β-Hydroxy-β-Methylbutyric Acid Promotes Repair of Sheep Myoblast Injury by Inhibiting IL-17/NF-κB Signaling
Abstract
1. Introduction
2. Results
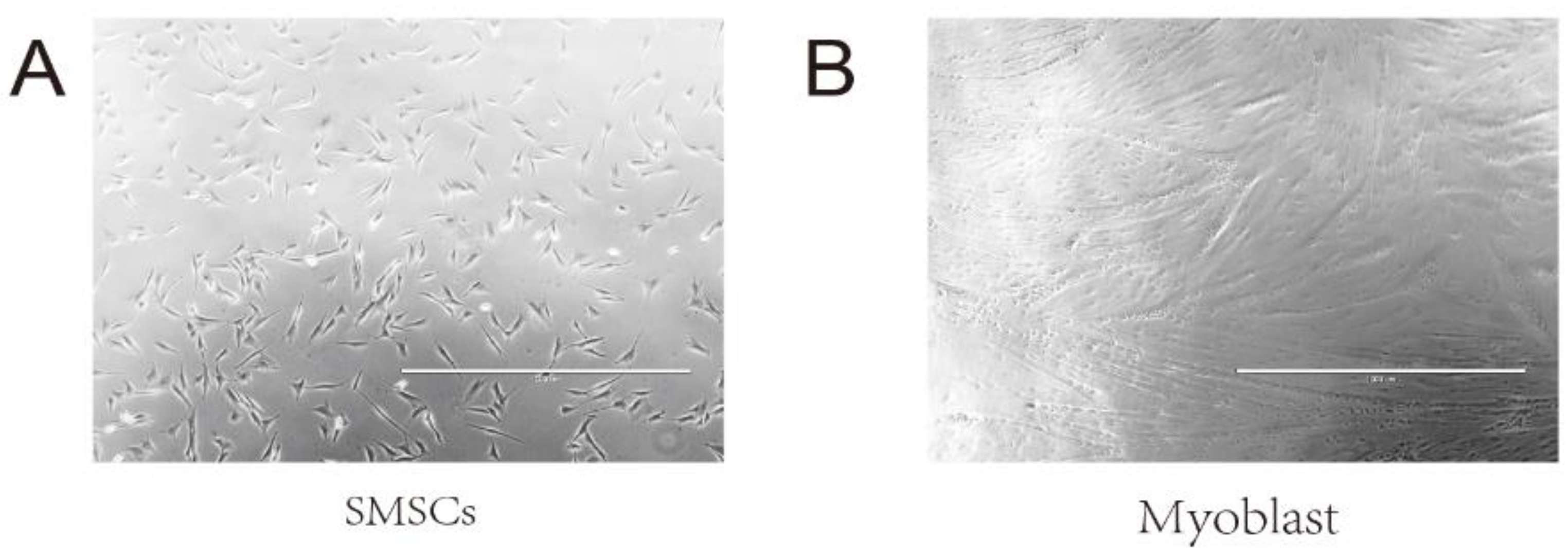
2.1. Immunofluorescence of SMSCs
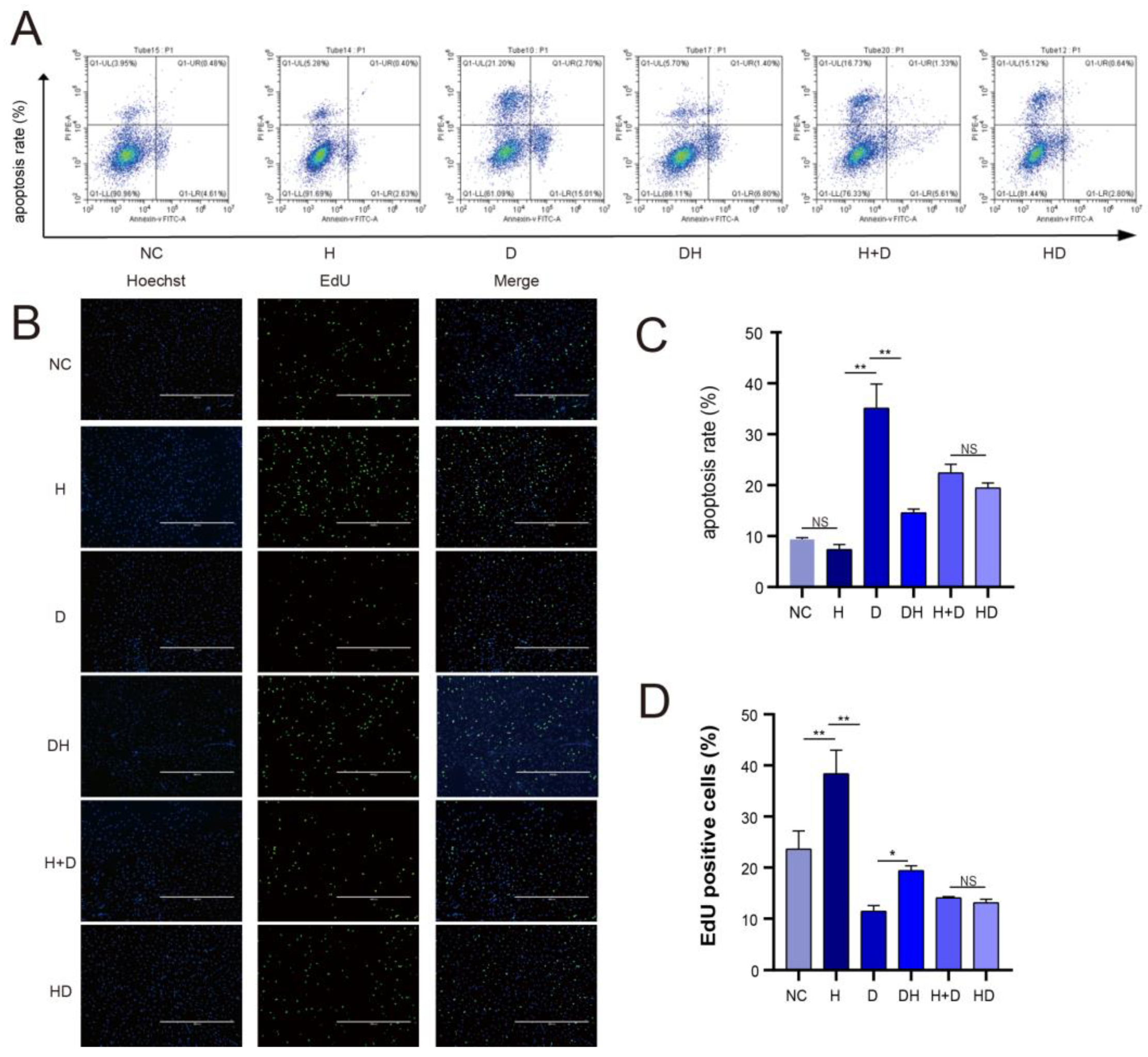
2.2. Cell Apoptosis and Proliferation
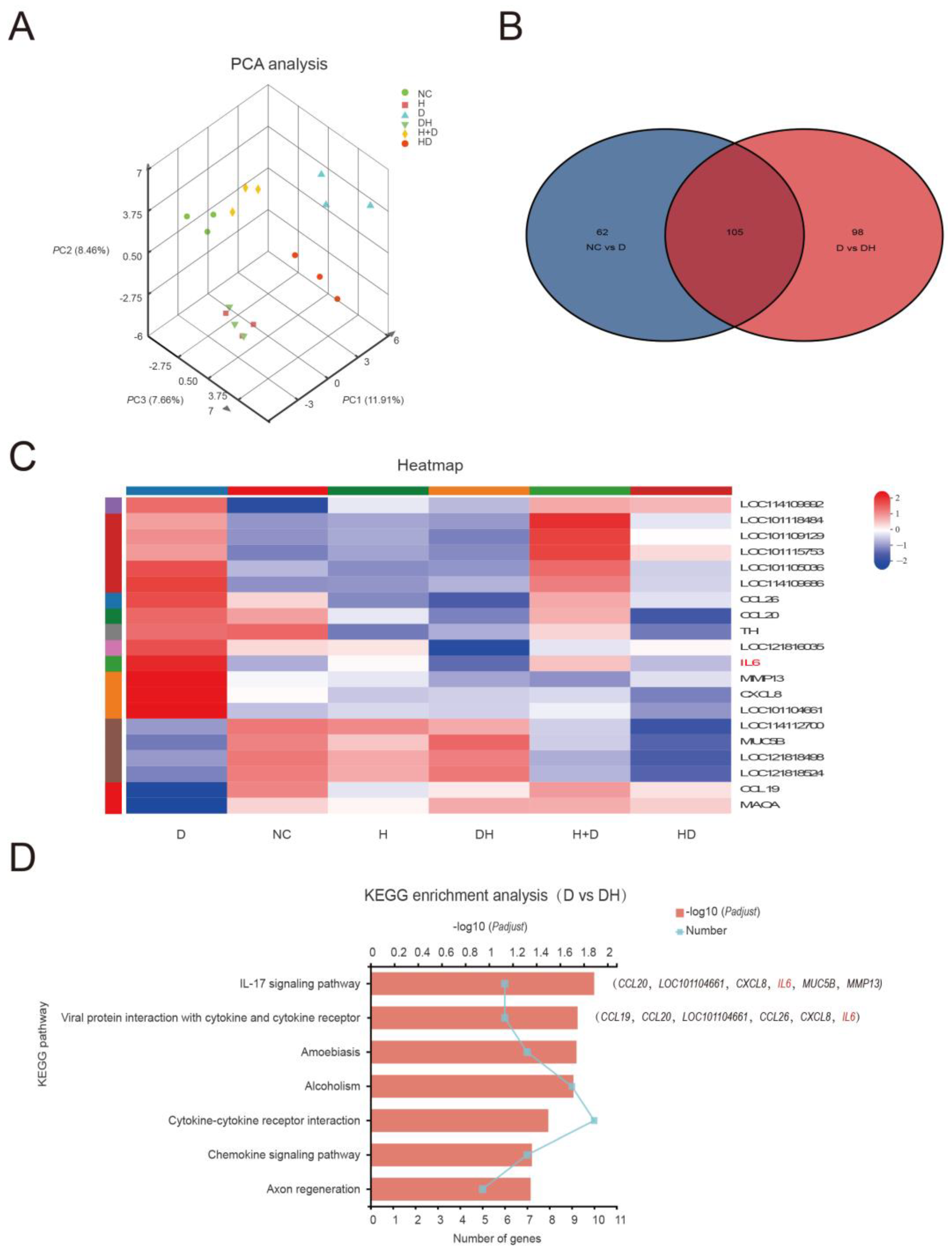
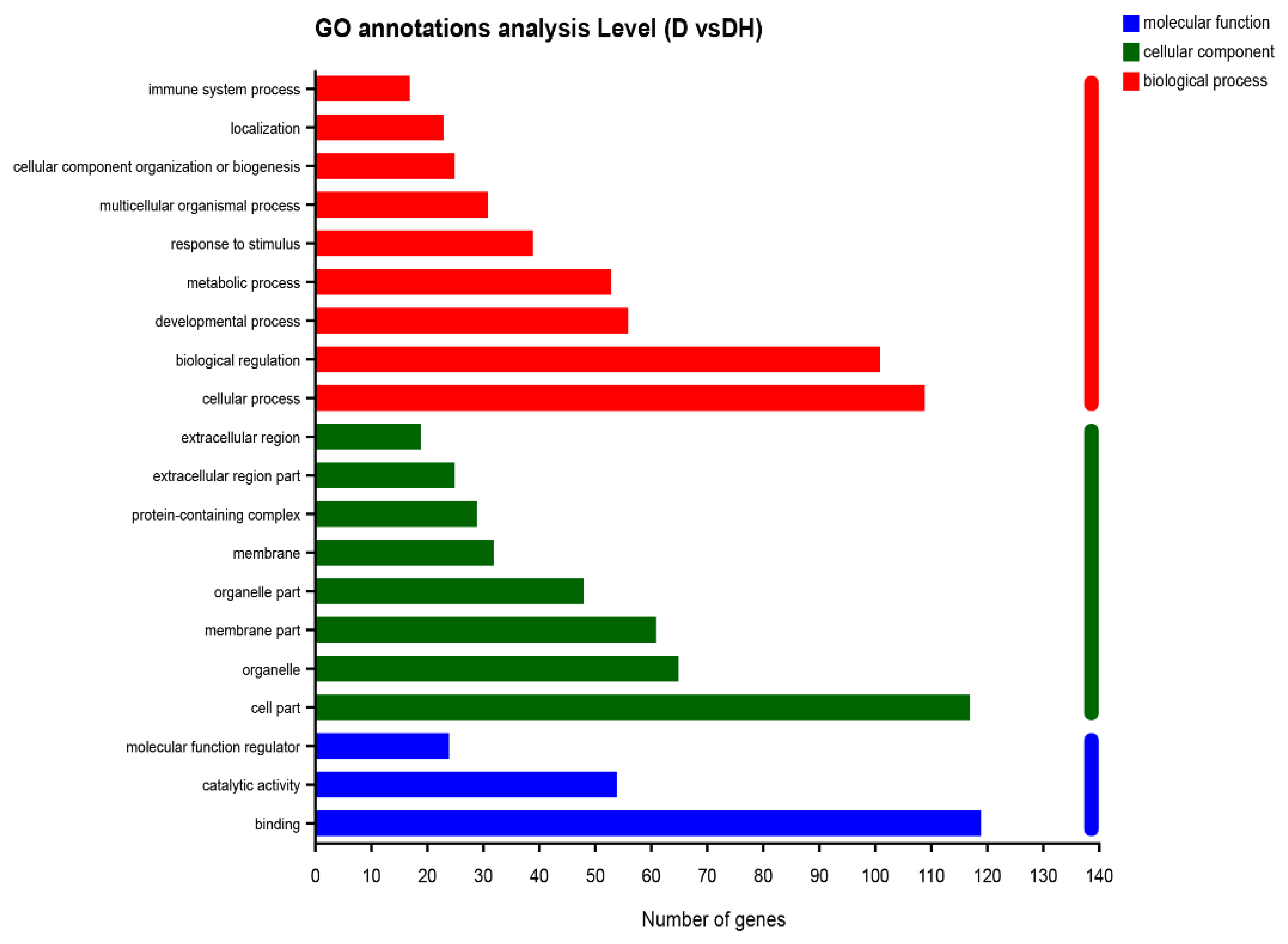
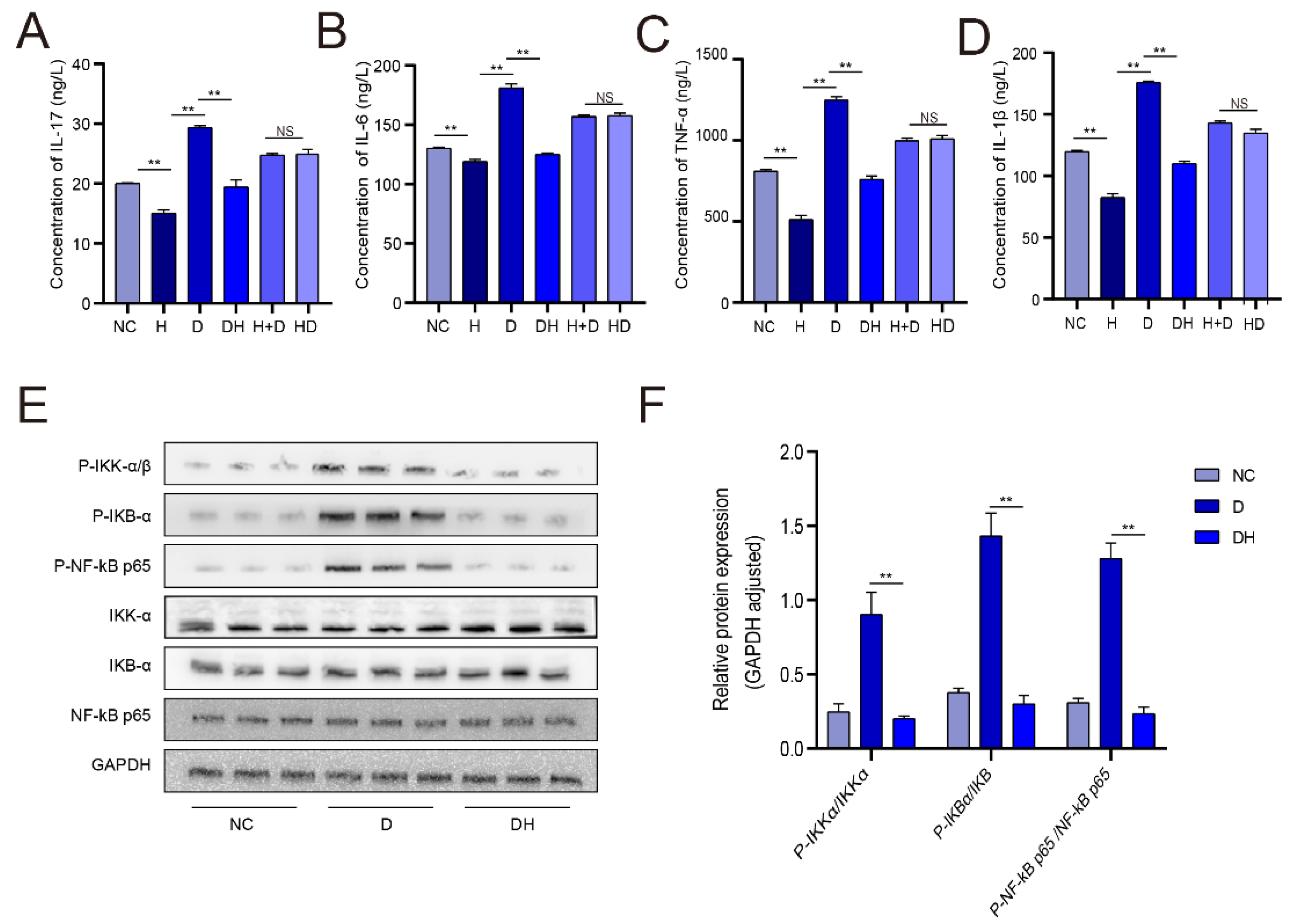
2.3. HMB Repairs Myoblast Injury by Blocking IL-17 and NF-κB Signaling
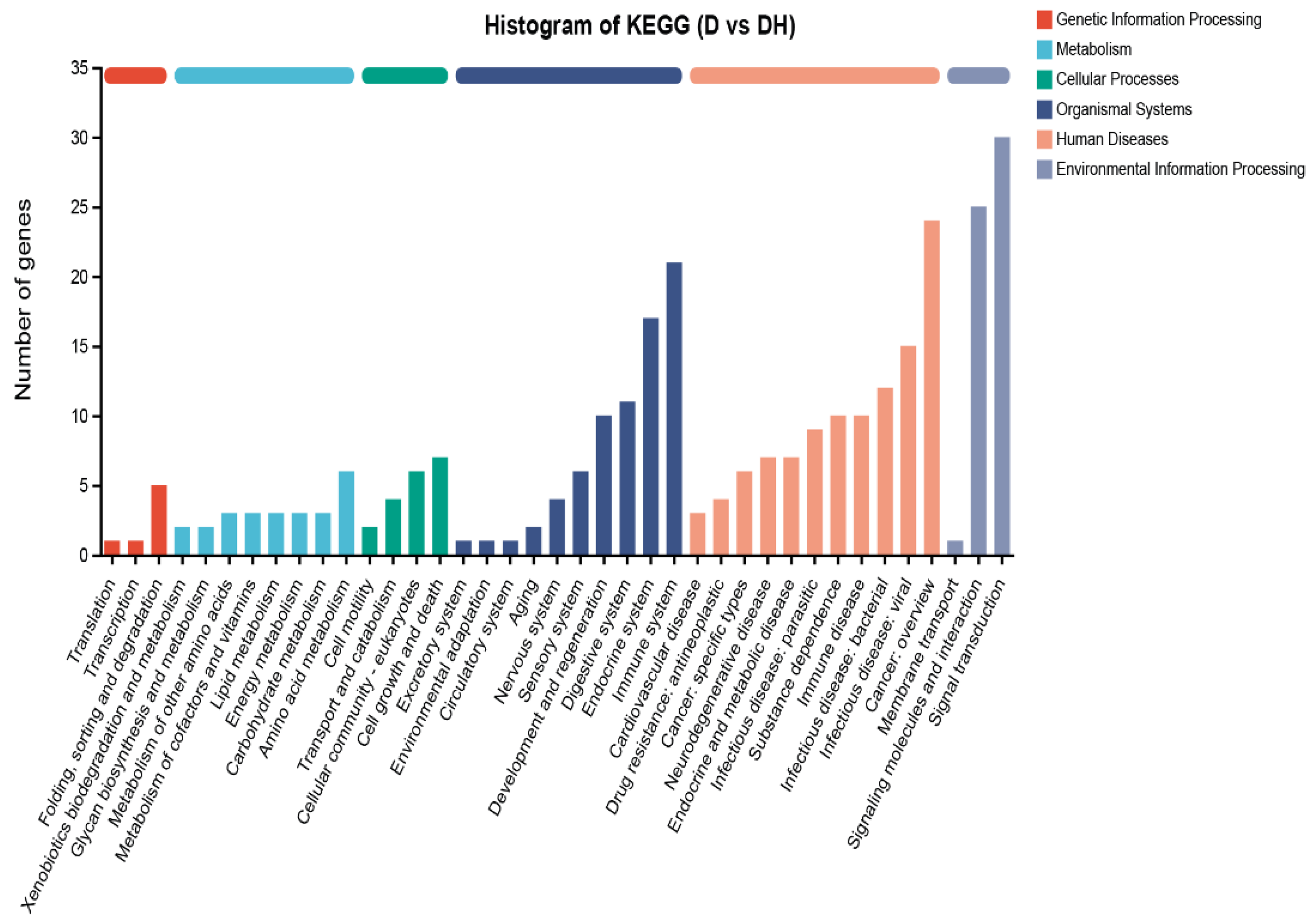
3. Discussion
4. Materials and Methods
4.1. Cell Isolation and Culture
4.2. Construction of Myoblast Injury Model
4.3. Detection of Cell Apoptosis by Flow Cytometry
4.4. EdU Assay
4.5. ELISA
4.6. Western Blot
4.7. RNA-Seq Analysis
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix B
| Sample | Raw Reads | Raw Bases | Clean Reads | Clean Bases | Error Rate (%) | Q20(%) | Q30(%) | GC Content |
|---|---|---|---|---|---|---|---|---|
| (%) | ||||||||
| NC1 | 6,100,7804 | 9,212,178,404 | 59,795,486 | 8,641,876,217 | 0.0252 | 97.86 | 94 | 53.63 |
| NC2 | 5,404,5836 | 8,160,921,236 | 52,765,256 | 7,614,406,901 | 0.0256 | 97.69 | 93.62 | 53.8 |
| NC3 | 5,465,8296 | 8,253,402,696 | 53,098,864 | 7,627,471,914 | 0.0254 | 97.77 | 93.87 | 53.77 |
| H1 | 58,538,782 | 8,839,356,082 | 57,553,362 | 8,395,202,659 | 0.0251 | 97.93 | 94.16 | 53.55 |
| H2 | 52,922,990 | 7,991,371,490 | 51,795,790 | 7,545,225,170 | 0.0257 | 97.66 | 93.54 | 53.5 |
| H3 | 49,916,272 | 7,537,357,072 | 48,886,386 | 7,108,693,973 | 0.0249 | 97.98 | 94.32 | 53.38 |
| D1 | 54,033,588 | 8,159,071,788 | 52,352,756 | 7,584,780,012 | 0.0253 | 97.8 | 93.95 | 53.79 |
