Validation of Six Commercial Antibodies for the Detection of Heterologous and Endogenous TRPM8 Ion Channel Expression
Abstract
1. Introduction
2. Results
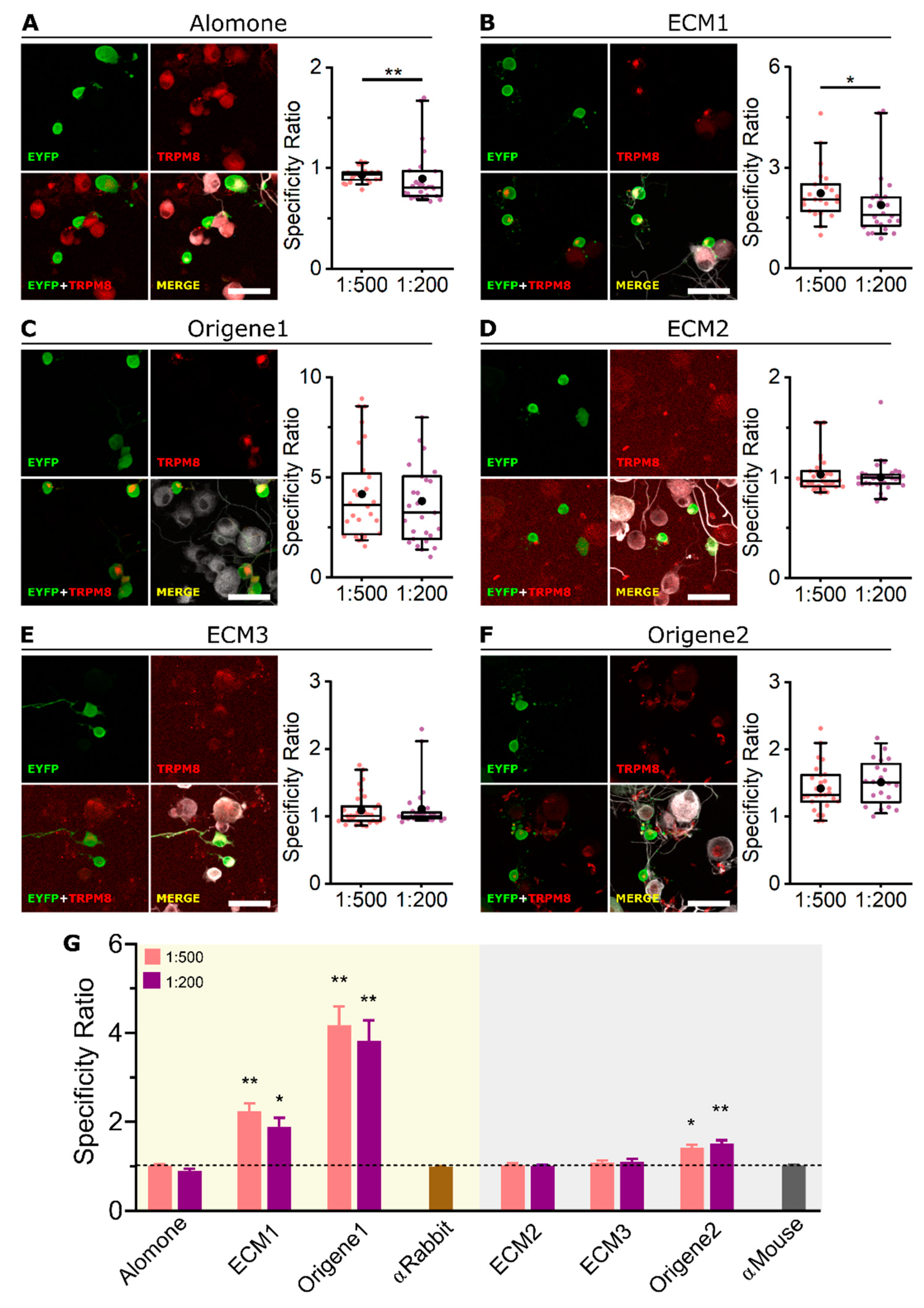
2.1. All Tested Antibodies Specifically Label Mouse TRPM8 Overexpressed in HEK-293 Cells
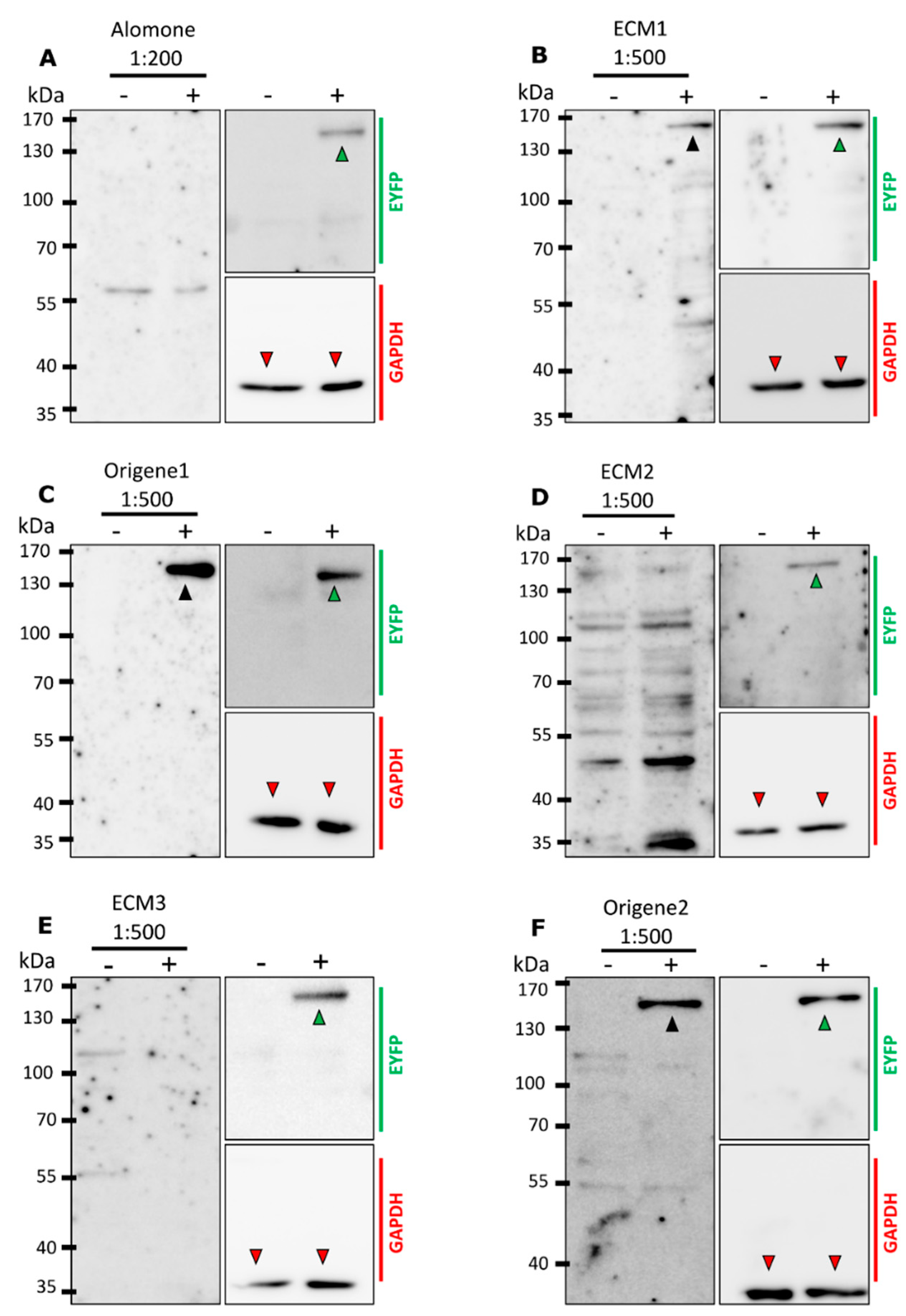
2.2. Not All Antibodies Detect mTRPM8 in Western Blotting
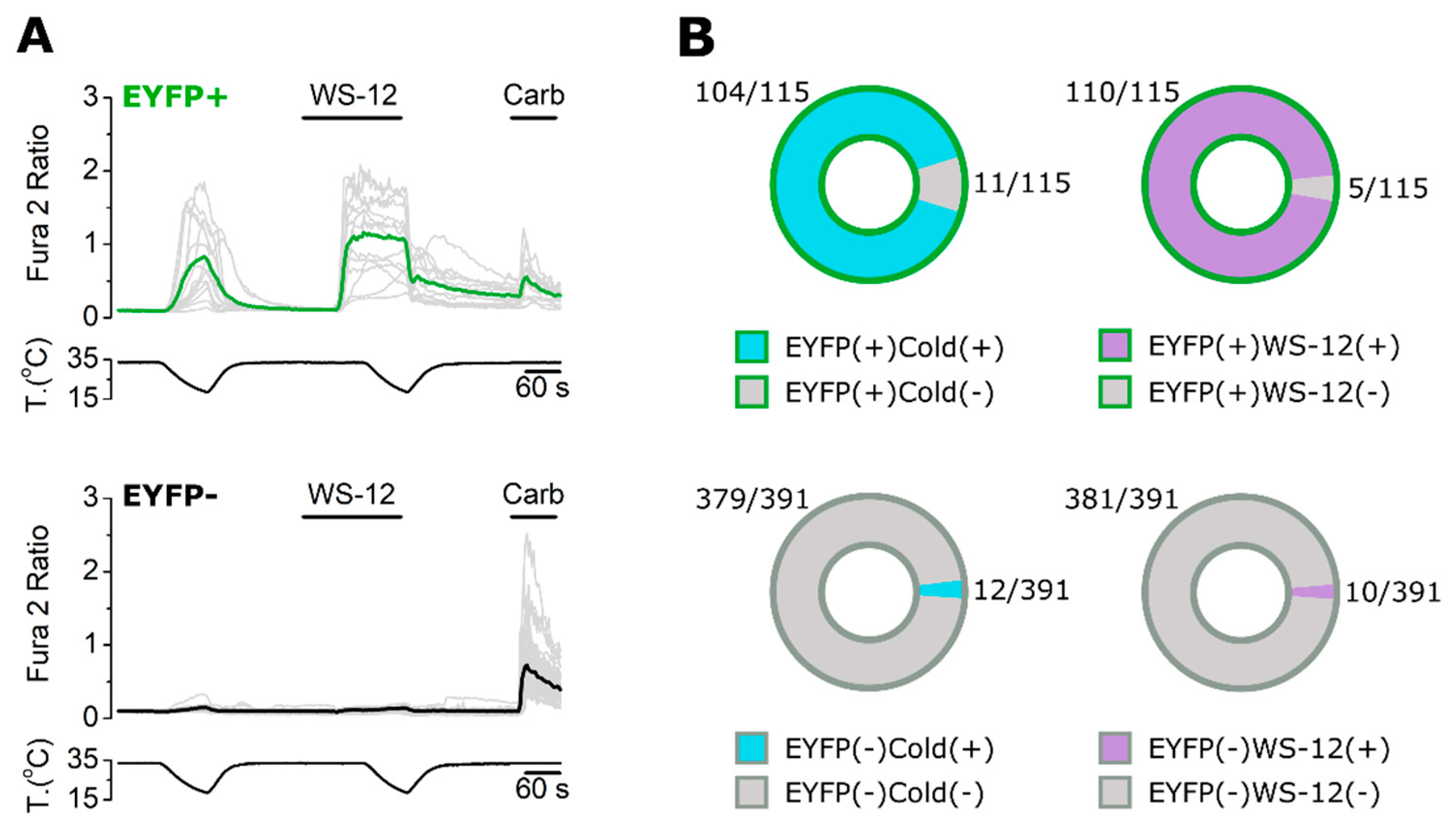
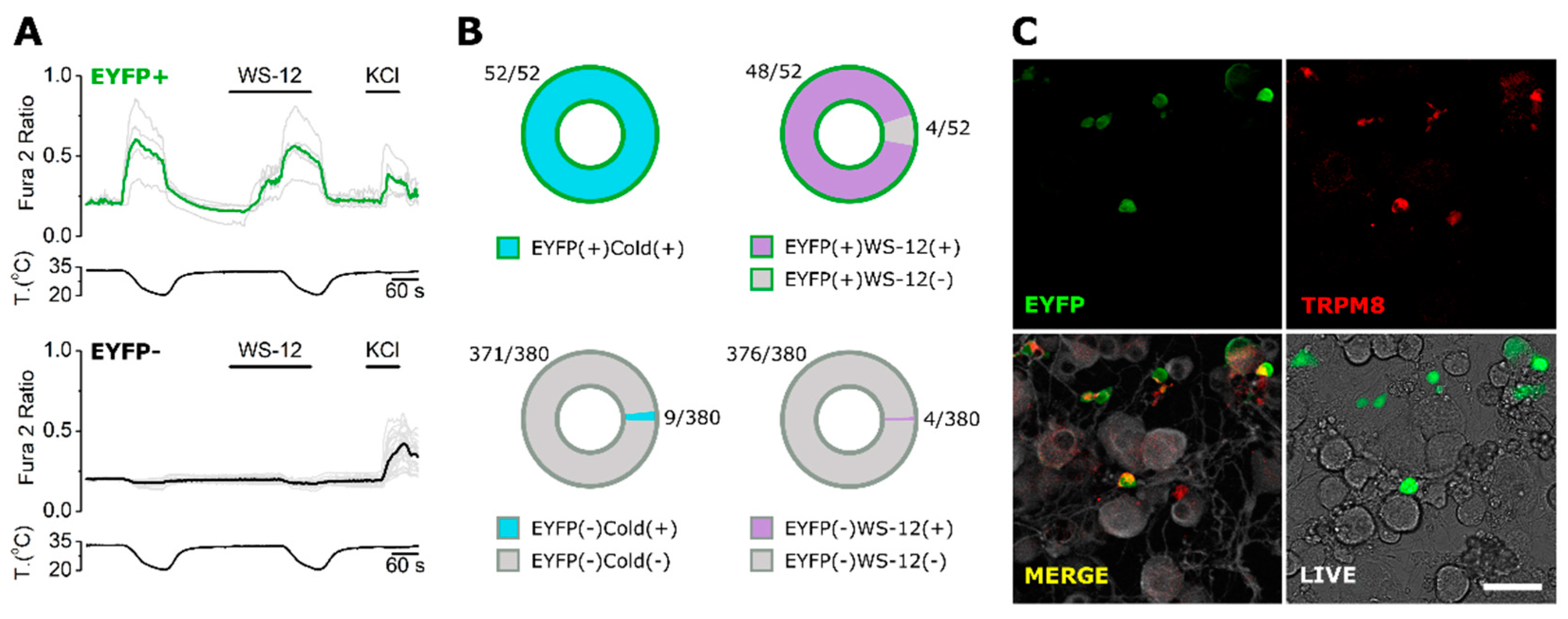
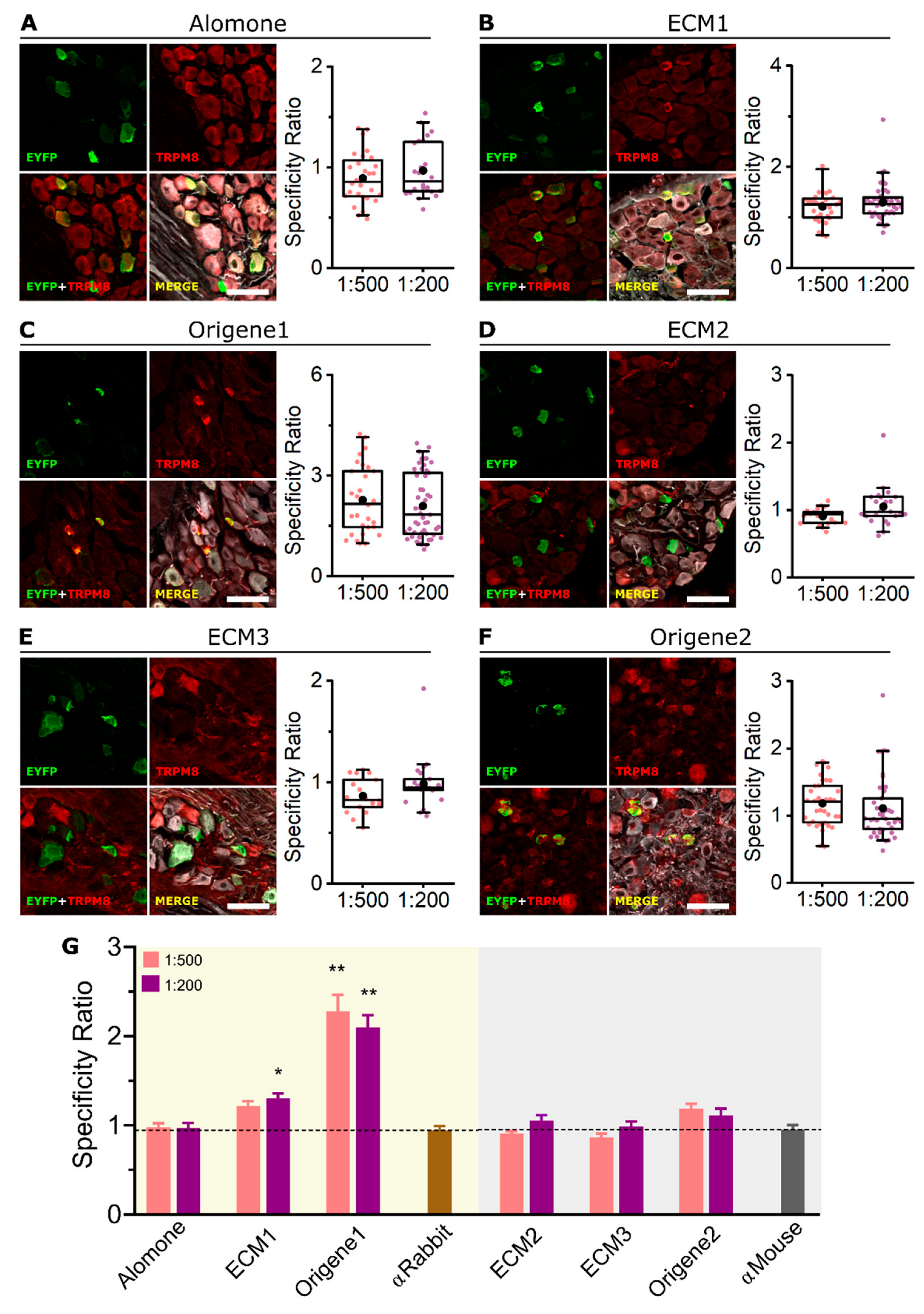
2.3. ECM1 and Origene1 Specifically Stain Endogenous TRPM8 in Mouse Dorsal Root Ganglion Sensory Neurons
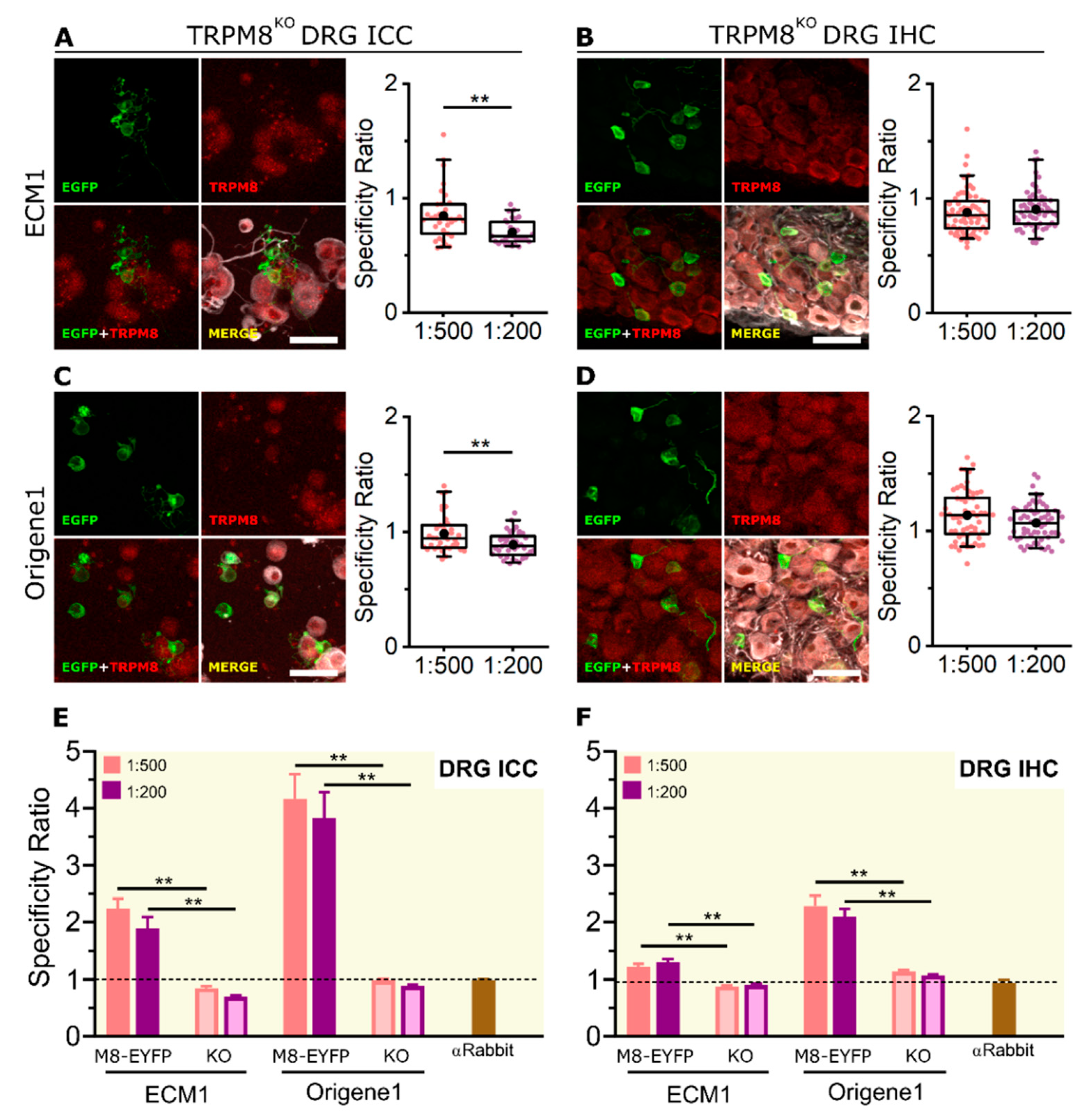
2.4. KO Validation of TRPM8 Antibody Specificity in KO Animals
3. Discussion
Limitations of the Study
4. Materials and Methods
4.1. Animals
Mouse Lines
4.2. Cell Line Culture and Transfection
4.3. Mouse DRG Extraction and Neuronal Culture
4.4. Calcium Microfluorometry
4.5. Western Blot
4.6. Immunocytochemistry
4.7. Immunohistochemistry
4.8. Image Analysis and Quantification
4.9. Image Display
4.10. Statistical Analysis
4.11. Antibodies Used in This Study
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peier, A.M.; Moqrich, A.; Hergarden, A.C.; Reeve, A.J.; Andersson, D.A.; Story, G.M.; Earley, T.J.; Dragoni, I.; McIntyre, P.; Bevan, S.; et al. A TRP Channel That Senses Cold Stimuli and Menthol. Cell 2002, 108, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Voets, T.; Owsianik, G.; Nilius, B. TRPM8. In Transient Receptor Potential (TRP) Channels; Handbook of Experimental Pharmacology; Flockerzi, V., Nilius, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; Volume 179, pp. 329–344. ISBN 978-3-540-34889-4. [Google Scholar]
- Sherkhelli, M.A.; Gisselmann, G.; Vogt-Eisele, A.K.; Doerner, J.F.; Hatt, H. Menthol Derivative WS-12 Selectively Activates Transient Receptor Potential Melastatin-8 (TRPM8) Ion Channels. Pak. J. Pharm. Sci. 2008, 21, 370–378. [Google Scholar]
- Almaraz, L.; Manenschijn, J.-A.; de la Peña, E.; Viana, F. TRPM8. Handb. Exp. Pharmacol. 2014, 222, 547–579. [Google Scholar] [CrossRef] [PubMed]
- McKemy, D.D.; Neuhausser, W.M.; Julius, D. Identification of a Cold Receptor Reveals a General Role for TRP Channels in Thermosensation. Nature 2002, 416, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Qin, F. Regulation of TRP Ion Channels by Phosphatidylinositol-4,5-Bisphosphate. Handb. Exp. Pharmacol. 2007, 179, 509–525. [Google Scholar] [CrossRef]
