Voltammetric Quantification of Anti-Cancer Antibiotic Bleomycin Using an Electrochemically Pretreated and Decorated with Lead Nanoparticles Screen-Printed Sensor
Abstract
1. Introduction
2. Results and Discussion
2.1. Sensor Characterization
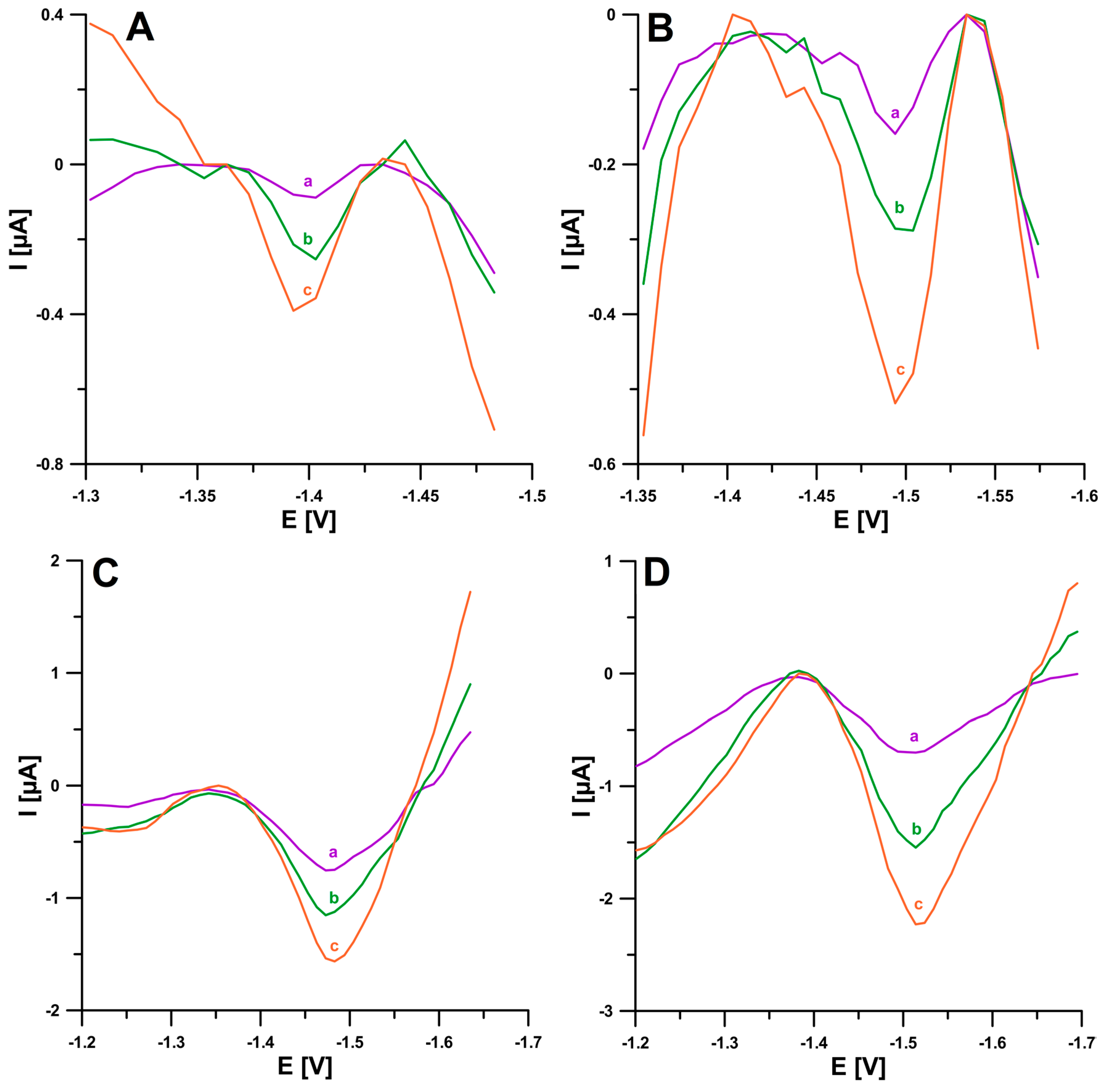
2.2. Supporting Electrolyte Composition
2.3. Voltammetric Behavior of BLM on the pSPCE/PbNPs
2.4. Optimization Step
2.5. Selectivity Studies and Sensor Reproducibility
2.6. Voltammetric Determination of BLM
2.7. Real Samples Analysis
3. Materials and Methods
3.1. Apparatus
3.2. Reagents and Solutions
3.3. Preparation of the pSPCE/PbNPs and Bleomycin (BLM) Analysis
3.4. Real Sample Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, S.; Yang, M.; Li, X.; Liu, X.; Yin, Y.; Cao, Y. Amplified detection of bleomycin based on an electrochemically driven recycling strategy. Anal. Methods 2014, 6, 5573–5577. [Google Scholar] [CrossRef]
- Shiu, G.K.; Goehl, T.J.; Pitlick, W.H. Rapid high-performance liquid chromatographic determination of bleomycin A2 in plasma. J. Pharm. Sci. 1979, 68, 232–234. [Google Scholar] [CrossRef] [PubMed]