| D2 | 62,382,402 | 9,419,742,702 | 61,289,950 | 8,929,993,888 | 0.0252 | 97.88 | 94.03 | 53.47 |
| D3 | 52,905,810 | 7,988,777,310 | 51,661,206 | 7,559,307,614 | 0.0253 | 97.8 | 93.91 | 53.46 |
| HD1 | 54,294,954 | 8,198,538,054 | 53,026,950 | 7,734,884,116 | 0.0255 | 97.75 | 93.78 | 53.74 |
| HD2 | 52,512,512 | 7,929,389,312 | 51,447,990 | 7,544,672,078 | 0.0251 | 97.93 | 94.18 | 53.33 |
| HD3 | 51934726 | 7,842,143,626 | 50,781,802 | 7,445,053,103 | 0.025 | 97.93 | 94.21 | 53.39 |
| D+H1 | 54,951,310 | 8,297,647,810 | 53,563,784 | 7,798,454,682 | 0.0255 | 97.72 | 93.71 | 53.69 |
| D+H2 | 52,651,856 | 7,950,430,256 | 50,706,446 | 7,391,244,697 | 0.0256 | 97.67 | 93.66 | 53.6 |
| D+H3 | 50,439,524 | 7,616,368,124 | 48,796,802 | 7,106,788,337 | 0.0254 | 97.8 | 93.9 | 53.71 |
| DH1 | 57,570,642 | 8,693,166,942 | 56,450,908 | 8,226,601,480 | 0.025 | 97.94 | 94.22 | 53.38 |
| DH2 | 57,144,710 | 8,628,851,210 | 55,878,600 | 8,101,309,031 | 0.0251 | 97.9 | 94.12 | 53.5 |
| DH3 | 46,672,916 | 7,047,610,316 | 45,545,154 | 6,640,024,249 | 0.0252 | 97.85 | 94.06 | 53.59 |
References
- Talebi, R.; Ghaffari, M.R.; Zeinalabedini, M.; Abdoli, R.; Mardi, M. Genetic basis of muscle-related traits in sheep: A review. Anim. Genet. 2022, 53, 723–739. [Google Scholar] [CrossRef]
- Georges, M.; Charlier, C.; Hayes, B. Harnessing genomic information for livestock improvement. Nat. Rev. Genet. 2019, 20, 135–156. [Google Scholar] [CrossRef]
- Liu, J.; Saul, D.; Böker, K.O.; Ernst, J.; Lehman, W.; Schilling, A.F. Current Methods for Skeletal Muscle Tissue Repair and Regeneration. Biomed Res. Int. 2018, 2018, 1984879. [Google Scholar] [CrossRef]