- Rohács, T.; Lopes, C.M.B.; Michailidis, I.; Logothetis, D.E. PI(4,5)P2 Regulates the Activation and Desensitization of TRPM8 Channels through the TRP Domain. Nat. Neurosci. 2005, 8, 626–634. [Google Scholar] [CrossRef]
- Vriens, J.; Nilius, B.; Voets, T. Peripheral Thermosensation in Mammals. Nat. Rev. Neurosci. 2014, 15, 573–589. [Google Scholar] [CrossRef]
- Parra, A.; Madrid, R.; Echevarria, D.; del Olmo, S.; Morenilla-Palao, C.; Acosta, M.C.; Gallar, J.; Dhaka, A.; Viana, F.; Belmonte, C. Ocular Surface Wetness Is Regulated by TRPM8-Dependent Cold Thermoreceptors of the Cornea. Nat. Med. 2010, 16, 1396–1399. [Google Scholar] [CrossRef]
- Robbins, A.; Kurose, M.; Winterson, B.J.; Meng, I.D. Menthol Activation of Corneal Cool Cells Induces TRPM8-Mediated Lacrimation but Not Nociceptive Responses in Rodents. Investig. Opthalmology Vis. Sci. 2012, 53, 7034. [Google Scholar] [CrossRef]
- Quallo, T.; Vastani, N.; Horridge, E.; Gentry, C.; Parra, A.; Moss, S.; Viana, F.; Belmonte, C.; Andersson, D.A.; Bevan, S. TRPM8 Is a Neuronal Osmosensor That Regulates Eye Blinking in Mice. Nat. Commun. 2015, 6, 7150. [Google Scholar] [CrossRef]
- Belmonte, C.; Gallar, J. Cold Thermoreceptors, Unexpected Players in Tear Production and Ocular Dryness Sensations. Investig. Opthalmology Vis. Sci. 2011, 52, 3888. [Google Scholar] [CrossRef] [PubMed]
- Belmonte, C.; Nichols, J.J.; Cox, S.M.; Brock, J.A.; Begley, C.G.; Bereiter, D.A.; Dartt, D.A.; Galor, A.; Hamrah, P.; Ivanusic, J.J.; et al. TFOS DEWS II Pain and Sensation Report. Ocul. Surf. 2017, 15, 404–437. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Yu, H.; Zhao, Z.; Luo, Z.; Chen, J.; Ni, Y.; Jin, R.; Ma, L.; Wang, P.; Zhu, Z.; et al. Activation of the Cold-Sensing TRPM8 Channel Triggers UCP1-Dependent Thermogenesis and Prevents Obesity. J. Mol. Cell Biol. 2012, 4, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Henström, M.; Hadizadeh, F.; Beyder, A.; Bonfiglio, F.; Zheng, T.; Assadi, G.; Rafter, J.; Bujanda, L.; Agreus, L.; Andreasson, A.; et al. TRPM8 Polymorphisms Associated with Increased Risk of IBS-C and IBS-M. Gut 2017, 66, 1725–1727. [Google Scholar] [CrossRef]
- Hosoya, T.; Matsumoto, K.; Tashima, K.; Nakamura, H.; Fujino, H.; Murayama, T.; Horie, S. TRPM8 Has a Key Role in Experimental Colitis-Induced Visceral Hyperalgesia in Mice. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2014, 26, 1112–1121. [Google Scholar] [CrossRef]
