR----PKC | +6 |
| − | [53] | |
| TkLTP2.2550-62 | PGCCARLRAQRA----C | +3 |
| IC50 = 45.0 μM against C. neoformans (100% germination inhibition at 100 μM); 13% germination inhibition of C. albicans at 300 μM; IC50 = 94.6 μM against C. michiganensis (95% germination inhibition at 300 μM); 36% germination inhibition of F. culmorum at 300 μM; 34% germination inhibition of F. oxysporum at 300 μM; 25% germination inhibition of P. savastanoi at 300 μM; 8% germination inhibition of P. carotovorum at 300 μM; no activity at 300 μM against F. solani and F. verticillioides. | [53] | |
| TkLTPd5.6 | --------------AGECGKTPADKMALKLAPCASAGQDP--KSAPSSGCCTAVHTIGKQSPKCLCAVMLSDT---AKS AGIKPEVAMSIPKRCNLVDRPVGYKCGAYTLP | +6 |
| − | [53] | |
| TkLTPd5.659-75 | SGCCTAVHTIGKQSPKC | +2 |
| 11% germination inhibition of C. neoformans at 300 μM; 24% germination inhibition of C. albicans at 300 μM; 23% germination inhibition of C. michiganensis at 300 μM; 18% germination inhibition of P. savastanoi at 300 μM; 2% germination inhibition of P. carotovorum at 300 μM; no activity at 300 μM against F. culmorum, F. oxysporum, F. solani, F. verticillioides. | [53] |
| Peptide | Amino Acid Sequence | Net Charge at pH 7.0 |
|---|---|---|
| Antimicrobial Activity | Reference | |
| SlMEG2 | APISQAKGSEMVPLIEPGKAEKMMIMLNNTRRKLGSFQICALCTCCGG KAVCLPTPCCYAINCNIPNRPFGYCSFTPKTCNCFGCHY | +6 |
| − | [46] | |
| γ92-104SlMEG2 | RPFGYCSFTPKTC | +2 |
| 97% growth inhibition of C. neoformans at 300 μM; 92% growth inhibition of C. michiganensis at 300 μM; 16% growth inhibition of P. savastanoi at 300 μM; 40% growth inhibition of B. cinerea at 300 μM; 36% growth inhibition of B. sorokiniana at 300 μM; no activity against C. albicans, P. carotovorum, F. culmorum, F. oxysporum, F. solani, F. verticillioides at 300 μM. | [46] | |
| TkThi1 | GWCDRACLFQCTHSGGQEDRCRTFCRCPNKSGERNALELCTSGCSSSIC GIINTVDGTEAGKHAAVGRCNEACASFCSKGEHGIQSVAT | 0 |
| − | [53] | |
| TkThi196-109 | VGRCNEACASFCSK | +1 |
| 13% germination inhibition of C. albicans at 300 μM; 40% germination inhibition of C. michiganensis at 300 μM; 7% germination inhibition of P. carotovorum at 300 μM; no activity at 300 μM against C. neoformans, F. culmorum, F. oxysporum, F. solani, F. verticillioides and P. savastanoi. | [53] | |
| Tk-AMP-K2 | PGCPVGQLMKCRTTFPCCGGRCVYC | +3 |
| − | [53] | |
| Tk-AMP-K210-23 | KCRTTFPCCGGRCV | +3 |
| 100% germination inhibition of C. neoformans at 300 μM; 49% germination inhibition of C. albicans at 300 μM; 71% germination inhibition of C. michiganensis at 150 μM; 42% germination inhibition of F. culmorum at 300 μM; 11% germination inhibition of F. oxysporum at 300 μM; 34% germination inhibition of F. solani at 150 μM; 34% germination inhibition of F. verticillioides at 300 μM; 85% germination inhibition of P. savastanoi at 300 μM; 70% germination inhibition of P. carotovorum at 300 μM. | [53] |
| Peptide | Amino Acid Sequence | Net Charge at pH 7.0 |
|---|---|---|
| Antimicrobial Activity | Reference | |
| WAMP-2 | AQRCGDQARGAKCPNCLCCGKYGFCGSGDAYCGKGSCQSQCRGCR | +5 |
| IC50 = 6.6 μM against B. sorokiniana; IC50 = 23.0 μM against A. alternata; IC50 = 8.0 μM against Cladosporium cucumerinum; IC50 = 8.8 μM against F. oxysporum; IC50 = 52.9 μM against F. culmorum. | [54] | |