- Morgan, J.E.; Partridge, T.A. Muscle satellite cells. Int. J. Biochem. Cell Biol. 2003, 35, 1151–1156. [Google Scholar] [CrossRef]
- Aicale, R.; Tarantino, D.; Maffulli, N. Overuse injuries in sport: A comprehensive overview. J. Orthop. Surg. Res. 2018, 13, 309. [Google Scholar] [CrossRef] [PubMed]
- Tatsumi, R.; Liu, X.; Pulido, A.; Morales, M.; Sakata, T.; Dial, S.; Hattori, A.; Ikeuchi, Y.; Allen, R.E. Satellite cell activation in stretched skeletal muscle and the role of nitric oxide and hepatocyte growth factor. Am. J. Physiol. Cell Physiol. 2006, 290, C1487–C1494. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.H.; Gil, J.H.; Quan, H.; Viet, D.H.; Kim, C.K. Effect of 8-week leucine supplementation and resistance exercise training on muscle hypertrophy and satellite cell activation in rats. Physiol. Rep. 2018, 6, e13725. [Google Scholar] [CrossRef]
- Demonbreun, A.R.; McNally, E.M. Muscle cell communication in development and repair. Curr. Opin. Pharmacol. 2017, 34, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Zammit, P.S. Function of the myogenic regulatory factors Myf5, MyoD, Myogenin and MRF4 in skeletal muscle, satellite cells and regenerative myogenesis. Semin. Cell Dev. Biol. 2017, 72, 19–32. [Google Scholar] [CrossRef]
- Eley, H.L.; Tisdale, M.J. Skeletal muscle atrophy, a link between depression of protein synthesis and increase in degradation. J. Biol. Chem. 2007, 282, 7087–7097. [Google Scholar] [CrossRef]