- Miura, T. The peroxidase activity of bleomycin-Fe3+ is associated with damage to biological components. J. Biochem. 2015, 157, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Galba, J.; Veizerová, L.; Piešťanský, J.; Mego, M.; Novotný, L.; Dokupilová, S.; Maráková, K.; Havránek, E.; Mikuš, P. HPLC-QTOF-MS method for identification and determination of bleomycin A2 and B2 Fractions. J. Liq. Chromatogr. Relat. Technol. 2015, 38, 294–302. [Google Scholar] [CrossRef]
- Falay, O.; Öztürk, E.; Bölükbaşı, Y.; Gümüş, T.; Örnek, S.; Özbalak, M.; Çetiner, M.; Demirkol, O.; Ferhanoğlu, B. Use of fluorodeoxyglucose positron emission tomography for diagnosis of bleomycin-induced pneumonitis in hodgkin lymphoma. Leuk. Lymphoma 2017, 58, 1114–1122. [Google Scholar] [CrossRef] [PubMed]
- Murray, V.; Chen, J.; Chung, L. The interaction of the metallo-glycopeptide anti-tumour drug bleomycin with DNA. Int. J. Mol. Sci. 2018, 19, 1372. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, C.; Zheng, J.; Qi, H. Ultrasensitive electrogenerated chemiluminescent DNA-based biosensing switch for the determination of bleomycin. Talanta 2013, 103, 8–13. [Google Scholar] [CrossRef]