| WAMP-N | AQRCGDQARGAKC | +2 |
| IC50 = 53.5 μM against B. sorokiniana (80.8% inhibition at 200 μg/mL); IC50 = 75.3 μM against A. alternata (77.2% inhibition at 200 μg/mL); IC50 = 205.5 μM against C. cucumerinum (43.6% inhibition at 200 μg/mL); IC50 = 174.6 μM against F. oxysporum (44.9% inhibition at 200 μg/mL); IC50 = 243.8 μM against F. culmorum (45.0% inhibition at 200 μg/mL); IC50 > 500 μM against F. avenaceum (40.4% inhibition at 400 μg/mL); IC50 = 161.5 μM against Parastagonospora nodorum (63.5% inhibition at 400 μg/mL). | [54] | |
| WAMP-G1 | LCCGKYGFCGSG | +1 |
| IC50 = 228.7 μM against B. sorokiniana (45.7% inhibition at 200 μg/mL); IC50 > 500 μM against A. alternata (16.7% inhibition at 200 μg/mL); IC50 > 500 μM against C. cucumerinum (19.2% inhibition at 400 μg/mL); IC50 > 500 μM against F. oxysporum (16.7% inhibition at 400 μg/mL); IC50 > 500 μM against F. culmorum (6.5% inhibition at 400 μg/mL); IC50 > 500 μM against P. nodorum (28.5% inhibition at 400 μg/mL); no activity against F. avenaceum at 400 μg/mL | [54] | |
| WAMP-G2 | CCGKYGFCGSGDAYC | 0 |
| IC50 = 127.3 μM against B. sorokiniana (54.3% inhibition at 200 μg/mL); IC50 = 94.9 μM against A. alternata (61.1% inhibition at 200 μg/mL); IC50 = 267.4 μM against C. cucumerinum (23.6% inhibition at 200 μg/mL); IC50 = 255.1 μM against F. oxysporum (35.5% inhibition at 200 μg/mL); IC50 > 500 μM against F. culmorum (26.2% inhibition at 400 μg/mL); IC50 = 393.1 μM against F. avenaceum (17.5% inhibition at 200 μg/mL); IC50 = 276.5 μM against P. nodorum (24.1% inhibition at 200 μg/mL). | [54] | |
| WAMP-C | GKGSCQSQCRGCR | +3 |
| IC50 = 313.6 μM against B. sorokiniana (48.7% inhibition at 400 μg/mL); IC50 = 401.9 μM against A. alternata (45.1% inhibition at 400 μg/mL); IC50 = 3.9 μM against C. cucumerinum (70.2% inhibition at 50 μg/mL); IC50 > 500 μM against F. oxysporum (11.5% inhibition at 400 μg/mL); IC50 > 500 μM against F. culmorum (23% inhibition at 400 μg/mL); IC50 = 240.7 μM against P. nodorum (59.9% inhibition at 400 μg/mL); no activity against F. avenaceum at 400 μg/mL. | [54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slezina, M.P.; Istomina, E.A.; Korostyleva, T.V.; Odintsova, T.I. The γ-Core Motif Peptides of Plant AMPs as Novel Antimicrobials for Medicine and Agriculture. Int. J. Mol. Sci. 2023, 24, 483. https://doi.org/10.3390/ijms24010483
Slezina MP, Istomina EA, Korostyleva TV, Odintsova TI. The γ-Core Motif Peptides of Plant AMPs as Novel Antimicrobials for Medicine and Agriculture. International Journal of Molecular Sciences. 2023; 24(1):483. https://doi.org/10.3390/ijms24010483
Chicago/Turabian StyleSlezina, Marina P., Ekaterina A. Istomina, Tatyana V. Korostyleva, and Tatyana I. Odintsova. 2023. "The γ-Core Motif Peptides of Plant AMPs as Novel Antimicrobials for Medicine and Agriculture" International Journal of Molecular Sciences 24, no. 1: 483. https://doi.org/10.3390/ijms24010483
APA StyleSlezina, M. P., Istomina, E. A., Korostyleva, T. V., & Odintsova, T. I. (2023). The γ-Core Motif Peptides of Plant AMPs as Novel Antimicrobials for Medicine and Agriculture. International Journal of Molecular Sciences, 24(1), 483. https://doi.org/10.3390/ijms24010483