- Jäger, R.; Kerksick, C.M.; Campbell, B.I.; Cribb, P.J.; Wells, S.D.; Skwiat, T.M.; Purpura, M.; Ziegenfuss, T.N.; Ferrando, A.A.; Arent, S.M.; et al. International Society of Sports Nutrition Position Stand: Protein and exercise. J. Int. Soc. Sports Nutr. 2017, 14, 20. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, E.K. Nutritional Strategies to Optimize Performanceand Recovery in Rowing Athletes. Nutrients 2020, 12, 1685. [Google Scholar] [CrossRef] [PubMed]
- Panton, L.B.; Rathmacher, J.A.; Baier, S.; Nissen, S. Nutritional role of the leucine metabolite β-hydroxy β-methylbutyrate (HMB)-Science Direct. J. Nutr. Biochem. 1997, 8, 300–311. [Google Scholar]
- Flummer, C.; Theil, P.K. Effect of β-hydroxy β-methyl butyrate supplementation of sows in late gestation and lactation on sow production of colostrum and milk and piglet performance. J. Anim. Sci. 2012, 90 (Suppl. 4), 372–374. [Google Scholar] [CrossRef] [PubMed]
- Supinski, G.S.; Callahan, L.A. β-hydroxy-β-methylbutyrate (HMB) prevents sepsis-induced diaphragm dysfunction in mice. Respir. Physiol. Neurobiol. 2014, 196, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Van Koevering, M.T.; Dolezal, H.G.; Gill, D.R.; Owens, F.N.; Strasia, C.A.; Buchanan, D.S.; Lake, R.; Nissen, S. Effects of beta-hydroxy-beta-methyl butyrate on performance and carcass quality of feedlot steers. J. Anim. Sci. 1994, 72, 1927–1935. [Google Scholar] [CrossRef]
- Zabek, K.; Wojcik, R.; Milewski, S.; Malaczewska, J.; Tanski, Z.; Siwicki, A.K. Effect of β-hydroxy-β-methylbutyrate acid on meat performance traits and selected indicators of humoral immunity in goats. Jpn. J. Vet. Res. 2016, 64, 247–256. [Google Scholar]