- Mack, J.M.; Peterson, E.C.; Crary, S.E.; Moran, J.H.; Neville, K.; Pierce, C.D.; Richter, G.T. Pharmacokinetics of bleomycin sclerotherapy in patients with vascular malformations. Pediatr. Blood Cancer 2022, 69, e29733. [Google Scholar] [CrossRef]
- Elson, M.K.; Oken, M.M.; Shafer, R.B.; Broughton, A.; Strong, J.; Braun, C.T.; Crooke, S.T. Comparison of two radioimmunoassays and a microbiologic assay for bleomycin. Med. Pediatr. Oncol. 1978, 5, 213–218. [Google Scholar] [CrossRef]
- Rajani, C.; Kincaid, J.R.; Petering, D.H. Resonance Raman studies of HOO−Co(III)bleomycin and Co(III)bleomycin: Identification of two important vibrational modes, ν(Co−OOH) and ν(O−OH). J. Am. Chem. Soc. 2004, 126, 3829–3836. [Google Scholar] [CrossRef]
- Yin, B.-C.; Wu, D.; Ye, B.-C. Sensitive DNA-based electrochemical strategy for trace bleomycin detection. Anal. Chem. 2010, 82, 8272–8277. [Google Scholar] [CrossRef] [PubMed]
- Zubair Malik, M.; Ahmad, M.; Muahammad, S. Rapid and simultaneous determination of adriamycin, bleomycin, vinblastine and dacarbazine in plasma of hodgkin’s lymphoma patients by a reversed phase HPLC method. J. Chil. Chem. Soc. 2013, 58, 1674–1677. [Google Scholar] [CrossRef]