- Ramachandran, R.; Hyun, E.; Zhao, L.; Lapointe, T.K.; Chapman, K.; Hirota, C.L.; Ghosh, S.; McKemy, D.D.; Vergnolle, N.; Beck, P.L.; et al. TRPM8 Activation Attenuates Inflammatory Responses in Mouse Models of Colitis. Proc. Natl. Acad. Sci. USA. 2013, 110, 7476–7481. [Google Scholar] [CrossRef]
- Gardiner, J.C.; Kirkup, A.J.; Curry, J.; Humphreys, S.; O’Regan, P.; Postlethwaite, M.; Young, K.C.; Kitching, L.; Ethell, B.T.; Winpenny, D.; et al. The Role of TRPM8 in the Guinea-Pig Bladder-Cooling Reflex Investigated Using a Novel TRPM8 Antagonist. Eur. J. Pharmacol. 2014, 740, 398–409. [Google Scholar] [CrossRef]
- Uvin, P.; Franken, J.; Pinto, S.; Rietjens, R.; Grammet, L.; Deruyver, Y.; Alpizar, Y.A.; Talavera, K.; Vennekens, R.; Everaerts, W.; et al. Essential Role of Transient Receptor Potential M8 (TRPM8) in a Model of Acute Cold-Induced Urinary Urgency. Eur. Urol. 2015, 68, 655–661. [Google Scholar] [CrossRef]
- Andersson, K.-E.; Gratzke, C.; Hedlund, P. The Role of the Transient Receptor Potential (TRP) Superfamily of Cation-Selective Channels in the Management of the Overactive Bladder. BJU Int. 2010, 106, 1114–1127. [Google Scholar] [CrossRef]
- Mukerji, G.; Yiangou, Y.; Corcoran, S.L.; Selmer, I.S.; Smith, G.D.; Benham, C.D.; Bountra, C.; Agarwal, S.K.; Anand, P. Cool and Menthol Receptor TRPM8 in Human Urinary Bladder Disorders and Clinical Correlations. BMC Urol. 2006, 6, 6. [Google Scholar] [CrossRef]
- Ordás, P.; Hernández-Ortego, P.; Vara, H.; Fernández-Peña, C.; Reimúndez, A.; Morenilla-Palao, C.; Guadaño-Ferraz, A.; Gomis, A.; Hoon, M.; Viana, F.; et al. Expression of the Cold Thermoreceptor TRPM8 in Rodent Brain Thermoregulatory Circuits. J. Comp. Neurol. 2021, 529, 234–256. [Google Scholar] [CrossRef] [PubMed]
- Reimúndez, A.; Fernández-Peña, C.; Ordás, P.; Hernández-Ortego, P.; Gallego, R.; Morenilla-Palao, C.; Navarro, J.; Martín-Cora, F.; Pardo-Vázquez, J.L.; Schwarz, L.A.; et al. The Cold-Sensing Ion Channel TRPM8 Regulates Central and Peripheral Clockwork and the Circadian Oscillations of Body Temperature. Acta Physiol. 2022, e13896. [Google Scholar] [CrossRef] [PubMed]
- Valero, M.; Morenilla-Palao, C.; Belmonte, C.; Viana, F. Pharmacological and Functional Properties of TRPM8 Channels in Prostate Tumor Cells. Pflugers Arch. 2011, 461, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Dhaka, A.; Murray, A.N.; Mathur, J.; Earley, T.J.; Petrus, M.J.; Patapoutian, A. TRPM8 Is Required for Cold Sensation in Mice. Neuron 2007, 54, 371–378. [Google Scholar] [CrossRef]