- Järvinen, T.A.; Järvinen, T.L.; Kääriäinen, M.; Kalimo, H.; Järvinen, M. Muscle injuries: Biology and treatment. Am. J. Sports Med. 2005, 33, 745–764. [Google Scholar] [CrossRef]
- Greising, S.M.; Corona, B.T.; Call, J.A. Musculoskeletal Regeneration, Rehabilitation, and Plasticity Following Traumatic Injury. Int. J. Sports Med. 2020, 41, 495–504. [Google Scholar] [CrossRef]
- Duan, Y.; Li, F.; Liu, H.; Li, Y.; Liu, Y.; Kong, X.; Zhang, Y.; Deng, D.; Tang, Y.; Feng, Z.; et al. Nutritional and regulatory roles of leucine in muscle growth and fat reduction. Front. Biosci. 2015, 20, 796–813. [Google Scholar]
- Duan, Y.; Zhong, Y.; Song, B.; Zheng, C.; Xu, K.; Kong, X.; Li, F. Suppression of protein degradation by leucine requires its conversion to β-hydroxy-β-methyl butyrate in C2C12 myotubes. Aging 2019, 11, 11922–11936. [Google Scholar] [CrossRef]
- Dumont, N.A.; Bentzinger, C.F.; Sincennes, M.C.; Rudnicki, M.A. Satellite Cells and Skeletal Muscle Regeneration. Compr. Physiol. 2015, 5, 1027–1059. [Google Scholar] [PubMed]
- Buckingham, M.; Relaix, F. PAX3 and PAX7 as upstream regulators of myogenesis. Semin. Cell Dev. Biol. 2015, 44, 115–125. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Chen, X.; Yu, X. Evaluation of New Monoclonal Anti-MyoD1 (MX049) for the Diagnosis of Rhabdomyosarcoma: Comparison with 5.8A, EP212, Anti-Desmin, Anti-Myogenin, and Fluorescence in situ Hybridization. Ann. Clin. Lab. Sci. 2020, 50, 412–416. [Google Scholar]
- Yoshioka, Y.; Kubota, Y.; Samukawa, Y.; Yamashita, Y.; Ashida, H. Glabridin inhibits dexamethasone-induced muscle atrophy. Arch. Biochem. Biophys. 2019, 664, 157–166. [Google Scholar] [CrossRef]