- Aszalos, A.; Crawford, J.; Vollmer, P.; Kantor, N.; Alexander, T. High-performance liquid chromatographic determination of components of bleomycin preparations. J. Pharm. Sci. 1981, 70, 878–880. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Hu, J.; Li, Q. Adsorptive stripping voltammetry of bleomycin. Analyst 1997, 122, 991–994. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Gai, P.; Li, H.; Li, F. Target-induced diffusivity enhancement for rapid and highly sensitive homogeneous electrochemical detection of BLM in human serum. Talanta 2018, 190, 492–497. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, X.; Zhang, J.; Zhang, H.; Li, Y. Simultaneous voltammetric determination of acetaminophen and isoniazid using MXene modified screen-printed electrode. Biosens. Bioelectron. 2019, 130, 315–321. [Google Scholar] [CrossRef]
- Bagherinasab, Z.; Beitollahi, H.; Yousefi, M.; Bagherzadeh, M.; Hekmati, M. Rapid sol gel synthesis of BaFe12O19 nanoparticles: An excellent catalytic application in the electrochemical detection of tramadol in the presence of acetaminophen. Microchem. J. 2020, 156, 104803. [Google Scholar] [CrossRef]
- Serrano, N.; Castilla, Ò.; Ariño, C.; Diaz-Cruz, M.; Díaz-Cruz, J. Commercial screen-printed electrodes based on carbon nanomaterials for a fast and cost-effective voltammetric determination of paracetamol, ibuprofen and caffeine in water samples. Sensors 2019, 19, 4039. [Google Scholar] [CrossRef]
- Tyszczuk-Rotko, K. Metal film electrodes prepared with a reversibly deposited mediator in voltammetric analysis of metal ions. Curr. Opin. Electrochem. 2019, 17, 128–133. [Google Scholar] [CrossRef]
- Kozak, J.; Tyszczuk-Rotko, K.; Wójciak, M.; Sowa, I.; Rotko, M. Electrochemically pretreated sensor based on screen-printed carbon modified with Pb nanoparticles for determination of testosterone. Materials 2022, 15, 4948. [Google Scholar] [CrossRef]
- Gosser, D.K. Cyclic Voltammetry, Simulation and Analysis of Reaction Mechanisms; Wiley VCH: New York, NY, USA, 1993. [Google Scholar]
- Grabarczyk, M.; Koper, A. How to determine uranium faster and cheaper by adsorptive stripping voltammetry in water samples containing surface active compounds. Electroanalysis 2011, 23, 1442–1446. [Google Scholar] [CrossRef]