- Takashima, Y.; Daniels, R.L.; Knowlton, W.; Teng, J.; Liman, E.R.; McKemy, D.D. Diversity in the Neural Circuitry of Cold Sensing Revealed by Genetic Axonal Labeling of Transient Receptor Potential Melastatin 8 Neurons. J. Neurosci. Off. J. Soc. Neurosci. 2007, 27, 14147–14157. [Google Scholar] [CrossRef]
- Kobayashi, K.; Fukuoka, T.; Obata, K.; Yamanaka, H.; Dai, Y.; Tokunaga, A.; Noguchi, K. Distinct Expression of TRPM8, TRPA1, and TRPV1 MRNAs in Rat Primary Afferent Neurons with Aδ/c-Fibers and Colocalization with Trk Receptors. J. Comp. Neurol. 2005, 493, 596–606. [Google Scholar] [CrossRef]
- Meissner, M.; Obmann, V.C.; Hoschke, M.; Link, S.; Jung, M.; Held, G.; Philipp, S.E.; Zimmermann, R.; Flockerzi, V. Lessons of Studying TRP Channels with Antibodies. In TRP Channels; Zhu, M.X., Ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2011; ISBN 978-1-4398-1860-2. [Google Scholar]
- Jemal, I.; Soriano, S.; Conte, A.L.; Morenilla, C.; Gomis, A. G Protein-Coupled Receptor Signalling Potentiates the Osmo-Mechanical Activation of TRPC5 Channels. Pflugers Arch. 2014, 466, 1635–1646. [Google Scholar] [CrossRef]
- Veliz, L.A.; Toro, C.A.; Vivar, J.P.; Arias, L.A.; Villegas, J.; Castro, M.A.; Brauchi, S. Near-Membrane Dynamics and Capture of TRPM8 Channels within Transient Confinement Domains. PLoS ONE 2010, 5, e13290. [Google Scholar] [CrossRef]
- Ghosh, D.; Pinto, S.; Danglot, L.; Vandewauw, I.; Segal, A.; Van Ranst, N.; Benoit, M.; Janssens, A.; Vennekens, R.; Vanden Berghe, P.; et al. VAMP7 Regulates Constitutive Membrane Incorporation of the Cold-Activated Channel TRPM8. Nat. Commun. 2016, 7, 10489. [Google Scholar] [CrossRef]
- Rivera, B.; Moreno, C.; Lavanderos, B.; Hwang, J.Y.; Fernández-Trillo, J.; Park, K.S.; Orio, P.; Viana, F.; Madrid, R.; Pertusa, M. Constitutive Phosphorylation as a Key Regulator of Trpm8 Channel Function. J. Neurosci. 2021, 41, 8475–8493. [Google Scholar] [CrossRef]
- Morenilla-Palao, C.; Luis, E.; Fernández-Peña, C.; Quintero, E.; Weaver, J.L.; Bayliss, D.A.; Viana, F. Ion Channel Profile of TRPM8 Cold Receptors Reveals a Role of TASK-3 Potassium Channels in Thermosensation. Cell Rep. 2014, 8, 1571–1582. [Google Scholar] [CrossRef]