- Ehling, S.; Reddy, T.M. Direct Analysis of Leucine and Its Metabolites β-Hydroxy-β-methylbutyric Acid, α-Ketoisocaproic Acid, and α-Hydroxyisocaproic Acid in Human Breast Milk by Liquid Chromatography-Mass Spectrometry. J. Agric. Food Chem. 2015, 63, 7567–7573. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J. Beta-Hydroxy-Beta-Methyl Butyrate (HMB): From Experimental Data to Clinical Evidence in Sarcopenia. Curr. Protein Pept. Sci. 2018, 19, 668–672. [Google Scholar] [CrossRef]
- Holeček, M. Beta-hydroxy-beta-methylbutyrate supplementation and skeletal muscle in healthy and muscle-wasting conditions. J. Cachexia Sarcopenia Muscle 2017, 8, 529–541. [Google Scholar] [CrossRef]
- Osuka, Y.; Kojima, N.; Sasai, H.; Wakaba, K.; Miyauchi, D.; Tanaka, K.; Kim, H. Effects of exercise and/or β-hydroxy-β-methylbutyrate supplementation on muscle mass, muscle strength, and physical performance in older women with low muscle mass: A randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 2021, 114, 1371–1385. [Google Scholar] [CrossRef]
- Song, X.; Qian, Y. The activation and regulation of IL-17 receptor mediated signaling. Cytokine 2013, 62, 175–182. [Google Scholar] [CrossRef]
- de Morales, J.M.G.R.; Puig, L.; Daudén, E.; Cañete, J.D.; Pablos, J.L.; Martín, A.O.; Juanatey, C.G.; Adán, A.; Montalbán, X.; Borruel, N.; et al. Critical role of interleukin (IL)-17 in inflammatory and immune disorders: An updated review of the evidence focusing in controversies. Autoimmun. Rev. 2020, 19, 102429. [Google Scholar] [CrossRef] [PubMed]
- Jimi, E.; Fei, H.; Nakatomi, C. NF-κB Signaling Regulates Physiological and Pathological Chondrogenesis. Int. J. Mol. Sci. 2019, 20, 6275. [Google Scholar] [CrossRef] [PubMed]