- Mocak, J.; Bond, A.M.; Mitchell, S.; Scollary, G. A statistical overview of standard (IUPAC and ACS) and new procedures for determining the limits of detection and quantification: Application to voltammetric and stripping techniques (technical report). Pure Appl. Chem. 1997, 69, 297–328. [Google Scholar] [CrossRef]
- Arafat, S.; Abdelmabood, A.A.; Mohamed, W.; El-Tagy, G.; El-Swify, A. Bleomycin intralesional injections of maxillofacial venous malformations in pediatric patients. Oral Maxillofac. Surg. Cases 2022, 8, 100256. [Google Scholar] [CrossRef]
- Reinert, T.; da Rocha Baldotto, C.S.; Nunes, F.A.P.; de Souza Scheliga, A.A. Bleomycin-induced lung injury. J. Cancer Res. 2013, 2013, 480608. [Google Scholar] [CrossRef]
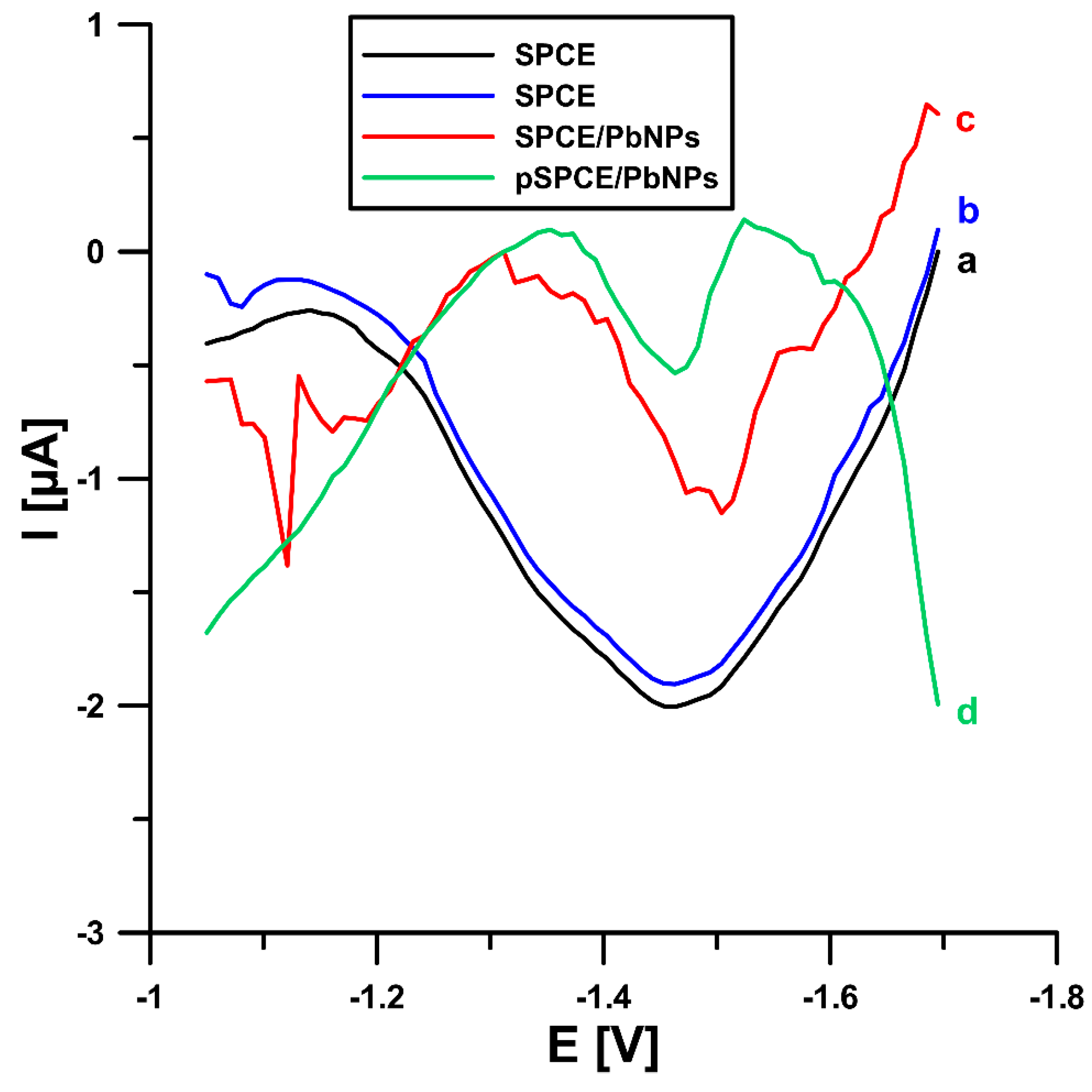
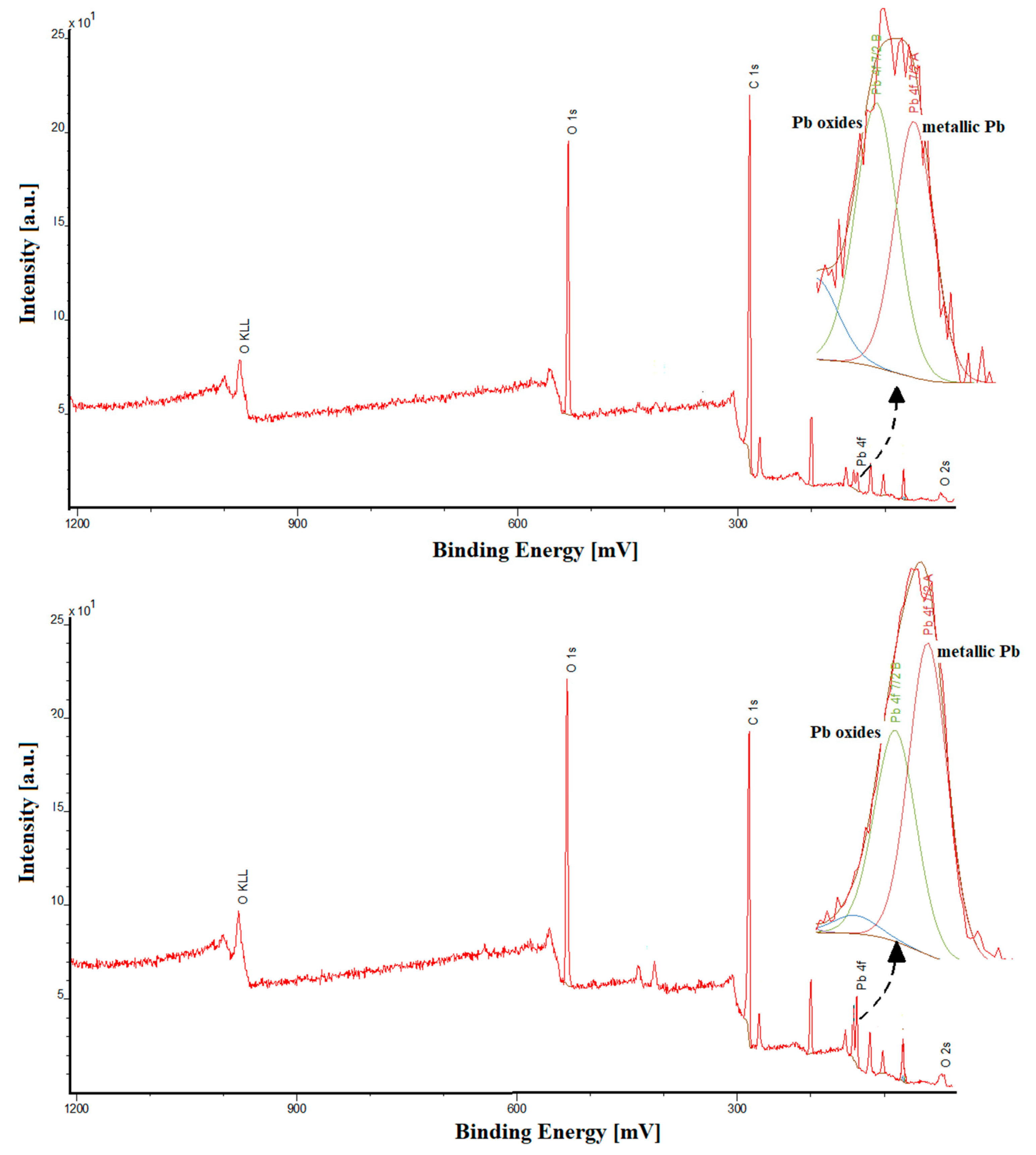
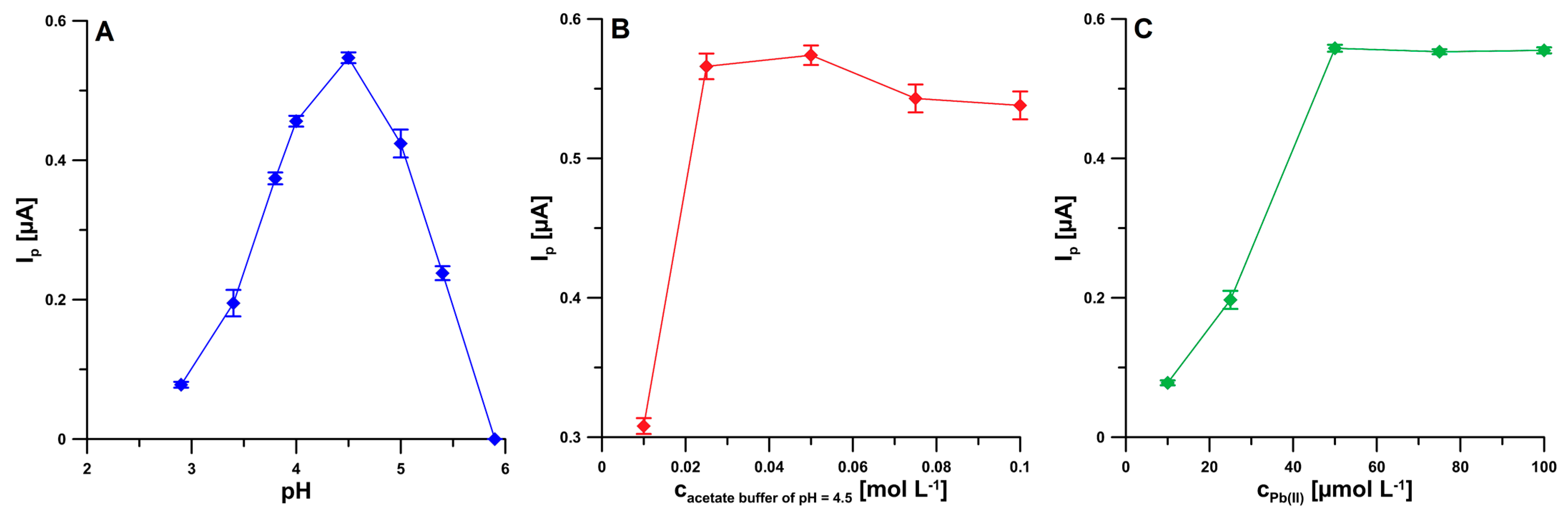
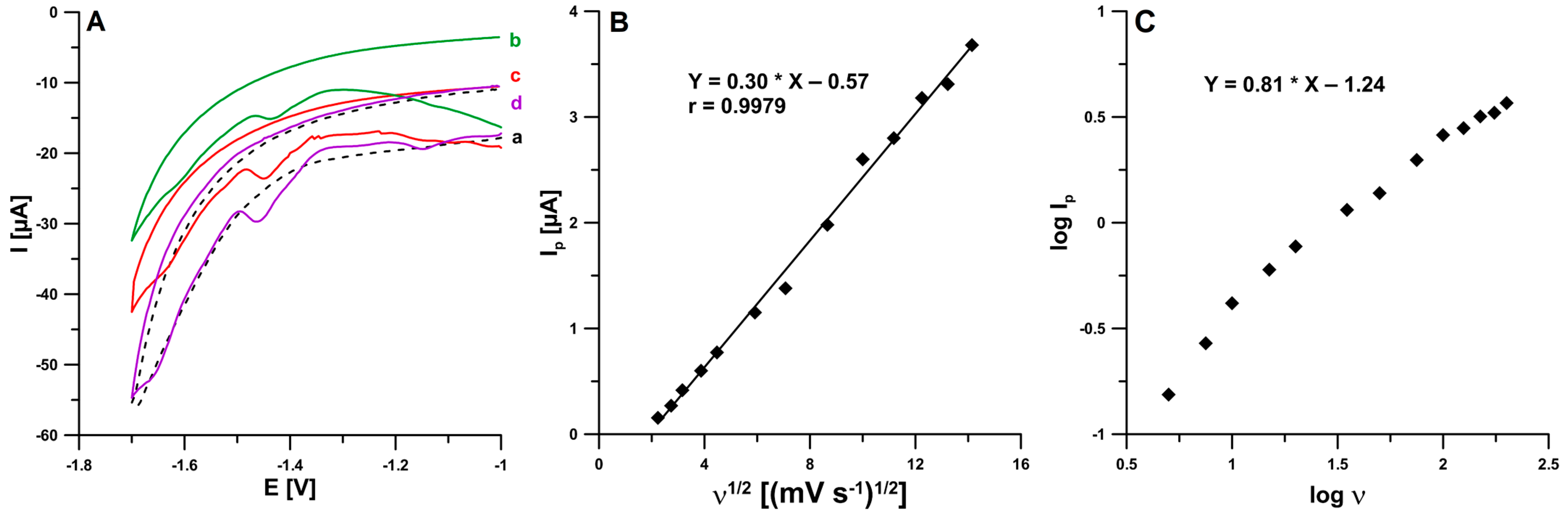
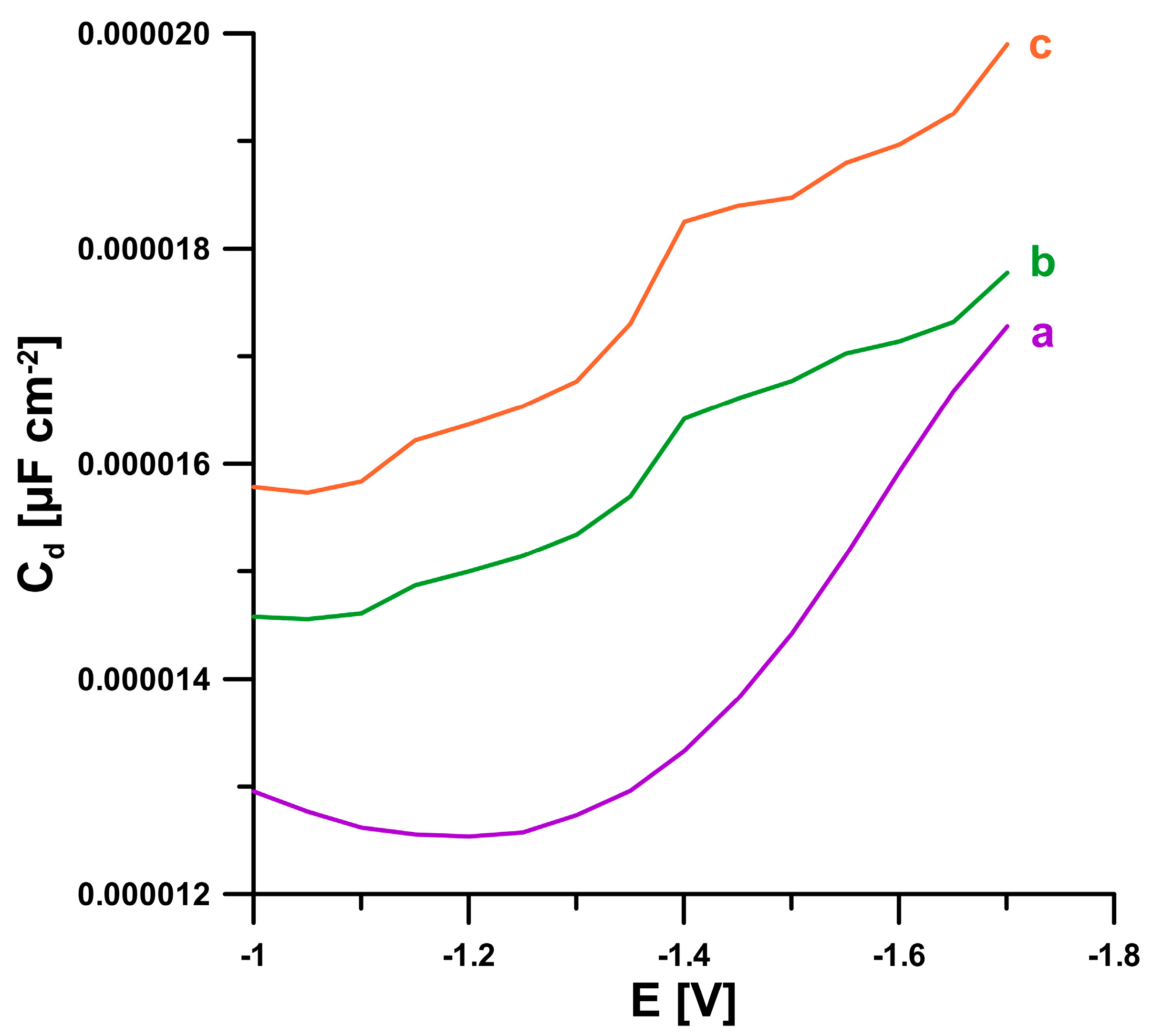
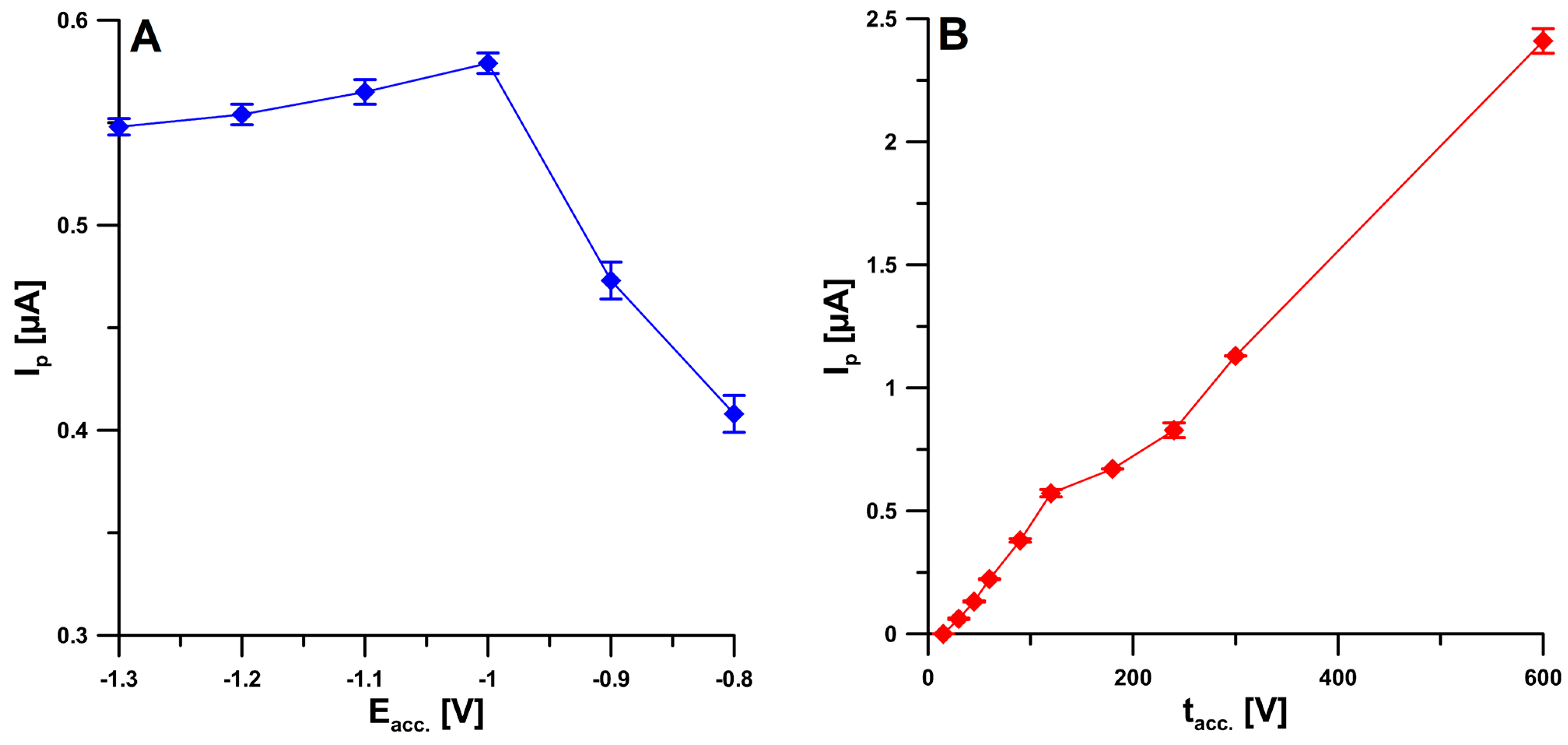
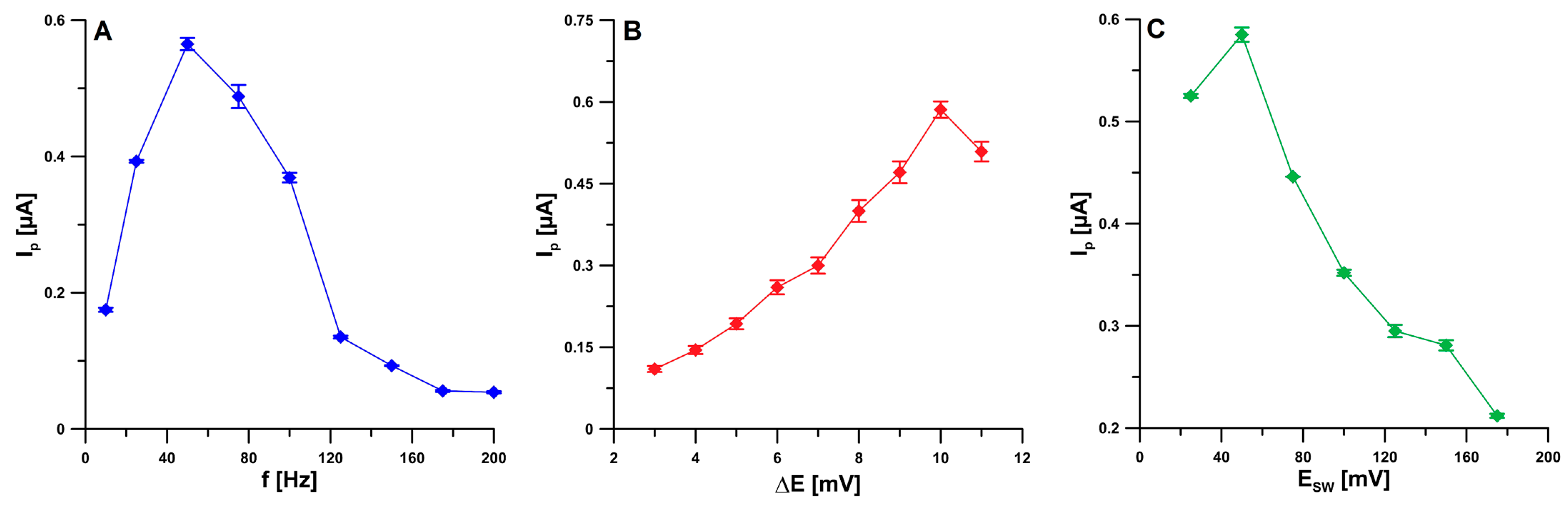
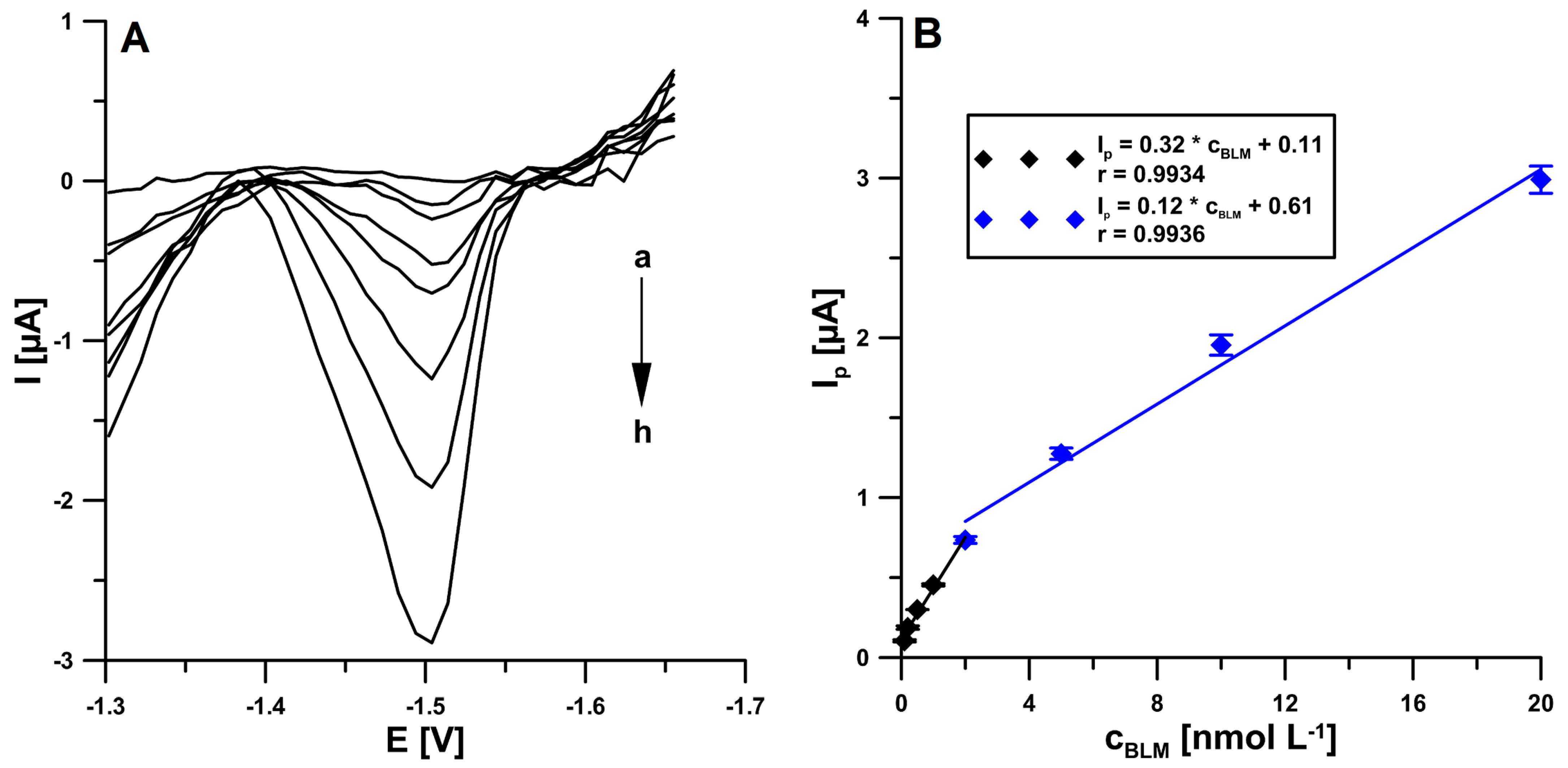
| Electrode | AS [cm2] | Rct [Ω cm2] | RSD [%] (n = 10) | Ref. |
|---|---|---|---|---|
| SPCE | 0.072 | 146.7 | - | [20] |
| SPCE/PbNPs | 0.23 | 121.5 | 17.74 | This work |