- Cattoretti, G.; Pileri, S.; Parravicini, C.; Becker, M.H.; Poggi, S.; Bifulco, C.; Key, G.; D’Amato, L.; Sabattini, E.; Feudale, E. Antigen Unmasking on Formalin-Fixed, Paraffin-Embedded Tissue Sections. J. Pathol. 1993, 171, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Krenacs, L.; Krenacs, T.; Stelkovics, E.; Raffeld, M. Heat-Induced Antigen Retrieval for Immunohistochemical Reactions in Routinely Processed Paraffin Sections. Methods Mol. Biol. Clifton NJ 2010, 588, 103–119. [Google Scholar] [CrossRef]
- Bordeaux, J.; Welsh, A.W.; Agarwal, S.; Killiam, E.; Baquero, M.T.; Hanna, J.A.; Anagnostou, V.K.; Rimm, D.L. Antibody Validation. BioTechniques 2010, 48, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Burry, R.W. Controls for Immunocytochemistry: An Update. J. Histochem. Cytochem. 2011, 59, 6–12. [Google Scholar] [CrossRef]
- Möller, M.; Möser, C.V.; Weiß, U.; Niederberger, E. The Role of AlphαSynuclein in Mouse Models of Acute, Inflammatory and Neuropathic Pain. Cells 2022, 11, 1967. [Google Scholar] [CrossRef]
- Genovesi, S.; Moro, R.; Vignoli, B.; De Felice, D.; Canossa, M.; Montironi, R.; Carbone, F.G.; Barbareschi, M.; Lunardi, A.; Alaimo, A. Trpm8 Expression in Human and Mouse Castration Resistant Prostate Adenocarcinoma Paves the Way for the Preclinical Development of TRPM8-Based Targeted Therapies. Biomolecules 2022, 12, 193. [Google Scholar] [CrossRef]
- Thapa, D.; Barrett, B.; Argunhan, F.; Brain, S.D. Influence of Cold-TRP Receptors on Cold-Influenced Behaviour. Pharmaceuticals 2021, 15, 42. [Google Scholar] [CrossRef]
- Vinuela-Fernandez, I.; Sun, L.; Jerina, H.; Curtis, J.; Allchorne, A.; Gooding, H.; Rosie, R.; Holland, P.; Tas, B.; Mitchell, R.; et al. The TRPM8 Channel Forms a Complex with the 5-HT1B Receptor and Phospholipase D That Amplifies Its Reversal of Pain Hypersensitivity. Neuropharmacology 2014, 79, 136–151. [Google Scholar] [CrossRef]
- Gkika, D.; Lemonnier, L.; Shapovalov, G.; Gordienko, D.; Poux, C.; Bernardini, M.; Bokhobza, A.; Bidaux, G.; Degerny, C.; Verreman, K.; et al. TRP Channel-Associated Factors Are a Novel Protein Family That Regulates TRPM8 Trafficking and Activity. J. Cell Biol. 2015, 208, 89–107. [Google Scholar] [CrossRef]
- Yudin, Y.; Lutz, B.; Tao, Y.-X.; Rohacs, T. Phospholipase C Δ4 Regulates Cold Sensitivity in Mice. J. Physiol. 2016, 594, 3609–3628. [Google Scholar] [CrossRef] [PubMed]