- Iwakura, Y.; Ishigame, H.; Saijo, S.; Nakae, S. Functional specialization of interleukin-17 family members. Immunity 2011, 34, 149–162. [Google Scholar] [CrossRef]
- De Simone, V.; Franze, E.; Ronchetti, G.; Colantoni, A.; Fantini, M.C.; Di Fusco, D.; Sica, G.S.; Sileri, P.; MacDonald, T.T.; Pallone, F.; et al. Th17-type cytokines, IL-6 and TNF-α synergistically activate STAT3 and NF-kB to promote colorectal cancer cell growth. Oncogene 2015, 34, 3493–3503. [Google Scholar] [CrossRef]
- Kang, S.; Narazaki, M.; Metwally, H.; Kishimoto, T. Historical overview of the interleukin-6 family cytokine. J. Exp. Med. 2020, 217, e20190347. [Google Scholar] [CrossRef]
- Heinrich, P.C.; Behrmann, I.; Haan, S.; Hermanns, H.M.; Müller-Newen, G.; Schaper, F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 2003, 374, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Unver, N.; McAllister, F. IL-6 family cytokines: Key inflammatory mediators as biomarkers and potential therapeutic targets. Cytokine Growth Factor Rev. 2018, 41, 10–17. [Google Scholar] [CrossRef]
- Kimura, A.; Kishimoto, T. IL-6: Regulator of Treg/Th17 balance. Eur. J. Immunol. 2010, 40, 1830–1835. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. Regulation of NF-κB by TNF family cytokines. Semin. Immunol. 2014, 26, 253–266. [Google Scholar] [CrossRef]