| pSPCE/PbNPs | 0.22 | 121.3 | 3.25 | [20] |
| Electrode | Method | Linear Range (M) | LOD (M) | Application | Ref. |
|---|---|---|---|---|---|
| AuE/DNA | DPV | 1.0 × 10−12–1.0 × 10−7 | 7.4 × 10−13 | Serum | [1] |
| AuE/DNA (E-DNA sensor) | SWV | 1.0 × 10−10–1.0 × 10−6 | 1.0 × 10−10 | Serum | [11] |
| HMDE | AdSV | 1.0 × 10−9–1.0 × 10−7 | 5.0 × 10−10 | Serum | [14] |
| ITO/MB-DNA | DPV | 1.0 × 10−10–1.0 × 10−7 | 3.3 × 10−11 | Serum | [15] |
| pSPCE/PbNPs | SWAdSV | 1.0 × 10−10–2.0 × 10−9 2.0 × 10−9–2.0 × 10−8 | 2.8 × 10−11 | Urine, wastewater | This work |
| Sample | BLM Concentration [µM] ± SD (n = 3) | Coefficient of Variation * [%] | Recovery ** [%] | ||
|---|---|---|---|---|---|
| Added | Found SWAdSV | Found in Electrochemical Cell | |||
| Purified wastewater | 0.005 0.02 | 0.0048 ± 0.00012 0.0193 ± 0.00035 | 0.00048 ± 0.000012 0.00193 ± 0.000035 | 2.13 1.16 | 103.5 99.0 |
| RM of human urine | 20.0 40.0 | 20.7 ± 0.44 39.6 ± 0.46 | 0.00207 ± 0.000044 0.00396 ± 0.000046 | 2.50 1.81 | 96.0 96.