- Chukyo, A.; Chiba, T.; Kambe, T.; Yamamoto, K.; Kawakami, K.; Taguchi, K.; Abe, K. Oxaliplatin-Induced Changes in Expression of Transient Receptor Potential Channels in the Dorsal Root Ganglion as a Neuropathic Mechanism for Cold Hypersensitivity. Neuropeptides 2018, 67, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Villalba-Riquelme, E.; de la Torre-Martínez, R.; Fernández-Carvajal, A.; Ferrer-Montiel, A. Paclitaxel in Vitro Reversibly Sensitizes the Excitability of IB4(-) and IB4(+) Sensory Neurons from Male and Female Rats. Br. J. Pharmacol. 2022, 179, 3693–3710. [Google Scholar] [CrossRef] [PubMed]
- Acharya, T.K.; Kumar, S.; Tiwari, N.; Ghosh, A.; Tiwari, A.; Pal, S.; Majhi, R.K.; Kumar, A.; Das, R.; Singh, A.; et al. TRPM8 Channel Inhibitor-Encapsulated Hydrogel as a Tunable Surface for Bone Tissue Engineering. Sci. Rep. 2021, 11, 3730. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.R.; Lee, J.Y.; Kim, H.B.; Lee, J.H.; Oh, S.B. TRPM8 Mediates Hyperosmotic Stimuli-Induced Nociception in Dental Afferents. J. Dent. Res. 2020, 99, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Majhi, R.K.; Saha, S.; Kumar, A.; Ghosh, A.; Swain, N.; Goswami, L.; Mohapatra, P.; Maity, A.; Kumar Sahoo, V.; Kumar, A.; et al. Expression of Temperature-Sensitive Ion Channel TRPM8 in Sperm Cells Correlates with Vertebrate Evolution. PeerJ 2015, 3, e1310. [Google Scholar] [CrossRef]
- Cornejo, V.H.; González, C.; Campos, M.; Vargas-Saturno, L.; de los Juricic, M.Á.; Miserey-Lenkei, S.; Pertusa, M.; Madrid, R.; Couve, A. Non-Conventional Axonal Organelles Control TRPM8 Ion Channel Trafficking and Peripheral Cold Sensing. Cell Rep. 2020, 30, 4505–4517.e5. [Google Scholar] [CrossRef]
- He, J.; Pham, T.L.; Kakazu, A.H.; Bazan, H.E.P. Remodeling of Substance P Sensory Nerves and Transient Receptor Potential Melastatin 8 (TRPM8) Cold Receptors After Corneal Experimental Surgery. Investig. Opthalmology Vis. Sci. 2019, 60, 2449. [Google Scholar] [CrossRef]
- Borowiec, A.; Sion, B.; Chalmel, F.; Rolland, A.D.; Lemonnier, L.; De Clerck, T.; Bokhobza, A.; Derouiche, S.; Dewailly, E.; Slomianny, C.; et al. Cold/Menthol TRPM8 Receptors Initiate the Cold-shock Response and Protect Germ Cells from Cold-shock–Induced Oxidation. FASEB J. 2016, 30, 3155–3170. [Google Scholar] [CrossRef]
- Ramos-Vara, J.A.; Miller, M.A. When Tissue Antigens and Antibodies Get Along: Revisiting the Technical Aspects of Immunohistochemistry—The Red, Brown, and Blue Technique. Vet. Pathol. 2014, 51, 42–87. [Google Scholar] [CrossRef]