- Hong, J.J.; Hadeler, E.K.; Mosca, M.L.; Brownstone, N.D.; Bhutani, T.; Liao, W.J. TNF-alpha inhibitors and ustekinumab for the treatment of psoriasis: Therapeutic utility in the era of IL-17 and IL-23 inhibitors. J. Psoriasis Psoriatic Arthritis 2022, 7, 79–92. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Mao, X.; Huang, J.; Ding, Y.; Wu, J.; Dong, S.; Kong, L.; Gao, G.; Li, C.Y.; Wei, L. KOBAS 2.0: A web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011, 39, W316–W322. [Google Scholar] [CrossRef]

| Medium | Ingredients |
|---|---|
| Complete medium | data High-glucose medium + 10%FBS + 1% penicillin/streptomycin (P/S) |
| Myoblast Differentiation medium | data High-glucose medium + 2%HS |
| Storage and transport | PBS + 5%P/S |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, J.; Li, B.; Yan, Y.; Huang, X.; Zhang, E. β-Hydroxy-β-Methylbutyric Acid Promotes Repair of Sheep Myoblast Injury by Inhibiting IL-17/NF-κB Signaling. Int. J. Mol. Sci. 2023, 24, 444. https://doi.org/10.3390/ijms24010444
Zheng J, Li B, Yan Y, Huang X, Zhang E. β-Hydroxy-β-Methylbutyric Acid Promotes Repair of Sheep Myoblast Injury by Inhibiting IL-17/NF-κB Signaling. International Journal of Molecular Sciences. 2023; 24(1):444. https://doi.org/10.3390/ijms24010444
Chicago/Turabian StyleZheng, Juan, Bo Li, Yiting Yan, Xiaoyu Huang, and Enping Zhang. 2023. "β-Hydroxy-β-Methylbutyric Acid Promotes Repair of Sheep Myoblast Injury by Inhibiting IL-17/NF-κB Signaling" International Journal of Molecular Sciences 24, no. 1: 444. https://doi.org/10.3390/ijms24010444
APA StyleZheng, J., Li, B., Yan, Y., Huang, X., & Zhang, E. (2023). β-Hydroxy-β-Methylbutyric Acid Promotes Repair of Sheep Myoblast Injury by Inhibiting IL-17/NF-κB Signaling. International Journal of Molecular Sciences, 24(1), 444. https://doi.org/10.3390/ijms24010444