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozak, J.; Tyszczuk-Rotko, K.; Metelka, R. Voltammetric Quantification of Anti-Cancer Antibiotic Bleomycin Using an Electrochemically Pretreated and Decorated with Lead Nanoparticles Screen-Printed Sensor. Int. J. Mol. Sci. 2023, 24, 472. https://doi.org/10.3390/ijms24010472
Kozak J, Tyszczuk-Rotko K, Metelka R. Voltammetric Quantification of Anti-Cancer Antibiotic Bleomycin Using an Electrochemically Pretreated and Decorated with Lead Nanoparticles Screen-Printed Sensor. International Journal of Molecular Sciences. 2023; 24(1):472. https://doi.org/10.3390/ijms24010472
Chicago/Turabian StyleKozak, Jędrzej, Katarzyna Tyszczuk-Rotko, and Radovan Metelka. 2023. "Voltammetric Quantification of Anti-Cancer Antibiotic Bleomycin Using an Electrochemically Pretreated and Decorated with Lead Nanoparticles Screen-Printed Sensor" International Journal of Molecular Sciences 24, no. 1: 472. https://doi.org/10.3390/ijms24010472
APA StyleKozak, J., Tyszczuk-Rotko, K., & Metelka, R. (2023). Voltammetric Quantification of Anti-Cancer Antibiotic Bleomycin Using an Electrochemically Pretreated and Decorated with Lead Nanoparticles Screen-Printed Sensor. International Journal of Molecular Sciences, 24(1), 472. https://doi.org/10.3390/ijms24010472