- Dhaka, A.; Earley, T.J.; Watson, J.; Patapoutian, A. Visualizing Cold Spots: TRPM8-Expressing Sensory Neurons and Their Projections. J. Neurosci. 2008, 28, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Pertusa, M.; González, A.; Hardy, P.; Madrid, R.; Viana, F. Bidirectional Modulation of Thermal and Chemical Sensitivity of TRPM8 Channels by the Initial Region of the N-Terminal Domain. J. Biol. Chem. 2014, 289, 21828–21843. [Google Scholar] [CrossRef] [PubMed]
- Caires, R.; Luis, E.; Taberner, F.J.; Fernandez-Ballester, G.; Ferrer-Montiel, A.; Balazs, E.A.; Gomis, A.; Belmonte, C.; De La Peña, E. Hyaluronan Modulates TRPV1 Channel Opening, Reducing Peripheral Nociceptor Activity and Pain. Nat. Commun. 2015, 6, 8095. [Google Scholar] [CrossRef] [PubMed]

| Antibody | Host | HEK-293 + TRPM8-EYFP | Mouse DRG | ||
|---|---|---|---|---|---|
| WB | ICC | ICC | IHC | ||
| Alomone | Rabbit | − | ++ | − | − |
| ECM1 | Rabbit | +++ | +++ | ++ | ++ |
| Origene1 | Rabbit | +++ | +++ | +++ | +++ |
| ECM2 | Mouse | − | ++ | − | − |
| ECM3 | Mouse | − | ++ | − | − |
| Origene2 | Mouse | +++ | ++ | + | − |
| Target | Name in This Study | Company | Ref. | Species | Use | Immunogen | Dilutions Used |
|---|---|---|---|---|---|---|---|
| TRPM8 | Alomone | Alomone | ACC-049 | Rabbit | Primary | Human TRPM8 917–929. Polyclonal | 1:200 (WB, ICC, ICH) 1:500 (ICC, ICH) |
| TRPM8 | ECM1 | ECM biosciences | TP5701 | Rabbit | Primary | Human TRPM8 extracellular domain. Polyclonal | 1:200 (ICC, ICH) 1:500 (WB, ICC, ICH) |
| TRPM8 | ECM2 | ECM biosciences | TM5711 | Mouse | Primary | Human TRPM8 extracellular domain. Monoclonal | 1:200 (ICC, ICH) 1:500 (WB, ICC, ICH) |
| TRPM8 | ECM3 | ECM biosciences | TM5721 | Mouse | Primary | Human TRPM8 extracellular domain. Monoclonal | 1:200 (ICC, ICH) 1:500 (WB, ICC, ICH) |
| TRPM8 | Origene1 | OriGene | TA307827 | Rabbit | Primary | Human TRPM8 extracellular domain. Monoclonal | 1:200 (ICC, ICH) 1:500 (WB, ICC, ICH) |
| TRPM8 | Origene2 | OriGene | TA811228 | Mouse | Primary | Human 1–300. Monoclonal | 1:200 (ICC, ICH) 1:500 (WB, ICC, ICH) |
| GFP/YFP | EYFP | Abcam | ab13970 | Chicken | Primary | GFP. Polyclonal | 1:2000 (ICC, IHC) |
| GFP/YFP | EGFP | Invitrogen | A6455 | Rabbit | Primary | Isolated directly from Jellyfish Aequorea Victoria Polyclonal | 1:1000 (WB) |
| Class III β-tubulin | βIIItub-M | Biolegend | 801201 | Mouse | Primary | Rat brain microtubules. Monoclonal | 1:1000 (ICC, IHC) |
| Class III β-tubulin | βIIItub-R | Biolegend | 802001 | Rabbit | Primary | Rat brain microtubules. Polyclonal | 1:1000 (ICC, IHC) |
| GAPDH | GAPDH | Sigma | G9545 | Rabbit | Primary | Synthetic peptide: residues 314–333 of mouse GAPDH | 1:5000 (WB) |
| Mouse | Mouse HRP | Sigma | A9044 | Rabbit | Secondary-HRP | Rabbit IgG fraction antiserum | 1:4000 (WB) |
| Mouse | MouseA598 | Invitrogen | A11005 | Goat | Secondary-Alexa598 | Mouse gamma globulin heavy and light chains | 1:1000 (ICC, IHC) |
| Mouse | MouseA647 | Invitrogen | A21237 | Goat | Secondary-Alexa647 | Mouse gamma globulin heavy and light chains | 1:1000 (ICC, IHC) |
| Chicken | ChickenA488 | Jackson IR | 703-545-155 | Donkey | Secondary-Alexa488 | Chicken gamma globulin | 1:1000 (ICC, IHC) |
| Rabbit | Rabbit HRP | Sigma | A9169 | Goat | Secondary-HRP | Goat IgG | 1:4000 (WB) |
| Rabbit | RabbitA555 | Abcam | ab150062 | Donkey | Secondary-Alexa555 | N/A | 1:1000 (ICC, IHC) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Ortego, P.; Torres-Montero, R.; de la Peña, E.; Viana, F.; Fernández-Trillo, J. Validation of Six Commercial Antibodies for the Detection of Heterologous and Endogenous TRPM8 Ion Channel Expression. Int. J. Mol. Sci. 2022, 23, 16164. https://doi.org/10.3390/ijms232416164
Hernández-Ortego P, Torres-Montero R, de la Peña E, Viana F, Fernández-Trillo J. Validation of Six Commercial Antibodies for the Detection of Heterologous and Endogenous TRPM8 Ion Channel Expression. International Journal of Molecular Sciences. 2022; 23(24):16164. https://doi.org/10.3390/ijms232416164
Chicago/Turabian StyleHernández-Ortego, Pablo, Remedios Torres-Montero, Elvira de la Peña, Félix Viana, and Jorge Fernández-Trillo. 2022. "Validation of Six Commercial Antibodies for the Detection of Heterologous and Endogenous TRPM8 Ion Channel Expression" International Journal of Molecular Sciences 23, no. 24: 16164. https://doi.org/10.3390/ijms232416164
APA StyleHernández-Ortego, P., Torres-Montero, R., de la Peña, E., Viana, F., & Fernández-Trillo, J. (2022). Validation of Six Commercial Antibodies for the Detection of Heterologous and Endogenous TRPM8 Ion Channel Expression. International Journal of Molecular Sciences, 23(24), 16164. https://doi.org/10.3390/ijms232416164






